1. Introduction
Exploration of water on celestial bodies such as the Moon and Mars has heightened, encouraged by the confirmed presence of ice particles and ice-cemented regolith [
1,
2]. These discoveries are not merely academic; they reshape our understanding of these bodies’ geological past and create tangible stepping stones toward future space exploration and potential colonization [
3]. The presence of water ice was notably confirmed at the Moon’s south pole by the Lunar Crater Observation and Sensing Satellite (LCROSS) mission and has been further evidenced by the observations of NASA’s Lunar Reconnaissance Orbiter (LRO) [
4,
5]. On Mars, the Mars Reconnaissance Orbiter and the Phoenix Lander have provided compelling evidence of water-ice, particularly within shaded craters and sub-surface layers [
6,
7]. Moreover, NASA’s Stratospheric Observatory for Infrared Astronomy (SOFIA) detected molecular water on the sunlit surface of the Moon’s Clavius Crater in October 2020, revealing that water molecules can withstand lunar surface conditions [
8,
9]. Building on this discovery, Liu et al. (2012) provided direct measurements of hydroxyl in the lunar regolith, offering further evidence of the persistence and stability of water molecules on the Moon’s surface [
10].
These findings contribute significantly to our understanding of the lunar hydrosphere and highlight the Moon’s potential for sustaining water-based activities necessary for future human and robotic missions. Water is a critical resource for human survival, mission operations, and as a fuel component, making the establishment of reliable water extraction technologies a crucial challenge [
11]. The unique conditions of the Moon, with water concentrations detected near the poles and in permanently shaded regions, make it a promising candidate for In-situ Resource Utilization (ISRU) [
12,
13]. Similarly, Mars presents unique challenges and opportunities, with its regolith believed to contain bound water molecules, which can be extracted to support long-duration human missions [
14].
Numerous techniques have been proposed to extract water from extraterrestrial bodies, employing a variety of thermal and physical processes tailored to the specific conditions and forms of water present [
15,
16,
17]. In scenarios where water exists as ice mixed with regolith, thermal sublimation is commonly applied. This method involves directly heating the ice-regolith mixtures to transform the ice into vapors, which are then captured [
18,
19]. The effectiveness of this technique has been demonstrated in controlled lunar simulations, illustrating how thermal energy can efficiently convert ice to vapor for collection.
Another promising technique is microwave heating, which has shown significant potential in laboratory settings. Unlike conventional heating methods, microwaves penetrate the regolith and directly excite water molecules, rapidly heating and sublimating the ice even when it is deeply embedded within the soil. This method is particularly advantageous for its efficiency, targeting water molecules specifically to minimize heat loss, and could be effectively adapted for lunar missions [
20].
Hydrothermal processing represents a third approach, utilizing hot water or steam to heat the regolith and release water molecules. This method adapts terrestrial geothermal recovery techniques for extraterrestrial environments, offering a versatile solution tailored to different regolith compositions and water content levels [
21]. By adjusting the temperature and pressure of the steam, hydrothermal processing can extract water from various forms of bound water in Martian permafrost or mineral hydrates.
Each of these techniques not only demonstrates the practicality of extracting water in space environments but also highlights the adaptability of Earth-based technologies for use on extraterrestrial bodies [
22]. The continued development and refinement of these methods are essential for advancing In-Situ Resource Utilization (ISRU) strategies, crucial for establishing sustainable human outposts on the Moon and Mars by leveraging local resources for life support, agriculture, and fuel production [
23].
The method proposed in this study involves filling an empty evaporator with a desired amount of icy-regolith, sealing the evaporator, and externally heating the reactor walls. As the temperature of the regolith rises during this heating phase, water vapor is released, which then accumulates on a cooled substrate, facilitating the collection of extracted water [
