Submitted:
29 April 2024
Posted:
01 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Etiopathogenesis
2.1. Age
2.2. Genetics
2.3. Nutrition
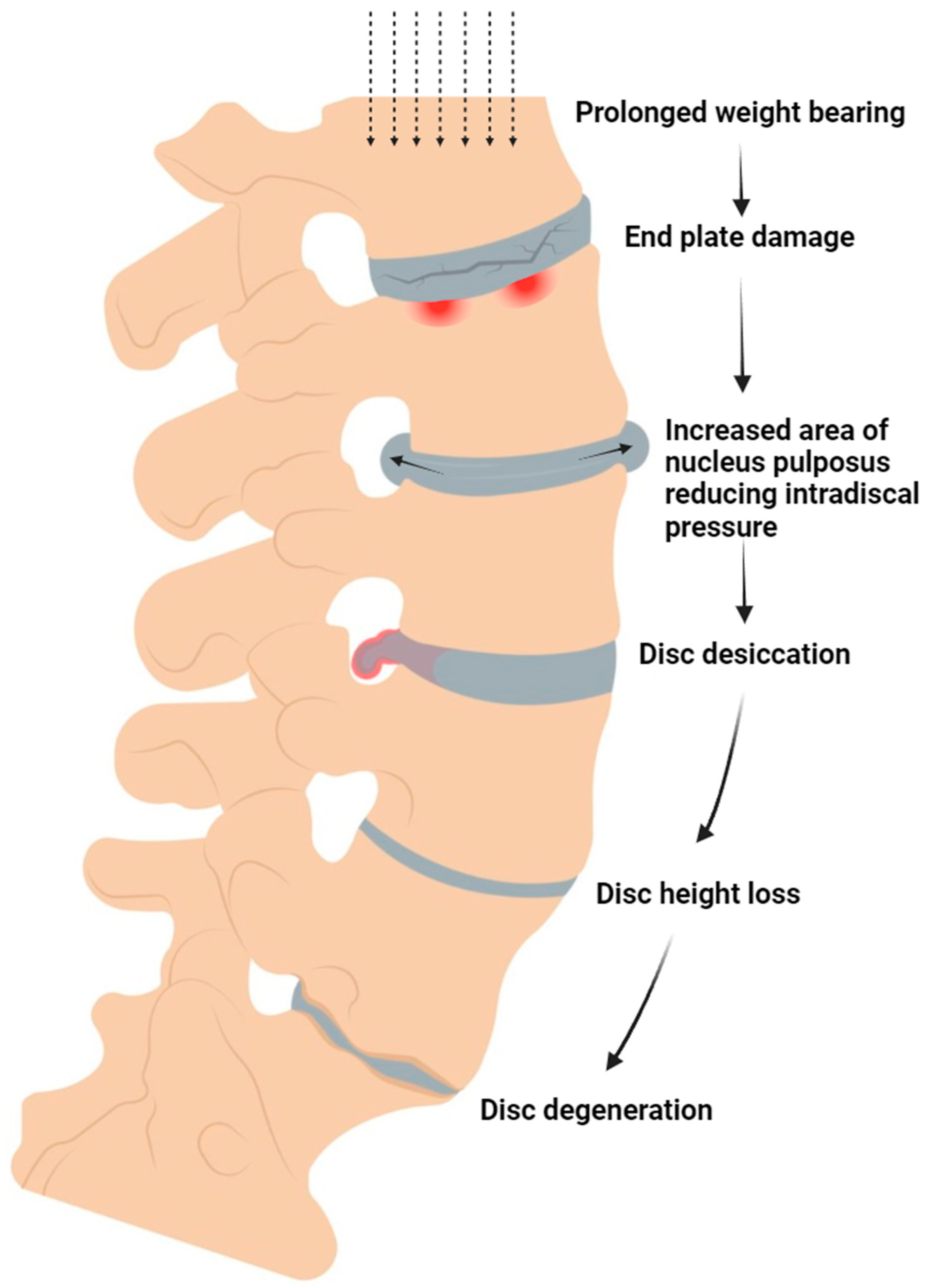
2.4. Mechanobiology
3. Orthobiologic Solutions
3.1. Platelet-Rich Plasma
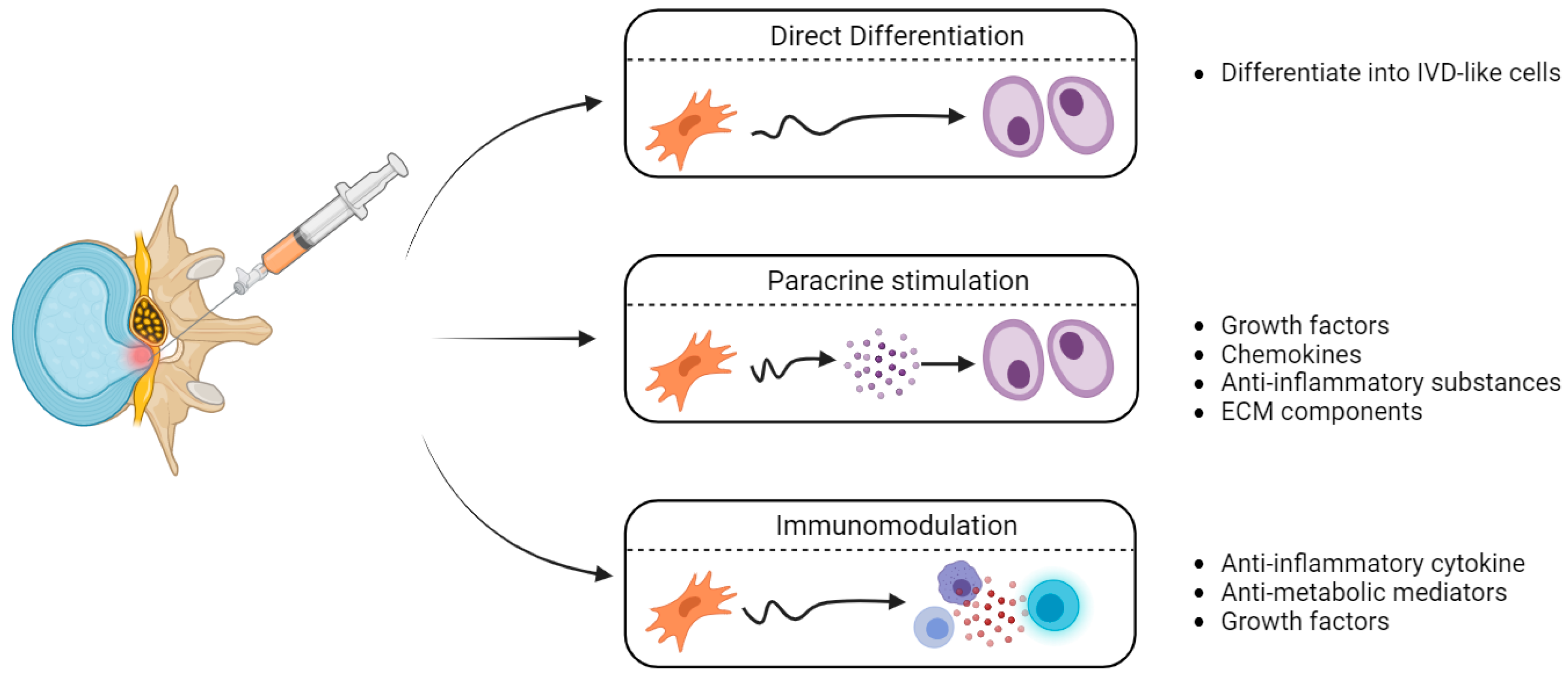
3.2. Mesenchymal Stem Cells
ECM Extracellular Matrix, IVD Intervertebral Disc
3.3. Adjunct Materials - Hyaluronic Acid
4. Clinical Evidence
5. Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alshami, A.M. Prevalence of Spinal Disorders and Their Relationships with Age and Gender. SMJ 2015, 36, 725–730. [Google Scholar] [CrossRef]
- Coenen, P.; Smith, A.; Paananen, M.; O’Sullivan, P.; Beales, D.; Straker, L. Trajectories of Low Back Pain From Adolescence to Young Adulthood: LBP Trajectories in Early Life. Arthritis Care & Research 2017, 69, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Van Boxem, K.; Van Zundert, J.; Patijn, J.; van Kleef, M. Pseudoradicular and Radicular Low-Back Pain: How to Diagnose Clinically? Pain 2008, 135, 311–312. [Google Scholar] [CrossRef]
- Raciborski, F.; Gasik, R.; Kłak, A. Disorders of the Spine. A Major Health and Social Problem. Reumatologia 2016, 54, 196–200. [Google Scholar] [CrossRef]
- Madigan, L.; Vaccaro, A.R.; Spector, L.R.; Milam, R.A. Management of Symptomatic Lumbar Degenerative Disk Disease. J Am Acad Orthop Surg 2009, 17, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Marcum, Z.A.; Hanlon, J.T. Recognizing the Risks of Chronic Nonsteroidal Anti-Inflammatory Drug Use in Older Adults. Annals of Long-Term Care.
- Setti, T.; Arab, M.G.L.; Santos, G.S.; Alkass, N.; Andrade, M.A.P.; Lana, J.F.S.D. The Protective Role of Glutathione in Osteoarthritis. Journal of Clinical Orthopaedics and Trauma 2020. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.; da Fonseca, L.F.; Azzini, G.; Santos, G.; Braga, M.; Cardoso Junior, A.M.; Murrell, W.D.; Gobbi, A.; Purita, J.; de Andrade, M.A.P. Bone Marrow Aspirate Matrix: A Convenient Ally in Regenerative Medicine. International Journal of Molecular Sciences 2021. [Google Scholar] [CrossRef] [PubMed]
- Ramires, L.C.; Jeyaraman, M.; Muthu, S.; Shankar A, N.; Santos, G.S.; da Fonseca, L.F.; Lana, J.F.; Rajendran, R.L.; Gangadaran, P.; Jogalekar, M.P.; et al. Application of Orthobiologics in Achilles Tendinopathy: A Review. Life 2022, 12, 399. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal Stem Cells as Trophic Mediators. Journal of Cellular Biochemistry 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant 2016, 25, 829–848. [Google Scholar] [CrossRef]
- Gallucci, M.; Limbucci, N.; Paonessa, A.; Splendiani, A. Degenerative Disease of the Spine. Neuroimaging Clin N Am 2007, 17, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Hadjipavlou, A.G.; Tzermiadianos, M.N.; Bogduk, N.; Zindrick, M.R. The Pathophysiology of Disc Degeneration. The Journal of Bone and Joint Surgery. British volume, 1261. [Google Scholar] [CrossRef]
- Gallucci, M.; Puglielli, E.; Splendiani, A.; Pistoia, F.; Spacca, G. Degenerative Disorders of the Spine. Eur Radiol 2005, 15, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.R.; Matsui, Y.; Wu, J.-J. Collagen Polymorphisms of the Intervertebral Disc. Biochemical Society Transactions 2002, 30, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Kawaguchi, Y.; Chiba, K.; Mikami, Y.; Kizawa, H.; Oya, T.; Mio, F.; Mori, M.; Miyamoto, Y.; Masuda, I.; et al. A Functional SNP in CILP, Encoding Cartilage Intermediate Layer Protein, Is Associated with Susceptibility to Lumbar Disc Disease. Nat Genet 2005, 37, 607–612. [Google Scholar] [CrossRef]
- Lana, J.F.; Macedo, A.; Ingrao, I.L.G.; Huber, S.C.; Santos, G.S.; Santana, M.H.A. Leukocyte-Rich PRP for Knee Osteoarthritis: Current Concepts. Journal of Clinical Orthopaedics and Trauma 2019. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.J.; Ferreira, J.R.; Cunha, C.; Corte-Real, J.V.; Bessa-Gonçalves, M.; Barbosa, M.A.; Santos, S.G.; Gonçalves, R.M. Macrophages Down-Regulate Gene Expression of Intervertebral Disc Degenerative Markers Under a Pro-Inflammatory Microenvironment. Front Immunol 2019, 10, 1508. [Google Scholar] [CrossRef]
