Submitted:
29 April 2024
Posted:
01 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
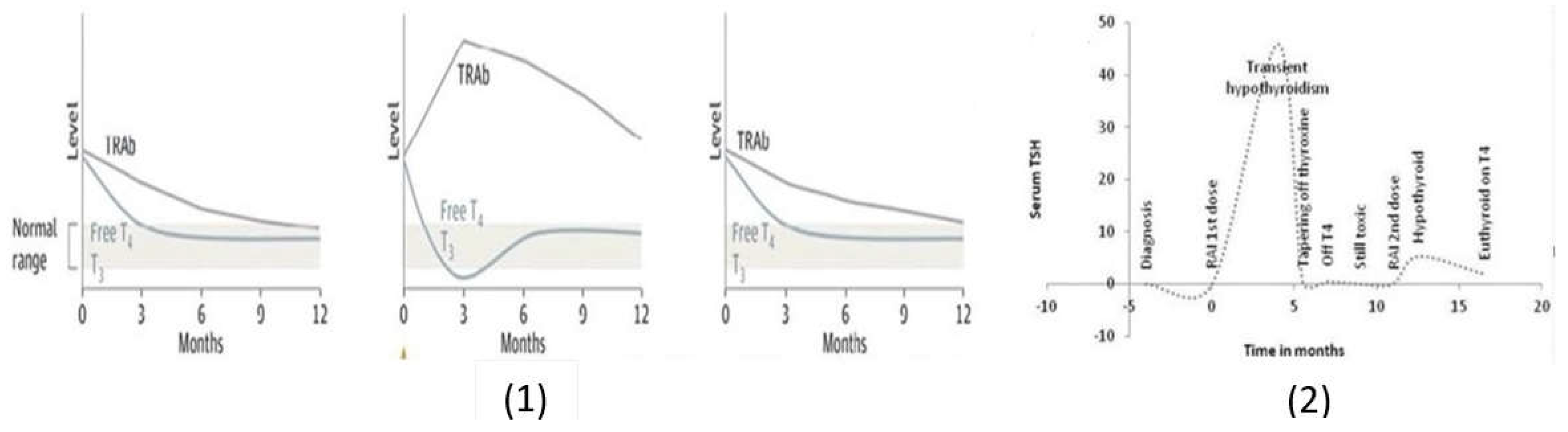
Antithyroid Drugs
Vicious Cycle in GD Patients
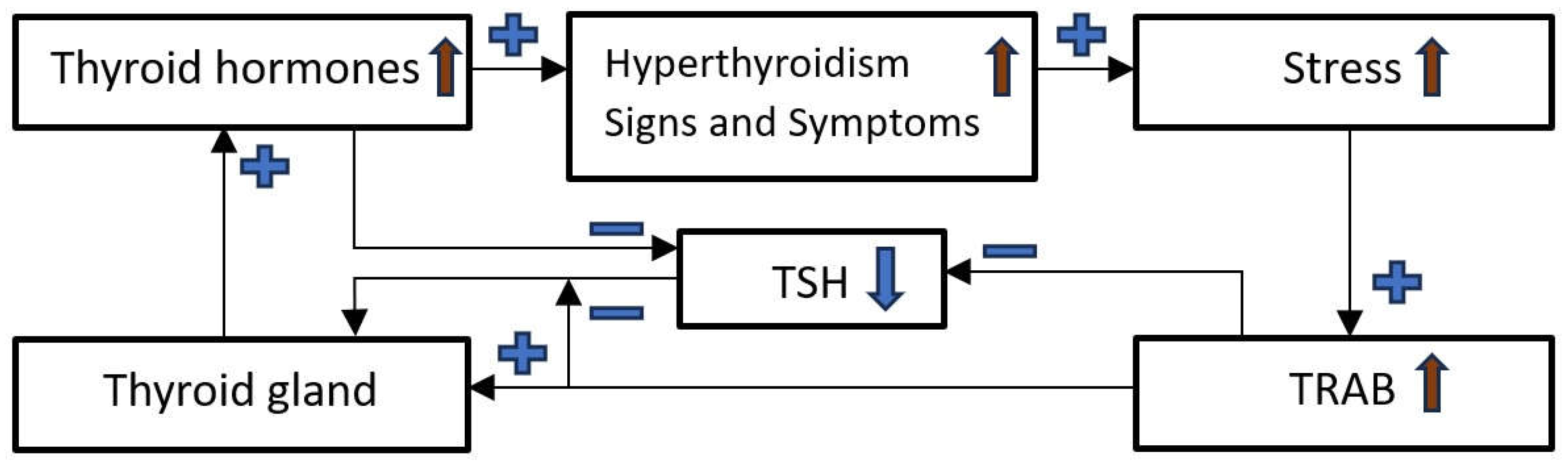
Breaking the Vicious Cycle
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Brokken, L. J. , Wiersinga, W. M., & Prummel, M. F. (2003). Thyrotropin receptor autoantibodies are associated with continued thyrotropin suppression in treated euthyroid Graves’ disease patients. The Journal of Clinical Endocrinology & Metabolism, 88(9), 4135-4138.
