You are currently viewing a beta version of our website. If you spot anything unusual, kindly let us know.
Preprint
Article
Patient Adherence to a Real-World Digital Asynchronous Weight-Loss Program in Australia That Combines Behavioural and GLP-1 RA Therapy: A Mixed Methods Study
Altmetrics
Downloads
127
Views
85
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Abstract
Increasingly large numbers of people are using digital weight-loss services (DWLSs) to treat overweight or obesity. Although it is widely agreed that digital modalities improve access to care in general, obesity stakeholders remain concerned that many DWLSs are not comprehensive or sustainable enough to deliver meaningful health outcomes. This study adopted a mixed-methods approach to assess why and after how long patients tend to discontinue Austral-ia’s largest DWLS, a program that combines behavioural and pharmacological therapy under the guidance of a multidisciplinary care team. We found that in a cohort of patients who commenced the Eucalyptus DWLS between January and June 2022 (n=5604), mean program adherence was 171.2 (±158.2). Inadequate supply of a patient’s desired Glucose-like peptide-1 receptor agonist medication was the most common reason for discontinuation (43.7%), followed by program cost (26.2%), result dissatisfaction (9.9%), and service dissatisfaction (7.2%). Statistical tests revealed that ethnicity and age both had a significant effect on patient adherence. These findings suggest that DWLSs have the potential to improve access to comprehensive, continuous obesity care, but that care models need to improve upon the one observed in the Eucalyptus Australia DWLS to maximise program adherence.
Keywords:
Subject: Public Health and Healthcare - Public Health and Health Services
1. Introduction
Obesity is a chronic disease that has reached epidemic proportions in Australia and many other countries throughout the world [1]. In recent times, Glucose-like peptide-1 receptor agonists (GLP-1 RAs) have shown promise in delivering significant weight-loss outcomes for people with overweight and obesity [2,3]. A growing number of people are using app-based services to manage their weight, many of which utilise GLP-1 RAs [4]. A plausible reason for this trend is the perception among patients that digital modalities improve their access to care without compromising on quality and safety, which an emerging body of research is starting to corroborate [5,6]. Yet while increasing care access is of vital importance to the global struggle against obesity, ensuring that care models are continuous and designed to engender sustainable behavioural change is equally critical given obesity’s status as a complex chronic disease [7,8].
The complexity of obesity is arguably best reflected in the variety of its determinants. Whereas the condition was regarded in bygone eras as a simple matter of individuals failing to control their eating behaviours and maintain a balance between caloric intake and expenditure (9), there is now general acceptance of the significant impact of social, economic, environmental and biological factors on weight management [10,11,12]. In recent times, stakeholders have drawn attention to an additional factor contributing to rising overweight and obesity levels: the difficulty of accessing quality weight management programs. According to the World Health Organization (WHO), weight management programs should connect patients with coordinated multidisciplinary care teams who provide ongoing treatment for their chronic condition [13]. Managing this type of care across various clinicians in primary care settings on a continual basis can be highly challenging for people with significant work and family commitments.
Digital care modalities have been proposed as a solution to this latter challenge. Proponents often point to findings of digital technologies improving care attendance rates among people living in rural and remote areas who typically have to travel long distances to access face-to-face (F2F) services [14,15]. Other scholars have argued digital care models can generate large financial savings; lead to significant improvements in resource allocation efficiency and data organization; and have the potential mitigate the access barrier to treatment for stigmatized conditions [16,17,18]. Yet despite the evidence of certain populations preferring the scheduling flexibility of telehealth consultations to F2F care and reporting higher check-in rates for other chronic conditions [19], scholars are yet to determine whether digital care models have improved access to quality weight-loss programs.
Compounding this uncertainty is the widespread concern that many digital weight-loss services (DWLSs) are providing substandard or even unethical care. This concern stems from the knowledge that several DWLSs are doing little more than forwarding patients GLP-1 RA scripts after one asynchronous telehealth consult with a previously unknown doctor [20]. Although large-scale randomized controlled trials have consistently shown GLP-1 RAs to have a good safety profile among patients with overweight and obesity [21,22], many stakeholders are sceptical of their safety and effectiveness in real-world DWLS settings, especially in cases where the medication is the central feature of the service. Among the reservations is the belief that few patients are willing to tolerate the physical and emotional challenges of GLP-1 RA obesity treatment for a meaningful period in real-world settings where everyday problems arise, such as family and work commitments, and competing financial demands [23,24]. At present, there does not appear to be any peer-reviewed evidence to support or counter this concern. The greatest concern, however, is that an increasing number of DWLSs and their purveyors are framing GLP-1 RAs as a panacea to obesity [25,26].
While recognising the unprecedented weight-loss efficacy of GLP-1 RAs, WHO and UK National Institute for Health and Care Excellence (NICE) stress that sustained behavioural interventions should always form the backbone of obesity programs [27,28]. This advice likely stems from the understanding that GLP-1 RA clinical trials reporting high efficacy in weight-loss patients all included a significant lifestyle intervention; the emerging knowledge of the post-GLP-1 RA therapy rebound effect; and the general uncertainty around the long-term impact of GLP-1 RA-induced weight loss. Both WHO and NICE also emphasise the importance of delivering comprehensive and continuous obesity care through multidisciplinary teams (MDTs), captured best in this excerpt of the NICE Semaglutide guidelines:
“Semaglutide should only be given alongside a suitably sustained programme of lifestyle interventions with multidisciplinary input…” [29] (pp.33)
Although DWLSs might increase access to obesity treatment, the concern that the treatment in such modalities compromises integral features of obesity management is yet to be refuted.
