1. Introduction
The anthropogenic climate change driven amplification of wildfire severity and frequency has been extensively experienced worldwide during the recent years, while also probed in scientific literature (Brown et al., 2023). The ascending global temperatures, extreme heatwaves and prolonged drought periods due to shifting precipitation dynamics are consequently positively correlated with an elevated susceptibility to wildfires, particularly in regions predisposed to arid conditions (Masson-Delmotte et al., 2022). Wildfires result in ecological catastrophe, human life loses and emerging long term public health impacts due to air pollution, and substantial economic loses (Aguilera et al., 2021; Thomas et al., 2017). It is therefore an imperative need to understand the causes of wildfires, as well as the effects posed on burned environments, and most importantly burned soils, in order to facilitate environmental regeneration and the long-term sustainability of burned environments (Souza-Alonso et al., 2022; Vogler et al., 2015).
A great number of factors affect the properties and function of soil after fire. Extensive reviews of the changes in soil physical, chemical and biological properties caused by fire have been carried out, highlighting the crucial impact of fire intensity on exerted changes (Certini, 2005; Verma and Jayakumar, 2012). Immediate effects concern the destruction or killing of part of the microbial microflora due to elevated temperatures and the loss of soil water (Francos et al., 2018a; Xue et al., 2014). Intermediate and long-term effects on burned soils reflect the interaction between the initial degree of change, revegetation, management practices and weather conditions on the one hand, and soil properties and topography on the other (Francos et al., 2018b; Francos Quijorna, 2019).
Particular attention has been paid on soil microbiological changes after fire. These changes may be reasonably explained by soil organic matter (SOM) loss, SOM transformation, soil structural changes that result in new surfaces to microbes, ash build up and pH increase (Castaldi et al., 2010). In turn, modified microbial composition and populations by fire may have significant impact on soil ecosystem processes and services, since the microbial biomass and activity act as a source or sink for labile nutrients and controls, among others, the overall content and chemical fractions of SOM (Duxbury et al., 1991; Singh et al., 1989).
The impacts of wildfire on soil biota diversity and activity are well elucidated (Certini et al., 2021). Wildfire in Spain resulted in significant bacteria population increase, whereas cyanobacteria and fungi population was diminished (Vázquez et al., 1993). As far as the fungal phyla and fungal functional groups is concerned, basidiomycetes and mycorrhizal taxa displayed particularly low tolerances in relation to fire severity in Alaska (Holden et al., 2016). Goberna et al. (2012) showed that archaea, bacteria and fungi responded differently following a Mediterranean shrublands wildfire, with the changes mainly reflected at the community structure rather the total number of microbial groups. Some microbial groups not only resisted but even benefited from fire, probably due to the increased abundance in labile forms of C, N and other macronutrients. While archaea decreased few days after fire, bacterial populations and microbial biomass were increased compared with pre-fire conditions (Goberna et al., 2012).
The study of soil respiration provides a powerful tool for assessing soil function and ecosystem services. Respiration is an index of nutrients contained in organic matter being converted to forms available to crops (e.g., nitrates, phosphates, sulfates). Soil respiration rates indirectly shows the level of soil fertility and functionality (Yiqi and Zhou, 2010). Therefore, soil respiration rates are constantly evaluated as a key indicator during soil quality and degradation studies (Paz-Ferreiro and Fu, 2016), and consequently constitute a key indicator in forest ecological restoration monitoring and research (Gatica-Saavedra et al., 2023).
Soil basal respiration was significantly lower in burned stands compared to unburned ones in a boreal forest ecosystem, with the deterioration being positively correlated with fire severity (Holden et al., 2016). Moreover, Castaldi et al. (2010) showed that the average daily field CO2 emissions were significantly lower in burned compared with the control plots one month after a wildfire in a tropical forest. Though, the difference between burned and unburned plots did not remain statistically significant eight months after fire (Castaldi et al., 2010). Prescribed fire did not exert any significant impacts on soil respiration, since a similar evolution between the burned and control plots in both the mixed and pure forest stands was shown (Plaza-Álvarez et al., 2017). Similarly, burning in a conifer site did not significantly affected soil respiration rates compared with unburned controls (Concilio et al., 2005). At the other end, soil respiration rates were even shown to be increased following wildfires, as increased rates of CO2 release were measured in the burnt areas compared with those in the unburnt control sites, with the differences being highly depended on the type of vegetation cover and time since fire (Fest et al., 2015; Muñoz-Rojas et al., 2016). Likewise, Tufeckioglu et al. (2010) showed that prescribed fire resulted in increased soil respiration rates in a Pinus brutia stand, compared with the control ones.
Nevertheless, studies measuring soil respiration rates in the field cannot easily isolate the contribution of heterotrophic soil microbial respiration from the autotrophic component of the soil respiration (plant roots) and CO2 release from litter decomposition (Wang et al., 2014). Estimates of basal respiration using soil incubations may overcome problems of spatial soil heterogeneity and variability related to the patchy distribution of vegetation (especially in Mediterranean type ecosystems) and perplexity due to after-fire changes of soil temperature and moisture regimes, which significantly affect soil CO2 fluxes (Yan et al., 2018).
The present study was carried out following a short duration but highly destructive wildfire in a mountainous area of Cyprus, and it is based on laboratory analyses and incubations. Microbial respiration was estimated few days after fire aiming at identifying the capacity of the burned soil to decompose soil organic matter. Measurements immediately after fire were also compared with respiration rates six months later. The effect of fire on microbial characteristics was also investigated by measuring soil respiration after fire ash application (with ash being known to contain macro and micro nutrients needed for microbial growth), and by measuring CO2 release after mixing soil samples with a typical, labile litter of Medicago sativa L. Respiration change findings were supported by directly analyzing bacterial population changes.
2. Materials and Methods
2.1. Study Sites and Sampling
A wildfire initiated at July 3rd 2021 at the mountain areas of Larnaca and Limassol districts of Cyprus, and in the course of a few hours burned about 55 km2 of land due to very strong winds. The fire burned pine forests, shrubland and cultivated areas.
Top soil (top 0 - 10 cm) was sampled from inter-canopy areas in burned and unburned control areas, immediately after (7 days), shortly after (one month, but before any autumn rains) and six months after the wildfire. Soil samples (approximately 8-10 kg) were collected as composite samples (constituted from 15-20 subsamples). Burned soil was sampled inside the burned site, whereas unburned control samples were collected from sites being either in close proximity (few meters) or far away (few hundreds of meters) from the fire front line. Control unburned samples collected in close proximity to the fire front could have been influenced by fire to a certain degree, while the ones far away provide the presumably unaffected control soil samples. Burned and the respective unburned control (close and far away from the fire front) samples were collected from three sites along the fire front: an olive grove, a pine forest and a shrubland macchia area. Top soil samples were transfer to the laboratory in plastic bags, passed through a 2 mm sieve for excluding litter and stored until the analysis of initial descriptive properties and incubations for quantifying soil respiration rates. Sieved samples collected from the burned sites were clearly darker compared with the unburned ones, despite being sieved.
The abbreviations of the sites included in the study are as follows: OGB: Olive Grove Burned, OGCn: Olive Grove Control near the fire front line OGCf: Olive Grove Control far away from the fire front line, MVB: Macchia Vegetation Burned, MVCn: Macchia Vegetation Control near the fire front line, MVCf: Macchia Vegetation Control far away from the fire front line, PFB: Pine Forest Burned PFC: Pine Forest Control (near the fire front).
2.2. Measurements of Soil Physicochemical Properties
The general physico-chemical properties of soil were measured in the three sets of samples (burned, unburned near, unburned far away). More precisely, soil texture, soil pH, electrical conductivity (EC), and total CaCO3 and total organic carbon (TOC) (using the Walkley-Black oxidation method) content were measured.
