3.1. Modeling Effect on Reactivity under Altitude and Sea Level Conditions
The initial phase of the study focused on model calibration to reproduce observed conversion efficiencies for CO and HC during steady-state tests conducted under warm sea level and cold altitude conditions, as referenced in [
16] and detailed in
Section 2.1. The calibration tests are detailed in
Table A1 for warm sea-level conditions (0 m and 20
C), and in
Table A2 for cold high-altitude conditions (2500 m and -7
C). Both tables are located in
Appendix A.
Due to the absence of
in these tests, calibration was restricted to reactions excluding this species. Specifically, it focused on HC adsorption and desorption (
and
), CO oxidation (
), and HC oxidation (
) reactions, as outlined in
Table 2. The calibration of kinetic constants for these reactions considered the effects of thermal transients on oxidation and the equilibrium of adsorption-desorption, as well as the progressive influence of HC accumulation on the reaction rate of adsorption-desorption. This methodology facilitated a steady-state representation at test completion, distinguishing between HC oxidation and adsorption conversion efficiencies under varying environmental conditions.
In this initial part of the calibration, the objective function, defined in Equation
5 and minimized using the simplex search method by Lagarias
et al. [
41], represented the weighted sum of cumulative errors in conversion efficiency (
) for CO and HC. It considered the modulus of the error up to each time point (
t) for each pollutant species (
p), CO and HC, ensuring balanced weighting for both pollutants.
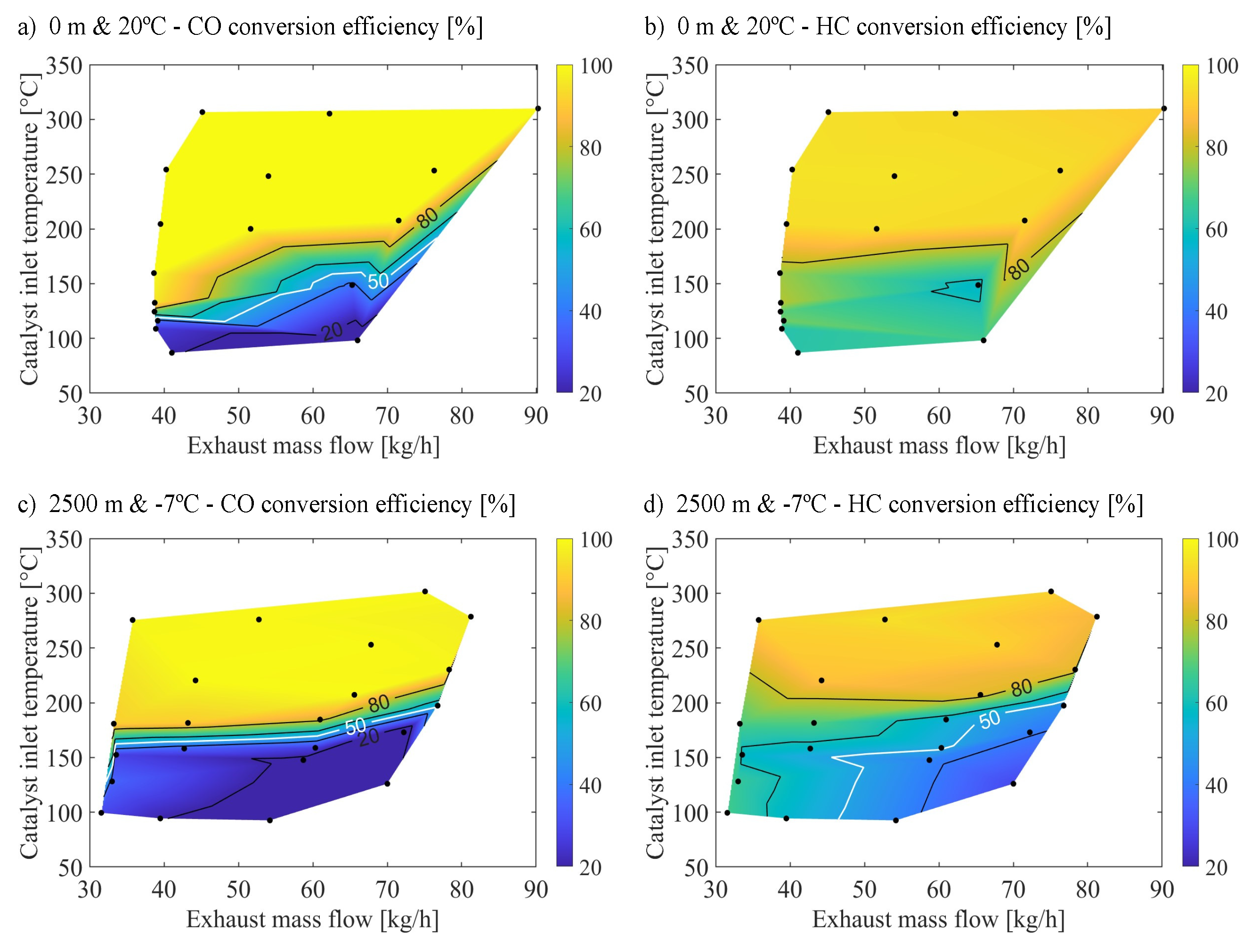
Figure 2 presents the modeling results of the steady-state tests under warm sea-level and cold altitude conditions. The comparison of the model results with experimental data, depicted in
Figure 1, underscores its efficacy in simulating CO and HC conversion efficiencies with notable accuracy.
The agreement between
Figure 2a and
Figure 1a validated the ability of the model to capture CO conversion behavior at warm-sea level. The model successfully predicted the low reactivity below 100
C OC inlet temperature. Additionally, it accurately captured the enhancement in CO conversion efficiency and its sensitivity to changes in exhaust mass flow as temperatures increased. Furthermore, the model accurately identified regions achieving 100% CO conversion.
In the context of CO conversion efficiency under cold altitude conditions, as illustrated in
Figure 2c, the model reflected the changes in conversion efficiencies caused by modifying environmental conditions. Notably, it reproduced the elevation in CO light-off temperature and the significant reduction in CO conversion efficiency at reduced exhaust mass flows, a trend more pronounced under cold altitude conditions than warm sea-level conditions.
The ability of the model to predict HC conversion efficiency under varying environmental conditions was further validated by comparing
Figure 2b,d with their corresponding experimental data of
Figure 1b,d. This analysis demonstrates the capacity of the model to capture the influence of exhaust mass flow and OC inlet temperature on HC conversion. Notably, the model accurately predicted the dominance of zeolite adsorption at lower temperatures [
16]. Additionally, it captured the dependence of conversion efficiency on both temperature and mass flow rate for oxidation reactions. However, the model predicted a slightly weaker dependence on these factors than the experimental data
The calibrated reactions allow CO and HC elimination prediction across diverse conditions, emphasizing the
oxidation pathway for lean combustion scenarios and accounting for HC accumulation. Nonetheless, these reactions cannot account for the influence of
present in exhaust gases under both warm altitude and sea level conditions, as elaborated in
Section 2.1. To address this gap, a targeted calibration of reactions that occur in the presence of
is essential.