At present, peer-reviewed research on the quality and safety of real-world DWLSs is scarce. Most studies in this field have investigated DWLSs providing stand-alone behavioural therapy [30,31], which while important in its own ways, does not come close to the scale of potential benefits, risks, and present-day uptake of GLP-1 RA-supported DWLSs. To our knowledge, less than a handful of peer-reviewed studies have been published on a service of the latter description, all of which pertain to the Eucalyptus (Juniper) DWLS. A 2024 study found that the Eucalyptus weight-loss program can deliver meaningful weight-loss outcomes to UK-based patients who adhere to it for 5 months, with a reasonable degree of MDT contact [32]. However, the study’s authors emphasised that the cohort’s high drop-out rate (77.3%) “was the most salient conclusion that could be drawn about the effectiveness of the Juniper UK service”. An analysis of Australian cohort of Eucalyptus DWLS patients found that program adherers achieved comparable weight-loss outcomes to the UK cohort but reported an even higher attrition rate (>95%) [33]. To address concerns about DWLS quality, GLP-1 RA-supported services like Eucalyptus need to demonstrate a lot more than meaningful weight-loss outcomes among engaged or ideal patients. If the average patient discontinues a program before it has any meaningful impact, one could not reasonably claim that the program adheres to WHO and NICE recommendations around care continuity, regardless of its design.
This study aims to conduct a focussed analysis of patient adherence to the Eucalyptus Australia weight-loss program. It is believed that an assessment of when and why Australian Eucalyptus weight-loss patients tend to discontinue their subscriptions will complement the earlier effectiveness studies and enable a more rounded foundational evaluation of the degree to which GLP-1 RA-supported DWLSs can follow international advice in delivering comprehensive and continuous care to good effect.
2. Materials and Methods
2.1. Study Design
The study adopted a mixed-methods design that combined observational quantitative analysis with survey-based research. Bellberry Limited approved the study’s ethics on 22 November 2022.
2.2. Program Overview
This investigation used data from the Eucalyptus digital health company. Eucalyptus has delivered a comprehensive DWLS (under the brand Juniper for women and Pilot for men) to over 70,000 patients across Australia, Germany, Japan, and the UK since 2021. The Eucalyptus DWLS is delivered asynchronously through a mobile application and identical platform that can be accessed via personal computers. The service is accredited by the Australian Council on Healthcare Standards (ACHS) and the UK Digital Technology Assessment Criteria (DTAC).
All Eucalyptus weight-loss patients are allocated a coordinated MDT, consisting of a doctor, a university-qualified dietitian, a pharmacist and a registered nurse, to guide them through personalised health coaching and GLP-1 RA therapy. Health coaching is informed by patient health data which is collected from pre-consultation questionnaires and every subsequent interaction between patients and their MDT. Pre-consultation questionnaires contain up to over 100 questions, including requests for test results and photos, and are used by doctors to determine patient eligibility for the Eucalyptus DWLS. Once MDTs develop personalised diet and exercise plans in consultation with patients, they send through a series of multimodal educational materials to assist them with their care journey. Dietitians message patients at fortnightly intervals to encourage them to upload data to the program progress tracker, and nurses send automated messages to patients every month to assess general health and wellbeing. Patients are free to solicit advice from any member of their MDT as often as they wish, which MDTs typically respond to within less than 24 hours. Patients are also free to request changes to their diet and exercise programs in consultation with their dietitian.
To supplement their behavioural treatment, Eucalyptus DWLS patients are sent a box of GLP-1 RA medications every month. Patients are sent three reminder messages in the lead-up to each order informing them that payment will be taken from their account. Transactions continue to be made unless a patient indicates to their MDT that they wish to cancel their subscription, or if they fail to attend a review or follow-up consult without communicating a reason within 50 days. Follow-up consults are held every three months by registered nurses to renew medication scripts. If patients report side effects, program dissatisfaction or less-than-desirable weight-loss outcomes, nurses will refer them to their prescribing doctor. Review consults are ad hoc and created by MDTs in response to adverse or substandard outcomes reported at any stage of a patient’s care journey. Monthly subscription to the Eucalyptus Australia DWLS costs $285 AUD, which covers every aspect of the service, including medication, app access, and all MDT consults.
All patient-MDT communication is stored on Eucalyptus’ central data repository on Metabase, including all questionnaire responses. MDTs have complete access to all communications concerning their patients through Metabase patient profiles to facilitate care coordination. All data on the Eucalyptus central data repository is encrypted and can only be accessed by MDTs, the Eucalyptus data analytics team, and the Eucalyptus clinical auditing team. When patients report side effects, the message not only goes directly to their MDT, but it also triggers an alarm in the Eucalyptus clinical auditing system. This allows the clinical auditing team to escalate the matter appropriately and ensure a timely intervention is made.
2.3. Participants
The study’s cohort consisted of all Juniper and Pilot weight-loss patients who started the services between 1 January and 29 June 2022. Patients who were already using the service at the start of the study period were excluded.
2.4. Measures
2.4.1. Demographics
Patients entered their baseline demographic data in their pre-consultation questionnaire, including their age, ethnicity, height, weight and gender. Patient Body Mass Index (BMI) is calculated by dividing their weight (in kilograms) by the square of their height (in metres).
