Submitted:
01 May 2024
Posted:
01 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction:
2. Mechanisms of Action:
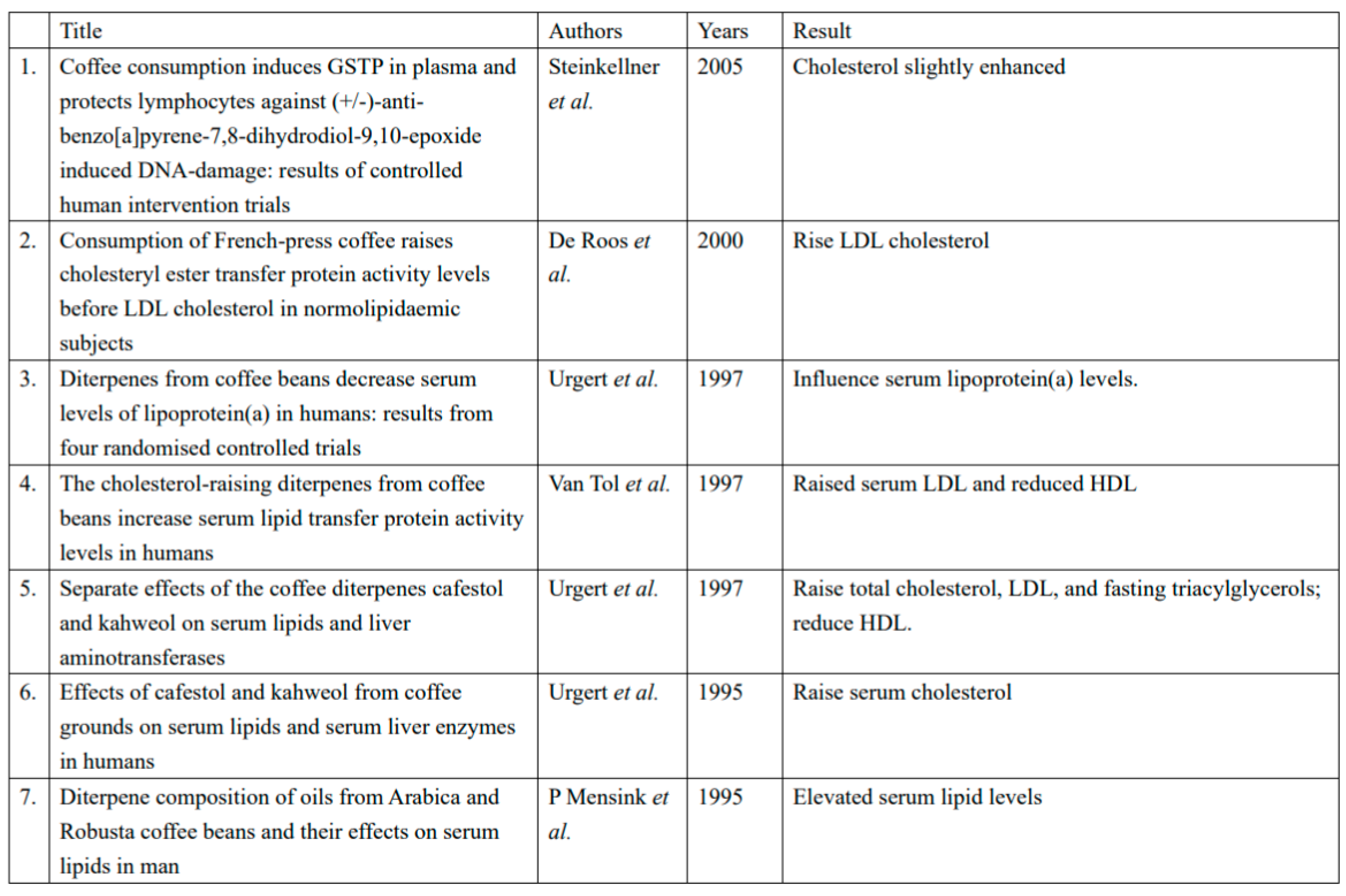
Effect on Lipid Metabolism:
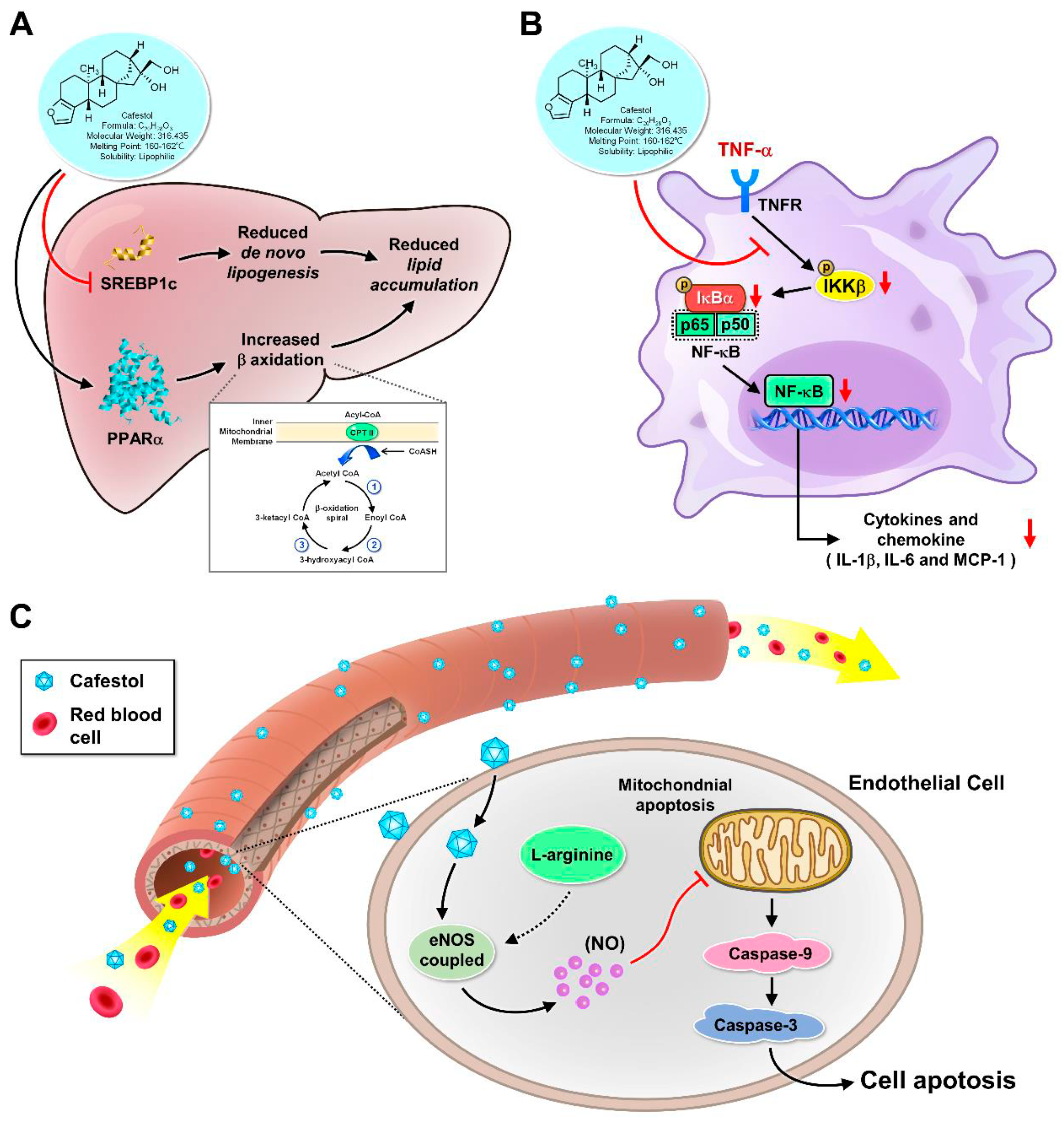
Impact on Inflammation:
Effects on Endothelial Function:
3. Epidemiological Evidence and Clinical Trials on Cafestol and Cardiovascular Health
4. Potential Mechanistic Insights:
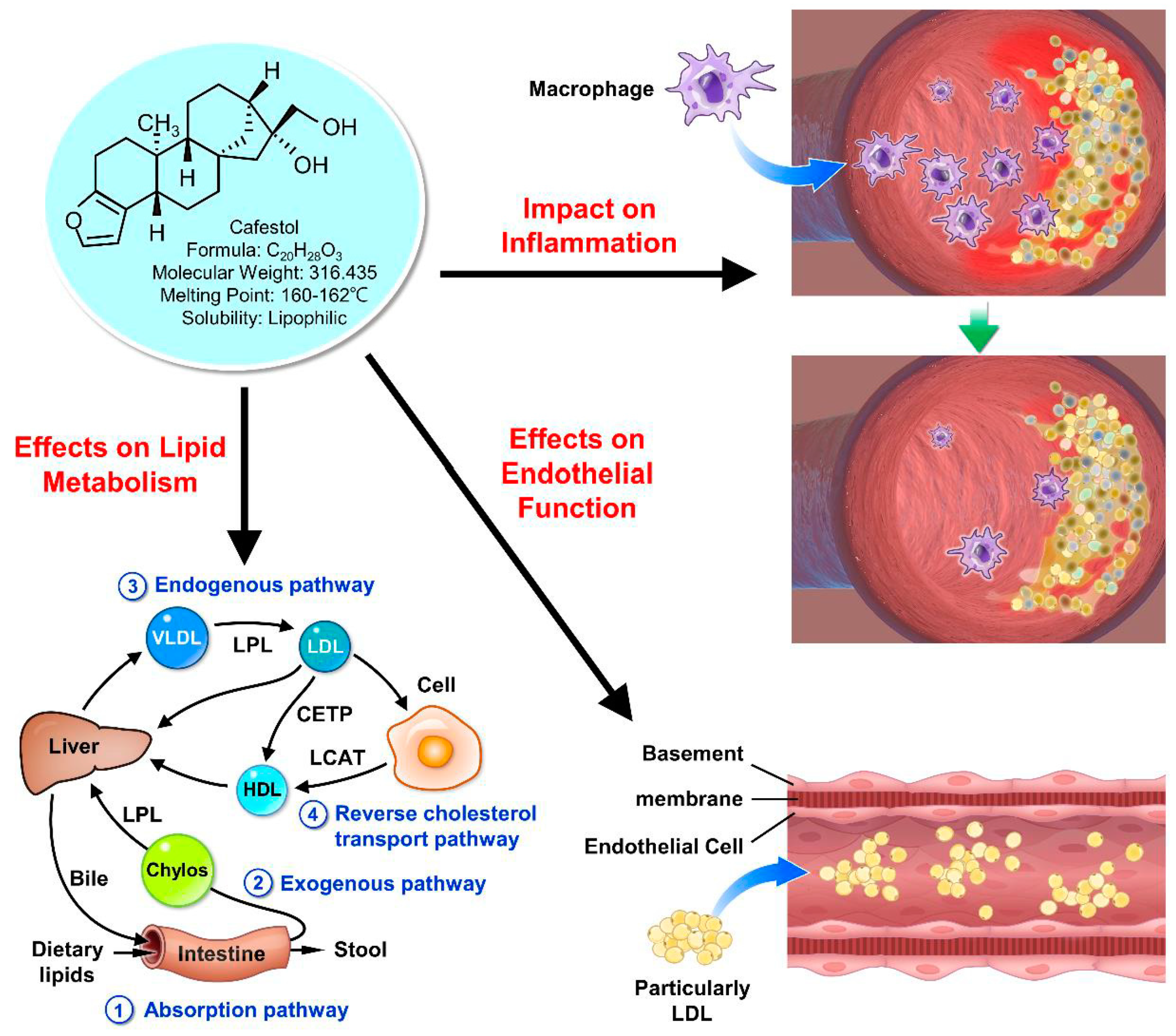
Role in Lipid Metabolism:
Role in Inflammation:
Role in Endothelial Function:
Clinical Translation and Future Directions:
5. Future Directions:
Elucidating the Dose-Response Relationship:
Impact of Coffee Brewing Methods and Types:
Long-Term Prospective Studies:
Well-Designed Clinical Trials:
6. Conclusion:
Acknowledgments
References
- Fiani, B.; Zhu, L.; Musch, B. L.; Briceno, S.; Andel, R.; Sadeq, N.; Ansari, A. Z. The Neurophysiology of Caffeine as a Central Nervous System Stimulant and the Resultant Effects on Cognitive Function. Cureus 2021, 13, e15032. [Google Scholar] [CrossRef]
- Socala, K.; Szopa, A.; Serefko, A.; Poleszak, E.; Wlaz, P. Neuroprotective Effects of Coffee Bioactive Compounds: A Review. Int J Mol Sci 2020, 22. [Google Scholar] [CrossRef]
- Liu, J. C.; Chen, P. Y.; Hao, W. R.; Liu, Y. C.; Lyu, P. C.; Hong, H. J. Cafestol Inhibits High-Glucose-Induced Cardiac Fibrosis in Cardiac Fibroblasts and Type 1-Like Diabetic Rats. Evid Based Complement Alternat Med 2020, 2020, 4503747. [Google Scholar] [CrossRef] [PubMed]
- Hao, W. R.; Sung, L. C.; Chen, C. C.; Hong, H. J.; Liu, J. C.; Chen, J. J. Cafestol Activates Nuclear Factor Erythroid-2 Related Factor 2 and Inhibits Urotensin II-Induced Cardiomyocyte Hypertrophy. Am J Chin Med 2019, 47, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y. T.; Sung, L. C.; Haw, W. R.; Chen, C. C.; Huang, S. F.; Liu, J. C.; Cheng, T. H.; Chen, P. Y.; Loh, S. H.; Tsai, C. S. Cafestol, a coffee diterpene, inhibits urotensin II-induced interleukin-8 expression in human umbilical vein endothelial cells. Eur J Pharmacol 2018, 820, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Hao, W. R.; Sung, L. C.; Chen, C. C.; Chen, P. Y.; Cheng, T. H.; Chao, H. H.; Liu, J. C.; Chen, J. J. Cafestol Inhibits Cyclic-Strain-Induced Interleukin-8, Intercellular Adhesion Molecule-1, and Monocyte Chemoattractant Protein-1 Production in Vascular Endothelial Cells. Oxid Med Cell Longev 2018, 2018, 7861518. [Google Scholar] [CrossRef]
- Farias-Pereira, R.; Park, C. S.; Park, Y. Mechanisms of action of coffee bioactive components on lipid metabolism. Food Sci Biotechnol 2019, 28, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Farias-Pereira, R.; Kim, E.; Park, Y. Cafestol increases fat oxidation and energy expenditure in via DAF-12-dependent pathway. Food Chem 2020, 307. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, M. L.; Boekschoten, M. V.; Kreeft, A. J.; Hooiveld, G. J.; Moen, C. J.; Muller, M.; Frants, R. R.; Kasanmoentalib, S.; Post, S. M.; Princen, H. M.; Porter, J. G.; Katan, M. B.; Hofker, M. H.; Moore, D. D. The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors. Mol Endocrinol 2007, 21, 1603–16. [Google Scholar] [CrossRef]
- Post, S. M.; de Roos, B.; Vermeulen, M.; Afman, L.; Jong, M. C.; Dahlmans, V. E.; Havekes, L. M.; Stellaard, F.; Katan, M. B.; Princen, H. M. Cafestol increases serum cholesterol levels in apolipoprotein E*3-Leiden transgenic mice by suppression of bile acid synthesis. Arterioscler Thromb Vasc Biol 2000, 20, 1551–6. [Google Scholar] [CrossRef]