Therefore, next phase of calibration focuses on fine-tuning the reactions involving
within the catalyst, specifically targeting reactions
to
as delineated in
Table 2. The goal is to refine the predictive capability of the model regarding the catalytic processes influenced by
. A new objective function for this calibration phase was added to reflect the selectivities (
) of CO and HC to reactions involving
and
pathways. These selectivities were detailed in a preceding investigation by Piqueras
et al. [
31], which examined the reactivity of an OC across different
concentrations. The objective of this calibration step was to ensure that the elimination of CO and HC occurred in the presence of
, following established pathways at each reaction temperature. Selectivity, defined in Equation
7, accounts for the contribution of each reaction (
) to the conversion of individual species, calculated as the proportion of a change in concentration of a species due to a specific reaction to the total change in that concentration, which is the sum of all reaction contributions.
A new error function was formulated based on the selectivities of each reaction, for both the simulation and the reference case, as described in Equation
8.
Equation
9 shows the objective function to calibrate the model in the second part of this study. The second term of this equation refers to the selectivity of CO to the oxidation with
(
) and the oxidation with ·OH (
). It also accounts for the selectivity of HC to the oxidation with
(
) and the oxidation with ·OH (
), without taking into account the adsorption/desorption processes taking place in the zeolite.
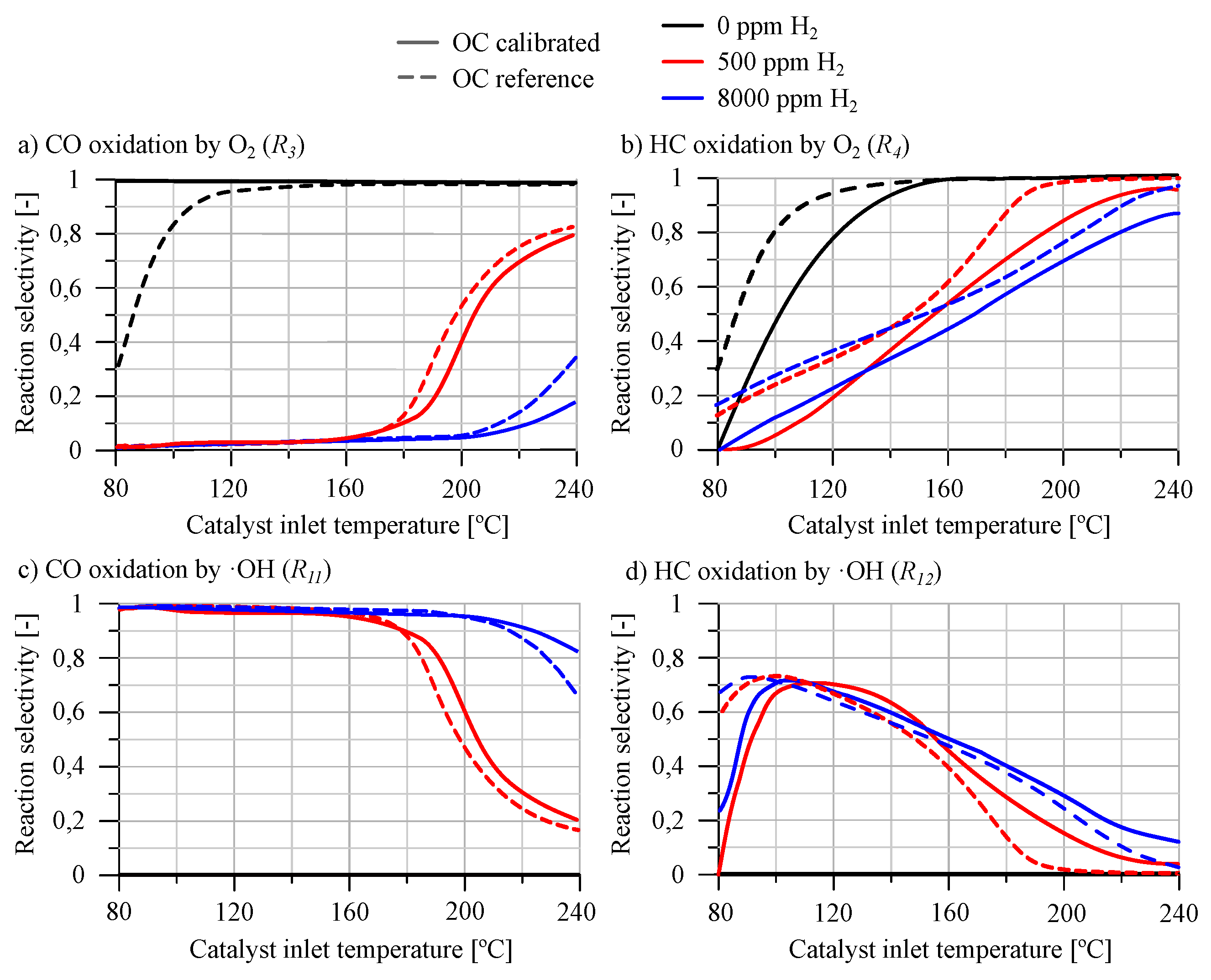
Results of the second part of the calibration are shown in
Figure 3, which compares the selectivities for CO and HC oxidation via
or ·OH with those reported by Piqueras
et al. [
31]. The analysis revealed that the presence of
in the exhaust gases yields a closely aligned response between the selectivity of the simulation and the reference.
For CO reactivity, as depicted in
Figure 3a,c, the model exhibited a high degree of consistency with the reference data in terms of selectivity. The selectivity towards ·OH, shown in
Figure 3c, indicated that at temperatures below 160
C for low
concentrations and below 200
C for high
concentrations, the selectivity was predominantly one, suggesting a preferred pathway. This trend is consistent in both the reference case and the results obtained by the model, with the selectivity being zero in the absence of
, indicating that this pathway does not occur in these conditions. Above these temperature thresholds, introducing
led to a decrease in CO selectivity from ·OH to the
oxidation pathway.
Regarding the selectivity of HC oxidation, as depicted in
Figure 3b,d, it showed the same type of trend as observed with CO upon the introduction of
. However, it did not reach the selectivity levels for the ·OH pathway observed in CO. The influence of
on HC conversion is weaker at low temperatures. While selectivity of ·OH pathway decreases with lower temperature, this decrease is more gradual and less affected by
concentration than for CO. Notably, the presence of adsorption reactions, which are significant under low-temperature conditions, means that the sum of selectivities at low temperatures for oxidation reactions does not total one, indicating that adsorption is an important reaction pathway at low temperature, especially in the absence of
.
