1. Introduction
Inflammatory bowel diseases (IBD), represented by Crohn's disease (CD) and ulcerative colitis (UC), are chronic and recurrent gastrointestinal disorders, characterized by alternating periods of symptom activation and remission, including abdominal pain, diarrhea and rectal bleeding. These conditions significantly compromise patients’ quality of life [
1,
2]
Epidemiological data indicate a rising global trend in new IBD cases, imposing a substantial burden on healthcare system [
3]. Despite ongoing research, the etiology of IBD remains incompletely understood. The interplay between genetic and environmental factors, oxidative stress, and an exacerbated, uncontrolled immune response is implicated in both the onset and progression of the inflammatory process [
4].
In inflammatory bowel diseases (IBD), a redox imbalance occurs, where the antioxidant defense is insufficient to counteract the excess of reactive oxygen and nitrogen species (RONS) generated. This contributes to impairment of the epithelial barrier and consequent increase in intestinal permeability, allowing the entry of luminal antigens that induce a dysregulated immune response characterized by elevated reactive species and pro-inflammatory cytokines. This results in oxidative damage to cellular components, further exacerbating the chronic inflammatory state [
5,
6].
Currently, drug therapy of IBD involves synthetic drugs and monoclonal antibodies designed to manage imminent inflammation. However, prolonged use of these therapies may lead adverse effects, limiting their effectiveness and causing many patients to become refractory to treatment, thereby increasing the risk of complications and surgical interventions [
7,
8].
To address these challenges, research has explored new therapeutic alternatives, with particular emphasis on the utilization of
Curcuma longa, commonly known as turmeric, and their three main curcuminoids — curcumin, demethoxycurcumin, and bisdemethoxycurcumin—with curcumin being the primary bioactive component [
9].
Curcumin, a hydrophobic polyphenol, exhibits potent antioxidant, anti-inflammatory, antitumor, antimicrobial, anti-glycating, anti-coagulant and healing properties, as evidenced by both experimental studies and clinical trials clinical trials in IBD [
10] and other clinical situations such as Type 2 diabetes [
11] and some cancers [
12]. However, its therapeutic potential is hindered by low bioavailability, attributed to reduced solubility, rapid metabolization and systemic elimination [
13,
14].
Piperine, a bioactive compound from black pepper (
Piper nigrum), emerges as a potentiation agent capable of inhibiting hepatic glucuronidation, thereby increasing curcumin bioavailability by up to 2000% [
15]. The combination of curcumin and piperine has shown successful outcomes in diseases where oxidative stress is a significant etiological factor, such as metabolic syndrome [
16,
17]. Despite the positive effects of curcumin or turmeric in IBD, the combined impact with piperine, along with its antioxidant effects and influence on inflammatory markers like fecal calprotectin and cytokines, remains unexplored in clinical trials [
18]. This highlights a crucial gap in understanding the comprehensive effects of the mixture, in patients with IBD.
Therefore, the objective of the present study was to clinically investigate the efficacy of using curcumin alone or in combination with piperine on markers of oxidative stress and inflammation in patients with IBD, in different matrixes (blood and feces).
