Submitted:
01 May 2024
Posted:
01 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Lactoperoxidase
2.2. Testing of LP in Liquid Broth
2.3. Testing of LP on Pork Cubes
2.4. Testing of LP in Meat Products
2.5. Microbial Analysis of Meat Products
2.6. TBARS
2.7. Statistical Analysis
3. Results
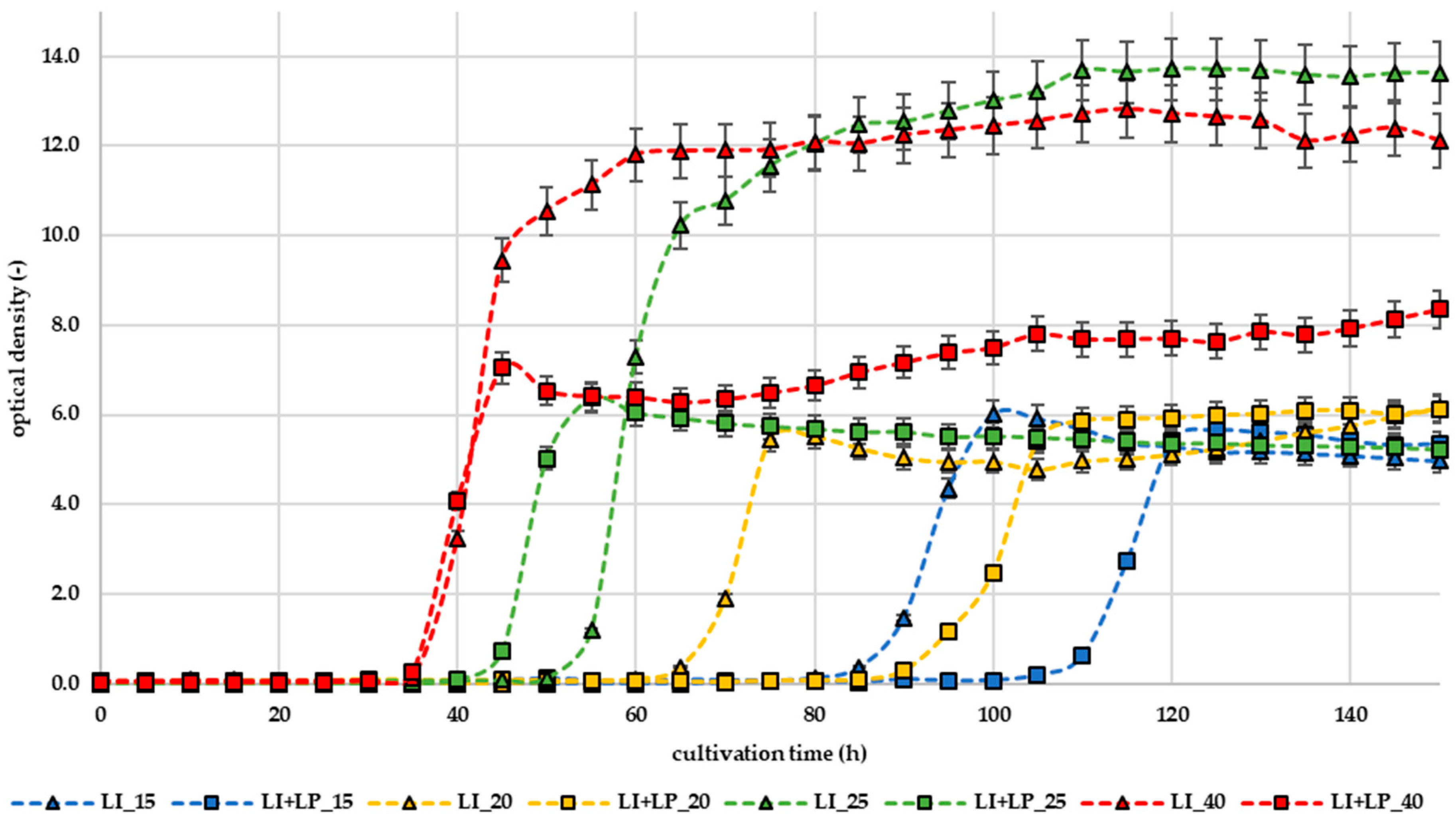
3.1. Effect of LP in Liquid Broth
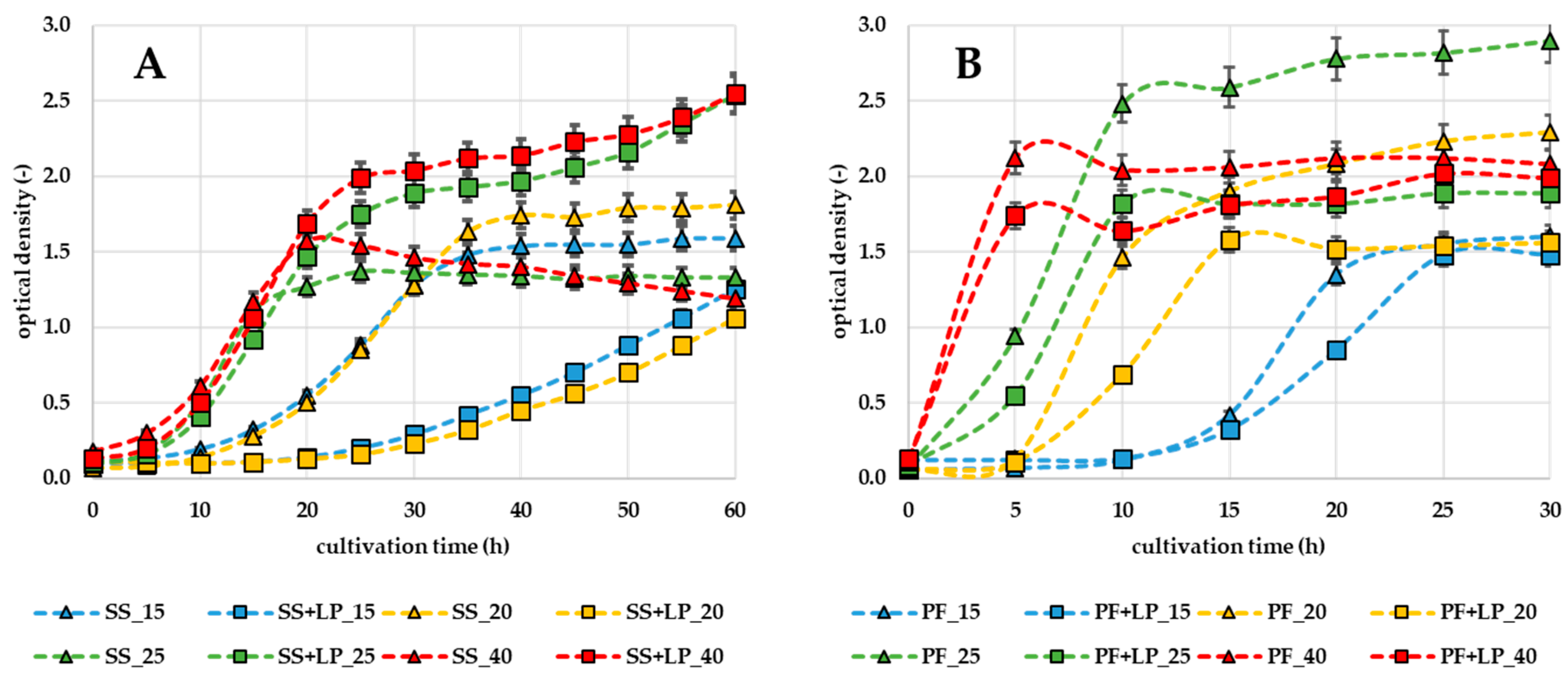
3.2. Effect of LP on the Surface of Pork Cubes
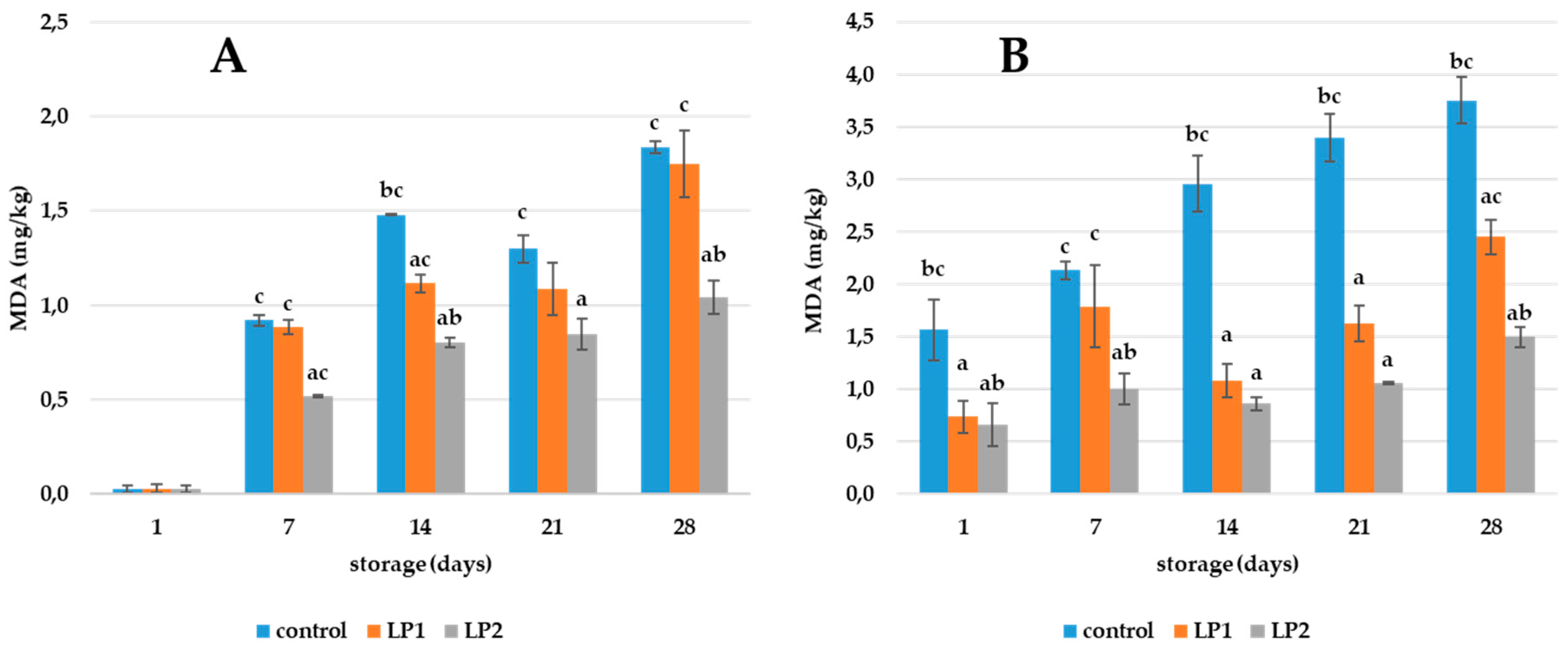
3.3. Effect of LP in Pork Ham and Pâté
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, M.; O´Rourke, A-L.; McLay, J.; Simmonds, R. Use of a ground beef model to assess the effect of the lactoperoxidase system on the growth of Escherichia coli O157:H7, Listeria monocytogenes and Staphylococcus aureus in red meat. International Journal of Food Microbiology 2000, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kaur, L.; Gupta, T.B.; Singh, J.; Bronlund, J. Multitarget preservation technologies for chemical-free sustainable meat processing. Journal of Food Science 2022, 4312–4328. [Google Scholar] [CrossRef] [PubMed]
- Crowe, W.; Elliot, CH.T.; Green, B.D. A Review of the In Vivo Evidence Investigating the Role of Nitrite Exposure from Processed Meat Consumption in the Development of Colorectal Cancer. Nutrients 2019, 2673. [Google Scholar] [CrossRef] [PubMed]