As a result of this calibration, the kinetic constants collected in
Table A3 -
Appendix B were obtained. These constants include the activation energies and pre-exponential factors of the reactions involved and the accumulation capacity of HC and ·OH in the catalyst.
Following the calibration of the model to assess environmental changes and the reaction pathways of CO and HC with
and
, these subsections examine the influence of
presence on the CO and HC conversion efficiency in both cold altitude and warm sea level conditions. The analysis involved simulating specific experimental conditions described in
Section 2.1, which were also employed in the first calibration phase of the model. These simulations incorporated varying mole fractions of
, namely 500, 1000, 5000, and 10000 ppm, in the gas mixture at the inlet of the OC.
3.2. CO Reactivity Analysis
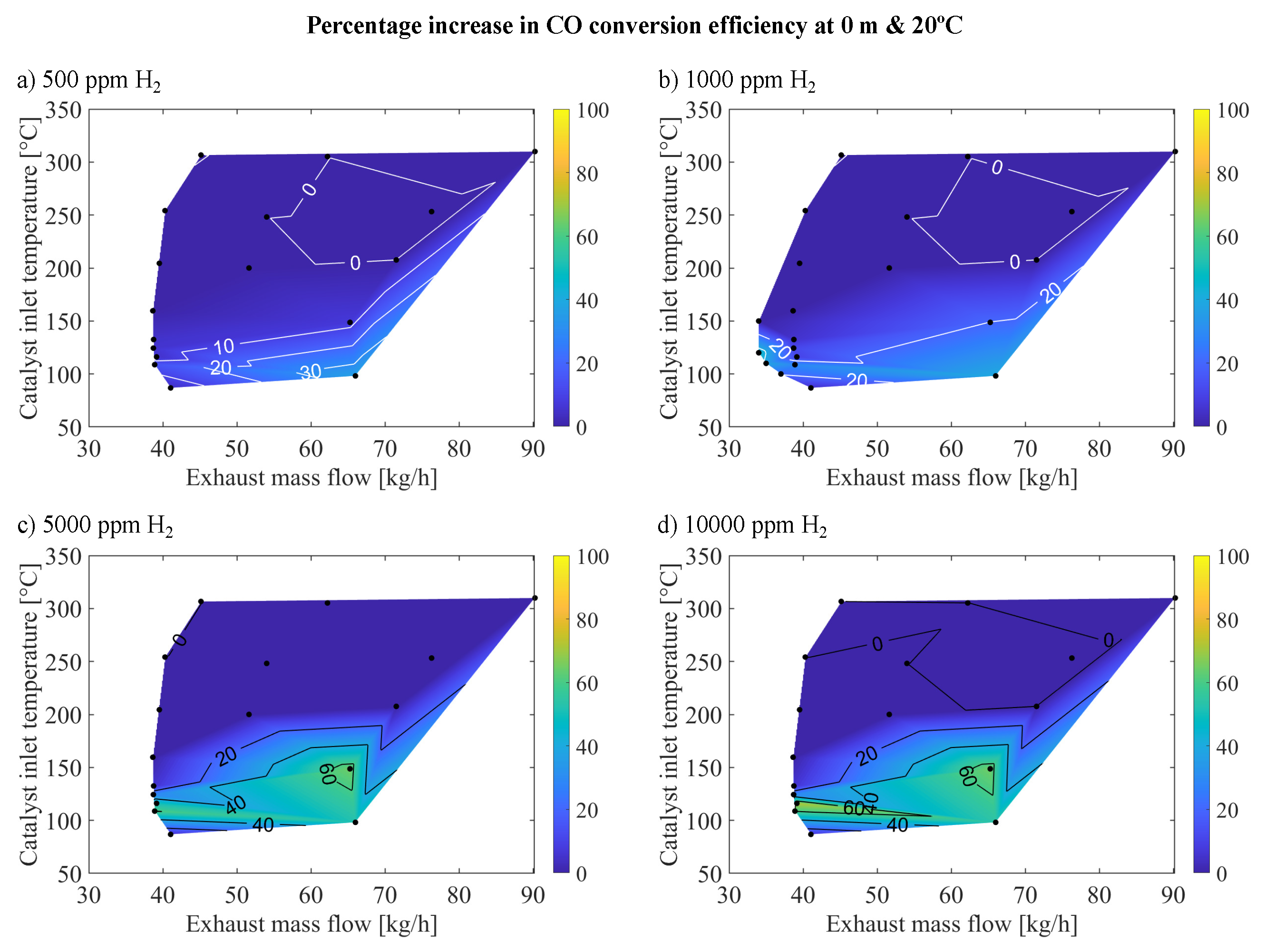
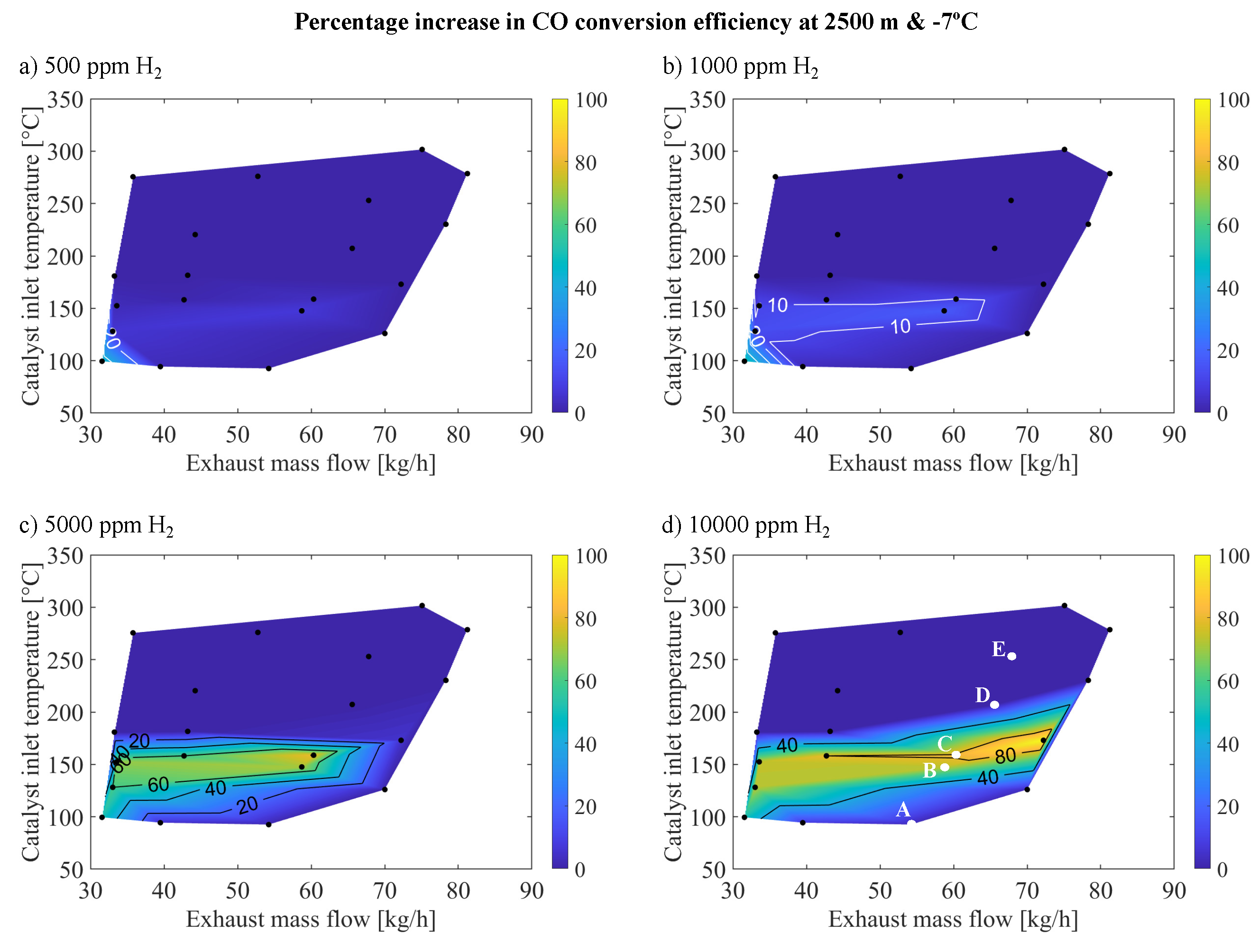
For the CO case,
Figure 4 shows the percentage increase in CO conversion efficiency relative to the baseline results shown in
Figure 2a under warm-sea level conditions, as a function of exhaust mass flow and catalyst inlet temperature. This analysis encompasses the introduction of 500, 1000, 5000, and 10000 ppm of
into the catalyst inlet gas, as detailed in
Section 2.
The findings indicated an enhancement in CO reactivity with increasing molar fractions of
, particularly evident within the low-temperature range under low flow conditions (around 100
C and 35-50 kg/h). This effect extended to higher temperatures as conditions transitioned to higher flow rates (around 150
C with 60-70 kg/h). The improvement in conversion efficiency was most pronounced in the operating points with notably low conversion efficiency in the absence of
. These points were characterized by elevated CO concentrations and temperatures insufficient to facilitate oxidation reactions with
, as shown in
Table A1 in
Appendix A.
The improvement in CO conversion efficiency consistently increased up to the introduction of 5000 ppm of , resulting in a significant improvement of over 60% at approximately 150C and an exhaust mass flow of 65 kg/h. This substantial increase differed from the 20% improvement observed at 1000 ppm of and less than 10% improvement at 500 ppm of , accompanied by an expansion of the enhanced region to higher temperatures. However, the addition of 10000 ppm of did not lead to a discernible improvement in reactivity beyond that achieved with 5000 ppm of , with the enhancement stabilized at 60%. This trend indicated a saturation point beyond which further increases in concentration did not significantly impact reactivity.
The effect of introducing
on improving CO conversion efficiency was also observed under cold altitude conditions.
Figure 5 shows the percentage increase in CO conversion efficiency relative to baseline results shown in
