Submitted:
30 April 2024
Posted:
30 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
- magnesia (MgO) (supplied by Grecian Magnesite SA, Greece, with >94.0% purity),
- calcium hydroxide (Ca(OH)2) (supplied by CaO Hellas, with >90% purity),
- huntite (Ca.Mg3(CO3)4) (supplied by Sibelco Ellas, Greece, with >95% purity),
- ferric chloro-sulfate (FeClSO4) (supplied by Feri-Tri SA, Greece, with 12,5% w/w content), and
- sodium aluminate (NaAlO2) (supplied by Loufakis Chemicals S.A., Greece, with content 25%w/w as Al2O3).
- GEH (supplied by GEH Wasserchemie GmbH & Co.KG, Osnabrück, Germany), consisting mainly form akaganeite.
- Bayoxide (supplied by Lanxess, Cologne, Germany), consisting mainly of goethite.
- AquAsZero (supplied by Loufakis Chemicals S.A., Greece), consisting mainly of tetravalent manganese feroxyhyte.
- the organic resin PuroliteA200 EMBCL (supplied by Purolite).
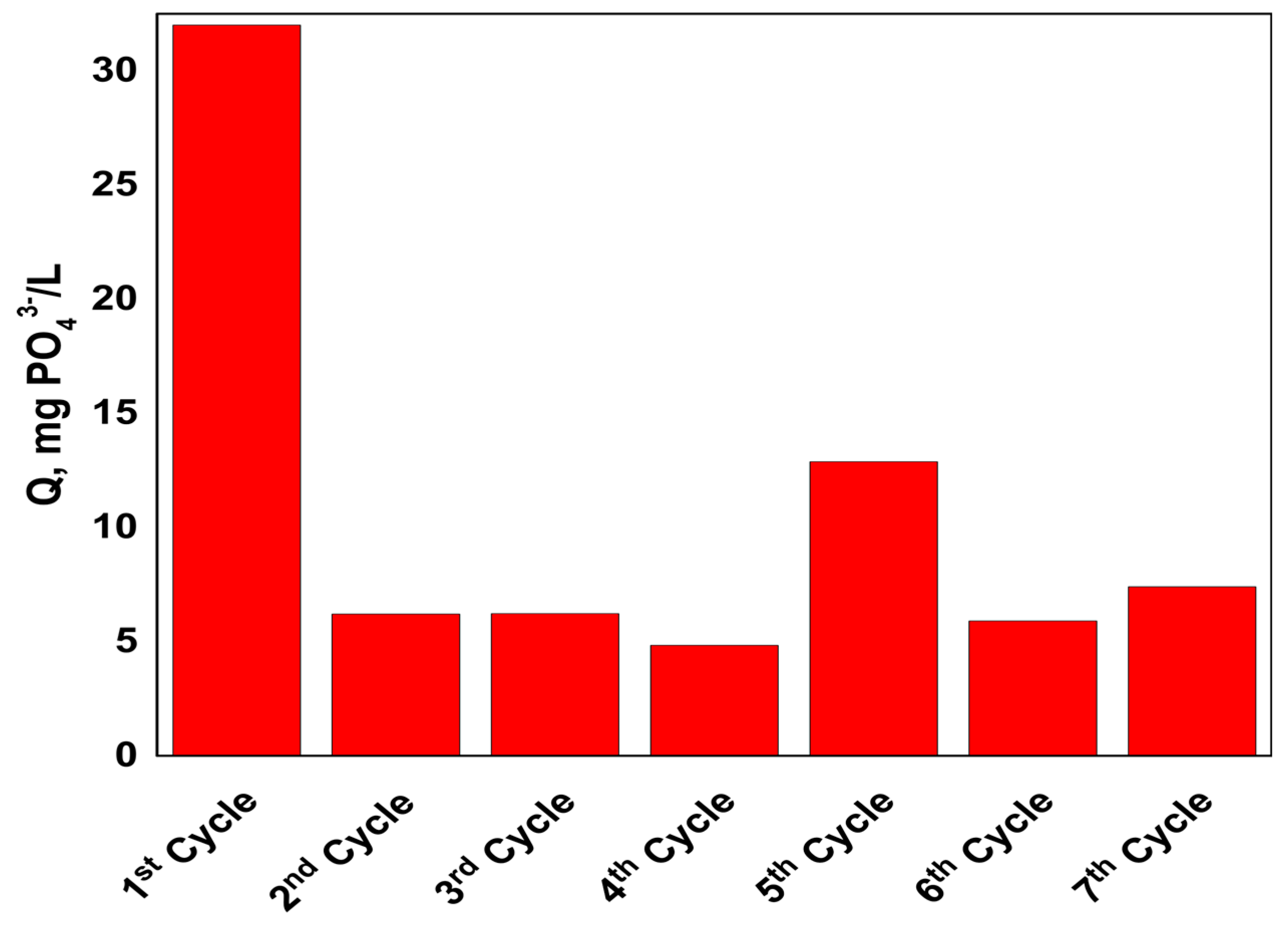
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johansson, K.; Perzon, M.; Fröling, M.; Mossakowska, A.; Svanström, M. Sewage sludge handling with phosphorus utilization – life cycle assessment of four alternatives, J. Clean. Prod. 2008, 16, 135–151. [Google Scholar] [CrossRef]
- Pradel, M.; Aissani, L. Environmental impacts of phosphorus recovery from a “product” Life Cycle Assessment perspective: Allocating burdens of wastewater treatment in the production of sludge-based phosphate fertilizers. Sci. Total Environ. 2019, 656, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Duan M, O'Dwyer E.; Stuckey D.C.; Guo M. Wastewater To Resource: Design of a Sustainable Phosphorus Recovery System. Chemistry Open 2019, 8, 1109–1120. [CrossRef] [PubMed]
- Linderholm, K.; Tillman, A.M.; Mattsson, J.E. Life cycle assessment of phosphorus alternatives for Swedish agriculture. Resour Conserv. Recycl. 2012, 66, 27–39. [Google Scholar] [CrossRef]
- Yan, H.; Shih, K. Effects of calcium and ferric ions on struvite precipitation: A new assessment based on quantitative X-ray diffraction analysis. Water Res. 2016, 95, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Kabdaşlı, I.; Kuşçuoğlu, S.; Tünay, O.; Siciliano, A. Assessment of K-Struvite Precipitation as a Means of Nutrient Recovery from Source Separated Human Urine. Sustainability 2022, 14, 1082. [Google Scholar] [CrossRef]
- Nijman, T.P.A.; Lemmens, M.; Lurling, M.; Kosten, S.; Welte, C.; Veraart, A.J. Phosphorus control and dredging decrease methane emissions from shallow lakes. Sci. Total Environ. 2022, 157584. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, R.; Ferreira, Q.; Rodrigues, G.C.; Oliveira, M. Nanofertilizer Use for Adaptation and Mitigation of the Agriculture/Climate Change Dichotomy Effects. Climate 2023, 11, 129. [Google Scholar] [CrossRef]