24]. The methodology involves a detailed analysis of three distinct substrate types: hydrophobic, hydrophilic, and grooved surfaces, under simulated lunar, Martian, and micro-gravity conditions. These substrates were chosen based on their unique properties, which influence the behavior of water molecules during the condensation process. The selection was informed by a comprehensive literature review, identifying these surfaces as promising candidates for enhancing water recovery rates [
25,
26,
27]. However, despite various proposed techniques, there is a lack of available studies on water extraction and recuperation under reduced gravity conditions. This paper seeks to bridge this gap by evaluating water recovery from regolith on the Moon, Mars, and under micro-gravity conditions through parabolic flight simulation. The experiment involves evaporating water introduced into synthetic regolith and recovering the resultant water vapor as condensed liquid. These experiments endeavor to provide valuable insights into developing sustainable water supplies for future manned missions, addressing a critical challenge for long-term space exploration and reducing the costs for space exploration.
2. Materials and Methods
2.1. Experimental Setup and Procedure
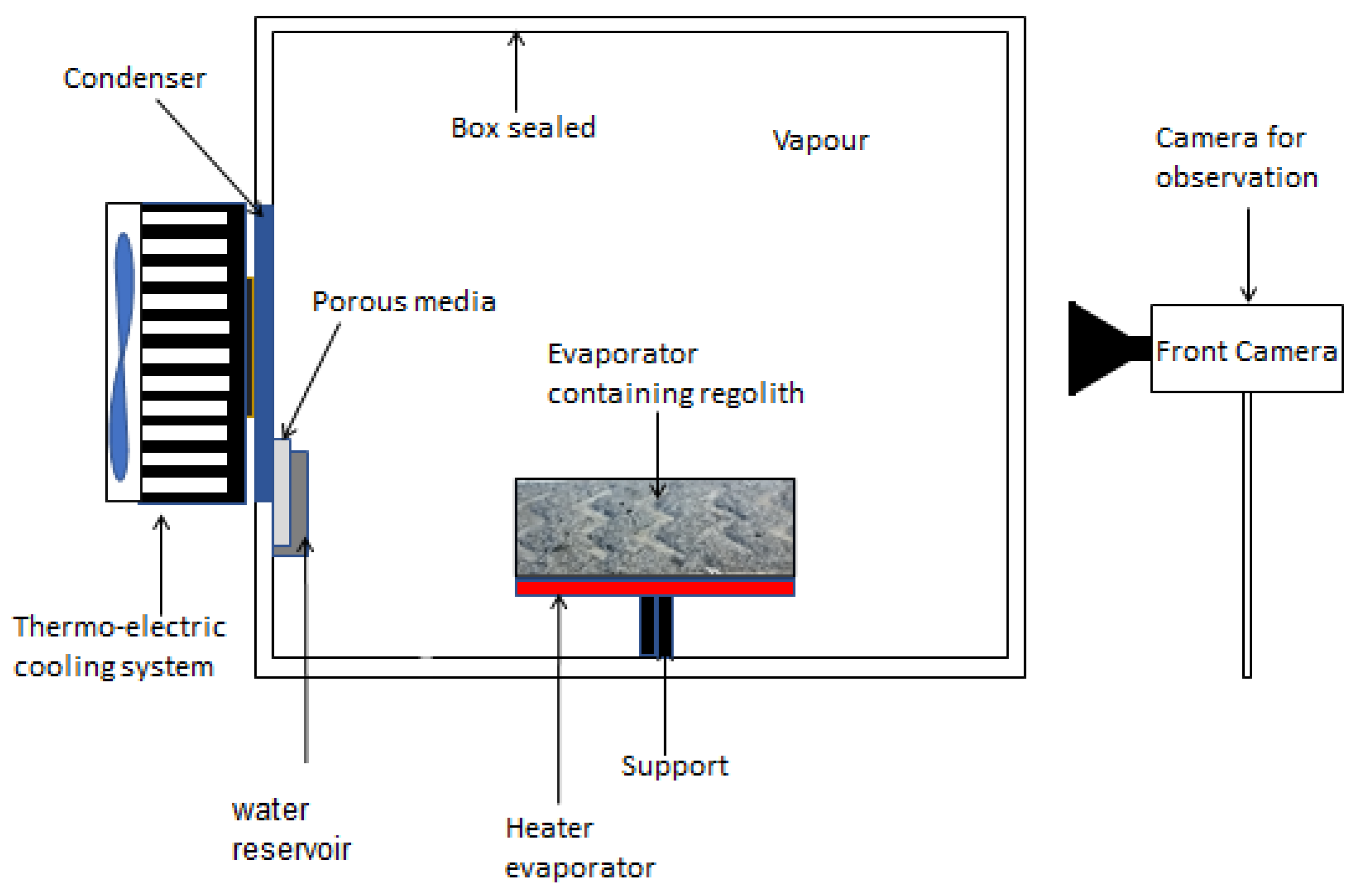
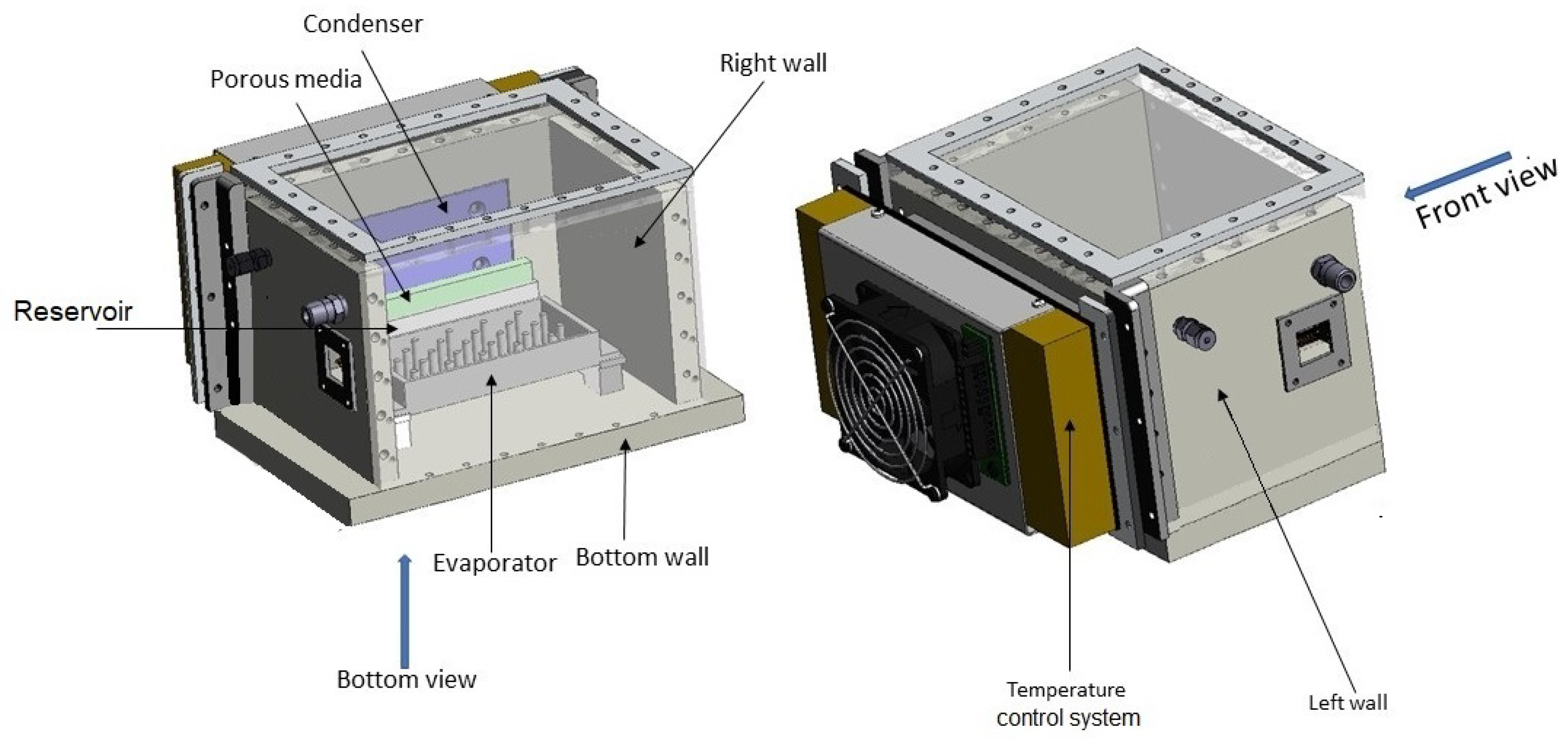
The experimental setup is shown in
Figure 1 while
Figure 2 offers a 3D view. The casing is constructed primarily from Polyetheretherketone (PEEK), with the exception of the top and frontal viewing panels, which are fabricated from Plexiglass to facilitate observation. This enclosure is hermetically sealed to prevent external contamination and to allow for precise control of internal pressure, crucial for experimental integrity. A heater is attached to the lower surface of the evaporator to ensure even heat distribution. Temperature and pressure sensors are strategically placed within the enclosure to monitor experimental conditions continuously.
A JAI GO-5000M camera is positioned externally, aligned with the condenser to document the condensation process. The dimensions of the enclosure are 210 x 170 x 160 mm, reinforcing its capability to shield the experiment from external variables and maintain the required internal conditions. Within this setup, 120 gr of highland regolith(OPRH3N, developed by Off Planet Research, LLC) was used, containing 80% anorthosite and 20% basaltic cinder mixed with 45 ml water evenly spread inside the evaporator surface. The grain sizes of the regolith used and mixed are shown in
Table 1. Initially, the environmental pressure inside the sealed case is set at 4 millibars, a value chosen to balance the need for low pressure against the practical constraints of parabolic flight preparations, where lower pressures would necessitate impractically extended preparation durations.
The flight campaign involved a series of 30 parabolas each day, simulating microgravity, lunar gravity, and Martian gravity conditions. The aircraft maneuvered through a controlled trajectory to achieve these conditions, carefully monitored by engine thrust. This dynamic flight pattern posed unique challenges for managing the condensed fluid. During hypergravity phases, fluid dispersal was chaotic, while in microgravity, condensate tended to float or form a thick film on the condenser plate, impeding condensation efficiency.
To address these challenges, porous media was strategically placed inside the reservoir below the condenser plate. This setup not only absorbed and channeled away fluid effectively but also prevented dispersion during hypergravity and inhibited thick film formation during microgravity phases. This ensured continuous and effective condensation across varying gravity conditions encountered during the parabolic flights.
The apparatus was equipped with a Peltier element, and a cooling system on the condenser plate to maintain desired temperature conditions, all controlled via a PID controller. Data from the sensors were logged using an Agilent 34970A data logger.
The methodology involved rigorous preparation of regolith simulants, assembly of the experimental apparatus, and control during flight to investigate water condensation dynamics effectively under varied gravitational conditions.
2.2. Parabolic Flight Conditions
In December 2020, the 74th ESA parabolic flight campaign provided our team the opportunity to study water recovery from lunar regolith in a simulated space environment. The experiments aimed to understand the performance of specific substrates under lunar, Martian, and microgravity conditions—key to future utilization of extraterrestrial resources.