Figure 2c, considering cold altitude scenarios as influenced by exhaust mass flow and catalyst inlet temperature. Similar to the previous scenario, the same molar fractions of
were introduced into the catalyst inlet gas: 500 ppm, 1000 ppm, 5000 ppm, and 10000 ppm. An enhancement band was observed at 150
C in the low-to-mid mass flow range (30-60 kg/h) for the cases of 1000, 5000 and 10000 ppm of
. Introducing 10000 ppm
further extends this band to the highest mass flow rates (70-80 kg/h). This differs from the warm sea-level case, where enhancement occurred at lower temperatures and mass flows (around 100
C and 35-50 kg/h) and at 150
C within the mid-mass flow region with high
molar fractions.
This difference in the increase of CO conversion efficiency profiles was primarily attributed to high CO raw concentration in cold altitude conditions (see
Table A2 in
Appendix A). This limited the effectiveness of
in enhancing CO reactivity. At lower molar fractions of
, such as 500 ppm, the enhancement in conversion efficiency was minimal and limited to regions with low exhaust mass flow and temperature, where the concentration of CO and inhibition was reduced. However, as
molar fraction increased, the enhancement region became more distinct and shifted towards higher exhaust mass flows, highlighting the role of the ·OH reaction pathway and the decrease in inhibition caused by CO consumption. Higher
molar fractions made this effect increasingly apparent. Unlike warm-sea level conditions, high molar fractions of
did not lead to saturation effects in cold altitude conditions in every operating point due to the inherently lower initial reactivity of the catalyst in the absence of
. Therefore, significantly greater reactivity was observed when
molar fraction was increased from 5000 to 10000 ppm, especially in areas of high mass flow, which were highly affected by inhibition.
To elucidate the improvement in CO reactivity observed in the regions depicted in
Figure 4 and
Figure 5 and to understand the variability in the response to
introduction across different operating points. The analysis was initiated by assessing the axial distribution of conversion efficiency throughout the catalyst. This involved identifying the cross-sectional areas of the OC that exhibit the highest reactivity at each operating point, as well as those areas most affected by the introduction of
. For this purpose, three sections along the length of the monolith have been selected. First, the catalyst inlet cross-section corresponds to 4.66 mm from the inlet of the monolith. Then, the middle cross-section was 69.99 mm, and the outlet cross-section was 135.34 mm. The study prioritizes the cold altitude case for a more detailed examination, given its more notable increase in reactivity attributed to higher levels of inhibition.
