1. Introduction
Traditional ice cream, while undeniably pleasurable, often presents a nutritional paradox. Its high sugar and fat content [
1,
2] can negate any potential health benefits. This observation coincides with the burgeoning popularity of functional foods, reflecting a change in basic assumptions in consumer preferences. Consumers are increasingly seeking food products that deliver synergistic benefits, offering both enjoyment and demonstrable health advantages beyond basic nutrition. This trend presents a challenge and an opportunity for modern food manufacturers. Their task lies in meticulously selecting ingredients that cater to the evolving needs of health-conscious consumers while maintaining the sensory appeal that defines their products. Probiotics have emerged as an exciting category of functional food ingredients due to their well-documented health benefits. These microorganisms provide numerous potential health benefits, including improved gut microbiota composition, enhanced gut barrier function, and modulation of the immune response [
3,
4]. However, probiotics are sensitive to factors such as freezing and storage conditions, posing a challenge for their incorporation into frozen desserts like ice cream. Recognizing this potential, researchers are actively developing innovative ice cream formulations that incorporate probiotics along with other health-promoting ingredients. This study explores the development of a novel probiotic-enriched ice cream formulation that leverages the combined health benefits of probiotics and fermented white kidney bean homogenate.
This study explores the development of a novel probiotic-enriched ice cream formulation that leverages the health benefits of fermented white kidney bean homogenate [
5,
6]. White kidney beans contain significant quantities of health-promoting compounds, including polyphenols and proteins [
7]. Polyphenols, natural defense systems within plants, possess potent antioxidant properties. By neutralizing free radicals, these antioxidants help mitigate the detrimental effects of oxidative stress, a cellular imbalance linked to chronic diseases. White kidney beans are particularly rich in polyphenols, offering a spectrum of health benefits such as antioxidant, antidiabetic, antiobesity, anti-inflammatory, antimutagenic, and anticarcinogenic properties [
8]. Fermentation, an age-old process, further unlocks the potential of these bioactive compounds by enhancing their bioavailability, thereby potentially amplifying their health-promoting effects [
9,
10]. By introducing specific microbial strains, fermentation can enhance the bioavailability of bioactive compounds within white kidney beans, unlocking greater health benefits. However, a knowledge gap persists regarding the impact of incorporating fermented white bean homogenate and probiotic bacteria on the overall functionality of ice cream. While ice cream remains a universally cherished dessert, it presents a unique challenge for incorporating functional ingredients like probiotics and antioxidants. The key challenges involve ensuring the survival of probiotic bacteria throughout ice cream production and storage while maintaining the crucial antioxidant activity of the incorporated compounds. Existing research explores probiotic ice creams, but there is a significant gap in exploring ice creams enriched with both probiotics and natural antioxidants from fermented plant sources, such as the white kidney bean homogenate used in this study. This innovative approach of integrating fermented white bean homogenate with probiotics in the ice cream matrix holds immense potential. The resulting product could be a true “multifunctional dessert,” offering a symbiotic blend of probiotics and antioxidants. Meanwhile, the retained antioxidant activity from the fermented white bean homogenate can further protect cells from oxidative stress. This research aims to bridge the knowledge gap by investigating the viability of probiotic strains throughout ice cream production and storage while evaluating the potential for enhanced antioxidant capacity due to the incorporation of fermented white kidney bean homogenate. Through this novel formulation exploration, we hope to contribute to the development of functional ice cream products that offer both indulgence and demonstrable health benefits.
This study aimed to comprehensively evaluate the potential health benefits associated with probiotic-enriched ice cream containing fermented white kidney bean homogenate. We evaluated the viability of various probiotic strains throughout the production and storage of the ice cream to ensure their presence and potential health benefits for consumers. Established methods such as DPPH, ABTS, FRAP, and TPC were employed to assess the overall antioxidant potential of the ice cream containing fermented bean homogenate and probiotic bacteria. These methods provided insights into the ability of these products to neutralize free radicals and potentially mitigate cellular damage. By combining the results on probiotic survival and enhanced antioxidant capacity, we aimed to explore the potential health benefits associated with consuming this type of ice cream compared to regular ice cream.
2. Materials and Methods
2.1. Materials
The biological materials used in this study included one commercial yogurt starter culture, YC-180 (Chr. Hansen, containing Streptococcus thermophilus, Lactobacillus delbrueckii subsp. bulgaricus), and five probiotic strains: Lactobacillus acidophilus La-5, Lactiplantibacillus plantarum 299v, Lacticaseibacillus rhamnosus GG, Lacticaseibacillus casei DN-114001, and Bifidobacterium animalis subsp. lactis Bb-12, all of which were deposited in the institute’s collection.
The ice cream samples were prepared using the following ingredients: 215 g of pasteurized milk with 3.2% fat content (OSM Łowicz, Poland), 45 g of UHT cream with 36% fat content (OSM Łowicz, Poland), 50 g of granulated sugar (Diamant Poland), 105 g of white bean homogenate (as specified in
Table 1), 5 mL of bacterial biomass, and 0.5 mL of vanilla flavoring (Delecta Trade, Poland). The white kidney beans of the “Piękny Jaś Karłowy” (
Phaseolus vulgaris) variety were procured from a local market.
2.2. Dairy-Fermented Beans Ice Cream
The ice cream recipe comprised the following ingredients: pasteurized milk (3.2% fat content) at 51.1%, UHT cream (36% fat content) at 10.7%, granulated beetroot sugar at 11.9%, white bean homogenate at 25.0%, bacterial biomass at 1.2%, and vanilla flavor at 0.1%.
The preparation of the white bean homogenate followed a previously established laboratory method [
11]. One hundred grams of whole, healthy beans were germinated in a kitchen germinator at 25 ± 0.5°C for 72 h, with regular moistening using tap water every 24 h. After germination, the beans, now weighing a combined 500 g with water, were transferred to a larger container filled with water. The mixture was then blended in a kitchen blender for 20 min at room temperature, resulting in a homogenate. This homogenate was brought to a boil and continuously stirred to gelatinize the bean starch. Subsequently, the beverage was filtered through a 0.1 mm sieve. The filtered homogenate was collected in a clean container, sterilized at 121°C for 20 min, and then cooled to 45 ± 0.5°C.
Before being used in the fermentation process, the probiotic strains were revived and propagated in MRS broth (for lactobacilli) or in BSM broth (for bifidobacteria) aliquots at 37 ± 0.5°C for 18 h. An 18-h broth culture was then centrifuged in an ultracentrifuge (model type 317a, Precision Mechanics Warsaw, Poland) for 5 min at 12,000×g at 4 ± 0.5°C. The number of viable bacterial cells in the culture broth was determined using the deep plate method (MRS agar for lactobacilli or BSM agar for bifidobacteria, 37 ± 0.5°C, 72 h, anaerobic conditions). The starter culture YC-180 was applied following the manufacturer’s instructions, including reconstitution in sterile water. The white bean homogenate samples were then stirred and maintained at 43 ± 0.5°C for 5 h to undergo the fermentation process. Subsequently, they were cooled to 4 ± 0.5°C to lower the temperature and stop the fermentation process.