- Elser, J.; Baker, J.; Boyer, T.; Grieger, K.; Liu, T.; Muenich, R.; Rittmann, B.; Saha, A. Creating an alternative future for Earth’s phosphorus cycle in the Anthropocene via eco-prospecting, eco-mining, and eco-refining. Earth Systems and Environmental Sciences 2023. [Google Scholar] [CrossRef]
- Cordell, D.; Rosemarin, A.; Schröder, J.J.; Smit, A.L. Towards Global Phosphorus Security: A Systems Framework for Phosphorus Recovery and Reuse Options. Chemosphere 2011, 84, 747–758. [Google Scholar] [CrossRef]
- Nanda, M.; Kansal, A.; Cordell, D. Managing Agricultural Vulnerability to Phosphorus Scarcity through Bottom-up Assessment of Regional-Scale Opportunities. Agric. Syst. 2020, 184, 102910. [Google Scholar] [CrossRef]
- Guaya, D.; Cobos, H.; Camacho, J.; López, C.M.; Valderrama, C.; Cortina, J.L. LTA and FAU-X Iron-Enriched Zeolites: Use for Phosphate Removal from Aqueous Medium. Materials 2022, 15, 5418. [Google Scholar] [CrossRef] [PubMed]
- Jupp, A.; Beijer, S.; Narain, G.; Schipper, W.; Slootweg, J. Phosphorus recovery and recycling-closing the loop. Chem. Soc. Rev. 2021, 50, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Bergfeldt, B.; Tomasi Morgano, M.; Leibold, H.; Richter, F.; Stapf, D. Recovery of Phosphorus and other Nutrients during Pyrolysis of Chicken Manure. Agriculture 2018, 8, 187. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Wybrandt, L.; Løkkegaard, H.; Antonsen, S.B.; Christensen, M.L. Pilot-scale study for phosphorus recovery by sludge acidification and dewatering. Environ. Technol. (United Kingdom) 2020, 41, 22. [Google Scholar] [CrossRef] [PubMed]
- Hollas, C.E.; Bolsan, A.C.; Venturin, B.; Bonassa, G.; Tápparo, D.C.; Cândido, D.; Antes, F.G.; Vanotti, M.B.; Szögi, A.A.; Kunz, A. Second-Generation Phosphorus: Recovery from Wastes towards the Sustainability of Production Chains. Sustainability 2021, 13, 5919. [Google Scholar] [CrossRef]
- Krishnamoorthy, N.; Dey, B.; Unpaprom, Y.; Ramaraj, R.; Maniam, G.P.; Govindan, N.; Jayaraman, S.; Arunachalam, T.; Paramasivan, B. Engineering principles and process designs for phosphorus recovery as struvite: A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 105579. [Google Scholar] [CrossRef]