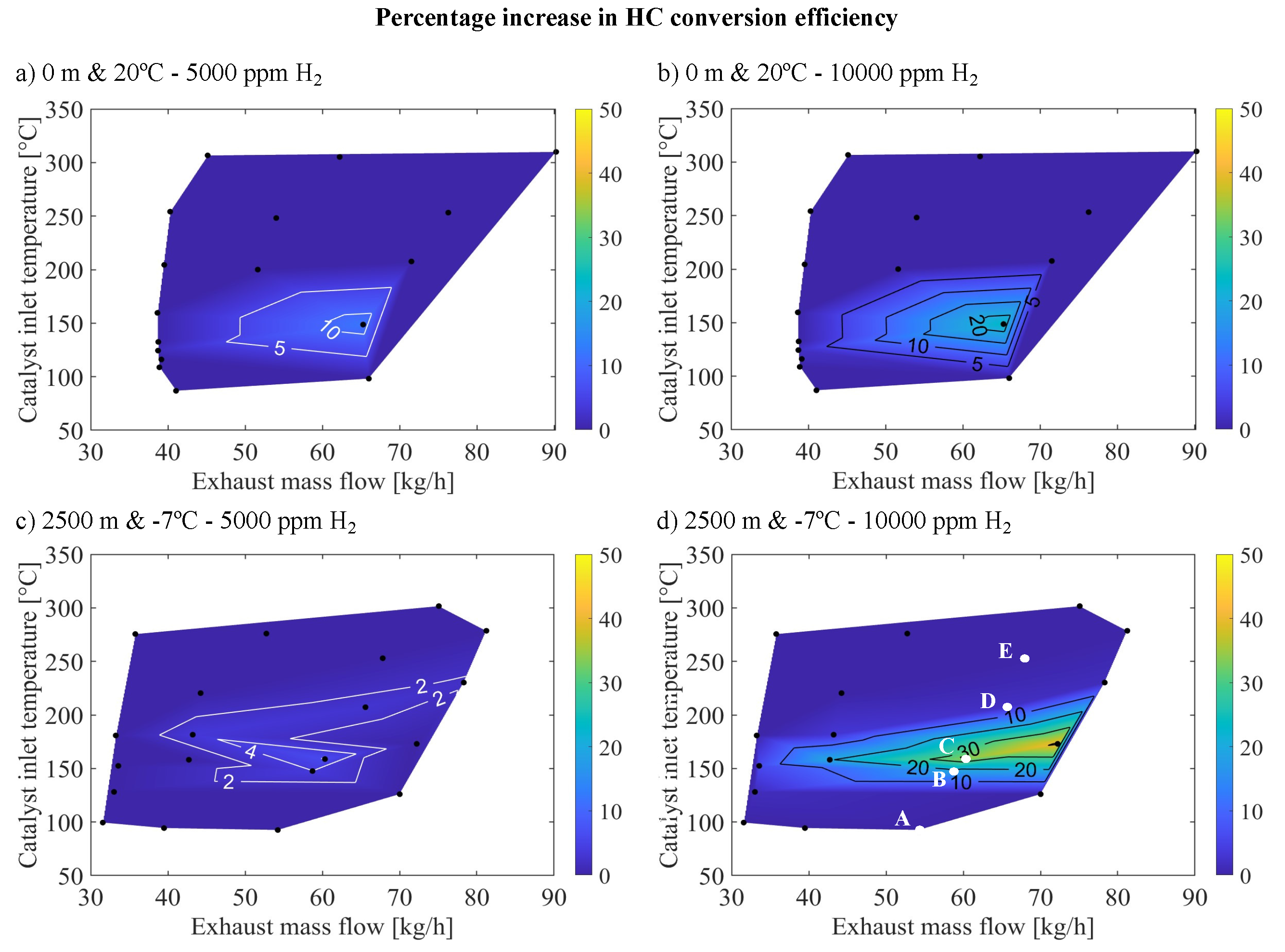
Table 3 presents points A-E from
Figure 5, representing different operating points of the region where a significant increase in reactivity is noted upon introducing
in the inlet gas mixture, arranged in increasing order by inlet gas temperature and exhaust mass flow to the OC.
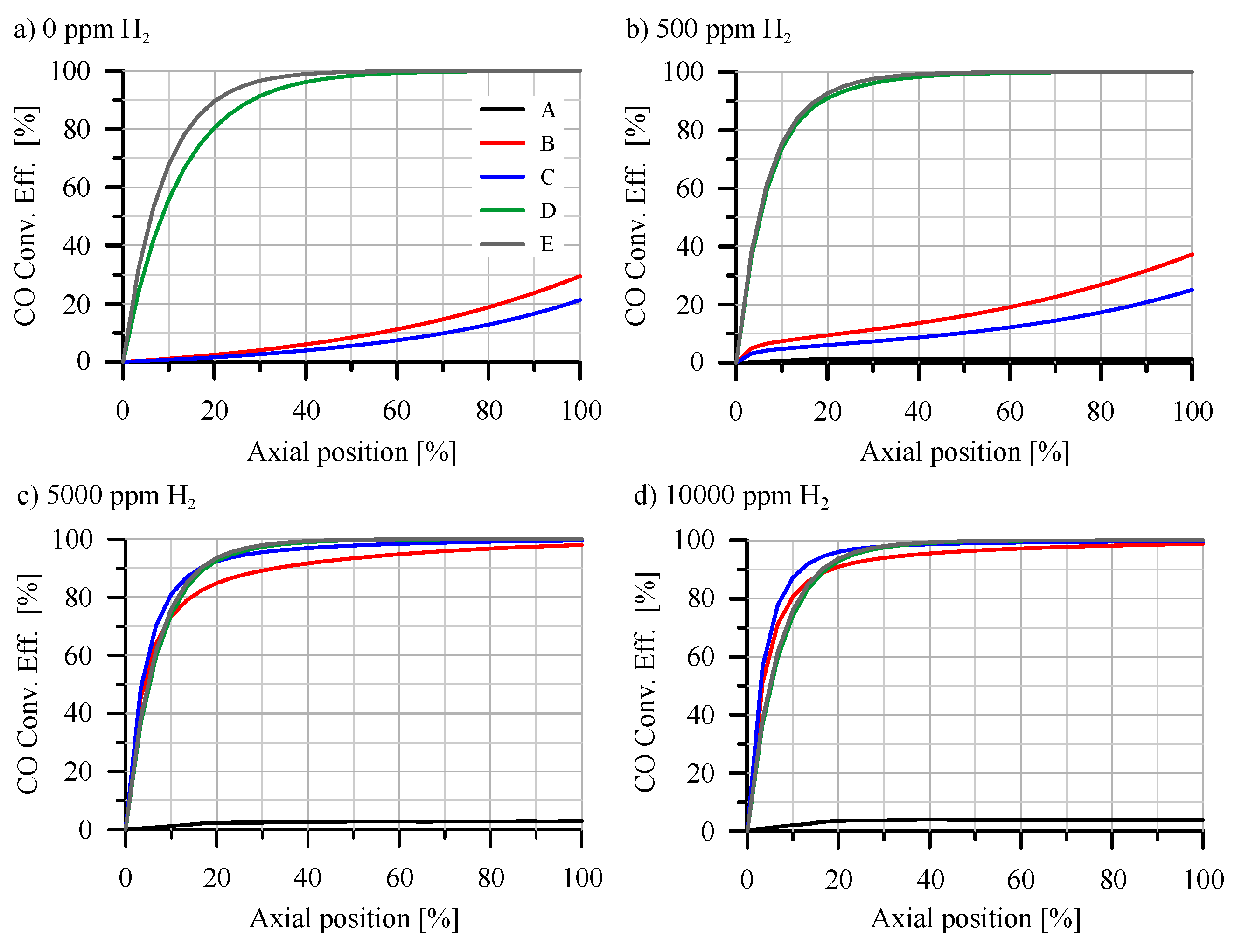
Thus,
Figure 6 presents the axial distribution of CO conversion efficiency along the monolith length for points A-E as outlined in
Table 3. Each subplot within this Figure corresponds to a different
molar fraction in the catalyst inlet gas. The results for 0 ppm of
shown in
Figure 6a, revealed three distinct trends across the five operational points presented. At operating point A, no reactivity was observed across any section of the monolith due to the low inlet gas temperature (92.52
C) and very high CO molar fraction (1194 ppm), resulting in significant inhibition. Points B and C exhibited higher inlet gas temperatures and high CO molar fractions (616 ppm and 461 ppm, respectively) but were lower than point A, indicating OC inhibition. However, the temperature facilitated the reaction of CO with
, resulting in heightened reactivity in the monolith cross-sections. As CO started to react at the inlet region of the monolith, its concentration decreased, thereby reducing inhibition in subsequent regions and enhancing overall reactivity along the monolith. In these scenarios, the entire length of the monolith contributed to the reaction, and an expanded catalyst volume would enhance CO conversion efficiency, contrasting with point A. Finally, points D and E, characterized by high inlet gas temperatures and low CO concentrations (with low inhibition), demonstrated conversion efficiencies near 100%, with most reactivity occurring at the inlet cross-section of the monolith.
