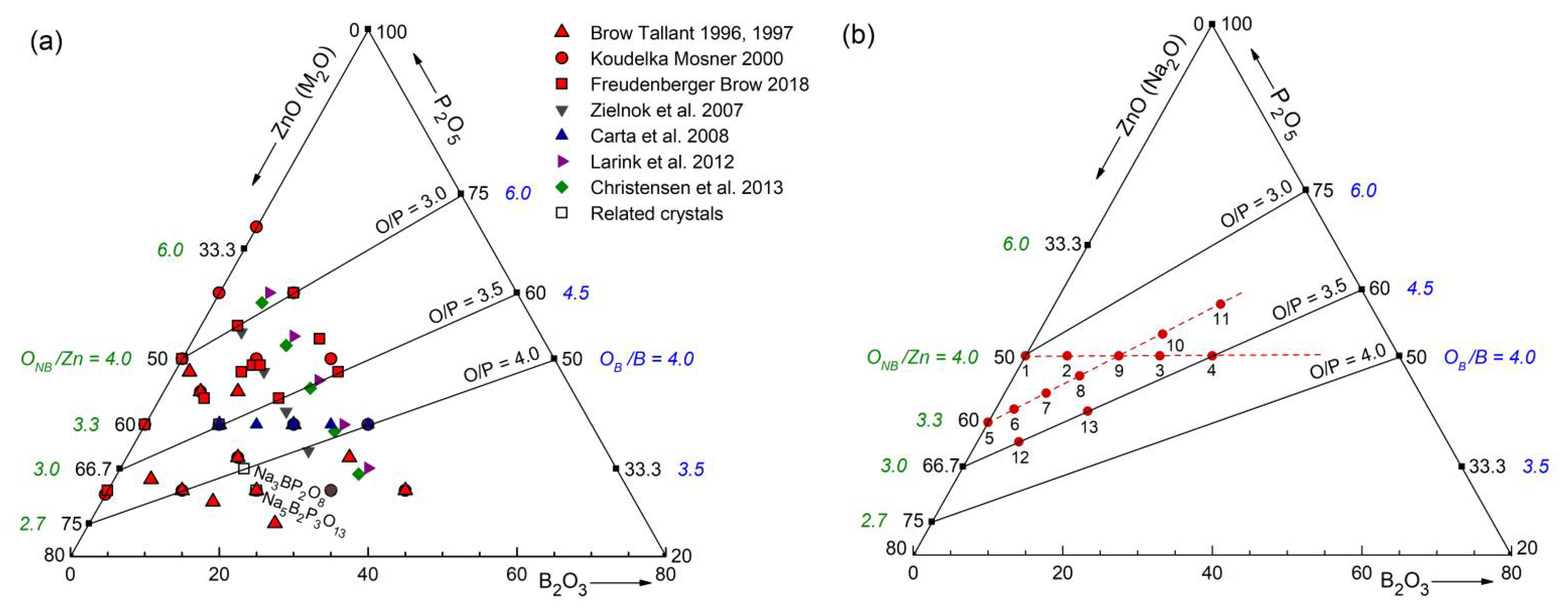
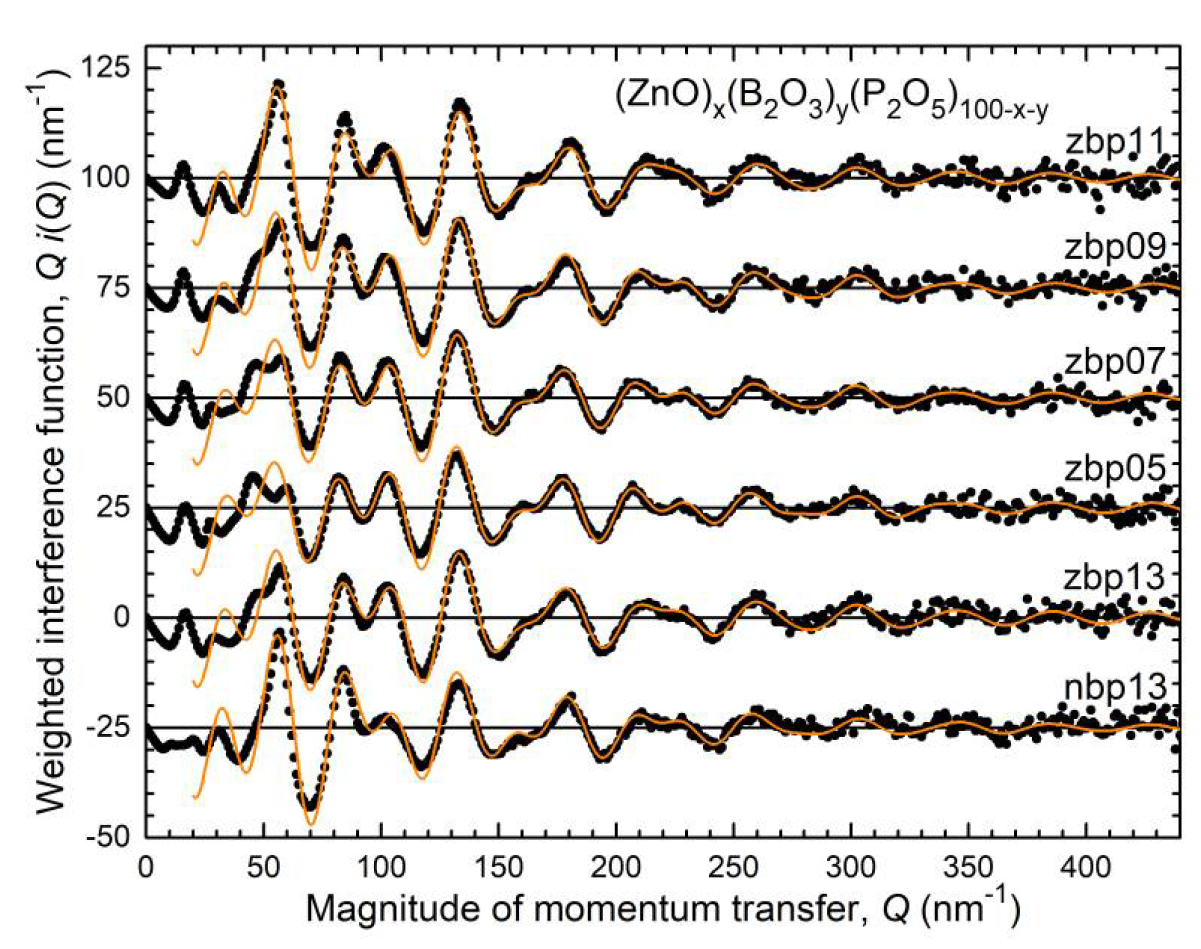
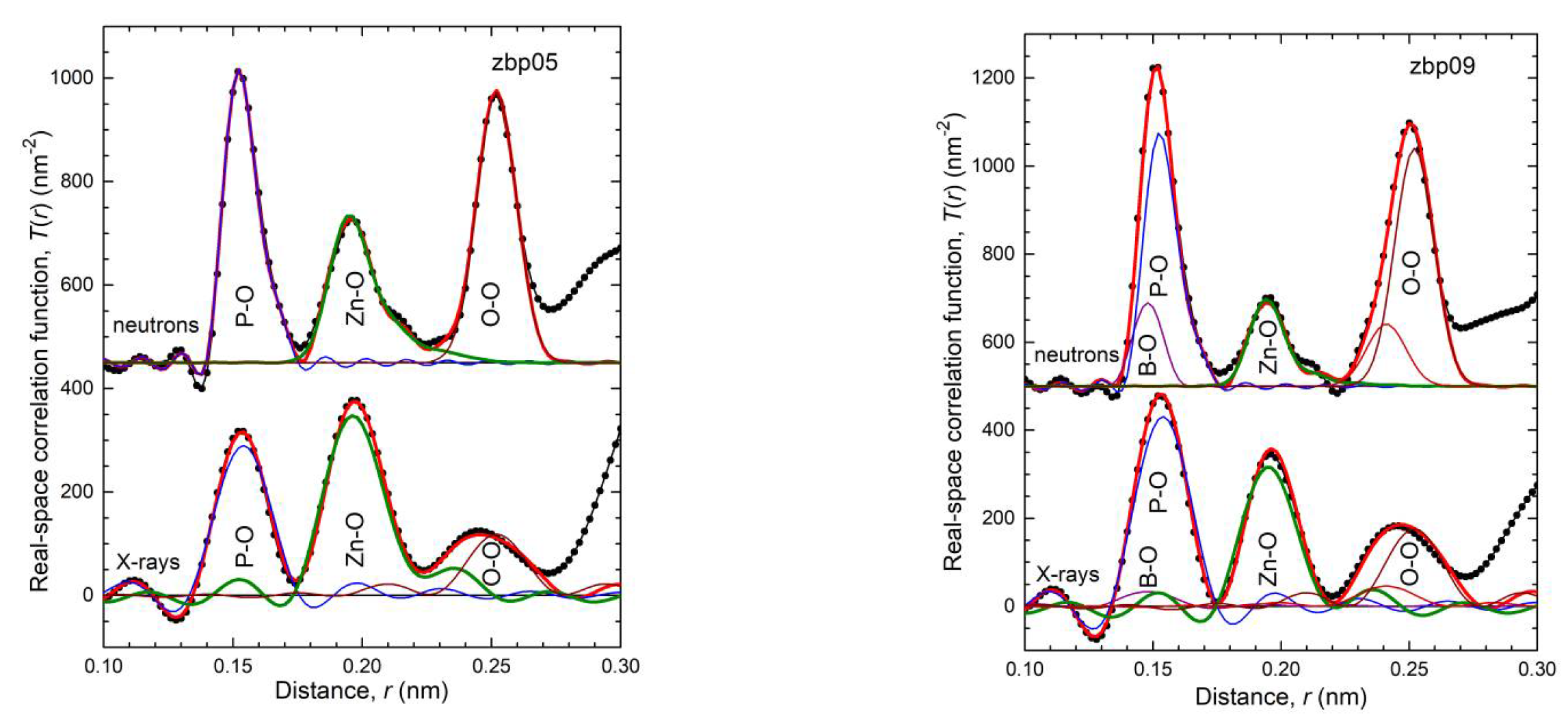
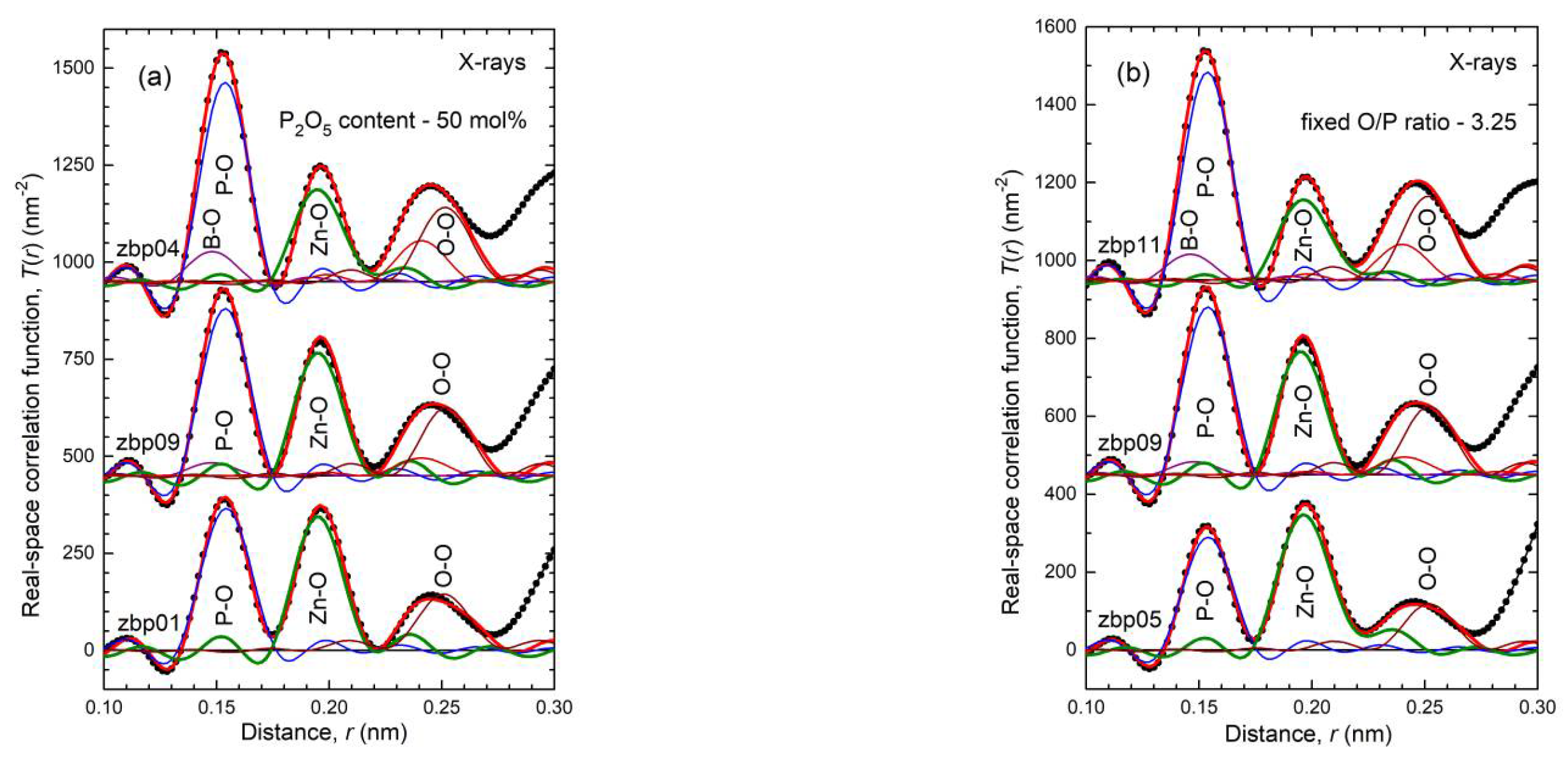
4.1. Fully Tetrahedral Networks and the Ratio ONB/Zn
The Zn borophosphate glasses have the potential to form continuous disordered networks of corner-connected tetrahedra with all oxygen atoms being two-fold coordinated. The Zn metaphosphate glass (50 mol% P
2O
5) has been considered a continuous tetrahedral network for a long time now [
40], which is manifested in its properties [
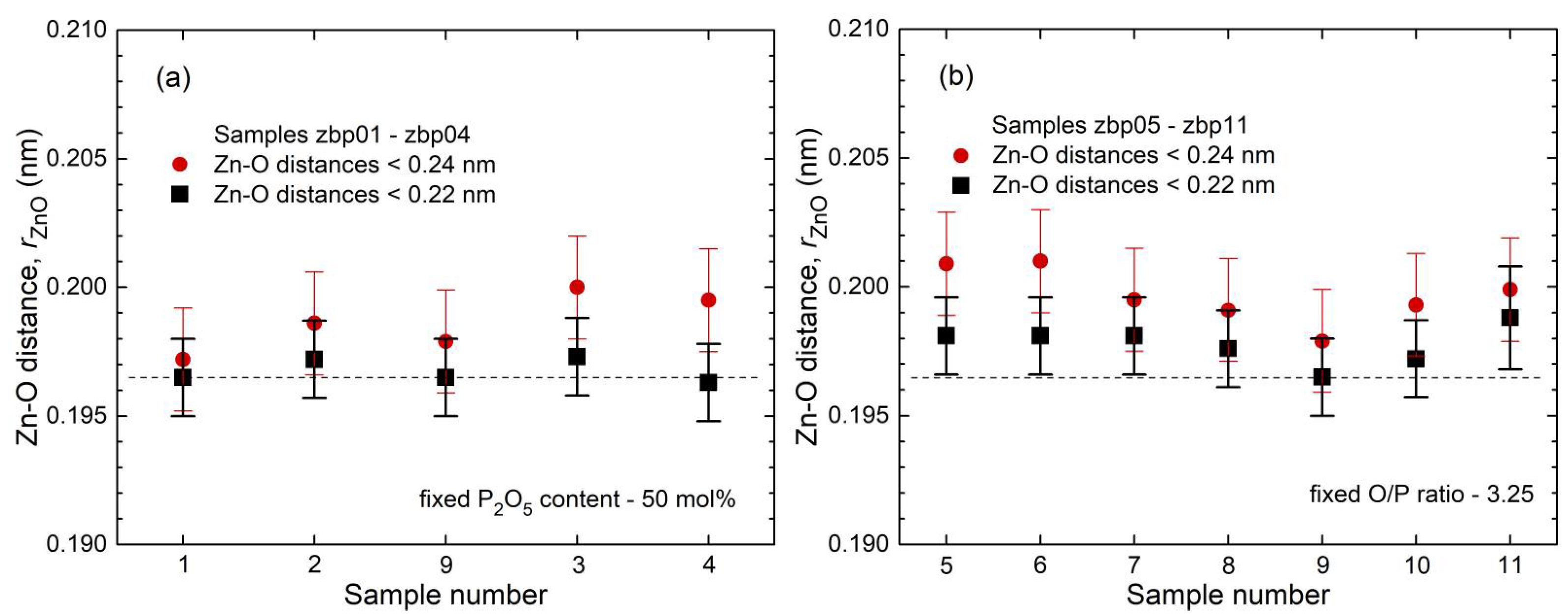
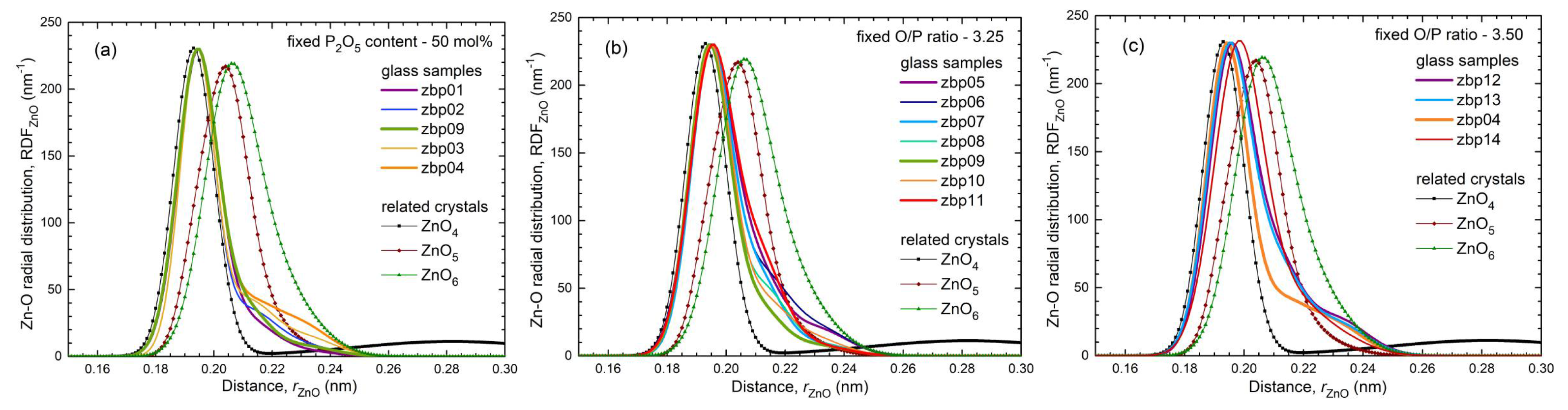
41]. This classification is not entirely accurate because the Zn−O coordination number is slightly larger than four, which is also reflected in the corresponding bond lengths (
Figure S4 and
Figure 5). The ZnO
4 units of the β-Zn(PO
3)
2 crystal [