2. Results
This section was divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
2.1. Baseline Characteristics
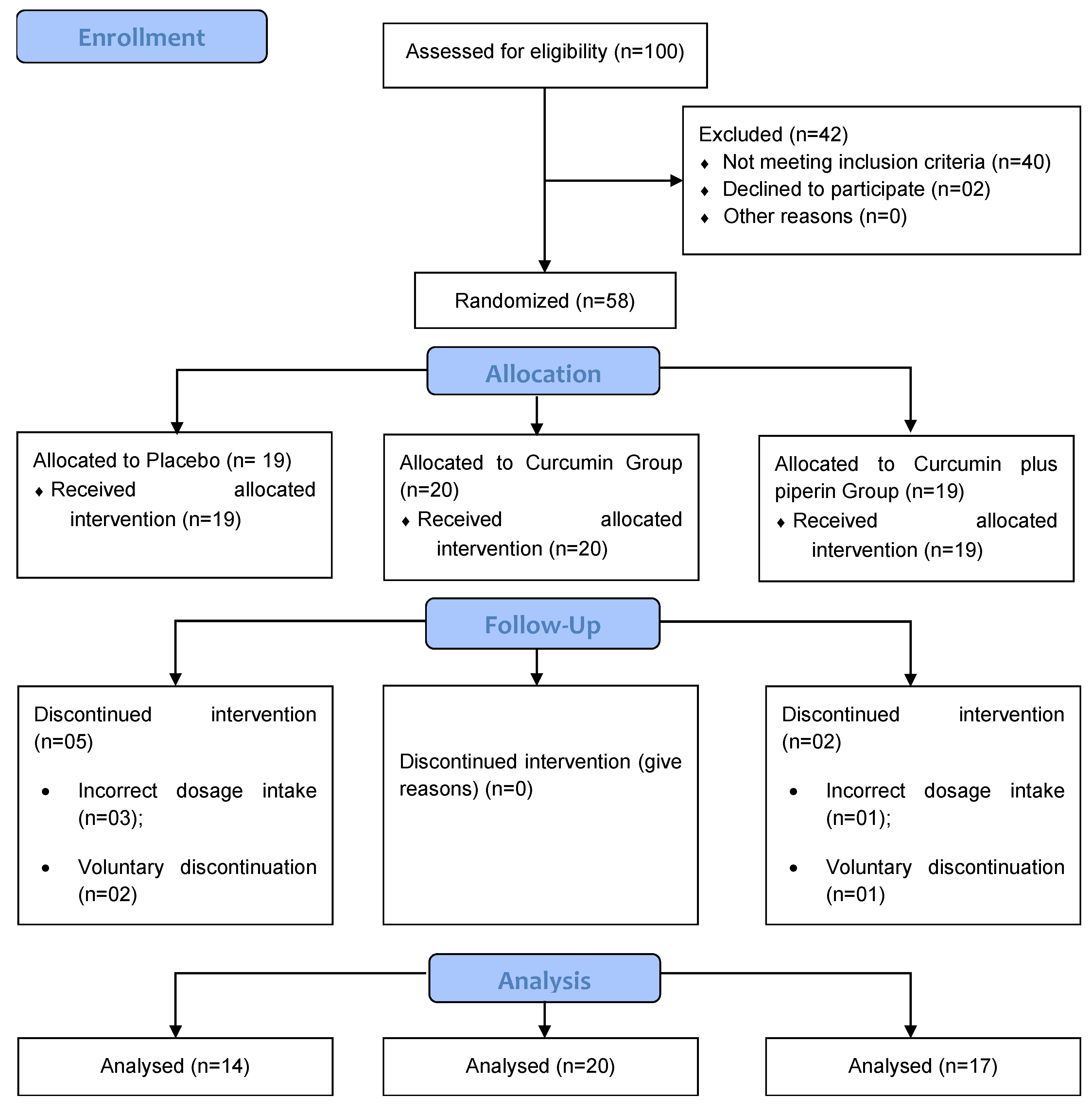
The present study was carried out between July 2021 and March 2023. Initially, fifty-eight (58) patients were enrolled in the study. Among them, fifty-one completed the 12 weeks of supplementation, 14 in the placebo group, 20 in the Curcumin group and 17 in the Curcumin + piperine group. Participants were excluded due to loss of contact during follow-up of the clinical study, voluntary discontinuation of the supplement and incorrect intake of the recommended dosage (
Figure 1).
The participants' mean age was 47.5 ± 15.5, with the majority being female (n=38; 65.5%) and in a stable relationship (n=44; 75.9%). None of the patients consumed alcoholic beverages, and physical activity was not a prevalent practice. Biologic therapy emerged as the primary pharmacological treatment among study participants (n=30; 51.7%), due to its effectiveness in better controlling the inflammatory condition. Notably, the observed data in
Table 1 underscore a low prevalence of non-communicable chronic diseases (n=17; 29.7%) and extraintestinal manifestations (n=5; 8.6%).
However, despite the randomization conducted at the beginning of the clinical trial, a higher prevalence of education exceeding 4 years was significantly more frequent in the placebo group (p=0.031), along with a greater number of patients with UC allocated to the Curcumin group (p=0.026). Nevertheless, both situations ceased to be statistically significant by the end of the experiment (data not shown).
Effects of Supplementation on Gastrointestinal Symptoms
In this study, as a secondary outcome, we assessed the impact of supplementation with Curcumin, with or without piperine, on various gastrointestinal complaints, common in patients with IBD. As depicted in supplementary 2, at baseline, there were no significant differences between the groups. However, at the study's conclusion, participants in the Curcumin + piperine group exhibited significantly higher complaints of heartburn compared to the placebo group. This distinction is evident in both the chi-square test (p=0.017) and the generalized estimating equation (group vs. time interaction), indicating 4.1 times higher (p=0,016; OR: 4.151; 95% IC, 1.309 – 13.169), likelihood of patients receiving this supplementation reporting heartburn compared to the placebo group (supplementary 1).