2.4.2. Adherence Period
Patient adherence data was retrieved from Eucalyptus’ central data repository on Metabase. Data will be summarised by the 4 possible discontinuation categories. 1/ Patient notified discontinuation – patient notifies their MCT that they are pausing or stopping their subscription; 2/ Consult drop-out – patient fails to attend review or follow-up consult and does not communicate the reason within 50 days (a period long enough for patient to sustain GLP-1 RA order if they stretch dosing schedule); 3/ Doctor decision – patient’s prescribing doctor decides to terminate patient subscription for medical reasons; 4/ Still active – patient has not paused their subscription at any point and is still an active user. For categories 1 and 3, the day the decision was communicated will be taken as the discontinuation date. For consult drop-outs, a patient’s last scheduled consult (the one they failed to attend) was considered their discontinuation date, and for still active patients, the date of analysis was used (5 March, 2024).
2.4.3. Discontinuation Reason
All patients in the cohort were emailed a link on 22 Feb 2024 to a one-question survey hosted by Typeform to establish their primary reason for discontinuing the program. The question was framed as follows: ‘We noticed you paused or stopped your Juniper/Pilot weight-loss program recently. What was the main reason for this decision?’ The order in which the list of responses appeared was randomised and patients could only select one response option. Free-text responses that were added to the ‘other (please specify)’ option were analysed and recoded using the Braun and Clarke thematic analysis method [34]. This method consists of a six-phase analytical procedure grounded in reflexivity. Phase one required the study’s investigators to familiarise themselves with the data, by reading and re-reading all free-text responses. Initial codes, effectively sub-codes in the context of our investigation, were generated in phase two, which were then reviewed and analysed in phase three to allow initial themes (codes) to be identified. The two investigators’ themes were reviewed and compared in phase four and then determined and named in phase five. For the sixth and final phase of the analysis, we completed a final inspection of the analysis and established a percentage-based order of the final codes to ensure all responses had been accounted for.
2.4.4. Endpoints
The coprimary endpoints were mean program adherence days (total days from program initiation to discontinuation) and the percentage distribution of discontinuation reason. Exploratory endpoints included a sub-group analysis of the coprimary endpoints, including gender, age, ethnicity, BMI and discontinuation categories.
2.5. Statistical Analyses
We ran an analysis of variance (ANOVA) to measure the effect of discontinuation category, a categorical variable with four levels, on adherence period, a continuous variable. Post hoc Tukey tests were then conducted to determine the precise levels across which significant differences were observed. To assess the relationship between adherence period and binary categorical predictor variables, such as gender and ethnicity (Caucasian/Non-Caucasian), two-sample T-tests were used. We ran Pearson correlation tests to measure the association between adherence period and continuous predictor variables, including age and BMI.
3. Results
5604 patients initiated a Eucalyptus weight-loss program within the study period, including 3339 women and 2266 men (all baseline data in Table 1). The mean adherence period observed for the entire cohort was 171.2 (±158.2) days. 2088 of these patients responded to the one-question survey on their discontinuation reason. Inadequate GLP-1 RA supply was the most common response (43.7%), followed by program cost (26.2%), dissatisfaction with results (9.9%) and dissatisfaction with the service (7.2%). All primary endpoint data are presented in Table 2. Four response categories were re-coded from open text responses in the ‘other (please specify)’ option, using the Braun and Clarke thematic analysis method, including ‘Inadequate GLP-1 RA supply’, ‘Dissatisfaction with Eucalyptus service’, ‘MDT encouraged me to discontinue’, and ‘Concerns about the long-term effect of GLP-1 RAs’. The ‘Inadequate GLP-1 RA supply’ category captured all responses that emphasised either the unavailability or shortage of a certain GLP-1 RA, or an unwillingness to change GLP-1 RA type (as necessitated by an inadequate supply). Examples of such responses include “Medication no longer available and alternative wasn’t for me”; “You ran out of product”; “I wanted to wait until you got stock of the original Ozempic brand”. In the few cases where open text responses referred to GLP-1 RA supply and another reason, responses were re-coded as the other reason, as the authors felt they were likely to have been more impactful e.g., the response “Poor supply and customer service” was re-coded as ‘Dissatisfaction with the service.”
The bulk of the cohort fell into one of two discontinuation categories. 49.9% paused or ended their subscription at a mean of 141.7(±136) days after initiation. 48.1% stopped following up with their MDT at a mean of 180.4 (±144) days post program commencement. 113 (2%) patients were still active users of their respective programs at the time of analysis, for whom the mean adherence period was 678 (±50.5) days. 1 patient was discontinued from the Juniper program by their doctor on day 166 due to medical reasons (Figure 1). The distribution of reasons given for discontinuation was similar across Juniper and Pilot programs. Inadequate Semaglutide supply was the most common reason (Juniper 43.2%; Pilot 45.1%), followed by program cost (Juniper 25.2%; Pilot 23.5%), dissatisfaction with results (Juniper 10.2%; Pilot 8.8%), and dissatisfaction with service (Juniper 6.6%; Pilot 6.9%).