35] show a fifth Zn−O bond of 0.28 nm length. Similarly, a few longer distances exist for the investigated glasses. Nevertheless, the metaphosphate glass zbp01 and the borophosphate glasses of 50 mol% P
2O
5 (zbp02, zbp09, zbp03, and zbp04) show the most narrow Zn−O peaks together with the smallest distance contributions at ~0.212 nm (
Figure 7a and
Figure S6), evidence that a great majority of Zn ions are in tetrahedral units that have the four corners in Zn−O−P linkages.
The Zn−O peaks of the samples having less than 50 mol% P
2O
5 show increased contributions at ~0.212 nm that indicate O
NBs shared by two Zn. A significant increase in the corresponding
NZnOs is not detected. Two samples (zbp10, zbp11) of the series with the fixed O/P ratio of 3.25 have more than 50 mol% P
2O
5. Sample zbp11 shows significantly increased values of
NZnO,
rZnO, and peak width (fwhm). In this case, the ratio O
NB/Zn is larger than four, and some larger units than ZnO
4 are formed. Similar to the binary ZnO-P
2O
5 system [
23,
24,
42], the borophosphate networks behave in such a way that avoids PO
4 units (Q
3, Q
2) with terminal P=O double bonds. The possible second bond partner of this oxygen is Zn but not B for reasons of bond valences. This is in contrast to what has been found for the Zn aluminophosphate glasses, where the Al
3+ cations form AlO
5 and AlO
6 units for the P
2O
5-rich and ZnO-poor compositions [
22]. This structural flexibility of Al allows the glass-forming range to reach the binary Al
2O
3-P
2O
5 border. However, similar to the BPO
4, the range of the AlPO
4 composition is excluded for its strong crystallization tendency.
On the side of the binary B
2O
3-P
2O
5 system, BPO
4 crystals [
12,
13] are known but disordered networks of PO
4 and BO
4 units are not formed. The structure of BPO
4 consists of
and
units, each connected via their four corners by P−O−B bridges.
