1. Introduction
Human hair constitutes the major appendage of the skin and is thought to play important roles in appearance, thermoregulation, sensory input and barrier protection [
1]. Throughout life, hair follicle undergoes constant cycles of regeneration producing hair shafts that can sometimes reach the length of several meters. The main components of hair are keratins, members of the intermediate filament family of proteins that are distinguished by a relatively high cysteine content. The cysteine residues form intra- and inter-molecular disulfide bonds as well as polysulfide bonds that collectively contribute to the mechanical strength and antioxidant capacity of hair fibre [
2,
3]. There are seventeen subtypes of keratins identified, which can be distinguished based on the isoelectric points, molecular weight and specific localization within the compartments of the hair shaft. In addition to the disulfide bonds, a significant number of hydrogen bonds contribute to the cross-linking of the keratins and the structure of hair fibres [
2,
3,
4].
It is thought that the shape of the hair fibre can be related to the geometric and molecular effects of keratins, particularly even distribution recorded in straight hair and asymmetric differentiation of the precortex giving rise to the curly shape of the hair shaft [
5]. The elasticity and strength of textured hair have been closely associated with the presence of disulfide bonds, as cross-linking structures supporting fibre shape, they are also major subjects of hair treatments requiring transient breaking and reformation. Other major factors that are thought to be linked to the phenotypic differences characterising textured hair include the formation of the structural unit through aggregation and folding of keratins and water-soluble keratin-associated proteins (KAPs) that contribute to the main hair compartments, the cuticle, cortex and medulla. The organisation of the fibres within the cortex is closely aligned with hair shaft shape and shows significant variability between straight and curly hair, with bilateral symmetry and cortical cell length playing a central role in fibre curvature. Other important components of the cuticle and the cortex are lipids, primarily 18-methyl eicosanoid acid (18-MEA) covalently bound to keratins by thioester bonds, which provide protection against mechanical and chemical stress and may vary in content among ethnic hair types [
6].
Relatively low content of fibrous proteins in African hair could be a contributing factor to the susceptibility to breakage [
7]. It has been demonstrated that textured hair might be more sensitive to environmental damage such as ultraviolet radiation (UVR), which affects particularly cuticle and hair surface through a decrease in lipid content and decreased tensile strength [
8]. UV exposure can also aggravate the bleaching process through oxidization and loss of the photoprotective function of melanin in the cortex [
7]. The variability of textured hair occurrence can be linked to skin ethnicity, for example, ranging from ~40% for wavy to ~12% for curly type in European and Asian populations and ~95% for curly type in African populations [
6]. A significant proportion of the worldwide population with textured hair also resides in geographical areas with high UV index, which can reach values of ~10 in West Africa, ~9 in South Asia and 15~ in South Africa [
9,
10,
11]. Curly hair can be also freezy and dry, whilst coily or kinky hair type, particularly of African origin, can be also susceptible to brittleness and breakage due to low tensile strength [
12,
13].
Hair styling practices such as chemical relaxing, mechanical or thermal factors carry particular risks [
14] The correct choice of hair-protective personalized products, with the increasing focus on textured hair, is an emerging trend in the current global research and industry market [
15,
16]. Conditioning products are formulated with a range of ingredients that can fulfil the requirements of different hair types, based on natural or synthetic polymers, mineral and vegetable oils and hydrolyzed proteins able to restore the charge, structure of the cuticle and moisture of hair fibre. At the same time, next-generation hair care products containing bioactive compounds with predicted or proven activity towards specific requirements of hair types are in high demand [
7,
16]. Formulation of such products requires the application of advanced technologies that can assist with screening and selection of active ingredients, which can be subsequently validated against many physical and biochemical characteristics of hair fibre. Currently, there is increased focus on the methodologies allowing fast and accurate evaluation of hair care products, including scanning electron microscopy, infrared spectroscopy, brightness and protein content [
17,
18].
In this study, we investigated the protective effect of novel conditioner against the damage of the hair caused by UVR. The conditioner has been based on a blend of three active ingredients with significant anti-oxidant and UVR-absorbing capacities; mangiferin, naringin and ferulic acid. Using several analytical approaches, we provide consistent evidence that curly hair shafts are more sensitive to UVR damage than straight hair. In particular, the alterations to textured hair by UVR appeared to be associated mostly with changes to the hair surface, permeability and keratin organization; these damaging effects were moreover significantly reduced by pre-treatment of the hair with conditioner. These data point to the importance of screening and selecting the right ingredients compatible with specific hair types and allowing the formulation of efficient and suitable hair care products.
2. Materials and Methods
2.1. Spectral Absorbance
Mangiferin (Mangifera indica), ferulic acid and naringin (from citrus fruit, Sigma) were dissolved in DMSO at 1% and diluted with deionized water to 0.005%. The hair conditioner was diluted to 0.1% (w/v) with deionized water. Alkaline hair extracts were diluted with deionized water to a concentration of 0.01%. The spectral absorbance of the samples was measured in SpectraMax iD3 microplate reader (Molecular Devices) at intervals of 5 nm.
2.2. Total Antioxidant Capacity
The total antioxidant capacity of the samples was measured according to the manufacturer’s protocol (Abcam, ab65329). Briefly, the Trolox standard was prepared to several dilutions containing 0-2 mM of Trolox/well. The samples and Trolox standards were transferred to the 96 microplate wells and incubated with 100 ml Cu2+ Working Solution for 90 minutes at room temperature in the dark. The colourimetric output was measured in a microplate reader (SpectraMax iD3 Microplate Reader, Molecular Devices) at OD 570 nm. Sample total antioxidant capacity (TAC) was calculated as TAC = (Ts/Sn)*D, where Ts = TAC amount in the sample well calculated from a standard curve, Sn = sample volume added in the sample wells (100 ml), D = sample dilution factor. For statistical analysis, the data comparing variation between the samples were validated using one-way Analysis Of Variance (ANOVA) with post-hoc Tukey HSD test, n=3. The graphs represent Mean ± SEM, with statistically significant outputs ** p < 0.01.
2.3. Hair Samples
Straight and textured hair samples were purchased from an online distributor of human hair as hair tresses). The samples were rinsed thoroughly with deionized water and dried at room temperature before processing for analysis. For the pre-treatment with conditioner, the hair was submerged for 10 minutes, rinsed with deionized water and dried for 3 hours at room temperature before exposure to UVR. For the Rhodamine B treatment, the compound was solubilized in ethanol at the concentration of 10 mg/mL and diluted to 0.005% with deionized water. The hair was submerged in the Rhodamine B solution for 3 minutes, rinsed briefly with deionized water and processed for analysis.
2.4. Extraction of Hair Keratins
Hair samples were powdered using a pestle and mortar. The hair powder was suspended in 1 M NaOH at a concentration of 10 mg/mL. The samples were heated to 95oC for 45 minutes until the hair powder was solubilised. The extracts were neutralized with HCl, centrifuged briefly at 12,000 rpm and diluted to 0.01% with deionized water for analysis.
2.5. UV Irradiation
For UVR treatment, the hair samples were exposed to a germicidal lamp (Philips TUV G30T8 30 W bulb) providing predominantly 254-nm light, for 15 and 30 minutes. UV dose (mJ/cm2) was calculated from lamp specification 125 mW/cm2 at a distance 1m x exposure time in seconds, 125 mW/cm2 x 900 sec. at 0.75 m distance= 150 mJ/cm2 and 125 mW/cm2 x 1800 sec. at 0.75 m distance= 300 mJ/cm2. All experiments were performed at room temperature.