- Chung, Y. J. , Lee, B. W., Kim, J. Y., Jung, J. H., Min, Y. K., Lee, M. S.,... & Chung, J. H. (2006). Continued suppression of serum TSH level may be attributed to TSH receptor antibody activity as well as the severity of thyrotoxicosis and the time to recovery of thyroid hormone in treated euthyroid Graves' patients. Thyroid, 16(12), 1251-1257.
- Barbesino, G. , & Tomer, Y. (2013). Clinical utility of TSH receptor antibodies. The Journal of Clinical Endocrinology & Metabolism, 98(6), 2247-2255.
- Eckstein, A. K. , Plicht, M., Lax, H., Neuhäuser, M., Mann, K., Lederbogen, S.,... & Morgenthaler, N. G. (2006). Thyrotropin receptor autoantibodies are independent risk factors for Graves’ ophthalmopathy and help to predict severity and outcome of the disease. The Journal of Clinical Endocrinology & Metabolism, 91(9), 3464-3470.
- Orgiazzi, J. , & Madec, A. M. (2002). Reduction of the risk of relapse after withdrawal of medical therapy for Graves' disease. Thyroid, 12(10), 849-853.
- Abdi, H. , Amouzegar, A., & Azizi, F. (2019). Antithyroid drugs. Iranian journal of pharmaceutical research: IJPR, 18(Suppl1), 1.
- Burch, H. B. , & Cooper, D. S. (2018). Antithyroid drug therapy: 70 years later. European Journal of Endocrinology, 179(5), R261-R274.
- Tötterman, T. H. , Karlsson, F. A., Bengtsson, M., & Mendel-Hartvig, I. B. (1987). Induction of circulating activated suppressor-like T cells by methimazole therapy for Graves' disease. New England Journal of Medicine, 316(1), 15-22.
- Laurberg, P. , Wallin, G., Tallstedt, L., Abraham-Nordling, M., Lundell, G., & Tørring, O. (2008). TSH-receptor autoimmunity in Graves' disease after therapy with anti-thyroid drugs, surgery, or radioiodine: a 5-year prospective randomized study. European Journal of Endocrinology, 158(1), 69-75.
- Törring, O. , Tallstedt, L., Wallin, G., Lundell, G., Ljunggren, J. G., Taube, A.,... & Hamberger, B. (1996). Graves' hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine--a prospective, randomized study. Thyroid Study Group. The Journal of Clinical Endocrinology & Metabolism, 81(8), 2986-2993.
- Reinwein, D. , Benker, G., Lazarus, J. H., & Alexander, W. D. (1993). A prospective randomized trial of antithyroid drug dose in Graves' disease therapy. European Multicenter Study Group on Antithyroid Drug Treatment. The Journal of Clinical Endocrinology & Metabolism, 76(6), 1516-1521.
- Lewandowski, K. C. , Marcinkowska, M., Skowrońska-Jóźwiak, E., Makarewicz, J., & Lewiński, A. (2008). New onset Graves' disease as a cause of an adrenal crisis in an individual with panhypopituitarism: brief report. Thyroid Research, 1, 1-5.
- Volpe, R. (1994). Evidence that the immunosuppressive effects of antithyroid drugs are mediated through actions on the thyroid cell, modulating thyrocyte-immunocyte signaling: a review. Thyroid, 4(2), 217-223.
- Kocjan, T. , Wraber, B., Kocijančič, A., & Hojker, S. (2004). Methimazole upregulates T-cell-derived cytokines without improving the existing Th1/Th2 imbalance in Graves’ disease. Journal of Endocrinological Investigation, 27, 302-307.
- Wenzel, K. W. , & Lente J. R. (1984). Similar effects of thionamide drugs and perchlorate on thyroid-stimulating immunoglobulins in Graves' disease: evidence against an immunosuppressive action of thionamide drugs. The Journal of Clinical Endocrinology & Metabolism, 58(1), 62-69.
- Stern, R. A. , Robinson, B., Thorner, A. R., Arruda, J. E., Prohaska, M. L., & Prange Jr, A. J. (1996). A survey study of neuropsychiatric complaints in patients with Graves' disease. Journal of Neuropsychiatry and Clinical Neurosciences, 8(2), 181-185.
- Bianchi, G. , Solaroli, E., Zaccheroni, V. A. A., Grossi, G., Bargossi, A. M., Melchionda, N., & Marchesini, G. (1999). Oxidative stress and anti-oxidant metabolites in patients with hyperthyroidism: effect of treatment. Hormone and Metabolic Research, 31(11), 620-624.