Soil texture was determined by the hydrometer method (Day, 1965), while soil pH was determined with a pH meter (Mettler Toledo, SevenGo SG2, Switzerland) in a 1:2.5 sample to water suspension. The same suspension was used for soil EC measurement using a benchtop conductivity meter (Mettler Toledo, Five Easy F30, Switzerland). The Bernard calcimeter was used for the assay of the percentage of total CaCO3. Soil inorganic N (NH4+-N and NO3--N) was extracted with 2M KCl and nitrate in extracts was determined calorimetrically by the copper-cadmium column method (Maynard et al., 1993). The NH4+-N estimation was based on the emerald green color formed when ammonia and sodium salicylate react in the presence of sodium hypochlorite at high pH. The color reaction was catalyzed by the presence of sodium nitroprusside, as previously described (Maynard et al., 1993). The soil Water Holding Capacity (WHC) was estimated as proposed by Grace et al. (2006). Oven-dried soil of known weight was placed in porous funnel and soaked with water to saturation. The water tension was then standardized by applying a suction of 0.1 MPa. The sample was then reweighed and WHC was determined as the percentage of oven dry soil.
2.3. Soil Respiration Rates
Soil respiration rates were determined by trapping the evolved CO2 from soil samples in NaOH. Soil samples were remoistened by adding water corresponding to 70% of their WHC and 50 g of subsamples were incubated in 2-L gas-tight jars at 25oC. A water film was constantly present in the bottom of the jars in order to maintain a vapor saturated atmosphere inside the jars. Evolved CO2 was captured in a vial containing 40 mL of 0.5 mol L-1 NaOH and the quantity of CO2-C absorbed in the alkali was determined by titration with 0.2 mol/L HCl. The rate of respiration was calculated by the method proposed by Alef (1995).
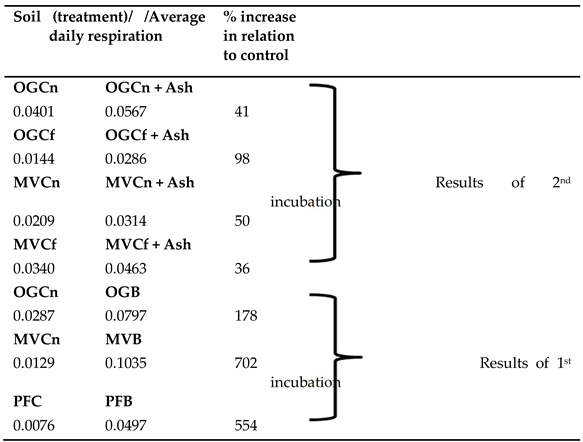
Five sets of incubation experiments were caried out at controlled conditions aiming at evaluating the respiration rates of certain batches of samples and for comparing different hypothesis concerning regeneration dynamics. Such an approach enabled the incorporation of the results of the initial incubations into the design of the followings. The first set of incubation was carried out using soil samples taken from the burned site close to the last front line of the wildfire, and their three respective non burned control areas at the other site of the fire front line. Samples concerned all three sites of this study (the olive grove, the pine forest and the Mediterranean macchia system). Each of the three samples that were incubated from each site represented a different sampling point in the field as samples were kept in separate bags. Samples were incubated immediately after transfer to the lab and first results of microbial respiration pointed to the need of a second sampling, which was carried out one month after fire, to include a second control area.
The second set of incubations were therefore carried out using two “unburned” controls: one from sites very close to the front line of the fire ensuring exactly the same soil type and management practices (for the case olive grove), and one far away from the fire front line to ensure conditions that were surely not affected by high temperatures. For the second incubation, soil respiration from samples coming from unburned sites near the fire line and unburned sites at a distance from the fire line was compared with the respiration of these soils mixed with ash coming from burned surface litter. The amount added depended on the SOC of the soil and it was such as to reach the SOC of the respective burned samples (
Table 1).
The third incubation set refers to the incubation of the ash used in the second incubation trial alone (without soil), and intended to estimate the carbon mineralization potential contained in it. A second treatment in this incubation included ash mixed with a very small amount of soil to boost microbial activity.
The fourth incubation intended to provide an indication of the capacity of the burned soils to decompose plant litter inputs. It was carried out using soil from two burned (olive grove and macchia) and their two respective unburned sites (near and far away from the fire front). Samples were incubated alone or mixed with 0.2 g of lucerne (Medicago sativa) litter.
The fifth set of incubation concerned samples collected six months after fire. Burned, unburned near and unburned far away from the fire soil samples were collected from the same areas as the initial samplings (but not exactly the same sampling points). Control blank incubations, constituted by jars containing the alkali trap but no soil were run in triplicates in all incubation sets. Soil samples were always prepared in double and one of them was used for the measurement of the initial pH (H2O) (1:2.5 soil to water ratio) and EC.
2.4. DNA Extraction and High-Throughput Sequencing
Soil DNA from burned and unburned samples was extracted using the DNeasy PowerSoil Kit (QIAGEN N.V., Venlo, Netherlands). Following the extraction process, the DNA samples were quantified using the Quant-iT PicoGreen dsDNA Assay Kit (Thermo Fisher Scientific Inc., MA, USA) and subsequently stored at -20°C for further analysis. To delineate the bacterial community composition, the V3-V4 variable region of the 16S rRNA gene was amplified using PCR primers 515/806. Pair-end (2x300 bp) sequencing using a MiSeq (Illumina, San Diego, CA, USA) platform was performed at the Genome Sequencing facilities of the Environmental Microbiology and Biotechnology Center (Agricultural Research Institute, Nicosia, Cyprus). DADA2 pipeline was used to quality-filter, de-noise, de-replicate, merge, and chimera checking of the raw sequencing data. Taxonomic assignment was performed with QIIME2 (Bolyen et al., 2019) sklearn-based classifier against SILVA rRNA database Release 132 at 99% identity. The process was performed using a Jupyter Notebook (Beg et al., 2021) web-based platform of the Bioinformatic Facility of the Environmental Microbiology and Biotechnology Center (Agricultural Research Institute, Nicosia, Cyprus). Data was uploaded to ENA (European Nucleotide Archive), supported by EMBL-EBI, with primary Accession PRJEB41189.
2.5. Data analysis
C-CO2 release data were analyzed to indicate changes of daily respiration rate and cumulative respiration over time. Fitting of exponential association functions (Y = Ymax X (1- e-kx) to cumulative CO2 data was initially attempted to designate rate constants (k) that could be used to the comparison between treatments, by using GraphPad Prism (GraphPad Software, Boston, MA, USA). However, as k co-varies with Ymax in a free fitting procedure, rate constant utilization would not be appropriate for such comparisons. Instead, average daily respiration estimates were used. These were calculated as the weighted mean of the daily respirations obtained after each incubation interval measurement. The comparison of results of CO2-C evolution for each incubation interval were subjected to analysis of variance (ANOVA) using GraphPad Prism (GraphPad Software, Boston, MA, USA).
All statistical computations regarding microbiome analysis were conducted using RStudio (Version 2022.07.2). Prior to analysis, taxa lacking sequence hits and ASVs present in less than 5% of samples were excluded. The α-diversity metrics, including the Fisher, Gini-Simpson, Inverse Simpson, and Shannon indices, were computed using the microbiome package. A two-way ANOVA was employed to investigate the effects of fire and ecosystem types on bacterial α-diversity. In this analysis, Fire (categorized as Yes or No) and Ecosystem (categorized as Pine forest, Olive grove, or Macchia vegetation) served as fixed factors, with their interaction also considered. The α-diversity metrics were treated as dependent variables. Data assumptions of normality and homoscedasticity were validated using the Shapiro and Levene's tests, respectively, facilitated by the rstatix package.