2.2. Effects of Supplementation on Redox Imbalance and Inflammation
Among the oxidative stress and inflammation markers (
Table 2 and
Table 3) evaluated in this study, supplementation with Curcumin and piperine for 3 months proved effective in significantly increasing the levels of SOD compared to the placebo group after adjusting for sex, age, and type of IBD (4346.9 ± 879.0 vs 3614.5 ± 731.5; p=0.02; IC95%: 102.262 – 1528.186). Furthermore, it demonstrated a more pronounced impact when comparing endline to baseline (Δ) (538.8 ± 1040.1 vs -126.8 ± 762.7, placebo group; p = 0.027; IC95%: -351.986 – 1094.626). The other redox markers (CAT, MDA, and H
2O
2 levels), disease activity (CalF), inflammation (MPO, TNF-α, IL-17A, and IL-22), and anti-inflammatory markers (IL-10) did not show significant changes after the experimental period.
3. Discussion
Up to our knowledge, this is the first randomized, double-blind clinical trial assessing the impact of Curcumin supplementation, either alone or in combination with piperine, on oxidative, inflammatory, and disease activity markers in patients with inflammatory bowel disease (IBD). Our findings allow to affirm that the standard commercially available powder, rich in curcumin and combined with piperine, exhibits significant antioxidant action by substantially enhancing the endogenous defense mechanism, as evidenced by a noteworthy increase in serum SOD levels among supplemented patients over the three-month intervention period. These unprecedented results for this polyphenol underscore its beneficial effects on patients with IBD. This is further supported by a recent meta-analysis published by our research group [
24].
The SOD family in mammals comprises three isoforms: SOD1 (Cu/ZnSOD, cytosolic), SOD2 (MnSOD, mitochondrial), and SOD3 (Cu/ZnSOD, extracellular). Their primary role is to scavenge superoxide radicals (O
2-•), reactive signaling molecules that can induce cell damage. SODs convert O
2-• into H
2O
2, a less reactive form that can be further detoxified by catalase or glutathione peroxidase (GPx) [
25,
26]. Additionally, SOD activity reduces iron release, inhibiting hydroxy radical (
•OH) formation, by Fenton reaction, and its downstream effects on lipids, proteins, and DNA [
27].
Conversely, reducing circulating levels of the hydroxy radical impact the generation of reactive nitrogen species (RNS), as the superoxide radical can directly react with nitric oxide (
•NO) to form peroxynitrite (ONOO
−), a potent oxidant and nitrating agent towards a wide range of macromolecules. Moreover, ONOO
− is relatively stable under physiological conditions and can diffuse across several cell diameters, rendering it more toxic [
26]. In this scenario, the increase of SOD levels not only diminishes peroxynitrite production, which also affects macromolecules, but also enables the utilization of nitric oxide as a vasodilator—an important factor in various biological contexts.
These results are corroborated by a randomized clinical trial in patients with metabolic syndrome, where the combination of curcumin plus piperine, supplemented at the same dosage used daily in this study (1000 mg + 10 mg), for 8 weeks, significantly increased SOD activity [
28]. Another study conducted by Panahi et al. [
29] also demonstrated the effectiveness of the Curcumin C3 Complex® formulation (1500 mg/day) in increasing SOD activity in patients with osteoarthritis, after 6 weeks [
30], thus demonstrating the effectiveness of curcumin on this marker. of redox imbalance.
The observed increase in SOD levels in this study among patients receiving Curcumin plus piperine, with no alterations in H
2O
2 and MDA levels, suggests a beneficial effect of this supplementation. It is noteworthy that oxidative stress in IBD is considered both an etiological factor and a trigger for exacerbations, negatively impacting the quality of life of individuals with IBD. Studies indicate significant changes in SOD activity among IBD patients. In addition to lower levels of this enzyme compared to individuals without the disease, these low levels are associated with an increase in the inflammatory process [
31]. Another change found in those individuals pertains to the expression of all three isoforms of SOD in the colonic mucosa. Kruidenier et al. [
32] concluded in their study with 29 IBD patients that both CD and UC are accompanied by increased intestinal Mn-SOD and decreased Cu/Zn-SOD and EC-SOD levels, particularly in the inflamed mucosal epithelium.