The ice cream mixture was homogenized using a Kenwood HB714P hand blender (Kenwood Poland, Warsaw, Poland). The ice cream base (excluding the white bean homogenate) was then pasteurized (75°C/30 s) and cooled to 20 ± 0.5°C before incorporating the white bean homogenate. Subsequently, the ice cream mixture was poured into an Unold type 8875 ice cream maker (Unold Poland, Gdynia, Poland). The ice cream production process took 50 min and involved freezing the mixture to a temperature of –22 ± 0.5°C. The experiment was performed in three independent repetitions. All ice cream samples were analyzed immediately after freezing and again after 1, 2, 3, 4, 5, and 6 months of storage at –25 ± 0.5°C.
2.3. Media and other Reagents
The MRS broth, MRS agar, M17 agar, BSM broth, and BSM agar were prepared in the correct quantities following the manufacturer’s instructions (Merck, Darmstadt, Germany). All reagents required for the analytical methods described below were obtained from local chemical reagent distributors and were of analytical grade.
2.4. Methods
2.4.1. Basic Chemical Composition
Dry matter (DM) determination: Oven-drying at 102 ± 0.5°C until a constant weight was achieved was employed [
11] for DM determination. All analyses were performed in triplicate and reported to two decimal places.
Determination of protein content: The Kjeldahl method was used to determine protein content, with crude protein content calculated as
N × 6.25 [
11]. All analyses were performed in triplicate and reported to two decimal places.
Determination of fat content: The Gerber method was utilized to determine fat content, with all analyses performed in triplicate and reported to two decimal places.
Determination of selected carbohydrates (lactose, glucose, galactose, sucrose, and raffinose-series oligosaccharides, RSO) content: High-performance liquid chromatography (HPLC) was employed for carbohydrate analysis, following the protocol described in our previous publications [
11,
12,
13], with slight modifications. A HPLC system was utilized for chromatographic analysis, consisting of the following components: DeltaChrom Pump Injector (S6020 Needle Injection Valve, Sykam, Germany), DeltaChrom Temperature Control Unit (Sykam, Germany), refractive index detector (S3580 RI Detector, Sykam, Germany), precolumn Guard Column Sugar-D (10 mm × 4.6 mm, 5 µm; Cosmosil, Nacalai Tesque, Japan), and Column Sugar-D (250 mm × 4.6 mm, 5 µm; Cosmosil). The separation parameters for the HPLC analysis were set as follows: flow rate: 1 mL/min, oven temperature: 30 ± 0.1°C, detector range: 10,000 mV, sampling rate: 2 Hz. The mobile phase employed was a 60:40 (v/v) mixture of HPLC-grade acetonitrile (Sigma-Aldrich, St. Louis, MI USA) and deionized water. All analyses were performed in triplicate and reported to two decimal places.
2.4.2. pH Measurement
The pH of the ice cream samples was measured using an Elmetron CPO-505 pH meter (Elmetron, Zabrze, Poland). The samples were initially thawed at 18°C for 15 min, and the pH reading was recorded after approximately 30 s, once the value had stabilized. All analyses were performed in triplicate and reported to two decimal places.
2.4.3. Ice Cream Overrun
The ice cream overrun is determined by measuring the volume of air present in a given volume of ice cream sample. The air volume in the ice cream is calculated by subtracting the volume of the ice cream sample from the volume obtained from the melted ice cream sample. The overrun is expressed as the percentage ratio of the air volume to the volume of the melted ice cream mixture.
One day before performing the ice cream overrun determination, cylindrical metal containers with a volume of 150 mL were placed in a freezer (–30 ± 0.5°C) to chill thoroughly. The containers were then carefully filled with ice cream samples (ensuring no empty spaces, air bubbles, or pressing down on the ice cream). Using a funnel, the entire ice cream sample was transferred from the container to a 250 mL measuring flask. After the ice cream had completely melted, the measuring flask was filled to the mark using distilled water measured from a burette. All analyses were performed in triplicate and reported to two decimal places. The ice cream overrun was calculated according to formula (1):
where
a represents the volume of the ice cream sample [mL], which is 150 mL;
V1 represents the capacity of the measuring flask [mL], which is 250 mL;
V2 represents the volume of added distilled water [mL]; and
P represents the ice cream overrun [%].
2.4.4. Determination of the Survival of Yogurt and Probiotic Bacteria
The total viable counts of yogurt bacteria and probiotic strains were enumerated using the plate technique [
1]. De Man, Rogosa, and Sharpe (MRS) agar (Merck, Darmstadt, Germany) were used to determine the counts of lactobacilli, M17 agar (Merck) was used to determine the counts of streptococci, and BSM agar (Sigma-Aldrich) was used to determine the counts of bifidobacteria. The plates inoculated with diluted samples were incubated at 37 ± 0.5°C for 72 h under aerobic conditions (for streptococci) or anaerobic conditions (for lactobacilli and bifidobacteria) using Anaerocult (Merck). After incubation, colony-forming units (CFU) per gram of the original beverage sample were determined by counting colonies and expressing the results as log CFU/g. All analyses were performed in triplicate and reported to two decimal places.
2.4.5. Determination of Antioxidant Capacity
DPPH: The 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay serves as a valuable tool for quantifying the antioxidant potential of milk-based ice cream. This assay relies on antioxidants’ ability to scavenge the stable DPPH radical, causing a reduction in its characteristic purple color. The level of DPPH radical scavenging correlates directly with the sample’s antioxidant activity. To conduct the assay, 0.2 mM 1,1-diphenyl-2-picrylhydrazyl (DPPH) in ethanol (0.8 mL) was mixed with 0.2 mL aliquots of each sample at varying concentrations (100–5,000 μg/mL). The resulting mixtures were vortexed and left to incubate in darkness for 10 min at 25 ± 0.5°C. Subsequently, the absorbance of the samples was measured at 492 nm using a Helios Gamma UV–Vis spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). All analyses were performed in triplicate and reported to two decimal places. The percentage of DPPH radical scavenged (RSA) was calculated using the following formula (2):
where
A1 represents the absorbance of the sample solution at 492 nm;
A0 represents the absorbance of the blank solution (0.8 mL ethanol added to 0.2 mL of sample solution); and RSA indicates the Radical Scavenging Activity [%].
ABTS: ABTS (2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) is a common method used to measure antioxidant activity in various food samples. In this study, the total antioxidant capacity of ice cream samples was evaluated using the ABTS method as described by [
14], with slight modifications. Our aim was to quantify the ice cream’s overall ability to scavenge free radicals, particularly in ice cream containing fermented white bean homogenate and probiotic bacteria. To prepare the ABTS radical stock solution, potassium persulfate (2.6 mM, Sigma-Aldrich) was added to an aqueous ABTS solution (7 mM, Sigma-Aldrich). This mixture was stored in the dark at room temperature for 12–16 h. Subsequently, the stock solution was diluted with methanol (POCH, Gliwice, Poland) to achieve a final absorbance of approximately 1.1 ± 0.02 at 734 nm, forming the working solution. Ice cream samples (0.3 mL) were then combined with the working solution (2.7 mL) and left to incubate at room temperature for 30 min. After incubation, the mixtures underwent centrifugation at 12,000×g for 2 min at 20 ± 0.5°C. The decrease in absorbance at 734 nm was measured against a methanol blank using a Helios Gamma UV–Vis spectrophotometer (Thermo Fisher Scientific). All analyses were performed in triplicate and results were reported to two decimal places. The results were expressed as Trolox equivalent (TE) antioxidant capacity (TEAC) per 100 mL of ice cream samples, with Trolox® (Sigma-Aldrich) serving as the reference standard.