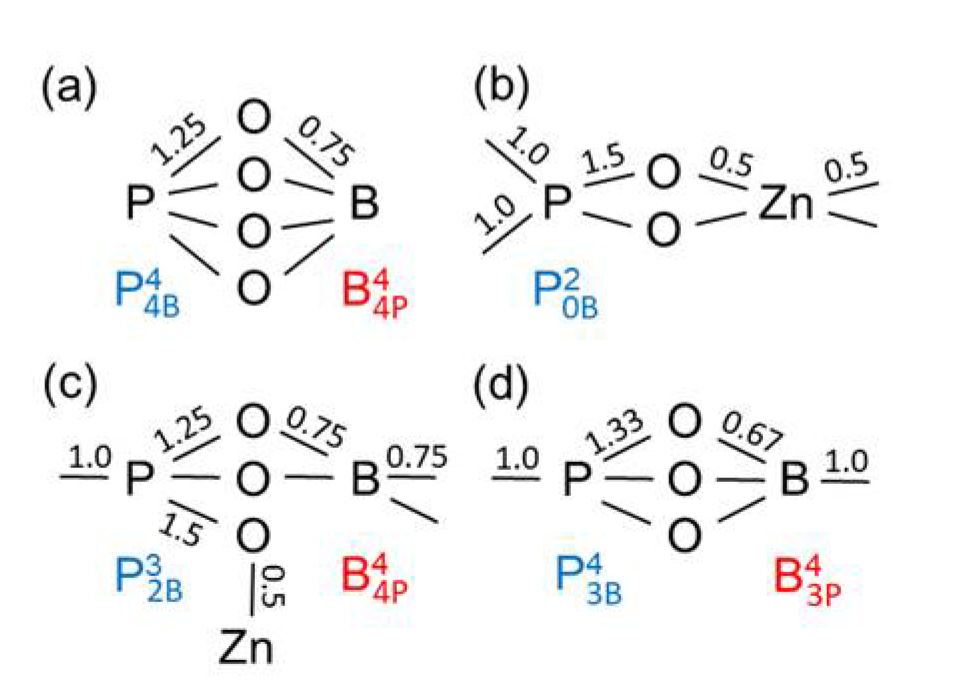
Figure 8a shows the bond valences in the BPO
4 crystals formed by these units. The tetrahedral network in the Zn(PO
3)
2 metaphosphate glass consists of
and ZnO
4 units (
Figure 8b). If one connects the Zn(PO
3)
2 and the BPO
4 compositions (50 mol% P
2O
5) in
Figure 1, their mixture could create networks of ZnO
4, BO
4, and PO
4 units whose corners are connected exclusively by twofold coordinated oxygens. That behavior gets support from the detected narrow Zn−O distance peaks (
Figure 7a). However, the question arises as to how the structures made of Zn(PO
3)
2 and BPO
4 with mixed
and
groups can be constructed, whereby the isolated ZnO
4 and BO
4 units must be provided with the necessary bond valences. Starting from the side of the ZnO-rich glasses, the problem is solved using a structural speciation reaction
where the oxygens of the
(a Q
1 unit) are shared with one ZnO
4, one PO
4, and two BO
4 units as shown in
Figure 8c. The Q
1 groups terminate the phosphate chains. Most ZnO
4 corners interact with the remaining Q
2 groups while the BO
4 tetrahedra connect the Q
1 and Q
0 groups. Neither the ZnO
4 nor the BO
4 could exist in isolated sites alone with only Q
1 neighbors (with
or
respectively). The ZnO
4 would have to share O
NBs and the BO
4 would not get sufficient bond valence. Approaching the composition of sample zbp04, nearly all Q
2 have changed to Q
1 groups. A high ordering of the ZnO
4 and BO
4 is required that both groups get the needed bond valences. Fortunately, a further solution exists for the bond valences, which stabilizes the disordered borophosphate networks, whereby some of the oxygens that, for example, would form P−O−B bonds instead are incorporated into B−O−B bonds, necessitating the concomitant formation of new P−O−P bond to produce a weak polymerization of the phosphate network [
11]. This rearrangement is described by
Figure 8d illustrates an arrangement of a
with a
unit.
11B MAS NMR has determined the fractions of the
besides the
units [
11]. Hence, the O/P ratio is no longer exactly related to the Q
n distribution. Four of the Zn borophosphate glasses used in [
11] have ~50 mol% P
2O
5. The glass with the highest B
2O
3 fraction (18.5 mol%) was found with the highest
fraction (88%). The other samples had ~35%
besides the
units. Reactions according to Equation 3 take place in glasses of high Q
2 fractions (high ZnO content) whereas reactions according to Equation 4 dominate when the Q
2 units are already in the minority (equal amounts of ZnO and B
2O
3). The fraction of oxygens that are available for coordinating the Zn
2+ cations is preserved when the fraction of
units changes. Equation 4 does not change the numbers of P−O and B−O bonds as illustrated in
Figure S8. That allows us to calculate the accurate O
NB/Zn without knowing the accurate
fraction and this ratio O
NB/Zn largely determines the Zn−O environments.
and units, (d) Zn borophosphate glass with and units. The numbers indicate the bond valences given in valence units (vu). Bond valences of 1.0 represent P−O−P or B−O−B bridges. The values of bond valences are not repeated for equivalent bonds.
Now it is discussed how one can divide the oxygen fractions to coordinate the Zn and B. Simply knowing the sample composition of the ternary phosphate glasses is not sufficient for that. The available amount of oxygens is derived by subtracting the number of P−O−P bonds per PO4 and that is calculated from the total O/P ratio. In analogy to the structural behavior of the Zn phosphate glasses (cf. Introduction), all oxygens will find two neighbors with decreasing P2O5 content (P, B, Zn) before threefold coordinated oxygen sites occur. Sophisticated considerations have to take into account the different properties of the two sorts of cations besides the P5+ such as charge balance, field strength, preference of definite oxygen polyhedra, or threefold coordinated oxygen sites.
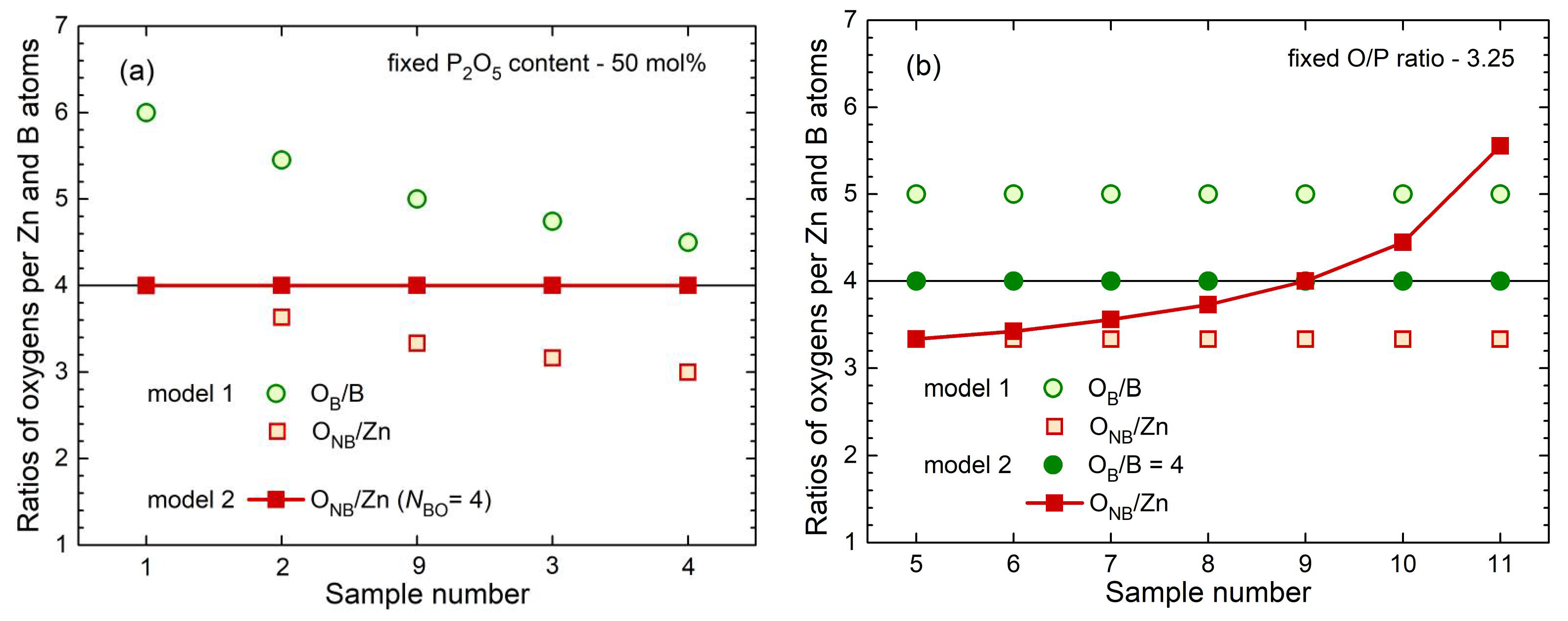
A general model (model 1) takes into account the different requirements for charge compensation of the Zn
2+ and B
3+ cations. It is assumed, that the two cationic species act independently of each other and are linked with the different Q
n by equal probability which is reasonable for a simple approach. The corresponding values O
NB/Zn and O
B/B are obtained with
where the
ci are the concentrations of the four sorts of atoms. This approach produces O
B/B ratios that are too high (> 4) for all samples as shown in
Figure 9 where the ratios are considered for the series with constant P
2O
5 contents of 50 mol% (a) and constant O/P ratios of 3.25 (b). The boron cannot meet this coordination behavior. In other words, the Zn−O environments and BO
4 units cannot form independently of each other. Preferences for special Q
n neighbors as shown in
Figure 8c,d must be effective. In the range of the glasses studied, the situation is quite simple because
11B MAS NMR detected only BO
4 (no BO
3) [
11]. When
NBO is fixed to the number four for BO
4 units, a well-defined fraction of oxygens is used as bridging ones by the borons. Then, the oxygens formed as non-bridging in the PO
4 units are used for the coordination of the zinc (model 2) with a ratio
For glasses with P
2O
5 contents of 50 mol%, model 2 predicts that O
NB/Zn = 4. This value is exactly what is necessary for isolated ZnO
4 units and a fully tetrahedral network (filled squares in
Figure 9a). Differently, the fixed O/P ratios of 3.25 produce a continuous increase of O
NB/Zn with increasing B
2O
3 content (cf.