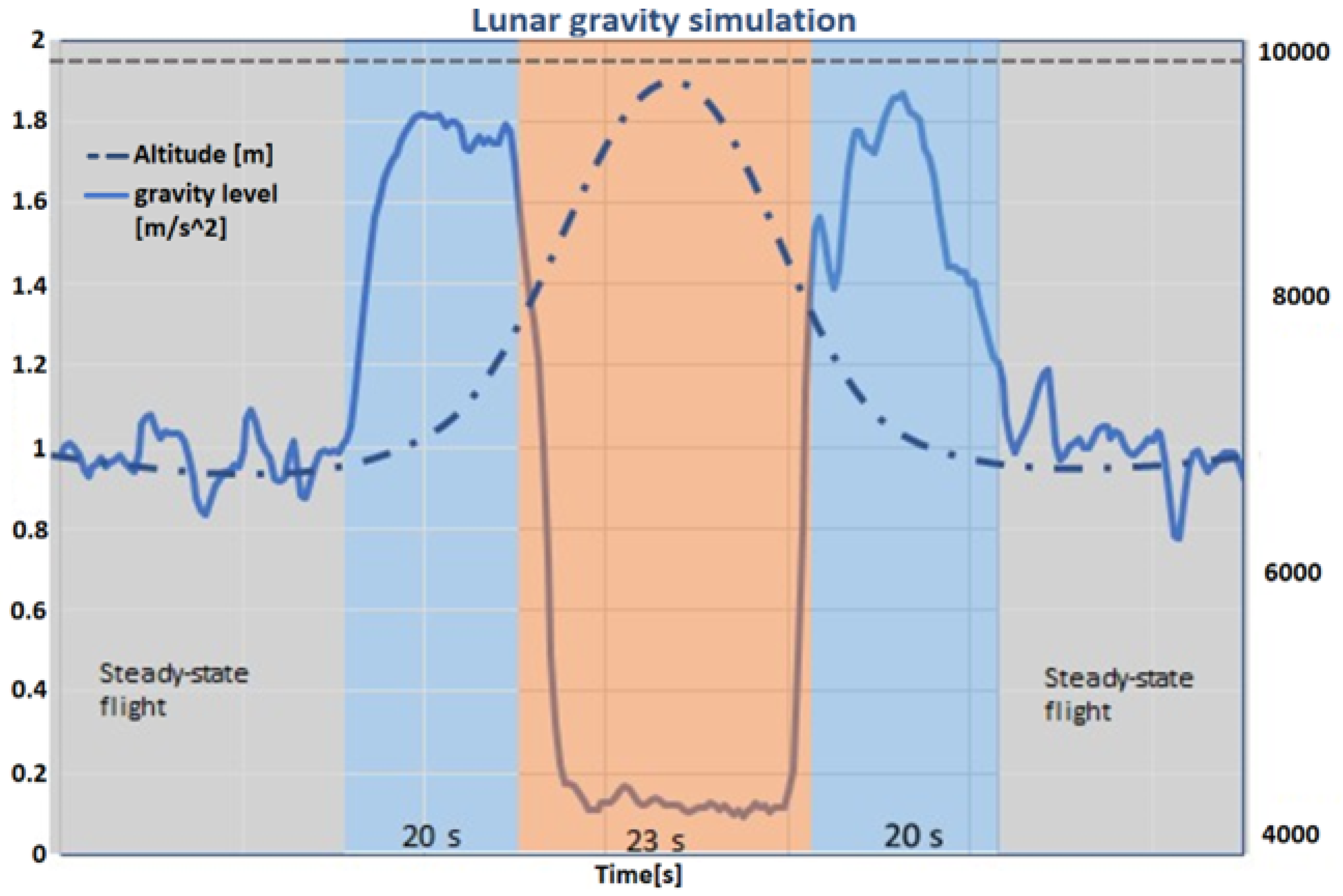
The experimental apparatus was housed in a robust container(Zargas aluminium case), monitored to preserve experimental conditions throughout the flight. The aircraft’s flight pattern was carefully planned to replicate different gravitational states. It began with a normal 1g phase, escalated to a 2g hypergravity during a sharp ascent, and transitioned into various reduced gravity states mimicking lunar and Martian gravities, and microgravity during the parabolic arcs.
Each of the parabolas executed daily involved precise control over ascent to a 2g state, maintenance of reduced gravity, and a return to 2g before stabilizing at 1g. The meticulous control over these phases ensured that each gravity level was sustained for specific durations necessary for the experiments.
Figure 3 in the documentation illustrates these transitions in gravity levels, altitude, and timing, showcasing the detailed management of experimental conditions.
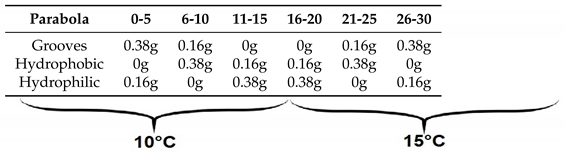
Each day, the temperature in the condenser plate was held at a stable value of around 10°C for the first fifteen parabolas and around 15°C for the second group of fifteen parabolas. Some variation in the temperature was still observed due to unstable conditions of the parabolic flights and the constant variations in gravity (g-jitters), which made it challenging to keep a fixed value. Each day, one of the substrates was used (grooved, hydrophobic, or hydrophilic). For each day (equivalent to one substrate choice),
Table 2 shows the composition of the flight corresponding to the reduced-gravity levels.
3. General Approach for the Image Analysis
3.1. Image Preparation
A Drop Shape Analyzed kruss DSA30 is used for precise droplet contact angle measurements. The results obtained helped in the analysis of the drop shape formed on the surface. Furthermore, as shown in
Figure 4, the contact angle was measured to be 50° for the hydrophilic surface, 119° for the grooved substrate, and 116° for the hydrophobic one. These values are found in harmony with the definitions found in the literature of hydrophobic and hydrophilic surfaces [
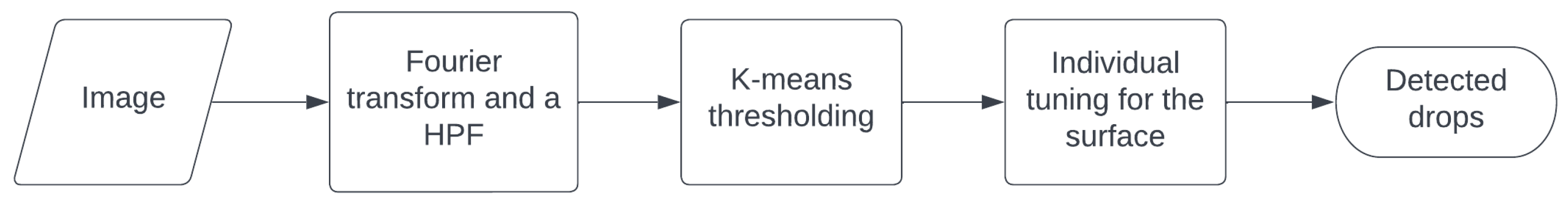
28]. It has been verified that by varying the contact angle around the mentioned respective values for each respective type of substrate, during the image processing, the obtained condensation values appeared to vary negligibly, and the general behavior of the analysis is thus considered to be preserved. Before resulting in the detection of condensed drops, the prepared images need to undergo individual tuning, depending on the type of substrate. The general process outlined in this section is illustrated in
Figure 5.
