Submitted:
30 April 2024
Posted:
30 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Natural Occurrence of Resveratrol and Related Compounds
3. Anti-Tumoral Properties
3.1. Leukemia and Lymphoma
3.2. Glioma, Glioblastoma and Neuroblastoma
3.3. Meningioma
3.4. Estrogen-Dependent Pituitary Tumor
3.5. Breast Cancer
3.6. Hepatocellular Carcinoma
3.7. Pancreatic Carcinoma
3.8. Esophageal Carcinoma
3.9. Gastric Cancer
3.10. Colorectal Carcinoma
3.11. Lung Cancer
3.12. Bladder Cancer
3.13. Cervical Carcinoma
3.14. Ovarian Cancer
3.15. Osteosarcoma
3.16. Epidermoid Carcinoma and Melanoma
3.17. Oral Squamous Cell Carcinoma
3.18. Nasopharyngeal Cancer
3.19. Prostate Cancer
4. Neuroprotective Activity
4.1. Neurodegenerative Disorders
4.2. Chemical-Induced Neurotoxicity
4.3. Scopolamine-Induced Cognitive Impairment
4.4. Resveratrol against Brain Injury
5. Metabolic Disturbances
5.1. Metabolic Syndrome
5.2. Antidiabetic Properties
5.3. Hyperlipidemia, Hepatic Steatosis, and Synaptic Impairment
5.4. Anti-Obesity and Anti-Atherosclerotic Effects
5.5. Polycystic Ovary Syndrome
5.6. Anti-Aging Properties
6. Hepatoprotective Effect
6.1. Liver Ischemia–Reperfusion
6.2. Non-Alcoholic Fatty Liver Disease
6.3. Anti-Hepatotoxic Activity
7. Cardioprotective Properties
7.1. Coronary Heart Disease
7.2. Myocardial Ischemia–Reperfusion
7.3. Resveratrol as Vascular Protective Natural Compound
7.4. Resveratrol and Calcium Fructoborate Supplementation against Stable Angina Pectoris
8. Immunostimulatory Activity
9. Antioxidant Effect
10. Bone Protection
11. Wound Healing Properties
12. Anti-Inflammatory Effect
13. Antimicrobial Activity
14. Antiviral Properties
15. UHPLC–MS Analysis of trans-Resveratrol in Romanian Wines
15.1. Chemicals and Reagents
15.2. Calibration Curve for Trans-Resveratrol Quantification
15.3. Wine Samples
15.4. UHPLC–MS Analysis
16. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, G.; Kaur, R.; Sodhi, G.K.; George, N.; Rath, S.K.; Walia, H.K.; Dwibedi, V.; Saxena, S. Stilbenes: A journey from folklore to pharmaceutical innovation. Arch. Microbiol. 2024, 206, 229. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.Y.; Gao, Y.; Shahidi, F. Stilbenoids: Chemistry, occurrence, bioavailability and health effects – a review. J. Food Bioact. 2021, 13, 20–31. [Google Scholar] [CrossRef]
- Pawlus, A.D.; Waffo-Téguo, P.; Shaver, J.; Mérillon, J.M. Stilbenoid chemistry from wine and the genus Vitis, a review. OENO One 2012, 46, 57–111. [Google Scholar] [CrossRef]
- Faisal, Z.; Mazhar, A.; Batool, S.A.; Akram, N.; Hassan, M.; Khan, M.U.; Afzaal, M.; Hassan, U.U.; Shah, Y.A.; Desta, D.T. Exploring the multimodal health-promoting properties of resveratrol: A comprehensive review. Food Sci. Nutr. 2024, 12, 2240–2258. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Bahroudi, Z.; Shoorei, H.; Hussen, B.M.; Talebi, S.F.; Baig, S.G.; Taheri, M.; Ayatollahi, S.A. Disease-associated regulation of gene expression by resveratrol: Special focus on the PI3K/AKT signaling pathway. Cancer Cell Int. 2022, 22, 298. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G. Nonalcoholic compounds of wine: The phytoestrogen resveratrol and moderate red wine consumption during menopause. Drugs Exp. Clin. Res. 1999, 25, 111–114. [Google Scholar] [PubMed]
- Huang, S.; Qi, B.; Yang, L.; Wang, X.; Huang, J.; Zhao, Y.; Hu, Y.; Xiao, W. Phytoestrogens, novel dietary supplements for breast cancer. Biomed. Pharmacother. 2023, 160, 114341. [Google Scholar] [CrossRef]
- Gehm, B.D.; McAndrews, J.M.; Chien, P.Y.; Jameson, J.L. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. Natl. Acad. Sci. U. S. A. 1997, 94, 14138–14143. [Google Scholar] [CrossRef] [PubMed]
- Alamolhodaei, N.S.; Tsatsakis, A.M.; Ramezani, M.; Hayes, A.W.; Karimi, G. Resveratrol as MDR reversion molecule in breast cancer: An overview. Food Chem. Toxicol. 2017, 103, 223–232. [Google Scholar] [CrossRef]
- Visioli, F.; Panaite, S.A.; Tomé-Carneiro, J. Wine’s phenolic compounds and health: A Pythagorean view. Molecules 2020, 25, 4105. [Google Scholar] [CrossRef]
- Rodríguez-Vaquero, M.J.; Vallejo, C.V.; Aredes-Fernández, P.A. Antibacterial, antioxidant and antihypertensive properties of polyphenols from Argentinean red wines varieties. Open J. Pharmacol. Pharmacother. 2020, 5, 1–6. [Google Scholar] [CrossRef]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent advances on the anti-inflammatory and antioxidant properties of red grape polyphenols: In vitro and in vivo studies. Antioxidants (Basel) 2019, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.J.; Jyostna, D.; Krupadanam, G.L.D.; Srimannarayana, G. Phenanthrene and stilbenes from Pterolobium hexapetallum. Phytochemistry 1988, 27, 3625–3626. [Google Scholar] [CrossRef]
- Duca, A.; Sturza, A.; Moacă, E.A.; Negrea, M.; Lalescu, V.D.; Lungeanu, D.; Dehelean, C.A.; Muntean, D.M.; Alexa, E. Identification of resveratrol as bioactive compound of propolis from Western Romania and characterization of phenolic profile and antioxidant activity of ethanolic extracts. Molecules 2019, 24, 3368. [Google Scholar] [CrossRef]
- Sun, N.J.; Woo, S.H.; Cassady, J.M.; Snapka, R.M. DNA polymerase and topoisomerase II inhibitors from Psoralea corylifolia. J. Nat. Prod. 1998, 61, 362–366. [Google Scholar] [CrossRef]
- Fuloria, S.; Sekar, M.; Khattulanuar, F.S.; Gan, S.H.; Rani, N.N.I.M.; Ravi, S.; Subramaniyan, V.; Jeyabalan, S.; Begum, M.Y.; Chidambaram, K.; et al. Chemistry, biosynthesis and pharmacology of viniferin: Potential resveratrol-derived molecules for new drug discovery, development and therapy. Molecules 2022, 27, 5072. [Google Scholar] [CrossRef]
- Pezet, R.; Perret, C.; Jean-Denis, J.B.; Tabacchi, R.; Gindro, K.; Viret, O. Delta-viniferin, a resveratrol dehydrodimer: One of the major stilbenes synthesized by stressed grapevine leaves. J. Agric. Food Chem. 2003, 51, 5488–5492. [Google Scholar] [CrossRef]
- He, S.; Jiang, L.; Wu, B.; Pan, Y.; Sun, C. Pallidol, a resveratrol dimer from red wine, is a selective singlet oxygen quencher. Biochem. Biophys. Res. Commun. 2009, 379, 283–287. [Google Scholar] [CrossRef]
- Guebailia, H.A.; Chira, K.; Richard, T.; Mabrouk, T.; Furiga, A.; Vitrac, X.; Monti, J.P.; Delaunay, J.C.; Mérillon, J.M. Hopeaphenol: The first resveratrol tetramer in wines from North Africa. J. Agric. Food Chem. 2006, 54, 9559–9564. [Google Scholar] [CrossRef]