Figure 9b). For completeness, the ratios O
NB/Zn according to model 2 are calculated for the samples zbp12 and zbp13 with 3.10 and 3.27, respectively.
The ratio O
NB/Zn becomes smaller than four for glasses with P
2O
5 contents less than 50 mol%. Then, two ZnO
4 units must share some of their O
NBs. The obtained ratios O
NB/Zn are still larger than three, which means a few connected ZnO
4 units, but not any interconnected ZnO
4 substructures. If one looks at the binary ZnO-B
2O
3 glasses [
17] that are obtained with 54 to 70 mol% ZnO, the ratios O
NB/Zn reach only 1.5 to 1.9 (
Table S3). That means for the ZnO
4 units existing in these glasses each O
NB is shared by at least two Zn and extended substructures of interconnected ZnO
4 exist. Octahedral Zn−O units were proposed for these glasses [
17]. However, EXAFS and X-ray diffraction suggest that ZnO
4 units are the dominant moiety [
43]. The
NZnO value of 4.3 for our Zn borate glass is similar to those of the borophosphate glasses. The mean bond length of 0.200 nm is the largest among those of the other glasses (cf. Fig. 7c). The Zn−O distances of zbp14 show large variations due to the massive need for shared O
NBs. Highly distorted ZnO
5 polyhedra co-exist beside the ZnO
4.
Fig. 9b shows strongly increasing ratios O
NB/Zn > 4 in the direction of increasing B
2O
3 contents. Thus, a significant increase in
NZnO is forced. According to the Zn−O distances (
Figure 5b and
Figure 7b), the
NZnO of sample zbp11 must be increased but it is still expected to be below five. Otherwise, in the case of
NZnO < O
NB/Zn, some terminal P=O double bonds must occur. According to the other limitations of sample zbp11 (
NPO = 3.6), its P
2O
5 content should be a little less than the nominal value. The formation of homogeneous glasses reaches the limit for this sample as reported for such compositions [
20].
Model 1 was introduced assuming equal preferences of B and Zn for the oxygens of the different Q
n. That means the B and Zn would form their environments independently of each other which is not possible with the limit
NBO ≤ 4. For the ZnO-Al
2O
3-P
2O
5 glasses, the Al−O coordination number can increase up to six and the changes of
NAlO according to model 1 are possible in a large concentration range. The fractions of AlO
4, AlO
5, and AlO
6 units were obtained by
27Al MAS NMR, and
NAlO values were calculated [
22]. One of the glasses is a compositional analog to that of sample zbp11. That Al−O coordination number was found to be 5.2 which is close to 5.0 as resulting from model 1 (
Figure 9b). The structural analysis of Na
2O-Al
2O
3-P
2O
5 glasses has shown that all oxygen is used for the breakage of the P−O−P bridges [
44]. The compositional dependence of
NAlO was found to follow other rules than simply a continuous increase with the O
NB fractions. Abrupt changes from
NAlO = 4 to 6 were found [
44] which indicates the greater stability of the AlO
4 and AlO
6 polyhedra if compared with the AlO
5 [
45]. The Na
+ coordinating the oxygens in Al−O−P bridges is essential for this behavior. Certainly, special preferences with the different Q
n groups exist.
In this work, the different preferences for the oxygens of the Q2, Q1, and Q0 units with the Zn and B polyhedra are due to the restrictions of the B to BO4 units (model 2). The different field strengths of Zn2+ and B3+ are not essential in this context. The maximum limit NBO = 4 made the model considerations comparably simple for the borophosphate glasses of this study. As long as the value NBO = 4 is less than the ratio OB/B of model 1, the ratio ONB/Zn can be calculated and it determines the Zn−O environments. The success of model 2 became obvious in the predicted behavior of the Zn−O distances with the narrow peaks along the line of 50 mol% P2O5.
4.2. The Stabilization of the BO4 Units and the Boron Anomaly
BO
4 tetrahedra and planar BO
3 triangles are the units in the crystalline forms of B
2O
3 [
46,
47]. The BO
4 unit is the variant of the densest packing and it is formed provided its bond valencies can be balanced. In the corresponding high-pressure B
2O
3 crystal [
46], the charge compensation is realized with threefold-linked oxygens. Usually, glasses are only formed when there is no or little threefold connected oxygen in the network. Other mechanisms cause also the required depletion of electron density from the B−O bonds in the BO
4 unit which then results in four bonds with the necessary valencies of ~0.75 vu. The occurrence of BO
4 tetrahedra in binary borate glasses was identified as the origin of non-continuous property changes [
10] which is widely known as the boron anomaly.
For the borophosphate glasses, the BPO
4 crystals [
12,
13] show the mutual benefit for the B and P atoms with a deficit and excess valence electron density in the B−O−P bridges of the tetrahedral networks (cf.
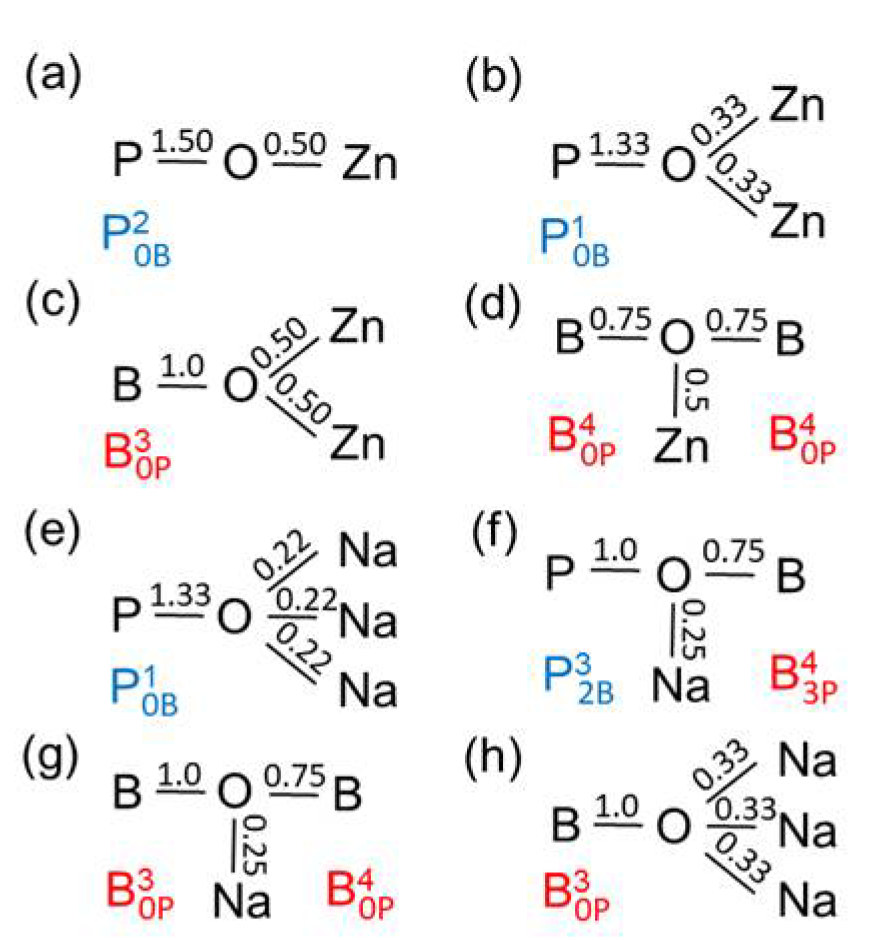
Figure 8a). This type of valence transfer is effective throughout the Zn borophosphate glasses studied whereby the BO
4 units do not form B−O−Zn bridges. The typical PO
4 – Zn interactions are shown in
Figure 10a,b which were discussed above. The interaction of the Zn with the oxygen sites in P−O−B bridges is rather improbable. Each Zn−O bond of a ZnO
4 needs a bond valence of ~0.50 vu which value cannot be shared with these oxygens. Similarly, the oxygen corner of a ZnO
4 unit cannot be that oxygen between two BO
3 units or a pair of BO
3 and BO
4 (analogously to
Figure 10g). These circumstances suggest a relation to the lack of glass formation in the B
2O
3-rich and P
2O
5-poor regions of the Zn borophosphate system. There is one exception, the oxygen sites in B−O−B between BO
4 pairs can coordinate a Zn (cf.