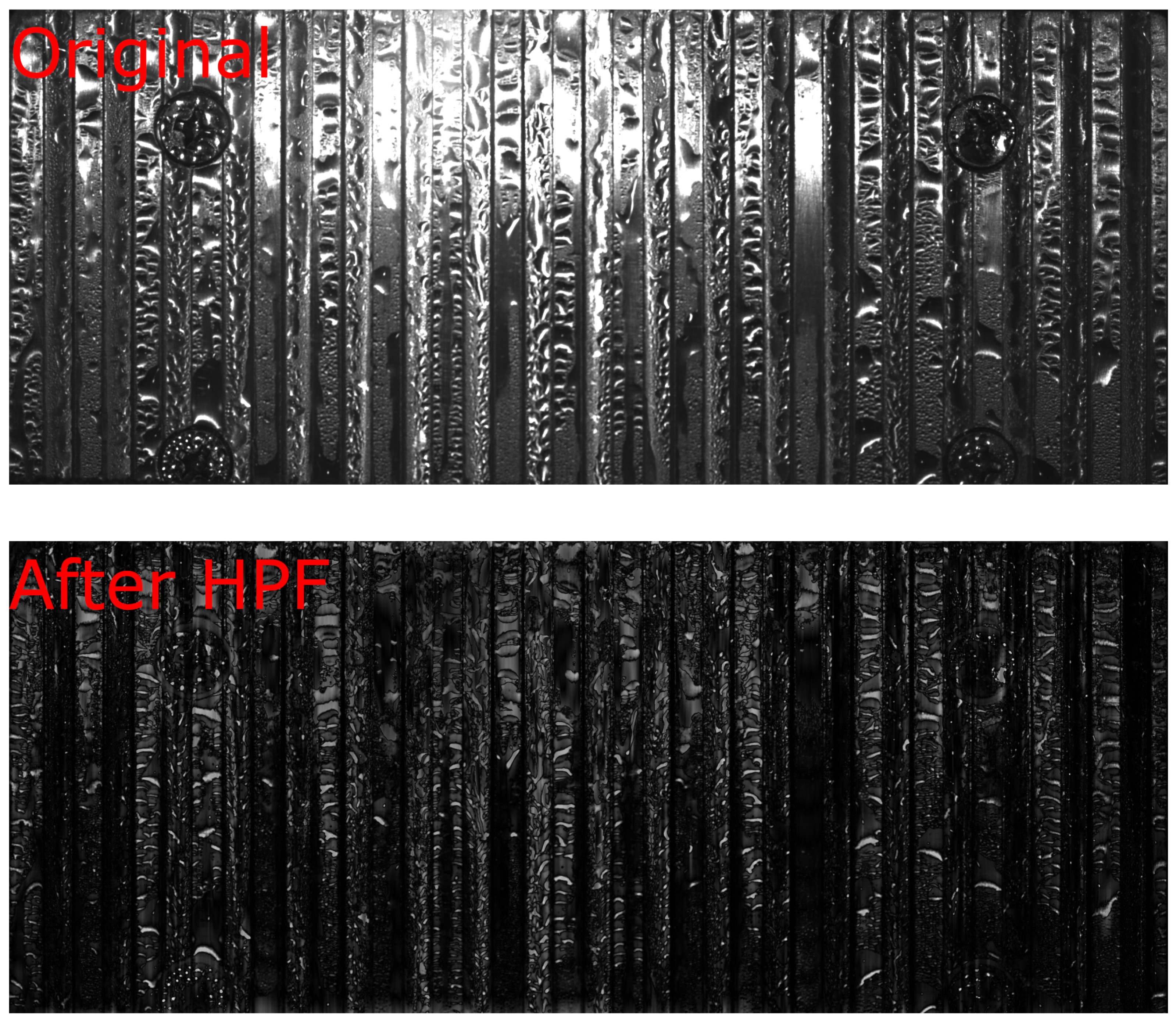
Figure 6 shows the grooves substrate, chosen for the capability of the channels to have the double effect of increasing the surface for heat exchange and the implemented capabilities of removing the condense through their channels. A camera showed water condensation for the substrates but no drop-wise condensation that was required for the analysis. The three substrates having uneven lighting, with drops not being uniform also caused trouble in this area. On the other hand, it can be seen that, at least in the short-axis direction, the individual droplets are by far the smallest feature in the picture, associated with much higher spatial frequencies than the lighting grooves or paths. To effectively filter out the lower spatial frequencies using a finely adjusted high-pass filter, the images were converted to the Fourier domain. Manual masking mitigated several challenges encountered in this approach, ensuring minimal impact on the final outcomes. This procedure was applied to the grooved substrate to illustrate the image analysis performed in this work. The same procedure was employed later on other substrates.
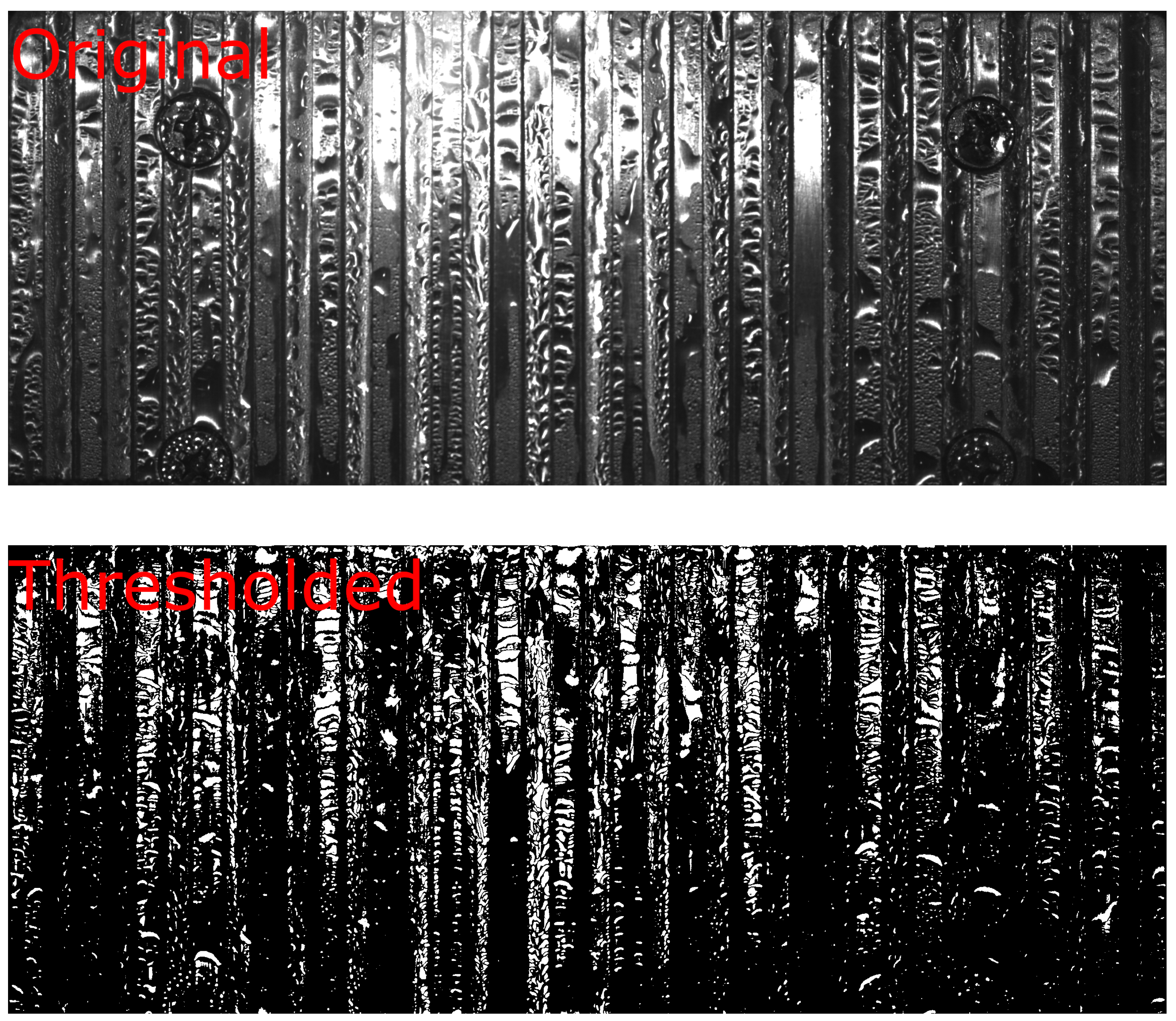
Figure 6 also provides a visual representation of this process, showcasing the application of a spatial high-pass filter in the y-direction. Several thresholding algorithms were compared by applying k-means clustering to partition the data into two distinct groups, which yielded the most favorable outcomes. In this context, ’k’ represents the number of clusters, determining the division of the data.
Figure 7 shows the thresholded image of the grooved substrate, allowing us to evaluate the areas of individual droplets. These areas can be further transformed into droplet volumes, where it was assumed that all droplets have a paraboloid shape, where the droplet area corresponds to the area of the paraboloid base.