- Aziz, M.H.; Kumar, R.; Ahmad, N. Cancer chemoprevention by resveratrol: In vitro and in vivo studies and the underlying mechanisms (review). Int. J. Oncol. 2003, 23, 17–28. [Google Scholar] [CrossRef]
- Clément, M.V.; Hirpara, J.L.; Chawdhury, S.H.; Pervaiz, S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood 1998, 92, 996–1002. [Google Scholar] [CrossRef]
- Fontecave, M.; Lepoivre, M.; Elleingand, E.; Gerez, C.; Guittet, O. Resveratrol, a remarkable inhibitor of ribonucleotide reductase. FEBS Lett. 1998, 421, 277–279. [Google Scholar] [CrossRef]
- Surh, Y.J.; Hurh, Y.J.; Kang, J.Y.; Lee, E.; Kong, G.; Lee, S.J. Resveratrol, an antioxidant present in red wine, induces apoptosis in human promyelocytic leukemia (HL-60) cells. Cancer Lett. 1999, 140, 1–10. [Google Scholar] [CrossRef]
- Bernhard, D.; Tinhofer, I.; Tonko, M.; Hübl, H.; Ausserlechner, M.J.; Greil, R.; Kofler, R.; Csordas, A. Resveratrol causes arrest in the S-phase prior to Fas-independent apoptosis in CEM-C7H2 acute leukemia cells. Cell Death Differ. 2000, 7, 834–842. [Google Scholar] [CrossRef]
- Park, J.W.; Choi, Y.J.; Jang, M.A.; Lee, Y.S.; Jun, D.Y.; Suh, S.I.; Baek, W.K.; Suh, M.H.; Jin, I.N.; Kwon, T.K. Chemopreventive agent resveratrol, a natural product derived from grapes, reversibly inhibits progression through S and G2 phases of the cell cycle in U937 cells. Cancer Lett. 2001, 163, 43–49. [Google Scholar] [CrossRef]
- Tsan, M.F.; White, J.E.; Maheshwari, J.G.; Bremner, T.A.; Sacco, J. Resveratrol induces Fas signalling-independent apoptosis in THP-1 human monocytic leukaemia cells. Br. J. Haematol. 2000, 109, 405–412. [Google Scholar] [CrossRef]
- Wieder, T.; Prokop, A.; Bagci, B.; Essmann, F.; Bernicke, D.; Schulze-Osthoff, K.; Dörken, B.; Schmalz, H.G.; Daniel, P.T.; Henze, G. Piceatannol, a hydroxylated analog of the chemopreventive agent resveratrol, is a potent inducer of apoptosis in the lymphoma cell line BJAB and in primary, leukemic lymphoblasts. Leukemia 2001, 15, 1735–1742. [Google Scholar] [CrossRef]
- Billard, C.; Izard, J.C.; Roman, V.; Kern, C.; Mathiot, C.; Mentz, F.; Kolb, J.P. Comparative antiproliferative and apoptotic effects of resveratrol, ε-viniferin and vine-shots derived polyphenols (vineatrols) on chronic B lymphocytic leukemia cells and normal human lymphocytes. Leuk. Lymphoma 2002, 43, 1991–2002. [Google Scholar] [CrossRef]
- Su, J.L.; Lin, M.T.; Hong, C.C.; Chang, C.C.; Shiah, S.G.; Wu, C.W.; Chen, S.T.; Chau, Y.P.; Kuo, M.L. Resveratrol induces FasL-related apoptosis through Cdc42 activation of ASK1/JNK-dependent signaling pathway in human leukemia HL-60 cells. Carcinogenesis 2005, 26, 1–10. [Google Scholar] [CrossRef]
- Ahmad, K.A.; Clement, M.V.; Hanif, I.M.; Pervaiz, S. Resveratrol inhibits drug-induced apoptosis in human leukemia cells by creating an intracellular milieu nonpermissive for death execution. Cancer Res. 2004, 64, 1452–1459. [Google Scholar] [CrossRef]
- Baatout, S.; Derradji, H.; Jacquet, P.; Ooms, D.; Michaux, A.; Mergeay, M. Enhanced radiation-induced apoptosis of cancer cell lines after treatment with resveratrol. Int. J. Mol. Med. 2004, 13, 895–902. [Google Scholar] [CrossRef]
- Lin, H.Y.; Tang, H.Y.; Davis, F.B.; Davis, P.J. Resveratrol and apoptosis. Ann. N. Y. Acad. Sci. 2011, 1215, 79–88. [Google Scholar] [CrossRef]
- Quoc Trung, L.; Espinoza, J.L.; Takami, A.; Nakao, S. Resveratrol induces cell cycle arrest and apoptosis in malignant NK cells via JAK2/STAT3 pathway inhibition. PLoS One 2013, 8, e55183. [Google Scholar] [CrossRef]
- Takashina, M.; Inoue, S.; Tomihara, K.; Tomita, K.; Hattori, K.; Zhao, Q.L.; Suzuki, T.; Noguchi, M.; Ohashi, W.; Hattori, Y. Different effect of resveratrol to induction of apoptosis depending on the type of human cancer cells. Int. J. Oncol. 2017, 50, 787–797. [Google Scholar] [CrossRef]
- Liontas, A.; Yeger, H. Curcumin and resveratrol induce apoptosis and nuclear translocation and activation of p53 in human neuroblastoma. Anticancer Res. 2004, 24, 987–998. [Google Scholar] [PubMed]
- Chen, Y.; Tseng, S.H.; Lai, H.S.; Chen, W.J. Resveratrol-induced cellular apoptosis and cell cycle arrest in neuroblastoma cells and antitumor effects on neuroblastoma in mice. Surgery 2004, 136, 57–66. [Google Scholar] [CrossRef]
- Tseng, S.H.; Lin, S.M.; Chen, J.C.; Su, Y.H.; Huang, H.Y.; Chen, C.K.; Lin, P.Y.; Chen, Y. Resveratrol suppresses the angiogenesis and tumor growth of gliomas in rats. Clin. Cancer Res. 2004, 10, 2190–2202. [Google Scholar] [CrossRef]
- Rahman, M.A.; Kim, N.H.; Kim, S.H.; Oh, S.M.; Huh, S.O. Antiproliferative and cytotoxic effects of resveratrol in mitochondria-mediated apoptosis in rat b103 neuroblastoma cells. Korean J. Physiol. Pharmacol. 2012, 16, 321–326. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, L.; Kuo, J.; Kuo, K.; Gautam, S.C.; Groc, L.; Rodriguez, A.I.; Koubi, D.; Hunter, T.J.; Corcoran, G.B.; et al. Resveratrol-induced apoptotic death in human U251 glioma cells. Mol. Cancer Ther. 2005, 4, 554–561. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Zhu, K.; Song, L.; Tao, M.; Huang, P.; Pan, Y. Resveratrol inhibits glioma cell growth via targeting LRIG1. J. BUON 2018, 23, 403–409. [Google Scholar]
- Yamamoto, M.; Suzuki, S.O.; Himeno, M. Resveratrol-induced autophagy in human U373 glioma cells. Oncol. Lett. 2010, 1, 489–493. [Google Scholar] [CrossRef]
- Zhang, W.; Murao, K.; Zhang, X.; Matsumoto, K.; Diah, S.; Okada, M.; Miyake, K.; Kawai, N.; Fei, Z.; Tamiya, T. Resveratrol represses YKL-40 expression in human glioma U87 cells. BMC Cancer 2010, 10, 593. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, H.; Liu, Y.; Guo, A.; Xu, X.; Qu, X.; Wang, S.; Zhao, J.; Li, Y.; Cao, Y. Resveratrol inhibits the invasion of glioblastoma-initiating cells via down-regulation of the PI3K/Akt/NF-κB signaling pathway. Nutrients 2015, 7, 4383–4402. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, Q.; Tang, Y.; Wang, C.; Wang, S.; Yu, D.; Hou, S.; Wang, Y.; Zhang, L.; Lin, N. Resveratrol ameliorates glioblastoma inflammatory response by reducing NLRP3 inflammasome activation through inhibition of the JAK2/STAT3 pathway. J. Cancer Res. Clin. Oncol. 2024, 150, 168. [Google Scholar] [CrossRef]
- Hu, S.A.; Wei, W.; Yuan, J.; Cheng, J. Resveratrol inhibits proliferation in HBL-52 meningioma cells. Onco Targets Ther. 2019, 12, 11579–11586. [Google Scholar] [CrossRef]