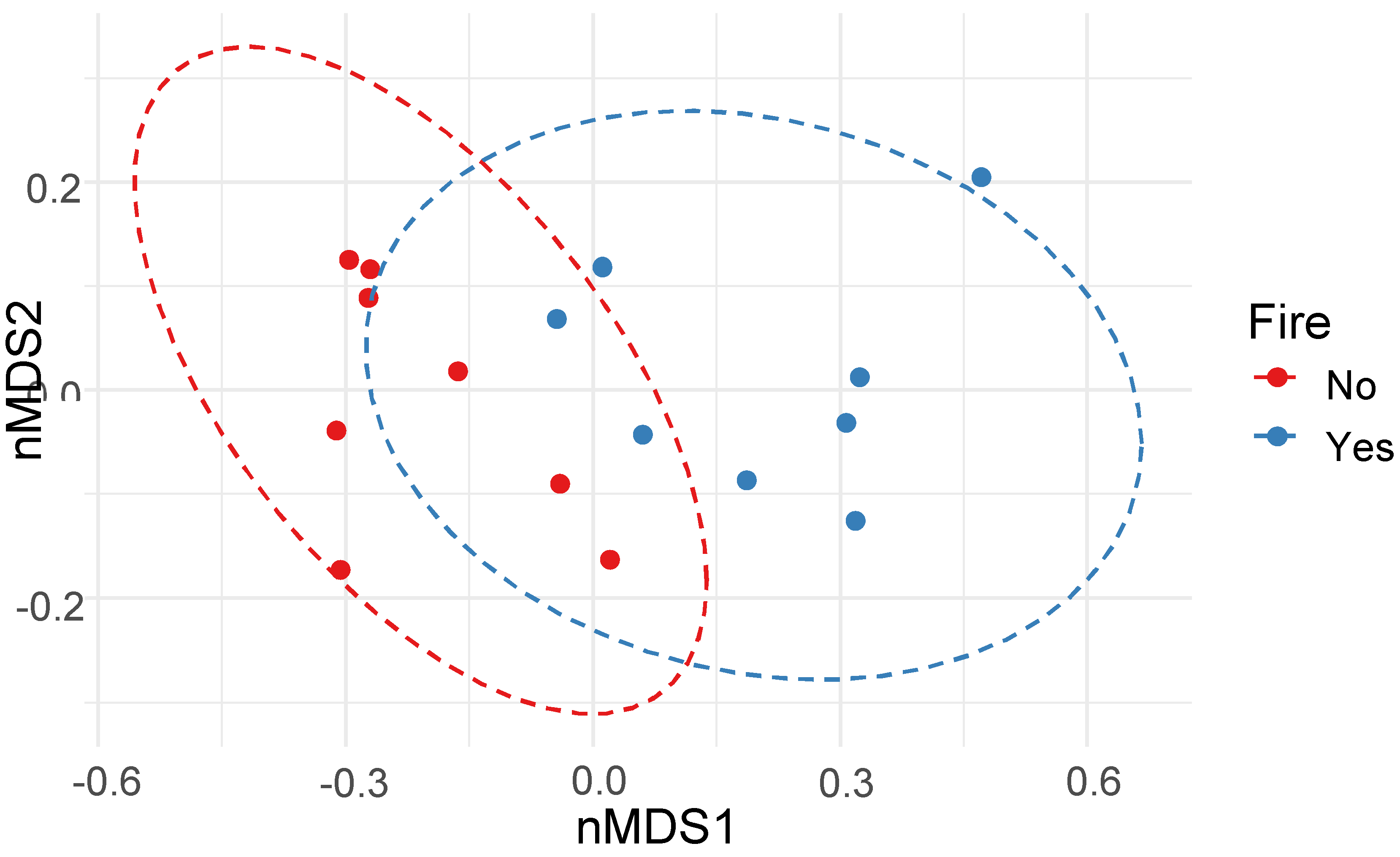
Bacterial β-diversity was ascertained using the Bray-Curtis distance metrics, and the resulting data were visualized using Nonmetric Multidimensional Scaling (NMDS). This was done after standardizing the data to the minimum number of reads observed across libraries (1250 reads). To compare the dissimilarity between various sample groups, PERMANOVA was applied using the “adonis2” function from the vegan package (Oksanen et al., 2007). An exploration of bacterial community composition distinctions between burned and unburned soil samples was executed and visually presented using the gplots and ggvenn packages.
3. Results
3.1. Results on Soil Physico-Chemical Characteristics as Affected by Wildfire
Analysis of the basic physico-chemical characteristics of sampled soil showed that burned soils had greater pH and EC values compared with control samples (
Table 1). This result was also confirmed by sample analyses of additional soils that were not used in incubations (results not shown). The increase was at the range of 0.24 to 0.64 units for pH and 0.20 to 0.66 dS m
-1 for EC. The content of CaCO
3 was always found greater in burned than unburned areas, a result probably attributed to the way that CaCO
3 concentration was measured (Bernard method) rather than to the effect of fire. An excess CO
2 release after hydrochloric acid application coming from the organic material of the burned soil samples would have resulted to an overestimation of CaCO
3.
The variance of data of inorganic N concentrations extracted six days after fire was great, thus hindering differences and making comparisons between burned and unburned sites non statistically significant. Mean values though, showed increased mineral N in the burned areas of both olive grove and macchia sites in relation to their respective unburned ones. Mineral N consisted mainly of ammonium while nitrates appeared only in traces. No effect of fire and no elevated soil ammonium concentrations have been shown six months after the wildfire.
3.2. Soil Respiration
Results of the first set on incubation experiment (
burned vs unburned) revealed that the average rate of CO
2 release ranged between 0.0076 and 0.1035 mg CO
2 g
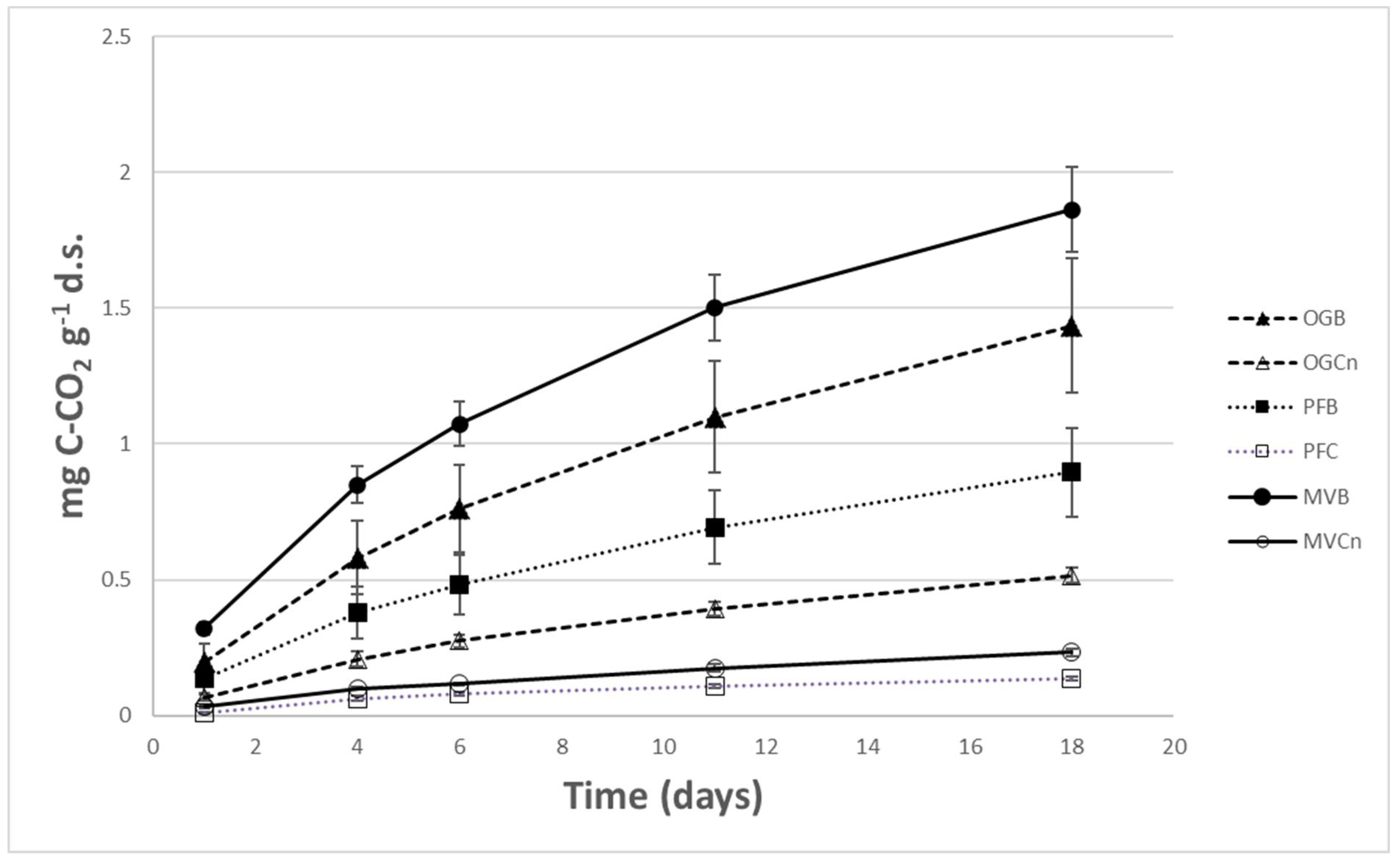
-1 dry soil. Microbial basal respiration from samples coming from burned sites was constantly greater than that from their respective unburned sites and differences were always statistically significant. This was true for all measurement time intervals and cumulative respiration results that are shown in
Figure 1. The greatest respiration was shown in macchia burned soils followed by olive grove burned soils and then by pine forest burned soils. The magnitude of difference of the average daily respiration between burned and unburned soils depended also on the type of vegetation in the studied sites, being greatest in the macchia site and followed by the pine forest and the olive grove.