- Tang, S.; Liang, J.; Xu, X.; Jin, Y.; Xuan, W.; Li, O.; Fang, L.; Li, Z. Targeting phosphorus transformation to hydroxyapatite through sewage sludge pyrolysis boosted by quicklime toward phosphorus fertilizer alternative with toxic metals compromised. Renew. Sustain. Energy Rev. 2023, 183. [Google Scholar] [CrossRef]
- Pinelli, D.; Bovina, S.; Rubertelli, G.; Martinelli, A.; Guida, S.; Soares, A.; Frascari, D. Regeneration and modelling of a phosphorous removal and recovery hybrid ion exchange resin after long term operation with municipal wastewater. Chemosphere 2022, 286, 1331581. [Google Scholar] [CrossRef]
- ESPP, German Phosphorus Platform, Netherlands Nutrient Platform. Catalogue of phosphorus recovery technologies. Eur. Sustain. Phosphorus Platf. 2023. Available online: https://phosphorusplatform.eu/images/download/ESPP-NNP-DPP_nutrient-recovery_tech_catalogue.pdf (accessed on 31 March 2024).
- Zhu, F.; Cakmak, E. K.; Cetecioglu, Z. Phosphorus recovery for circular Economy: Application potential of feasible resources and engineering processes in Europe. J. Chem. Eng. 2023, 454, 140153. [Google Scholar] [CrossRef]
- Raptopoulou, C.; Kalaitzidou, K.; Tolkou, A.; Palasantza, P.-A.; Mitrakas, M.; Zouboulis, A. Phosphate Removal from Effluent of Secondary Wastewater Treatment: Characterization of Recovered Precipitates and Potential Re-use as Fertilizer. Waste Biomass Valorization 2016, 7, 850–860. [Google Scholar] [CrossRef]
- Raptopoulou, C.; Palasantza, P.A.; Mitrakas, M.; Kalaitzidou, K.; Tolkou, A.; Zouboulis, A. Statistical variation of nutrient concentrations and biological removal efficiency of a wastewater treatment plant. Water Util. J. 2016, 14, 5–17. [Google Scholar]
- Kalaitzidou, K.; Mitrakas, M.; Raptopoulou, C.; Tolkou, A.; Palasantza, P.-A.; Zouboulis, A. Pilot-Scale Phosphate Recovery from Secondary Wastewater Effluents. Environ. Process. 2016, 3, 5–22. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Zouboulis, A.; Mitrakas, M. Thermodynamic Study of Phosphate Adsorption and Removal from Water Using Iron Oxyhydroxides. Water 2022, 14, 1163. [Google Scholar] [CrossRef]
- Vu, M.T.; Duong, H.C.; Wang, Q.; Ansari, A.; Cai, Z.; Hoang, N.B.; Nghiem, L.D. Recent technological developments and challenges for phosphorus removal and recovery toward a circular economy. Environ. Technol. Innov. 2023, 30. [Google Scholar] [CrossRef]
- Lu, S.; Pei, L.; Bai, X. Study on method of domestic wastewater treatment through new-type multi-layer artificial wetland. Int. J. Hydrogen Energy 2015, 40, 11207–11214. [Google Scholar] [CrossRef]
- EEC Council Council Directive of 21 May 1991 concerning urban waste water treatment (91/271/EEC). Off. J. Eur. Communities 1991, L135.