In this context, it becomes clear that finding strategies, whether pharmacological or not, to improve the activity of this enzyme is crucial for the clinical therapy of the patients. The use of SOD or SOD mimetics has been tested as a pharmacological strategy in various
in vivo and
in vitro models [
33], including induced colitis either through direct administration [
34], or by stimulating the production of this enzyme in
Lactobacillus [
35]. However, these interventions are not yet considered a viable/safe option in humans, and hence, randomized clinical trials testing these formulations are not readily available. Considering this, antioxidant therapy with natural or synthetic substances has been the subject of numerous investigations.
Recently, in a meta-analysis published by our group, evaluating clinical trials that investigated antioxidant or anti-inflammatory actions in their treatments for IBD, only SOD was significantly modulated, while markers such as MDA, like our findings, and total antioxidant capacity showed no significant relevance [
18].
As mentioned earlier, despite Curcumin being a widely studied polyphenol in IBD, its antioxidant and anti-inflammatory roles have only been confirmed in animal models of ulcerative colitis and not in clinical trials, underscoring the novelty of our results.
Regarding the impact of supplementation on the inflammatory profile, in the present study, no significant changes were found in the plasma levels of the investigated markers (TNF-α, IL-22, IL-17, IL-10, and MPO), nor in CalF, reflecting the inflammatory activity of the intestine. These results align with the study by Sadeghi et al. (2020), conducted in patients with UC, where the administration of 1500 mg/day of curcumin for 8 weeks did not cause changes in TNF-α levels [
36].
While we acknowledge certain limitations in this study, such as the loss of five patients in the placebo group, the short duration of supplementation (3 months), and the inclusion of patients from a single follow-up center, it is crucial to emphasize that both treatment groups remain representative in relation to the calculated sample size. The brief supplementation period was strategically based on the standard interval between medical consultations in the department itself, and the single follow-up center serves as the state reference for patients with IBD. We employed common markers, enabling effective comparisons with other studies and strengthening the generalizability and applicability of the results.
Despite the higher incidence of heartburn complaints in the Curcumin plus piperine supplemented group, it is noteworthy that no patient reported the necessity to discontinue the supplementation. This suggests that the combination is well-tolerated and does not result in significant adverse effects. This self-reported symptom may be related to curcumin's ability to prominently inhibit the activity of cyclooxygenase-1 and 2, causing effects similar to some nonsteroidal anti-inflammatory drugs. The consequent blockade in prostaglandin synthesis, which acts as cytoprotective to the gastric mucosa, leads to increased acid production and reduced mucus in the stomach [
37,
38].
4. Materials and Methods
Study Type and Design
This randomized, double-blind, placebo-controlled clinical study was conducted at the Coloproctology sector of Hospital Universitário Professor Alberto Antunes, Maceió-AL, Brazil, from July 2021 to March 2023.
Eligible participants were diagnosed with mild to moderate CD or UC, aged ≥18 years, and undergoing conventional drug therapy for IBD (aminosalicylates, immunosuppressants, biological therapy) or without a specific medication prescription for IBD, with preserved kidney and liver function. Exclusion criteria encompassed individuals with one or more of the following conditions: (1) fistulas, abscesses and strictures; (2) extensive resections of the small intestine (< 200 cm of remaining intestine); (3) patients admitted for acute treatment, in serious general condition; (4) cancer; (5) HIV positive; (6) Pregnant and breastfeeding women; and (7) hospitalization for IBD in the last three months. The exclusion of the clinical trial occurred if: (1) gestation; (2) change or withdrawal of conventional dosage/medication for IBD; (3) HIV-positive patients and renal function and compromised liver; (4) patients who voluntarily discontinued supplementation; and (5) hospitalization for IBD during clinical trial. All participants signed an Informed Consent Form.
This study was registered at ensaiosclinicos.gov.br as RBR-89q4ydz on July 20, 2023. It was approved by the Ethics Committee of the Federal University of Alagoas under the process no. 7829516.5.0000.5013, on February 28, 2019.
Intervention
The Curcumin powder, along with piperine, was administered at doses of 1000 mg/day and 10 mg/day, respectively were prescribed to participants who maintained their clinical therapeutic regimen prescribed by a medical professional in the sector. These supplements were encapsulated in two capsules, meticulously designed to minimize color distortion. Patients were instructed to take these capsules after lunch consistently over a 12-week period. The co-administration of Curcumin (doses of 1000 mg/day) or Curcumin + piperine with lipid-containing meals was strategically employed to improve the bioavailability of these antioxidants, thereby facilitating optimal absorption. It is noteworthy that these capsules were gastro-resistant and free from lactose and gluten.