- Zhu, P.; Li, J.; Fujino, M.; Zhuang, J.; Li, X.-K. Development and Treatments of Inflammatory Cells and Cytokines in Spinal Cord Ischemia-Reperfusion Injury. Mediators Inflamm 2013, 2013, 701970. [Google Scholar] [CrossRef]
- Hellenbrand, D.J.; Quinn, C.M.; Piper, Z.J.; Morehouse, C.N.; Fixel, J.A.; Hanna, A.S. Inflammation after Spinal Cord Injury: A Review of the Critical Timeline of Signaling Cues and Cellular Infiltration. Journal of Neuroinflammation 2021, 18, 284. [Google Scholar] [CrossRef]
- Tokuhara, C.K.; Santesso, M.R.; Oliveira, G.S.N. de; Ventura, T.M. da S.; Doyama, J.T.; Zambuzzi, W.F.; Oliveira, R.C. de Updating the Role of Matrix Metalloproteinases in Mineralized Tissue and Related Diseases. J Appl Oral Sci 2019, 27, e20180596. [Google Scholar] [CrossRef]
- Haefeli, M.; Kalberer, F.; Saegesser, D.; Nerlich, A.G.; Boos, N.; Paesold, G. The Course of Macroscopic Degeneration in the Human Lumbar Intervertebral Disc. Spine 2006, 31, 1522–1531. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan Form and Function: A Comprehensive Nomenclature of Proteoglycans. Matrix Biol 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Roberts, S.; Evans, E.H.; Kletsas, D.; Jaffray, D.C.; Eisenstein, S.M. Senescence in Human Intervertebral Discs. Eur Spine J 2006, 15, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Gruber, H.E.; Ingram, J.A.; Norton, H.J.; Hanley, E.N.J. Senescence in Cells of the Aging and Degenerating Intervertebral Disc: Immunolocalization of Senescence-Associated β-Galactosidase in Human and Sand Rat Discs. Spine 2007, 32, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Haro, H.; Wakabayashi, Y.; Kawa-uchi, T.; Komori, H.; Shinomiya, K. The Association of Degeneration of the Intervertebral Disc with 5a/6a Polymorphism in the Promoter of the Human Matrix Metalloproteinase-3 Gene. The Journal of Bone and Joint Surgery. British volume. [CrossRef]
- Persikov, A.V.; Ramshaw, J.A.M.; Brodsky, B. Collagen Model Peptides: Sequence Dependence of Triple-Helix Stability. Biopolymers 2000, 55, 436–450. [Google Scholar] [CrossRef]
- Solovieva, S.; Kouhia, S.; Leino-Arjas, P.; Ala-Kokko, L.; Luoma, K.; Raininko, R.; Saarela, J.; Riihimäki, H. Interleukin 1 Polymorphisms and Intervertebral Disc Degeneration. Epidemiology 2004, 15, 626–633. [Google Scholar] [CrossRef]
- De Geer, C.M. Intervertebral Disk Nutrients and Transport Mechanisms in Relation to Disk Degeneration: A Narrative Literature Review. J Chiropr Med 2018, 17, 97–105. [Google Scholar] [CrossRef]
- Grunhagen, T.; Wilde, G.; Soukane, D.M.; Shirazi-Adl, S.A.; Urban, J.P.G. Nutrient Supply and Intervertebral Disc Metabolism. J Bone Joint Surg Am 2006, 88 Suppl 2, 30–35. [Google Scholar] [CrossRef]
- Azzini, G.O.M.; Santos, G.S.; Visoni, S.B.C.; Azzini, V.O.M.; Santos, R.G. dos; Huber, S.C.; Lana, J.F. Metabolic Syndrome and Subchondral Bone Alterations: The Rise of Osteoarthritis – A Review. Journal of Clinical Orthopaedics and Trauma 2020. [Google Scholar] [CrossRef] [PubMed]
- Stokes, I.A.F.; Iatridis, J.C. Mechanical Conditions That Accelerate Intervertebral Disc Degeneration: Overload Versus Immobilization. Spine (Phila Pa 1976) 2004, 29, 2724–2732. [Google Scholar] [CrossRef]
- Chan, S.C.W.; Ferguson, S.J.; Gantenbein-Ritter, B. The Effects of Dynamic Loading on the Intervertebral Disc. Eur Spine J 2011, 20, 1796–1812. [Google Scholar] [CrossRef] [PubMed]
- Neidlinger-Wilke, C.; Würtz, K.; Urban, J.P.G.; Börm, W.; Arand, M.; Ignatius, A.; Wilke, H.-J.; Claes, L.E. Regulation of Gene Expression in Intervertebral Disc Cells by Low and High Hydrostatic Pressure. Eur Spine J 2006, 15, 372–378. [Google Scholar] [CrossRef]