Figure 10d) as found in several Zn borate crystals [
48,
49,
50,
51]. However, this feature is not sufficient to stabilize any glasses poor in P
2O
5 but it can occur in the glasses of moderate P
2O
5 content until not quite 50 mol%.
Binary Zn borate glasses are obtained in a small range rich in ZnO [
17] where already a significant fraction of O
NBs in BO
3-triangles exists (O
NB/Zn ≥ 1.5). Two Zn
2+ cations share each O
NB (
Figure 10c) which means the ZnO
4 or ZnO
5 use three such O
NB corners at minimum. Our diffraction results of the Zn borate glass (zbp14) suggest a fraction of ZnO
5 units besides the ZnO
4. The ZnO
5 have bonds of unequal lengths whose two more distant corners can coordinate the oxygens in any B−O−B bridge. The corner of a ZnO
4 can coordinate only the oxygen in a bridge between two BO
4 units (cf.
Figure 10d).
Similar to the ZnO
4 units, the Na
+ cations have total coordination numbers close to four (
Table S2). The cation oxygen distance is larger for Na
+ and the width (fwhm) of the Na−O peak (
Figure S6) is ~0.042 nm, much more than the ~0.017 nm of the Zn−O peak (
Figure 6). These parameters express the large flexibility of the Na
+ cations to form distorted oxygen environments.
Figure 10e,h show the interaction of the Na
+ with the O
NBs of the P or B atoms which is similar to the Zn−O bonds in
Figure 10a,b,c. Here, the Na−O bonds are marked with bond valences of ~0.25 vu as belonging to a NaO
4 tetrahedron. This value can vary according to the distortions of the NaO
m polyhedra including the variations in
m. The Na−O bonds are dominantly ionic but it is better not to mix the bond valences and electronic charges in the schematic presentations. The weak Na−O interaction allows the Na
+ to approach the bridging oxygens in the P−O−B and B−O−B bridges (cf.
Figure 10f,g). The corresponding oxygens are still quasi-twofold linked. The third partner Na
+ forms a flexible bond that maintains sufficient flexibility to the disordered network as needed for glass formation.
It was emphasized that the BO
4 units in the Zn borophosphate glasses with P
2O
5 contents ≥50 mol% are charge-balanced by PO
4 units (
Figure 8). The Zn
2+ cations can approach oxygens in B−O−B bridges only for glasses of P
2O
5 content <50 mol% and then contribute also to the BO
4 stabilization. Of course, the BO
4 fractions in the binary Zn borate glasses [
17] are charge-balanced by the Zn
2+ cations only. What about the Na borophosphate glasses? One can compare with the known crystal structures. The Na
2B
8O
13 crystal free of phosphate [
52] shows two mechanisms for BO
4 stabilization through Na
+. The lengths of the B−O bonds suggest that the surplus negative charge of the BO
4 is not only balanced by the Na
+ coordinating the BO
4 corners but it is also transferred to the neighboring BO
3 triangles. The bond lengths in the BO
3 units are strengthened in B−O−B bridges to the BO
4 (~0.134 nm) whereas they are elongated in bridges to other BO
3 (~0.138 nm). Thus, the Na
+ cations act also across the BO
3 triangles as neighbors of the BO
4. It is to be presumed that there is no serious difference between the depletion effects of electron density from the bonds in the BO
4 in the direction of the PO
4 units or the Zn
2+ and Na
+ cations.
4.3. Different Effects of Zn and Na on the P−O Bond Lengths
The lengths of the P−O bonds in the glasses zbp13 and nbp13 of equivalent compositions with an O/P ratio of 3.5 reveal the different effects of the Zn
2+ and Na
+ cations. Binary phosphate glasses of the same O/P ratio (pyrophosphates with O
NB/O
B = 6:1) show usually two different lengths of P−O bonds, the P−O
NB and P−O
B bonds with a frequency ratio of 3:1 [
21,
27,
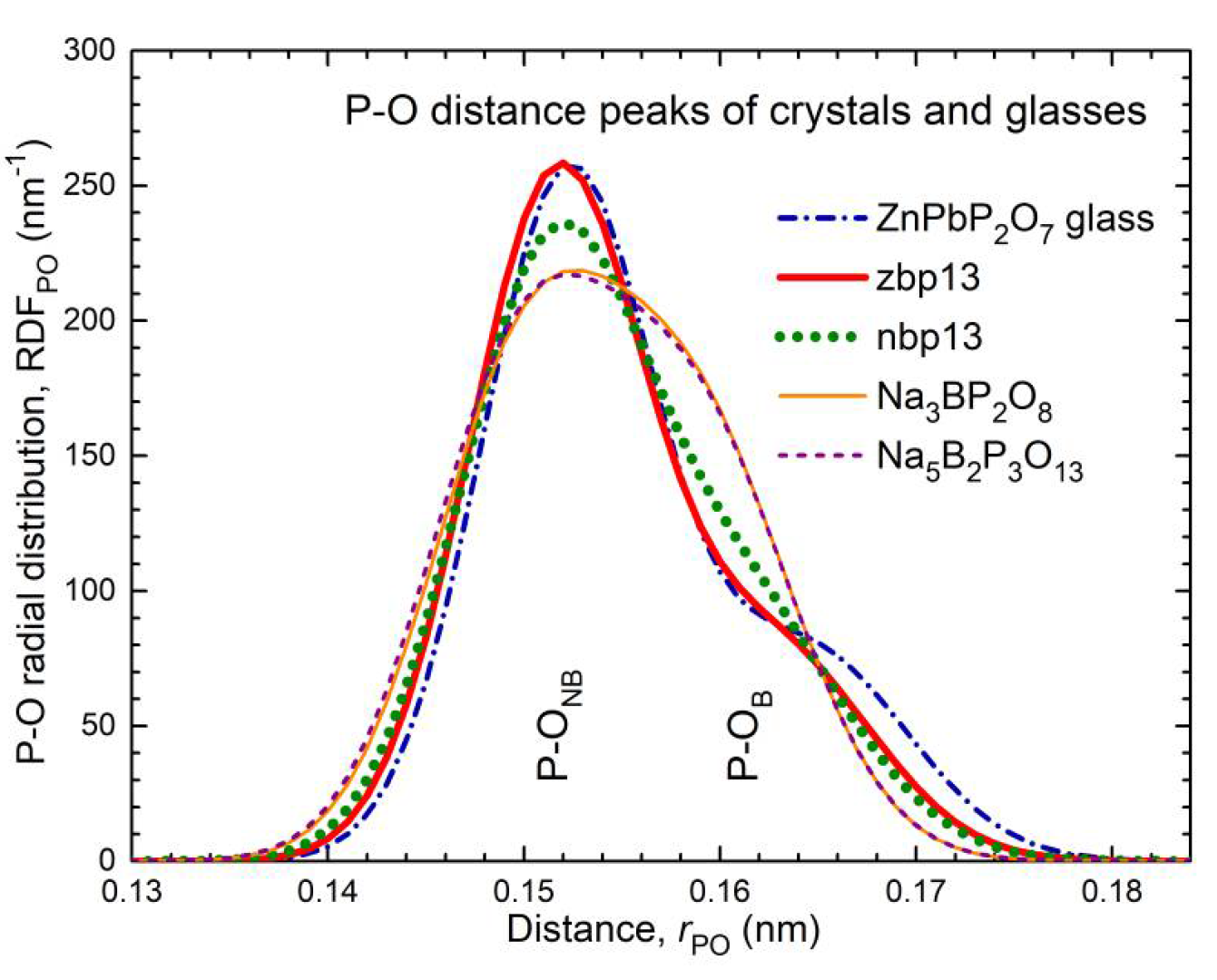
53]. The neutron diffraction results of zbp13 and nbp13 also show two different types of P−O bonds (cf. Figures S3, S4). Their P−O model peaks composed of two Gaussians are compared in
Figure 11 with that of a phosphate glass (ZnPbP
2O
7 [
53]) of the same O/P ratio. The frequency numbers of the two P−O bonds are obtained with 2.8 and 1.0 for zbp13 and 2.5 and 1.3 for nbp13 (cf.