2.6. Biometric Analysis of Hair Fibres
The light and fluorescent images were captured using an Olympus CK40 microscope and photographed with an Olympus U-PMTVC Camera and Infinity Capture v 6.5.7 software (Lumenera) with a lens magnification of 10x. Biometric data were collected manually based on the images using ImageJ software (ImageJ 1.36b, National Institute of Health, USA). From the light images, the measurements of hair thickness were performed on three consecutive images by tracing alongside a straight line. The collected measurements of length units were then converted to microns, based on the 1392 pixels in the ImageJ corresponding to 605 microns. For densitometry analysis of hair darkness in light images and Rhodamine uptake in fluorescent images, the intensities of the signals were measured in a defined area on three consecutive images for each treatment using ImageJ software and adjusted to control untreated sample values equal to 1. For statistical analysis, the data were validated using ANOVA with post-hoc Tukey HSD test, n=3. The graphs represent the mean ± standard deviation, with statistically significant outputs ** p < 0.01.
2.7. ATR-FTIR Analysis
Cleaned hair samples were powdered using a pestle and mortar to approximately 1-0.5 mm particles. The solid optically dense powder was transferred to the FTIR spectrometer (PerkinElmer- UATR Two) fitted with Diamond ATR crystal. The spectrum of the sample was recorded in the wavelength range of 4000 cm-1 to 450 cm-1; the software used was Spectrum IR. For the presentation of the data on graphs, the transmittance (%T) values were converted to absorbance (Abs) based on the equation Abs= 2-LOG (%T).
2.8. Scanning Electron Microscopy (SEM)
The hair samples were mounted onto the SEM specimen stubs and coated with gold using a sputter coating machine. SEM was performed using a TESCAN VEGA3 scanning electron microscope at SEM HV: 8 kV. The image data were recorded at SEM magnifications 200x, 1.00 kx and 5.00 kx and assembled in PowerPoint.
3. Results
3.1. The Protective Effects of the Conditioner on the Structure of Straight and Curly Hair
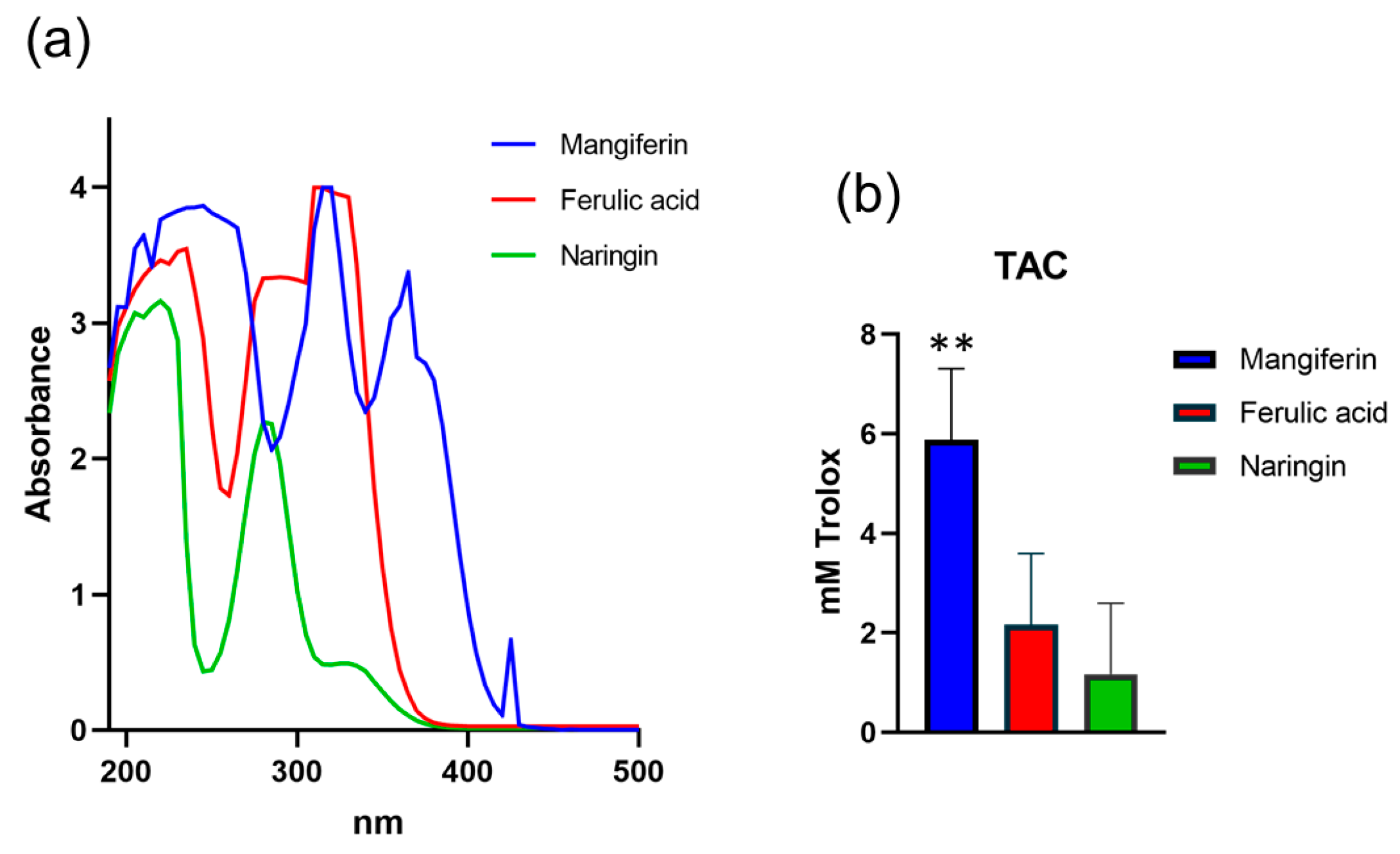
The hair shaft is a complex macromolecular structure that is constantly exposed to various environmental factors causing alterations to the surface and physical characteristics affecting its appearance. One such factor with a major impact on hair fibre is ultraviolet radiation (UVR), pointing to the requirement for applications of novel and effective hair products with protective and restoring properties. To test the beneficial effects of selected bioactive compounds on the hair fibre, we formulated a conditioner containing three major ingredients of natural origin, mangiferin (C19H18O11), ferulic acid (C10H10O4) and naringin (C15H12O5). For the spectral analysis purpose, each compound was solubilized in dimethylsulfoxide (DMSO) and diluted with water to the concentration of 0.005%. When analysed across the 200-500 nm UV-VIS absorbance spectrum, each compound demonstrated a strong UVR absorbance capacity, with noticeable differences in the absorbance peaks. The spectral absorbance of naringin was characterized by the presence of two peaks, at 220 nm and 280 nm; similarly, the absorbance of ferulic acid was also defined by the presence of two peaks, at 235 nm and 290 nm, which corresponds to the UVC-UVB range. In contrast, spectral absorbance for mangiferin was characterized by the presence of three peaks at 245 nm, 320 nm and 365 nm, indicative that mangiferin is also protective against the UVA range (
Figure 1).
It is well documented that the molecular damage caused by UVR is closely accompanied by oxidative stress generated through impaired balance between the generation of free radicals (ROS) and the ability to neutralize them by antioxidant mechanisms [
19]. When analyzed for total antioxidant capacity (TAC), all three compounds showed significant antioxidant potential at 1% concentration. The antioxidant power for mangiferin was 5.88 mM, for ferulic acid 2.17 mM and naringin 1.17 mM of Trolox power, indicative of a strong protective potential of the ingredients against UVR-induced oxidative damage (
Figure 1).