The capsules were prepared by an outsourced compounding pharmacy with Curcumin and piperine powders, depending on the treatment group (produced with gastro-resistant capsules, free from lactose and sucrose). Notably, the placebo capsules replicated all the physical characteristics (color, texture and appearance) of the treatment capsules, and were composed of starch + orange food coloring. The supplementation routine adhered to the standard procedures of the hospital's monitoring and medication delivery department, ensuring consistency in the delivery process to the patients.
Ingredient Characterization
The Curcumin and Piperine powder was obtained from the company Fragon® with curcumin making up a total of 98% of the curcuminoids. Chromatographic analysis was performed as previously described [
19], with slight modifications. Curcumin and piperine extracts (0.25 mg/mL) and their combination (9.9:0.1 w/w), diluted in acetonitrile, were used to obtain the chromatograms. The mobile phase was composed from deionized water, acidified with phosphoric acid (0.1% v/v) (solvent A) and acetonitrile (solvent B). The chromatogram was obtained with phase A + B (30:70 v/v), in 9 min, with a flow of 1.0 mL/min and temperature of 35 °C and chromatograms were recorded at 345 nm (Supplementary 1).
Blood Collection and Sample Preparation
Participants' blood was collected in tubes containing EDTA and centrifuged at 4,000 x g at 4 ºC, for 10 minutes. Subsequently, the plasma was aspirated and stored in aliquots a -80 ºC in a biofreezer for analysis of inflammatory and oxidative stress markers (primary outcome).
General Date and Anthropometric Measurements
Socioeconomic data, clinical history, lifestyle and gastrointestinal symptoms (secondary outcome) were collected through a customized questionnaire. Anthropometric assessment was conducted by a trained professional both before and after the intervention period, encompassing measurements weight (kg) and height (m) to calculate the body mass index (BMI).
Fecal Calprotectin Analysis (CalF)
CalF levels were assessed using Bühlmann's Quantum Blue® test, according to the manufacturer's information. Elevated CalF values were considered when reached ≥ 200 μg/g of feces, indicative of active disease.
Inflammation and Oxidative Stress Analyses
Malondialdehyde (MDA) levels were measured using reversed-phase ion-pair high performance liquid chromatography (HPLC) with ultraviolet detection at 270 nm, expressed as ng/µL [
20]. Superoxide dismutase (SOD) activity was quantified using the SOD Assay Kit per the manufacturer’s instructions, measured at 450 nm absorbance in a spectrophotometer, and results expressed in U/µL. Hydrogen peroxide (H
2O
2) was measured according to the protocol established by Pick e Keisari (1980), and the results read at 610 nm and expressed as ng/µL [
21]. Catalase (CAT) activity was determined following the colorimetric method adapted by Aebi (1974), monitored at 240 nm and was expressed in U/µL [
22].
Myeloperoxidase (MPO) activity was measured by adapting the previously proposed method by Bradley et al. (1982), and results were expressed in U/µL. One unit of MPO was defined as the amount of enzyme required to decompose 1 μmol H
2O
2 [
23].
Levels of tumor necrosis factor alfa (TNF-α), interleukin 17A (IL-17A), IL-22 and IL-10) were determined by enzyme-linked immunosorbent assay (ELISA), using the PeproTech® kit, according to the manufacturer’s instructions. Absorbance reading was performed at 450 nm in an ELISA plate reader, and the results were expressed in pg/µL.
Furthermore, as a secondary outcome, the effect of curcumin supplementation, with or without piperine, was assessed concerning the primary gastrointestinal symptoms in patients with IBD.
Sample Size
The decision to explore the impact of curcumin supplementation on SOD levels was influenced by studies conducted within our research group, specifically in animal models. This choice was made because there is a lack of human studies, both randomized controlled trials (RCTs) and non-RCTs, that have assessed the effects of curcumin or curcumin + piperine on oxidative stress markers in individuals with inflammatory bowel disease (IBD). The relevant experimental clinical trial, on which the sample size calculation was based, is available in the thesis repository of the Universidade Federal de Alagoas at the following link:
https://www.repositorio.ufal.br/handle/riufal/5281. The rationale behind using this study was driven by the absence of existing research in human subjects, making our animal studies a valuable reference. Considering this information and the patient population registered at the Coloproctology outpatient clinic at Hospital Universitário Professor Alberto Antunes (HUPAA), a minimum sample size of 19 patients in each group was calculated, aiming for 80% power to detect a significant difference between groups at a 5% significance level. Notably, HUPAA serves as the primary referral center for individuals with IBD in the state of Alagoas. The sample size calculation was based on the data of the 200 registered patients at the hospital's Coloproctology outpatient clinic.