Upon introducing 500 ppm of
, as shown in
Figure 6b, the conversion efficiency results were largely consistent with the 0 ppm of
scenario, with only minor differences. Notably, at points B and C, there was a marginal increase in conversion efficiency in the initial cross-section of the monolith, which exhibited higher local conversion efficiency than subsequent sections. This indicated an enhanced conversion efficiency in the initial zone, attributed to new reaction pathways facilitated by the presence of
, particularly through the formation of ·OH groups on the surface. At this relatively low
concentration, the generation of these surface groups was limited, resulting in only a modest reactivity increase at the monolith inlet. Points D and E also experienced a boost in reactivity from the
presence at the inlet, with point D showing slightly higher reactivity due to the additional
, which offsets the lower inlet temperature. This resulted in better conversion efficiency for point D compared to point E, due to its lower inlet mass flow and longer residence time. However, since both points already achieved near 100% conversion efficiency, no significant improvement was noted in the conversion efficiency increase map (
Figure 5a). For point A, the presence of
fails to enhance conversion efficiency due to the inlet temperature being too low for reactivity through the new reaction pathway.
The enhancement effects were more pronounced when the
molar fraction was increased to 5000 and 10000 ppm of
, as illustrated in
Figure 6c,d. At points B and C, the increase in reactivity resulting from the high molar fraction of
led to greater availability of ·OH groups at the monolith inlet, consequently increasing the conversion efficiency to near 100% in these inlet regions. For operating points D and E, the enhanced reactivity improved conversion efficiency in the inlet region, but only marginally, as these points already face limitations due to mass transfer rather than reactivity. The superior reactivity of point C, coupled with its lower inlet flow, allows for higher residence time and conversion efficiency at the inlet compared to points D and E. Furthermore, the elevated availability of ·OH groups with this high
concentration boosts the reactivity at the inlet for operating point A. However, this increase in conversion efficiency was modest due to the low temperature, which does not sufficiently reduce CO concentration to overcome inhibition. Consequently, despite this initial increase, the conversion efficiency throughout the rest of the monolith remained negligible due to the persistent inhibition and low gas temperature.
The findings depicted in
Figure 6 demonstrated that
predominantly influences the inlet cross-section of the monolith, with the observed variations in reactivity in subsequent sections resulting from alterations in CO concentration initiated in these inlet cross-sections. To understand why the impact of
presence was primarily at the inlet of the monolith, it is crucial to examine the selectivity of CO reactions with
and ·OH across different operating points and cross-sections of the monolith.
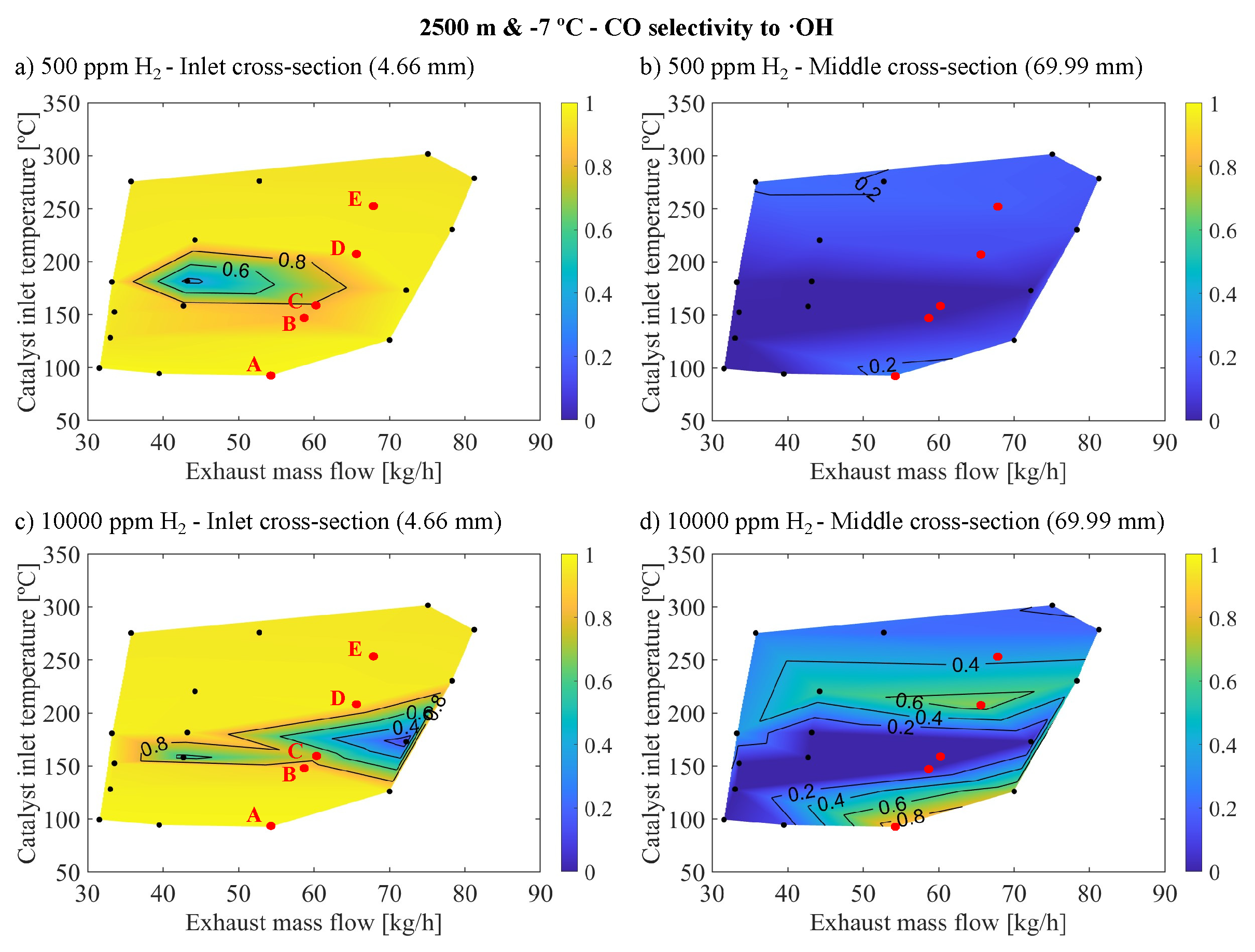
Figure 7 illustrates the selectivity of CO towards ·OH in the context of cold altitude conditions with 500 ppm of
(
Figure 5a,b) and 10000 ppm of
(
Figure 5c,d), for the inlet and medium cross-sections of the monolith.