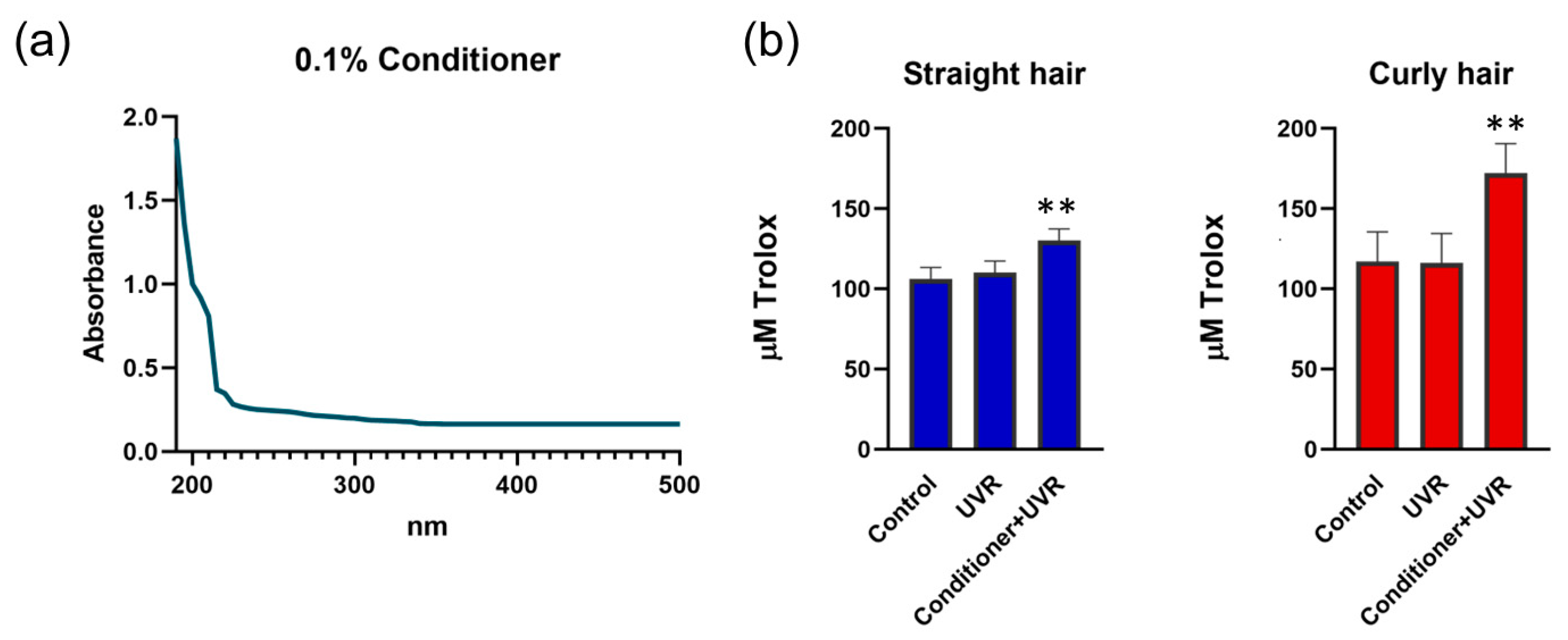
When blended in the conditioner, the absorbance profile of the 0.1% formulation revealed peak values within 190-220 nm and the absorbance was still detectable within the range of 220-350 nm, covering the UVC, UVB and UVA, before reaching a plateau at 350 nm. This indicates a protective effect of the conditioner against UVR and absorption of the harmful wavelengths, particularly in shorter ranges (
Figure 2).
We next sought to determine whether the conditioner would affect the antioxidant power of the hair shaft. We selected two types of dark hair, straight and curly, which was pre-treated with the formulation, rinsed before irradiation with 300 mJ/cm
2 UVR and subjected to alkaline extraction of keratins as described before [
20]. TAC analysis of 0.01% extracts obtained from control and UV-irradiated hair showed similar values of ~110 microM Trolox power. Pre-treatment of hair with the conditioner resulted in a similar increase in TAC to ~130 microM Trolox power in both hair types, however, the TAC was increased further to ~170 microM Trolox power in the extract from curly hair. These results suggested that the conditioner affected slightly the antioxidant capacity of the hair shaft and this effect was more pronounced after exposure of curly hair to UVR (
Figure 2).
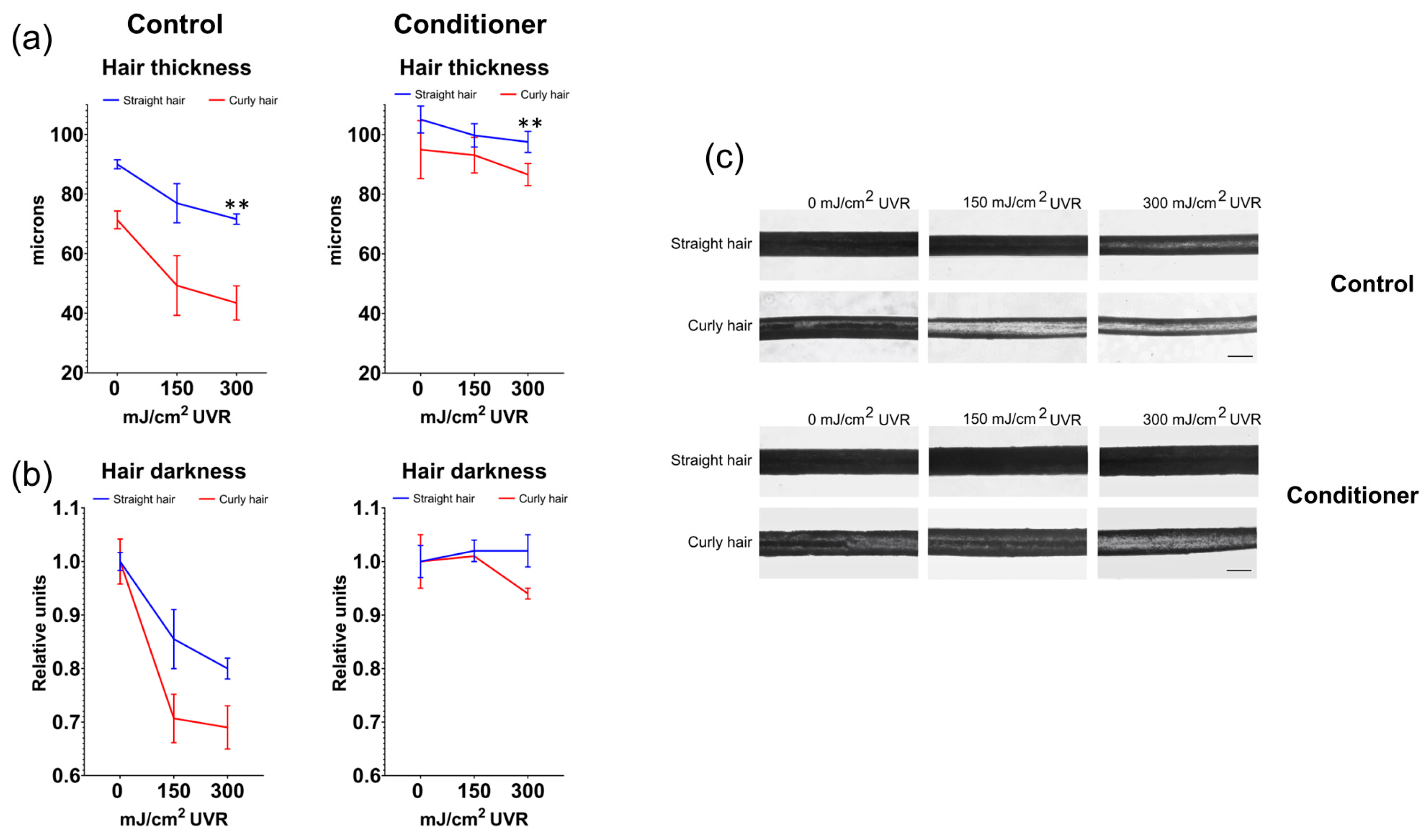
To expand on these observations further, we analyzed the effects of UVR and the conditioner on hair morphology. Initially, we measured the thickness of the hair fibres from light microscopic images. The initial thickness of straight hair was ~90 microns, and curly hair was ~75 microns. Irradiation of the samples with UV with two doses of 150 mJ/cm
2 and 300 mJ/cm
2 resulted in significant thinning of the hair fibre, to ~75 microns for straight hair and to ~45 microns for curly hair. The decrease in hair thickness after a higher UVR dose was also more pronounced for curly hair than straight hair (~ 40% and ~21%, respectively), indicative that curly hair is much more prone to UVR-induced damage. Pre-treatment of the hair with conditioner resulted in increased hair thickness, up to ~110 microns for straight hair and ~95 microns for curly hair. The conditioner also slightly prevented UVR-induced hair thinning by ~10 microns in both hair types (
Figure 3).