Randomization, Blinding and Allocation
The participants' randomization process took place using the “runif” command of the “R” software (v. 4.1.3, The R Project Team, Vienna, Austria). A sequence of random numbers between 0 and 2 was requested to generate the list with a random sequence. This list was kept by a researcher without contact with the participants. They were included sequentially to guarantee the allocation concealment. By simple rounding of the generated random numbers, participants assigned with the number “0” were placed in the placebo group, those assigned with the number “1” in the Curcumin experimental group, and those assigned with the number “2” in the Curcumin + piperine experimental group. An independent evaluator, who had no contact at any point with the participants and researchers of the study, was responsible for conducting the statistical analyses.
Statistical Analysis
All analyses were conducted using the Statistical Package for Social Science (SPSS®), version 26.0 (SPSS, Chicago, IL, USA). Descriptive results were expressed as mean and standard deviation (SD) or median (interquartile range), and frequencies (n and %). Variance homogeneity was assessed using the Levene's test. Variables that were not homogeneous were logarithmized. Delta (Δ = endline -T2 - minus baseline – T1) was calculated only on homogeneous variables. The comparison of categorical outcomes between treatment groups was made using the chi-square test/Fisher's exact test. The comparison of categorical outcomes was made using Generalized estimating equations (GEE), adjusted by sex, age and IBD type. In continuous outcomes, it was utilized ANCOVA test, followed by Bonferroni test, adjusted for sex, age, and type of IBD. Results were considered significant with a p-value < 0.05.
5. Conclusions
In summary, the findings of this study are unprecedent and highly relevant to the scientific community and individuals with IBD. The results provide new insights into the use of a natural therapeutic strategy and underscore the effectiveness of its combination with piperine, in enhancing the antioxidant effects of Curcumin. This approach offers therapeutic alternatives without significant adverse effects, expanding the possibilities for individualized treatment and supporting the effective continuity of the treatment.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Table S1: curcumin and piperine characterization; Table S2: Gastrointestinal complaints of patients with Inflammatory Bowel Disease according to the treatment group: baseline data (T1) and endline data (T2).
Author Contributions
Conceptualization, data curation and methodology, NBB, M.O.F.G. and F.A.M; investigation, ASPM, ORPA, ASG, FLCA, JKGV, JIRJ, ITC, M.O.F.G. and F.A.M; Collection of data and materials, ASPM, ORPA, ASG, FLCA, JKGV, JIRJ, ITC; writing—original draft preparation, ASPM, ORPA, ASG, FLCA, JKGV, JIRJ, ITC; writing—review and editing, ASPM, M.O.F.G. and F.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support of the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) [435704/2018-4], INCT-Bioanalítica (Instituto Nacional de Ciências e Tecnologia em Bioanalítica) [465389/2014-7], CAPES/RENORBIO/PROAP (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), CAPES – DS (social demand), and FAPEAL/PPSUS (Fundação de Amparo à Pesquisa do Estado de Alagoas/Programa Pesquisa para o SUS, Ministério da Saúde) [60030-00879].
Institutional Review Board Statement
This study was registered at ensaiosclinicos.gov.br as RBR-89q4ydz on July 20, 2023. It was approved by the Ethics Committee of the Federal University of Alagoas under process no. 7829516.5.0000.5013 on February 28, 2019.
Informed Consent Statement
All participants in this study signed the Informed Consent Form (ICF).
Data Availability Statement
This is an unpublished work, not under submission process in any other scientific journal. All data is privately accessible.