An analysis of variance (ANOVA) revealed significant variation among the discontinuation categories, F(2, 5601) = [817.8], p < 0.01), and a post hoc Tukey test showed that all three groups (excluding doctor cancellations) differed significantly from one another at p <0.01. Although a slightly higher mean adherence period was observed among Juniper patients (M =173.0, SD = 160) compared to Pilot patients (M = 168.5, SD = 155), a two-sample t-test revealed that this difference was not statistically significant, t(5603) =1.04, p=0.3. A Pearson correlation test showed that patient adherence was positively associated with age (r (5601) = .09, p <0.01) to a small, yet statistically significant degree; and that BMI had no significant effect on adherence (r (5602) = 0.01, p >0.4. Due to the sample’s low representation across non-Caucasian ethnic groups, we created a Caucasian/non-Caucasian binary variable for ethnicity. A two-sample t-test revealed that Caucasian patients (M=175.8, SD = 160) tended to adhere to the Eucalyptus program for longer than non-Caucasian patients (M = 148.7, SD = 149) and that this difference was statistically significant, t(5603) = 4.84, p <0.01.
4. Discussion
To our knowledge, this study represents the first focussed investigation of patient adherence to a real-world digital GLP-1 RA-supported weight-loss program. A mixed-methods approach was used to discover why and after how long Australian patients tend to discontinue the Eucalyptus (Juniper and Pilot) DWLS. The quantitative analysis found that mean adherence to the Eucalyptus weight-loss program (Juniper and Pilot) was 171.2 (±158.2) days for patients who commenced their subscription between January and June 2022. However, data were widely dispersed and therefore the median adherence result of 115 days may be more informative. We also found that over two-thirds of patients who completed the survey, discontinued for reasons concerning an inadequate supply of their desired GLP-1 RA medication or program cost. A significant portion of patients dropped out of the program due to their dissatisfaction with their weight loss outcomes or the Eucalyptus DWLS in general. Ethnicity and age were both observed to have had a statistically significant effect on patient adherence. These findings suggest that DWLSs have the potential to mitigate a significant factor in global overweight and obesity levels, but that care models need to improve upon the one observed in the Eucalyptus Australia DWLS.
4.1. The Significance of Patient Adherence to DWLSs
The difficulty of accessing continuous coordinated multidisciplinary obesity care has contributed to the steady rise in the disease’s incidence over the past 3 decades. Many care providers have responded to this access barrier by launching DWLSs. Yet while the logic of digital modalities improving initial access to obesity care is broadly accepted, prior to this study, no one had attempted to investigate whether DWLSs improve access to the type of care obesity requires. In other words, no study had investigated whether patients can adhere to DWLSs for long enough for them to have a meaningful effect. Previous studies had indicated that a high proportion of patients drop out of the Eucalyptus DWLS before 5- and 7-month weight-loss measurements [35,36]. However, those were effectiveness studies whose discontinuation rates were significantly impacted by strict inclusion criteria. This study, on the other hand, was a focussed adherence analysis that included every single patient who started the Juniper DWLS within the study window.
Although the observed mean adherence period [171.2 (±158.2) days] for the Eucalyptus DWLS appears reasonable, it is difficult to assess without any comparable data available. WHO emphasises Obesity's chronicity and the importance of ongoing comprehensive care. While some DWLSs might be tempted to argue that many patients who supplement their lifestyle treatment with GLP-1 RA therapy can achieve enough weight loss to move into a healthy BMI range within 171 days, until there is more knowledge around the sustainability of GLP-1 RA-induced weight loss, DWLSs should be underpinned by long-term lifestyle interventions. It is unlikely WHO or other major health institutions would consider 171 days long enough for an individual to adopt sustainable behavioural changes. Nevertheless, researchers now have an initial benchmark for ongoing research in patient adherence to GLP-1 RA-supported DWLSs.
Discoveries of the cohort’s skewed adherence distribution and the distribution of discontinuation reasons were arguably more informative than the mean adherence rate. Given the relatively large sample (5604), the asymmetrical adherence distribution can be interpreted as a reliable reflection of the varied experiences among Eucalyptus DWLS patients rather than random noise. The broad range of discontinuation reason responses enriches this data by illuminating the many factors that impact adherence to real-world GLP-1 RA supported DWLSs. In addition to highlighting the importance of program cost, service quality, and side effect management, the survey responses indicate that a high percentage of patients will drop out of a DWLS if their desired GLP-1 RA dose becomes unavailable, even if the service is supported by a behavioural intervention. This problem may be specific to care models such as Eucalyptus’ that only offer GLP-1-supported programs, rather than services with standalone behavioural options for which a program transfer could be available. It is understandable that many Eucalyptus patients were not willing to tolerate anything less than their most desired GLP-1 RA medication given the service’s high monthly subscription fee of $285 AUD. The direct and indirect impact of cost on DWLS adherence is likely to be significant in any GLP-1 RA-supported program that is not subsidised.
4.2. Future Research
The ultimate goal of this study was to assess average patient adherence to the Eucalyptus DWLS and the service’s key discontinuation factors. This research question represents one half of the question as to whether DWLSs have the potential to increase access to the type of care people with obesity need, and one part of the broader series of questions that need to be addressed around DWLS quality and safety. While this study generated some findings around average adherence to the Eucalyptus Australia program and patient discontinuation reasons, similar research on several other DWLSs and F2F obesity services is needed before strong conclusions around adherence standards and safeguards can be made. Regarding the other half of the obesity care access question, future studies should seek to conduct deep analyses of real-world DWLS care models. Although this study assumed that the Eucalyptus DWLS delivered holistic multidisciplinary care underpinned by behavioural therapy to the investigated cohort (based on the service’s claims and its ACHS and DTAC accreditations), it did not attempt to systematically verify this. Future research might, for example, endeavour to quantify the number of behaviour-related messages a DWLS provides in a certain period or assess the precise behavioural changes patients adopt over the course of a program. Researchers should also consider prospective analyses of real-world DWLSs that measure the association between adherence, engagement, and effectiveness outcomes across segmented adherence periods.