- Kalaitzidou, K.; Tolkou, A.; Mitrakas, M.; Zouboulis, A. Phosphorus Removal from Aqueous Solutions and Wastewater samples by Chemical Precipitation. Proceedings of IWA Balkan young water professionals, Thessaloniki, Greece, 10-12 May 2015. [Google Scholar]
- Amy, G.; Chen, H.W.; Drizo, A.; von Gunten, U.; Brandhuber, P.; Hund, R.; Chowdhury, Z.; Kommineni, S.; Sinha, S.; Jekel, M.; Banerjee, K. Adsorbent Treatment Technologies for Arsenic Removal. AWWA Research Foundation and American Water Works Association, Washington D.C. 2005. [Google Scholar]
- Tresintsi, S.; Simeonidis, K.; Pliatsikas, N.; Vourlias, G.; Patsalas, P.; Mitrakas, M. The role of SO42- surface distribution in arsenic removal by iron oxy-hydroxides. J. Solid State Chem. 2014, 145–151. [Google Scholar] [CrossRef]
- Guo, Y.; Sanjaya, E.H.; Wang, T.; Rong, C.; Luo, Z.; Xue, Y.; Chen, H.; Li, Y.Y. The phosphorus harvest from low-temperature mainstream wastewater through iron phosphate crystallization in a pilot-scale partial nitritation/anammox reactor. Sci. Total Environ. 2023, 862, 160750. [Google Scholar] [CrossRef]
- Palasantza, P.-A.; Germanidis, G.; Tolkou, A.; Mitrakas, M.; Kalaitzidou, K.; Tsimpoukas, N.; Noula, K.; Zouboulis, A. Phosphorus Recovery from Effluent of WWTP in Greece: Design, Built and Operation of a Pilot Plant. In Proceedings of the IWA Specialists Conference on Nutrient removal and recovery: moving innovation into practice, Gdansk, Polland, 18-21 May 2015. [Google Scholar]