- Urgert, R.; Essed, N.; van der Weg, G.; Kosmeijer-Schuil, T. G.; Katan, M. B. Separate effects of the coffee diterpenes cafestol and kahweol on serum lipids and liver aminotransferases. The American journal of clinical nutrition 1997, 65, 519–24. [Google Scholar] [CrossRef]
- de Roos, B.; Caslake, M. J.; Stalenhoef, A. F.; Bedford, D.; Demacker, P. N.; Katan, M. B.; Packard, C. J. The coffee diterpene cafestol increases plasma triacylglycerol by increasing the production rate of large VLDL apolipoprotein B in healthy normolipidemic subjects. The American journal of clinical nutrition 2001, 73, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Urgert, R.; Weusten-van der Wouw, M. P.; Hovenier, R.; Meyboom, S.; Beynen, A. C.; Katan, M. B. Diterpenes from coffee beans decrease serum levels of lipoprotein(a) in humans: results from four randomised controlled trials. Eur J Clin Nutr 1997, 51, 431–6. [Google Scholar] [CrossRef]
- Beynen, A. C.; Weusten-Van der Wouw, M. P.; de Roos, B.; Katan, M. B. Boiled coffee fails to raise serum cholesterol in hamsters and rats. Br J Nutr 1996, 76, 755–64. [Google Scholar] [CrossRef] [PubMed]
- Arauz, J.; Moreno, M. G.; Cortes-Reynosa, P.; Salazar, E. P.; Muriel, P. Coffee attenuates fibrosis by decreasing the expression of TGF-beta and CTGF in a murine model of liver damage. J Appl Toxicol 2013, 33, 970–9. [Google Scholar] [CrossRef]
- Halvorsen, B.; Ranheim, T.; Nenseter, M. S.; Huggett, A. C.; Drevon, C. A. Effect of a coffee lipid (cafestol) on cholesterol metabolism in human skin fibroblasts. J Lipid Res 1998, 39, 901–12. [Google Scholar] [CrossRef]
- Ranheim, T.; Halvorsen, B.; Huggett, A. C.; Blomhoff, R.; Drevon, C. A. Effect of a coffee lipid (cafestol) on regulation of lipid metabolism in CaCo-2 cells. J Lipid Res 1995, 36, 2079–89. [Google Scholar] [CrossRef]