4.3. Strengths and Limitations
The study used a large sample of real-world patients and did not exclude anyone who initiated the Eucalyptus DWLS within the specified analysis period. Data were collected from a commercially available, ACHS-accredited data repository, and the collection and analysis of these data did not interfere with Eucalyptus patient experiences in any way. To our knowledge, the research question is the first of its kind and thus the findings set an initial benchmark for ongoing research into real-world DWLS adherence.
The study also contained several limitations. Firstly, survey responses were collected from less than half the cohort (37.3%), and were sent to most patients long after they had discontinued (22 Feb 2024) the Eucalyptus program. It is feasible that many non-responders did not feel as strongly about their previous discontinuation reason as those who could no longer receive their desired GLP-1 RA medication or afford the program. Consequently, the reported discontinuation reasons may not have been representative of the cohort. Secondly, survey data could not be linked to other discontinuation data due to privacy restrictions and so a deeper investigation of the observed impact of ethnicity and age on adherence could not be conducted. This issue may have been resolvable had patient income data been collected, but these data were also unavailable. A third limitation of the study was its predominantly Caucasian sample. Finally, investigators had to estimate the discontinuation date for patients who failed to attend or review follow-up consults. Although the method for estimating this date (50 days after the last attended consult) seemed logical given the standard coverage period of GLP-1 RA orders and historical consultation intervals of Eucalyptus weight-loss patients, it is possible that a high percentage of this category of discontinuers stopped their therapy earlier than the estimated date.
5. Conclusions
Increasingly large numbers of people are using DWLSs to treat overweight and obesity, yet stakeholders remain sceptical of these services’ capability to provide continuous and comprehensive care. To assess the validity of these concerns, scholars need to investigate patient adherence to real-world comprehensive DWLSs. This study adopted a mixed methods approach to measure why and after how long patients of the Eucalyptus Australia DWLS discontinue the service. Our findings set an important foundation in the emerging literature on modern weight-loss interventions. They indicate that patient adherence to digital GLP-1 RA-supported weight-loss programs vary significantly in real-world, non-subsidised settings, and that medication supply and program cost are two of the key determinants of this adherence. To deepen understanding of the potential of DWLSs in mitigating global obesity rates, similar adherence data needs to be obtained from numerous other DWLSs. Future research should also consider exploring the relationship between adherence, MDT engagement and weight-loss effectiveness across segmented adherence periods.
Author Contributions
Conceptualization, L.T., and M.V.; methodology, L.T; software, L.T.; validation, L.T and M.V.; formal analysis, L.T., and M.V.; investigation, L.T.; resources, L.T., and M.V.; data curation, L.T.; writing—original draft preparation, L.T.; writing—review and editing, L.T. and M.V.; visualization, L.T.; supervision, M.V.; project administration, L.T.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Bellberry Ethics Committee (# 2023-05-563-A-1, 22 November 2023).
Informed Consent Statement
This study received exemption status from the Bellberry Ethics Committee (# 2023-05-563-A-1, 22 November 2023) for retrospective analyses of de-identified data. Eucalyptus patients consent to the service’s privacy policy at subscription, which includes permission to use their de-identified data for research.
Data Availability Statement
The data presented in this study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to thank all patients and clinicians involved in the Eucalyptus weight-loss program over the study period.
Conflicts of Interest
LT and MV are paid a salary by Eucalyptus.
References
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217–155217. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, X., Astrup, A., Fujioka, K., et al. A randomized, controlled trial of 3.0mg of Liragltuide in weight management. N Engl J Med 2015, 373, 11–22. [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Irvin, L.; Madden, L.A.; Marshall, P.; Vince, R.V. Digital Health Solutions for Weight Loss and Obesity: A Narrative Review. Nutrients 2023, 15, 1858. [Google Scholar] [CrossRef]
- Hinchliffe, N.; Capehorn, M.S.; Bewick, M.; Feenie, J. The Potential Role of Digital Health in Obesity Care. Adv. Ther. 2022, 39, 4397–4412. [Google Scholar] [CrossRef] [PubMed]
- Irvin, L.; Madden, L.A.; Marshall, P.; Vince, R.V. Digital Health Solutions for Weight Loss and Obesity: A Narrative Review. Nutrients 2023, 15, 1858. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Health service delivery framework for prevention and management of obesity, 2023. Geneva: WHO.
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. European Guidelines for Obesity Management in Adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef]
- Hall, K.D.; Heymsfield, S.B.; Kemnitz, J.W.; Klein, S.; Schoeller, D.A.; Speakman, J.R. Energy balance and its components: implications for body weight regulation. Am. J. Clin. Nutr. 2012, 95, 989–994. [Google Scholar] [CrossRef]
- Javed, Z.; Valero-Elizondo, J.; Maqsood, M.H.; Mahajan, S.; Taha, M.B.; Patel, K.V.; Sharma, G.; Hagan, K.; Blaha, M.J.; Blankstein, R.; et al. Social determinants of health and obesity: Findings from a national study of US adults. Obesity 2022, 30, 491–502. [Google Scholar] [CrossRef]
- Verde, L.; Frias-Toral, E.; Cardenas, D. Editorial: Environmental factors implicated in obesity. Front. Nutr. 2023, 10. [Google Scholar] [CrossRef]
- Hall, K.D.; Farooqi, I.S.; Friedman, J.M.; Klein, S.; Loos, R.J.F.; Mangelsdorf, D.J.; O’rahilly, S.; Ravussin, E.; Redman, L.M.; Ryan, D.H.; et al. The energy balance model of obesity: beyond calories in, calories out. Am. J. Clin. Nutr. 2022, 115, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Health service framework for prevention and management of obesity. Geneva, 2023.