The reactivity of CO with ·OH varied significantly between the inlet and middle cross-sections. Notably, the selectivity towards CO with ·OH was considerably higher in the inlet cross-section of the monolith for 500 ppm and 10000 ppm of , approaching a value of 1 for most operating points, except in areas showing the most significant increases in conversion efficiency. This selectivity sharply declined along the monolith length, with much lower values in the middle cross-sections, reaching its peak at approximately 0.6 for 500 ppm of and 0.8 for 10000 ppm of introduced into the inlet gas. Interestingly, these peaks in selectivity occurred in regions with minimal increases in conversion efficiency. The areas of high selectivity in the middle cross-section aligned with areas of low temperature where there was no reactivity (point A) and, to a lesser degree, with areas of high temperature where conversion efficiency was already elevated without (points D and E). In both scenarios, reactivity in these areas remained low due to unfavourable conditions that avoid oxidation (point A) or oxidation has already occurred in the inlet cross-section, leaving no residual CO to react (points D and E). This selectivity pattern may seem counterintuitive since the areas with the most pronounced improvements in conversion efficiency, namely points B and C, upon introduction, appeared least affected by the CO with ·OH reaction pathway. Moreover, the selectivity for this reaction was more significant with 500 ppm of than with 10000 ppm since, in the latter case, the improvement is much more substantial, but the selectivity is significantly lower.
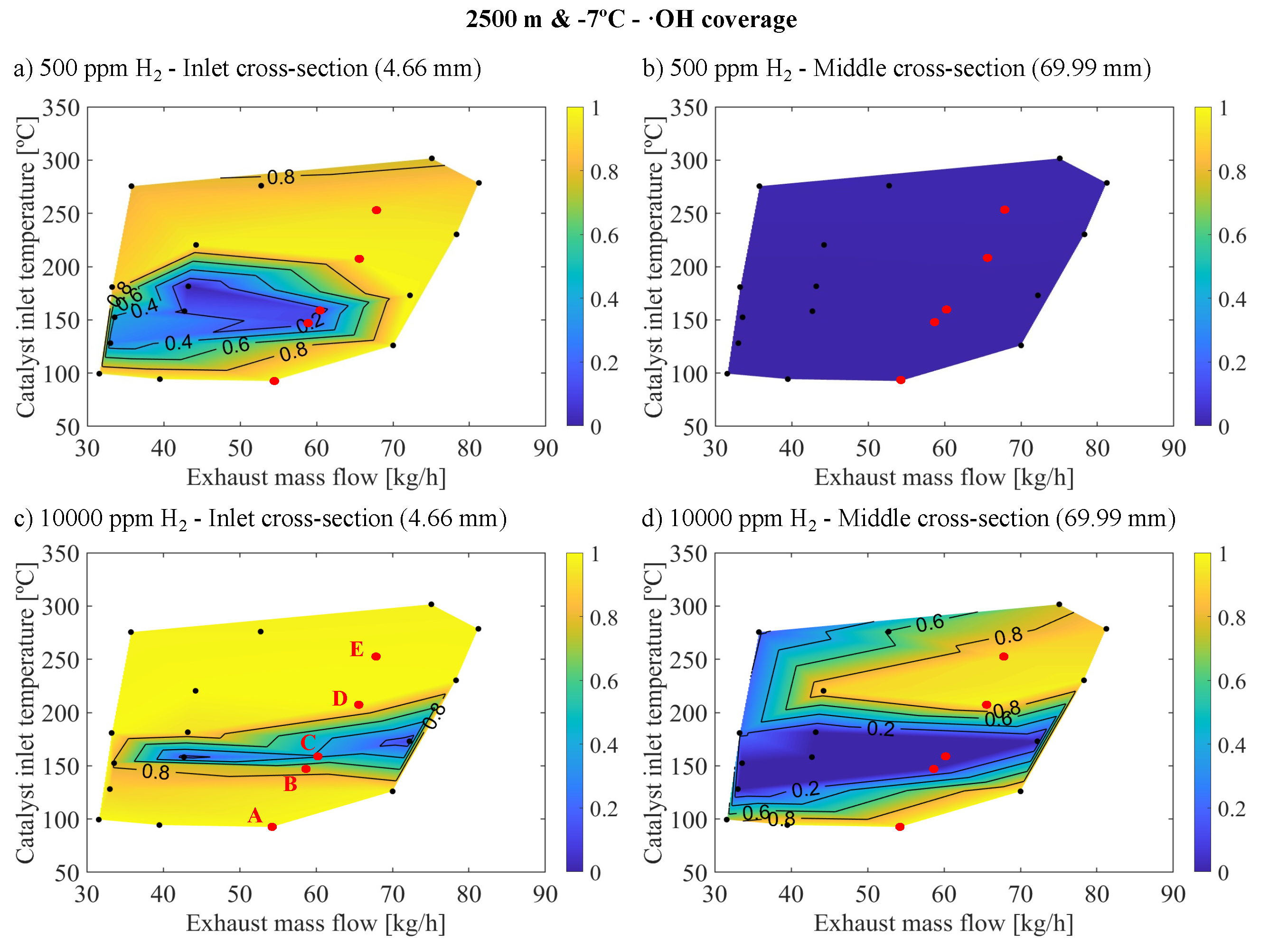
This seemingly contradictory behavior can be elucidated by examining the interplay between oxidation reactions and inhibition dynamics, alongside ·OH coverage, as demonstrated in
Figure 8. This figure illustrates ·OH coverage at both 500 and 10000 ppm of
, focusing on the inlet and middle cross-sections of the monolith under cold altitude conditions. At points D and E, the variation in CO concentration was less pronounced compared to points B and C, attributed to their lower initial CO concentrations. This facilitated the replacement of CO oxidation with
by oxidation with ·OH, enabled by the relative abundance of
, even at 500 ppm, which allowed for significant·OH coverage.