- Kim, J. Y.; Jung, K. S.; Jeong, H. G. Suppressive effects of the kahweol and cafestol on cyclooxygenase-2 expression in macrophages. FEBS letters 2004, 569, 321–6. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Wu, L.; Feng, J.; Mo, W.; Wu, J.; Yu, Q.; Li, S.; Zhang, J.; Dai, W.; Xu, X.; Mao, Y.; Xu, S.; Chen, K.; Li, J.; Guo, C. Cafestol preconditioning attenuates apoptosis and autophagy during hepatic ischemia-reperfusion injury by inhibiting ERK/PPARgamma pathway. Int Immunopharmacol 2020, 84, 106529. [Google Scholar] [CrossRef]
- Lee, K. J.; Jeong, H. G. Protective effects of kahweol and cafestol against hydrogen peroxide-induced oxidative stress and DNA damage. Toxicol Lett 2007, 173, 80–7. [Google Scholar] [CrossRef]
- Islam, M. T.; Tabrez, S.; Jabir, N. R.; Ali, M.; Kamal, M. A.; da Silva Araujo, L.; De Oliveira Santos, J. V.; Da Mata, A.; De Aguiar, R. P. S.; de Carvalho Melo Cavalcante, A. A. An Insight into the Therapeutic Potential of Major Coffee Components. Curr Drug Metab 2018, 19, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Savoia, C. New Insights into Endothelial Dysfunction in Cardiometabolic Diseases: Potential Mechanisms and Clinical Implications. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Wang, S.; Yoon, Y. C.; Sung, M. J.; Hur, H. J.; Park, J. H. Antiangiogenic properties of cafestol, a coffee diterpene, in human umbilical vein endothelial cells. Biochem Biophys Res Commun 2012, 421, 567–71. [Google Scholar] [CrossRef]
- Moeenfard, M.; Cortez, A.; Machado, V.; Costa, R.; Luis, C.; Coelho, P.; Soares, R.; Alves, A.; Borges, N.; Santos, A. Anti-Angiogenic Properties of Cafestol and Kahweol Palmitate Diterpene Esters. J Cell Biochem 2016, 117, 2748–2756. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y. Coffee and Endothelial Function: A Coffee Paradox? Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Makiso, M. U.; Tola, Y. B.; Ogah, O.; Endale, F. L. Bioactive compounds in coffee and their role in lowering the risk of major public health consequences: A review. Food Sci Nutr 2024, 12, 734–764. [Google Scholar] [CrossRef] [PubMed]
- Whitton, C.; Ramos-Garcia, C.; Kirkpatrick, S. I.; Healy, J. D.; Dhaliwal, S. S.; Boushey, C. J.; Collins, C. E.; Rollo, M. E.; Kerr, D. A. A Systematic Review Examining Contributors to Misestimation of Food and Beverage Intake Based on Short-Term Self-Report Dietary Assessment Instruments Administered to Adults. Adv Nutr 2022, 13, 2620–2665. [Google Scholar] [CrossRef]
- Bonita, J. S.; Mandarano, M.; Shuta, D.; Vinson, J. Coffee and cardiovascular disease: in vitro, cellular, animal, and human studies. Pharmacol Res 2007, 55, 187–98. [Google Scholar] [CrossRef] [PubMed]
- Svatun, A. L.; Lochen, M. L.; Thelle, D. S.; Wilsgaard, T. Association between espresso coffee and serum total cholesterol: the Tromso Study 2015-2016. Open Heart 2022, 9. [Google Scholar] [CrossRef]
- Gross, G.; Jaccaud, E.; Huggett, A. C. Analysis of the content of the diterpenes cafestol and kahweol in coffee brews. Food Chem Toxicol 1997, 35, 547–54. [Google Scholar] [CrossRef]
- Naidoo, N.; Chen, C.; Rebello, S. A.; Speer, K.; Tai, E. S.; Lee, J.; Buchmann, S.; Koelling-Speer, I.; van Dam, R. M. Cholesterol-raising diterpenes in types of coffee commonly consumed in Singapore, Indonesia and India and associations with blood lipids: a survey and cross sectional study. Nutr J 2011, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- De Roos, B.; Van Tol, A.; Urgert, R.; Scheek, L. M.; Van Gent, T.; Buytenhek, R.; Princen, H. M.; Katan, M. B. Consumption of French-press coffee raises cholesteryl ester transfer protein activity levels before LDL cholesterol in normolipidaemic subjects. J Intern Med 2000, 248, 211–6. [Google Scholar] [CrossRef] [PubMed]