- Tolkou, A.; Raptopoulou, C.; Zouboulis, A.; Kalaitzidou, K.; Mitrakas, M.; Palasantza, P.-A.; Noula, K.; Christodoulou, K. Phosphorus recovery in wastewater treatment: moving from lab to pilot scale. In Proceedings of the World Congress on New Technologies (NewTech 2015), Barcelona, Spain, 15-17 July, 2015. [Google Scholar]
- Kalaitzidou, K.; Zouboulis, A.; Mitrakas, M. Cost evaluation for Se(IV) removal, by applying common drinking water treatment processes: Coagulation/precipitation or adsorption. J. Environ. Chem. Eng. 2020, 8, 104209. [Google Scholar] [CrossRef]
- Akinnaw, O.S. Eutrophication: Causes, consequences, physical, chemical and biological techniques for mitigation strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar] [CrossRef]
- Sahu, A.K.; Mir, S.; Nayak,Β.; Baitharu, I. In Water Resources Management for Rural Development; Elsevier, Chapter 13 - Sustainable management of eutrophication and problems associated with the algal toxin in ponds and lakes of rural areas 2024, 155-170. [CrossRef]
- Papp, L.A.; Cardinali-Rezende, J.; Júdice, W.A. de S.; Sanches, M.B.; Araújo, W.L. Total phosphorus contents currently found in the raw wastewater – Problems and technical solutions for its removal in full-scale wastewater treatment plants. Resour. Conserv. Recycl. 2023, 196, 107026. [Google Scholar] [CrossRef]
- Egle, L.; Rechberger, H.; Krampe, J.; Zessner, M. Phosphorus recovery from municipal wastewater: An integrated comparative technological, environmental and economic assessment of P recovery technologies. Sci. Total Environ. 2016, 571, 522–542. [Google Scholar] [CrossRef] [PubMed]
- Otieno, B.; Funani, C. K.; Khune, S.M.; Kabuba, J.; Osifo, P. Struvite recovery from anaerobically digested waste-activated sludge: A short review. J. Mater. Res. 2023, 38, 3815–3826. [Google Scholar] [CrossRef]