Acknowledgments
Professor Alberto Antunes University Hospital, Natural Resources Chemistry Research Laboratory (LPqRN), Cellular and Molecular Biology Laboratory (BCM) and the Institute for Multidisciplinary Skills in Gut Microbiota (InHaMMI).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chan W, Chen A, Tiao D, Selinger C, Leong R. Medication adherence in inflammatory bowel disease. Intest Res. 2017 Oct;15(4):434-45. [CrossRef]
- Karthikeyan A, Young KN, Moniruzzaman M, Beyene AM, Do K, Kalaiselvi S, et al. Curcumin and Its Modified Formulations on Inflammatory Bowel Disease (IBD): The Story So Far and Future Outlook. Pharmaceutics. 2021 Apr 2;13(4). [CrossRef]
- Flynn S, Eisenstein S. Inflammatory Bowel Disease Presentation and Diagnosis. Surg Clin North Am. 2019 Dec;99(6):1051-62. [CrossRef]
- Mehta M, Ahmed S, Dryden G. Immunopathophysiology of inflammatory bowel disease: how genetics link barrier dysfunction and innate immunity to inflammation. Innate Immun. 2017 Aug;23(6):497-505. [CrossRef]
- Balmus IM, Ciobica A, Trifan A, Stanciu C. The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: Clinical aspects and animal models. Saudi journal of gastroenterology : official journal of the Saudi Gastroenterology Association. 2016 Jan-Feb;22(1):3-17. [CrossRef]
- Caer C, Wick MJ. Human Intestinal Mononuclear Phagocytes in Health and Inflammatory Bowel Disease. Front Immunol. 2020;11:410. [CrossRef]
- Jain S, Ahuja V, Limdi JK. Optimal management of acute severe ulcerative colitis. Postgrad Med J. 2019 Jan;95(1119):32-40. [CrossRef]
- Martins ASP, Campos SBG, Goulart MOF, Moura FA. Extraintestinal manifestations of inflammatory bowel disease, nitroxidative stress and dysbiosis: What is the link between them? 2021;45(3):461--81.
- Sabir SM, Zeb A, Mahmood M, Abbas SR, Ahmad Z, Iqbal N. Phytochemical analysis and biological activities of ethanolic extract of Curcuma longa rhizome. Braz J Biol. 2021 Jul-Sep;81(3):737-40. [CrossRef]
- Alves MC, Santos MO, Bueno NB, Araújo ORP, Goulart MOF, Moura FA. Efficacy of oral consumption of curcumin/ for symptom improvement in inflammatory bowel disease: A systematic review of animal models and a meta-analysis of randomized clinical trials. Biocell. 2022;46(9):2015--47.
- Panahi Y, Khalili N, Sahebi E, Namazi S, Reiner Z, Majeed M, et al. Curcuminoids modify lipid profile in type 2 diabetes mellitus: A randomized controlled trial. Complementary therapies in medicine. 2017 Aug;33:1-5. [CrossRef]
- Khan K, Quispe C, Javed Z, Iqbal MJ, Sadia H, Raza S, et al. Resveratrol, curcumin, paclitaxel and miRNAs mediated regulation of PI3K/Akt/mTOR pathway: go four better to treat bladder cancer. Cancer Cell Int. 2020 Nov 23;20(1):560. [CrossRef]
- Peng Y, Ao M, Dong B, Jiang Y, Yu L, Chen Z, et al. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug design, development and therapy. 2021;15:4503-25. [CrossRef]
- Martins ASP, Alves MC, Araújo ORP, Camatari FOdS, Goulart MOF, Moura FA. Curcumin in inflammatory bowel diseases: Cellular targets and molecular mechanisms. Biocell. 2023;47(11):2547--66. [CrossRef]
- Hewlings SJ, Kalman DS. Curcumin: A Review of Its Effects on Human Health. Foods. 2017 Oct 22;6(10). [CrossRef]
- Heidari H, Bagherniya M, Majeed M, Sathyapalan T, Jamialahmadi T, Sahebkar A. Curcumin-piperine co-supplementation and human health: A comprehensive review of preclinical and clinical studies. Phytotherapy research : PTR. 2023 Apr;37(4):1462-87. [CrossRef]
- Hosseini H, Ghavidel F, Panahi G, Majeed M, Sahebkar A. A systematic review and meta-analysis of randomized controlled trials investigating the effect of the curcumin and piperine combination on lipid profile in patients with metabolic syndrome and related disorders. Phytotherapy research : PTR. 2023 Mar;37(3):1212-24. [CrossRef]
- Rodrigues Junior JI, Vasconcelos JKG, Xavier L, Gomes ADS, Santos JCF, Campos SBG, et al. Antioxidant Therapy in Inflammatory Bowel Disease: A Systematic Review and a Meta-Analysis of Randomized Clinical Trials. Pharmaceuticals (Basel). 2023 Sep 28;16(10).