- Urgert, R.; Schulz, A. G.; Katan, M. B. Effects of cafestol and kahweol from coffee grounds on serum lipids and serum liver enzymes in humans. The American journal of clinical nutrition 1995, 61, 149–54. [Google Scholar] [CrossRef] [PubMed]
- van Tol, A.; Urgert, R.; de Jong-Caesar, R.; van Gent, T.; Scheek, L. M.; de Roos, B.; Katan, M. B. The cholesterol-raising diterpenes from coffee beans increase serum lipid transfer protein activity levels in humans. Atherosclerosis 1997, 132, 251–4. [Google Scholar] [CrossRef]
- van Rooij, J.; van der Stegen, G. H.; Schoemaker, R. C.; Kroon, C.; Burggraaf, J.; Hollaar, L.; Vroon, T. F.; Smelt, A. H.; Cohen, A. F. A placebo-controlled parallel study of the effect of two types of coffee oil on serum lipids and transaminases: identification of chemical substances involved in the cholesterol-raising effect of coffee. The American journal of clinical nutrition 1995, 61, 1277–83. [Google Scholar] [CrossRef]
- Hofman, M. K.; Weggemans, R. M.; Zock, P. L.; Schouten, E. G.; Katan, M. B.; Princen, H. M. CYP7A1 A-278C polymorphism affects the response of plasma lipids after dietary cholesterol or cafestol interventions in humans. The Journal of nutrition 2004, 134, 2200–4. [Google Scholar] [CrossRef]
- Grubben, M. J.; Boers, G. H.; Blom, H. J.; Broekhuizen, R.; de Jong, R.; van Rijt, L.; de Ruijter, E.; Swinkels, D. W.; Nagengast, F. M.; Katan, M. B. Unfiltered coffee increases plasma homocysteine concentrations in healthy volunteers: a randomized trial. The American journal of clinical nutrition 2000, 71, 480–4. [Google Scholar] [CrossRef]