FRAP: The ferric reducing antioxidant power (FRAP) provides a complementary method to evaluate the overall antioxidant potential of ice cream, in conjunction with other assays such as DPPH and ABTS. FRAP specifically focuses on the capacity of antioxidant compounds to reduce metal ions, a common mechanism through which antioxidants neutralize free radicals. The FRAP assay, originally described by [
15], was employed to evaluate the antioxidant capacity of the samples. This assay hinges on the reduction of a tripyridyltriazine (TPTZ) complex, causing a color change in the presence of antioxidants. Fresh FRAP reagent was prepared by mixing 5 mL of 10 mmol/L TPTZ solution in 40 mmol/L HCl with 5 mL of 20 mmol/L FeCl
3 solution and 50 mL of 0.3 mol/L acetate buffer, maintaining it at 37 ± 0.5°C. Aliquots of sample supernatant (50 μL) were mixed with 1.5 mL of FRAP reagent and incubated at 37 ± 0.5°C for 10 min. The absorbance of the resultant mixture was then measured at 593 nm using a spectrophotometer. A calibration curve was constructed using eight FeSO
4×7H
2O concentrations (100–1000 mmol/L). The absorbance of the calibration standards was measured akin to the samples. Results were expressed as antioxidant concentration/100 mL of ice cream samples, with ferric reducing capacity equivalent to 1 mmol/L FeSO
4 [
15]. All analyses were triplicated and reported to two decimal places.
Total polyphenol content (TPC) using the Folin–Ciocalteu method: TPC is measured using a colorimetric assay that quantifies the reducing capacity of phenolic compounds, a type of antioxidant found in plants. TPC provides a measurement of the overall phenolic content, which can enhance the ice cream’s antioxidant. In addition, TPC values can reveal whether the fermented white bean homogenate and probiotic bacteria contribute to the ice cream’s phenolic content. A calibration curve was prepared using a standard solution of gallic acid (Pol–Aura, Poland, with reagent purity ≥99%). Calibration solutions of the acid were prepared in 25 mL measuring flasks with concentrations of 0, 0.5, 1.0, 1.5, 2.0, and 2.5 mg/mL. Absorbance was measured at a wavelength of 765 nm against a blank (0 mg/mL gallic acid solution) using plastic cuvettes (Bionovo Poland, Legnica, Poland) and a Helios Gamma UV–Vis spectrophotometer (Thermo Fisher Scientific). Based on the measured absorbances, a calibration curve was plotted and utilized to calculate the TPC in the analyzed ice cream samples in terms of gallic acid equivalents (GAE). After establishing the calibration curve, the absorbance of the ice cream samples’ test solutions was measured. Thawed ice cream samples (thawed at 18 ± 0.5℃ for 30 min) were weighed in 30 g portions into Falcon tubes, capped, and centrifuged using an MPW-350 laboratory centrifuge (MPW Med. Instruments, Spółdzielnia Pracy, Warsaw, Poland) at 10,000×g for 20 min at 4 ± 0.5°C. Following centrifugation, the tubes exhibited three layers: oil at the top, supernatant in the middle, and sediment at the bottom. 0.25 mL of the supernatant layer was withdrawn and transferred to 25 mL measuring flasks. Then, 15 mL of distilled water and 1.25 mL of Folin–Ciocalteu reagent were added, mixed, and left to stand for 3 min at room temperature. Subsequently, 3.75 mL of saturated sodium carbonate solution was added, the solution was topped up with distilled water, mixed, and placed in a 40℃ thermostat for 30 min before measuring the absorbance at a wavelength of 765 nm. The TPC in the ice cream was calculated based on the calibration curve and expressed in terms of gallic acid equivalent. All analyses were performed in triplicate and reported to two decimal places, with results expressed as GAE per 100 mL of ice cream samples.
2.5. Statistical Analysis
The entire experiment was conducted in three independent replicates, with each analysis performed in two replicates for each experiment, resulting in an average of nine replicates per result. The data underwent a two-way analysis of variance, and mean differences among the statistical groups were assessed at a significance level of α = 0.05 using Tukey’s test within the ANOVA method. Statistical analysis of the results was conducted using the Statgraphics Centurion XVII program (Statgraphics Technologies Inc., The Plains, VA, USA).
3. Results and Discussion
3.1. Basic Chemical Composition
Our research findings regarding the basic chemical composition align well with the expected properties of ice cream. The average protein content seems similar across all samples, ranging from 3.00% to 3.03% (
Table 2). Similarly, the fat content also appears comparable between the control and other samples, ranging from 5.59% to 5.66%. The dry matter content shows a slightly wider range, with the control sample having the highest value (26.92%) and ics1–ics6 samples having the lowest (26.60%). However, the error margins are large enough that there may not be statistically significant differences.
The lactose content remains consistent across all samples, ranging from 26.71 to 28.36 mg/kg (
Table 3). On the other hand, sucrose content is notably higher, ranging from 110.26 to 116.97 mg/kg. Statistically significant differences were observed between the ics4 sample and the other samples (ics1–ics3, ics5, ics6 samples), indicating a potential sucrose decomposition during ice cream production or storage due to enzymatic activity from the microorganisms used (white bean homogenate fermented by
L. rhamnosus GG was utilized in the ics4 samples). Glucose, the third most prevalent sugar, also displays slight variations among samples (ranging from 4.99 to 5.26 mg/kg). These sugars’ content is generally low, averaging below 0.15 mg/kg. The lowest glucose content was found in the ics2 sample with the addition of white bean homogenate fermented by
L. acidophilus La-5. Carbohydrates such as raffinose, stachyose, and verbascose had low contents, with only stachyose showing statistically significant differences between samples fermented with different probiotics.
Dry matter, primarily composed of milk solids–not–fat and sugars, provides the solid structure of ice cream [
16], preventing it from becoming excessively icy or runny. A higher dry matter content results in denser and firmer ice cream. Additionally, it aids in trapping air bubbles incorporated during churning, creating the light and fluffy texture we all enjoy. Insufficient dry matter would lead to the disappearance of these air bubbles, resulting in a dense, potentially icy product. Although milk protein constitutes a smaller portion of ice cream compared to other ingredients, it plays a crucial role [
17]. Acting like tiny whisks, milk proteins evenly distribute fat droplets throughout the mixture. Like dry matter, protein also helps stabilize air bubbles for a delightful texture. Our research demonstrates the advantage of using white bean homogenate, as it does not significantly reduce protein content, contributing to an optimal recipe formulation. While the protein content in our ice cream formulations may not qualify them as a significant source of dietary protein, the inclusion of white beans introduces additional fiber and other potentially health-promoting compounds.
Milk fat is another key ingredient that influences both the taste and quality of ice cream [
18,
19]. When freezing occurs, milk fat crystallizes, forming a network that traps air bubbles and hinders the formation of large ice crystals. Studies have shown that higher fat content results in lower overrun (less air incorporated) [
20]. Additionally, fat content can contribute to satiety, but the type of fat must be considered. Our emphasis on using healthy fats, such as those found in milk and white beans, can offer certain health benefits.
While fat, sugar content, and bacterial count do not directly affect ice cream’s acidity, they can influence its pH and viscosity [
21]
. Our research confirms that yogurt bacteria and probiotic strains generally have minimal impact on the overall carbohydrate content of ice cream. Although they might utilize some sugars during fermentation, the amount is negligible compared to the total sugar content. However, probiotics may influence the activity of enzymes that break down carbohydrates (such as alpha-amylase and alpha-glucosidase) [
22]. The cold storage temperature of ice cream significantly hinders bacterial activity. In fact, some research suggests that optimizing sugar concentration might even improve probiotic survival [
23].