- Benneker, L.M.; Heini, P.F.; Alini, M.; Anderson, S.E.; Ito, K. 2004 Young Investigator Award Winner: Vertebral Endplate Marrow Contact Channel Occlusions and Intervertebral Disc Degeneration. Spine 2005, 30, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Rade, M.; Määttä, J.H.; Freidin, M.B.; Airaksinen, O.; Karppinen, J.; Williams, F.M. Vertebral Endplate Defect as Initiating Factor in Intervertebral Disc Degeneration; Strong Association between Endplate Defect and Disc Degeneration in the General Population. Spine (Phila Pa 1976) 2018, 43, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Lotz, J.C.; Fields, A.J.; Liebenberg, E.C. The Role of the Vertebral End Plate in Low Back Pain. Global Spine J 2013, 3, 153–164. [Google Scholar] [CrossRef]
- Tang, P.; Zhu, R.; Ji, W.-P.; Wang, J.-Y.; Chen, S.; Fan, S.-W.; Hu, Z.-J. The NLRP3/Caspase-1/Interleukin-1β Axis Is Active in Human Lumbar Cartilaginous Endplate Degeneration. Clin Orthop Relat Res 2016, 474, 1818–1826. [Google Scholar] [CrossRef]
- Marjoram, T. The Endplate and Trabecular Bone in Lumbar Degenerative Disc Disease: A Narrative Review. SN Compr. Clin. Med. 2020, 2, 332–337. [Google Scholar] [CrossRef]
- Fields, A.J.; Ballatori, A.; Liebenberg, E.C.; Lotz, J.C. Contribution of the Endplates to Disc Degeneration. Curr Mol Biol Rep 2018, 4, 151–160. [Google Scholar] [CrossRef]
- Ferrari, M.; Zia, S.; Valbonesi, M.; Henriquet, F.; Venere, G.; Spagnolo, S.; Grasso, M.A.; Panzani, I. A New Technique for Hemodilution, Preparation of Autologous Platelet-Rich Plasma and Intraoperative Blood Salvage in Cardiac Surgery. Int J Artif Organs 1987, 10, 47–50. [Google Scholar] [CrossRef]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Stojanovic, P. Platelet Rich Plasma: A Short Overview of Certain Bioactive Components. Open Med (Wars) 2016, 11, 242–247. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Santos, G.S.; Alkass, N.; Chiesa, T.L.; Azzini, G.O.; da Fonseca, L.F.; Dos Santos, A.F.; Rodrigues, B.L.; Mosaner, T.; Lana, J.F. The Regenerative Mechanisms of Platelet-Rich Plasma: A Review. Cytokine 2021, 144, 155560. [Google Scholar] [CrossRef]
- Akeda, K.; An, H.S.; Okuma, M.; Attawia, M.; Miyamoto, K.; Thonar, E.J.-M.A.; Lenz, M.E.; Sah, R.L.; Masuda, K. Platelet-Rich Plasma Stimulates Porcine Articular Chondrocyte Proliferation and Matrix Biosynthesis. Osteoarthritis Cartilage 2006, 14, 1272–1280. [Google Scholar] [CrossRef]
- Yang, H.; Yuan, C.; Wu, C.; Qian, J.; Shi, Q.; Li, X.; Zhu, X.; Zou, J. The Role of TGF-Β1/Smad2/3 Pathway in Platelet-Rich Plasma in Retarding Intervertebral Disc Degeneration. J Cell Mol Med 2016, 20, 1542–1549. [Google Scholar] [CrossRef]
- Liu, M.-C.; Chen, W.-H.; Wu, L.-C.; Hsu, W.-C.; Lo, W.-C.; Yeh, S.-D.; Wang, M.-F.; Zeng, R.; Deng, W.-P. Establishment of a Promising Human Nucleus Pulposus Cell Line for Intervertebral Disc Tissue Engineering. Tissue Eng Part C Methods 2014, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Lo, W.-C.; Lee, J.-J.; Su, C.-H.; Lin, C.-T.; Liu, H.-Y.; Lin, T.-W.; Lin, W.-C.; Huang, T.-Y.; Deng, W.-P. Tissue-Engineered Intervertebral Disc and Chondrogenesis Using Human Nucleus Pulposus Regulated through TGF-Β1 in Platelet-Rich Plasma. Journal of Cellular Physiology 2006, 209, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Mietsch, A.; Neidlinger-Wilke, C.; Schrezenmeier, H.; Mauer, U.M.; Friemert, B.; Wilke, H.-J.; Ignatius, A. Evaluation of Platelet-Rich Plasma and Hydrostatic Pressure Regarding Cell Differentiation in Nucleus Pulposus Tissue Engineering. Journal of Tissue Engineering and Regenerative Medicine 2013, 7, 244–252. [Google Scholar] [CrossRef]
- Drengk, A.; Zapf, A.; Stürmer, E.K.; Stürmer, K.M.; Frosch, K.-H. Influence of Platelet-Rich Plasma on Chondrogenic Differentiation and Proliferation of Chondrocytes and Mesenchymal Stem Cells. Cells Tissues Organs 2009, 189, 317–326. [Google Scholar] [CrossRef]
- Pirvu, T.N.; Schroeder, J.E.; Peroglio, M.; Verrier, S.; Kaplan, L.; Richards, R.G.; Alini, M.; Grad, S. Platelet-Rich Plasma Induces Annulus Fibrosus Cell Proliferation and Matrix Production. Eur Spine J 2014, 23, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Hondke, S.; Cabraja, M.; Krüger, J.P.; Stich, S.; Hartwig, T.; Sittinger, M.; Endres, M. Proliferation, Migration, and ECM Formation Potential of Human Annulus Fibrosus Cells Is Independent of Degeneration Status. Cartilage 2020, 11, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Nagae, M.; Ikeda, T.; Mikami, Y.; Hase, H.; Ozawa, H.; Matsuda, K.-I.; Sakamoto, H.; Tabata, Y.; Kawata, M.; Kubo, T. Intervertebral Disc Regeneration Using Platelet-Rich Plasma and Biodegradable Gelatin Hydrogel Microspheres. Tissue Eng 2007, 13, 147–158. [Google Scholar] [CrossRef]