However, at points B and C, which had higher initial CO concentrations, the introduction of 500 ppm of
enhanced CO reactivity via the ·OH pathway, as evidenced by observed increases in conversion efficiency and selectivity. Yet, this
concentration was insufficient to sustain high ·OH coverages, limiting the reaction rate, particularly in the inlet cross-section as shown in
Figure 8a, which is crucial for overall reactivity. With 10000 ppm of
, the ability to generate and maintain ·OH coverage improved significantly in the inlet section (
Figure 8c), leading to a substantial reduction in CO concentration and decreased inhibition, thus facilitating oxidation via the
pathway. This resulted in high activity but low selectivity for the ·OH reaction.
After analyzing all cases, it was found that there is a significant correlation between the coverage of ·OH and selectivity, confirming that in operating points where conversion efficiency improved, most notably under altitude conditions, the reaction rate was constrained by the capability to stabilize ·OH coverage. This elucidates why no saturation in conversion efficiency enhancement was observed with higher molar fractions at altitude, as the increased molar fraction boosted the ·OH coverage in the most positively affected operating points. With 10000 ppm of , selectivity saturation had not been reached indicating a higher selectivity for reactions with but the reaction with ·OH effectively reduced CO enough to decrease inhibition, thereby enabling reactivity with and achieving conversion efficiency saturation.
Moreover, at the highest temperature points, ·OH coverage neared saturation at the inlet (approaching saturation in intermediate regions) due to diminished reactivity from lower CO concentrations at the inlet. These areas did not benefit from further
introduction. Conversely, low-temperature regions also reached ·OH saturation, but the prevailing temperatures were too low for significant reactivity, hence not benefiting from increased
concentration. Therefore, it is evident that certain operating points do not gain from
introduction, whereas those that do require higher
molar fractions for cold altitude conditions compared to warm sea level conditions. This is mainly due to the higher CO concentrations in these conditions necessitating higher
mole fractions to stabilize sufficient ·OH coverage to guarantee the elimination of CO in this way. Notably, ·OH coverages decrease abruptly along the channel in the axial direction, indicating that the presence of
primarily enhances reactivity in the inlet sections. This localized improvement is partially attributable to the reduction in the inhibition of oxidation with
. Furthermore, this reduction in inhibition is the principal factor behind the reactivity enhancement in downstream regions, as evidenced by the low selectivity of the ·OH reaction pathway (
Figure 7b,d), which is a consequence of the limited ·OH coverage (
Figure 8b,d).
3.3. HC Reactivity Analysis
In the analysis of the impact of
on HC reactivity,
Figure 9 shows an improvement in HC conversion efficiency under warm sea-level and cold altitude scenarios. This figure illustrates the percentage increase in HC conversion efficiency based on the mass flow rate of exhaust gas and the inlet temperature to the OC, particularly at high molar fractions of
(5000 and 10000 ppm) in the exhaust gas for warm-sea level and cold altitude cases. It is observed that the most significant enhancements in HC conversion efficiency induced by
corresponded with the conditions that are also most affected by
in terms of CO conversion efficiency, specifically at temperatures around 140
C - 150
C and an exhaust mass flow of 60 to 65 kg/h. However, in the case of HC, this improvement was less significant, as it aligned with zones where adsorption by reaction
from
Table 2 was dominant in the absence of
. In this area, oxidation with
by reaction
was not yet significant, but already showed moderate HC conversion efficiencies, as shown in
Figure 2b,d.
Moreover, introducing
gradually increased reactivity under warm sea-level environmental conditions as the concentration of
rises. This was manifested by the fact that introducing 5000 ppm
(
Figure 9a) led to maximum improvements of around 10% while introducing 10000 ppm of
resulted in a maximum improvement exceeding 20%. On the other hand, in cold altitude conditions, a significant enhancement in reactivity only occurred with the introduction of 10000 ppm of
, as shown in
Figure 9d, with conversion efficiency improvements exceeding 30% at point B, whereas introducing 5000 ppm (
Figure 9c) yields only minimal changes (maximum improvement of around 5%).
This phenomenon of improvement and the varying behavior between different altitude conditions can be attributed to two complementary factors. Firstly, increased reactivity was facilitated by the reaction pathway between HC and ·OH, following the reaction
detailed in
Table 2. Given the inherently lower reactivity of HC, significant coverage of
was required, requiring higher concentrations of
. Secondly, greater efficiency in the CO conversion reaction reduced inhibition by this species, facilitating the oxidation of HC with
[
16].
In cold altitude conditions, as depicted in
Figure 9c,d, an improvement in reactivity only occurred in the presence of 10000 ppm of
. Under these conditions, ·OH coverage reached significant levels at the catalyst inlet, as illustrated in
Figure 8c, indicating an excess of this intermediary that facilitates the
reaction. Less notably, this phenomenon also occurred at warm-sea level, where HC reactivity increased more gradually because lower amounts of CO at the catalyst inlet required less
to stabilize the necessary ·OH groups.
Finally, the fact that the improvement in the efficiency of the HC reaction was more moderate compared to that of CO is because not only an increase in reactivity but also a change in the adsorption pathway is taking place (
) to that of oxidation, both with ·OH (
) and with
(
) (the latter due to the reduction in inhibition). This replacement of one reaction pathway by another was reflected in the global selectivity (integrated across all conditions) of the
reaction, depicted in
Figure 10 for warm sea-level conditions with 0 ppm of
(plot a) and with 10000 ppm of
(plot b), and for cold altitude conditions with 0 ppm of
(plot c) and with 10000 ppm of
(plot d).
The analysis of
Figure 10 showed a decrease in the selectivity for adsorption in HC upon the introduction of
, with this reaction accounting for 100% of the reactivity when
is not introduced in regions where improvements occur upon its introduction, and also in colder regions. However, this reaction becomes irrelevant for both environmental conditions once the light-off temperature for the
reaction is reached. When 10000 ppm of
was introduced, the selectivity for the
reaction significantly decreased in the area with temperatures around 140 - 150
C and exhaust mass flow of 60 to 65 kg/h, falling from 100% with 0 ppm of
in both environmental conditions to 2% with 10000 ppm of
for the warm sea-level case and to 39% for the cold altitude case. This replacement of one reaction pathway by another is the most significant phenomenon related to the effect of
on the reactivity of HC in these conditions, not implying a particularly relevant change in the overall conversion efficiency of the HC into the OC.