- Magacz, M.; Kędziora, K.; Sapa, J.; Krzyściak, W. The Significance of Lactoperoxidase System in Oral Health: Application and Efficacy in Oral Hygiene Products. International Journal of Molecular Sciences 2019, 1443. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, A.K.; Kaushik, S.; Sinha, M.; Singh, R.P.; Sharma, P.; Sirohi, H.; Kaur, P.; Singh, T.P. Lactoperoxidase: structural insights into the function, ligand binding and inhibition. International Journal of Biochemistry and Molecular Biology 2013, 108–128. [Google Scholar]
- Hoogendoorn, H.; Piessens, J.P.; Scholtes, W.; Stoddard, L.A. Hypothiocyanite ion; the inhibitor formed by the system lactoperoxidase-thiocyanate-hydrogen peroxide. I. Identification of the inhibiting compound. Caries Research 1977, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Seifu, E.; Buys, E.M.; Donkin, E.F. Significance of lactoperoxidase system in the dairy industry and its potential applications: a review. Trends in Food Science and Technology 2005, 137–154. [Google Scholar] [CrossRef]
- Reiter, B.; Härnulv, G. Lactoperoxidase Antibacterial System: Natural Occurrence, Biological Functions and Practical Applications. Journal of Food Protection 1984, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Elliot, R.M.; McLay, J.C.; Kennedy, M.J.; Simmonds, R.S. Inhibition of foodborne bacteria by the lactoperoxidase systemin a beef cube system. International Journal of Food Microbiology 2004, 73–81. [Google Scholar] [CrossRef]
- Bafort, F.; Parisi, O.; Perraudin, J.P.; Jijakli, M.H. Mode of action of lactoperoxidase as related to its antimicrobial activity: a review. Enzyme Research 2014, 517164, 1–13. [Google Scholar] [CrossRef]
- Al-Baarri, A.N.; Ogawa, M.; Hayakawa, S. Scale-up studies on immobilisation of lactoperoxidase using milk whey for producing antimicrobial agent. Journal of the Indonesian Tropical Animal Agriculture 2010, 35, 185–191. [Google Scholar] [CrossRef]
- Whitaker, J.R., Voragen, A.G.J., Wonf, D.W.S. Handbook of Food Enzymology, CRP Press 2003.
- Almehdar, H.A.; El-Fakharany, E.M.; Uversky, V.N.; Redwan, E.M. Disorder in milk proteins: structure, functional disorder, and biocidal potentials of lactoperoxidase. Current Protein Peptide Science 2015, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.G.; Delgadillo, I.; Saraiva, J.A. Effect of thermal pasteurisation and high-pressure processing on immunoglobulin content and lysozyme and lactoperoxidase activity in human colostrum. Food Chemistry 2014, 79–85. [Google Scholar] [CrossRef]