One additional effect of UV irradiation recorded in this assay was the bleaching of hair fibre. Quantification of the units corresponding to relative hair darkness from microscopic images revealed a decrease in pigmentation by ~20% for straight hair and ~30% for curly hair, respectively, after UV irradiation (
Figure 2B, left panel). In contrast, pre-treatment with the conditioner had a clear protective effect, with dark hair retaining the same darkness and curly hair reduced in darkness by only ~5% after 300 mJ/cm
2 irradiation (
Figure 3).
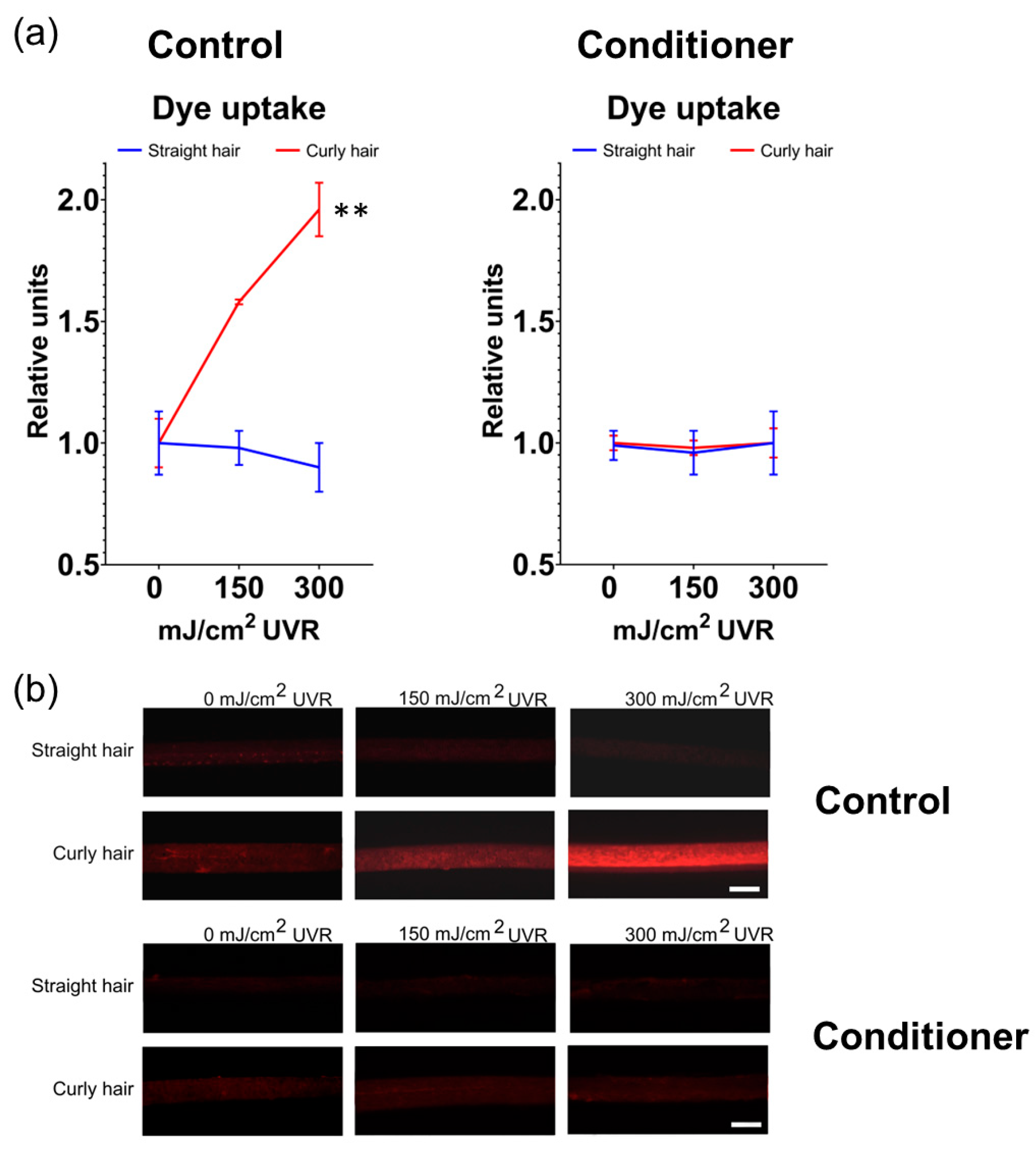
To expand on these observations further, we subsequently assessed the degree of damage by measuring the intensity of the signal from the penetration of fluorescent dye Rhodamine B (C28H31CIN2O3) into the hair fibre. Rhodamine B is widely used as a probe for assessment of the structural integrity of hair fibre, with the dye diffusing more efficiently both the cuticle and cortex of damaged hair [
21]. Irradiation of hair samples with 0, 150 and 300 mJ/cm2 UV followed by 3 minutes incubation with 0.005% aqueous solution of Rhodamine B and image analysis revealed that straight hair was relatively resistant to the penetration of the compound. In contrast, textured hair was significantly more permeable, with the dye uptake and fluorescence intensity increased two-fold after irradiation with 300 mJ/cm
2 UV compared to unirradiated control. Treatment of both hair types with conditioner before UV irradiation had a protective effect, which was evident, particularly for textured hair where the Rhodamine B uptake was reduced to the same levels as straight hair and unirradiated control (
Figure 4).
These data indicated that the conditioner had a beneficial effect on the hair shaft, increasing its thickness, as well as likely protective action against cuticle damage resulting in bleaching and increased permeability. Such an effect was particularly evident for textured hair, which also demonstrated increased sensitivity to UVR damage.