The addition of ash in control unburned soils (second set of incubation experiments) resulted in increased microbial respiration. The percent increase associated to ash addition, though, was much smaller in relation to the percent difference in respiration of burned and unburned samples obtained at the previous incubation (
Table 2). Burned olive grove soil, for example, showed 178% increase in average daily respiration in relation to the respective unburned soil, whereas when this soil was mixed with fire ash it showed an increase of only 41% in average daily respiration. Mixing with ash resulted also in increased soil pH: 7.21 for OGCn + Ash, 7.33 for OGCf + Ash, 7.74 MVCf + Ash and 7.62 for MVCn + Ash. Ash by itself, with or without a soil inoculum showed no measurable CO
2 release at all but one incubation internal, which even then was very small and lasted for only two days (third set of incubation). Respiration of ash was considered, therefore, as negligible and results are not presented.
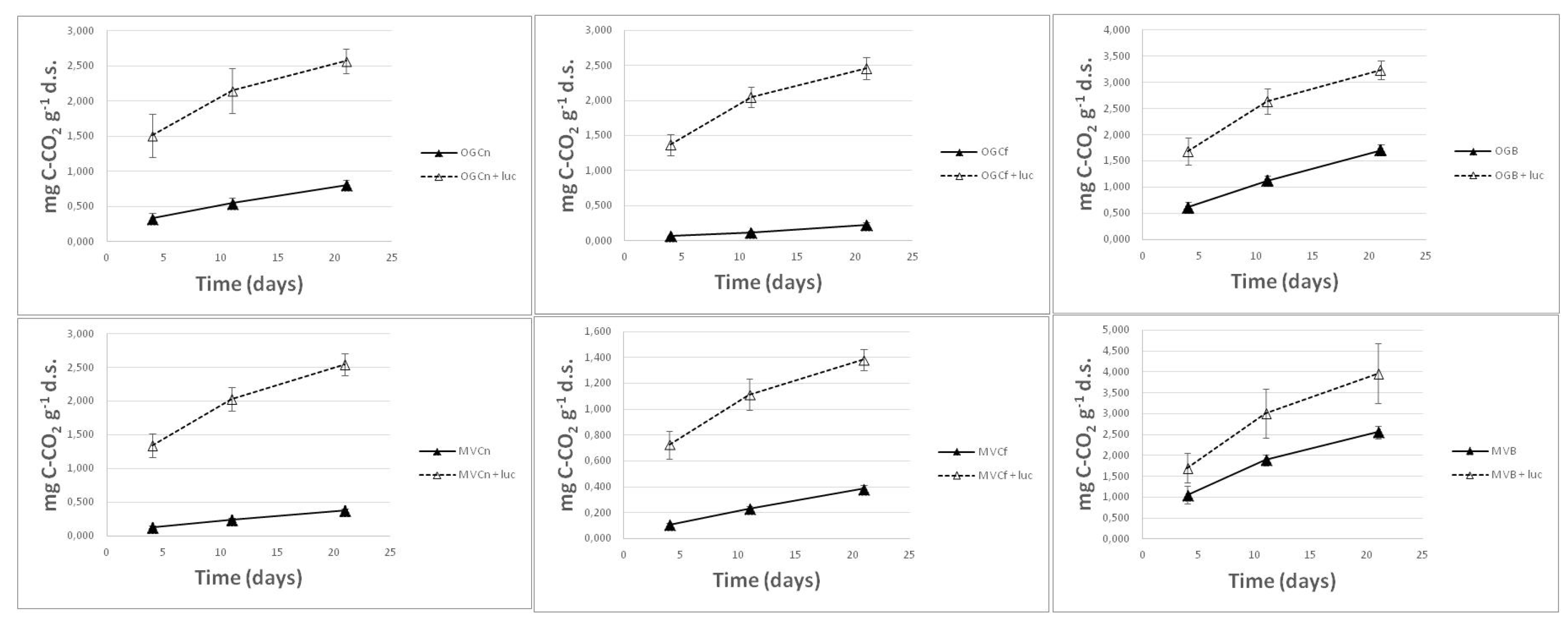
The addition of dry plant material of lucerne in soil samples significantly boosted microbial respiration in all burned and unburned soils. The microbial respiration rate of soil samples amended with plant litter was always higher in burned soils compared with their respective unburned control samples. However, by subtracting CO
2 due to soil only emissions, respiration data indicated that the decomposition of
Medicago sativa at the same temperature and moisture conditions and for the same period of time was greatest in the unburned soils. Indicatively, the total amount of released CO
2 was 89 % higher in the burned olive grove soil samples receiving alfalfa litter and incubated for 21 days, but 219 and 989% higher when the litter was incorporated in the respective unburned control samples (
Figure 2).
The fifth set of incubation experiments, concerning samples collected six months after the wildfire, revealed that the absolute values of CO
2 release rates were constantly lower compared with the respective ones measured in samples collected just few days after fire. The comparison of respiration between burned and unburned areas six months after fire showed essentially the same result patterns as those immediately after fire. Samples coming from burned areas respired with a greater rate than from unburned areas. For the olive grove in particular, even the percent difference between burned and unburned samples seems to have not been changed after six months. For the macchia vegetation though, this difference was much restricted (
Table 3).