- Iwamoto, H.; Izumi, K.; Natsagdorj, A.; Naito, R.; Makino, T.; Kadomoto, S.; Hiratsuka, K.; Shigehara, K.; Kadono, Y.; Narimoto, K.; Saito, Y.; Nakagawa-Goto, K.; Mizokami, A. Coffee diterpenes kahweol acetate and cafestol synergistically inhibit the proliferation and migration of prostate cancer cells. Prostate 2019, 79, 468–479. [Google Scholar] [CrossRef]
- Huber, W. W.; Teitel, C. H.; Coles, B. F.; King, R. S.; Wiese, F. W.; Kaderlik, K. R.; Casciano, D. A.; Shaddock, J. G.; Mulder, G. J.; Ilett, K. F.; Kadlubar, F. F. Potential chemoprotective effects of the coffee components kahweol and cafestol palmitates via modification of hepatic N-acetyltransferase and glutathione S-transferase activities. Environ Mol Mutagen 2004, 44, 265–76. [Google Scholar] [CrossRef]
- Kalthoff, S.; Ehmer, U.; Freiberg, N.; Manns, M. P.; Strassburg, C. P. Coffee induces expression of glucuronosyltransferases by the aryl hydrocarbon receptor and Nrf2 in liver and stomach. Gastroenterology 2010, 139, 1699–710, 1710 e1-2. [Google Scholar] [CrossRef] [PubMed]
- Shokouh, P.; Jeppesen, P. B.; Hermansen, K.; Norskov, N. P.; Laustsen, C.; Jacques Hamilton-Dutoit, S.; Qi, H.; Stodkilde-Jorgensen, H.; Gregersen, S. A Combination of Coffee Compounds Shows Insulin-Sensitizing and Hepatoprotective Effects in a Rat Model of Diet-Induced Metabolic Syndrome. Nutrients 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C. A.; Oluwagbemigun, K.; Nothlings, U. Advances in dietary pattern analysis in nutritional epidemiology. Eur J Nutr 2021, 60, 4115–4130. [Google Scholar] [CrossRef] [PubMed]
- Nosal, B. M.; Sakaki, J. R.; Kim, D. O.; Chun, O. K. Impact of coffee preparation on total phenolic content in brewed coffee extracts and their contribution to the body's antioxidant status. Food Sci Biotechnol 2022, 31, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