- Sawamura, K.; Ikeda, T.; Nagae, M.; Okamoto, S.; Mikami, Y.; Hase, H.; Ikoma, K.; Yamada, T.; Sakamoto, H.; Matsuda, K.; et al. Characterization of in Vivo Effects of Platelet-Rich Plasma and Biodegradable Gelatin Hydrogel Microspheres on Degenerated Intervertebral Discs. Tissue Eng Part A 2009, 15, 3719–3727. [Google Scholar] [CrossRef]
- Gullung, G.B.; Woodall, J.W.; Tucci, M.A.; James, J.; Black, D.A.; McGuire, R.A. Platelet-Rich Plasma Effects on Degenerative Disc Disease: Analysis of Histology and Imaging in an Animal Model. Evid Based Spine Care J 2011, 2, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Z.; JIN, J.-Y.; GUO, Y.-D.; MA, L.-Y.; CHANG, Q.; PENG, X.-G.; GUO, F.-F.; ZHANG, H.-X.; HU, X.-F.; WANG, C. Intervertebral Disc Regeneration Using Platelet-Rich Plasma-Containing Bone Marrow-Derived Mesenchymal Stem Cells: A Preliminary Investigation. Mol Med Rep 2016, 13, 3475–3481. [Google Scholar] [CrossRef]
- Noriega, D.C.; Ardura, F.; Hernández-Ramajo, R.; Martín-Ferrero, M.Á.; Sánchez-Lite, I.; Toribio, B.; Alberca, M.; García, V.; Moraleda, J.M.; Sánchez, A.; et al. Intervertebral Disc Repair by Allogeneic Mesenchymal Bone Marrow Cells: A Randomized Controlled Trial. Transplantation 2017, 101, 1945–1951. [Google Scholar] [CrossRef]
- Pettine, K.A.; Suzuki, R.K.; Sand, T.T.; Murphy, M.B. Autologous Bone Marrow Concentrate Intradiscal Injection for the Treatment of Degenerative Disc Disease with Three-Year Follow-Up. Int Orthop 2017, 41, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Pettine, K.; Suzuki, R.; Sand, T.; Murphy, M. Treatment of Discogenic Back Pain with Autologous Bone Marrow Concentrate Injection with Minimum Two Year Follow-Up. Int Orthop 2016, 40, 135–140. [Google Scholar] [CrossRef]
- Pettine, K.A.; Murphy, M.B.; Suzuki, R.K.; Sand, T.T. Percutaneous Injection of Autologous Bone Marrow Concentrate Cells Significantly Reduces Lumbar Discogenic Pain through 12 Months. Stem Cells 2015, 33, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Orozco, L.; Soler, R.; Morera, C.; Alberca, M.; Sánchez, A.; García-Sancho, J. Intervertebral Disc Repair by Autologous Mesenchymal Bone Marrow Cells: A Pilot Study. Transplantation 2011, 92, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Haufe, S.M.W.; Mork, A.R. Intradiscal Injection of Hematopoietic Stem Cells in an Attempt to Rejuvenate the Intervertebral Discs. Stem Cells Dev 2006, 15, 136–137. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Ueda, Y.; Miyazaki, K.; Koizumi, M.; Takakura, Y. Disc Regeneration Therapy Using Marrow Mesenchymal Cell Transplantation: A Report of Two Case Studies. Spine 2010, 35, E475. [Google Scholar] [CrossRef]
- Elabd, C.; Centeno, C.J.; Schultz, J.R.; Lutz, G.; Ichim, T.; Silva, F.J. Intra-Discal Injection of Autologous, Hypoxic Cultured Bone Marrow-Derived Mesenchymal Stem Cells in Five Patients with Chronic Lower Back Pain: A Long-Term Safety and Feasibility Study. Journal of Translational Medicine 2016, 14, 253. [Google Scholar] [CrossRef]
- Centeno, C.; Markle, J.; Dodson, E.; Stemper, I.; Williams, C.J.; Hyzy, M.; Ichim, T.; Freeman, M. Treatment of Lumbar Degenerative Disc Disease-Associated Radicular Pain with Culture-Expanded Autologous Mesenchymal Stem Cells: A Pilot Study on Safety and Efficacy. J Transl Med 2017, 15, 197. [Google Scholar] [CrossRef]
- Blanco, J.F.; Villarón, E.M.; Pescador, D.; da Casa, C.; Gómez, V.; Redondo, A.M.; López-Villar, O.; López-Parra, M.; Muntión, S.; Sánchez-Guijo, F. Autologous Mesenchymal Stromal Cells Embedded in Tricalcium Phosphate for Posterolateral Spinal Fusion: Results of a Prospective Phase I/II Clinical Trial with Long-Term Follow-Up. Stem Cell Res Ther 2019, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ruiz, V.; Blanco, J.F.; Villarón, E.M.; Fidalgo, H.; López-Parra, M.; Sánchez-Guijo, F. Autologous Mesenchymal Stem Cell Transplantation for Spinal Fusion: 10 Years Follow-up of a Phase I/II Clinical Trial. Stem Cell Research & Therapy 2023, 14, 78. [Google Scholar] [CrossRef]