- Scheer, J.; Areias, A.C.; Molinos, M.; Janela, D.; Moulder, R.; Lains, J.; Bento, V.; Yanamadala, V.; Correia, F.D.; Costa, F. Engagement and Utilization of a Complete Remote Digital Care Program for Musculoskeletal Pain Management in Urban and Rural Areas Across the United States: Longitudinal Cohort Study. JMIR mHealth uHealth 2023, 11, e44316. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, D., & Marciniuk, D. Rural and remote care: Overcoming the challenges of distance. Chronic respiratory disease 2016, 13, 192–203.
- Sweet, C.C.; Jasik, C.B.; Diebold, A.; DuPuis, A.; Jendretzke, B. Cost Savings and Reduced Health Care Utilization Associated with Participation in a Digital Diabetes Prevention Program in an Adult Workforce Population. J. Heal. Econ. Outcomes Res. 2023, 7, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Ekman, B. Cost Analysis of a Digital Health Care Model in Sweden. PharmacoEconomics - Open 2017, 2, 347–354. [Google Scholar] [CrossRef]
- Naslund, J.; Deng, D. Addressing mental health stigma in low-income and middle-income countries: A new frontier for digital mental health. Ethic- Med. Public Heal. 2021, 19. [Google Scholar] [CrossRef] [PubMed]
- Quisel, T., Foschini, L., Zbikowski, S., et al. The association between medication adherence for chronic conditions and digital health activity tracking: Retrospective analysis. J Med Internet Res 2019, 21, e11486. [CrossRef]
- Talay, L. , Alvi, O. Digital healthcare solutions to better achieve the weight loss outcomes expected by payors and patients. Diabetes, Obesity and Metabolism.
- Pi-Sunyer, X., Astrup, A., Fujioka, K., et al. A randomized, controlled trial of 3.0mg of Liragltuide in weight management. N Engl J Med 2015, 373, 11–22. [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Carls, G.S.; Tuttle, E.; Tan, R.-D.; Huynh, J.; Yee, J.; Edelman, S.V.; Polonsky, W.H. Understanding the Gap Between Efficacy in Randomized Controlled Trials and Effectiveness in Real-World Use of GLP-1 RA and DPP-4 Therapies in Patients With Type 2 Diabetes. Diabetes Care 2017, 40, 1469–1478. [Google Scholar] [CrossRef]
- Palanca, A.; Ampudia-Blasco, F.J.; Calderón, J.M.; Sauri, I.; Martinez-Hervás, S.; Trillo, J.L.; Redón, J.; Real, J.T. Real-World Evaluation of GLP-1 Receptor Agonist Therapy Persistence, Adherence and Therapeutic Inertia Among Obese Adults with Type 2 Diabetes. Diabetes Ther. 2023, 14, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Fallows, E.; Ells, L.; Anand, V. Semaglutide and the future of obesity care in the UK. Lancet 2023, 401, 2093–2096. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K. The role of commercial weight loss programmes. In: Haslam, D., Malhotra, A., Capehorn, S. (eds) Bariatric surgery in clinical practice. In clinical practice. Springer. 2022.
- World Health Organization. Health service framework for prevention and management of obesity. Geneva, 2023.
- National Institute for Health and Care Excellence. Semaglutide for managing overweight and obesity, Sep 2023.
- National Institute for Health and Care Excellence. Semaglutide for managing overweight and obesity, Sep 2023.
- Gemesi, K., Winkler, S., Schmidt-Tesch, S., et al. Efficacy of an app-based multimodal lifestyle intervention on bodyweight in persons with obesity: results from a randomised controlled trial. International Journal of Obesity, Nov 2023, 48, 118–126.
- upila, S., Joki, A., Suojanen, L, et al. The effectiveness of eHealth interventions for weight loss and weight loss maintenance in adults with overweight and obesity: A systematic review of systematic reviews. Curr Obes Rep;12, 371-394.
- Talay, L. , & Vickers, M. Effectiveness and care continuity in an app-based, glucagon-like peptide-1 receptor agonist-supported weight-loss service for women with overweight and obesity in the UK: A real-world retrospective cohort analysis. Diabetes, Obesity and Metabolism.
- Talay, L. , Alvi, O. Digital healthcare solutions to better achieve the weight loss outcomes expected by payors and patients. Diabetes, Obesity and Metabolism.
- Braun, V.; Clarke, V. Thematic Analysis: A Practical Guide. Qmip Bull. 2022, 1, 46–50. [Google Scholar] [CrossRef]
- Talay, L. , & Vickers, M. Effectiveness and care continuity in an app-based, glucagon-like peptide-1 receptor agonist-supported weight-loss service for women with overweight and obesity in the UK: A real-world retrospective cohort analysis. Diabetes, Obesity and Metabolism.
- Talay, L. , Alvi, O. Digital healthcare solutions to better achieve the weight loss outcomes expected by payors and patients. Diabetes, Obesity and Metabolism.
Figure 1.
Adherence distribution and mean by discontinuation category.