- Silva, E.; Oliveira, J.; Silva, Y.; Urbano, S.; Sales, D.; Moraes, E.; Rangel, A.; Anaya, K. Lactoperoxidase system in the dairy industry: Challenges and opportunities. Czech Journal of Food Science 2020, 337–346. [Google Scholar] [CrossRef]
- de Wit, J.N.; van Hooydonk, A.C.M. Structure, functions and applications of lactoperoxidase in natural antimicrobial systems. Netherlands Milk and Dairy Journal 1996, 227–244. [Google Scholar]
- Dumitrascu, L.; Stanciuc, N.; Stanciu, S.; Râpeanu, G. Thermal inactivation of lactoperoxidase in goat, sheep and bovine milk e a comparative kinetic and thermodynamic study. Journal of Food Engineering 2012, 47–52. [Google Scholar] [CrossRef]
- Marks, N.E.; Grandison, A.S.; Lewis, M.J. Challenge testing of the lactoperoxidase system in pasteurised skim milk. Journal of Applied Microbiology 2001, 735–741. [Google Scholar] [CrossRef]
- Decree No. 69/2016 Coll. Czech Republic (2016): Decree on requirements for meat, meat product, fishery and aquaculture products and products thereof, eggs and products thereof. Collection of laws Czech Republic, 26 (2016): 714-759.