3.3. Influence of Fire on Bacterial Phyla and Their Correlation with Vegetation
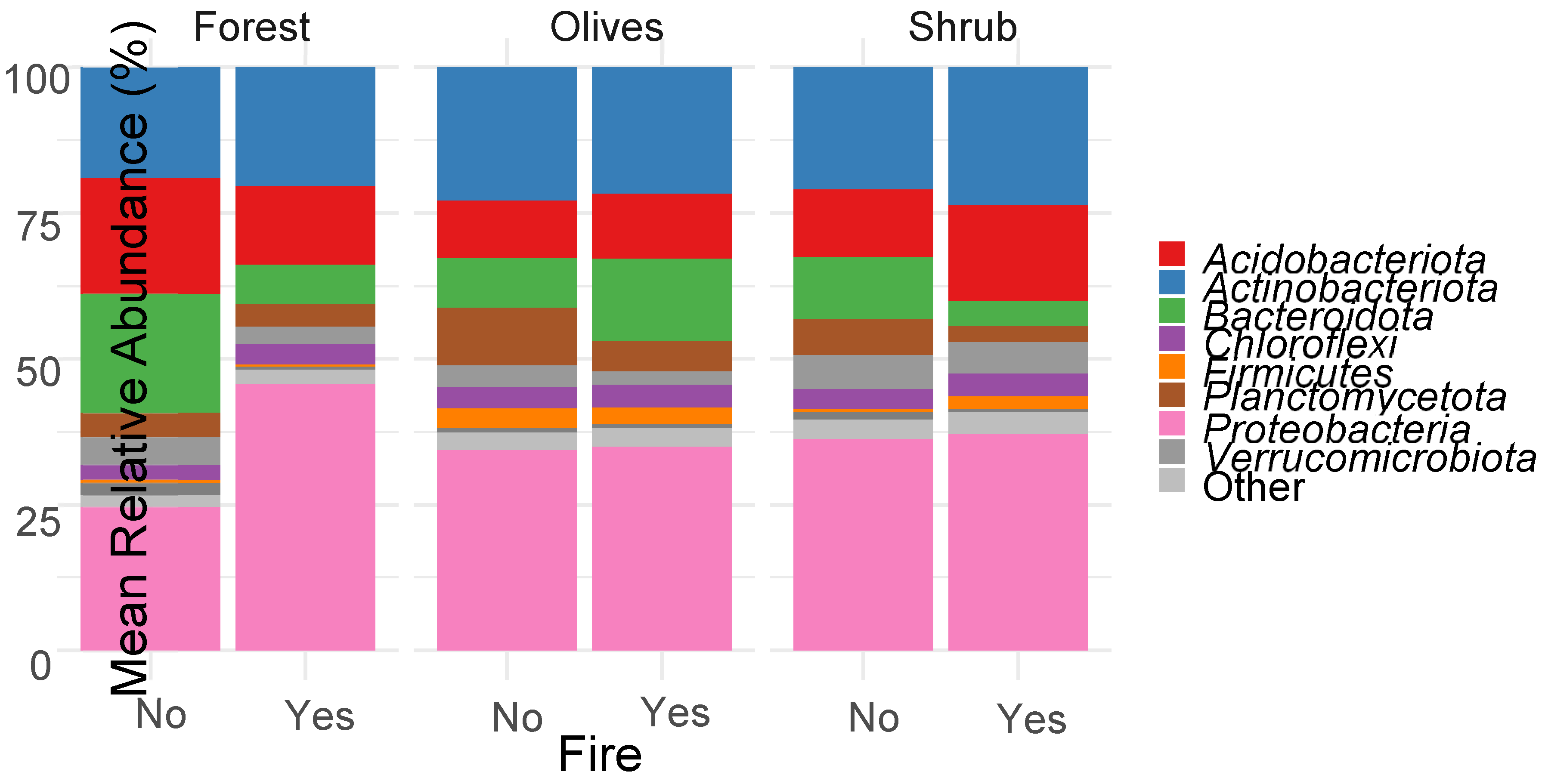
From the data collected, we obtained a total of 3,317,501 quality sequences, with each sample ranging from 1,816 to 8,247 sequences (average 5,045). The dominant phylum observed was Proteobacteria (~36%), followed by Actinobacteriota (~24%), Acidobacteriota (~12%), Bacteroidota (~11%), Planctomycetota (~5%), Chloroflexi (~4.5%), Verrucomicrobiota (~3.5%), and Firmicutes (~1.5%) (
Figure 3). Both fire and vegetation type significantly influenced the abundance of bacterial phyla (
Figure 3). For instance, the macchia ecosystem witnessed a notable increase in the abundance of Acidobacteriota and Firmicutes, while the olive grove saw an uptick in soil Cyanobacteria. In contrast, burned soils from the macchia ecosystem exhibited a marked reduction in the relative abundance of Bacteroidota.
3.4. Wildfire influence on Bacterial Community Composition and Diversity
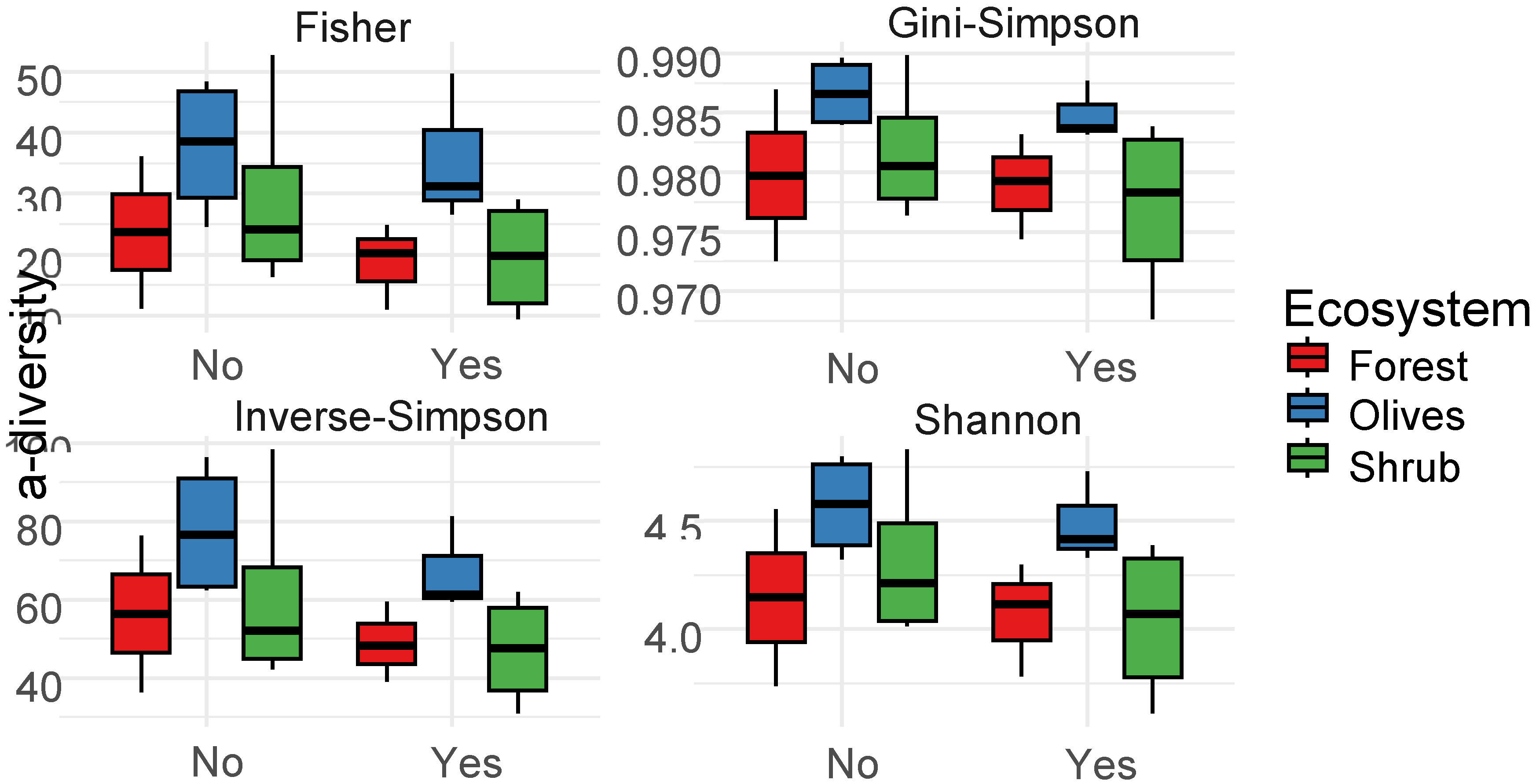
Across all tested vegetation types, fire did not appear to impact the richness or evenness of the bacterial community. Across indicators, both unburned and burned soil samples exhibited comparable values (
Figure 4). In contrast, when analyzing β-diversity based on the Bray-Curtis distance for taxa with a prevalence of 40% of the samples, fire was found to induce significant compositional shifts in bacterial communities across all ecosystems (PERMANOVA: R
2= 12.34%, p=0.023) (
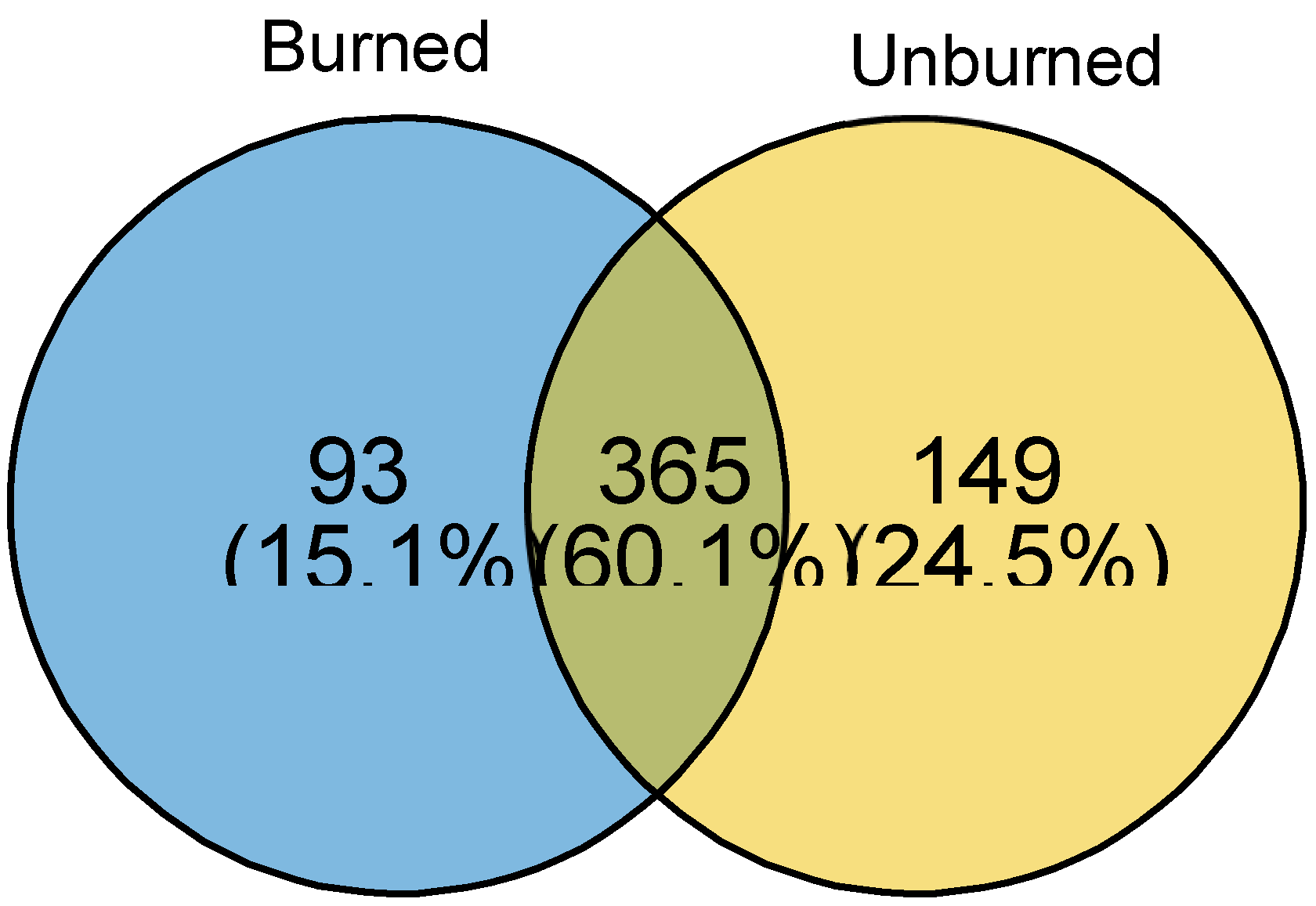
Figure 5). Further analysis of the distance metrics revealed that these observed differences in the soil bacterial community were more attributable to the fire itself rather than random distribution patterns or specific ecosystems. Upon examining taxa present in 20% of the samples with a relative abundance greater than 1%, we observed that regardless of vegetation type, both burned and unburned soils shared 60% of the ASVs. Notably, burned soils had fewer unique ASVs (15%) compared to the 25% unique taxa identified in unburned samples (
Figure 6).
4. Discussion
The effect of fire on soil properties has quite extensively been studied especially in Mediterranean type of ecosystems where wildfires occur very often during the summer period (Francos et al., 2018a; Francos et al., 2018b). Studies have been undertaken aiming at evaluating the impact of fire on physical properties, such as soil water repellency, infiltration and aggregate stability, and chemical properties, such as pH, electrical conductivity and concentrations of C, N and P. The former can largely indicate after-fire erosion risks and the latter revegetation potential (Certini et al., 2021; Souza-Alonso et al., 2022).
Soil respiration studies are less frequent. Variables affecting soil respiration are associated to either positive or negative microbial responses due to fire. The thermal shock could kill microorganisms and reduce soil respiration whereas increased nutrient availability in deposited ash would favor microbial activity and CO2 release (Sánchez-García et al., 2021; Zhang et al., 2021b). Increases in pH and dissolved organic carbon, especially in low severity fires may enhance microbial respiration, whereas the formation of material characterized by high aromaticity and recalcitrance would result in a drop of microbial activity (Hu et al., 2020).