Although there is limited data on the direct effects of probiotics and yogurt bacteria on the carbohydrate content of ice cream, there is potential for the metabolic properties of probiotics to impact the composition and characteristics of dairy products enriched with these microorganisms. Further investigation is necessary to thoroughly understand these effects, especially within the low-temperature environment of ice cream. Changes in fermentative activity could result in reduced levels of unfavorable carbohydrates, such as oligosaccharides that cause flatulence, through breakdown processes. Probiotics, like
L. plantarum, might influence fermentation in white bean products used as ice cream ingredients, potentially altering carbohydrate content by converting simple and complex sugars [
11,
13]. Incorporating probiotics into white bean-based products for ice cream formulation could impact their nutritional value, including carbohydrate content, by improving digestibility and potentially stimulating the growth of desirable bacteria that ferment dietary fiber and other carbohydrates [
24]. This suggests potential health benefits associated with the consumption of these ice creams, warranting further investigation.
3.2. pH Value
In our experiments, the ice cream recipe incorporating fermented white bean homogenate (ics1–ics6 samples) showed a statistically significant decrease in pH compared to the control samples (ics0) containing nonfermented white bean homogenate (
Table 6). However, most ice cream samples maintained stable pH values over the 6-month storage period, except for the ics2 and ics3 samples which exhibited variations. These observed pH variations might influence the survival of probiotics and the antioxidant properties of the ice cream, topics that will be discussed in the following sections.
The pH value of milk ice cream varies depending on ingredients and production methods, typically ranging from 6.0 to 6.8 [
21,
25,
26,
27,
28,
29]. This range has a significant impact on various aspects of ice cream quality. However, limited information is available on how pH directly affects features such as bacterial survival, overrun, carbohydrate content, or antioxidant properties in ice cream. While some probiotic strains thrive in acidic environments, others struggle [
30]. The pH level of fermented ice cream significantly impacts probiotic survival, with variations depending on the bacterial strains used. For example, L. acidophilus thrives in slightly acidic environments (pH 5.5–6.0), whereas bifidobacteria prefer a more neutral range (pH 6.0–7.0). L. acidophilus demonstrates better tolerance to acidity compared to bifidobacteria, which experience significantly slowed growth below pH 5.5. Furthermore, even among Bifidobacterium species, tolerance to acidity can vary between strains. Therefore, precise pH management during ice cream fermentation is crucial. If the pH falls below 5.5, it can significantly reduce viable probiotic bacteria.
While pH does not directly affect overrun, it does influence the structure and stability of the ice cream’s protein matrix, impacting its ability to retain air during mixing and freezing. Balanced pH is important for achieving the desired ice cream texture. The direct effect of pH on carbohydrate content is limited. However, pH value can affect the stability of bioactive compounds in ice cream, including their antioxidant properties. Lower pH levels generally tend to increase the antioxidant activity of certain ingredients, but this effect varies depending on the specific compound. pH values can also affect the availability of phenols and other antioxidants in foods.
3.3. Ice Cream Overrun
Overrun, which refers to the amount of air incorporated during churning, plays a crucial role in determining ice cream texture, volume, and consumer acceptance. Higher overrun results in a lighter texture but can also strain probiotics. In our experiments, the overrun of ice cream was significantly affected by the type of white bean homogenate and the probiotic strain used for fermentation (
Table 5). Notably, ice cream produced with white bean homogenate fermented with
L. acidophilus La-5 (ics2) and
L. plantarum 299v (ics3) exhibited the highest overrun. This suggests that both the composition of the white bean homogenate and the bacterial strains employed in fermentation have a significant impact on ice cream overrun.
While there is limited data on overrun specifically for dairy and probiotic ice cream, some general observations can be made. Studies have shown that incorporating plant milk into probiotic ice cream without fermentation can improve sensory aspects and probiotic survival [
31]. Additionally, substituting skimmed milk with sweet potatoes in probiotic ice cream has been found to have no significant impact on overrun [
32]. Conversely, other research has found no impact of probiotic addition on overrun [
33,
34]. Further research is necessary to determine the exact mechanisms through which white bean homogenate and lactic acid bacteria influence ice cream overrun. Our findings suggest that overrun plays a crucial role in balancing the desired textural properties of probiotic ice cream with the viability of probiotic cultures, thereby impacting the potential health benefits for consumers. While higher overrun contributes to a lighter texture, it may also strain the probiotics, potentially reducing their survival during processing and storage [
35]. Previous research has highlighted the sensitivity of
Bifidobacterium bacteria to oxygen in dairy products [
36,
37,
38,
39]. While further research is required to determine the exact mechanisms, our research supports the idea that ice cream with lower overrun could provide a more conducive environment for probiotic survival [
40,
41]. Our study aligns with these observations by showing that ice cream with lower overrun levels tended to maintain a higher number of viable probiotic bacteria after processing and storage compared to those with high overrun. Future studies can investigate the interplay between overrun, probiotic strains, and ice cream formulation to optimize both sensory qualities and potential health benefits.
3.4. Survival of Yoghurt and Probiotic Bacteria
Probiotic ice cream offers a novel approach to delivering beneficial bacteria to the gut, potentially merging enjoyment with probiotic supplementation [
31,
42,
43]. Maintaining probiotic viability during processing and storage, while maintaining sensory and nutritional qualities, is crucial for these functional ice creams.
Table 4 shows the viability (colony forming units, CFU) of yogurt and probiotic bacteria strains in ice cream throughout the production process and during frozen storage. The data is expressed in logarithmic units (log CFU/mL) for easier comparison. The result confirms that the ice cream production process itself does not introduce any contaminating bacteria. All probiotic strains (ics1–ics6 samples) showed a statistically significant decrease in viability over the storage period. However, freezing the ice cream appears to have minimal impact on the initial bacterial count for most strains (when comparing values at 0 months). Overall, the results suggest that probiotic bacteria can survive in ice cream during production and frozen storage, although their viability declines over the 6-month storage period. The rate of decline varies among strains, as expected due to freezing and storage stress.
L. casei DN-114001 (ics5 samples) displayed the highest resilience, whereas
L. delbrueckii subsp.
bulgaricus (ics1 samples) showed the most significant decline. This data allows for comparing the survival of different probiotic strains (ics1–ics6 samples) in ice cream, which is valuable for selecting appropriate strains for ice cream products. Our findings regarding probiotic survival during storage, which generally exceeded the recommended minimum of 6 log CFU/g, suggest that this ice cream formulation holds promise for delivering viable probiotics to the gut, potentially enhancing health benefits. The observed differences in probiotic survival rates suggest that selecting strains with higher viability after storage (such as
L. plantarum 299v) could enhance the potential health benefits associated with consuming probiotic ice cream.
Previous research has shown that probiotics can survive in ice cream for up to 6 months when stored frozen at temperatures ranging from –18 to –28°C, with viable cell counts exceeding the recommended minimum of 6 log CFU/g [
23,
27,
44]. Factors influencing survival include the type of strain, production methods, storage temperature and duration, and the composition of the product (including bulking agents, sweeteners, and fat content) [
30]. Our findings align with Salem et al. [
41], who observed a decrease in live cell counts for various probiotic strains in ice cream stored at –26°C. Despite this decline, their ice cream remained a probiotic food with viable cell counts above the minimum threshold. Similarly, our study showed a decrease in live cell counts, albeit with different bacterial strains and ice cream formulations, highlighting the influence of both bacterial and ice cream composition on survival rates. Further research involving a wider range of formulations and storage conditions could provide additional insights into how these variables influence probiotic viability.