- Vali, N.; Combres, A.; Hosseinian, A.; Pettersson, A. The Effect of the Elemental Composition of Municipal Sewage Sludge on the Phosphorus Recycling during Pyrolysis, with a Focus on the Char Chemistry—Modeling and Experiments. Separations 2023, 10, 31. [Google Scholar] [CrossRef]
- Ottosen, L.M.; Kirkelund, G.M.; Jensen, P.E.; Pedersen, K.B. Extraction of Phosphorus from Sewage Sludge Ash—Influence of Process Variables on the Electrodialytic Process. Sustainability 2023, 15, 13953. [Google Scholar] [CrossRef]
- Fan, X.; Wu, Y.; He, Y.; Liu, H.; Guo, J.; Li, B.; Peng, H. Efficient removal of phosphorus by adsorption. Phosphorus Sulfur Silicon Relat. Elem. 2023, 198, 375–384. [Google Scholar] [CrossRef]
- Deng, L.; Dhar, B.R. Phosphorus recovery from wastewater via calcium phosphate precipitation: A critical review of methods, progress, and insights. Chemosphere 2023, 330, 138685. [Google Scholar] [CrossRef]
- Fu, J.; Lin, Z.; Zhao, P.; Wang, Y.; He, L.; Zhou, J. Establishment and efficiency analysis of a single-stage denitrifying phosphorus removal system treating secondary effluent. Bioresour. Technol. 2019, 288, 121520. [Google Scholar] [CrossRef]
- Zhang, C.; Guisasola, A.; Baeza, J.A. A review on the integration of mainstream P-recovery strategies with enhanced biological phosphorus removal. Water Res. 2022, 212, 118102. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.D.; He, S.; Su, D.; Abu Amr, S.S.; Toh, P.Y.; Bashir, M.J.K. Sewage Water Treatment Using Chlorella Vulgaris Microalgae for Simultaneous Nutrient Separation and Biomass Production. Separations 2023, 10, 229. [Google Scholar] [CrossRef]