Table S2). For sample zbp13, the ratio is close to 3:1, and the P−O peak agrees with that of the ZnPbP
2O
7 glass. The sample nbp13 has a significantly larger fraction of P−O bonds with lengths of ~0.160 nm. An effect that could increase this P−O fraction is the formation of B−O−B bridges between two BO
4 according to Equation 4 which is accompanied by additional P−O−P bridges. A borophosphate glass of composition close to that of zbp13 has 27.7% boron in
units [
11]. According to the glass composition of zbp13 and excluding B−O−B bridges, the P−O bonds can be divided into 25% in P−O−P bridges, 30% in P−O−B bridges, and 45% in P−O
NB. 27.7% of the B in
units means an increase of the bonds in P−O−P bridges from 25% to 27%. Hence, the corresponding increase in the number of the longer P−O
B bonds is insignificant (<0.1).
The Na borophosphate glass nbp13 should be almost free of B−O−B bridges as it was reported for a similar glass (sample
x = 0.125 in [
6]). The structures of the related Na
3BP
2O
8 [
14] and Na
5B
2P
3O
13 [
15] crystals help to understand the larger fraction of the longer P−O bonds although the crystal’s compositions differ somewhat from that of nbp13 (cf.
Figure 1). The Na borophosphate crystals have isolated PO
4 units (Q
0). From the point of view of binary phosphates [
21], a single P−O distance should occur. However, two distances become obvious. Half of the oxygens of the PO
4 units participate in P−O−B bridges and these oxygens have a Na
+ cation in their close vicinity as illustrated in
Figure 10f. These Na
+ stabilize the BO
4 but also reduce the bond valence in the adjacent PO
4 (elongation of the P−O bond). The other two PO
4 corners coordinate only Na
+ cations as shown in
Figure 10e and the corresponding short P−O bonds carry the surplus bond valence taken over from the others. The difference in both P−O bond strengths in these crystals is a little smaller than that in the zbp13 and ZnPbP
2O
7 glasses, the latter with bond valences of 1.33 and 1.0 vu (cf.
Figure 11). For the glass nbp13, the changes in bond lengths appear less pronounced than in the crystals. Again, 25% of the P−O bonds are in P−O−P bridges, 30% in P−O−B bridges, and 45% in P−O
NB. To achieve the right ratio of the short and long P−O bonds of sample nbp13 (2.5:1.3), the fraction of the P−O−B bridges is split into two parts, those with a Na
+ close nearby and those free of any Na
+ neighbor. Then, 30% of the P−O bonds in the P−O−B bridges are elongated indicating the effect of a Na
+ cation. The other 70% of P−O bonds in P−O−B bridges are not affected by any Na
+ cations and have lengths similar to those of typical P−O
NB bonds. This difference to zbp13 means that the BO
4 units in the glass nbp13 undergo charge compensation equally by Na
+ cations and PO
4 tetrahedra. The ZnO
4 units in zbp13 are less contributing because their corners do not participate in P−O−B bridges.
Two species of P−O−B bridges have been suggested for nbp13 that seem to be distinguishable easily. One is coordinated to a Na
+ cation, and the other is not. On the other hand, a clear separation of the P−O−B bridges into those with and without a Na
+ neighbor might be questionable due to the large width of the Na−O distance peak. Both P−O peak components overlay with each other. The analyses of the O 1s spectra (XPS) did not distinguish two different types of P−O−B bridges in the Na and Zn borophosphate glasses [
6,
18]. Nevertheless, the effect of the Na
+ on the P−O−B is real and it is not effective for Zn
2+. Another approach supports the fact that only part of the P−O−B bridges of nbp13 can have Na
+ neighbors. The total Na−O coordination number is calculated assuming all oxygens in the P−O
NB bonds or P−O−B bridges with three or one Na
+ neighbors as shown in
Figure 10e,f, respectively. Then for nbp13, a value
NNaO of ~6.0 is obtained which noticeably exceeds the value (4.0) obtained from diffraction. Accordingly, only part of the P−O−B can have a Na
+ neighbor (30% as estimated for nbp13). Also, the O
NBs shown in
Figure 10e can have only 2.3 Na
+ neighbors on average instead of three. These conditions mean that only a third of the Na
+ can coordinate the oxygen in a P−O−B bridge. All other oxygens in the Na−O bonds belong to the P−O
NB bonds. Here, only the behavior of a single sample is discussed. The corresponding relations will strongly change with the glass compositions.
The weak forces of the Na
+ are not sufficient to take profit from the maximum capabilities of the modifier cation’s coordination. The Na−O distance peaks in
Figure S7 show that the values
NNaO of the glasses are smaller than those of the related crystals [
14,
15], accompanied by smaller bond lengths. Similarly, the number and bond valences of the P−O
NB bonds in zbp13 (cf.
Figure 10b) would allow the Zn
2+ to form ZnO
6 octahedra, which does not occur. The strong bonds in the PO
4 and BO
4 units together with the comparably high cross-link density of the borophosphate networks prevent the modifier cations from forming compact oxygen polyhedra.
The relation between the network structure and the modifier environments was also used to interpret the mixed network-former effect. The average number of O
Bs per glass-forming unit,
BO, calculated for the
yNa
2O-
xB
2O
3-(1-
x)P
2O
5 glasses characterizes the stiffness of the network [
4,
8]. This value’s behavior correlates well with the
Tg values. A larger
BO means an increasing network stiffness, that is accompanied by more disordered modifier environments, here the NaO
m polyhedra. In addition, the ratio O
NB/Na decreases (Equation 7). These changes explain the reduced activation energies for ionic transport when the B
2O
3 content increases while the Na
2O content is constant [
4,
8]. The average number of bridging corners per glass-forming unit,
BCGFU, is a more plausible value with
BCGFU = 2
BO [
45]. For the borophosphate glasses, it is calculated with
The
BCGFU value is two for the
chain units in the NaPO
3 glass. It increases for sample nbp13 to 2.6. Maximum values of ≥3 are reached for larger B
2O
3 content till the potential of the PO
4 units to stabilize the BO
4 is exhausted and BO
3 triangles occur. The Na−O distances of three glass compositions are shown in
Figure S7. A significant change in the Na−O peak is not observed. The small scattering power of Na if compared with Zn and the overlap with the O−O distances at 0.25 nm impede an accurate distance analysis.