3.2. Tuning for Individual Surfaces
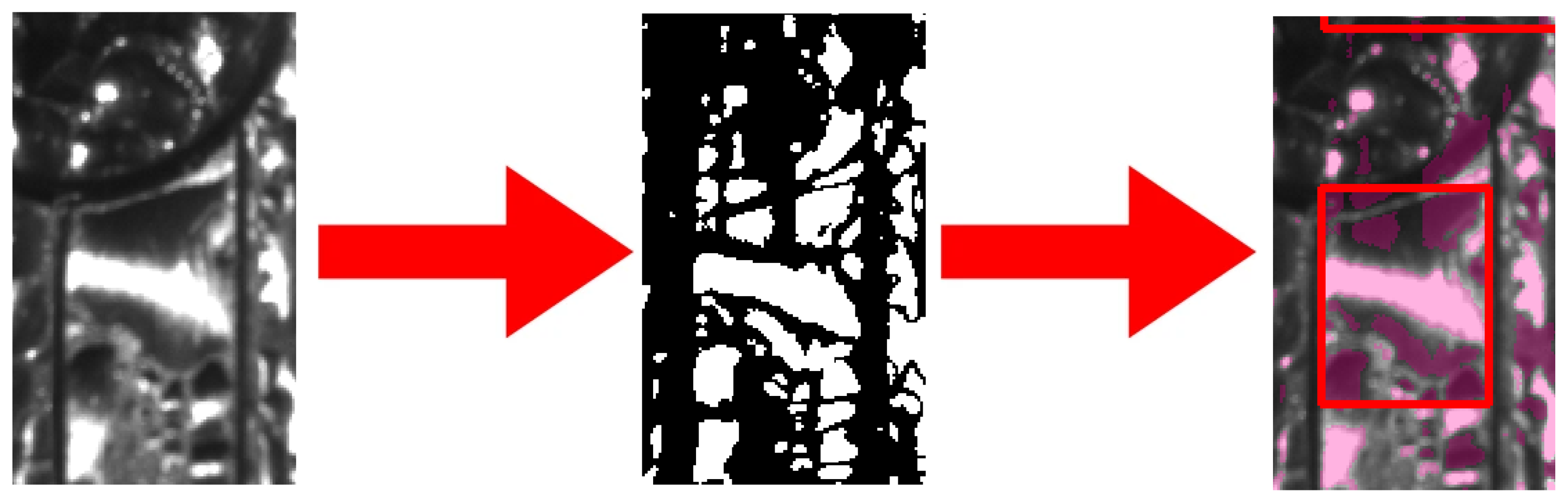
There are no features in the y-direction with a similar spatial frequency to the droplets. In the x-direction (horizontal), however, the grooves have a spatial frequency not far from the droplets. Therefore, in the Fourier domain, a high-pass filter is constructed such that it only considers the spatial frequencies in the y-direction. Almost all the larger drops are not detected as one drop but as two separate segments with different colours very close to each other. This is a consequence of directional lighting. Consequently, drops appear as smaller drops and the volume is underestimated. To mitigate this effect, a k-means algorithm is used. Larger drops (empirically found to be defined as over 0.7 mm
2 in the area) are counted, with n-big drops being the total number and then grouped into n-big drops/2 clusters. Such clusters subsequently enter volume calculations as whole droplets. This is illustrated in
Figure 8. An example of the overall result of the process is shown in
Figure 9.
Similarly, to the grooves, there is a feature with a similar x-direction spatial frequency to the droplets, but one must filter out the paths of the fallen droplets. The solution is to apply a high-pass filter devised in a way that only uses the y-direction. Another problem encountered was the bolts getting often detected with no droplets on them. Masking them manually solved this. Sometimes unrealistically large areas around the edges were also detected, and therefore, all drops with a size above an experimentally found threshold (found to be 115 mm
2) were filtered out. A typical process result on a hydrophobic surface is shown in
Figure 10. In the case of a hydrophilic surface, all the features are very similar in the x and y-directions. Therefore, a high-pass filter is used to take both directions into account. However, bolts have to be masked as they are getting detected. Areas above an empirically established threshold (70 mm
2) were filtered out. It was also found that better results were achieved if a morphological operation of dilation was applied after the thresholding. For the dilation, a 3x3 kernel was used. The typical results are shown in
Figure 11.
4. Results
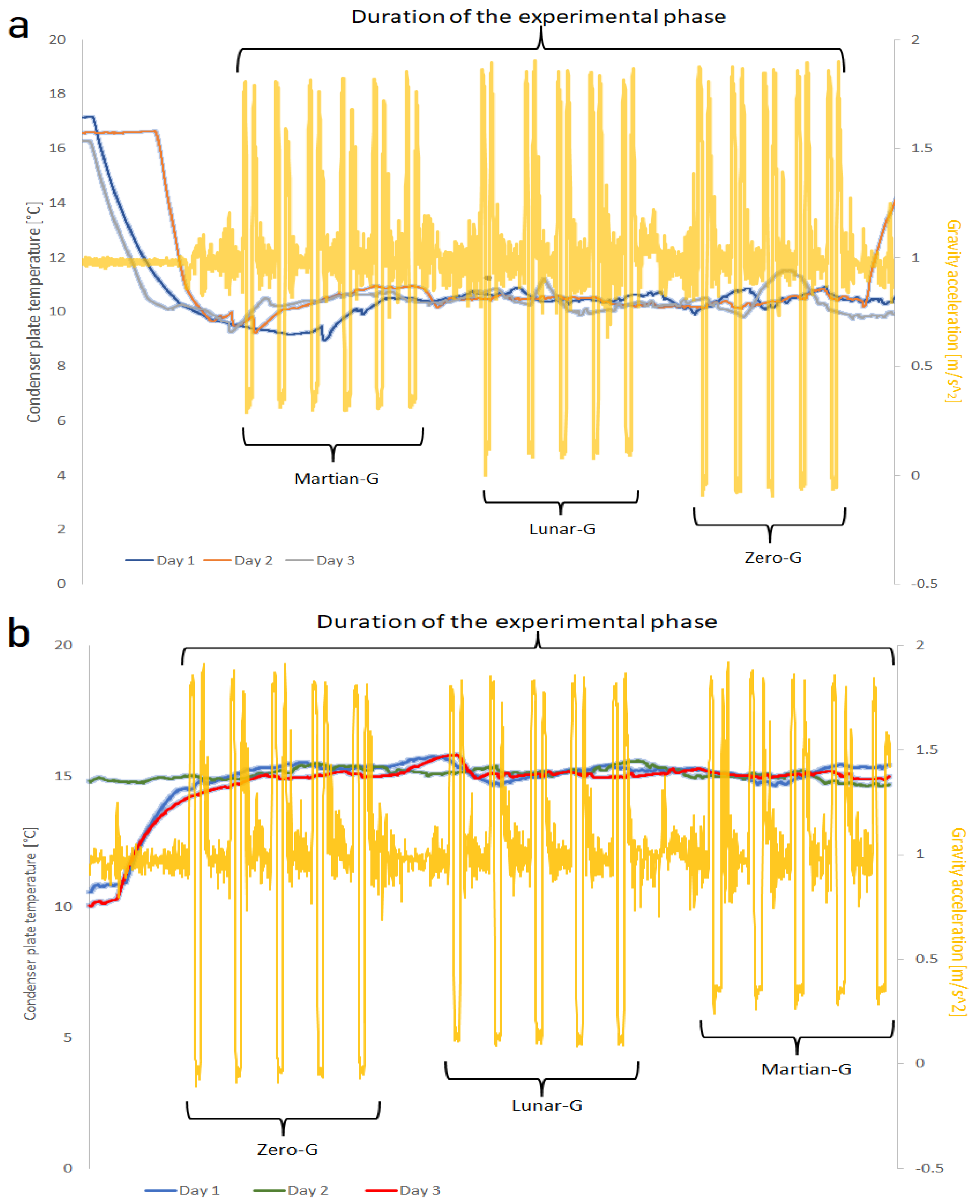
Figure 12(a) shows the measured temperature over the condensing surfaces during the first set of parabolas, while
Figure 12(b) shows the temperature trend over the condensing surfaces during the second set of parabolas. As can be seen, the first set is indeed kept at about 10°C, and the second at 15 °C. However, it can also be seen that fluctuations in the temperature of up to 2°C are possible. As an example, the gravity levels corresponding to the first day (with the grooved substrate) are drawn in
Figure 12 as well. During the hypergravity phase, the removal of the liquid film formed on the face of the condenser was impacted, leading to less heat being pumped outwards. The removal of the drops caused temperatures to decline and a downward oscillation. The stationary conditions re-established at the end of the parabola caused temperatures to vary upwards, explaining the reason for the recorded temperature fluctuations.