- Stojanovich, L., & Marisavljevich, D. (2008). Stress as a trigger of autoimmune disease. Autoimmunity Reviews, 7(3), 209-213.
- Sharif, K. , Watad, A., Coplan, L., Lichtbroun, B., Krosser, A., Lichtbroun, M.,... & Shoenfeld, Y. (2018). The role of stress in the mosaic of autoimmunity: an overlooked association. Autoimmunity Reviews, 17(10), 967-983.
- Vita, R. , Lapa, D., Trimarchi, F., & Benvenga, S. (2015). Stress triggers the onset and the recurrences of hyperthyroidism in patients with Graves’ disease. Endocrine, 48, 254-263.
- Gulseren, S. , Gulseren, L., Hekimsoy, Z., Cetinay, P., Ozen, C., & Tokatlioglu, B. (2006). Depression, anxiety, health-related quality of life, and disability in patients with overt and subclinical thyroid dysfunction. Archives of medical research, 37(1), 133-139.
- Larsen, C. B. , Riis, K. R., Winther, K. H., Larsen, E. L., Ellervik, C., Hegedüs, L.,... & Bonnema, S. J. (2021). Treatment of hyperthyroidism reduces systemic oxidative stress, as measured by markers of RNA and DNA damage. The Journal of Clinical Endocrinology & Metabolism, 106(7), e2512-e2520.
- Vita, R. , Lapa, D., Vita, G., Trimarchi, F., & Benvenga, S. (2009). A patient with stress-related onset and exacerbations of Graves’ disease. Nature Clinical Practice Endocrinology & Metabolism, 5(1), 55-61.
- Benvenga, S. (1996). Benzodiazepine and remission of Graves' disease. Thyroid, 6(6), 659-660.
- Wang, L. , Wang, B., Chen, S. R., Hou, X., Wang, X. F., Zhao, S. H.,... & Wang, Y. G. (2016). Effect of selenium supplementation on recurrent hyperthyroidism caused by Graves’ disease: a prospective pilot study. Hormone and Metabolic Research, 48(09), 559-564.
- Shahida, B. , Tsoumani, K., Planck, T., Modhukur, V., Asp, P., Sundlöv, A.,... & Lantz, M. (2022). Increased risk of Graves´ ophthalmopathy in patients with increasing TRAb after radioiodine treatment and the impact of CTLA4 on TRAb titres. Endocrine, 75(3), 856-864.
- Burch, H. B. , & Cooper, D. S. (2015). Management of Graves disease: a review. JAMA, 314(23), 2544-2554.
- Sheehan, M. T. , & Doi, S. A. (2016). Transient hypothyroidism after radioiodine for Graves’ disease: challenges in interpreting thyroid function tests. Clinical Medicine & Research, 14(1), 40-45.
- Abalovich, M. , Llesuy, S., Gutierrez, S., & Repetto, M. (2003). Peripheral parameters of oxidative stress in Graves’ disease: the effects of methimazole and 131 iodine treatments. Clinical Endocrinology, 59(3), 321-327.
- Stan, M. N. , Durski, J. M., Brito, J. P., Bhagra, S., Thapa, P., & Bahn, R. S. (2013). Cohort Study on Radioactive Iodine–Induced Hypothyroidism: Implications for Graves' Ophthalmopathy and Optimal Timing for Thyroid Hormone Assessment. Thyroid, 23(5), 620-625.
- Perros, P. , Kendall-Taylor, P., Neoh, C., Frewin, S., & Dickinson, J. (2005). A prospective study of the effects of radioiodine therapy for hyperthyroidism in patients with minimally active Graves’ ophthalmopathy. The Journal of Clinical Endocrinology & Metabolism, 90(9), 5321-5323.
- Aizawa, Y. , Yoshida, K., Kaise, N., Fukazawa, H., Kiso, Y., Sayama, N.,... & Abe, K. (1997). The development of transient hypothyroidism after iodine-131 treatment in hyperthyroid patients with Graves' disease: prevalence, mechanism and prognosis. Clinical Endocrinology, 46(1), 1-5.
- Chakrabarti, S. K., Ghosh, S., Banerjee, S., Mukherjee, S., & Chowdhury, S. (2016). Oxidative stress in hypothyroid patients and the role of antioxidant supplementation. Indian Journal of Endocrinology and Metabolism, 20(5), 674-678.
- Tallstedt, L. , Lundell, G., Blomgren, H., & Bring, J. (1994). Does early administration of thyroxine reduce the development of Graves' ophthalmopathy after radioiodine treatment? European Journal of Endocrinology, 130(5), 494-497.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