- Chrispim, M.C.; Scholz, M.; Nolasco, M.A. Phosphorus recovery from municipal wastewater treatment: Critical review of challenges and opportunities for developing countries. J. Environ. Manag. 2019, 248, 109268. [Google Scholar] [CrossRef]
- Carrillo, V.; Fuentes, B.; Gómez, G.; Vidal, G. Characterization and recovery of phosphorus from wastewater by combined technologies. Rev. Environ. Sci. Biotechnol. 2020, 19, 389–418. [Google Scholar] [CrossRef]
- Petruzzelli, D.; Dell’Erba, A.; Liberti, L.; Notarnicola, M.; Sengupta, A.K. A phosphate-selective sorbent for the REM NUT® process: Field experience at Massafra Wastewater Treatment Plant. React. Funct. Polym. 2004, 60, 195–202. [Google Scholar] [CrossRef]
- Drenkova-Tuhtan, A.; Schneider, M.; Franzreb, M.; Meyer, C.; Gellermann, C.; Sextl, G.; Mandel, K.; Steinmetz, H. Pilot-scale removal and recovery of dissolved phosphate from secondary wastewater effluents with reusable ZnFeZr adsorbent @ Fe3O4/SiO2 particles with magnetic harvesting. Water Res. 2017, 109, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Guida, S.; Rubertelli, G.; Jefferson, B.; Soares, A. Demonstration of ion exchange technology for phosphorus removal and recovery from municipal wastewater. Chem. Eng. J. 2021, 420, 129913. [Google Scholar] [CrossRef]
- Cheng, M.; Shi, C.; Hao, L.; Wang, X.; Guo, X.; Liu, R.; Hao, X. Sustainable development of phosphorus recovery: From a product perspective. Sustain. Prod. Consum. 2023, 41, 275–290. [Google Scholar] [CrossRef]