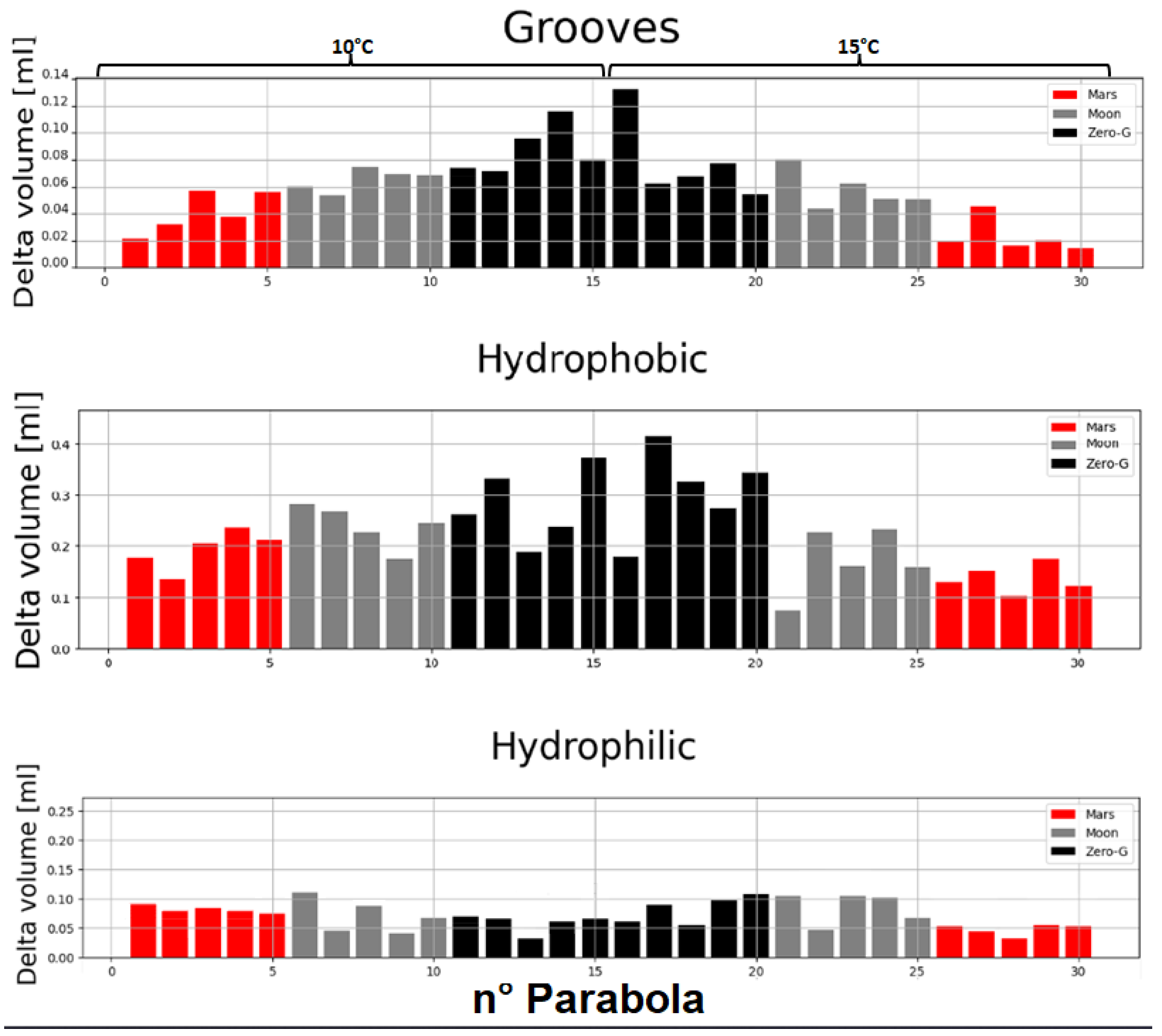
Figure 13 shows the measured condensed water during the reduced-gravity intervals for the three substrates for all the parabolas. For each temperature (10 and 15°C), each gravity level (Martian, lunar, microgravity), and each substrate (grooved, hydrophobic, and hydrophilic), the total amount of condensed water is reported in
Table 3.
First, we look at the overall results.
Figure 13 shows that, for the grooved and hydrophobic substrates, a maximum amount of condensed water occurs at microgravity, whereas this amount decreases as gravity increases. The overall tendency appears to be that the lower the gravity, the more water is condensed. A numerical study shows that gravity plays a role in the vapor-liquid distribution [
29]. A lower gravity seems, according to the numerical study, to enhance heat transfer and increase the amount of condensation as is observed in this work. Ground experiments were also performed, which seem to confirm, in general, that higher gravity levels result in less condensation. Interestingly, we can see that the amount of condensed water on the ground is the lowest also for the hydrophilic substrate, which confirms that there is an effect of gravity. Temperature fluctuations have been observed during the runs at 10 °C for the hydrophilic substrate. It is believed that this causes the exception to the observed overall tendency for the hydrophilic substrate at 10°C. We can imagine that these temperature fluctuations change the vapor saturation conditions and, therefore, also the condensation process. Knowing the existence of these temperature fluctuations and the confirmation of the overall gravity tendency on condensation from the result on the ground, we may conclude that, indeed, gravity tends to lower the amount of condensed water. However, it is worth mentioning that the effect of gravity on the amount of condensed water seems to be much lower for the hydrophilic substrate with respect to the other substrates. It may be so that the gravity effect on condensed water for the hydrophilic substrate is perturbed by the film-wise condensation. It would, therefore, be beneficial to verify the values for the condensed water on the hydrophilic substrate at 10°C by other reduced-gravity experiments. It has been mentioned that the hydrophilic substrate exhibits relatively low condensation with respect to the other substrates. Indeed, the hydrophobic substrate is shown to give the highest amount of condensed water. This can be easily understood by noting that in hydrophobic conditions, the condensation is typically of a drop-wise type, which is known to exhibit higher heat transfer [
30]. The grooved substrate exhibits an intermediary behavior.
Finally, the effect of temperature is quite clear.
Table 3 shows that a higher substrate temperature results in less condensed water. This is understood by reminding us that at a higher temperature, the saturation pressure is also higher, resulting in a thermodynamic equilibrium favoring more vapor in the gas phase and, therefore, less condensate.
5. Discussions
The successful execution of in-situ resource utilization (ISRU) technologies hinges on understanding fluid dynamics under varied gravitational conditions. Our study, conducted during the 74th ESA parabolic flight campaign, explored the efficiency of water recovery from regolith analogs in simulated lunar and Martian gravities. These types of findings are important for the development of sustainable extraterrestrial habitats.
Our experiments highlighted the influence of gravity on condensation efficiency, with lower gravity environments favoring higher condensation rates. This enhanced condensation in microgravity can be attributed to decreased mechanical resistance against the phase transition of water molecules.
Among the substrates tested—hydrophobic, hydrophilic, and grooved—the hydrophobic surface demonstrated the highest water recovery efficiency. This substrate’s tendency to promote drop-wise condensation, which is more effective for heat transfer and, thus, water recovery, points to the potential of material engineering in optimizing ISRU technologies.
