Submitted:
30 April 2024
Posted:
01 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
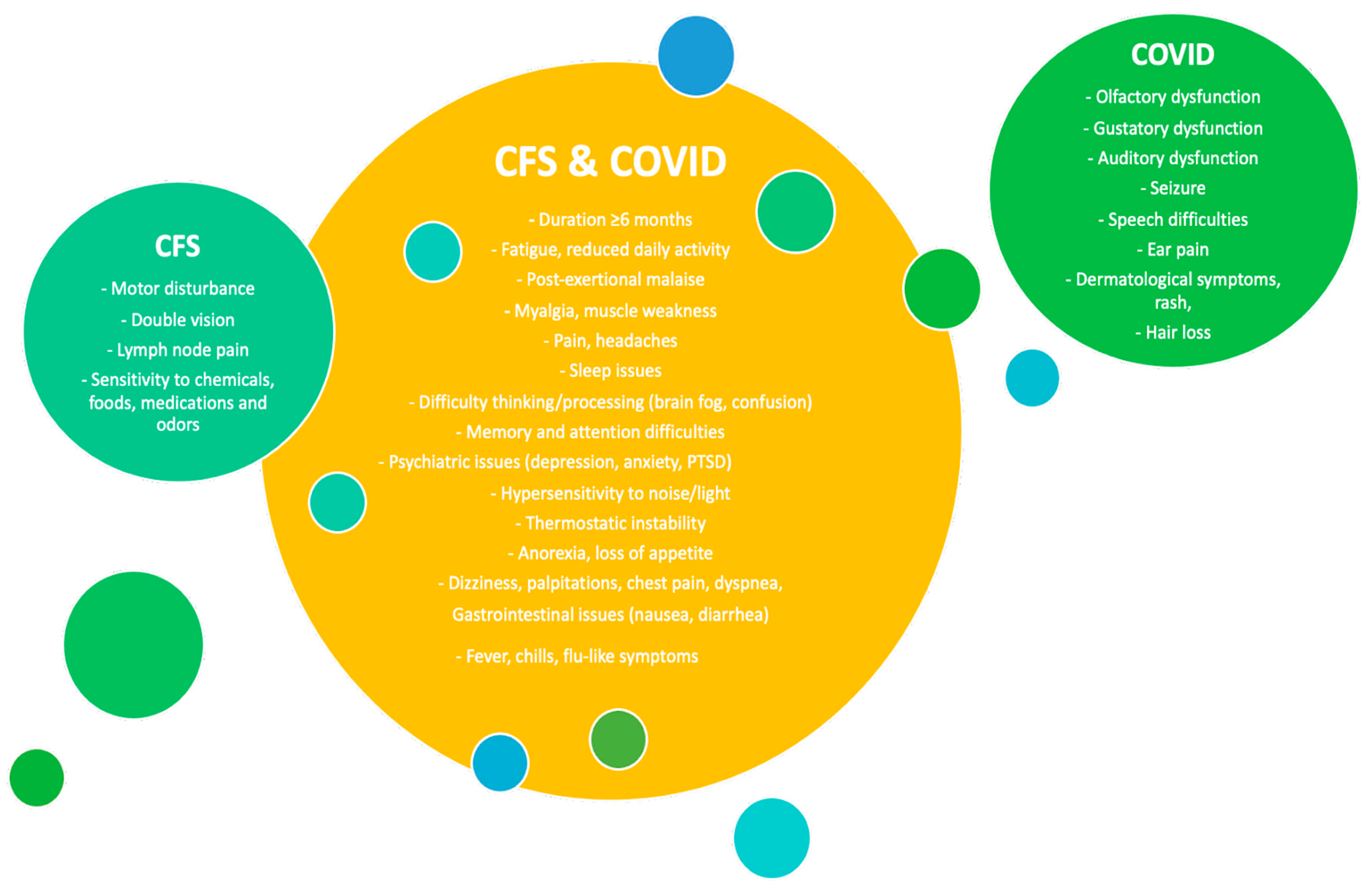
2. Role of the Alerted Gut Microbiome in ME/CFS and Long COVID Pathogenesis
3. Use of Probiotic in the Management of ME/CFS and Long COVID Symptoms
3.1. Effects of Probiotics on Fatigue and Inflammation in ME/CFS and Long COVID
3.2. Effects of Probiotics on Psychiatric Symptoms (Psychobiotics)
3.3. Effects of Probiotics on Gastrointestinal Symptoms in ME/CFS and Long COVID
4. Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Germain A, Barupal DK, Levine SM, Hanson MR. Comprehensive Circulatory Metabolomics in ME/CFS Reveals Disrupted Metabolism of Acyl Lipids and Steroids. Metabolites. 2020 Jan 14;10(1):34. [CrossRef] [PubMed]
- König RS, Albrich WC, Kahlert CR, Bahr LS, Löber U, Vernazza P, Scheibenbogen C, Forslund SK. The Gut Microbiome in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Front Immunol. 2022 Jan 3;12:628741. Erratum in: Front Immunol. 2022 Mar 30;13:878196. [CrossRef] [PubMed]
- Lakhan SE, Kirchgessner A. Gut inflammation in chronic fatigue syndrome. Nutr Metab (Lond). 2010 Oct 12;7:79. [CrossRef] [PubMed]
- Mirin AA, Dimmock ME, Jason LA. Research update: The relation between ME/CFS disease burden and research funding in the USA. Work. 2020;66(2):277-282. [CrossRef] [PubMed]
- Sandler CX, Lloyd AR. Chronic fatigue syndrome: progress and possibilities. Med J Aust. 2020 May;212(9):428-433. [CrossRef] [PubMed]
- Jason LA, McManimen S, Sunnquist M, Brown A, Newton JL, Strand EB. Examining the institute of medicine’s recommendations regarding chronic fatigue syndrome: Clinical versus research criteria. Journal of neurology and psychology. 2015;2015(Suppl 2).
- Barry PW, Kelley K, Tan T, Finlay I. NICE guideline on ME/CFS: robust advice based on a thorough review of the evidence. Journal of Neurology, Neurosurgery & Psychiatry. 2024 Feb 28.