3.2. Straight and Textured Hair Demonstrate Differences in Keratin Organization and the Responses to Conditioner at the Molecular Level
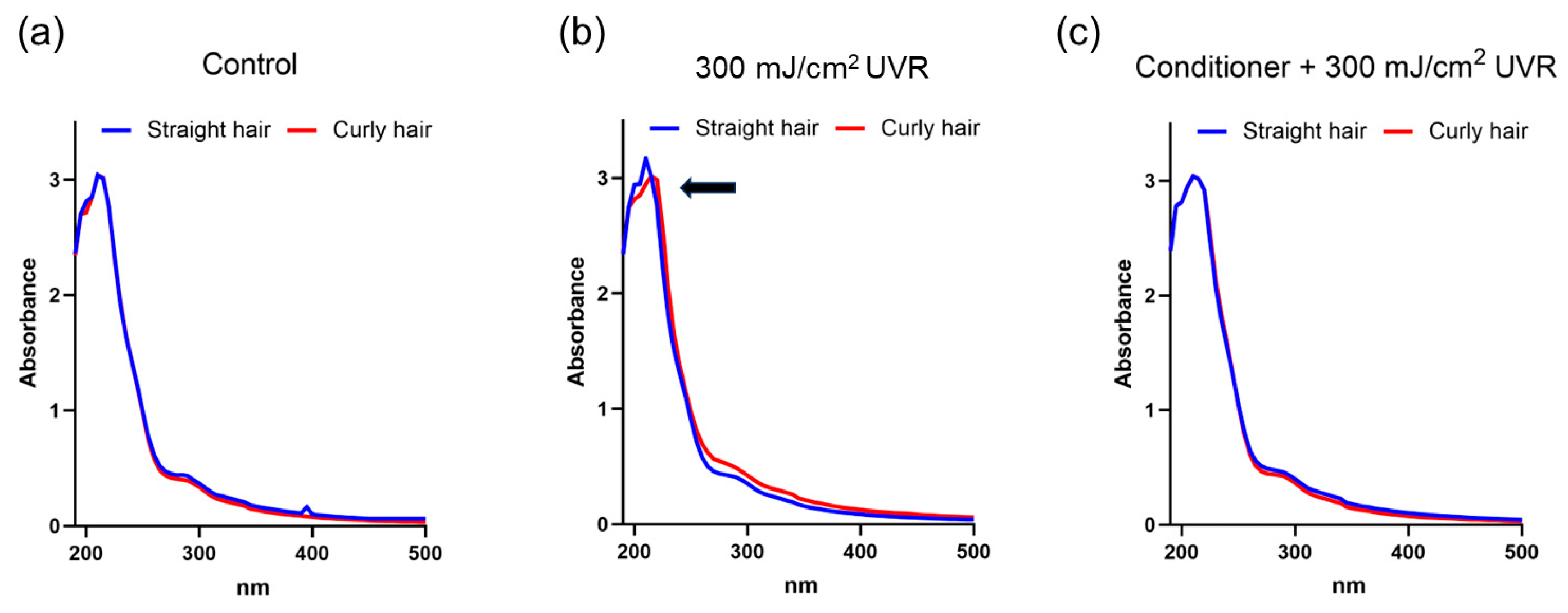
To obtain more insights into the molecular basis of the microscopic changes recorded in the assays, we analysed the spectral absorbance of the keratin extracts obtained from control and UVR-treated straight and curly hair fibres with or without pre-treatment with the conditioner. The hair samples were irradiated with UV at a dose of 300 mJ/cm
2 and subjected to alkaline extraction as described before, which extracts mostly keratins [
20]. Measurements of the spectral absorbance of the 0.01% extracts within the 200-500 nm spectrum revealed similar profiles for both control straight and curly hair, with a peak in absorbance of 210 nm corresponding to hair keratins within the region of 200-250 nm as described before [
22]. Irradiation of hair fibres with UVR resulted in visible differences in spectral absorbance profiles, characterised by a significantly reduced 210 nm peak in textured hair extract, indicative of the presence of structural changes to keratin caused by UVR exposure. Significantly, these changes were absent when the hair was pre-treated with the conditioner before irradiation, with both spectral absorbance profiles for straight and textured hair appearing similar (
Figure 5).
These results suggested that the keratin from textured hair could be more sensitive to UVR-induced damage than from straight hair and the likely protective effect of the formulation, particularly on textured hair.
To expand on this observation further, we performed the Attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy analysis of hair samples. ATR-FTIR spectroscopy allows for obtaining detailed biochemical profiles of the samples analysed based on infrared (IR) light and ATR crystal with high refractive index [
23]. The average sample from both straight and textured hair demonstrated prominent peaks of protein absorbance at 1639-1636 cm
-1 (Amide I, C=O stretch and C=C stretch), 1532-1529 cm
-1 (Amide II, N-O stretch, N-H bend) and 1241 cm
-1 (Amide III, N-H bend, C-N stretch from amino acids) corresponding to keratins across the spectrum of 4000- 450 cm
-1 as described before [
24,
25]. The analysis revealed the differences in absorbance as well as repositioning between the bands of the groups, which represents alterations in the biochemical bonds of mostly keratins and to some extent lipids. Comparison between the straight and textured hair samples indicated a general pattern of decreased absorbance from textured hair within these ranges, pointing to the differences in the functional molecular groups that are responsible for the structural characteristics of the two hair types (
Figure 6).
Irradiation with UV changed the profiles visibly, with the absorbance of straight hair samples decreased within a range of 3000-2000 cm
-1, which corresponds to C-H stretching (alkane groups at 2925-2924 cm
-1 and 2858-2853 cm
-1 representing lipid acyl CH2 groups). In contrast, the absorbance of textured hair samples was increased within a range of 4000-3000 cm
-1, which corresponds to O-H stretching (carboxyl and hydroxyl groups at 3287-3281 cm
-1). The second range increase in absorbance was 1700-1100 cm
-1, which includes Amide I, Amide II, Amide III and C-H bending (methyl groups in lipids and proteins at 1442-1428 cm
-1). There were less pronounced changes in the groups containing Sulphur, including S=O stretch (cysteine monoxide group at 1077-1073 cm
-1), -SO3 stretch (cysteic acid group at 1049-1040 cm
-1) and S-OR esters (detected at 857-853 cm
-1,
Figure 4E). Pre-treatment of both hair types with the conditioner resulted in profiles similar to the un-irradiated controls, with the changes in absorbance of textured hair more pronounced than straight hair. This pattern was particularly evident for the hydroxyl group (3287-3281 cm
-1) as well as Amide I, Amide II and Amide III within the 1639-1241 cm
-1 range, corresponding to keratins. Within Amide I, the structural changes were reflected by a peak of absorbance at 1644 cm
-1, which is represented by the random coil structure (1649-1640 cm
-1), indicating the presence of the most pronounced changes in this secondary structure of keratin. The S-OR ester group within the 857-853 cm
-1 range and cysteic acid group (-SO3 stretch at 1049-1040 cm
-1) were also more affected by UVR and protected by the conditioner in textured hair (
Figure 6).
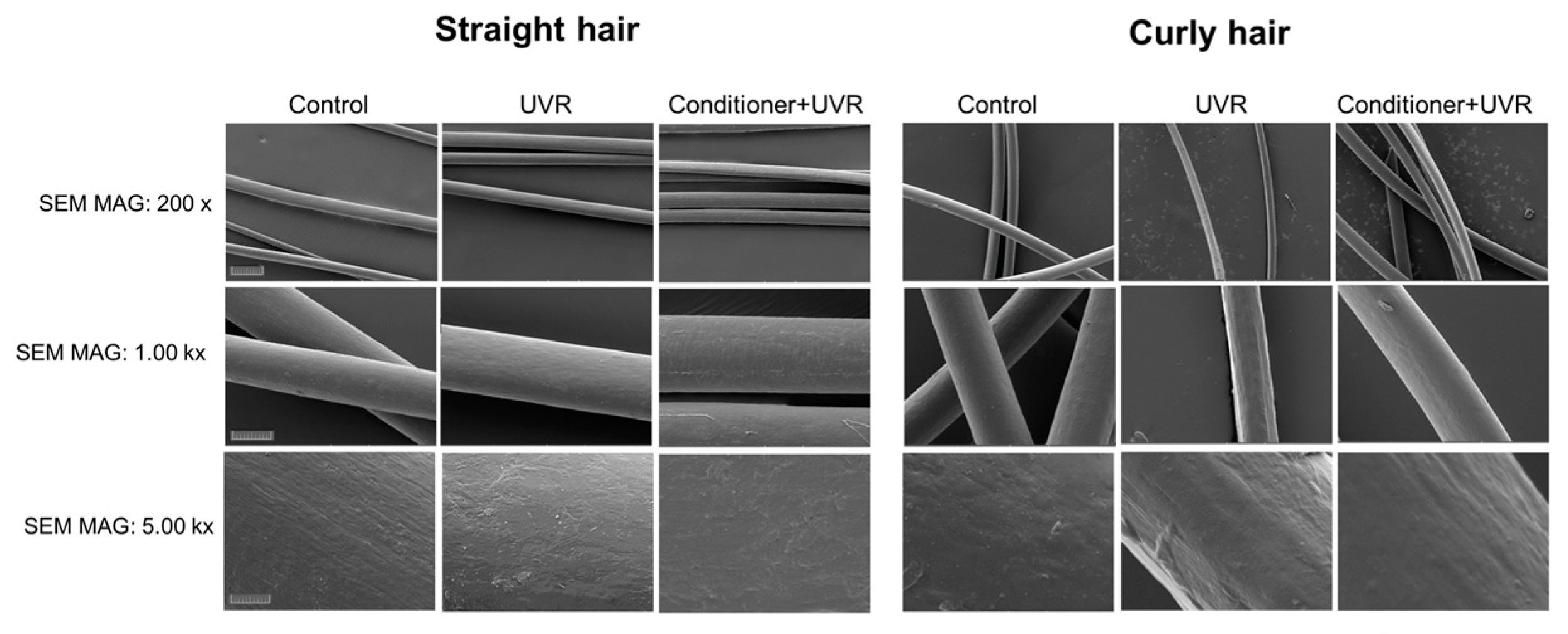
3.3. Evaluation of the Effect of UVR and the Conditioner on Straight and Textured Hair by SEM
The text continues here. To gain more insight into the morphology and ultrastructural appearance of the surface of the hair samples, we performed scanning electron microscopy (SEM) on control, UVR-damaged and condition-treated straight and textured hair. For this purpose, hair samples were irradiated with 300 mJ/cm
2 UVR dose or pre-treated with the conditioner before irradiation. At SEM magnification of 200 x, it was evident that the thickness of straight hair was not affected significantly by UVR. In contrast, there was a pronounced thinning of the structured hair after exposure to the UVR, which was also prevented by pre-treatment by the conditioner (
Figure 7).