Furthermore, the incorporation of porous media effectively managed fluid dispersal across varying gravity phases, maintaining the integrity and consistency of water recovery. This strategic integration highlights the necessity for advanced system engineering in ISRU technologies to address the complexities of fluid management in space.
Future research should aim to refine these systems through extended material studies and advanced prototypes, potentially in actual space missions. Testing these technologies under real space conditions will validate their long-term stability and efficiency, crucial for developing reliable life support systems on the Moon and Mars.
This research contributes significantly to the field of ISRU by advancing our understanding of water recovery in space. It offers a foundational step towards achieving long-term human presence on extraterrestrial bodies.
6. Conclusion
The proposed experiment focuses on extracting and condensing water as a preliminary step towards improving the efficiency of water extraction from lunar regolith. To determine the most efficient surface, three types of substrates were selected and evaluated (groove, hydrophobic, and hydrophilic). A thermal extraction was implemented, with each component undergoing initial testing under terrestrial conditions for parabolic flight suitability. This process not only validated the feasibility of the water collection system but also confirmed the potential use of these surfaces for future water extraction. Future implementations will require the addition of a water retraction pump and a reclaimed water storage container. During the parabolic flight, the condensation surfaces (grooves, hydrophobic, hydrophilic) were tested while maintaining an identical global configuration across three days. Observable differences in condensing capacity led to the conclusion that a hydrophobic substrate demonstrated a superior ability to convert vapor into liquid under the same experimental conditions.
Subsequent ground experiments conducted after the parabolic flight yielded lower water vapor condensation results compared to the flight experiments. The fluctuation in temperature occurred due to the box being sealed. However, the hydrophobic surface generally exhibited the best condensation performance. Additionally, the results indicated that condensation efficiency was independent of the surface properties of the condenser and as well as the gravity level experienced during the experiment. Three gravity levels were used for the experimental tests: Martian, lunar, and micro-gravity. The highest condensation was observed in micro-gravity conditions, followed by lunar gravity and then Martian gravity. The ground experiment further confirmed the tendency that a higher gravity level results in less condensation. This straightforward approach using a thermal condenser holds promise as an in-situ resource utilization (ISRU) method for water extraction. However, various factors, such as the heating method, power level, and ice fraction, influence the extraction process. The experimental setup presented in this study could be implemented for extracting water from lunar regolith, and the insights gained from the results contribute to our understanding of thermal water extraction as an ISRU approach. In conclusion, this experiment demonstrated the efficiency of a thermal condenser in extracting water from lunar regolith under different gravity levels and surface properties. The findings provide valuable insights into the parameters impacting condensation efficiency and rate, which can guide future research and development of thermal condensers for water extraction. The values of
Table 3 are considered accurate as they belong in the domain of expected values and tolerance. Further investigation will delve into the analysis of sublimation under various gravities using different condensation substrates.
Author Contributions
“Conceptualization, C.S.I., P.Q, C.M, H.M, and D.F.; methodology, C.S.I., P.Q, C.M, H.M, D.F.; software, P.Q. C.M; validation, C.S.I., P.Q, C.M, H.M, and D.F.; formal analysis, C.S.I., P.Q, C.M, H.M, and D.F; investigation, C.S.I., P.Q, C.M, H.M, and D.F.; resources, C.S.I; data curation, D.F.; writing—original draft preparation, D.F., and H.M.; writing—review and editing, D.F., C.S.I and H.M.; visualization, D.F., C.M, and P.Q.; supervision, C.S.I.; project administration, C.S.I.; funding acquisition, C.S.I. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to express their gratitude to the European Space Agency (ESA) for funding this project and for organizing the 74th Parabolic Flight Campaign. Special thanks also to NoveSpace’s team for meticulously arranging all preparatory steps. We extend our appreciation to the entire CREST team for their resilience and unity, which enable us to overcome every challenge with determination. We are particularly grateful to Andrey Glushchuk for his substantial support in preparing the setup for the parabolic flight. Special thanks are due to our dedicated students, Nadine Karaouni and Ondřej Dvořák, for their persistent assistance and unwavering commitment throughout this project.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ISRU |
In-Situ Resource Utilization |
| LCROSS |
Lunar Crater Observation and Sensing Satellite |
| LRO |
Lunar Reconnaissance Orbiter |
| SOFIA |
Stratospheric Observatory for Infrared Astronomy |
| MTCS |
Methyltrichlorosilane |
| PVA |
Polyvinyl Alcohol |
| MMT |
Montmorillonite |
| PMA |
Polyvinyl Alcohol-Montmorillonite Aerogel |
| HPMA |
Highly Porous Montmorillonite Aerogel |
| CVD |
Chemical Vapor Deposition |
| ESA |
European Space Agency |
| NASA |
National Aeronautics and Space Administration |
| ATM |
Aero-Thermo-Mechanics |
| ULB |
Université Libre de Bruxelles |
| CREST |
Centre for Research and Engineering in Space Technologies |
| DSA30 |
Drop Shape Analyzer 30 |
References
- Shackelford, A.; Donaldson Hanna, K.L.; Horton, M.; Noce, D. Morphological and Spectral Characterization of Lunar Regolith Breakdown due to Water Ice. The Planetary Science Journal 2024, 5(1), 1. [CrossRef]
- Pitcher, C.; Kömle, N.; Leibniz, O.; Morales-Calderon, O.; Gao, Y.; Richter, L. Investigation of the properties of icy lunar polar regolith simulants. Advances in Space Research 2016, 57(5), 1197-1208. [CrossRef]
- Anton, A.; Elliott, J.; Schetsche, M. Meeting Extraterrestrials: Scenarios of First Contact from the Perspective of Exosociology. Acta Astronautica 2024.
- Clark, R. N. Detection of Adsorbed Water and Hydroxyl on the Moon. Science 2009, 326, 562-564.
- Levrard, B.; Forget, F.; Montmessin, F.; Laskar, J. Recent ice-rich deposits formed at high latitudes on Mars by sublimation of unstable equatorial ice during low obliquity. Nature 2004, 431(7012), 1072-1075. [CrossRef]
- Arvidson, R.E. Aqueous history of Mars as inferred from landed mission measurements of rocks, soils, and water ice. Journal of Geophysical Research: Planets 2016.
- Paige, D.A.; Siegler, M.A.; Zhang, J.A.; Hayne, P.O.; Foote, E.J.; Bennett, K.A.; Vasavada, A.R.; Greenhagen, B.T.; Schofield, J.T., et al. Diviner lunar radiometer observations of cold traps in the Moon’s south polar region. Science 2010, 330(6003), 479–482.
- Li, S.; Milliken, R. E. Direct Evidence of Surface Exposed Water Ice in the Lunar Polar Regions. PNAS 2017, 114, 201701147.
- Honniball, C.I.; Lucey, P.G.; Li, S., et al. Molecular water detected on the sunlit Moon by SOFIA. Nature Astronomy 2021, 5, 121–127.
- Liu, Y.; Guan, Y.; Zhang, Y.; Rossman, G.R.; Eiler, J.M. Direct Measurement of Hydroxyl in the Lunar Regolith and the Origin of Lunar Surface Water. Nature Geoscience 2012, 5, 779–782.
- Peslier, A. H.; Hauri, E. H.; Saal, A. E. Water in the Moon’s Interior: Truth and Consequences. Earth and Planetary Science Letters 2010, 292, 181-189.
- Ricardo, D.; Hodgkinson, J.; Rhamdhani, M.A.; Brooks, G. A review on the preparation techniques and geotechnical behaviour of icy lunar regolith simulants. Advances in Space Research 2023. Received July 25, 2023; Accepted September 15, 2023; Available online September 20, 2023; Version of Record October 20, 2023. [CrossRef]
- Chin, G.; Brylow, S.; Foote, M.; Garvin, J.; Kasper, J.; Keller, J.; Litvak, M.; Mitrofanov, I.; Paige, D.; Raney, K., et al. Lunar reconnaissance orbiter overview: The instrument suite and mission. Space Science Reviews 2007, 129(4), 391–419.