- Beňo, F.; Horsáková, I.; Kmoch, M.; Petrzik, K.; Krátká, G.; Ševčík, R. Bacteriophages as a Strategy to Protect Potato Tubers against Dickeya dianthicola and Pectobacterium carotovorum Soft Rot. Microorganisms 2022, 2369. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Cegielska-Radziejewska, R.; Szablewski, T.; Zabielski, J. TBARS and Microbial Growth Predicative Models of Pork Sausage Stored at Different Temperatures. Czech Journal of Food Science 2015, 320–325. [Google Scholar] [CrossRef]
- Yu, H.H.; Chin, Y.-W.; Paik, H.-D. Application of Natural Preservatives for Meat and Meat Products against Food-Borne Pathogens and Spoilage Bacteria: A Review. Foods 2021, 2418. [Google Scholar] [CrossRef]
- Jooyandeh, H.; Aberoumand, A.; Nasehi, B. Application of lactoperoxidase system in fish and food products: a review. American – Eurasian Journal of Agricultural and Environmental Sciences 2011, 89–96. [Google Scholar]
- Aprodu, J.; Stanciuc, N.; Dumitrascu, L.; Râpeanu, G.; Stanciu, S. Investigations towards understanding the thermal denaturation of lactoperoxidase. International Dairy Journal 2014, 47–54. [Google Scholar] [CrossRef]
- Lara-Aguilar, S.; Alcaine, D.S. Lactose oxidase: a novel activator of the lactoperoxidase system in milk for improved shelf life. Journal of Dairy Science 2019, 1–10. [Google Scholar] [CrossRef]
- Min, S.; Harris, L.J.; Krochta, J.M. Inhibition of Salmonella enterica and Escherichia coli O157: H7 on roasted turkey by edible whey protein coatings incorporating the lactoperoxidase system. Journal of Food Protection 2006, 784–793. [Google Scholar] [CrossRef]
- Ehsani, A.; Hashemi, M.; Afshari, A.; Aminzare, M.; Raeisi, M.; Tayeheb, Z. Effect of different types of active biodegradable films containing lactoperoxidase system or sage essential oil on the shelf life of fish burger during refrigerated storage. LWT 2020, 108633. [Google Scholar] [CrossRef]
- Bensid, A.; Ucar, Y.; Bendeddouche, B.; Özogul, F. Effect of the icing with thyme, oregano and clove extracts on quality parameters of gutted and beheaded anchovy (Engraulis encrasicholus) during chilled storage. Food Chemistry 2014, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Gómez, M. Shelf life of fresh foal meat under MAP, overwrap and vacuum packaging conditions. Meat Science 2012, 610–618. [Google Scholar] [CrossRef]
- Farshidi, M.; Yousefi, M.; Ehsani, A. The combined effects of lactoperoxidase system and whey protein coating on microbial, chemical, textural, and sensory quality of shrimp (Penaeus merguiensis) during cold storage (4 ± 1°C). Food Science and Nutrition 2018, 1378–1386. [Google Scholar] [CrossRef]