3.5. Antioxidant Capacity
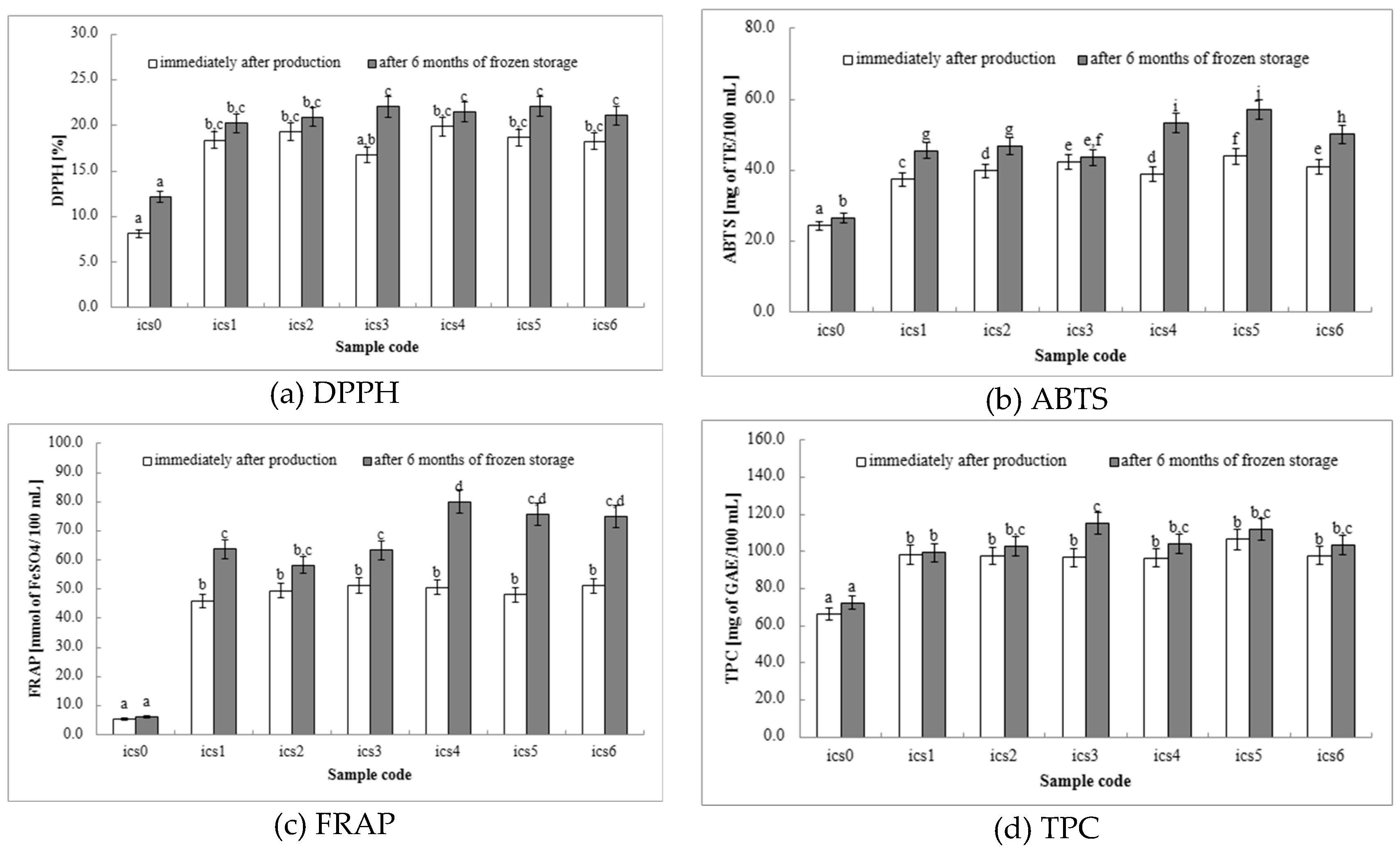
DPPH: The DPPH assay measures a product’s ability to neutralize free radicals, which are harmful molecules linked to cellular damage and various diseases. Milk-based ice cream containing fermented white bean homogenate serves as a source of antioxidant compounds, including polyphenols and proteins derived from milk, white beans, and other ingredients. Through the DPPH assay, we can gauge the effectiveness of these compounds in scavenging free radicals. A higher level of antioxidant activity suggests that the ice cream might provide health benefits by protecting cells against oxidative stress. All ice cream samples with fermented white bean homogenate (ics1–ics6 samples) exhibited significantly higher DPPH activity compared to the control group (ics0 samples) at both time points (
Figure 1a), indicating that fermented white bean homogenate successfully enhances the ice cream’s antioxidant properties. Among the fermented white bean homogenate samples (ics1–ics6 samples), no statistically significant differences in DPPH values were observed after production or after 6 months of storage, except for the sample with
L. plantarum 299v (ics3 samples), where storage time had a significant effect. Our findings demonstrate that incorporating fermented white bean homogenate with probiotic bacteria into milk-based ice cream represents a promising approach for enhancing its antioxidant potential. This increase in antioxidant activity suggests potential health benefits, such as reducing cellular damage associated with oxidative stress. Further research could explore the specific contributions of different bean types, fermentation parameters, and storage conditions on the overall antioxidant profile of the ice cream.
ABTS: The DPPH and ABTS assays measure a product’s ability to neutralize free radicals, which can damage cells and contribute to various diseases. These assays help evaluate the efficacy of these compounds in scavenging free radicals. Higher antioxidant activity suggests potential health benefits by protecting cells from oxidative stress. In the DPPH assay, all ice cream samples with fermented white bean homogenate (ics1–ics6 samples) exhibited significantly higher ABTS values than the control (ics0 samples) both during production and after 6 months of storage (
Figure 1b). This affirms that fermented white bean homogenate effectively enhances the ice cream’s overall antioxidant capacity. Interestingly, the ABTS assay suggests some variation in the impact of different probiotic strains.
L. rhamnosus GG (ics4 samples) and
L. casei DN-114001 (ics5 samples) showed the highest values, while
L. plantarum 299v (ics3 samples) had a smaller increase. This suggests these strains might have a more substantial impact on the overall antioxidant profile, warranting further investigation into the specific mechanisms and compounds responsible. Our findings demonstrate that incorporating fermented white bean homogenate with probiotic bacteria is a promising strategy to improve the antioxidant potential of milk-based ice cream. This increase in antioxidant activity, as measured by both DPPH and ABTS assays, suggests potential health benefits by potentially reducing cellular damage caused by free radicals. Further research could explore the reasons for the observed strain-specific differences and identify the specific components contributing to the ice cream’s antioxidant properties.
FRAP: As observed in the DPPH and ABTS assays (sections 3.5.1 and 3.5.2), all ice cream samples containing fermented white bean homogenate (ics1–ics6 samples) exhibited significantly higher FRAP values compared to the control (ics0 samples) both at production and after 6 months of storage (
Figure 1c). This confirms that fermented white bean homogenate effectively enhances the ice cream’s overall antioxidant capacity, as measured by all three assays (DPPH, ABTS, and FRAP). Interestingly, the FRAP assay suggests some variability in the impact of different probiotic strains. Apart from ics0 and ics2, all samples showed increased FRAP values after storage, with
L. rhamnosus GG (ics4 samples) showing the most significant increase. This suggests that
L. rhamnosus GG may have a more significant impact on ferric reducing power, potentially by influencing the types of antioxidant compounds present in the ice cream. These findings, however, differ somewhat from the observations related to DPPH values, highlighting the potential for strain-specific effects on various aspects of antioxidant activity. Further investigation is warranted to identify the mechanisms driving these differences and to optimize probiotic selection for maximizing the antioxidant benefits of probiotic ice cream. The increased ferric reducing power observed with the FRAP assay suggests a potential for these ice creams to contribute to increased antioxidant activity in the body, potentially reducing cellular damage caused by free radicals.