- Devasahayam A, Lawn T, Murphy M, White PD. Alternative diagnoses to chronic fatigue syndrome in referrals to a specialist service: service evaluation survey. JRSM Short Rep. 2012 Jan;3(1):4. [CrossRef] [PubMed]
- Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020 Aug 11;324(6):603-605. [CrossRef] [PubMed]
- Komaroff AL, Lipkin WI. ME/CFS and Long COVID share similar symptoms and biological abnormalities: road map to the literature. Front Med (Lausanne). 2023 Jun 2;10:1187163. [CrossRef] [PubMed]
- Wong TL, Weitzer DJ. Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)-A Systemic Review and Comparison of Clinical Presentation and Symptomatology. Medicina (Kaunas). 2021 Apr 26;57(5):418. [CrossRef] [PubMed]
- White PD, Thomas JM, Amess J, Crawford DH, Grover SA, Kangro HO, Clare AW. Incidence, risk and prognosis of acute and chronic fatigue syndromes and psychiatric disorders after glandular fever. The British Journal of Psychiatry. 1998 Dec;173(6):475-81.
- Tate WP, Walker MO, Peppercorn K, Blair AL, Edgar CD. Towards a better understanding of the complexities of myalgic encephalomyelitis/chronic fatigue syndrome and long COVID. International Journal of Molecular Sciences. 2023 Mar 7;24(6):5124.
- Mandarano AH, Maya J, Giloteaux L, Peterson DL, Maynard M, Gottschalk CG, Hanson MR. Myalgic encephalomyelitis/chronic fatigue syndrome patients exhibit altered T cell metabolism and cytokine associations. The Journal of clinical investigation. 2020 Mar 2;130(3):1491-505.
- Wang EY, Mao T, Klein J, Dai Y, Huck JD, Jaycox JR, Liu F, Zhou T, Israelow B, Wong P, Coppi A. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021 Jul 8;595(7866):283-8.
- Ryabkova VA, Gavrilova NY, Poletaeva AA, Pukhalenko AI, Koshkina IA, Churilov LP, Shoenfeld Y. Autoantibody correlation signatures in fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome: association with symptom severity. Biomedicines. 2023 Jan 18;11(2):257.
- Sotzny F, Blanco J, Capelli E, Castro-Marrero J, Steiner S, Murovska M, Scheibenbogen C. Myalgic encephalomyelitis/chronic fatigue syndrome–evidence for an autoimmune disease. Autoimmunity reviews. 2018 Jun 1;17(6):601-9.
- Maksoud R, Magawa C, Eaton-Fitch N, Thapaliya K, Marshall-Gradisnik S. Biomarkers for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a systematic review. BMC medicine. 2023 ;21(1):189. 24 May.
- Patarca, R. Cytokines and chronic fatigue syndrome. Annals of the New York Academy of Sciences. 2001 Mar;933(1):185-200.
- Low RN, Low RJ, Akrami A. A review of cytokine-based pathophysiology of Long COVID symptoms. Frontiers in Medicine. 2023 Mar 31;10:1011936.
- Montoya JG, Holmes TH, Anderson JN, Maecker HT, Rosenberg-Hasson Y, Valencia IJ, Chu L, Younger JW, Tato CM, Davis MM. Cytokine signature associated with disease severity in chronic fatigue syndrome patients. Proceedings of the National Academy of Sciences. 2017 Aug 22;114(34):E7150-8.
- Missailidis D, Annesley SJ, Fisher PR. Pathological mechanisms underlying myalgic encephalomyelitis/chronic fatigue syndrome. Diagnostics. 2019 Jul 20;9(3):80.
- Hatziagelaki E, Adamaki M, Tsilioni I, Dimitriadis G, Theoharides TC. Myalgic encephalomyelitis/chronic fatigue syndrome—metabolic disease or disturbed homeostasis due to focal inflammation in the hypothalamus?. Journal of Pharmacology and Experimental Therapeutics. 2018 Oct 1;367(1):155-67.
- Al-Hakeim HK, Al-Rubaye HT, Al-Hadrawi DS, Almulla AF, Maes M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: a proof of concept and mechanism study. Molecular Psychiatry. 2023 Feb;28(2):564-78.
- Walker MO, Hall KH, Peppercorn K, Tate WP. The significance of oxidative stress in the pathophysiology of Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Medical Research Archives. 2022 Sep 20;10(9).
- Stufano A, Isgrò C, Palese LL, Caretta P, De Maria L, Lovreglio P, Sardanelli AM. Oxidative Damage and Post-COVID Syndrome: A Cross-Sectional Study in a Cohort of Italian Workers. International Journal of Molecular Sciences. 2023 Apr 18;24(8):7445.
- Tahaghoghi-Hajghorbani S, Zafari P, Masoumi E, Rajabinejad M, Jafari-Shakib R, Hasani B, Rafiei A. The role of dysregulated immune responses in COVID-19 pathogenesis. Virus research. 2020 Dec 1;290:198197.
- Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami ME, Katsaounou P, Ntaganou M. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell host & microbe. 2020 Jun 10;27(6):992-1000.
- Paul BD, Lemle MD, Komaroff AL, Snyder SH. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proceedings of the National Academy of Sciences. 2021 Aug 24;118(34):e2024358118.
- Tanaka S, Kuratsune H, Hidaka Y, Hakariya Y, Tatsumi KI, Takano T, Kanakura Y, Amino N. Autoantibodies against muscarinic cholinergic receptor in chronic fatigue syndrome. International journal of molecular medicine. 2003 Aug 1;12(2):225-30.
- Freitag H, Szklarski M, Lorenz S, Sotzny F, Bauer S, Philippe A, Kedor C, Grabowski P, Lange T, Riemekasten G, Heidecke H. Autoantibodies to vasoregulative G-protein-coupled receptors correlate with symptom severity, autonomic dysfunction and disability in myalgic encephalomyelitis/chronic fatigue syndrome. Journal of Clinical Medicine. 2021 Aug 19;10(16):3675.