Previous diffraction work on Na
2O-B
2O
3-P
2O
5 glasses [
54,
55] did not reach that large
Qmax which is needed to resolve different P−O bonds. The structural analysis was made by the Reverse Monte Carlo (RMC) method which was aimed at the elucidation of the mixed network former effect. A main point was the description of the medium-range order which includes the migration pathways for the Na
+ cations. The analysis of the Na−O environments in the model configurations gave total
NNaO values of ~4.5 with very broad distributions of the distinct Na sites ranging from two- to eightfold coordination. The two distances of 0.152 and 0.156 nm given in [
55] for the P−O
NB and P−O
B bonds are simply calculated from the RMC configurations but are not resolved in the measuring results and thus cannot be compared with our data.
4.4. The Evolution of the Properties of the Zn Borophosphate Glasses
The figures with the mass densities
ρ of the ZnO-B
2O
3-P
2O
5 glasses in [
20] show a continuous increase with fixed ZnO and increasing B
2O
3 contents. Glass transition temperatures
Tg show similar trends while the expansion coefficients α change in opposite directions. The values of
ρ and
Tg in [
11] show similar trends though the comparisons are made along constant O/P ratios. It needs to be remembered that the Zn phosphate glasses have been classified as showing anomalous behavior [
40]. Minima of mass density at 50 mol% ZnO [
23,
40,
56] or
Tg values [
11,
27,
56] at ~60 mol% ZnO are characteristic of the binary Zn phosphate glasses. Below, the mass densities that were published for the full range of glass formation in the ZnO-B
2O
3-P
2O
5 system are discussed [
20]. Packing densities are better suited for comparisons whereby the influence of atomic size and mass is widely eliminated. Such comparisons were made for the ZnO-P
2O
5 glasses recently [
25] by using the ionic radii from [
57].
A packing density is the filled volume fraction assuming the atoms as spheres of known ionic radii. Since the BO
4 tetrahedra have small O−O distances there is some overlap of the oxygen spheres with the ionic radius of 0.135 nm, to a lesser extent also for the PO
4 tetrahedra. A correction was made for this overlap. The packing densities of the Zn borophosphate glasses obtained from the mass densities given in [
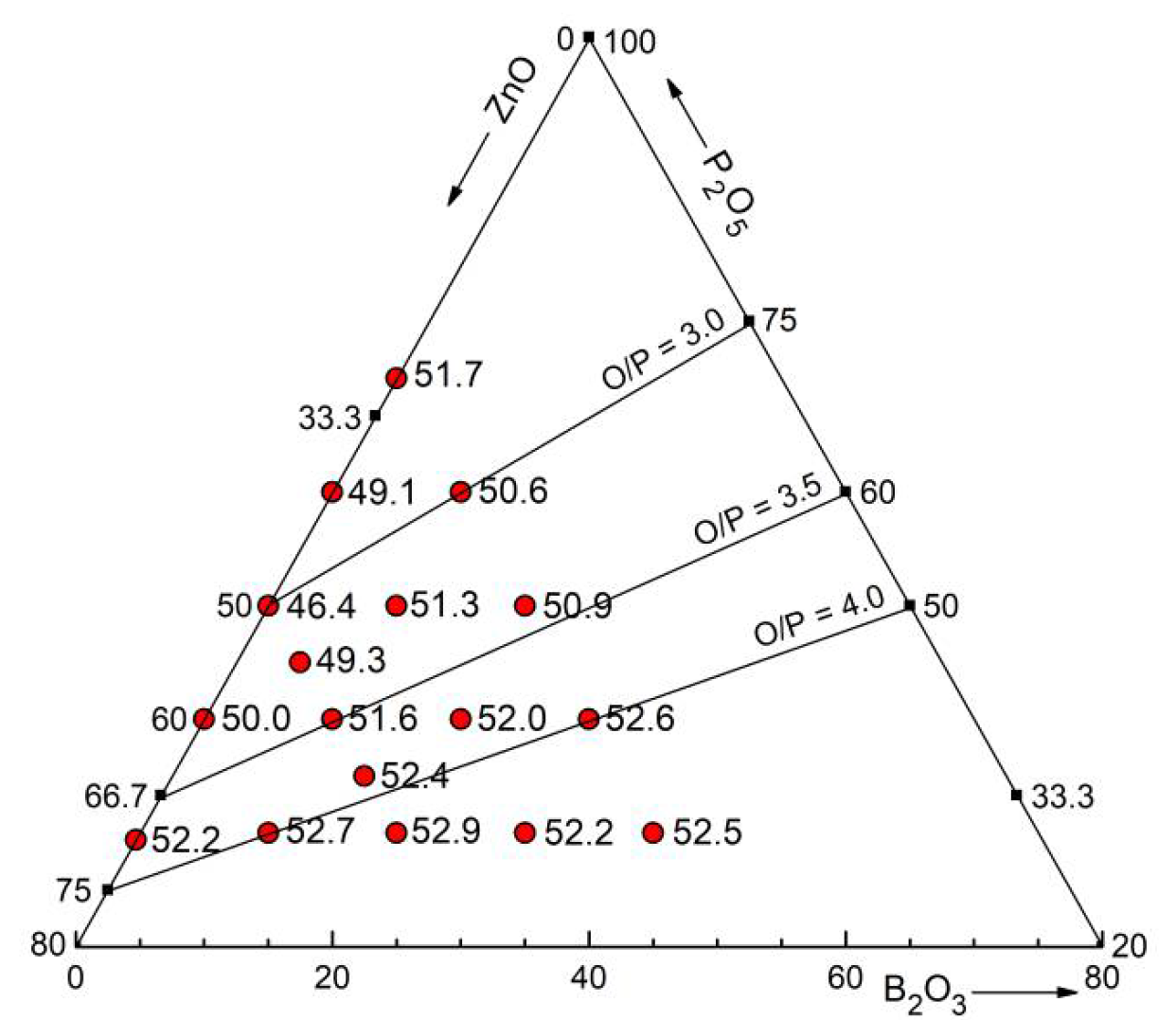
20] are shown in
Figure 12 as numbers close to the sample positions in the concentration triangle. The absolute minimum at 50 mol% ZnO is found with 46.4% packing density. This behavior was attributed to being caused by the network of corner-connected PO
4 and ZnO
4 tetrahedra with all oxygens in bridges (
Figure 8b), a network that fills the space quite inefficiently [
23,
27].
Above, it was shown that the tetrahedral character of the network does not change when B
2O
3 replaces the ZnO while 50 mol% P
2O
5 is fixed. A plateau of ~51% packing density is reached with 10% B
2O
3 (
Figure 12). This increase is due to the exchange of the rather open ZnO
4 unit for the more compact BO
4 unit. There exists a further subtle increase of the packing density up to ~52.5% in the direction of decreasing P
2O
5 contents. This fact is interpreted with the change from P−O−Zn bridges (
Figure 10a) to the first O
NBs shared by two Zn (
Figure 10b) and the exchange of PO
4 for BO
4 units. When finally only isolated PO
4 units exist then also BO
3 units with O
NBs shared by two Zn are formed (
Figure 10c) and the packing densities are still increasing. The (ZnO)
x(B
2O
3)
1-x glasses with 0.54 ≤
x ≤ 0.67 [
17] with few BO
4 connected with BO
3 have packing efficiencies of 55% to 53.5% where the O
NBs of the BO
3 are shared by two Zn. For the reachable glass compositions, the BO
3 units of the Zn borate glasses have one or two O
NBs.