TPC:
Figure 1d displays the TPC, expressed in milligrams of Gallic Acid Equivalents (mg GAE) per 100 mL of ice cream. All ice cream samples containing fermented white bean homogenate (ics1–ics6 samples) exhibited significantly higher TPC values compared to the control group (ics0 samples) both at production and after 6 months of storage. This indicates a greater abundance of phenolic compounds in the ice cream with added probiotic bacteria. The control group showed minimal changes in TPC, suggesting a low inherent phenolic content. These findings suggest that all yogurt and probiotic strains (ics1–ics6 samples) contribute to the ice cream’s overall phenolic content, potentially due to the presence of phenolics in the fermented white bean homogenate itself or those produced during fermentation. Interestingly, ics3 samples (
L. plantarum 299v fermentation) showed the most substantial and statistically significant increase in TPC at 6 months. This suggests that
L. plantarum 299v might have a more significant impact on the overall phenolic content, warranting further investigation into the specific types of phenolics produced and their contribution to antioxidant activity. The increased phenolic content, as measured by TPC, suggests a greater potential for antioxidant activity. Phenolic compounds can function as free radical scavengers, potentially conferring health benefits by reducing oxidative stress in the body. Further research could identify the specific types of phenolic compounds present in the ice cream and determine their individual contributions to the overall antioxidant profile.
The antioxidant capacity of ice cream is influenced by the presence and concentration of ingredients known for their antioxidant properties. Milk-based ice creams derive benefits from the inherent nutrients in milk, such as vitamins and proteins, while plant-based options often utilize fruits, nuts, and seeds that are rich in natural antioxidants, including vitamins and phenolics [
31,
45,
46,
47,
48]. Our study specifically focused on milk-based ice cream and investigated how incorporating fermented white bean homogenate with probiotic bacteria could enhance its antioxidant capacity compared to regular ice cream. Fermented white bean homogenate is likely to contribute natural antioxidants like polyphenols (e.g., flavonoids, phenolic acid) and proteins (e.g., albumins, globulins, and phaseolin) [
49,
50,
51,
52]. Processes like soaking, sprouting, and fermentation can influence the polyphenol content of white beans, with fermentation potentially converting complex polyphenols into simpler, more bioavailable forms [
9,
10]. Studies on other legumes, such as soybeans, suggest that fermentation can influence enzymatic activities and potentially affect polyphenol content [
53].
All assays (DPPH, ABTS, FRAP, TPC) demonstrated a significant increase in antioxidant activity in ice cream containing fermented white bean homogenate compared to the control. Fermentation can lead to the formation of bioactive compounds, including plant-derived polyphenols known for their antioxidant properties [
54,
55,
56]. White beans naturally contain polyphenols, and fermentation by lactic acid bacteria or bifidobacteria may release these compounds, potentially increasing the overall antioxidant capacity as observed in fermented soy milk [
57]. This suggests a greater ability to neutralize free radicals and potentially protect cells from oxidative stress.
It is important to consider factors influencing antioxidant capacity, such as the specific probiotic strain, fermentation conditions, and bean quality. L. rhamnosus GG (ics4 samples) consistently displayed high values across most assays and showed the greatest increase in FRAP over storage, suggesting a significant impact. Further research is necessary to identify the specific components responsible for the observed increase in antioxidant activity and how this process affects the health benefits of fermented white bean homogenate in ice cream. Storage time generally led to an increase in antioxidant activity as measured by ABTS and FRAP, with the exception of DPPH values. This suggests potential benefits from ongoing fermentation processes even during storage. The overall increase in antioxidant capacity observed in our study suggests potential health benefits for consumers, such as reduced cellular damage from free radicals.
4. Conclusion
This study investigated the viability of probiotic bacteria in ice cream enriched with fermented white bean homogenate during storage. Although all strains showed a decrease in viability over 6 months, certain strains, particularly L. plantarum 299v and L. casei DN-114001, demonstrated promising survival rates. These findings suggest the potential of probiotic ice cream to deliver viable bacteria to the gut, potentially influencing gut microbiota composition and promoting digestive health, reducing inflammation, and enhancing immune system function, which are established benefits associated with probiotic consumption.
The incorporation of fermented white bean homogenate significantly increased the overall antioxidant activity of the ice cream, as measured by DPPH, ABTS, and FRAP assays. Interestingly, L. rhamnosus GG and L. plantarum 299v exhibited a more pronounced effect on the ice cream’s antioxidant properties. This suggests a potential synergistic effect between probiotic strains and the bioactive compounds found in fermented white beans. The increased antioxidant activity could provide additional health benefits by potentially mitigating oxidative stress, a factor linked to various chronic diseases. The combination of viable probiotic bacteria and increased antioxidant activity derived from fermented white beans in this ice cream formulation shows promise in supporting gut health and mitigating oxidative stress.
While this study demonstrates the potential health benefits of probiotic ice cream containing fermented white bean homogenate, further research is necessary to fully understand the specific health effects and determine the optimal dosage for consumption. Optimizing probiotic survival during storage and identifying strains with maximized viability and contributions to antioxidant activity is crucial for maximizing potential health benefits. Finally, exploring the sensory attributes and consumer acceptance of this functional ice cream is essential for its successful commercialization.
Author Contributions
Conceptualization, M.Z.; formal analysis, M.Z., P.C., E.K., and D.Z.; investigation, M.Z., P.C., E.K., and D.Z.; writing—original draft preparation, M.Z. and D.Z.; writing—review and editing, M.Z., and D.Z.; visualization, M.Z., P.C., E.K., and D.Z.; supervision, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author (M.Z.) upon reasonable request.
Acknowledgments
The authors gratefully acknowledge the Institute of Food Sciences of Warsaw University of Life Sciences WULS–SGGW for supporting and providing the necessary infrastructure and research staff.
Conflict of Interest
The authors declare no conflict of interest.
References
- Gasheva, M.A. Selection Of Probiotic Starter Cultures For The Production Of Fermented Ice Cream With Prescribed Functional Properties. New Technol. 2022, 18, 17–23. [Google Scholar] [CrossRef]
- Wang, W.; Wang, M.; Xu, C.; Liu, Z.; Gu, L.; Ma, J.; Jiang, L.; Jiang, Z.; Hou, J. Effects of Soybean Oil Body as a Milk Fat Substitute on Ice Cream: Physicochemical, Sensory and Digestive Properties. Foods 2022, 11, 1504. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Arslaner, A.; Salık, M.A. Functional Ice Cream Technology. Agric. Food Sci. 2020. [Google Scholar] [CrossRef]
- Hayat, I.; Ahmad, A.; Masud, T.; Ahmed, A.; Bashir, S. Nutritional and Health Perspectives of Beans ( Phaseolus Vulgaris L.): An Overview. Crit. Rev. Food Sci. Nutr. 2014, 54, 580–592. [Google Scholar] [CrossRef]
- Abirami; Kaur, J. Kidney Beans (Phaseolus Vulgaris L.) Its Nutrient Profile, Health Benefits, Value–Added Products and Anti–Nutritional Properties. Pharma Innov. 2023, 12, 1524–1528. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol–Rich Dry Common Beans (Phaseolus Vulgaris L.) and Their Health Benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef] [PubMed]
- Orwat, J. Phenolic Antioxidant–Linked Bioactive Enrichment in Black Beans (Phaseolus Vulgaris l.) to Screen for Health Benefits and Enhancement of Salinity Resilience.; 2016.