- Maes M, Twisk FN, Johnson C. Myalgic encephalomyelitis (ME), chronic fatigue syndrome (CFS), and chronic fatigue (CF) are distinguished accurately: results of supervised learning techniques applied on clinical and inflammatory data. Psychiatry research. 2012 Dec 30;200(2-3):754-60.
- Yamamoto S, Ouchi Y, Nakatsuka D, Tahara T, Mizuno K, Tajima S, Onoe H, Yoshikawa E, Tsukada H, Iwase M, Yamaguti K. Reduction of [11C](+) 3-MPB binding in brain of chronic fatigue syndrome with serum autoantibody against muscarinic cholinergic receptor. PloS one. 2012 Dec 11;7(12):e51515.
- Fischer DB, William AH, Strauss AC, Unger ER, Jason LA, Marshall Jr GD, Dimitrakoff JD. Chronic fatigue syndrome: the current status and future potentials of emerging biomarkers. Fatigue: biomedicine, health & behavior. 2014 Apr 3;2(2):93-109.
- Patil S, Choudhari S, Raka V, Narkar S, Dahiphale J, Gondhali G. Neurological and systemic manifestations in Long covid: Underestimated sequel of covid’s pandora. World Journal of Advanced Pharmaceutical and Medical Research. 2023;4(01):042-52.
- Uhde M, Indart AC, Green PH, Yolken RH, Cook DB, Shukla SK, Vernon SD, Alaedini A. Suppressed immune and metabolic responses to intestinal damage-associated microbial translocation in myalgic encephalomyelitis/chronic fatigue syndrome. Brain, Behavior, & Immunity-Health. 2023 Jul 1;30:100627.
- Cliff JM, King EC, Lee JS, Sepúlveda N, Wolf AS, Kingdon C, Bowman E, Dockrell HM, Nacul L, Lacerda E, Riley EM. Cellular immune function in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Frontiers in immunology. 2019 Apr 16;10:422277.
- Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM, Schatzberg AF. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Molecular psychiatry. 2017 Apr;22(4):527-36.
- Meierovics A, Yankelevich WJ, Cowley SC. MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proceedings of the National Academy of Sciences. 2013 Aug 13;110(33):E3119-28.
- Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nature Reviews Microbiology. 2023 Mar;21(3):133-46.
- Guo C, Che X, Briese T, Ranjan A, Allicock O, Yates RA, Cheng A, March D, Hornig M, Komaroff AL, Levine S. Deficient butyrate-producing capacity in the gut microbiome is associated with bacterial network disturbances and fatigue symptoms in ME/CFS. Cell host & microbe. 2023 Feb 8;31(2):288-304.
- Mendes de Almeida V, Engel DF, Ricci MF, Cruz CS, Lopes ÍS, Alves DA, d’Auriol M, Magalhães J, Machado EC, Rocha VM, Carvalho TG. Gut microbiota from patients with COVID-19 cause alterations in mice that resemble post-COVID symptoms. Gut Microbes. 2023 Dec 18;15(2):2249146.
- Nijs J, Crombez G, Meeus M, Knoop H, Van Damme S, Van Cauwenbergh D, Bleijenberg G. Pain in patients with chronic fatigue syndrome: time for specific pain treatment?. Pain physician. 2012;15(5):E677-86.
- Koc HC, Xiao J, Liu W, Li Y, Chen G. Long COVID and its Management. International Journal of Biological Sciences. 2022;18(12):4768.
- Varesi A, Deumer US, Ananth S, Ricevuti G. The emerging role of gut microbiota in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): current evidence and potential therapeutic applications. Journal of Clinical Medicine. 2021 Oct 29;10(21):5077.
- Thomas N, Gurvich C, Huang K, Gooley PR, Armstrong CW. The underlying sex differences in neuroendocrine adaptations relevant to myalgic encephalomyelitis chronic fatigue syndrome. Frontiers in neuroendocrinology. 2022 Jul 1;66:100995.
- Aron-Wisnewsky J, Clément K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nature Reviews Nephrology. 2016 Mar;12(3):169-81.
- Lazar V, Ditu LM, Pircalabioru GG, Picu A, Petcu L, Cucu N, Chifiriuc MC. Gut microbiota, host organism, and diet trialogue in diabetes and obesity. Frontiers in nutrition. 2019 Mar 13;6:21.
- Fujimura KE, Slusher NA, Cabana MD, Lynch SV. Role of the gut microbiota in defining human health. Expert review of anti-infective therapy. 2010 Apr 1;8(4):435-54.
- Iacob S, Iacob DG, Luminos LM. Intestinal microbiota as a host defense mechanism to infectious threats. Frontiers in Microbiology. 2019 Jan 23;9:426119.
- Kho ZY, Lal SK. The human gut microbiome–a potential controller of wellness and disease. Frontiers in microbiology. 2018 Aug 14;9:356589.
- DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current understanding of dysbiosis in disease in human and animal models. Inflammatory bowel diseases. 2016 ;22(5):1137-50. 1 May.
- Tisza BB, Iván G, Keczeli V, Kóró M, Szántóri P, Varga ZG, Müller H, Pribéli O, Szabó Z, Verzár Z, Gyuró MS. A Review of Possible Supplements to Relieve the Symptoms of Fatigue after COVID-19.
- Shukla SK, Cook D, Meyer J, Vernon SD, Le T, Clevidence D, Robertson CE, Schrodi SJ, Yale S, Frank DN. Changes in gut and plasma microbiome following exercise challenge in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). PloS one. 2015 Dec 18;10(12):e0145453.
- Navaneetharaja N, Griffiths V, Wileman T, Carding SR. A role for the intestinal microbiota and virome in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)?. Journal of clinical medicine. 2016 Jun 6;5(6):55.
- Nagy-Szakal D, Williams BL, Mishra N, Che X, Lee B, Bateman L, Klimas NG, Komaroff AL, Levine S, Montoya JG, Peterson DL. Fecal metagenomic profiles in subgroups of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. 2017 Dec;5:1-7.