At the SEM magnification of 1.00 kx the thickness and the surface of irradiated straight hair appeared unaffected compared to control. Furthermore, pre-treatment of straight hair with the conditioner before UV irradiation led to an increase in hair volume. Similar to straight hair, control textured hair showed a smooth surface, however after UV irradiation there was visible damage to the surface alongside a significant thinning of the hair fibre. Pre-treatment with conditioner UVR exposure resulted in remarkable preservation of hair structure, including smooth surface and thickness. At SEM magnification of 5.00 kx, straight hair demonstrated a smooth surface, with tightly overlapping and intact cuticle scales, that was only slightly affected by UVR. In contrast, whilst control textured hair had also a smooth surface, it was damaged by UVR exposure resulting in uneven distribution of cuticle scales, likely exposure of the cortical cells and visible changes to the cuticle edge. This structural damage could be prevented by pre-treatment with the conditioner, resulting in significant restoration of the smooth hair surface (
Figure 7). These data indicated that consistent with other assays, curly hair was more prone to damage by UVR than straight hair and the conditioner had a significant protective effect.
4. Discussion
In this study, we analysed the effect of UV irradiation on the morphology and molecular structure of straight and curly hair and provided evidence of the protective capacity of the hair conditioner that was created based on natural UVR-absorbing and antioxidant ingredients, mangiferin, ferulic acid and naringin. All ingredients demonstrated strong UV absorbance, mostly within the UVC and UVB range, with mangiferin showing an additional peak at 365 nm, indicative of a strong capacity to also absorb the UVA. The UV absorbance was also recorded in the conditioner formulation tested at 0.1%. All ingredients had a significant total antioxidant capacity (TAC), which was most pronounced for mangiferin. It is well-established that all three compounds are potent antioxidants, able to neutralize and prevent the formation of reactive oxygen species (ROS) and reduce oxidative stress [
26,
27,
28]. The ingredients could therefore have a protective effect on the hair shaft by absorbing UVR, reducing free radicals and breaking in disulfide bonds. Pre-treatment of hair samples with the conditioner before UV irradiation and subsequent analysis of hair extracts revealed increased TAC, which was additionally higher for textured hair compared with straight hair. This indicates that the conditioner would penetrate the hair likely protecting the keratins from oxidative damage.
Hair morphology can be defined by the parameters such as thickness and darkness. In this study, we recorded that curly hair was significantly more sensitive to UVR exposure than straight hair, resulting in more pronounced and progressive thinning and bleaching of the fibre after irradiation with 150 mJ/cm
2 and 300 mJ/cm
2 UV. These parameters were significantly restored by pre-treatment with conditioner before irradiation; resulting in increased thickness and darkness of both hair types compared to UVR only. Hair fibre and its proteins are usually protected by pigment molecules that filter and absorb the UVR, however, such molecules are also degraded by exposure to radiation [
29]. Preservation of hair darkness during UVR exposure indicates the protective effect of the conditioner due to the UV filtering capacity of its ingredients.
The protective effect of the conditioner was additionally confirmed by the Rhodamine B assay. The dye penetrated the curly UV-irradiated hair more efficiently than straight hair, indicative of more pronounced damage to the structural integrity of the fibre that corresponded to the UVR dose. Pre-treatment with conditioner before UVR exposure prevented the diffusion of Rhodamine B, particularly in structured hair, suggestive of a protective effect on the hair cuticle and perhaps the cortex.
The hair shaft is composed of mainly keratins, the intermediate filament proteins with high cysteine content responsible for the mechanical strength and the antioxidant capacity of hair fibre [
2,
3]. Extract of the keratins obtained from curly hair after UV irradiation demonstrated a visibly decreased peak at 210-220 nm in the spectral absorbance analysis compared to straight hair. This suggested more profound changes in the keratins in curly hair caused by UVR, which could also be prevented by pre-treatment with the conditioner. The ATR-FTIR spectroscopy analysis revealed prominent peaks of protein absorbance corresponding to keratins; Amide I, Amide II and Amide III; within Amide I the secondary structure of keratin was most strongly represented by the random coil. All three peaks were reduced in curly control hair compared to straight hair; similar absorbance profiles were obtained for the hair pre-treated with the conditioner before UVR exposure. UV irradiation caused profound alterations in the biochemical bonds of keratins in untreated curly hair, with the increase in Amids I, II and III absorptions likely caused by changes in molecular densities and conformation of keratins linked to the thinning of hair fibre. Another bond strongly affected in curly hair was the O-H stretching characterizing carboxyl and hydroxyl groups. Carboxyl groups in aspartic acid and glutamine contribute to a negative surface charge of the hair. It is recognized that damaged hair accumulates more carboxylate from oxidative cleavage of peptide bonds, the altered hair surface properties can be also associated with changes in hydroxyl groups, which is consistent with the absorbance profiles observed in this study [
30,
31].
Alkane CH2 groups representing fatty acids and lipid acyl molecules were also affected by UV irradiation in this study. It has been reported previously that, despite similar lipid composition, textured hair of African origin contains fewer integral lipids and free fatty acids than straight hair of Asian origin. The integral lipids could moreover contribute to the protection against hair shaft damage by UVR [
32]. The reduced peaks corresponding to alkane groups in curly hair recorded in this study could also represent the reduced lipid content. Interestingly, cosmetic oil-based formulations containing alkanes have applications in hair care [
33]
. Finally, the -SO3 stretch and S-OR ester group were also more affected by UVR in curly hair compared to straight hair. The hair that is damaged due to bleaching was shown to contain higher quantities of sulfonate -SO3 groups with negatively charged surface groups corresponding to cysteic acid. Such alterations were due to the cleavage of thioester bonds and cysteine oxidation [
34]. The increase in peak absorbance represented by these groups in UV- UV-irradiated curly hair in the present study is consistent with increased oxidative damage involving cysteine. The protective effect of the conditioner was evident through the absorbance profiles, which were similar to the un-irradiated controls in both hair types, however, the changes were more pronounced in curly hair indicative of a stronger effect of the conditioner.
These results were subsequently confirmed by SEM analysis, indicating more extensive damage to the fibre surface of UV- UV-irradiated curly hair than straight hair, which was associated with alterations of the cuticle edge and cuticle scales, together with significant loss of fibre volume. The visible effects of the conditioner were mostly observed in increased hair volume in both hair types and a smooth surface of the fibre in curly hair.
Recent studies led to a better understanding of the molecular and biophysical differences between straight and textured hair, which opens up and supports the growing trends in personalized hair care. Curly hair, particularly of African origin (94.9% population), has a smaller diameter at bending points, elliptical cross-section, a thinner cuticle layer, decreased water retention and is more prone to mechanical breakage than straight and wavy hair of European and Asian origin [
16,
35]. Hair curliness is associated with fibre diameter and tensile strength or elasticity, showing an inverse correlation with both factors, which could explain the propensity of very curly hair to break [
35].