- Navani, A.; Ambach, M.; Calodney, A.; Rosenthal, R.; Li, G.; Mahoney, C.B.; Everts, P.A. The Safety and Effectiveness of Orthobiologic Injections for Discogenic Chronic Low Back Pain: A Multicenter Prospective, Crossover, Randomized Controlled Trial with 12 Months Follow-Up. Pain Physician 2024, 27, E65–E77. [Google Scholar]
- Pang, X.; Yang, H.; Peng, B. Human Umbilical Cord Mesenchymal Stem Cell Transplantation for the Treatment of Chronic Discogenic Low Back Pain. Pain Physician 2014, 17, 525–530. [Google Scholar] [CrossRef]
- Comella, K.; Silbert, R.; Parlo, M. Effects of the Intradiscal Implantation of Stromal Vascular Fraction plus Platelet Rich Plasma in Patients with Degenerative Disc Disease. Journal of Translational Medicine 2017, 15, 12. [Google Scholar] [CrossRef]
- Kumar, H.; Ha, D.-H.; Lee, E.-J.; Park, J.H.; Shim, J.H.; Ahn, T.-K.; Kim, K.-T.; Ropper, A.E.; Sohn, S.; Kim, C.-H.; et al. Safety and Tolerability of Intradiscal Implantation of Combined Autologous Adipose-Derived Mesenchymal Stem Cells and Hyaluronic Acid in Patients with Chronic Discogenic Low Back Pain: 1-Year Follow-up of a Phase I Study. Stem Cell Res Ther 2017, 8, 262. [Google Scholar] [CrossRef]
- Tuakli-Wosornu, Y.A.; Terry, A.; Boachie-Adjei, K.; Harrison, J.R.; Gribbin, C.K.; LaSalle, E.E.; Nguyen, J.T.; Solomon, J.L.; Lutz, G.E. Lumbar Intradiskal Platelet-Rich Plasma (PRP) Injections: A Prospective, Double-Blind, Randomized Controlled Study. PM&R 2016, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Levi, D.; Horn, S.; Tyszko, S.; Levin, J.; Hecht-Leavitt, C.; Walko, E. Intradiscal Platelet-Rich Plasma Injection for Chronic Discogenic Low Back Pain: Preliminary Results from a Prospective Trial. Pain Medicine 2016, 17, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, M.A.; Evans, N.E.; Bae, H.; Kamrava, E.; Calodney, A.; Remley, K.; Benyamin, R.; Franc, D.; Peterson, M.R.; Lovine, J.; et al. Safety and Efficacy of Platelet Rich Plasma for Treatment of Lumbar Discogenic Pain: A Prospective, Multicenter, Randomized, Double-Blind Study. Pain Physician 2022, 25, 29–34. [Google Scholar] [PubMed]
- Goyal, T.; Paswan, A.K.; Jain, D.; Verma, N.; Dubey, R.K. Comparative Evaluation of Efficacy of Percutaneous Intradiscal Radiofrequency Ablation and Platelet Rich Plasma Injection for Discogenic Low Back Pain: A Prospective Randomized Trial. J. Musculoskelet. Res. 2022, 25, 2250009. [Google Scholar] [CrossRef]
- Akeda, K.; Ohishi, K.; Takegami, N.; Sudo, T.; Yamada, J.; Fujiwara, T.; Niimi, R.; Matsumoto, T.; Nishimura, Y.; Ogura, T.; et al. Platelet-Rich Plasma Releasate versus Corticosteroid for the Treatment of Discogenic Low Back Pain: A Double-Blind Randomized Controlled Trial. Journal of Clinical Medicine 2022, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Akeda, K.; Fujiwara, T.; Takegami, N.; Yamada, J.; Sudo, A. Retrospective Analysis of Factors Associated with the Treatment Outcomes of Intradiscal Platelet-Rich Plasma-Releasate Injection Therapy for Patients with Discogenic Low Back Pain. Medicina 2023, 59, 640. [Google Scholar] [CrossRef] [PubMed]
- Bodor, M.; Toy, A.; Aufiero, D. Disc Regeneration with Platelets and Growth Factors. In Platelet-Rich Plasma: Regenerative Medicine: Sports Medicine, Orthopedic, and Recovery of Musculoskeletal Injuries; Lana, J.F.S.D., Andrade Santana, M.H., Dias Belangero, W., Malheiros Luzo, A.C., Eds.; Lecture Notes in Bioengineering; Springer: Berlin, Heidelberg, 2014; ISBN 978-3-642-40117-6. [Google Scholar]
- Navani, A.; Hames, A. Platelet-Rich Plasma Injections for Lumbar Discogenic Pain: A Preliminary Assessment of Structural and Functional Changes. Techniques in Regional Anesthesia and Pain Management 2015, 19, 38–44. [Google Scholar] [CrossRef]