- Guo C, Yi B, Wu J, Lu J. The microbiome in post-acute infection syndrome (PAIS). Comput Struct Biotechnol J. 2023 Aug 5;21:3904-3911. [CrossRef] [PubMed]
- Silva CF, Motta JM, Teixeira FC, Gomes AM, Vilanova E, Kozlowski EO, Borsig L, Pavão MS. Non-anticoagulant heparan sulfate from the ascidian phallusia nigra prevents colon carcinoma metastasis in mice by disrupting platelet-tumor cell interaction. Cancers. 2020 May 26;12(6):1353.
- Estaki M, Pither J, Baumeister P, Little JP, Gill SK, Ghosh S, Ahmadi-Vand Z, Marsden KR, Gibson DL. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016 Dec;4:1-3.
- Giloteaux L, Goodrich JK, Walters WA, Levine SM, Ley RE, Hanson MR. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. 2016 Dec;4:1-2.
- Dumas A, Bernard L, Poquet Y, Lugo-Villarino G, Neyrolles O. The role of the lung microbiota and the gut–lung axis in respiratory infectious diseases. Cellular microbiology. 2018 Dec;20(12):e12966.
- Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM. Emerging pathogenic links between microbiota and the gut–lung axis. Nature Reviews Microbiology. 2017 Jan;15(1):55-63.
- Wang RX, Zhou M, Ma HL, Qiao YB, Li QS. The role of chronic inflammation in various diseases and anti-inflammatory therapies containing natural products. ChemMedChem. 2021 May 18;16(10):1576-92.
- Binda C, Lopetuso LR, Rizzatti G, Gibiino G, Cennamo V, Gasbarrini A. Actinobacteria: a relevant minority for the maintenance of gut homeostasis. Digestive and Liver Disease. 2018 May 1;50(5):421-8.
- Simpson CA, Diaz-Arteche C, Eliby D, Schwartz OS, Simmons JG, Cowan CS. The gut microbiota in anxiety and depression–A systematic review. Clinical psychology review. 2021 Feb 1;83:101943.
- Alenazy MF, Aljohar HI, Alruwaili AR, Daghestani MH, Alonazi MA, Labban RS, El-Ansary AK, Balto HA. Gut microbiota dynamics in relation to long-COVID-19 syndrome: role of probiotics to combat psychiatric complications. Metabolites. 2022 Sep 27;12(10):912.
- Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY, Chung AC, Cheung CP, Tso EY, Fung KS, Chan V. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021 Apr 1;70(4):698-706.
- Chen J, Hall S, Vitetta L. Altered gut microbial metabolites could mediate the effects of risk factors in Covid-19. Reviews in Medical Virology. 2021 Sep;31(5):1-3.
- Tian Y, Ran H, Wen X, Fu G, Zhou X, Liu R, Pan T. Probiotics improve symptoms of patients with COVID-19 through gut-lung axis: a systematic review and meta-analysis. Front Nutr. 2023 May 22;10:1179432. [CrossRef] [PubMed]
- Ferreira C, Viana SD, Reis F. Gut microbiota dysbiosis–immune hyperresponse–inflammation triad in coronavirus disease 2019 (COVID-19): Impact of pharmacological and nutraceutical approaches. Microorganisms. 2020 Oct 1;8(10):1514.
- Zuo T, Wu X, Wen W, Lan P. Gut microbiome alterations in COVID-19. Genomics, proteomics & bioinformatics. 2021 Oct 1;19(5):679-88.
- Liu Q, Mak JW, Su Q, Yeoh YK, Lui GC, Ng SS, Zhang F, Li AY, Lu W, Hui DS, Chan PK. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut. 2022 Mar 1;71(3):544-52.
- Zhang D, Zhou Y, Ma Y, Chen P, Tang J, Yang B, Li H, Liang M, Xue Y, Liu Y, Zhang J. Gut microbiota dysbiosis correlates with long COVID-19 at one-year after discharge. Journal of Korean Medical Science. 2023 Apr 4;38(15).
- Alenazy MF, Aljohar HI, Alruwaili AR, Daghestani MH, Alonazi MA, Labban RS, El-Ansary AK, Balto HA. Gut Microbiota Dynamics in Relation to Long-COVID-19 Syndrome: Role of Probiotics to Combat Psychiatric Complications. Metabolites. 2022 Sep 27;12(10):912. [CrossRef] [PubMed]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature reviews Gastroenterology & hepatology. 2014.
- Shahbazi R, Yasavoli-Sharahi H, Alsadi N, Ismail N, Matar C. Probiotics in treatment of viral respiratory infections and neuroinflammatory disorders. Molecules. 2020 Oct 22;25(21):4891.
- Sandionigi A, De Giani A, Tursi F, Michelotti A, Cestone E, Giardina S, Zampolli J, Di Gennaro P. Effectiveness of multistrain probiotic formulation on common infectious disease symptoms and gut microbiota modulation in flu-vaccinated healthy elderly subjects. BioMed Research International. 2022 Jan 27;2022.
- Farzi A, Fröhlich EE, Holzer P. Gut microbiota and the neuroendocrine system. Neurotherapeutics. 2018 Jan 1;15(1):5-22.
- Buglione-Corbett R, Deligiannidis KM, Leung K, Zhang N, Lee M, Rosal MC, Moore Simas TA. Expression of inflammatory markers in women with perinatal depressive symptoms. Archives of women's mental health. 2018 Dec;21:671-9.
- Aziz T, Naveed M. Integrated genome based evaluation of safety and probiotic characteristics of Lactiplantibacillus plantarum YW11 isolated from Tibetan kefir. Frontiers in Microbiology. 2023 Apr 20;14:1157615.
- Rao AV, Bested AC, Beaulne TM, Katzman MA, Iorio C, Berardi JM, Logan AC. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut pathogens. 2009 Dec;1:1-6.
- Venturini L, Bacchi S, Capelli E, Lorusso L, Ricevuti G, Cusa C. Modification of immunological parameters, oxidative stress markers, mood symptoms, and well-being status in CFS patients after probiotic intake: observations from a pilot study. Oxidative medicine and cellular longevity. 2019 Nov 23;2019.