- Ongol, M.P.; Owino, J.; Lung’aho, M.; Dusingizimana, T.; Vasanthakaalam, H. Micro–Mineral Retention and Anti–Nutritional Compounds Degradation During Bean Cooking Process. Curr. Res. Nutr. Food Sci. J. 2018, 6, 526–535. [Google Scholar] [CrossRef]
- Sharma, R.; Diwan, B.; Singh, B.P.; Kulshrestha, S. Probiotic Fermentation of Polyphenols: Potential Sources of Novel Functional Foods. Food Prod. Process. Nutr. 2022, 4, 21. [Google Scholar] [CrossRef]
- Ziarno, M.; Zaręba, D.; Maciejak, M.; Veber, A.L. The Impact of Dairy Starter Cultures on Selected Qualitative Properties of Functional Fermented Beverage Prepared from Germinated White Kidney Beans. J. Food Nutr. Res. 2019, 58, 167–176. [Google Scholar]
- Kowalska, E.; Maliszewska, B.; Ziarno, M. Characterization of Fermented Milks After the Passaging Process of Starter Cultures. Postępy Tech. Przetwórstwa Spoż. 2021, 2, 11–22. [Google Scholar]
- Cichońska, P.; Ziarno, M. Effect Of Germination On Content Of Selected Carbohydrates And Total Polyphenols In White Kidney Bean Beverages. Zywnosc Nauka Technol. JakoscFood Sci. Technol. Qual. 2021, 128, 86–94. [Google Scholar] [CrossRef]
- ERTAN, K.; BAYANA, D.; GÖKÇE, O.; ALATOSSAVA, T.; YILMAZ, Y.; GÜRSOY, O. Total Antioxidant Capacity and Phenolic Content of Pasteurized and UHT–Treated Cow Milk Samples Marketed in Turkey. Akad. Gıda 2017, 15, 103–108. [Google Scholar] [CrossRef]
- Sadeghi, N.; Behzad, M.; Jannat, B.; Oveisi, M.R.; Hajimahmoodi, M.; Kashanipour, A.H. Total Phenolic Compounds Content and Antioxidant Activity in Packed and Bulk Milk in Different Regions of Tehran, Iran. J Food Safe Hyg 2018, 4, 8–12. [Google Scholar]
- Tong, P.S.; Berner, L.A. Dairy Processing and Products. In Reference Module in Food Science; Elsevier, 2016 ISBN 978–0–08–100596–5.
- Goff, H.D. Milk Proteins in Ice Cream. In Advanced Dairy Chemistry: Volume 1B: Proteins: Applied Aspects; McSweeney, P.L.H., O’Mahony, J.A., Eds.; Springer: New York, NY, 2016; pp. 329–345. ISBN 978–1–4939–2800–2. [Google Scholar]
- Małkowska, M.; Staniewski, B.; Ziajka, J. Analyses of Milk Fat Crystallization and Milk Fat Fractions. Int. J. Food Prop. 2021, 24, 325–336. [Google Scholar] [CrossRef]
- Méndez–Velasco, C.; Goff, H.D. Fat Structure in Ice Cream: A Study on the Types of Fat Interactions. Food Hydrocoll. 2012, 29, 152–159. [Google Scholar] [CrossRef]
- Alamprese, C.; Foschino, R.; Rossi, M.; Pompei, C.; Savani, L. Survival of Lactobacillus Johnsonii La1 and Influence of Its Addition in Retail–Manufactured Ice Cream Produced with Different Sugar and Fat Concentrations. Int. Dairy J. 2002, 12, 201–208. [Google Scholar] [CrossRef]
- Turgut, T.; Cakmakci, S. Investigation of the Possible Use of Probiotics in Ice Cream Manufacture. Int. J. Dairy Technol. 2009, 62, 444–451. [Google Scholar] [CrossRef]
- Talearngkul, R.; Sae–tan, S.; Sirivarasai, J. Effect of Yogurt Ice Cream on the Viability and Antidiabetic Potential of the Probiotics Lactobacillus Acidophilus, Lacticaseibacillus Rhamnosus, and Bifidobacterium Animalis Subsp. Lactis after In Vitro Digestion. Foods 2023, 12, 4373. [Google Scholar] [CrossRef]
- Akın, M.B.; Akın, M.S.; Kırmacı, Z. Effects of Inulin and Sugar Levels on the Viability of Yogurt and Probiotic Bacteria and the Physical and Sensory Characteristics in Probiotic Ice–Cream. Food Chem. 2007, 104, 93–99. [Google Scholar] [CrossRef]
- Purwijantiningsih, E. Pengaruh Jenis Prebiotik Terhadap Kualitas Yogurt Probiotik. Biota J. Ilm. Ilmu–Ilmu Hayati 2007, 177–185. [Google Scholar] [CrossRef]
- Hidayat, S.; Z, W.N.H.; Kuntoro, B. Melting Rate, pH and Glucose Content of Goat Milk Ice Cream Stabilize by Grass Jelly Leaves Gel (Cyclea Barbata Miers) in Different Concentration. J. Peternak. 2019, 16, 61–65. [Google Scholar] [CrossRef]
- Saputro, A.E.; Yanti, R.; Rahayu, E.S. Microbiological, Physicochemical, and Sensory Characters of Synbiotic Ice Cream from Fermented Milk Using Lactiplantibacillus Plantarum Subsp. Plantarum Dad–13 Combined with Inulin. Curr. Res. Nutr. Food Sci. J. 2023, 11, 1363–1373. [Google Scholar] [CrossRef]
- Favaro–Trindade, C.S.; Bernardi, S.; Bodini, R.B.; De Carvalho Balieiro, J.C.; De Almeida, E. Sensory Acceptability and Stability of Probiotic Microorganisms and Vitamin C in Fermented Acerola (Malpighia Emarginata DC.) Ice Cream. J. Food Sci. 2006, 71, S492–S495. [Google Scholar] [CrossRef]
- Haynes, I.N.; Playne, M.J. Survival of Probiotic Cultures in Low–Fat Ice–Cream. Aust. J. Dairy Technol. 2002, 57, 10. [Google Scholar]
- Miano, T.F. Effect Of Various Ingredients On The Physico Chemical Properties Of Ice Cream. Pak. J. Sci. 2021, 73. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mortazavian, A.M.; Khosrokhavar, R.; da Cruz, A.G. Probiotic Ice Cream: Viability of Probiotic Bacteria and Sensory Properties. Ann. Microbiol. 2011, 61, 411–424. [Google Scholar] [CrossRef]
- Elsamani, M.O. Probiotics,Organoleptic and Physicochemical Properties of Vegetable Milk Based Bio–Ice Cream Supplemented with Skimmed Milk Powder. Int. J. Nutr. Food Sci. 2016, 5, 361–366. [Google Scholar] [CrossRef]
- Nurliyani, N.; Indratiningsih, I.; Putri, K.B. Substitution Effect of Skim Milk With Sweet Potato (Ipomoea Batatas) on Probiotic Ice Cream Characteristics. Agric. Food Sci. 2013. [Google Scholar]
- Abghari, A.; Sheikh–Zeinoddin, M.; Soleimanian–Zad, S. Nonfermented Ice Cream as a Carrier for Lactobacillus Acidophilus and Lactobacillus Rhamnosus. Int. J. Food Sci. Technol. 2011, 46, 84–92. [Google Scholar] [CrossRef]