Accordingly, contrasting results on basal microbial respiration have been reported in the literature. De Marco et al. (2005) attributed greater respiration rates of soils in the months following fire to increased availability of nutrients. Similarly, Guerrero et al. (2005) found that the solubilization of carbon due to heating and increased nutrients results in increased colonization by bacteria and increased microbial respiration. However, the exhaustion of easily mineralizable compounds eventually resulted in a decrease of respiration rates with increasing time post fire. On the contrary, in situ measurements of soil C emissions have often been found lower after fire in forest stands (Uribe et al., 2013). Francos Quijorna (2019) found that basal soil respiration, and basal soil respiration /microbial C values at the fire-affected sites of his study were significantly lower than those recorded at his control, thus attributing the reduction to the highly recalcitrant charcoal produced at fire, which is more resistant to microbial decomposition, or the release of organic pollutants and heavy metals. Lower basal respiration and microbial biomass-C has been also reported by Hernandez et al. (1997) for a Mediterranean pine forest, 9 months after the fire.
In this study, differences of soil respiration between burned and unburned sites were, however, clear and statistically significant. In laboratory incubation conditions, burned soils respired more than unburned ones. The positive effect of fire on CO2 soil fluxes were documented in all three ecosystem types that were selected along the front line of the wildfire. All soils in the studied area were young and poorly developed with low organic matter contents. Olive grove soils respired more than macchia soils, which respired more than Pine forest soils. Differences in respiration rates between sites, however, should be attributed to a combination of effects by topography/erosion variability and by vegetation cover. Fire effects though, as they were expressed by the difference in CO2 release between burned and unburned samples were ranked as follows: Macchia > Pine forest > Olive grove. Respiration decreased six months after fire, but the difference between burned and unburned soil samples persisted. Apparently, the soil six months after fire that was used in incubations was more exhausted in decomposable organic matter in relation to the soil immediately after fire, as fresh above ground or root litter had not been incorporated yet. The percent increase due to fire in macchia decreased and approached that of olive grove.
Ash addition to unburned soil samples provoked an increase in CO2 release indicating organic C supply or enhanced nutrient provision to soil microbes. However, when incubated alone, ash did not provide any evidence of decomposition. Hence, the contribution to basal respiration of burned soils of eventual not fully stable forms of C contained in ash cannot be considerable. Better nutrient status was often used as an explanation for the proliferation of some microbial groups and the increase of saprophytic and decrease of autotrophic microorganisms after fire (Vázquez et al., 1993). The important role of ash in post-fire soil respiration was also emphasized by Sánchez-García et al. (2021) working in a savanna environment. Adding wildland fire ash enhanced CO2 emissions by up to 3 times compared with pre- and post-fire soils without ash, suggesting that this was the result of the high content of readily available nutrients in the ash.