- Williamson CB, Burns CM, Gossard CM, Pizano JM, Dolan KE, Finley HJ, Gasta MG, Parker EC, Lipski EA. Probiotics and disease: A comprehensive summary—Part 3, Cardiometabolic disease and fatigue syndromes. Integrative Medicine: A Clinician's Journal. 2017 Feb;16(1):30.
- Rathi A, Jadhav SB, Shah N. A randomized controlled trial of the efficacy of systemic enzymes and probiotics in the resolution of post-COVID fatigue. Medicines. 2021 Aug 30;8(9):47.
- Xu L, Yang CS, Liu Y, Zhang X. Effective regulation of gut microbiota with probiotics and prebiotics may prevent or alleviate COVID-19 through the gut-lung axis. Frontiers in Pharmacology. 2022 Apr 25;13:895193.
- Sohail A, Cheema HA, Mithani MS, Shahid A, Nawaz A, Hermis AH, Chinnam S, Nashwan AJ, Cherrez-Ojeda I, Awan RU, Ahmad S. Probiotics for the prevention and treatment of COVID-19: a rapid systematic review and meta-analysis. Frontiers in Nutrition. 2023;10.
- Jason LA, Natelson BH, Bonilla H, Sherif ZA, Vernon SD, Gutierrez MV, O’Brien L, Taylor E. What Long COVID investigators can learn from four decades of ME/CFS research. Brain Behavior and Immunity Integrative. 2023 Dec 1; 4: 100022.Jason LA, Natelson BH, Bonilla H, Sherif ZA, Vernon SD, Gutierrez MV, O’Brien L, Taylor E. What Long COVID investigators can learn from four decades of ME/CFS research. Brain Behavior and Immunity Integrative. 2023 Dec 1;4:100022. [Google Scholar]
- Roman P, Estévez AF, Miras A, Sánchez-Labraca N, Cañadas F, Vivas AB, Cardona D. A pilot randomized controlled trial to explore cognitive and emotional effects of probiotics in fibromyalgia. Scientific reports. 2018 Jul 19;8(1):10965.
- Groeger D, O’Mahony L, Murphy EF, Bourke JF, Dinan TG, Kiely B, Shanahan F, Quigley EM. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut microbes. 2013 Jul 12;4(4):325-39.
- Sullivan Å, Nord CE, Evengård B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutrition journal. 2009 Jan 26;8(1):4.
- Tomkinson S, Triscott C, Schenk E, Foey A. The potential of probiotics as ingestible adjuvants and immune modulators for antiviral immunity and management of SARS-CoV-2 infection and COVID-19. Pathogens. 2023 Jul 11;12(7):928.
- Santinelli L, Laghi L, Innocenti GP, Pinacchio C, Vassalini P, Celani L, Lazzaro A, Borrazzo C, Marazzato M, Tarsitani L, Koukopoulos AE. Oral bacteriotherapy reduces the occurrence of chronic fatigue in COVID-19 patients. Frontiers in nutrition. 2022 Jan 12;8:1139.
- Caprioli F, Marinoni B, Rimondi A, Bottaro F, Ciafardini C, Amoroso C, Muia M, Caridi B, Noviello D, Bandera A, Gori A. The Role of VSL# 3 in the Treatment of Fatigue and Other Symptoms in Long Covid-19 Syndrome: a Randomized, Double-blind, Placebo-controlled Pilot Study (DELong# 3). medRxiv. 2023:2023-06.
- Khokhlova E, Colom J, Simon A, Mazhar S, García-Lainez G, Llopis S, Gonzalez N, Enrique-López M, Álvarez B, Martorell P, Tortajada M. Immunomodulatory and antioxidant properties of a novel potential probiotic Bacillus clausii CSI08. Microorganisms. 2023 Jan 18;11(2):240.
- Thomas R, Williams M, Aldous J, Yanagisawa Y, Kumar R, Forsyth R, Chater A. A randomised, double-blind, placebo-controlled trial evaluating concentrated phytochemical-rich nutritional capsule in addition to a probiotic capsule on clinical outcomes among individuals with COVID-19—the UK Phyto-V study. COVID. 2022 Mar 22;2(4):433-49.
- Hinchado MD, Quero-Calero CD, Otero E, Gálvez I, Ortega E. Synbiotic Supplementation Improves Quality of Life and Inmunoneuroendocrine Response in Patients with Fibromyalgia: Influence of Codiagnosis with Chronic Fatigue Syndrome. Nutrients. 2023 Mar 25;15(7):1591.
- Obermoser K, Brigo N, Schroll A, Monfort-Lanzas P, Gostner JM, Engl S, Geisler S, Knoll M, Schennach H, Weiss G, Fuchs D. Positive effects of probiotic therapy in patients with post-infectious fatigue. Metabolites. 2023 May 8;13(5):639.
- Ross, K. Psychobiotics: Are they the future intervention for managing depression and anxiety? A literature review. Explore. 2023 Sep 1;19(5):669-80.
- Warda AK, Rea K, Fitzgerald P, Hueston C, Gonzalez-Tortuero E, Dinan TG, Hill C. Heat-killed lactobacilli alter both microbiota composition and behaviour. Behavioural Brain Research. 2019 Apr 19;362:213-23.
- Zielińska D, Karbowiak M, Brzezicka A. The Role of Psychobiotics to Ensure Mental Health during the COVID-19 Pandemic—A Current State of Knowledge. International Journal of Environmental Research and Public Health. 2022 Sep 3;19(17):11022.
- Sharma R, Gupta D, Mehrotra R, Mago P. Psychobiotics: The next-generation probiotics for the brain. Current microbiology. 2021 Feb;78:449-63.
- Miyaoka T, Kanayama M, Wake R, Hashioka S, Hayashida M, Nagahama M, Okazaki S, Yamashita S, Miura S, Miki H, Matsuda H. Clostridium butyricum MIYAIRI 588 as adjunctive therapy for treatment-resistant major depressive disorder: a prospective open-label trial. Clinical neuropharmacology. 2018 Sep 1;41(5):151-5.