- Stahl, S.; Chun, T.Y.; Gray, W.G. Phytoestrogens act as estrogen agonists in an estrogen-responsive pituitary cell line. Toxicol. Appl. Pharmacol. 1998, 152, 41–48. [Google Scholar] [CrossRef]
- Behroozaghdam, M.; Dehghani, M.; Zabolian, A.; Kamali, D.; Javanshir, S.; Hasani Sadi, F.; Hashemi, M.; Tabari, T.; Rashidi, M.; Mirzaei, S.; et al. Resveratrol in breast cancer treatment: From cellular effects to molecular mechanisms of action. Cell. Mol. Life Sci. 2022, 79, 539. [Google Scholar] [CrossRef]
- Quarta, A.; Gaballo, A.; Pradhan, B.; Patra, S.; Jena, M.; Ragusa, A. Beneficial oxidative stress-related trans-resveratrol effects in the treatment and prevention of breast cancer. Appl. Sci. 2021, 11, 11041. [Google Scholar] [CrossRef]
- Gu, Y.; Fei, Z. Mesoporous silica nanoparticles loaded with resveratrol are used for targeted breast cancer therapy. J. Oncol. 2022, 2022, 8471331. [Google Scholar] [CrossRef]
- Khazaei, M.R.; Bozorgi, M.; Khazaei, M.; Aftabi, M.; Bozorgi, A. Resveratrol nanoformulation inhibits invasive breast cancer cell growth through autophagy induction: An in vitro study. Cell J. 2024, 26, 112–120. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Chen, T.; Guo, W.; Bao, X.; Wang, D.; Ren, B.; Wang, H.; Li, Y.; Wang, Y.; et al. Anticancer effects of resveratrol-loaded solid lipid nanoparticles on human breast cancer cells. Molecules 2017, 22, 1814. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Song, C.; Zhang, J.; Zhao, X. Targeted delivery and apoptosis induction activity of peptide-transferrin targeted mesoporous silica encapsulated resveratrol in MCF-7 cells. J. Pharm. Pharmacol. 2023, 75, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.P.L.; Pezzuto, J.M. Cancer chemopreventive activity of resveratrol. Ann. N. Y. Acad. Sci. 2002, 957, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Kurzava Kendall, L.; Ma, Y.; Yang, T.; Lubecka, K.; Stefanska, B. Epigenetic effects of resveratrol on oncogenic signaling in breast cancer. Nutrients 2024, 16, 699. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Choi, B.T.; Lee, Y.T.; Park, D.I.; Rhee, S.H.; Park, K.Y.; Choi, Y.H. Resveratrol inhibits cell proliferation and induces apoptosis of human breast carcinoma MCF-7 cells. Oncol. Rep. 2004, 11, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Laux, M.T.; Aregullin, M.; Berry, J.P.; Flanders, J.A.; Rodriguez, E. Identification of a p53-dependent pathway in the induction of apoptosis of human breast cancer cells by the natural product, resveratrol. J. Altern. Complement. Med. 2004, 10, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.Y.; Chiang, E.P.I.; Sun, Y.C. Resveratrol inhibits heregulin-β1-mediated matrix metalloproteinase-9 expression and cell invasion in human breast cancer cells. J. Nutr. Biochem. 2008, 19, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, Q.M.; Lu, Y.Y.; Zhang, H.; Chen, Q.L.; Zhao, M.; Su, S.B. Resveratrol inhibits the migration and metastasis of MDA-MB-231 human breast cancer by reversing TGF-β1-induced epithelial–mesenchymal transition. Molecules 2019, 24, 1131. [Google Scholar] [CrossRef] [PubMed]
- da Costa, P.S.; Ramos, P.S.; Ferreira, C.; Silva, J.L.; El-Bacha, T.; Fialho, E. Pro-oxidant effect of resveratrol on human breast cancer MCF-7 cells is associated with CK2 inhibition. Nutr. Cancer 2022, 74, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Karabekir, S.C.; Özgörgülü, A. Possible protective effects of resveratrol in hepatocellular carcinoma. Iran. J. Basic Med. Sci. 2020, 23, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Pan, C.; Zhao, S.; Wang, Z.; Zhang, H.; Wu, W. Resveratrol inhibits tumor necrosis factor-alpha-mediated matrix metalloproteinase-9 expression and invasion of human hepatocellular carcinoma cells. Biomed. Pharmacother. 2008, 62, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Chong, S.A.; Nam, M.J. Resveratrol induces apoptosis in human SK-HEP-1 hepatic cancer cells. Cancer Genomics Proteomics 2009, 6, 263–268. [Google Scholar]
- Moghadam, D.; Zarei, R.; Vakili, S.; Ghojoghi, R.; Zarezade, V.; Veisi, A.; Sabaghan, M.; Azadbakht, O.; Behrouj, H. The effect of natural polyphenols Resveratrol, Gallic acid, and Kuromanin chloride on human telomerase reverse transcriptase (hTERT) expression in HepG2 hepatocellular carcinoma: Role of SIRT1/Nrf2 signaling pathway and oxidative stress. Mol. Biol. Rep. 2023, 50, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna, M.; Adamiec-Organisciok, M.; Hudy, D.; Dziedzic, A.; Los, L.; Skladany, L.; Grgurevic, I.; Filipec-Kanizaj, T.; Jagodzinski, M.; Kukla, M.; et al. Hepatocellular cancer cell lines, Hep-3B and Hep-G2 display the pleiotropic response to resveratrol and berberine. Adv. Med. Sci. 2022, 67, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zong, L.; Chen, X.; Jiang, Z.; Nan, L.; Li, J.; Duan, W.; Lei, J.; Zhang, L.; Ma, J.; et al. Resveratrol in the treatment of pancreatic cancer. Ann. N. Y. Acad. Sci. 2015, 1348, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Sun, R.; Yu, Y.; Gou, S.; Zhao, G.; Wang, C. Antiproliferative effect of resveratrol in pancreatic cancer cells. Phytother. Res. 2010, 24, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, L.; Tian, W.; Li, J.; Liu, J.; Zhu, M.; Zhang, Y.; Yang, Y.; Liu, F.; Zhang, Q.; et al. Resveratrol plays dual roles in pancreatic cancer cells. J. Cancer Res. Clin. Oncol. 2014, 140, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, L.; Yang, Y.; Wang, M.; Liu, T.; Ji, W.; Liu, Y.; Lv, H.; Zhao, Y.; Chen, X.; et al. Polydopamine-based resveratrol–hyaluronidase nanomedicine inhibited pancreatic cancer cell invasive phenotype in hyaluronic acid enrichment tumor sphere model. ACS Pharmacol. Transl. Sci. 2024, 7, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, G.; Chen, Y.; Mu, Z.; Zhou, H.; Zhang, L. Resveratrol pre-treatment alleviated caerulein-induced acute pancreatitis in high-fat diet-feeding mice via suppressing the NF-κB proinflammatory signaling and improving the gut microbiota. BMC Complement. Med. Ther. 2022, 22, 189. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Hong, T.; Shimada, Y.; Komoto, I.; Kawabe, A.; Ding, Y.; Kaganoi, J.; Hashimoto, Y.; Imamura, M. Suppression of N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by resveratrol. Carcinogenesis 2002, 23, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.B.; Yan, Y.; Sun, Y.N.; Zhu, J.R. Resveratrol induces apoptosis in human esophageal carcinoma cells. World J. Gastroenterol. 2003, 9, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Rafiei, H.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Anti-tumor activity of resveratrol against gastric cancer: A review of recent advances with an emphasis on molecular pathways. Cancer Cell Int. 2021, 21, 66. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, Y.; Zhu, B.; Liu, Q.; Yao, Q.; Zhao, G. Resveratrol induces apoptosis in SGC-7901 gastric cancer cells. Oncol. Lett. 2018, 16, 2949–2956. [Google Scholar] [CrossRef] [PubMed]