- Alamprese, C.; Foschino, R.; Rossi, M.; Pompei, C.; Corti, S. Effects of Lactobacillus Rhamnosus GG Addition in Ice Cream. Int. J. Dairy Technol. 2005, 58, 200–206. [Google Scholar] [CrossRef]
- Ferraz, J.L.; Cruz, A.G.; Cadena, R.S.; Freitas, M.Q.; Pinto, U.M.; Carvalho, C.C.; Faria, J.A.F.; Bolini, H.M.A. Sensory Acceptance and Survival of Probiotic Bacteria in Ice Cream Produced with Different Overrun Levels. J. Food Sci. 2012, 77, S24–28. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.–B.; Hwang, H.–J.; Park, J.–H. Physiological Responses of Oxygen–Tolerant Anaerobic Bifidobacterium Longum under Oxygen. J. Microbiol. Biotechnol. 2001, 11, 443–451. [Google Scholar]
- Bolduc, M.–P.; Raymond, Y.; Fustier, P.; Champagne, C.P.; Vuillemard, J.–C. Sensitivity of Bifidobacteria to Oxygen and Redox Potential in Non–Fermented Pasteurized Milk. Int. Dairy J. 2006, 16, 1038–1048. [Google Scholar] [CrossRef]
- Cruz, A.G.; Cadena, R.S.; Faria, J.A.F.; Bolini, H.M.A.; Dantas, C.; Ferreira, M.M.C.; Deliza, R. PARAFAC: Adjustment for Modeling Consumer Study Covering Probiotic and Conventional Yogurt. Food Res. Int. 2012, 45, 211–215. [Google Scholar] [CrossRef]
- Fortin, M.–H.; Champagne, C.P.; St–Gelais, D.; Britten, M.; Fustier, P.; Lacroix, M. Effect of Time of Inoculation, Starter Addition, Oxygen Level and Salting on the Viability of Probiotic Cultures during Cheddar Cheese Production. Int. Dairy J. 2011, 21, 75–82. [Google Scholar] [CrossRef]
- Goktas, H.; Dikmen, H.; Bekiroglu, H.; Cebi, N.; Dertli, E.; Sagdic, O. Characteristics of Functional Ice Cream Produced with Probiotic Saccharomyces Boulardii in Combination with Lactobacillus Rhamnosus GG. LWT 2022, 153, 112489. [Google Scholar] [CrossRef]
- Salem, M.M.E.; Fathi, F.A.; Awad, R.A. Production Of Probiotic Ice Cream. Pol. J. Food Nutr. Sci. 2005, 55, 267–271. [Google Scholar]
- Pimentel, T.C.; de Oliveira, L.I.G.; de Souza, R.C.; Magnani, M. Probiotic Ice Cream: A Literature Overview of the Technological and Sensory Aspects and Health Properties. Int J Dairy Technol 2022, 75, 59–76. [Google Scholar] [CrossRef]
- Aboulfazli, F.; Shori, A.B.; Baba, A.S. Effects of the Replacement of Cow Milk with Vegetable Milk on Probiotics and Nutritional Profile of Fermented Ice Cream. LWT 2016, 70, 261–270. [Google Scholar] [CrossRef]
- Kailasapathy, K.; Sultana, K. Survival and [Beta]–D–Galactosidase Activity of Encapsulated and Free Lactobacillus Acidophilus and Bifidobacterium Lactis in Ice–Cream. Aust. J. Dairy Technol. 2003, 58, 223. [Google Scholar]
- Patil, A.G.; Banerjee, S. Variants of Ice Creams and Their Health Effects. MOJ Food Process. Technol. 2017, 4, 58–64. [Google Scholar] [CrossRef]
- Kumari, J.; Dubey, R.P. Development of Nutritious Ice–Creams from Soymilk Andpumpkin Seed Milk and Evaluation of Their Acceptability. Food Sci Res J 2016, 7, 96–100. [Google Scholar] [CrossRef]
- Taspinar, T.; Yazici, G.N.; Güven, M. Evaluating the Potential of Using Plant–Based Milk Substitutes in Ice Cream Production. Biol. Life Sci. Forum 2023, 26, 21. [Google Scholar] [CrossRef]
- Aboulfazli, F.; Baba, A.S.; Misran, M. The Rheology and Physical Properties of Fermented Probiotic Ice Creams Made with Dairy Alternatives. Int. J. Food Eng. 2015, 11, 493–504. [Google Scholar] [CrossRef]
- Giusti, F.; Capuano, E.; Sagratini, G.; Pellegrini, N. A Comprehensive Investigation of the Behaviour of Phenolic Compounds in Legumes during Domestic Cooking and in Vitro Digestion. Food Chem. 2019, 285, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.J.; Tako, E.; Kochian, L.V.; Glahn, R.P. Identification of Black Bean (Phaseolus Vulgaris L.) Polyphenols That Inhibit and Promote Iron Uptake by Caco–2 Cells. J. Agric. Food Chem. 2015, 63, 5950–5956. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Sathe, S.K.; Salunkhe, D.K.; Cornforth, D.P. Effects of Dehulling on Phytic Acid, Polyphenols, and Enzyme Inhibitors of Dry Beans (Phaseolus Vulgaris L.). J. Food Sci. 1982, 47, 1846–1850. [Google Scholar] [CrossRef]
- Kajiwara, V.; Moda–Cirino, V.; dos Santos Scholz, M.B. Studies on Nutritional and Functional Properties of Various Genotypes of Andean Beans. J. Food Sci. Technol. 2022, 59, 1468–1477. [Google Scholar] [CrossRef]
- de Reu, J. c.; Rombouts, F. m.; Nout, M. j. r. Influence of Acidity and Initial Substrate Temperature on Germination of Rhizopus Oligosporus Sporangiospores during Tempe Manufacture. J. Appl. Bacteriol. 1995, 78, 200–208. [Google Scholar] [CrossRef]
- Saranraj, P. Lactic Acid Bacteria and Its Antimicrobial Properties A Review. Int. J. Pharm. Biol. Arch. 2014. [Google Scholar]
- Verni, M.; Rizzello, C.G. The Antioxidant Potential of Fermented Foods: Challenges and Future Trends. Fermentation 2023, 9, 790. [Google Scholar] [CrossRef]
- Aziz, T.; Xingyu, H.; Sarwar, A.; Naveed, M.; Shabbir, M.A.; Khan, A.A.; Ulhaq, T.; Shahzad, M.; Zhennai, Y.; Shami, A.; et al. Assessing the Probiotic Potential, Antioxidant, and Antibacterial Activities of Oat and Soy Milk Fermented with Lactiplantibacillus Plantarum Strains Isolated from Tibetan Kefir. Front. Microbiol. 2023, 14, 1265188. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. –C.; Yu, R.–C.; Chou, C.–C. Antioxidative Activities of Soymilk Fermented with Lactic Acid Bacteria and Bifidobacteria. Food Microbiol. 2006, 23, 128–135. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).