- Diop L, Guillou S, Durand H. Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: a double-blind, placebo-controlled, randomized trial. Nutrition research. 2008 Jan 1;28(1):1-5.
- Eskandarzadeh S, Effatpanah M, Khosravi-Darani K, Askari R, Hosseini AF, Reisian M, Jazayeri S. Efficacy of a multispecies probiotic as adjunctive therapy in generalized anxiety disorder: a double blind, randomized, placebo-controlled trial. Nutritional Neuroscience. 2021 Feb 1;24(2):102-8.
- Mohammadi AA, Jazayeri S, Khosravi-Darani K, Solati Z, Mohammadpour N, Asemi Z, Adab Z, Djalali M, Tehrani-Doost M, Hosseini M, Eghtesadi S. The effects of probiotics on mental health and hypothalamic–pituitary–adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutritional neuroscience. 2016 Nov 8;19(9):387-95.
- Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, Jafari P, Akbari H, Taghizadeh M, Memarzadeh MR, Asemi Z, Esmaillzadeh A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition. 2016 Mar 1;32(3):315-20.
- Marotta A, Sarno E, Del Casale A, Pane M, Mogna L, Amoruso A, Felis GE, Fiorio M. Effects of probiotics on cognitive reactivity, mood, and sleep quality. Frontiers in psychiatry. 2019 Mar 27;10:427235.
- Ait-Belgnaoui A, Colom A, Braniste V, Ramalho L, Marrot A, Cartier C, Houdeau E, Theodorou V, Tompkins T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterology & Motility. 2014 Apr;26(4):510-20.
- Andersson H, Tullberg C, Ahrné S, Hamberg K, Lazou Ahrén I, Molin G, Sonesson M, Håkansson Å. Oral administration of Lactobacillus plantarum 299v reduces cortisol levels in human saliva during examination induced stress: a randomized, double-blind controlled trial. International journal of microbiology. 2016 Oct;2016.
- Lalitsuradej E, Sirilun S, Sittiprapaporn P, Sivamaruthi BS, Pintha K, Tantipaiboonwong P, Khongtan S, Fukngoen P, Peerajan S, Chaiyasut C. The effects of synbiotics administration on stress-related parameters in thai subjects—a preliminary study. Foods. 2022 Mar 6;11(5):759.
- Ghorbani Z, Nazari S, Etesam F, Nourimajd S, Ahmadpanah M, Jahromi SR. The effect of synbiotic as an adjuvant therapy to fluoxetine in moderate depression: a randomized multicenter trial. Archives of neuroscience. 2018 Apr 30;5(2).
- Lennartsson AK, Theorell T, Kushnir MM, Bergquist J, Jonsdottir IH. Perceived stress at work is associated with attenuated DHEA-S response during acute psychosocial stress. Psychoneuroendocrinology. 2013 Sep 1;38(9):1650-7.
- Morris G, Anderson G, Maes M. Hypothalamic-pituitary-adrenal hypofunction in myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS) as a consequence of activated immune-inflammatory and oxidative and nitrosative pathways. Molecular neurobiology. 2017 Nov;54:6806-19.
- Lau HC, Ng SC, Yu J. Targeting the Gut Microbiota in Coronavirus Disease 2019: Hype or Hope? Gastroenterology. 2022 Jan;162(1):9-16. [CrossRef] [PubMed]
- Alenazy MF, Aljohar HI, Alruwaili AR, Daghestani MH, Alonazi MA, Labban RS, El-Ansary AK, Balto HA. Gut Microbiota Dynamics in Relation to Long-COVID-19 Syndrome: Role of Probiotics to Combat Psychiatric Complications. Metabolites. 2022 Sep 27;12(10):912. [CrossRef] [PubMed]
- Thye AY, Law JW, Tan LT, Pusparajah P, Ser HL, Thurairajasingam S, Letchumanan V, Lee LH. Psychological Symptoms in COVID-19 Patients: Insights into Pathophysiology and Risk Factors of Long COVID-19. Biology (Basel). 2022 Jan 2;11(1):61. [CrossRef] [PubMed]
- Del Toro-Barbosa M, Hurtado-Romero A, Garcia-Amezquita LE, García-Cayuela T. Psychobiotics: mechanisms of action, evaluation methods and effectiveness in applications with food products. Nutrients. 2020 Dec 19;12(12):3896.
- Corbitt M, Campagnolo N, Staines D, Marshall-Gradisnik S. A systematic review of probiotic interventions for gastrointestinal symptoms and irritable bowel syndrome in chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). Probiotics and antimicrobial proteins. 2018 Sep;10:466-77.
- Choudhury A, Tariq R, Jena A, Vesely EK, Singh S, Khanna S, Sharma V. Gastrointestinal manifestations of long COVID: A systematic review and meta-analysis. Therap Adv Gastroenterol. 2022 Aug 19;15:17562848221118403. [CrossRef] [PubMed]
- Chakraborty C, Sharma AR, Bhattacharya M, Dhama K, Lee SS. Altered gut microbiota patterns in COVID-19: Markers for inflammation and disease severity. World J Gastroenterol. 2022 Jul 7;28(25):2802-2822. [CrossRef] [PubMed]
- Lymberopoulos E, Gentili GI, Budhdeo S, Sharma N. COVID-19 severity is associated with population-level gut microbiome variations. Front Cell Infect Microbiol. 2022 Aug 23;12:963338. [CrossRef] [PubMed]
- d'Ettorre G, Ceccarelli G, Marazzato M, Campagna G, Pinacchio C, Alessandri F, Ruberto F, Rossi G, Celani L, Scagnolari C, Mastropietro C. Challenges in the management of SARS-CoV2 infection: the role of oral bacteriotherapy as complementary therapeutic strategy to avoid the progression of COVID-19. Frontiers in medicine. 2020 Jul 7;7:389.
- Sfera A, Osorio C, Hazan S, Kozlakidis Z, Maldonado JC, Zapata-Martín del Campo CM, Anton JJ, Rahman L, Andronescu CV, Nicolson GL. Long COVID and the Neuroendocrinology of Microbial Translocation Outside the GI Tract: Some Treatment Strategies. Endocrines. 2022 Nov 7;3(4).