- Rojo, D.; Madrid, A.; Martín, S.S.; Párraga, M.; Silva Pinhal, M.A.; Villena, J.; Valenzuela-Valderrama, M. Resveratrol decreases the invasion potential of gastric cancer cells. Molecules 2022, 27, 3047. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xia, L. Resveratrol inhibits the proliferation, invasion, and migration, and induces the apoptosis of human gastric cancer cells through the MALAT1/miR-383-5p/DDIT4 signaling pathway. J. Gastrointest. Oncol. 2022, 13, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Li, L.; Zhou, E.; Li, H.; Wu, S.; Cao, Z. Resveratrol downregulates miR-155-5p to block the malignant behavior of gastric cancer cells. Biomed Res. Int. 2022, 2022, 6968641. [Google Scholar] [CrossRef] [PubMed]
- Ostwal, V.; Ramaswamy, A.; Bhargava, P.; Srinivas, S.; Mandavkar, S.; Chaugule, D.; Peelay, Z.; Baheti, A.; Tandel, H.; Jadhav, V.K.; et al. A pro-oxidant combination of resveratrol and copper reduces chemotherapy-related non-haematological toxicities in advanced gastric cancer: Results of a prospective open label phase II single-arm study (RESCU III study). Med. Oncol. 2022, 40, 17. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Zhai, J.; Huan. X.K.; Xu, W.W.; Tian, J.; Farhood, B. A systematic review of the therapeutic potential of resveratrol during colorectal cancer chemotherapy. Mini Rev. Med. Chem. 2023, 23, 1137–1152. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Akao, Y.; Tanaka, T.; Iinuma, M.; Nozawa, Y. Vaticanol C, a novel resveratrol tetramer, inhibits cell growth through induction of apoptosis in colon cancer cell lines. Biol. Pharm. Bull. 2002, 25, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.E.; Wenzel, U.; Daniel, H.; Planas, J.M. Resveratrol induces apoptosis through ROS-dependent mitochondria pathway in HT-29 human colorectal carcinoma cells. J. Agric. Food Chem. 2008, 56, 4813–4818. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Luo, H.; Deng, Y.; Yao, X.; Zhang, J.; He, B. Resveratrol inhibits proliferation and induces apoptosis via the Hippo/YAP pathway in human colon cancer cells. Biochem. Biophys. Res. Commun. 2022, 636, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Brown, V.A.; Jones, D.J.L.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef] [PubMed]
- Czapla, J.; Drzyzga, A.; Matuszczak, S.; Pilny, E.; Cichoń, T.; Stojecki, K.; Smolarczyk, R. The complex composition of trans-resveratrol, quercetin, vitamin E and selenium inhibits the growth of colorectal carcinoma. Anticancer Res. 2022, 42, 4763–4772. [Google Scholar] [CrossRef] [PubMed]
- Khayat, M.T.; Zarka, M.A.; El-Telbany, D.F.A.; El-Halawany, A.M.; Kutbi, H.I.; Elkhatib, W.F.; Noreddin, A.M.; Khayyat, A.N.; El-Telbany, R.F.A.; Hammad, S.F.; et al. Intensification of resveratrol cytotoxicity, pro-apoptosis, oxidant potentials in human colorectal carcinoma HCT-116 cells using zein nanoparticles. Sci. Rep. 2022, 12, 15235. [Google Scholar] [CrossRef] [PubMed]
- Momchilova, A.; Pankov, R.; Staneva, G.; Pankov, S.; Krastev, P.; Vassileva, E.; Hazarosova, R.; Krastev, N.; Robev, B.; Nikolova, B.; et al. Resveratrol affects sphingolipid metabolism in A549 lung adenocarcinoma cells. Int. J. Mol. Sci. 2022, 23, 10870. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Iyer, A.K.V.; Yakisich, J.S.; Azad, N. Anti-tumorigenic effects of resveratrol in lung cancer cells through modulation of c-FLIP. Curr. Cancer Drug Targets 2017, 17, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, F.; Wang, F.; Li, J.; Lin, C.; Du, J. Resveratrol inhibits the proliferation of A549 cells by inhibiting the expression of COX-2. Onco Targets Ther. 2018, 11, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, R.; Chang, Z.; Wang, X. Resveratrol activates CD8+ T cells through IL-18 bystander activation in lung adenocarcinoma. Front. Pharmacol. 2022, 13, 1031438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chiu, J.; Zhang, H.; Qi, T.; Tang, Q.; Ma, K.; Lu, H.; Li, G. Autophagic cell death induced by resveratrol depends on the Ca2+/AMPK/mTOR pathway in A549 cells. Biochem. Pharmacol. 2013, 86, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, Y.; Zhang, Y.; Liu, Y.; Yu, Y.; Ma, M. Resveratrol induces autophagy and apoptosis in non-small-cell lung cancer cells by activating the NGFR-AMPK-mTOR pathway. Nutrients 2022, 14, 2413. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Liang, C.; Wang, R.; Yi, K.; Zhou, X.; Li, X.; Chen, Y.; Miao, D.; Zhong, C.; Zhu, J. Resveratrol suppresses lung cancer by targeting cancer stem-like cells and regulating tumor microenvironment. J. Nutr. Biochem. 2022, 112, 109211. [Google Scholar] [CrossRef] [PubMed]
- Davoodvandi, A.; Darvish, M.; Borran, S.; Nejati, M.; Mazaheri, S.; Reza Tamtaji, O.; Hamblin, M.R.; Masoudian, N.; Mirzaei, H. The therapeutic potential of resveratrol in a mouse model of melanoma lung metastasis. Int. Immunopharmacol. 2020, 88, 106905. [Google Scholar] [CrossRef] [PubMed]