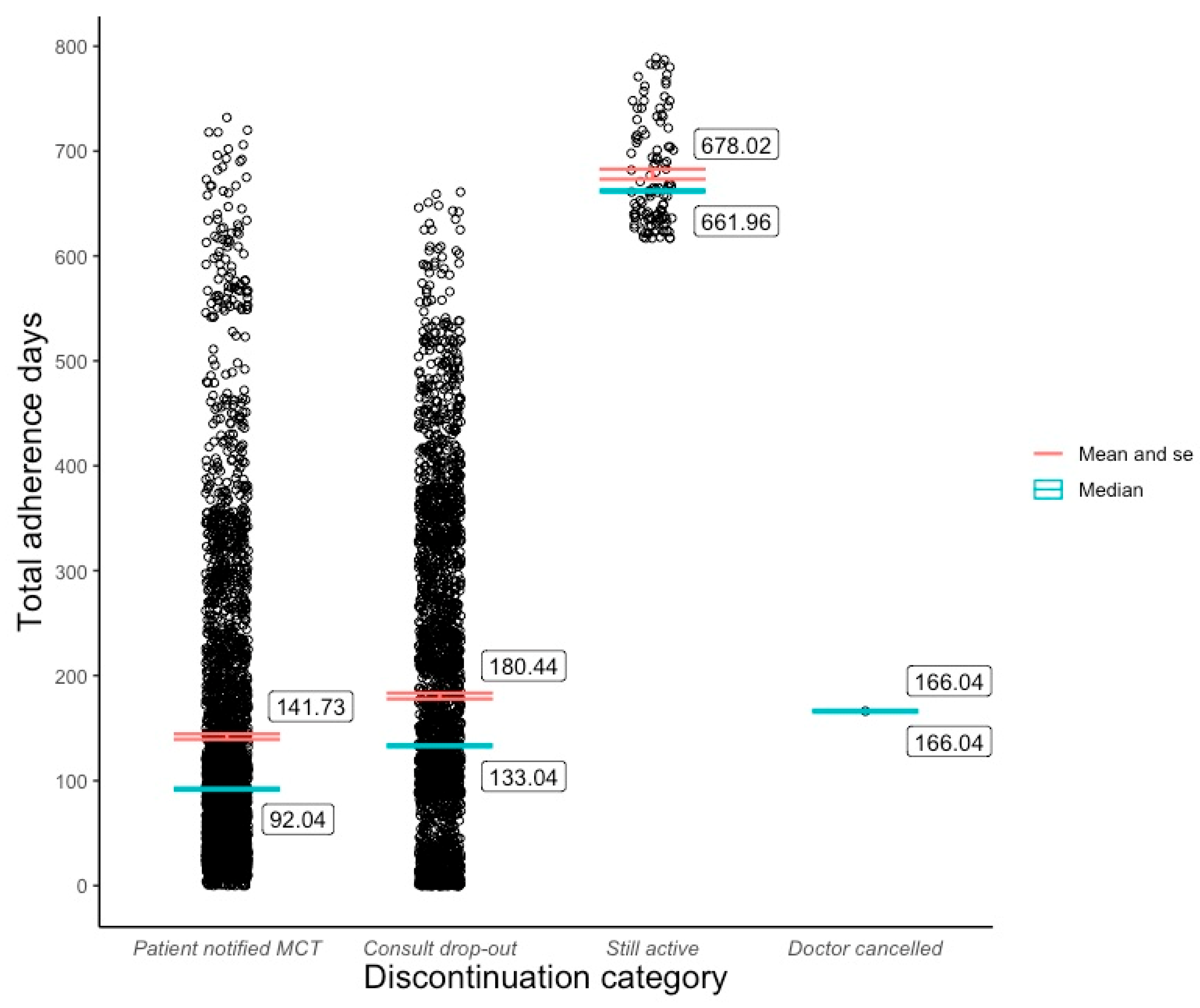
Table 1.
Patient characteristics.
| Demographic information | |
|---|---|
| Age – mean (SD) | 41.6 (10.12) |
| Gender – no. (%) | |
| Female (Juniper) | 3339 (59.6) |
| Male (Pilot) | 2266 (40.4) |
| Ethnicity – no. (%) | |
| Caucasian | 4645 (82.9) |
| Asian (Inc. subcontinent) | 162 (5.48) |
| Not listed | 298 (5.32) |
| Indigenous Australian | 137 (2.44) |
| Baseline clinical information – mean (SD) | |
| BMI | 35.17(8.8) |
Table 2.
Primary endpoint data.