- Akeda, K.; Ohishi, K.; Masuda, K.; Bae, W.C.; Takegami, N.; Yamada, J.; Nakamura, T.; Sakakibara, T.; Kasai, Y.; Sudo, A. Intradiscal Injection of Autologous Platelet-Rich Plasma Releasate to Treat Discogenic Low Back Pain: A Preliminary Clinical Trial. Asian Spine J 2017, 11, 380–389. [Google Scholar] [CrossRef]
- Strassburg, S.; Richardson, S.M.; Freemont, A.J.; Hoyland, J.A. Co-Culture Induces Mesenchymal Stem Cell Differentiation and Modulation of the Degenerate Human Nucleus Pulposus Cell Phenotype. Regenerative Medicine 2010, 5, 701–711. [Google Scholar] [CrossRef]
- Svanvik, T.; Barreto Henriksson, H.; Karlsson, C.; Hagman, M.; Lindahl, A.; Brisby, H. Human Disk Cells from Degenerated Disks and Mesenchymal Stem Cells in Co-Culture Result in Increased Matrix Production. Cells Tissues Organs 2009, 191, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Esquijarosa Hechavarria, M.; Richard, S.A. Edifying the Focal Factors Influencing Mesenchymal Stem Cells by the Microenvironment of Intervertebral Disc Degeneration in Low Back Pain. Pain Research and Management 2022, 2022, e6235400. [Google Scholar] [CrossRef]
- Mochida, J.; Sakai, D.; Nakamura, Y.; Watanabe, T.; Yamamoto, Y.; Kato, S. Intervertebral Disc Repair with Activated Nucleus Pulposus Cell Transplantation: A Three-Year, Prospective Clinical Study of Its Safety. Eur Cell Mater 2015, 29, 202–212. [Google Scholar] [CrossRef]
- Ekram, S.; Khalid, S.; Bashir, I.; Salim, A.; Khan, I. Human Umbilical Cord-Derived Mesenchymal Stem Cells and Their Chondroprogenitor Derivatives Reduced Pain and Inflammation Signaling and Promote Regeneration in a Rat Intervertebral Disc Degeneration Model. Mol Cell Biochem 2021, 476, 3191–3205. [Google Scholar] [CrossRef]
- Huang, H.; Liu, X.; Wang, J.; Suo, M.; Zhang, J.; Sun, T.; Zhang, W.; Li, Z. Umbilical Cord Mesenchymal Stem Cells for Regenerative Treatment of Intervertebral Disc Degeneration. Front Cell Dev Biol 2023, 11, 1215698. [Google Scholar] [CrossRef]
- See, E.Y.-S.; Toh, S.L.; Goh, J.C.H. Simulated Intervertebral Disc-like Assembly Using Bone Marrow-Derived Mesenchymal Stem Cell Sheets and Silk Scaffolds for Annulus Fibrosus Regeneration. Journal of Tissue Engineering and Regenerative Medicine 2012, 6, 528–535. [Google Scholar] [CrossRef]
- Risbud, M.V.; Albert, T.J.; Guttapalli, A.; Vresilovic, E.J.; Hillibrand, A.S.; Vaccaro, A.R.; Shapiro, I.M. Differentiation of Mesenchymal Stem Cells Towards a Nucleus Pulposus-like Phenotype In Vitro: Implications for Cell-Based Transplantation Therapy. Spine 2004, 29, 2627. [Google Scholar] [CrossRef]
- Shim, E.-K.; Lee, J.-S.; Kim, D.-E.; Kim, S.K.; Jung, B.-J.; Choi, E.-Y.; Kim, C.-S. Autogenous Mesenchymal Stem Cells from the Vertebral Body Enhance Intervertebral Disc Regeneration via Paracrine Interaction: An in Vitro Pilot Study. Cell Transplant 2016, 25, 1819–1832. [Google Scholar] [CrossRef]
- Teixeira, G.Q.; Pereira, C.L.; Ferreira, J.R.; Maia, A.F.; Gomez-Lazaro, M.; Barbosa, M.A.; Neidlinger-Wilke, C.; Goncalves, R.M. Immunomodulation of Human Mesenchymal Stem/Stromal Cells in Intervertebral Disc Degeneration: Insights From a Proinflammatory/Degenerative: Ex Vivo: Model. Spine 2018, 43, E673. [Google Scholar] [CrossRef]
- Liang, C.; Li, H.; Tao, Y.; Zhou, X.; Li, F.; Chen, G.; Chen, Q. Responses of Human Adipose-Derived Mesenchymal Stem Cells to Chemical Microenvironment of the Intervertebral Disc. Journal of Translational Medicine 2012, 10, 49. [Google Scholar] [CrossRef]
- Merceron, C.; Mangiavini, L.; Robling, A.; Wilson, T.L.; Giaccia, A.J.; Shapiro, I.M.; Schipani, E.; Risbud, M.V. Loss of HIF-1α in the Notochord Results in Cell Death and Complete Disappearance of the Nucleus Pulposus. PLOS ONE 2014, 9, e110768. [Google Scholar] [CrossRef]
- Stoyanov, J.V.; Gantenbein-Ritter, B.; Bertolo, A.; Aebli, N.; Baur, M.; Alini, M.; Grad, S. Role of Hypoxia and Growth and Differentiation Factor-5 on Differentiation of Human Mesenchymal Stem Cells towards Intervertebral Nucleus Pulposus-like Cells. Eur Cell Mater 2011, 21, 533–547. [Google Scholar] [CrossRef]