- Stocco, B.; Toledo, K.; Salvador, M.; Paulo, M.; Koyama, N.; Torqueti Toloi, M.R. Dose-dependent effect of resveratrol on bladder cancer cells: Chemoprevention and oxidative stress. Maturitas 2012, 72, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, H.; Zeng, X.; Ye, D.; Liu, J. Resveratrol inhibits proliferation, migration and invasion via Akt and ERK1/2 signaling pathways in renal cell carcinoma cells. Biomed. Pharmacother. 2018, 98, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.C.; Melo, A.S.; Lima, A.P.B.; Branquinho, R.T.; da Silva, G.N. Resveratrol induces the production of reactive oxygen species, interferes with the cell cycle, and inhibits the cell migration of bladder tumour cells with different TP53 status. Nat. Prod. Res. 2023, 37, 3838–3843. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; AlSharif, D.; Mazza, C.; Syar, P.; Al Sharif, M.; Fata, J.E. Resveratrol and pterostilbene exhibit anticancer properties involving the downregulation of HPV oncoprotein E6 in cervical cancer cells. Nutrients 2018, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qiu, R.L.; Lin, Y.; Cai, Y.; Bian, Y.; Fan, Y.; Gao, X.J. Resveratrol suppresses human cervical carcinoma cell proliferation and elevates apoptosis via the mitochondrial and p53 signaling pathways. Oncol. Lett. 2018, 15, 9845–9851. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liu, Z.; Zhou, X.; Peng, F.; Wang, Z.; Li, F.; Li, M. Resveratrol induces apoptosis, suppresses migration, and invasion of cervical cancer cells by inhibiting the Hedgehog signaling pathway. Biomed Res. Int. 2022, 2022, 8453011. [Google Scholar] [CrossRef] [PubMed]
- Stakleff, K.S.; Sloan, T.; Blanco, D.; Marcanthony, S.; Booth, T.D.; Bishayee, A. Resveratrol exerts differential effects in vitro and in vivo against ovarian cancer cells. Asian Pac. J. Cancer Prev. 2012, 13, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Qin, Z.; Li, F.; Zhang, H.; Fang, Z.; Hao, E. Apoptotic cell death induced by resveratrol is partially mediated by the autophagy pathway in human ovarian cancer cells. PLoS One 2015, 10, e0129196. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Zheng, G.Z.; Xie, P.; Zhang, Q.H.; Lin, F.X.; Chang, B.; Hu, Q.X.; Du, S.X.; Li, X.D. Antitumor activity of resveratrol against human osteosarcoma cells: A key role of Cx43 and Wnt/β-catenin signaling pathway. Oncotarget 2017, 8, 111419–111432. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Marinheiro, D.; Bastos, V.; Daniel-da-Silva, A.L.; Vieira, R.; Oliveira, H.; Almeida, J.C.; Ferreira, B.J.M.L. Resveratrol-loaded polydimethylsiloxane–silica hybrid materials: Synthesis, characterization, and antitumoral activity. Polymers (Basel) 2024, 16, 879. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bäckesjö, C.M.; Haldosén, L.A.; Lindgren, U. Resveratrol inhibits proliferation and promotes apoptosis of osteosarcoma cells. Eur. J. Pharmacol. 2009, 609, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Wang, L.; Fu, S.; Jiang, B. Resveratrol is cytotoxic and acts synergistically with NF-κB inhibition in osteosarcoma MG-63 cells. Arch. Med. Sci. 2020, 17, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Adhami, V.M.; Ahmad, N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol. Appl. Pharmacol. 2003, 186, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Synowiec-Wojtarowicz, A.; Krawczyk, A.; Kimsa-Dudek, M. The effect of resveratrol and static magnetic field interactions on the oxidation–reduction parameters of melanoma malignant cells. Appl. Sci. 2023, 13, 8042. [Google Scholar] [CrossRef]
- Kim, A.L.; Zhu, Y.; Zhu, H.; Han, L.; Kopelovich, L.; Bickers, D.R.; Athar, M. Resveratrol inhibits proliferation of human epidermoid carcinoma A431 cells by modulating MEK1 and AP-1 signalling pathways. Exp. Dermatol. 2006, 15, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Osmond, G.W.; Augustine, C.K.; Zipfel, P.A.; Padussis, J.; Tyler, D.S. Enhancing melanoma treatment with resveratrol. J. Surg. Res. 2012, 172, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, B.; E, C.; Liu, J.; Zhang, Q.; Liu, J.; Chen, N.; Chen, R.; Zhu, R. Resveratrol inhibits the proliferation of human melanoma cells by inducing G1/S cell cycle arrest and apoptosis. Mol. Med. Rep. 2015, 11, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.D.; Cao, Y.; Wang, K.F.; Xu, S.F.; Han, R. [Chemopreventive effect of resveratrol to cancer]. Ai Zheng 2004, 23, 869–873. [Google Scholar] [PubMed]
- Kim, S.E.; Shin, S.H.; Lee, J.Y.; Kim, C.H.; Chung, I.K.; Kang, H.M.; Park, H.R.; Park, B.S.; Kim, I.R. Resveratrol induces mitochondrial apoptosis and inhibits epithelial–mesenchymal transition in oral squamous cell carcinoma cells. Nutr. Cancer 2018, 70, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Yang, G.; Xiang, W.; Pei-Jun, W.; Bin, Z. Effects of resveratrol on oral squamous cell carcinoma (OSCC) cells in vitro. J. Cancer Res. Clin. Oncol. 2014, 140, 371–374. [Google Scholar] [CrossRef] [PubMed]
- ElAttar, T.M.; Virji, A.S. Modulating effect of resveratrol and quercetin on oral cancer cell growth and proliferation. Anticancer Drugs 1999, 10, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Pilankar, A.; Singhavi, H.; Raghuram, G.V.; Siddiqui, S.; Khare, N.K.; Jadhav, V.; Tandel, H.; Pal, K.; Bhattacharjee, A.; Chaturvedi, P.; et al. A pro-oxidant combination of resveratrol and copper down-regulates hallmarks of cancer and immune checkpoints in patients with advanced oral cancer: Results of an exploratory study (RESCU 004). Front. Oncol. 2022, 12, 1000957. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Cheng, J.; Jiang, S.; Wen, J.; Jian, Y.; Wei, L.; Zhe, Z.; Fu-Qiang, J.; Peng, X. The antitumor effect of resveratrol on nasopharyngeal carcinoma cells. Front. Biosci. (Landmark Ed.) 2019, 24, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhao, L.; Qin, Y.; Zhang, M.; He, Y. Resveratrol inhibits proliferation and induces apoptosis of nasopharyngeal carcinoma cell line C666-1 through AMPK activation. Pharmazie 2015, 70, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.R.; O’Brian, C.A. Resveratrol antagonizes EGFR-dependent Erk1/2 activation in human androgen-independent prostate cancer cells with associated isozyme-selective PKC alpha inhibition. Invest. New Drugs 2004, 22, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Hicks, C.; Levenson, A.S. Resveratrol and prostate cancer: Promising role for microRNAs. Mol. Nutr. Food Res. 2011, 55, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Jajoo, S.; Kaur, T.; Mukherjea, D.; Sheehan, K.; Rybak, L.P.; Ramkumar, V. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PLoS One 2012, 7, e51655. [Google Scholar] [CrossRef] [PubMed]
- Nassir, A.M.; Shahzad, N.; Ibrahim, I.A.A.; Ahmad, I.; Md, S.; Ain, M.R. Resveratrol-loaded PLGA nanoparticles mediated programmed cell death in prostate cancer cells. Saudi Pharm. J. 2018, 26, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.N.; Upadhyay, P.K.; Dewangan, H.K. Development, evaluation, pharmacokinetic and biodistribution estimation of resveratrol loaded solid lipid nanoparticles for prostate cancer targeting. J. Microencapsul. 2022, 39, 563–574. [Google Scholar] [CrossRef] [PubMed]