- Geirnaert A, Calatayud M, Grootaert C, Laukens D, Devriese S, Smagghe G, De Vos M, Boon N, Van de Wiele T. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Scientific reports. 2017 Sep 13;7(1):11450.
- Homayouni-Rad A, Soleimani RA, Khani N. Can postbiotics prevent or improve SARS-CoV-2?. Current Nutrition & Food Science. 2023 Oct 1;19(8):756-7.
- Johnson D, Thurairajasingam S, Letchumanan V, Chan KG, Lee LH. Exploring the role and potential of probiotics in the field of mental health: Major depressive disorder. Nutrients. 2021 ;13(5):1728. 20 May.
- El Dib R, Periyasamy AG, de Barros JL, França CG, Senefonte FL, Vesentini G, Alves MG, da Silva Rodrigues JV, Gomaa H, Júnior JR, Costa LF. Probiotics for the treatment of depression and anxiety: A systematic review and meta-analysis of randomized controlled trials. Clinical nutrition ESPEN. 2021 Oct 1;45:75-90.
- Zhou J, Li M, Chen Q, Li X, Chen L, Dong Z, Zhu W, Yang Y, Liu Z, Chen Q. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nature Communications. 2022 Jun 14;13(1):3432.
- Kumar LS, Pugalenthi LS, Ahmad M, Reddy S, Barkhane Z, Elmadi J. Probiotics in irritable bowel syndrome: a review of their therapeutic role. Cureus. 2022 Apr 18;14(4).
- Haarhuis JE, Kardinaal A, Kortman GA. Probiotics, prebiotics and postbiotics for better sleep quality: A narrative review. Beneficial Microbes. 2022 Aug 3;13(3):169-82.
- Castro-Marrero J, Faro M, Aliste L, Sáez-Francàs N, Calvo N, Martínez-Martínez A, de Sevilla TF, Alegre J. Comorbidity in chronic fatigue syndrome/myalgic encephalomyelitis: a nationwide population-based cohort study. Psychosomatics. 2017 Sep 1;58(5):533-43.
- Castro-Marrero J, Domingo JC, Cordobilla B, Ferrer R, Giralt M, Sanmartín-Sentañes R, Alegre-Martín J. Does coenzyme Q10 plus selenium supplementation ameliorate clinical outcomes by modulating oxidative stress and inflammation in individuals with myalgic encephalomyelitis/chronic fatigue syndrome?
- Barletta MA, Marino G, Spagnolo B, Bianchi FP, Falappone PC, Spagnolo L, Gatti P. Coenzyme Q10+ alpha lipoic acid for chronic COVID syndrome. Clinical and Experimental Medicine. 2023 Jul;23(3):667-78.
| Participants (n) | Intervention (dose) |
Duration | Outcomes | References | |
|---|---|---|---|---|---|
| ME/CFS patients (n=83) |
Single probiotic: Lactobacillus casei Shirota |
8 weeks | Reduced anxiety scores, Changed the faecal composition |
[88] | |
| ME/CFS patients (n=83) |
Single probiotic: Bifidobacterium infantis 35624 |
8 weeks | Reduced inflammatory biomarkers (CRP, IL-6) | [88] | |
| ME/CFS patients (n=9) |
Probiotic protocol: Enterelle (given in week 1, 4-8): Saccharomyces cerevisiae sub. Boilardii MTCC-5375, Saccharomyces cerevisiae sub. Boilardii SP92, Enterococcus faecium UBEF41, Lactobacillus acidophilus LA14 Bifiselle (given in week 2): Bifidobacterium lactis BL04, Bifidobacterium breve BB03, Bifidobacterium bifidum BB06, Bifidobacterium longum BL05 Citogenex (given in week 4-8): Saccharomyces spp extract titrated in 1-3 beta glucans, Vitamin C, Lactobacillus casei LC11, Bifidobacterium lactis BL04, Lactobacillus acidophilus LA14 Ramnoselle (given in week 3, 4-8): Lactobacillus acidophilus LA14, Lactobacillus rhamnosus LR32, Lactobacillus rhamnosus HN001 |
2 caps/a day Each strain > 1 × 109 CFU |
8 weeks | Reduction of fatigue on the Chadler’s scale, Improvement of physical and mental conditions, Overall increase in the quality of life on the SF-36 score, Improvement of mood on PCS score, Reduced depression symptoms on BDI-I and II scores Reduced inflammation (CRP); Improvement in immune defense (increase in IgM, reduction in CD4/CD8 ratio); Inconsistent effects on oxidative status |
[82] |
| ME/CFS (n=15) |
Multistrain probiotic: Lactobacillus F19 Lactobacillus acidophilus NCFB 1748 Bifidobacterium lactis Bb12 |
× 108 CFU/ml | weeks | Improvement in neurocognitive functions measured as the VAS mean, Inconsistent effects fatigue, mental and physical health, No major changes in GI microbiota |
[90] |
| Hospitalized COVID-19 patients (n=24) |
Multistrain probiotic (SLAB51; Sivomixx800®): Streptococcus thermophilus DSM 32245®, Bifidobacterium lactis DSM 32246®, Bifidobacterium lactis DSM 32247®, Lactobacillus acidophilus DSM 32241®, Lactobacillus helveticus DSM 32242®, Lactobacillus paracasei DSM 32243®, Lactobacillus plantarum DSM 32244®, Lactobacillus brevis DSM 27961® |
3 equal doses of a total of 2.400 × 1011 CFU billion bacteria per day | 23 days | Reduced fatigue on FAS score, Minor changes in metabolomic profile - increased levels of arginine, asparagine, lactate, and decreased levels of 3-Hydroxyisobutyrate following probiotic intake, |
[92] |
| Long COVID patients (19) |
Multistrain probiotic (VSL#3®): Streptococcus thermophilus BT01, Bifidobacterium breve BB02, Bifidobacterium animalis subsp. lactis BL03, Bifidobacterium animalis subsp. lactis BI04, Lactobacillus acidophilus BA05, Lactobacillus plantarum BP06, Lactobacillus paracasei BP07, Lactobacillus helveticus BD08 |