- Chen, P.; Ning, L.; Qiu, P.; Mo, J.; Mei, S.; Xia, C.; Zhang, J.; Lin, X.; Fan, S. Photo-Crosslinked Gelatin-Hyaluronic Acid Methacrylate Hydrogel-Committed Nucleus Pulposus-like Differentiation of Adipose Stromal Cells for Intervertebral Disc Repair. Journal of Tissue Engineering and Regenerative Medicine 2019, 13, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, G.; Russo, F.; Musumeci, M.; D’Este, M.; Cattani, C.; Catanzaro, G.; Tirindelli, M.C.; Lazzari, L.; Alini, M.; Giordano, R.; et al. Clinically Relevant Hydrogel-Based on Hyaluronic Acid and Platelet Rich Plasma as a Carrier for Mesenchymal Stem Cells: Rheological and Biological Characterization. J Orthop Res 2017, 35, 2109–2116. [Google Scholar] [CrossRef]
- Ohnishi, T.; Homan, K.; Fukushima, A.; Ukeba, D.; Iwasaki, N.; Sudo, H. A Review: Methodologies to Promote the Differentiation of Mesenchymal Stem Cells for the Regeneration of Intervertebral Disc Cells Following Intervertebral Disc Degeneration. Cells 2023, 12, 2161. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan: From Extracellular Glue to Pericellular Cue. Nat Rev Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef]
- Altman, R.; Bedi, A.; Manjoo, A.; Niazi, F.; Shaw, P.; Mease, P. Anti-Inflammatory Effects of Intra-Articular Hyaluronic Acid: A Systematic Review. CARTILAGE 2019, 10, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Stergar, J.; Gradisnik, L.; Velnar, T.; Maver, U. Intervertebral Disc Tissue Engineering: A Brief Review. Bosn J Basic Med Sci 2019, 19, 130–137. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, S.; Gao, M.; Li, B.; He, Z.; Tang, T.; Zhu, Z.; Liu, S.; Zhou, Z. Hyaluronic Acid Ameliorates Intervertebral Disc Degeneration via Promoting Mitophagy Activation. Front Bioeng Biotechnol 2022, 10, 1057429. [Google Scholar] [CrossRef] [PubMed]
- Häckel, S.; Zolfaghar, M.; Du, J.; Hoppe, S.; Benneker, L.M.; Garstka, N.; Peroglio, M.; Alini, M.; Grad, S.; Yayon, A.; et al. Fibrin-Hyaluronic Acid Hydrogel (RegenoGel) with Fibroblast Growth Factor-18 for In Vitro 3D Culture of Human and Bovine Nucleus Pulposus Cells. Int J Mol Sci 2019, 20, 5036. [Google Scholar] [CrossRef]
- Gansau, J.; Buckley, C.T. Incorporation of Collagen and Hyaluronic Acid to Enhance the Bioactivity of Fibrin-Based Hydrogels for Nucleus Pulposus Regeneration. J Funct Biomater 2018, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Isa, I.L.M.; Srivastava, A.; Tiernan, D.; Owens, P.; Rooney, P.; Dockery, P.; Pandit, A. Hyaluronic Acid Based Hydrogels Attenuate Inflammatory Receptors and Neurotrophins in Interleukin-1β Induced Inflammation Model of Nucleus Pulposus Cells. Biomacromolecules 2015, 16, 1714–1725. [Google Scholar] [CrossRef] [PubMed]
- Mohd Isa, I.L.; Abbah, S.A.; Kilcoyne, M.; Sakai, D.; Dockery, P.; Finn, D.P.; Pandit, A. Implantation of Hyaluronic Acid Hydrogel Prevents the Pain Phenotype in a Rat Model of Intervertebral Disc Injury. Sci Adv 2018, 4, eaaq0597. [Google Scholar] [CrossRef]
- Watanabe, A.; Mainil-Varlet, P.; Decambron, A.; Aschinger, C.; Schiavinato, A. Efficacy of HYADD®4-G Single Intra-Discal Injections in a Rabbit Model of Intervertebral Disc Degeneration. Biomed Mater Eng 2019, 30, 403–417. [Google Scholar] [CrossRef]
- Peeters, M.; Detiger, S.E.L.; Karfeld-Sulzer, L.S.; Smit, T.H.; Yayon, A.; Weber, F.E.; Helder, M.N. BMP-2 and BMP-2/7 Heterodimers Conjugated to a Fibrin/Hyaluronic Acid Hydrogel in a Large Animal Model of Mild Intervertebral Disc Degeneration. Biores Open Access 2015, 4, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Kazezian, Z.; Sakai, D.; Pandit, A. Hyaluronic Acid Microgels Modulate Inflammation and Key Matrix Molecules toward a Regenerative Signature in the Injured Annulus Fibrosus. Advanced Biosystems 2017, 1, 1700077. [Google Scholar] [CrossRef]
- Food and Drug Administration Intent To Consider the Appropriate Classification of Hyaluronic Acid Intra-Articular Products Intended for the Treatment of Pain in Osteoarthritis of the Knee Based on Scientific Evidence Available online:. Available online: https://www.federalregister.gov/documents/2018/12/18/2018-27351/intent-to-consider-the-appropriate-classification-of-hyaluronic-acid-intra-articular-products (accessed on 14 February 2024).