- El-Benhawy, S.A.; Morsi, M.I.; Fahmy, E.I.; Soula, M.A.; Khalil, F.A.Z.F.; Arab, A.R. Role of resveratrol as radiosensitizer by targeting cancer stem cells in radioresistant prostate cancer cells (PC-3). Asian Pac. J. Cancer Prev. 2021, 22, 3823–3837. [Google Scholar] [CrossRef] [PubMed]
- Han, D.S.; Lee, H.J.; Lee, E.O. Resveratrol suppresses serum-induced vasculogenic mimicry through impairing the EphA2/twist-VE-cadherin/AKT pathway in human prostate cancer PC-3 cells. Sci. Rep. 2022, 12, 20125. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Z.; Li, H.; Cao, J.; Nan, N.; Zhai, X.; Liu, Y.; Chong, T. Resveratrol inhibits TRAF6/PTCH/SMO signal and regulates prostate cancer progression. Cytotechnology 2022, 74, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Yadav, E.; Yadav, P.; Khan, M.M.U.; Singh, H.; Verma, A. Resveratrol: A potential therapeutic natural polyphenol for neurodegenerative diseases associated with mitochondrial dysfunction. Front. Pharmacol. 2022, 13, 922232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, H.; Fu, H. Protective effect of resveratrol combined with levodopa against oxidative damage in dopaminergic neurons. Cell Biochem. Biophys.2024, Apr 15. [CrossRef] [PubMed]
- Shamsher, E.; Khan, R.S.; Davis, B.M.; Dine, K.; Luong, V.; Cordeiro, M.F.; Shindler, K.S. Intranasal resveratrol nanoparticles enhance neuroprotection in a model of multiple sclerosis. Int. J. Mol. Sci. 2024, 25, 4047. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.A.Q.; Silva, J.P.B.; Romeiro, C.F.R.; Dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Mendes, P.F.S.; Varela, E.L.P.; Monteiro, M.C. Neuroprotective mechanisms of resveratrol in Alzheimer’s disease: Role of SIRT1. Oxid. Med. Cell. Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef] [PubMed]
- Bartra, C.; Yuan, Y.; Vuraić, K.; Valdés-Quiroz, H.; Garcia-Baucells, P.; Slevin, M.; Pastorello, Y.; Suñol, C.; Sanfeliu, C. Resveratrol activates antioxidant protective mechanisms in cellular models of Alzheimer’s disease inflammation. Antioxidants (Basel) 2024, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.D.; Chen, X.X.; Yang, L.J.; Gao, X.R.; Xia, Q.R.; Qi, C.C.; Ge, J.F. Resveratrol ameliorates learning and memory impairments induced by bilateral hippocampal injection of streptozotocin in mice. Neurochem. Int. 2022, 159, 105385. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Yeo, M.A.; Kim, S.J.; Moon, H.I. Neuroprotective effects of resveratrol derivatives from the roots of Vitis thunbergii var. sinuata against glutamate-induced neurotoxicity in primary cultured rat cortical cells. Hum. Exp. Toxicol. 2011, 30, 1404–1408. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gupta, L.K.; Mediratta, P.K.; Bhattacharya, S.K. Effect of resveratrol on scopolamine-induced cognitive impairment in mice. Pharmacol. Rep. 2012, 64, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhao, X.Y.; Meng, Q.P.; Teng, D.; Deng, K.; Lin, N. Resveratrol activates the SIRT1/PGC-1 pathway in mice to improve synaptic-related cognitive impairment after TBI. Brain Res. 2022, 1796, 148109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, X.B.; Zhao, J.C.; Gao, X.F.; Zhang, X.N.; Hou, K. Neuroprotective effect of resveratrol against radiation after surgically induced brain injury by reducing oxidative stress, inflammation, and apoptosis through NRf2/HO-1/NF-κB signaling pathway. J. Biochem. Mol. Toxicol. 2020, 34, e22600. [Google Scholar] [CrossRef] [PubMed]
- Reda, D.; Elshopakey, G.E.; Mahgoub, H.A.; Risha, E.F.; Khan, A.A.; Rajab, B.S.; El-Boshy, M.E.; Abdelhamid, F.M. Effects of resveratrol against induced metabolic syndrome in rats: Role of oxidative stress, inflammation, and insulin resistance. Evid. Based Complement. Alternat. Med. 2022, 2022, 3362005. [Google Scholar] [CrossRef] [PubMed]
- Ciddi, V.; Dodda, D. Therapeutic potential of resveratrol in diabetic complications: In vitro and in vivo studies. Pharmacol. Rep. 2014, 66, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.K.; Kang, H.S. Anti-diabetic effect of cotreatment with quercetin and resveratrol in streptozotocin-induced diabetic rats. Biomol. Ther. (Seoul) 2018, 26, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.J.; Rimando, A.M.; Mizuno, C.S.; Mathews, S.T. α-Glucosidase inhibitory effect of resveratrol and piceatannol. J. Nutr. Biochem. 2017, 47, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Liao, W.; Xia, H.; Wang, S.; Sun, G. The effect of resveratrol on blood lipid profile: A dose-response meta-analysis of randomized controlled trials. Nutrients 2022, 14, 3755. [Google Scholar] [CrossRef] [PubMed]
- Su, C.H.; Wang, H.L.; Tsai, M.L.; Lin, Y.C.; Liao, J.M.; Yen, C.C.; Ting, H.C.; Yu, C.H. Protective effect of microorganism biotransformation-produced resveratrol on the high fat diet-induced hyperlipidemia, hepatic steatosis and synaptic impairment in hamsters. Int. J. Med. Sci. 2022, 19, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Liu, J.L.; Du, A.L. Cardioprotective effect of resveratrol on atherogenic diet-fed rats. Int. J. Clin. Exp. Pathol. 2014, 7, 7899–7906. [Google Scholar] [PubMed]
- Ohara, K.; Kusano, K.; Kitao, S.; Yanai, T.; Takata, R.; Kanauchi, O. ε-Viniferin, a resveratrol dimer, prevents diet-induced obesity in mice. Biochem. Biophys. Res. Commun. 2015, 468, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.W.; Nakamoto, Y.; Hisatome, T.; Yoshida, S.; Miyazaki, H. Resveratrol and its dimers ε-viniferin and δ-viniferin in red wine protect vascular endothelial cells by a similar mechanism with different potency and efficacy. Kaohsiung J. Med. Sci. 2020, 36, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhou, Y.; Xie, X. Resveratrol inhibiting TGF/ERK signaling pathway can improve atherosclerosis: Backgrounds, mechanisms and effects. Biomed. Pharmacother. 2022, 155, 113775. [Google Scholar] [CrossRef] [PubMed]