2 shashets a day, A total of 4.5 × 1011 CFU per sachet | 4 weeks | Reduced fatigue (Chalder Fatigue Scale), Significant improvement on physical functioning on SF-36 scale, Reduction in GI symptoms on SAGIS score), No significant difference in specific symptoms of acid regurgitation, nausea and vomiting, constipation, epigastric pain and IBS symptoms vs placebo, No effects on psychiatric symptoms of anxiety, depression, performance and somatization symptoms on SCL-12 scale, |
[93] |
| Fatigue subjects (9) |
Single probiòtic: Lactobacillus paracasei HII01 |
4 × 1010 CFU/g | 12 weeks | Decreased salivary levels of cortisol, No effects on DHEA-S concentration, Decreased ratio cortisol: DHEA-S, |
[110] |
| Hospitalized COVID patients (28) |
Multistrain probiotic (Sivomixx®): Streptococcus thermophilus DSM 32345, Lactobacillus acidophilus DSM 32241, Lactobacillus helveticus DSM 32242, Lacticaseibacillus paracasei DSM 32243, Lactobacillus plantarum DSM 32244, Lactobacillus brevis DSM 27961, Bifidobacterium lactis DSM 32246, Bifidobacterium lactis DSM 32247 |
2.5 × 1010 CFU/a day | 1 week | Amelioration of in diarrhea within 3-7 days, Reduction in other symptoms, including fever, asthenia, headache, myalgia, and dyspnea within 2 days, Improvement in respiratory function – 8-fold reduced risk of respiratory failure, |
[122] |
| Multi-ingredient microbial preparations | |||||
| Long COVID patients (100) |
ImmunoSEB formulation: Probiotics - ProbioSEB CSC3: Bacillus coagulans LBSC (DSM 17654), Bacillus subtilis PLSSC (ATCC SD 7280), Bacillus clausii 088AE (MCC 0538) Bioactives: Peptizyme SP: enteric coated serratiopeptidase, bromelain, amylase, lysozyme, peptidase, catalase, papain, glucoamylase, lactoferrin |
4 capsules a day: One capasule: ImmunoSEB: 500g/capsule + ProbioSEB CSC3: 5 × 1010 CFU/capsule |
2 weeks | Significant reduction of fatigue determined by by the CFQ-11 score (87% of patients were fatigue free in the end of interevention), Significant reduction in all individual measures of physical fatigue (tiredness, need to rest, drowsiness, ability to do things, energy level, muscle strength and feeling of weakness), Significant reduction in mental fatigue (concentration, focus and memory), No adverse events reported, including nausea, vomiting or diarrhea at any stage of study |
[84] |
| Long COVID patients (126) | Phytochemical-rich concentrated food capsule: Probiotic: Lactobacillus plantarum, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Lactococcus lactis Lactobacillus paracasei Prebiotic: Inulin fibre Bioactives: Citrus Sinensis fruit, Chamomile (Matricaria recutita L. flower), Curcuma Longa, Pomegranate (Punica granatum L.), Resveratrol (Polygonum cuspidatum root) |
1 capsule given twice a day Probiotic: 10 × 1010 CFU/total in capsule Prebiotic: 200mg/capsule Phytochemical-rich whole food capsule (PC): Citrus Sinensis fruit: 400 mg, inc. 70 mg of bioflavonoids, Chamomile (Matricaria recutita L. flower): 1000 mg, Curcuma Longa: 23.8mg of curcuminoid, Pomegranate (Punica granatum L.): 1000mg, incl. 10mg ellagic acid, Polygonum cuspidatum root: 100 mg of resveratrol |
2 weeks | 2-fold reduction in fatigue measured on the Chalder Fatigue Scale vs placebo, Reduction in other symtoms associated with infection, including cough, Improved overall well-being measured on SWB score, |
[95] |
| Fibromyalgia (FM) patients co-diagnosed with ME/CFS (15) | Synbiotic (Gasteel Plus®) formulation: Probiotics: Bifidobacterium lactis CBP-001010, Lactobacillus rhamnosus CNCM I-4036 Bifidobacterium longum ES1 Prebiotic: Fructooligosaccharides Bioactives: Zinc, Selenium, Vitamin D |
Each synbiotic bar (300mg): Probiotic: 1 × 109 CFU/total in bar Prebiotic: 200mg/bar Bioactives: Zinc: 1.5mg Selenium: 8.25mg Vitamin D: 0.75g |
2 weeks | No changes in the objective perception of activity/sedentarism and sleep, Significant improvements in depression, stress, anxiety and fatigue in FM patients, Significant improvement in anxiety and fatigue in FM patients with ME/CFS, Reduced inflammation (decrease in IL-8, increse in IL-10) among FM patients, Increased ratio of cortisol/DHEA in all participants, Overall no significant benefits for improved pain, sleep quality and gastrointestinal health of the participants. |
[96] |
| Long COVID patients (70) | OMNi-BiOTiC®STRESS Repair 9” formulation: Probiotics: Lactobacillus casei W56, Lactobacillus acidophilus W22, Lactobacillus paracasei W20, Bifidobacterium lactis W51, Lactobacillus salivarius W24, Lactococcus lactis W19, Bifidobacterium lactis W52, Lactobacillus plantarum W62, Bifidobacterium bifidum W23 Prebiotic: Fructooligosaccharides, inulin Bioactives: Enzymes (amylases), Potassium chloride, Manganese sulfate, Vitamin B2 , Vitamin B6 , Vitamin B12 |
Each sachet: 3g Probiotic: 7.5 × 109 CFU/total in sachet Prebiotic: N/A Bioactives: Enzymes (amylases), Potassium chloride, Manganese sulfate, Vitamin B2 Vitamin B6 Vitamin B12 |
24 weeks | Significant reduction in the fatigue on the FSS score vs placebo, Significant improvement in the severity of depression, Significant improvement in the quality of life, including physical functioning and general Health. Improvement of digestion and reduction of GI complaints. No effect on immune parameters. |
[97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