- Binch, A.L.A.; Fitzgerald, J.C.; Growney, E.A.; Barry, F. Cell-Based Strategies for IVD Repair: Clinical Progress and Translational Obstacles. Nat Rev Rheumatol 2021, 17, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Maloney, J.; Strand, N.; Wie, C.; Pew, S.; Dawodu, A.; Dunn, T.; Johnson, B.; Eells, A.; Viswanath, O.; Freeman, J.; et al. Current Review of Regenerative Medicine Therapies for Spine-Related Pain. Curr Pain Headache Rep 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dong, H.; Xia, Q.; Wang, Y.; Zhu, L.; Hu, Z.; Xia, J.; Mao, Q.; Weng, Z.; Yi, J.; et al. A New Strategy for Intervertebral Disc Regeneration: The Synergistic Potential of Mesenchymal Stem Cells and Their Extracellular Vesicles with Hydrogel Scaffolds. Biomedicine & Pharmacotherapy 2024, 172, 116238. [Google Scholar] [CrossRef]
- Sakai, D.; Andersson, G.B.J. Stem Cell Therapy for Intervertebral Disc Regeneration: Obstacles and Solutions. Nat Rev Rheumatol 2015, 11, 243–256. [Google Scholar] [CrossRef]
- Thorpe, A.A.; Bach, F.C.; Tryfonidou, M.A.; Le Maitre, C.L.; Mwale, F.; Diwan, A.D.; Ito, K. Leaping the Hurdles in Developing Regenerative Treatments for the Intervertebral Disc from Preclinical to Clinical. JOR Spine 2018, 1, e1027. [Google Scholar] [CrossRef]
| Treatment | First author, year | Total participant number | Trial design | Outcome |
|---|---|---|---|---|
| BMMSC | [56] | 24 | Randomized controlled trial – single intradiscal injection of BMMSCs or sham injection (unspecified anesthetic) | Reduced pain and degeneration at 12 months follow-upFeasibility and safety confirmed |
| BMMSC | [57,58,59] | 26 | Prospective, open-label nonrandomized trial – intradiscal injection of BMMSCs at one IVD (n=13) or two adjacent IVDs (n=13). | Reduced pain at 1,2 and 3 years follow up |
| BMMSC | [60] | 10 | Single treatment group – single intradiscal injection of BMMSCs | Feasibility and safety confirmed. Reduced pain and disability at 3 months. IVD water content increased at 12 months |
| BMMSC | [61] | 10 | Single treatment group – single intradiscal injections of BMMSCs followed by 2 weeks of hyperbaric oxygen therapy | No pain reduction at 12 months follow up |
| BMMSC | [62] | 2 | Single treatment group – single intradiscal injection of collagen scaffold soaked in BMMSCs | Reduced pain and vacuum phenomenon (gas in IVD) at 24 months follow-up |
| BMMSC | [63] | 5 | Single treatment group – single intradiscal injection of hypoxic-cultured BMMSCs. | No adverse outcomes. Improved mobility and strength reported for 4 patients at 4-6 year follow-up |
| BMMSC | [64] | 33 | Single treatment group – single intradiscal injection of BMMSCs. | Safety confirmed. Pain reduction at 3-6 years follow-up. 85% of the 20 patients who underwent post-treatment MRI also had reduced disc bulge size |
| BMMSC | [65,66] | 11 | Single treatment group – single lumbar intradiscal injection of BMMSCs embedded in tricalcium pohosphate | Reduced pain and disability at 5 and 10 years follow-up. All imaged patients demonstrated lumbar fusion. |
| BMMSC & PRP | [67] | 40 | Multicenter randomized controlled trial – single intradiscal injection of BMMSCs, PRP or saline (placebo control) | PRP reduced pain and improved function at 1 year follow-up when compared to placebo. BMAC reduced pain and improved function at 1 year follow-up when compared to placebo. No significant differences between PRP and BMMSC treatments were detected. |
| UCMSC | [68] | 2 | Single treatment group – single injection of UCMSCs | No severe adverse events following treatment. Reduced pain at 24 months follow-up |
| AMSC | [69] | 15 | Single treatment group – single injection of AMSCs | No severe adverse events following treatment. Reduced pain and disability at 12 months follow-up |
| AMSC & HA | [70] | 10 | Single treatment group – single injection of AMSCs combined with a HA derivative | No severe adverse events following treatment. Reduced pain at 1 year follow-up. Three patients demonstrated increased IVD water content in 1 year follow-up MRI. |
| PRP | [71] | 47 | Double-blind, randomized controlled trial. Single intradiscal injection of PRP (n=29) or contrast agent (placebo control; n=18). | Statistically significant pain reduction at 8 weeks follow-up for PRP treatment group when compared with placebo group. |
| PRP | [72] | 22 | Single treatment group – intradiscal injection of PRP in two IVDs (n=10), three IVDs (n=2) or five IVDs (n=1). | Reduced pain and disability at 6 months follow-up. |
| PRP | [73] | 26 | Double-blind, randomized controlled trial. Single intradiscal injection of PRP (n=18) or saline (placebo control; n=8) | No significant differences in pain or disability reduction seen between PRP and placebo groups. |
| PRP | [74] | 48 | Double-blind, randomized controlled trial. Single intradiscal injection of PRP Percutaneous intradiscal radiofrequency ablation | Statistically significant reduction in pain and disability at 3 and 6 months follow-up, however no statistically significant difference in pain/disability reduction between PRP and radiofrequency ablation groups. |
| PRP | [75,76] | 16 | Double-blind, randomized controlled trial. Single intradiscal injection of PRP releasate (n=9) or betamethasone sodium phosphate (a corticosteroid; n=7). 15 patients also received an additional, optional PRP injection 8 weeks after treatment. | Significant improvement in disability and walking ability in PRP releasate group when compared to corticosteroid group at 26 weeks follow-up. Both treatment groups had significant reduction in pain, however no significant differences in pain reduction between groups were detected. |
| PRP | [77] | 5 | Single treatment group – single intradiscal injection of PRP | Gradual pain and disability reduction up to and including at 1 year follow-up |
| PRP | [78] | 6 | Single treatment group – single intradiscal injection of PRP | Pain reduction at approximately monthly follow-ups for 6 months for all patients. 6-months post MRI demonstrated structural improvements in disc anatomy for some patients. |
| PRP | [79] | 14 | Single treatment group – single intradiscal injection of PRP releasate | No adverse effects observed following treatment. Statistically significant pain reduction at 1 and 6-month follow-ups. No significant differences detected in follow-up MRI T2 quantification. |
| Treatment | ClinicalTrials.gov ID | Total participant number | Protocol | Trial Status at March, 2024 |
|---|---|---|---|---|
| PRP | NCT05287867 | 42 (28 treatment, 14 sham control) | Single-blind, randomized, placebo-controlled study. 2 treatments, 4 weeks apart of intradiscal PRP (or sham injection) | Actively recruiting |
| PRP | NCT04816747 | 50 (estimated) | Single group assignment, single intradiscal PRP injection | Not yet recruiting |
| PRP | NCT02983747 | 112 (estimated) | Randomised controlled trial (PRP intradiscal injection compared to thrice weekly oral NSAID (loxoprofen)) | Recruiting |
| UCMSC | NCT04414592 | 20 (estimated) | Single group assignment, single intradiscal UCMSCs injection | Status unknown |
| AMSC | NCT05011474 | 4 (estimated) | Single group assignment (AMSC intradiscal injection enriched with the ECM protein matrilin-3) | Status unknown |
| BMMSC | NCT05066334 | 52 (estimated) | Randomized controlled trial (intradiscal injection of BMMSCs vs sham control of local anesthesia) | Status unknown |
| BMMSC | NCT04759105 | 48 | Randomized control trial (intradiscal injection of BMMSCs vs sham control of local anesthesia) | Active, not recruiting |
| BMMSC | NCT04042844 | 99 (estimated) | Double-blind, randomized controlled trial (intradiscal injection of BMMSC vs saline) | Actively recruiting |
| BMMSC | NCT04735185 | 106 (estimated) | Randomized controlled trial of single intradiscal injection (intradiscal injection of BMMSCs, methylprednisolone or local anesthethic (bupivacaine) control) | Suspended (awaiting sponsor and FDA feedback) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