Burned soils did not lose their capacity to decompose plant residues. Hence, it can be reasonably alleged that services provided by soil microbial activity are not fully disrupted by fire. The addition of alfalfa multiplied CO2 release in incubated soils. The overall respiration of burned soils continued to be greater than that of the unburned ones even after the addition of litter. However, the subtraction of CO2 corresponding solely to soil samples (not amended with organic matter) showed smaller decomposition potential at the burned soils. If greater basal respiration of burned soils was simply a result of soil nutrient availability contained in ash, the addition of the same (qualitative and quantitively) litter in burned soils would result in similar or even higher CO2 release compared with that from unburned soils. The results of the incubation with alfalfa, therefore, provide a strong indication of either a shift in microbial community composition after fire or a decrease in enzymes activity due to elevated pH values. The exoenzymes of the otherwise similar in capacity microbes may operate less effectively at the post-fire conditions of elevated pH (Schill, 2022; Zhang et al., 2021a). Alternatively, heat-resistant microorganisms dominating soil microbiome after fire may well be capable of decomposing exogenous organic material, but not at the rate of the undisturbed unburned microbial community.
DNA analysis has illuminated the intricate bacterial dynamics in response to environmental variables such as fire events. Previous studies on Mediterranean ecosystems after wildfires have documented an upsurge in the abundance of Proteobacteria, Actinobacteria, and Firmicutes, alongside a decline in Bacteroidetes, echoing our results (Lucas-Borja et al., 2019; Rodríguez et al., 2018). In the current study, Proteobacteria, were dominant in post-fire environments followed by Actinobacteriota and Acidobacteriota. Notably, these bacterial groups possess characteristics that may confer advantages in burned soils. For instance, Nelson et al. (2022) observed a pronounced increase in the abundance of Firmicutes and Actinobacteria in soils affected by wildfires. These findings further highlight the enhanced expression of thermal resistance genes, such as those for sporulation and heat shock proteins, suggesting these as adaptive strategies against wildfire heat. This upregulated function was notably associated with taxa within the Actinobacteria and Firmicutes phyla. Beyond the impact of fire, vegetation type can significantly influence bacterial communities, stemming from variations in soil characteristics and pre-existing bacterial assemblies before the fire event (Stephanou et al., 2021). Our observation of increased Acidobacteriota and Firmicutes abundance in the shrub ecosystem post-fire only, suggests that specific bacterial groups thrive under certain ecosystem conditions.
Current results indicate that while fire does not significantly alter the richness or evenness of bacterial communities across different ecosystems, it does induce compositional shifts in these communities. This observation aligns with prior research which found that bacterial α-diversity remains largely unchanged in soils subjected to either controlled or natural wildfires (Lucas-Borja et al., 2019; Weber et al., 2014). The short duration of the wildfire under investigation, combined with soil temperature thresholds, likely preserved bacterial richness and evenness across the examined ecosystems. The observation that burned soils had fewer unique ASVs compared to unburned samples is particularly intriguing (
Figure 6). The observed response might be due to the potential reduction in the abundance of specific bacterial taxa sensitive to high temperatures by fire, resulting in fewer unique ASVs in burned soils. Such shifts, as highlighted by Rodríguez et al. (2018) can be attributed to the direct and indirect effects of fire, including alterations in soil properties, nutrient availability, and the physical structure of the habitat.
5. Conclusions
Basal respiration of burned soils was higher than of the unburned ones. Part of the difference should be attributed to ash, which apparently provides nutrients for microbial growth. When litter ash was added to soil, CO2 release during incubations significantly increased in all types of soil used. Additionally, soil DNA extraction and 16S rRNA gene analysis revealed changes in microbial community composition and shift in dominance of bacterial species, which should be also considered as responsible for the increase in soil respiration after fire. Soils after fire do not lose their ability to decompose fresh litter despite these changes, although the rate of this decomposition is smaller than at the unburned samples. More extended research is needed in order to reveal whether these decomposition process changes are the result of ecosystem adaptation to fire so that SOM content is more rapidly replenished.
References
- Aguilera, R., Corringham, T., Gershunov, A., Benmarhnia, T., 2021. Wildfire smoke impacts respiratory health more than fine particles from other sources: observational evidence from Southern California. Nature communications 12, 1493. [CrossRef]
- Alef, K., 1995. Estimation of soil respiration, in: Alef, K., Nannipieri, P. (Eds.), Methods in applied soil microbiology and biochemistry. Academic, London, pp. 215-216.
- Beg, M., Taka, J., Kluyver, T., Konovalov, A., Ragan-Kelley, M., Thiéry, N.M., Fangohr, H., 2021. Using Jupyter for reproducible scientific workflows. Computing in Science & Engineering 23, 36-46. [CrossRef]
- Bolyen, E., Rideout, J.R., Dillon, M.R., Bokulich, N.A., Abnet, C.C., Al-Ghalith, G.A., Alexander, H., Alm, E.J., Arumugam, M., Asnicar, F., 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nature biotechnology 37, 852-857. [CrossRef]
- Brown, P.T., Hanley, H., Mahesh, A., Reed, C., Strenfel, S.J., Davis, S.J., Kochanski, A.K., Clements, C.B., 2023. Climate warming increases extreme daily wildfire growth risk in California. Nature 621, 760-766. [CrossRef]
- Castaldi, S., De Grandcourt, A., Rasile, A., Skiba, U., Valentini, R., 2010. CO2, CH4 and N2O fluxes from soil of a burned grassland in Central Africa. Biogeosciences 7, 3459-3471. [CrossRef]
- Certini, G., 2005. Effects of fire on properties of forest soils: a review. Oecologia 143, 1-10. [CrossRef]
- Certini, G., Moya, D., Lucas-Borja, M.E., Mastrolonardo, G., 2021. The impact of fire on soil-dwelling biota: A review. Forest Ecology and Management 488, 118989. [CrossRef]
- Concilio, A., Ma, S., Li, Q., LeMoine, J., Chen, J., North, M., Moorhead, D., Jensen, R., 2005. Soil respiration response to prescribed burning and thinning in mixed-conifer and hardwood forests. Canadian journal of forest research 35, 1581-1591. [CrossRef]
- Day, P.R., 1965. Fractionation and particle size analysis, in: Black, C.A. (Ed.), Methods of Soil Analysis. Part I. Agronomy 9. American Society of Agronomy, Inc, Madison, Wisconsin, pp. 545-567.
- De Marco, A., Gentile, A.E., Arena, C., De Santo, A.V., 2005. Organic matter, nutrient content and biological activity in burned and unburned soils of a Mediterranean maquis area of southern Italy. International Journal of Wildland Fire 14, 365-377.
- Duxbury, J., Lauren, J., Fruci, J., 1991. Measurement of the biologically active soil nitrogen fraction by a 15N technique. Agriculture, Ecosystems & Environment 34, 121-129. [CrossRef]
- Fest, B.J., Livesley, S.J., von Fischer, J.C., Arndt, S.K., 2015. Repeated fuel reduction burns have little long-term impact on soil greenhouse gas exchange in a dry sclerophyll eucalypt forest. Agricultural and Forest Meteorology 201, 17-25. [CrossRef]
- Francos, M., Pereira, P., Alcañiz, M., Úbeda, X., 2018a. Post-wildfire management effects on short-term evolution of soil properties (Catalonia, Spain, SW-Europe). Science of the Total Environment 633, 285-292. [CrossRef]
- Francos, M., Úbeda, X., Pereira, P., Alcañiz, M., 2018b. Long-term impact of wildfire on soils exposed to different fire severities. A case study in Cadiretes Massif (NE Iberian Peninsula). Science of the Total Environment 615, 664-671. [CrossRef]
- Francos Quijorna, M., 2019. Wildfire and forest management effects on soil properties.
- Gatica-Saavedra, P., Aburto, F., Rojas, P., Echeverría, C., 2023. Soil health indicators for monitoring forest ecological restoration: A critical review. Restoration Ecology 31, e13836. [CrossRef]
- Goberna, M., García, C., Insam, H., Hernández, M., Verdú, M., 2012. Burning fire-prone Mediterranean shrublands: immediate changes in soil microbial community structure and ecosystem functions. Microbial ecology 64, 242-255. [CrossRef]
- Grace, C., Hart, M., Brookes, P.C., 2006. Laboratory manual of the soil microbial biomass group. Rothamsted research 65.