- Khismatrao A, Bhairy S, Hirlekar R. Development and Validation of Rp-Hplc Method for Simultaneous Estimation of Curcumin and Piperine. International Journal of Applied Pharmaceutics. 2018;10(5):43. [CrossRef]
- Karatas F, Karatepe M, Baysar a. Determination of free malondialdehyde in human serum by high-performance liquid chromatography. Analytical biochemistry. 2002;311(1):76-9. [CrossRef]
- Pick E, Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. Journal of immunological methods. 1980;38(1505):161-70. [CrossRef]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121-26.
- Bradley PP, Christensen RD, Rothstein G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood. 1982 Sep;60(3):618-22.
- Alves MdC, Santos MO, Bueno NB, Araújo ORPd, Goulart MOF, Moura FA. Efficacy of oral consumption of curcumin/ for symptom improvement in inflammatory bowel disease: A systematic review of animal models and a meta-analysis of randomized clinical trials. 2022;46(9):2015--47.
- McCord JM, Edeas MA. SOD, oxidative stress and human pathologies: a brief history and a future vision. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2005 May;59(4):139-42. [CrossRef]
- Wang Y, Branicky R, Noe A, Hekimi S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. 2018 Jun 4;217(6):1915-28. [CrossRef]
- Liochev SI, Fridovich I. Superoxide and iron: partners in crime. IUBMB Life. 1999 Aug;48(2):157-61.
- Panahi Y, Hosseini MS, Khalili N, Naimi E, Majeed M, Sahebkar A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin Nutr. 2015 Dec;34(6):1101-8. [CrossRef]
- Panahi Y, Ghanei M, Hajhashemi A, Sahebkar A. Effects of Curcuminoids-Piperine Combination on Systemic Oxidative Stress, Clinical Symptoms and Quality of Life in Subjects with Chronic Pulmonary Complications Due to Sulfur Mustard: A Randomized Controlled Trial. Journal of dietary supplements. 2016;13(1):93-105. [CrossRef]
- Panahi Y, Rahimnia AR, Sharafi M, Alishiri G, Saburi A, Sahebkar A. Curcuminoid treatment for knee osteoarthritis: a randomized double-blind placebo-controlled trial. Phytotherapy research : PTR. 2014 Nov;28(11):1625-31. [CrossRef]
- Mohammadi E, Qujeq D, Taheri H, Hajian-Tilaki K. Evaluation of Serum Trace Element Levels and Superoxide Dismutase Activity in Patients with Inflammatory Bowel Disease: Translating Basic Research into Clinical Application. Biol Trace Elem Res. 2017 Jun;177(2):235-40. [CrossRef]
- Kruidenier L, Kuiper I, van Duijn W, Marklund SL, van Hogezand RA, Lamers CB, et al. Differential mucosal expression of three superoxide dismutase isoforms in inflammatory bowel disease. The Journal of pathology. 2003 Sep;201(1):7-16. [CrossRef]
- Younus H. Therapeutic potentials of superoxide dismutase. International journal of health sciences. 2018 May-Jun;12(3):88-93.
- Segui J, Gironella M, Sans M, Granell S, Gil F, Gimeno M, et al. Superoxide dismutase ameliorates TNBS-induced colitis by reducing oxidative stress, adhesion molecule expression, and leukocyte recruitment into the inflamed intestine. J Leukoc Biol. 2004 Sep;76(3):537-44. [CrossRef]
- Hou CL, Zhang J, Liu XT, Liu H, Zeng XF, Qiao SY. Superoxide dismutase recombinant Lactobacillus fermentum ameliorates intestinal oxidative stress through inhibiting NF-kappaB activation in a trinitrobenzene sulphonic acid-induced colitis mouse model. J Appl Microbiol. 2014 Jun;116(6):1621-31.
- Sadeghi N, Mansoori A, Shayesteh A, Hashemi SJ. The effect of curcumin supplementation on clinical outcomes and inflammatory markers in patients with ulcerative colitis. Phytotherapy research : PTR. 2020 May;34(5):1123-33. [CrossRef]
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as "Curecumin": from kitchen to clinic. Biochem Pharmacol. 2008 Feb 15;75(4):787-809.
- Faki Y, Er A. Different Chemical Structures and Physiological/Pathological Roles of Cyclooxygenases. Rambam Maimonides Med J. 2021 Jan 19;12(1). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).