- Banaszewska, B.; Wrotyńska-Barczyńska, J.; Spaczynski, R.Z.; Pawelczyk, L.; Duleba, A.J. Effects of resveratrol on polycystic ovary syndrome: A double-blind, randomized, placebo-controlled trial. J. Clin. Endocrinol. Metab. 2016, 101, 4322–4328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhuang, L.; Gai, S.; Shan, Y.; Wang, S.; Li, F.; Chen, L.; Zhao, D.; Liu, X. Beneficial phytoestrogenic effects of resveratrol on polycystic ovary syndrome in rat model. Gynecol. Endocrinol. 2021, 37, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Malvasi, A.; Tinelli, A.; Lupica, G.; Vimercati, A.; Spyropoulou, K.; Dellino, M.; Mynbaev, O. Effects of a combination of resveratrol and alpha-lipoic acid on body weight and adipose composition in women with PCOS: A preliminary pilot study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 6578–6582. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Shah, M.; Malik, M.O.; Ehtesham, E.; Habib, S.H.; Rauf, B. Treatment with combined resveratrol and myoinositol ameliorates endocrine, metabolic alterations and perceived stress response in women with PCOS: A double-blind randomized clinical trial. Endocrine 2023, 79, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Huo, P.; Li, M.; Le, J.; Zhu, C.; Yao, J.; Zhang, S. Resveratrol improves follicular development of PCOS rats via regulating glycolysis pathway and targeting SIRT1. Syst. Biol. Reprod. Med. 2023, 69, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, C.X.; Liu, Y.M.; Chen, K.L.; Chen, G. A comparative study of anti-aging properties and mechanism: Resveratrol and caloric restriction. Oncotarget 2017, 8, 65717–65729. [Google Scholar] [CrossRef] [PubMed]
- Leis, K.; Pisanko, K.; Jundziłł, A.; Mazur, E.; Mêcińska-Jundziłł, K.; Witmanowski, H. Resveratrol as a factor preventing skin aging and affecting its regeneration. Postepy Dermatol. Alergol. 2022, 39, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Totonchi, H.; Mokarram, P.; Karima, S.; Rezaei, R.; Dastghaib, S.; Koohpeyma, F.; Noori, S.; Azarpira, N. Resveratrol promotes liver cell survival in mice liver-induced ischemia–reperfusion through unfolded protein response: A possible approach in liver transplantation. BMC Pharmacol. Toxicol. 2022, 23, 74. [Google Scholar] [CrossRef] [PubMed]
- Theodotou, M.; Fokianos, K.; Moniatis, D.; Kadlenic, R.; Chrysikou, A.; Aristotelous, A.; Mouzouridou, A.; Diakides, J.; Stavrou, E. Effect of resveratrol on non-alcoholic fatty liver disease. Exp. Ther. Med. 2019, 18, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Tejada, S.; Capó, X.; Mascaró, C.M.; Monserrat-Mesquida, M.; Quetglas-Llabrés, M.M.; Pons, A.; Tur, J.A.; Sureda, A. Hepatoprotective effects of resveratrol in non-alcoholic fatty live disease. Curr. Pharm. Des. 2021, 27, 2558–2570. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Yang, L.; Zhong, W.; Hu, Y.; Tan, Y.; Ren, Z.; Ban, Q.; Yang, C.S.; Wang, Y.; Wang, Z. Polydatin, a glycoside of resveratrol, is better than resveratrol in alleviating non-alcoholic fatty liver disease in mice fed a high-fructose diet. Front. Nutr. 2022, 9, 857879. [Google Scholar] [CrossRef] [PubMed]
- Chupradit, S.; Bokov, D.; Zamanian, M.Y.; Heidari, M.; Hakimizadeh, E. Hepatoprotective and therapeutic effects of resveratrol: A focus on anti-inflammatory and antioxidative activities. Fundam. Clin. Pharmacol. 2022, 36, 468–485. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.M.; Shaw, L.H.; Chang, P.J.; Tung, S.Y.; Chang, T.S.; Shen, C.H.; Hsieh, Y.Y.; Wei, K.L. Hepatoprotective effect of resveratrol against ethanol-induced oxidative stress through induction of superoxide dismutase in vivo and in vitro. Exp. Ther. Med. 2016, 11, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Gokkaya, E.O.; Yesilot, S.; Ozgocmen, M.; Aslankoc, R.; Aydin Acar, C. Protective effects of resveratrol and avocado oil against paracetamol-induced hepatotoxicity in rats. Drug Chem. Toxicol. 2022, 45, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Pace-Asciak, C.R.; Hahn, S.; Diamandis, E.P.; Soleas, G.; Goldberg, D.M. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: Implications for protection against coronary heart disease. Clin. Chim. Acta 1995, 235, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Duan, Q.J.; Shen, J. [Resveratrol pretreatment improves mitochondrial function and alleviates myocardial ischemia–reperfusion injury by up-regulating mi R-20b-5p to inhibit STIM2]. Zhongguo Zhong Yao Za Zhi 2022, 47, 4987–4995. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Liu, C.; Zhang, Z.; Huang, K.; Wang, T.; Chen, S.; Li, Z. Progress in the preclinical and clinical study of resveratrol for vascular metabolic disease. Molecules 2022, 27, 7524. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, X.; Liao, Y.; Wang, C.; Wang, Y. Resveratrol alleviates Ang II-induced vascular smooth muscle cell senescence by upregulating E2F1/SOD2 axis. Toxicol. Res. (Camb.) 2022, 11, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Militaru, C.; Donoiu, I.; Craciun, A.; Scorei, I.D.; Bulearca, A.M.; Scorei, R.I. Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris: Effects on lipid profiles, inflammation markers, and quality of life. Nutrition 2013, 29, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Baolin, L.; Inami, Y.; Tanaka, H.; Inagaki, N.; Iinuma, M.; Nagai, H. Resveratrol inhibits the release of mediators from bone marrow-derived mouse mast cells in vitro. Planta Med. 2004, 70, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wu, S.L.; Zhang, M.; Pan, C.E. [Effect of resveratrol alone and its combination with cyclosporin A on the immune function of human peripheral blood T lymphocytes]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2003, 19, 549–551. [Google Scholar] [PubMed]