- Guerrero, C., Mataix-Solera, J., Gómez, I., García-Orenes, F., Jordán, M.M., 2005. Microbial recolonization and chemical changes in a soil heated at different temperatures. International Journal of Wildland Fire 14, 385-400.
- Hernández, T., Garcia, C., Reinhardt, I., 1997. Short-term effect of wildfire on the chemical, biochemical and microbiological properties of Mediterranean pine forest soils. Biology and fertility of soils 25, 109-116. [CrossRef]
- Holden, S.R., Rogers, B.M., Treseder, K.K., Randerson, J.T., 2016. Fire severity influences the response of soil microbes to a boreal forest fire. Environmental Research Letters 11, 035004. [CrossRef]
- Hu, M., Song, J., Li, S., Li, Z., Hao, Y., Di, M., Wan, S., 2020. Understanding the effects of fire and nitrogen addition on soil respiration of a field study by combining observations with a meta-analysis. Agricultural and Forest Meteorology 292, 108106. [CrossRef]
- Lucas-Borja, M., Miralles, I., Ortega, R., Plaza-Álvarez, P., Gonzalez-Romero, J., Sagra, J., Soriano-Rodríguez, M., Certini, G., Moya, D., Heras, J., 2019. Immediate fire-induced changes in soil microbial community composition in an outdoor experimental controlled system. Science of the Total Environment 696, 134033. [CrossRef]
- Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., 2022. Global Warming of 1.5 C: IPCC special report on impacts of global warming of 1.5 C above pre-industrial levels in context of strengthening response to climate change, sustainable development, and efforts to eradicate poverty. Cambridge University Press.
- Maynard, D., Kalra, Y., Crumbaugh, J., 1993. Nitrate and exchangeable ammonium nitrogen. Soil sampling and methods of analysis 1, 25-38.
- Muñoz-Rojas, M., Lewandrowski, W., Erickson, T.E., Dixon, K.W., Merritt, D.J., 2016. Soil respiration dynamics in fire affected semi-arid ecosystems: Effects of vegetation type and environmental factors. Science of the Total Environment 572, 1385-1394. [CrossRef]
- Nelson, A.R., Narrowe, A.B., Rhoades, C.C., Fegel, T.S., Daly, R.A., Roth, H.K., Chu, R.K., Amundson, K.K., Young, R.B., Steindorff, A.S., 2022. Wildfire-dependent changes in soil microbiome diversity and function. Nature microbiology 7, 1419-1430.
- Oksanen, J., Kindt, R., Legendre, P., O’Hara, B., Stevens, M.H.H., Oksanen, M.J., Suggests, M., 2007. The vegan package. Community ecology package 10, 719.
- Paz-Ferreiro, J., Fu, S., 2016. Biological indices for soil quality evaluation: perspectives and limitations. Land Degradation & Development 27, 14-25. [CrossRef]
- Plaza-Álvarez, P.A., Lucas-Borja, M.E., Sagra, J., Moya, D., Fontúrbel, T., De las Heras, J., 2017. Soil respiration changes after prescribed fires in Spanish black pine (Pinus nigra Arn. ssp. salzmannii) monospecific and mixed forest stands. Forests 8, 248.
- Rodríguez, J., González-Pérez, J.A., Turmero, A., Hernández, M., Ball, A.S., González-Vila, F.J., Arias, M.E., 2018. Physico-chemical and microbial perturbations of Andalusian pine forest soils following a wildfire. Science of the Total Environment 634, 650-660. [CrossRef]
- Sánchez-García, C., Santin, C., Doerr, S.H., Strydom, T., Urbanek, E., 2021. Wildland fire ash enhances short-term CO2 flux from soil in a Southern African savannah. Soil Biology and Biochemistry 160, 108334. [CrossRef]
- Schill, M.L., 2022. Severe Wildfires Reduce Soil Microbial Exoenzyme Production and Fungal Abundances in the Southern Appalachian Mountains. The University of Texas at San Antonio.
- Singh, J., Raghubanshi, A., Singh, R., Srivastava, S., 1989. Microbial biomass acts as a source of plant nutrients in dry tropical forest and savanna. Nature 338, 499-500. [CrossRef]
- Souza-Alonso, P., Saiz, G., García, R.A., Pauchard, A., Ferreira, A., Merino, A., 2022. Post-fire ecological restoration in Latin American forest ecosystems: Insights and lessons from the last two decades. Forest Ecology and Management 509, 120083. [CrossRef]
- Stephanou, C., Omirou, M., Philippot, L., Zissimos, A.M., Christoforou, I.C., Trajanoski, S., Oulas, A., Ioannides, I.M., 2021. Land use in urban areas impacts the composition of soil bacterial communities involved in nitrogen cycling. A case study from Lefkosia (Nicosia) Cyprus. Scientific Reports 11, 8198. [CrossRef]
- Thomas, D., Butry, D., Gilbert, S., Webb, D., Fung, J., 2017. The costs and losses of wildfires. NIST Special Publication 1215.
- Tüfekçioğlu, A., Küçük, M., Bilmiş, T., Altun, L., Yılmaz, M., 2010. Soil respiration and root biomass responses to burning in Calabrian pine (Pinus brutia) stands in Edirne, Turkey. Journal of Environmental Biology.
- Uribe, C., Inclán, R., Sánchez, D., Clavero, M., Fernández, A., Morante, R., Cardeña, A., Blanco, A., Van Miegroet, H., 2013. Effect of wildfires on soil respiration in three typical Mediterranean forest ecosystems in Madrid, Spain. Plant and soil 369, 403-420. [CrossRef]
- Vázquez, F.J., Acea, M.J., Carballas, T., 1993. Soil microbial populations after wildfire. FEMS Microbiology Ecology 13, 93-103. [CrossRef]
- Verma, S., Jayakumar, S., 2012. Impact of forest fire on physical, chemical and biological properties of soil: A review. proceedings of the International Academy of Ecology and Environmental Sciences 2, 168.
- Vogler, K.C., Ager, A.A., Day, M.A., Jennings, M., Bailey, J.D., 2015. Prioritization of forest restoration projects: tradeoffs between wildfire protection, ecological restoration and economic objectives. Forests 6, 4403-4420. [CrossRef]
- Wang, X., Liu, L., Piao, S., Janssens, I.A., Tang, J., Liu, W., Chi, Y., Wang, J., Xu, S., 2014. Soil respiration under climate warming: differential response of heterotrophic and autotrophic respiration. Global change biology 20, 3229-3237. [CrossRef]
- Weber, C.F., Lockhart, J.S., Charaska, E., Aho, K., Lohse, K.A., 2014. Bacterial composition of soils in ponderosa pine and mixed conifer forests exposed to different wildfire burn severity. Soil Biology and Biochemistry 69, 242-250. [CrossRef]
- Xue, L., Li, Q., Chen, H., 2014. Effects of a wildfire on selected physical, chemical and biochemical soil properties in a Pinus massoniana forest in South China. Forests 5, 2947-2966.
- Yan, Z., Bond-Lamberty, B., Todd-Brown, K.E., Bailey, V.L., Li, S., Liu, C., Liu, C., 2018. A moisture function of soil heterotrophic respiration that incorporates microscale processes. Nature communications 9, 2562. [CrossRef]
- Yiqi, L., Zhou, X., 2010. Soil respiration and the environment. Elsevier.
- Zhang, J., Ling, L., Singh, B.P., Luo, Y., Jeewani, P.H., Xu, J., 2021a. Decomposition of substrates with recalcitrance gradient, primed CO2, and its relations with soil microbial diversity in post-fire forest soils. Journal of Soils and Sediments 21, 3007-3017. [CrossRef]
- Zhang, Y., Yan, C., Liu, H., Pu, S., Chen, H., Zhou, B., Yuan, R., Wang, F., 2021b. Bacterial response to soil property changes caused by wood ash from wildfire in forest soils around mining areas: relevance of bacterial community composition, carbon and nitrogen cycling. Journal of Hazardous Materials 412, 125264. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).