- Shokri, S.; Ehsani, A.; Jasour, M.S. Efficacy of lactoperoxidase system-whey protein coating on shelf-life extension of rainbow trout fillets during cold storage (4°C). Food and Bioprocess Technology 2015, 54–62. [Google Scholar] [CrossRef]
- Jasour, M.S.; Ehsani, A.; Mehryar, L.; Naghibi, S.S. Chitosan coating incorporated with the lactoperoxidase system: An active edible coating for fish preservation. Journal of the Science of Food and Agriculture 2015, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Rostami, H.; Abbaszadeh, S.; Shokri, S. Combined effects of lactoperoxidase system-whey protein coating and modified atmosphere packaging on the microbiological, chemical and sensory attributes of Pike-Perch fillets. Journal of Food Science and Technology 2017, 3243–3250. [Google Scholar] [CrossRef] [PubMed]
- Dimova, M.; Tugai, A.; Tugai, T.; Iutynska, G.; Dordevic, D.; Kushkevych, I. Molecular Research of Lipid Peroxidation and Antioxidant Enzyme Activity of Comamonas testosteroni Bacterial Cells under the Hexachlorobenzene Impact. International Journal of Molecular Sciences 2022, 11415. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. Biological Effect of Protein Modifications by Lipid Peroxidation Products. Chemistry and Physics of Lipids 2019, 46–52. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Total viable count (log10 CFU/g ± SD1) | ||
|---|---|---|---|
| day 1 | day 4 | day 7 | |
| control | 5.2c ± 0.1 | 7.7bc ± 0.2 | 9.1bc ± 0.2 |
| control + L. innocua | 5.3c ± 0.1 | 8.2ac ± 0.1 | 9.8ac ± 0.0 |
| L. innocua + LP | 4.7ab ± 0.1 | 6.9ab ± 0.1 | 8.8ab ± 0.1 |
| Meat product | Storage (days) | Count (log10 CFU/g ± SDa) | |||||
|---|---|---|---|---|---|---|---|
| TVC | LAB | ||||||
| control | LP1 | LP2 | control | LP1 | LP2 | ||
| Pork ham | 1 | 4.0bc ± 0.1 | 3.7ac ± 0.1 | 3.4ab ± 0.1 | 2.7bc ± 0.1 | 2.4a ± 0.0 | 2.5a ± 0.1 |
| 7 | 4.5c ± 0.1 | 4.4c ± 0.0 | 3.9ab ± 0.2 | 4.0c ± 0.2 | 3.8 ± 0.1 | 3.7a ± 0.1 | |
| 14 | 5.0c ± 0.2 | 4.5 ± 0.0 | 4.3a ± 0.1 | 4.5bc ± 0.2 | 3.8a ± 0.0 | 3.8a ± 0.1 | |
| 21 | 5.2bc ± 0.1 | 5.1a ± 0.1 | 4.9a ± 0.1 | 5.1c ± 0.1 | 4.9 ± 0.1 | 4.7a ± 0.1 | |
| 28 | 6.3bc ± 0.1 | 6.2ac ± 0.1 | 5.9ab ± 0.1 | 6.1c ± 0.1 | 5.9 ± 0.1 | 5.8a ± 0.0 | |
| Pork pâté | 1 | 4.2c ± 0.2 | 4.0 ± 0.1 | 3.8a ± 0.1 | < 1.0 | < 1.0 | < 1.0 |
| 7 | 5.0c ± 0.0 | 4.8c ± 0.2 | 4.5a ± 0.1 | < 1.0 | < 1.0 | < 1.0 | |
| 14 | 5.9bc ± 0.1 | 5.4ac ± 0.0 | 4.7ab ± 0.2 | < 1.0 | < 1.0 | < 1.0 | |
| 21 | 6.3c ± 0.2 | 6.2 ± 0.1 | 5.1a ± 0.0 | < 1.0 | < 1.0 | < 1.0 | |
| 28 | 6.6bc ± 0.1 | 6.1ac ± 0.1 | 5.6ab ± 0.0 | < 1.0 | < 1.0 | < 1.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