- Gallbrecht, M.M.; Cervone, A.; Vincent-Bonnieu, S. The Moon as an effective propellant source: A comprehensive exergy analysis from extraction to depot. Acta Astronautica 2024, 52, days ago.
- Ethridge, E.; Kaukler, W. Extraction of Water from Polar Lunar Permafrost with Microwaves - Dielectric Property Measurements. Presented at GPSE-6: Physical Sciences for Exploration II. Published Online: June 15, 2012. [CrossRef]
- Hauri, E. H.; Saal, A. E.; Rutherford, M. J.; Van Orman, J. A. Water in the Moon’s Interior: Truth and Consequences. Earth and Planetary Science Letters 2015, 409, 252-264.
- Schlüter, L.; Cowley, A. Review of Techniques for In-Situ Oxygen Extraction on the Moon. Planetary and Space Science 2019, 104753. [CrossRef]
- Colaprete, A.; Schultz, P.; Heldmann, J.; Wooden, D., et al. Detection of water in the LCROSS ejecta plume. Science 2010, 330, 463–468.
- Purrington, C.; Sowers, G.; Dreyer, C. Thermal Mining of Volatiles in Lunar Regolith Simulant. Planetary and Space Science 2022, 222, 105550. [CrossRef]
- Ethridge, E.C. Microwave Heating of Lunar Regolith for Water Extraction. Journal of Aerospace Engineering 2009, 22(1), 53–61.
- Zhang, X. Hydrothermal Processes in Martian Regolith: Experiments and Applications. Journal of Geophysical Research: Planets 2008, 113, E06003.
- Sanders, G.; Larson, W.; Sacksteder, K., et al. NASA in-situ resource utilization (ISRU) project: Development and implementation. AIAA SPACE 2008 Conference & Exposition 2008.
- Kawamoto, H.; Hata, K.; Shibata, T. Vertical Transport of Lunar Regolith and Ice Particles Using Electrodynamic Traveling Wave. Journal of Aerospace Engineering 2021, 34(4). [CrossRef]
- Sowers, G.F. A cislunar transportation system fueled by lunar resources. Space Policy 2016, 37, 103–109.
- Barakhovskaia, E.; Glushchuk, A.; Queeckers, P.; Iorio, C.S. Stabilisation of condensate flow from curvilinear surfaces by means of porous media for space applications. Experimental Thermal and Fluid Science 2021, 121, 110283. Available online: https://www.sciencedirect.com/science/article/pii/S0894177720307858. [CrossRef]
- Berto, A.; Azzolin, M.; Lavieille, P.; Glushchuk, A.; Queeckers, P.; Bortolin, S.; Iorio, C.S.; Miscevic, M.; Del Col, D. Experimental investigation of liquid film thickness and heat transfer during condensation in microgravity. International Journal of Heat and Mass Transfer 2022. [CrossRef]
- Shakeri Bonab, M.; Minetti, C.; Iorio, C.S.; Zhao, D.; Liu, Q.-S.; Ou, J.; Kempers, R.; Amirfazli, A. Experimental investigation of dropwise condensation shedding by shearing airflow in microgravity using different surface coatings. Langmuir 2022. [CrossRef]
- Duta, L.; Popescu, A.; Zgura, I.; Preda, N.; Mihailescu, I. Wettability of nanostructured surfaces. Wetting and Wettability 2015, 8, 207–252.
- Gu, X.; Wen, J.; Tian, J.; Li, C.; Liu, H.; Wang, S. Role of gravity in condensation flow of R1234ze(E) inside horizontal mini/macro-channels. Experimental and Computational Multiphase Flow 2019, 1, 219–229.
- Hu, H.; Tang, G.; Niu, D. Experimental investigation of condensation heat transfer on hybrid wettability finned tube with large amount of non-condensable gas. International Journal of Heat and Mass Transfer 2015, 85, 513–523.
- Shkuratov, Y.G.; Bondarenko, N.V. Regolith layer thickness mapping of the Moon by radar and optical data. Icarus 2001, 149(2), 329–338.
Figure 1.
Scheme of the Experiment
Figure 1.
Scheme of the Experiment
Figure 2.
View of the setup used for the parabolic flight campaign
Figure 2.
View of the setup used for the parabolic flight campaign
Figure 3.
Gravity profile during the parabolic flight with respect to Earth Gravity
Figure 3.
Gravity profile during the parabolic flight with respect to Earth Gravity
Figure 4.
Contact angle (CA) measured on the top of the groove, as well as for the hydrophobic and hydrophilic
Figure 4.
Contact angle (CA) measured on the top of the groove, as well as for the hydrophobic and hydrophilic
Figure 5.
Generalization of the Droplet Volume Quantification Process
Figure 5.
Generalization of the Droplet Volume Quantification Process
Figure 6.
Image of the condenser before and after applying the high-pass filter
Figure 6.
Image of the condenser before and after applying the high-pass filter
Figure 7.
Image of the Condenser before and after Applying the High-Pass Filter and Thresholder by K-means
Figure 7.
Image of the Condenser before and after Applying the High-Pass Filter and Thresholder by K-means
Figure 8.
Sequence of Clustering the Drop Parts Starting From the Original Grooves Surface Image
Figure 8.
Sequence of Clustering the Drop Parts Starting From the Original Grooves Surface Image
Figure 9.
Example of the volume quantification on grooves
Figure 9.
Example of the volume quantification on grooves
Figure 10.
Example of the volume quantification on a hydrophobic surface
Figure 10.
Example of the volume quantification on a hydrophobic surface
Figure 11.
Example of the volume quantification on a hydrophilic surface
Figure 11.
Example of the volume quantification on a hydrophilic surface
Figure 12.
(a)The difference in temperature maintained during the experiment between the condenser plate and heat extraction system of the three days at a set temperature of 10°C. (b) The difference in temperature maintained during the experiment between the condenser plate and heat extraction system of the three days at a set temperature of 15°C.
Figure 12.
(a)The difference in temperature maintained during the experiment between the condenser plate and heat extraction system of the three days at a set temperature of 10°C. (b) The difference in temperature maintained during the experiment between the condenser plate and heat extraction system of the three days at a set temperature of 15°C.
Figure 13.
Water condensed in ml for each parabola experienced for each substrate
Figure 13.
Water condensed in ml for each parabola experienced for each substrate
Table 1.
Grain sizes of the used regolith simulant
Table 1.
Grain sizes of the used regolith simulant
| Layer |
Grain Size |
| Layer 1 |
Grain size 0.125 mm to 0.105 mm |
| Layer 2 |
Grain size 0.250 mm to 0.177 mm |
| Layer 3 |
Grain size 2 mm to 0.500 mm |
Table 2.
Days flight composition
Table 2.
Days flight composition
Table 3.
Volume of water condensed on the substrate after a series of 5 parabolas. Results are in ml of water condensed. The first table refers to condensation with the temperature of the substrate at 10°C, and the second table refers to the temperature of the substrate at 15 °C Volume of water in ml
Table 3.
Volume of water condensed on the substrate after a series of 5 parabolas. Results are in ml of water condensed. The first table refers to condensation with the temperature of the substrate at 10°C, and the second table refers to the temperature of the substrate at 15 °C Volume of water in ml
| 10 °C |
Grooves |
Hydrophobic |
Hydrophilic |
| Martian |
0.18 |
0.97 |
0.45 |
| Lunar |
0.33 |
1.15 |
0.37 |
| Zero-g |
0.39 |
1.36 |
0.32 |
| Ground exp. |
0.13 |
0.31 |
0.14 |
| 15 °C |
Grooves |
Hydrophobic |
Hydrophilic |
| Martian |
0.13 |
0.66 |
0.23 |
| Lunar |
0.29 |
0.83 |
0.38 |
| Zero-g |
0.38 |
1.26 |
0.43 |
| Ground exp. |
0.1 |
0.27 |
0.12 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).