Hair fibre is also highly responsive to environmental factors such as chemical exposure or irradiation causing structural and molecular damage, with a significant percentage of individuals with curly hair also residing in geographical areas with high UV index. Current strategies to protect and repair damaged hair include cosmetic treatments that are compatible with hair shaft structure and biophysical properties. Modern hair conditioners can lubricate the cuticle whilst increasing the hydrophobic properties and neutralising the negative charge of the hair fibre. Based on the molecular weight, the active ingredients can penetrate the surface only or the cortex. Hair conditioners are formulated to strengthen the cuticle, decrease frizz, detangle the hair and increase its smoothness and shine [
16]. The hair conditioner and the active ingredients described in this study offer all these benefits, with an additional focus on relevant factors such as ethnicity and hair type. Development of similar new hair products will require an acknowledgement and a deeper understanding of individual variations in hair fibres present in different ethnicities.
Author Contributions
Conceptualization, E.M. and O.C.I.; methodology, E.M and O.C.I; software, O.C.I.; validation, E.M; formal analysis, E.M.; investigation, E.M and O.C.I.; resources, O.C.I.; data curation, E.M.; writing—original draft preparation, E.M.; writing—review and editing, E.M. and O.C.I; visualization, E.M.; supervision, O.C.I.; project administration, O.C.I.; funding acquisition, O.C.I. Authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
Authors wish to acknowledge the Spectroscopy and Elemental Analysis Facilities and Analytical Services (Newcastle University) and for technical support with ATR-FTIR and SEM analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yesudian, P. Human hair - an evolutionary relic? Int J Trichology 2011, 3, 69. [Google Scholar] [CrossRef]
- Yu, J.; Yu, D.; Checkla, D. M, Irwin M. Freedberg, I.M.; Arthur P. Bertolino, A.P. Human hair keratins. J Investig Dermatol, 1993, 101, S56-S59. [CrossRef]
- Hirai, T.; Ikeda-Imafuku, M.; Tasaka, N.; Chuang, V.T.C.; Xian, M.; Ishida, T. ; Akaike. ; T, Ishima, Y. Human hair keratin responds to oxidative stress via reactive sulfur and supersulfides. Adv Redox Res 2024, 10, 100091. [Google Scholar] [CrossRef]
- Langbein, L.; Rogers, M.A.; Praetzel-Wunder, S.; Böckler, D.; Schirmacher, P.; Schweizer, J. Novel type I hair keratins K39 and K40 are the last to be expressed in differentiation of the hair: completion of the human hair keratin catalog. J Invest Dermatol 2007, 127, 1532–1535. [Google Scholar] [CrossRef]
- Thibaut, S.; Barbarat, P.; Leroy, F.; Bernard, B.A. Human hair keratin network and curvature. Int J Dermatol, 2007, 46, Suppl 1:7-10. [CrossRef]
- Cloete, E.; Khumalo, N.P.; Ngoepe, M.N. The what, why and how of curly hair: a review. Proc Math Phys Eng Sci 2019, 475, 20190516. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Medronho, B.; Alves, L.; Rasteiro, M.G. On Hair Care Physicochemistry: From Structure and Degradation to Novel Biobased Conditioning Agents. Polymers (Basel) 2023, 15, 608. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.H.; Park, T.S.; Lee, H.J.; Kim, Y.D.; Pi, L.Q.; Jin, X.H.; Lee, W.S. The ethnic differences of the damage of hair and integral hair lipid after ultra violet radiation. Ann Dermatol 2013, 25, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Egbuim, T.C.; Onyeuwaoma, N.D.; Okere, B.I.; Ezenwugo, M.H.; Chukwudi, A.O.; Uhiene, G.O.; Ugwuozor, N.D.; Shaibu, B.I.; Ugboma, E.A.; Ewim, D.R.E. Erythemal UV radiation across Nigeria: where do we stand? Heliyon 2022, 8, e10158. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.M.; Bencherif, H.; Portafaix, T.; Lamy, K.; Ncongwane, K.; Coetzee, G.J.R.; Wright, C.Y. Comparison of Ground-Based and Satellite-Derived Solar UV Index Levels at Six South African Sites. Int J Environ Res Public Health 2017, 14, 1384. [Google Scholar] [CrossRef]
- Harinarayan, C.V.; Holick, M.F.; Prasad, U.V.; Vani, P.S.; Himabindu, G. Vitamin D status and sun exposure in India. Dermatoendocrinol 2013, 5, 130–141. [Google Scholar] [CrossRef]
- Mayo, T.T.; Callender, V.D. The art of prevention: It's too tight-Loosen up and let your hair down. Int J Womens Dermatol 2021, 7, 174–179. [Google Scholar] [CrossRef]
- Sinclair, R.D. Healthy Hair: What Is it? J Investig Dermatol Symp Proc 2007, 12, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Asbeck, S.; Riley-Prescott, C.; Glaser, E.; Tosti, A. Afro-Ethnic Hairstyling Trends, Risks, and Recommendations. Cosmetics 2022, 9, 17. [Google Scholar] [CrossRef]
- Collins, H.N.; Johnson, P.I.; Calderon, N.M. et al. Differences in personal care product use by race/ethnicity among women in California: implications for chemical exposures. J Expo Sci Environ Epidemiol 2023, 33, 292–300. [CrossRef]
- Gavazzoni Dias, M.F. Hair cosmetics: an overview. Int J Trichology 2015, 7, 2–15. [Google Scholar] [CrossRef]
- Da Gama, R.M.; Baby, A.R.; Velasco, M.V.R. In Vitro Methodologies to Evaluate the Effects of Hair Care Products on Hair Fiber. Cosmetics 2017, 4, 2. [Google Scholar] [CrossRef]
- Pande, C.M.; Yang, B.; Squibb, B.M. Near-infrared spectroscopy: applications in hair research. J Cosmet. Sci 2000, 51, 183–192. [Google Scholar]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S,S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv Exp Med Biol 2017, 996, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.L. Solubility of structurally complicated materials: 3. Hair. Sci World J 2009, 9, 255–271. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Silva, A.L.; Joekes, I. Rhodamine B diffusion in hair as a probe for structural integrity. Colloids Surf B Biointerfaces 2005, 40, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Ge, N.W.; Zhang, Y.; Zhang, H.; & Cheng, L.; Shi, X. (2021). Extraction and Characterization of Keratin and
Keratin Hydrogels from Wasted Rabbit Hair. JPCS 2021, 1790. 012008. [CrossRef]
- Tiernan, H.; Byrne, B.; Kazarian, S.G. ATR-FTIR spectroscopy and spectroscopic imaging for the analysis of biopharmaceuticals. Spectrochim Acta A Mol Biomol Spectrosc 2020, 241, 118636. [Google Scholar] [CrossRef]
- Mujeeb, M.A. and Zafar, M.K.M. FTIR spectroscopic analysis on human hair. IJIRSET 2017, 6, 9327–9332. [Google Scholar]
- Ehteshamuddin, M.A.; Ahmed, J.K; Adeel, A. FTIR spectroscopic analysis of keratinised tissue- the hair. Int. J. Eng. Sci 2017, 6, 105–107. [Google Scholar]
- Imran, M.; Arshad, M.S.; Butt, M.S.; Kwon, J.H.; Arshad, M.U. ; Sultan, M,T. Mangiferin: a natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis, 2017, 16, 84. [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic Acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr 2007, 40, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and Naringenin: Their Mechanisms of Action and the Potential Anticancer Activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef] [PubMed]
- Santos Nogueira, A.C.; Joekes, I. Hair color changes and protein damage caused by ultraviolet radiation. J. Photochem. Photobiol. B Biol 2004, 74, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Guo, X.; Xu, G.; Zou, S.; Wu, Y.; Hu, C.; Chang, K.; Wang, J. Improving the Mechanical Properties of Damaged Hair Using Low-Molecular Weight Hyaluronate. Molecules 2022, 27, 7701. [Google Scholar] [CrossRef] [PubMed]
- Labarre, L.; Squillace, O.; Liu, Y.; Fryer, P.J.; Kaur, P.; Whitaker, S.; Marsh, J.M.; Zhang, ZJ. Hair surface interactions against different chemical functional groups as a function of environment and hair condition. Int J Cosmet Sci 2023, 45, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.H.; Park, T.S.; Lee, H.J.; Kim, Y.D.; Pi, L.Q.; Jin, X.H.; Lee, W.S. The ethnic differences of the damage of hair and integral hair lipid after ultra violet radiation. Ann Dermatol 2013, 25, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Duprat-de-Paule, S.; Guilbot, J.; Roso, A.; Cambos, S.; Pierre, A. Augmented bio-based lipids for cosmetics. OCL 2018, 25, D503. [Google Scholar] [CrossRef]
- Korte, M.; Akari, S.; Kühn, H.; Baghdadli, N.; Möhwald, H.; Luengo, G.S. Distribution and localization of hydrophobic and ionic chemical groups at the surface of bleached human hair fibers. Langmuir 2014, 30, 12124–12129. [Google Scholar] [CrossRef]
- Cloete, E.; Khumalo, N.P.; Ngoepe, M.N. The what, why and how of curly hair: a review. Proc Math Phys Eng Sci 2019, 475, 20190516. [Google Scholar] [CrossRef]
Figure 1.