- Ferrero, M.E.; Bertelli, A.E.; Fulgenzi, A.; Pellegatta, F.; Corsi, M.M.; Bonfrate, M.; Ferrara, F.; De Caterina, R.; Giovannini, L.; Bertelli, A. Activity in vitro of resveratrol on granulocyte and monocyte adhesion to endothelium. Am. J. Clin. Nutr. 1998, 68, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Kasdallah-Grissa, A.; Mornagui, B.; Aouani, E.; Hammami, M.; Gharbi, N.; Kamoun, A.; El-Fazaa, S. Protective effect of resveratrol on ethanol-induced lipid peroxidation in rats. Alcohol Alcohol. 2006, 41, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Chanvitayapongs, S.; Draczynska-Lusiak, B.; Sun, A.Y. Amelioration of oxidative stress by antioxidants and resveratrol in PC12 cells. Neuroreport 1997, 8, 1499–1502. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Li, S.Y.; Huang, Q.; Zeng, F.F.; Li, B.L.; Cao, W.T.; Chen, Y.M. Greater habitual resveratrol intakes were associated with lower risk of hip fracture – a 1:1 matched case-control study in Chinese elderly. Phytother. Res. 2023, 37, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Bilgic, T. Comparison of the effect of local and systemic injection of resveratrol on cutaneous wound healing in rats. Int. J. Low. Extrem. Wounds 2021, 20, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Yoksa, D.T.; Abba, Y.; Shamaki, B.U.; Satumari, N.A. Effects of resveratrol topical ointment on wound healing of full-thickness cutaneous burns in albino rats. J. Wound Care 2022, 31, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Dong, Y.; Xu, P.; Pan, Q.; Jia, K.; Jin, P.; Zhou, M.; Xu, Y.; Guo, R.; Cheng, B. A composite hydrogel containing resveratrol-laden nanoparticles and platelet-derived extracellular vesicles promotes wound healing in diabetic mice. Acta Biomater. 2022, 154, 212–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, B.; Zheng, H.; Xu, G.; Jiang, H. Resveratrol promotes diabetic wound healing by inhibiting Notch pathway. J. Surg. Res. 2024, 297, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.B.; Wang, Y.J.; Wan, W.J.; Wu, J.; Wang, B.J.; Zhu, H.L.; Xie, M.; Liu, L. Resveratrol ameliorates oxaliplatin-induced neuropathic pain via anti-inflammatory effects in rats. Exp. Ther. Med. 2022, 24, 586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, H.; Lin, Q.; Liu, X.; Cheng, Y.; Deng, B. Anti-inflammatory effect of resveratrol attenuates the severity of diabetic neuropathy by activating the Nrf2 pathway. Aging (Albany N.Y.) 2021, 13, 10659–10671. [Google Scholar] [CrossRef] [PubMed]
- Ashafaq, M.; Intakhab Alam, M.; Khan, A.; Islam, F.; Khuwaja, G.; Hussain, S.; Ali, R.; Alshahrani, S.; Antar Makeen, H.; Alhazmi, H.A.; et al. Nanoparticles of resveratrol attenuates oxidative stress and inflammation after ischemic stroke in rats. Int. Immunopharmacol. 2021, 94, 107494. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, E.; Tanaka, D.; Glogauer, M.; Tenenbaum, H.C.; Ikeda, Y. Healing effects of monomer and dimer resveratrol in a mouse periodontitis model. BMC Oral Health 2022, 22, 460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, S.; Xu, W.; Zhou, Y.; Luan, H.; Wang, D. Resveratrol decreases local inflammatory markers and systemic endotoxin in patients with aggressive periodontitis. Medicine (Baltimore) 2022, 101, e29393. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Okamoto-Shibayama, K.; Yoshida, A.; Ishihara, K. Inhibitory effect of resveratrol on Candida albicans biofilm formation. Bull. Tokyo Dent. Coll. 2021, 62, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Liang, R.; Duan, J.; Song, S.; Pan, Y.; Liu, H.; Zhu, M.; Li, L. Synergistic antibacterial and anti-biofilm activities of resveratrol and polymyxin B against multidrug-resistant Pseudomonas aeruginosa. J. Antibiot. (Tokyo) 2022, 75, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Daroch, F.; Hoeneisen, M.; González, C.L.; Kawaguchi, F.; Salgado, F.; Solar, H.; García, A. In vitro antibacterial activity of Chilean red wines against Helicobacter pylori. Microbios 2001, 104, 79–85. [Google Scholar] [PubMed]
- Spósito, L.; Fonseca, D.; Gonçalves Carvalho, S.; Miguel Sábio, R.; Marena, G.D.; Maria Bauab, T.; Bagliotti Meneguin, A.; Parreira, P.; Martins, M.C.L.; Chorilli, M. Engineering resveratrol-loaded chitosan nanoparticles for potential use against Helicobacter pylori infection. Eur. J. Pharm. Biopharm. 2024, 199, 114280. [Google Scholar] [CrossRef] [PubMed]
- Heredia, A.; Davis, C.; Redfield, R. Synergistic inhibition of HIV-1 in activated and resting peripheral blood mononuclear cells, monocyte-derived macrophages, and selected drug-resistant isolates with nucleoside analogues combined with a natural product, resveratrol. J. Acquir. Immune Defic. Syndr. 2000, 25, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Docherty, J.J.; Smith, J.S.; Fu, M.M.; Stoner, T.; Booth, T. Effect of topically applied resveratrol on cutaneous herpes simplex virus infections in hairless mice. Antiviral Res. 2004, 61, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Evers, D.L.; Wang, X.; Huong, S.M.; Huang, D.Y.; Huang, E.S. 3,4’,5-Trihydroxy-trans-stilbene (resveratrol) inhibits human cytomegalovirus replication and virus-induced cellular signaling. Antiviral Res. 2004, 63, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liao, D.; Zhou, G.; Zhu, Z.; Cui, Y.; Pu, R. Antiviral activities of resveratrol against rotavirus in vitro and in vivo. Phytomedicine 2020, 77, 153230. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Li, J.; Lin, W.; Long, G. Effects of resveratrol on hepatitis B virus replication: In vitro and in vivo experiments. Intervirology 2022, 65, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Vlase, L.; Kiss, B.; Leucuta, S.E.; Gocan, S. A rapid method for determination of resveratrol in wines by HPLC-MS. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 2105–2121. [Google Scholar] [CrossRef]
- Svilar, L.; Martin, J.C.; Defoort, C.; Paut, C.; Tourniaire, F.; Brochot, A. Quantification of trans-resveratrol and its metabolites in human plasma using ultra-high performance liquid chromatography tandem quadrupole–orbitrap mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1104, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yuan, H.; Liu, Y.; Wang, B.; Xu, X.; Xu, X.; Hussain, D.; Ma, L.; Chen, D. Current analytical strategies for the determination of resveratrol in foods. Food Chem. 2024, 431, 137182. [Google Scholar] [CrossRef] [PubMed]
- Crăciun, A.L.; Gutt, G. Optimization of experimental parameters in the solvent extraction of trans-resveratrol from pruning waste of Vitis vinifera, Fetească Neagră variety. Appl. Sci. 2023, 13, 823. [Google Scholar] [CrossRef]
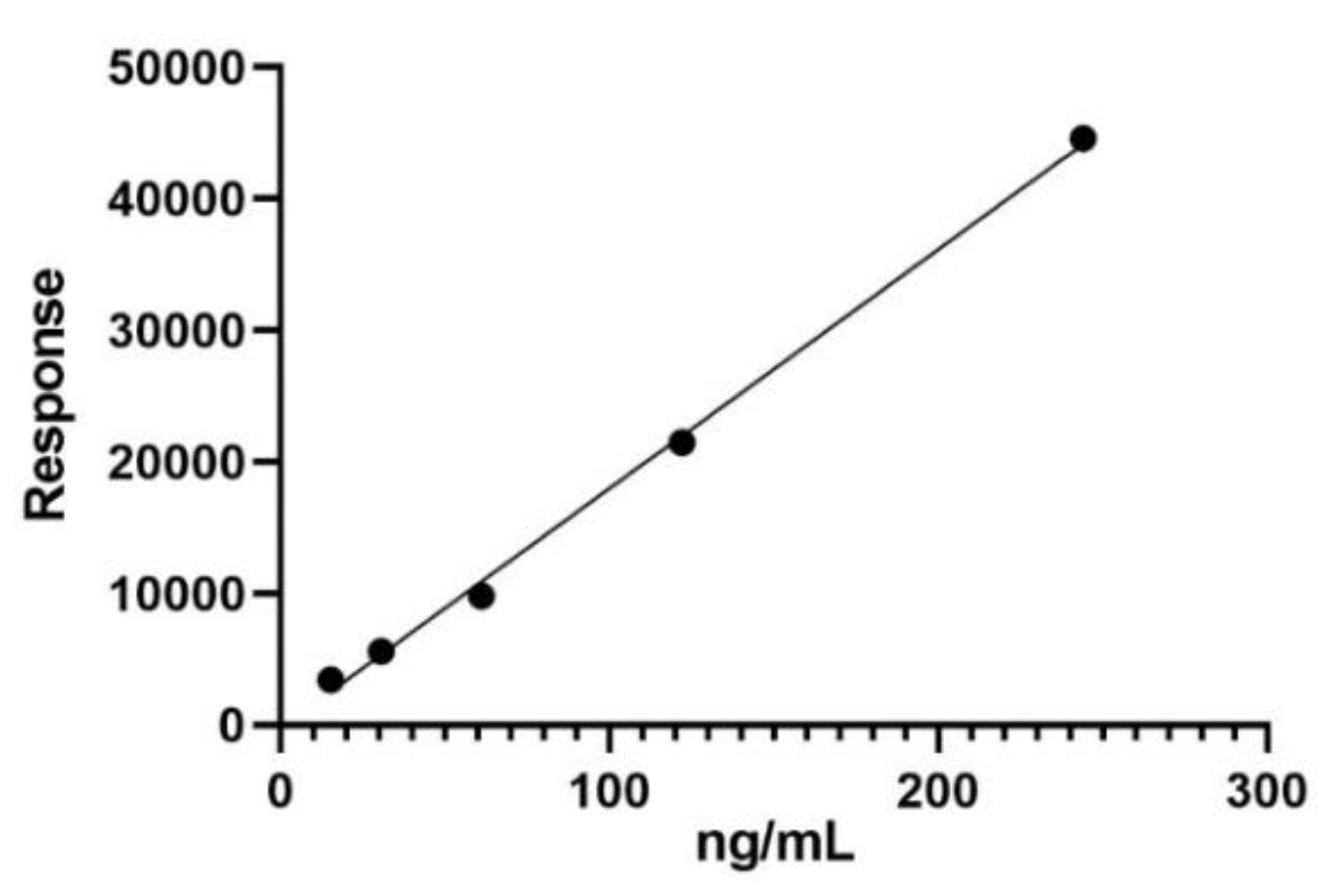
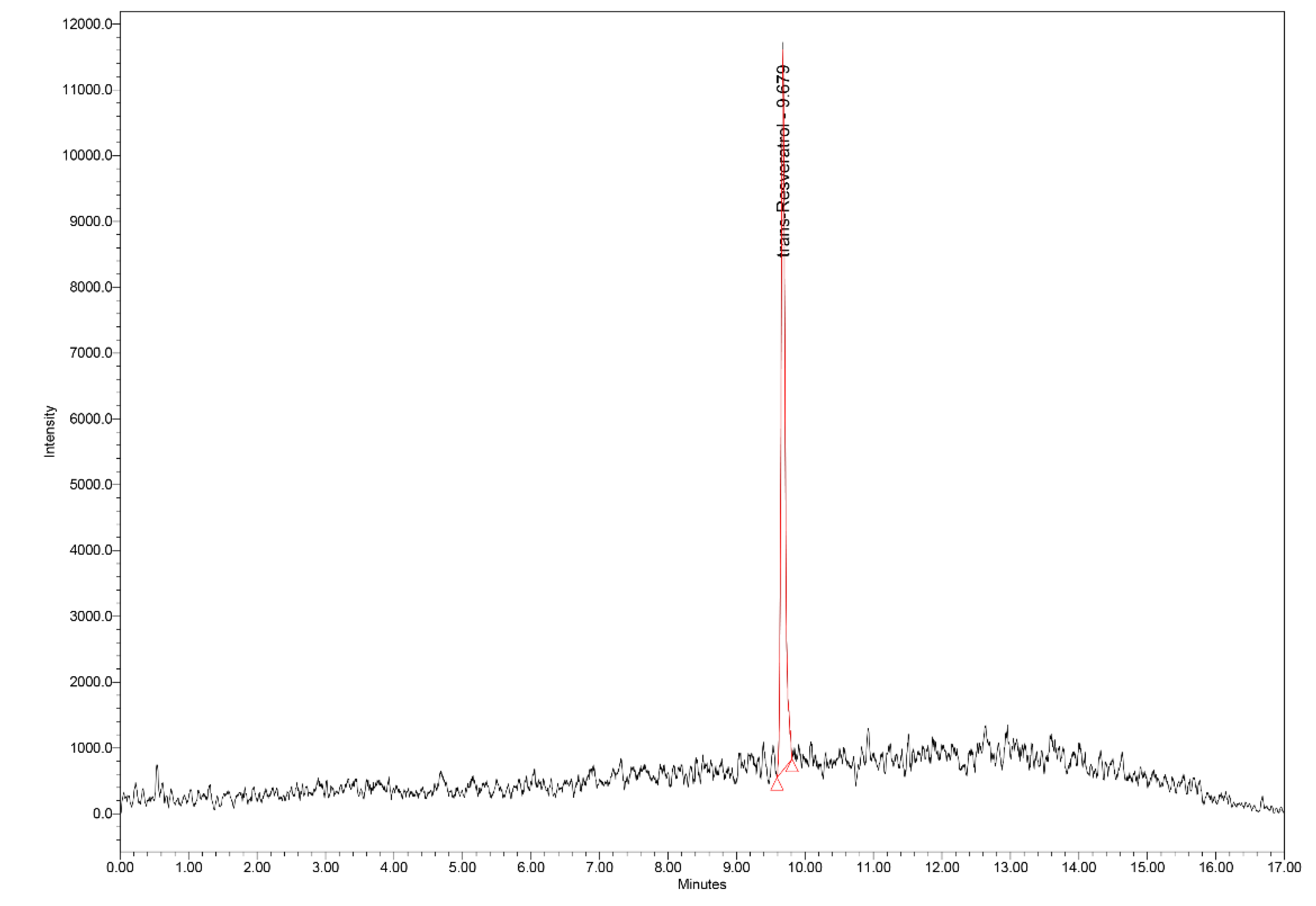
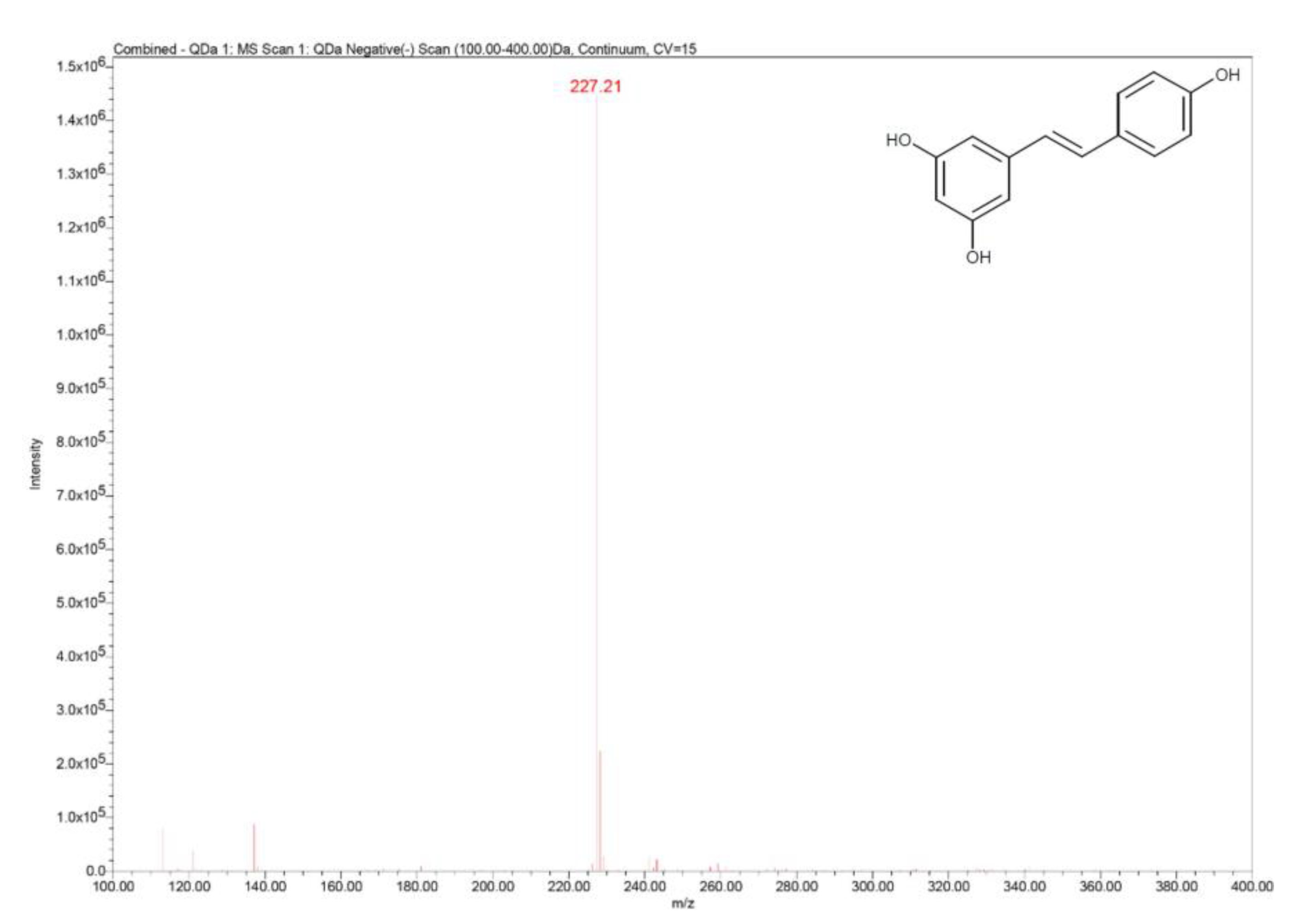
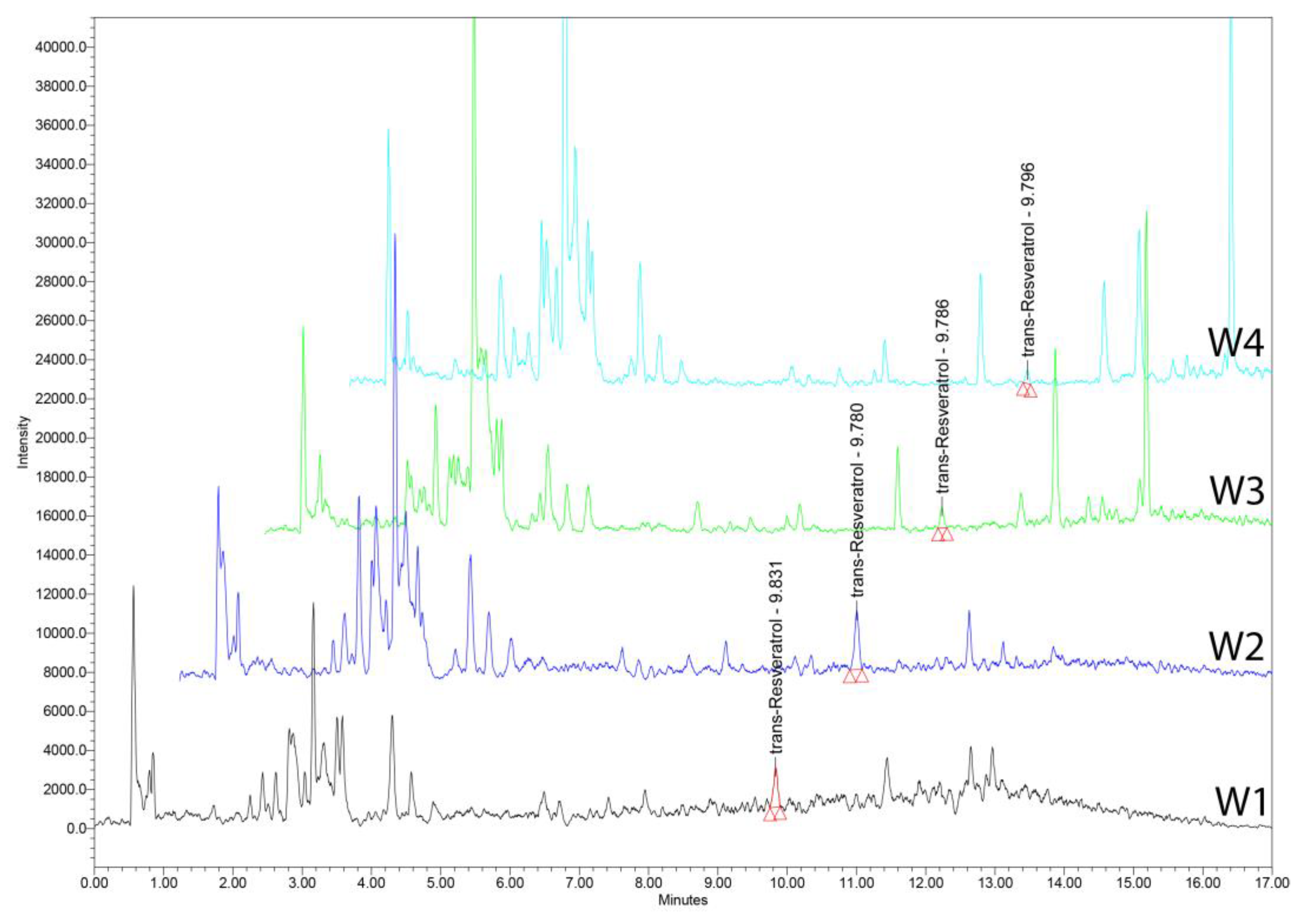
| Parameter | Values |
|---|---|
| Linearity range [ng/mL] | 15.259–244.141 |
| R2 | 0.9979 |
| Equation | Y = 181.7X - 197.6 |
| LOD [ng/mL] | 16.197 |
| LOQ [ng/mL] | 49.081 |
| Sample | trans-Resveratrol amount [ng/mL] |
|---|---|
| W1 | 243.291±2.51 |
| W2 | 372.042±2.75 |
| W3 | 108.487±1.98 |
| W4 | 69.166±1.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