- Ekman Burgman, L.; Wallsten, B. Should the Sludge Hit the Farm? – How Chemo-Social Relations Affect Policy Efforts to Circulate Phosphorus in Sweden. Sustain. Prod. Consum. 2021, 27, 1488–1497. [Google Scholar] [CrossRef]
- Slocombe, S. P.; Zúñiga-Burgos, T.; Chu, L. , Wood, N. J.; Camargo-Valero, M. A.; Baker, A. Fixing the Broken Phosphorus Cycle: Wastewater Remediation by Microalgal Polyphosphates. Front Plant Sci. 2020, 11, 982. [Google Scholar] [CrossRef]
- Geissler, B.; Hermann, L.; Mew, M.C.; Steiner, G. Striving Toward a Circular Economy for Phosphorus: The Role of Phosphate Rock Mining. Minerals 2018, 8, 395. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Pagona, E.; Zouboulis, A.; Mitrakas, M. Exploitation of the fine rejected run of mine (ROM 0–4 mm) material to produce refractories in combination with the mining by-products of magnesite mine. Mater. Chem. Phys. 2022, 292, 126743. [Google Scholar] [CrossRef]
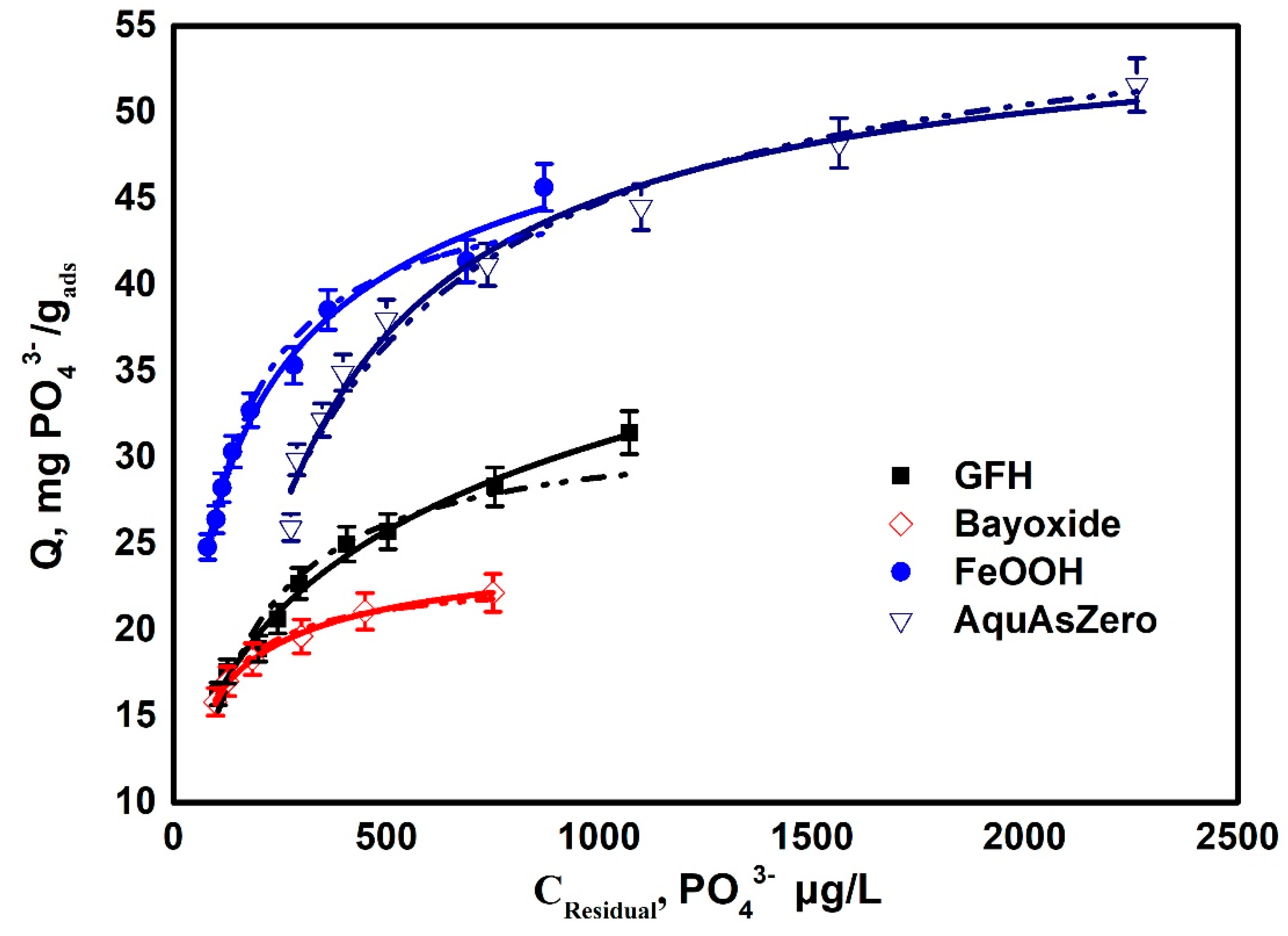
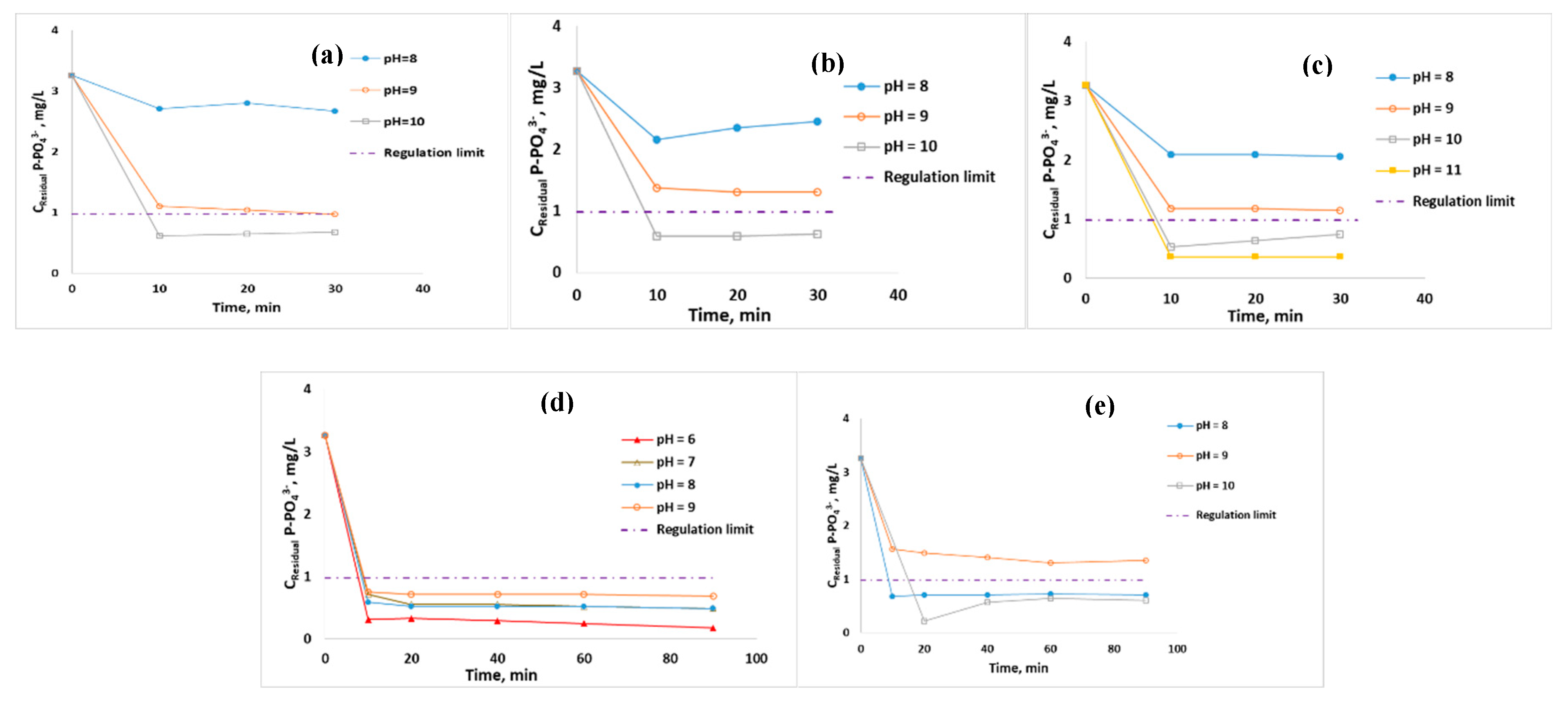
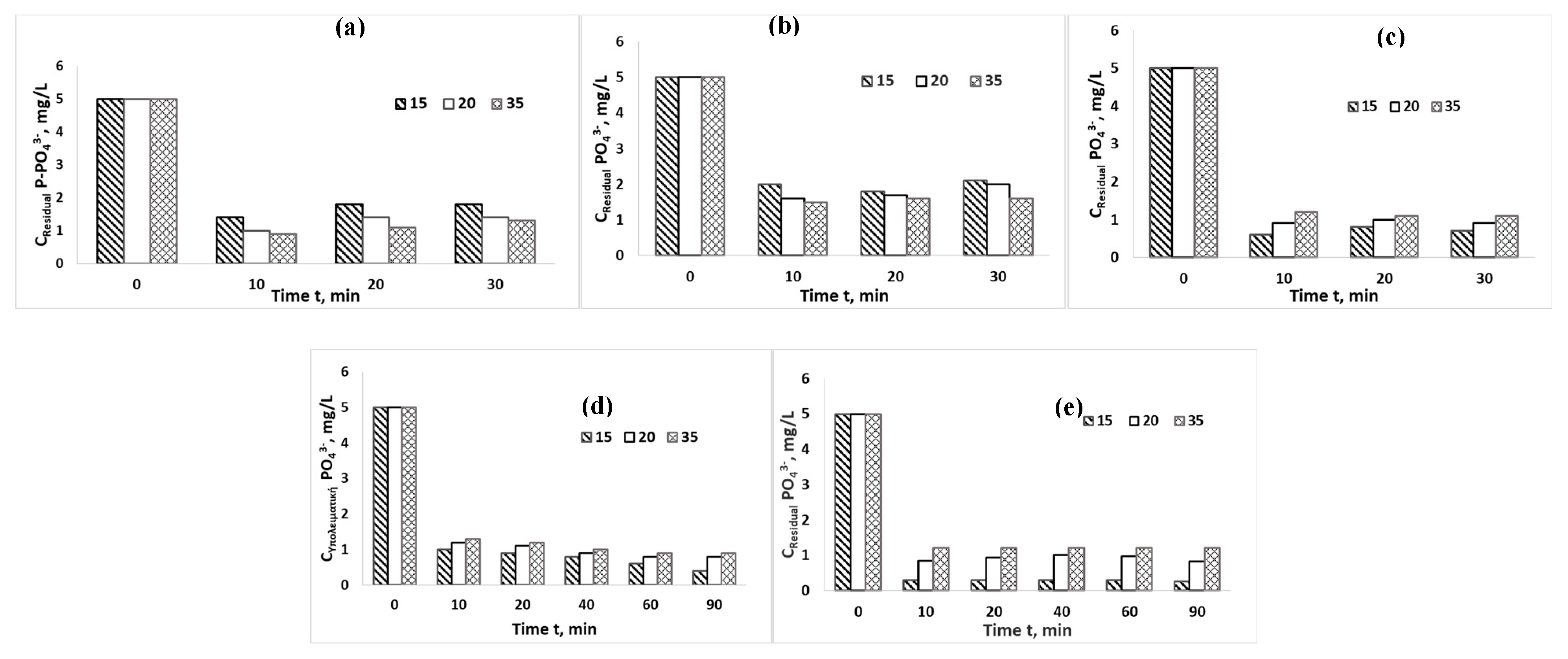
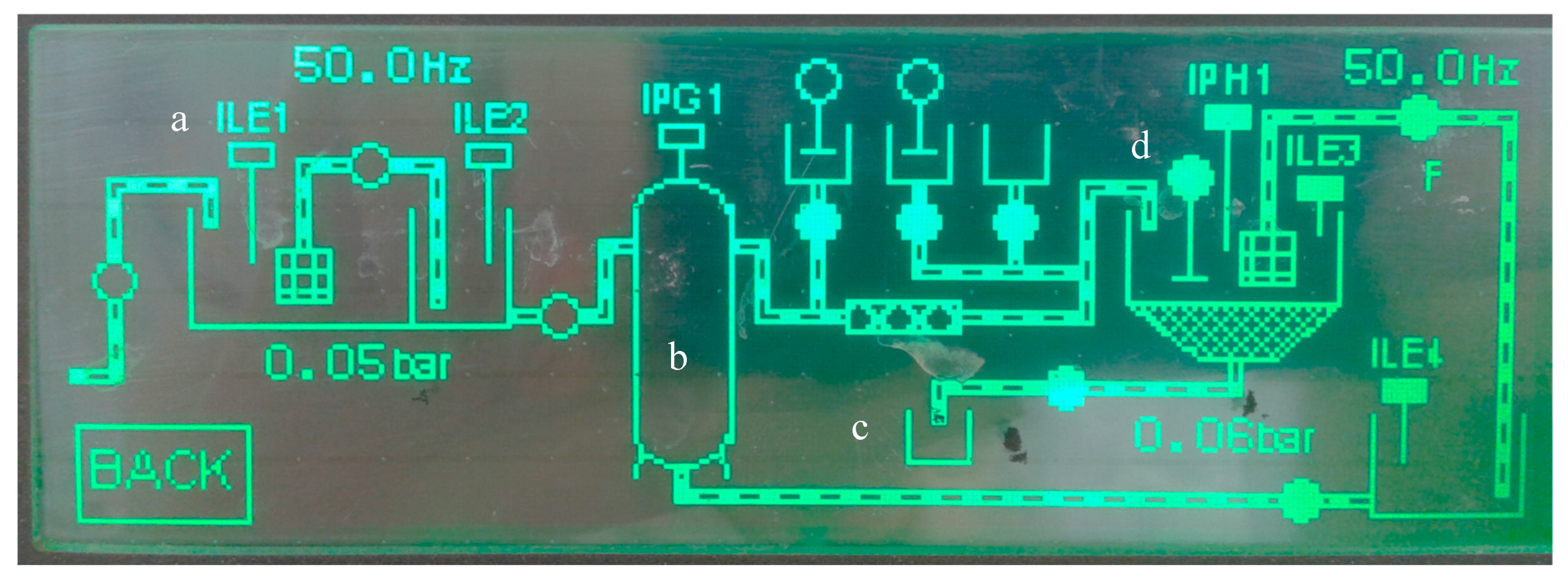

| Parameter | Potable | NSF Water | WWTP treated Effluent |
|---|---|---|---|
| pH | 7.5±0.1 | - | 7.4±0.3 |
| ConductivityμS/cm | 590±10 | - | 4400±200 |
| Hardness | 30±1 | - | 750±50 |
| HCO3-mg CaCO3/L | 341.6±5 | 138 | 500±50 |
| NO2- mg/L | ND | ND | 0.45±0.1 m |
| ΝH4+ mg/L | ND | ND | 0.6±0.2 |
| PO43- mg/L | 0.055 | 0.04 | 12-17 |
| Ca2+ mg/L | 50.2±2 | 40 | 120±10 |
| Mg2+ mg/L | 28.1±1 | 12.7 | 100±10 |
| Na+ mg/L | 8.1±0.5 | 88.8 | 585±10 |
| K+ mg/L | 2.4±0.5 | ND | 37±5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