UV-absorbing and antioxidant capacities of natural ingredients of novel hair conditioner. (a) Spectral absorbance profiles of 0.005% mangiferin, ferulic acid and naringin; (b) Total antioxidant capacities (TAC) of the ingredients at 1% concentration. Error bars are Mean ± SEM, n=3, with statistically significant outputs ** p < 0.01 in one-way ANOVA with post-hoc Tukey HSD test.
Figure 1.
UV-absorbing and antioxidant capacities of natural ingredients of novel hair conditioner. (a) Spectral absorbance profiles of 0.005% mangiferin, ferulic acid and naringin; (b) Total antioxidant capacities (TAC) of the ingredients at 1% concentration. Error bars are Mean ± SEM, n=3, with statistically significant outputs ** p < 0.01 in one-way ANOVA with post-hoc Tukey HSD test.
Figure 2.
UV-protective capacities of the conditioner. (a) Spectral absorbance profile of 0.1% conditioner; (b) TAC of hair keratin extracts prepared from control, UV-irradiated and conditioner-treated hair demonstrating the increased antioxidant effect of the conditioner on curly hair. Error bars are Mean ± SEM, n=3, with statistically significant outputs ** p < 0.01 in one-way ANOVA with post-hoc Tukey HSD test.
Figure 2.
UV-protective capacities of the conditioner. (a) Spectral absorbance profile of 0.1% conditioner; (b) TAC of hair keratin extracts prepared from control, UV-irradiated and conditioner-treated hair demonstrating the increased antioxidant effect of the conditioner on curly hair. Error bars are Mean ± SEM, n=3, with statistically significant outputs ** p < 0.01 in one-way ANOVA with post-hoc Tukey HSD test.
Figure 3.
Protective capacities of the conditioner against structural damage of hair fibre by UVR. (a) A decrease in hair thickness after exposure to increasing doses of UVR, particularly prominent in curly hair, is prevented by pre-treatment of both hair types by the conditioner; (b) Bleaching of the hair caused by UVR is also more pronounced in curly hair, pre-treatment of both hair types with the conditioner prevents the loss of hair darkness due to irradiation. Error bars are Mean ± SD, n=3, with statistically significant outputs ** p < 0.01 in one-way ANOVA with post-hoc Tukey HSD test; (c) Light microscopy images of hair samples (10x lens magnification, scale bar is 100 μm).
Figure 3.
Protective capacities of the conditioner against structural damage of hair fibre by UVR. (a) A decrease in hair thickness after exposure to increasing doses of UVR, particularly prominent in curly hair, is prevented by pre-treatment of both hair types by the conditioner; (b) Bleaching of the hair caused by UVR is also more pronounced in curly hair, pre-treatment of both hair types with the conditioner prevents the loss of hair darkness due to irradiation. Error bars are Mean ± SD, n=3, with statistically significant outputs ** p < 0.01 in one-way ANOVA with post-hoc Tukey HSD test; (c) Light microscopy images of hair samples (10x lens magnification, scale bar is 100 μm).
Figure 4.
Enhanced penetration of harmful molecules to damaged textured hair is prevented by the conditioner. (a) Uptake of Rhodamine B after irradiation of hair with increasing doses of UV is significantly increased in curly hair compared to straight hair. Both hair types are resistant to Rhodamine B after pre-treatment with the conditioner before UV irradiation. Error bars are Mean ± SD, n=3, with statistically significant outputs ** p < 0.01 in one-way ANOVA with post-hoc Tukey HSD test; (b) Fluorescence microscopy images of hair samples (10x lens magnification, scale bar is 100 μm).
Figure 4.
Enhanced penetration of harmful molecules to damaged textured hair is prevented by the conditioner. (a) Uptake of Rhodamine B after irradiation of hair with increasing doses of UV is significantly increased in curly hair compared to straight hair. Both hair types are resistant to Rhodamine B after pre-treatment with the conditioner before UV irradiation. Error bars are Mean ± SD, n=3, with statistically significant outputs ** p < 0.01 in one-way ANOVA with post-hoc Tukey HSD test; (b) Fluorescence microscopy images of hair samples (10x lens magnification, scale bar is 100 μm).
Figure 5.
Spectral absorbance profiles of alkaline extracts from straight and curly hair with a peak of absorbance at 210 nm corresponding to keratins. (a) Control hair; (b) UV-irradiated hair; (c) Hair pre-treated with the conditioner before irradiation.
Figure 5.
Spectral absorbance profiles of alkaline extracts from straight and curly hair with a peak of absorbance at 210 nm corresponding to keratins. (a) Control hair; (b) UV-irradiated hair; (c) Hair pre-treated with the conditioner before irradiation.
Figure 6.
ATR-FTIR spectra of hair samples with defined absorption bands related to specific molecules and chemical bonds. The main absorption bands were detected as hydroxyl groups (3287-3281 cm-1), alkane groups (2925-2924 cm-1), lipid acyl CH2 groups (2858-2853 cm-1), Amide I (1639-1636 cm-1), Amide II (1532-1529 cm-1) and Amide III (1241 cm-1) corresponding to keratins and S-OR esters (857-853 cm-1). Alterations in the molecular arrangement and quantities of the chemical bonds are reflected by differences in absorbance and band displacement. (a) Control hair; (b) UV-irradiated hair; (c) Hair pre-treated with the conditioner before irradiation.
Figure 6.
ATR-FTIR spectra of hair samples with defined absorption bands related to specific molecules and chemical bonds. The main absorption bands were detected as hydroxyl groups (3287-3281 cm-1), alkane groups (2925-2924 cm-1), lipid acyl CH2 groups (2858-2853 cm-1), Amide I (1639-1636 cm-1), Amide II (1532-1529 cm-1) and Amide III (1241 cm-1) corresponding to keratins and S-OR esters (857-853 cm-1). Alterations in the molecular arrangement and quantities of the chemical bonds are reflected by differences in absorbance and band displacement. (a) Control hair; (b) UV-irradiated hair; (c) Hair pre-treated with the conditioner before irradiation.
Figure 7.
Scanning electron microscopy (SEM) images of straight and textured hair after exposure to UV irradiation alone or pre-treatment with the conditioner. The images were recorded at SEM magnification of 200 x, 1.00 kx and 5.00 kx. Scale bars are 200 μm for the upper panel, 50 μm for the middle panel and 10 μm for the lower panel.
Figure 7.
Scanning electron microscopy (SEM) images of straight and textured hair after exposure to UV irradiation alone or pre-treatment with the conditioner. The images were recorded at SEM magnification of 200 x, 1.00 kx and 5.00 kx. Scale bars are 200 μm for the upper panel, 50 μm for the middle panel and 10 μm for the lower panel.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).