| Adherence period – mean (SD); median | |
|---|---|
| Juniper (female) | 173.0 (160) days; 117 days |
| Pilot (male) | 168.5 (155) days; 113 days |
| Total cohort | 171.2 (158.2) days; 115 days |
| Discontinuation reasons – no. (%) | |
| Inadequate GLP-1 RA supply* | 912 (43.7) |
| “It was too expensive” | 547 (26.2) |
| “I was not seeing results” | 206 (9.9) |
| Dissatisfaction with the service | 150 (7.2) |
| “My side effects were intolerable” | 79 (3.8) |
| “Weight loss was no longer a priority for me” | 76 (3.6) |
| “I found a better digital weight-loss service” | 49 (2.3) |
| “I found a better in-person weight loss service” | 35 (1.7) |
| MDT encouraged me to discontinue | 19 (0.9) |
| Concerns about the long-term effect of GLP-1 RAs | 15 (0.7) |
* The four non-italicised response categories were re-coded from open text responses in the ‘other (please specify)’ category, using the Braun and Clarke thematic analysis method.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Submitted:
01 May 2024
Posted:
01 May 2024
You are already at the latest version
Alerts
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
01 May 2024
Posted:
01 May 2024
You are already at the latest version
Alerts
Abstract
Increasingly large numbers of people are using digital weight-loss services (DWLSs) to treat overweight or obesity. Although it is widely agreed that digital modalities improve access to care in general, obesity stakeholders remain concerned that many DWLSs are not comprehensive or sustainable enough to deliver meaningful health outcomes. This study adopted a mixed-methods approach to assess why and after how long patients tend to discontinue Austral-ia’s largest DWLS, a program that combines behavioural and pharmacological therapy under the guidance of a multidisciplinary care team. We found that in a cohort of patients who commenced the Eucalyptus DWLS between January and June 2022 (n=5604), mean program adherence was 171.2 (±158.2). Inadequate supply of a patient’s desired Glucose-like peptide-1 receptor agonist medication was the most common reason for discontinuation (43.7%), followed by program cost (26.2%), result dissatisfaction (9.9%), and service dissatisfaction (7.2%). Statistical tests revealed that ethnicity and age both had a significant effect on patient adherence. These findings suggest that DWLSs have the potential to improve access to comprehensive, continuous obesity care, but that care models need to improve upon the one observed in the Eucalyptus Australia DWLS to maximise program adherence.
Keywords:
Subject: Public Health and Healthcare - Public Health and Health Services
1. Introduction
Obesity is a chronic disease that has reached epidemic proportions in Australia and many other countries throughout the world [1]. In recent times, Glucose-like peptide-1 receptor agonists (GLP-1 RAs) have shown promise in delivering significant weight-loss outcomes for people with overweight and obesity [2,3]. A growing number of people are using app-based services to manage their weight, many of which utilise GLP-1 RAs [4]. A plausible reason for this trend is the perception among patients that digital modalities improve their access to care without compromising on quality and safety, which an emerging body of research is starting to corroborate [5,6]. Yet while increasing care access is of vital importance to the global struggle against obesity, ensuring that care models are continuous and designed to engender sustainable behavioural change is equally critical given obesity’s status as a complex chronic disease [7,8].
The complexity of obesity is arguably best reflected in the variety of its determinants. Whereas the condition was regarded in bygone eras as a simple matter of individuals failing to control their eating behaviours and maintain a balance between caloric intake and expenditure (9), there is now general acceptance of the significant impact of social, economic, environmental and biological factors on weight management [10,11,12]. In recent times, stakeholders have drawn attention to an additional factor contributing to rising overweight and obesity levels: the difficulty of accessing quality weight management programs. According to the World Health Organization (WHO), weight management programs should connect patients with coordinated multidisciplinary care teams who provide ongoing treatment for their chronic condition [13]. Managing this type of care across various clinicians in primary care settings on a continual basis can be highly challenging for people with significant work and family commitments.
Digital care modalities have been proposed as a solution to this latter challenge. Proponents often point to findings of digital technologies improving care attendance rates among people living in rural and remote areas who typically have to travel long distances to access face-to-face (F2F) services [14,15]. Other scholars have argued digital care models can generate large financial savings; lead to significant improvements in resource allocation efficiency and data organization; and have the potential mitigate the access barrier to treatment for stigmatized conditions [16,17,18]. Yet despite the evidence of certain populations preferring the scheduling flexibility of telehealth consultations to F2F care and reporting higher check-in rates for other chronic conditions [19], scholars are yet to determine whether digital care models have improved access to quality weight-loss programs.
Compounding this uncertainty is the widespread concern that many digital weight-loss services (DWLSs) are providing substandard or even unethical care. This concern stems from the knowledge that several DWLSs are doing little more than forwarding patients GLP-1 RA scripts after one asynchronous telehealth consult with a previously unknown doctor [20]. Although large-scale randomized controlled trials have consistently shown GLP-1 RAs to have a good safety profile among patients with overweight and obesity [21,22], many stakeholders are sceptical of their safety and effectiveness in real-world DWLS settings, especially in cases where the medication is the central feature of the service. Among the reservations is the belief that few patients are willing to tolerate the physical and emotional challenges of GLP-1 RA obesity treatment for a meaningful period in real-world settings where everyday problems arise, such as family and work commitments, and competing financial demands [23,24]. At present, there does not appear to be any peer-reviewed evidence to support or counter this concern. The greatest concern, however, is that an increasing number of DWLSs and their purveyors are framing GLP-1 RAs as a panacea to obesity [25,26].
While recognising the unprecedented weight-loss efficacy of GLP-1 RAs, WHO and UK National Institute for Health and Care Excellence (NICE) stress that sustained behavioural interventions should always form the backbone of obesity programs [27,28]. This advice likely stems from the understanding that GLP-1 RA clinical trials reporting high efficacy in weight-loss patients all included a significant lifestyle intervention; the emerging knowledge of the post-GLP-1 RA therapy rebound effect; and the general uncertainty around the long-term impact of GLP-1 RA-induced weight loss. Both WHO and NICE also emphasise the importance of delivering comprehensive and continuous obesity care through multidisciplinary teams (MDTs), captured best in this excerpt of the NICE Semaglutide guidelines:
“Semaglutide should only be given alongside a suitably sustained programme of lifestyle interventions with multidisciplinary input…” [29] (pp.33)
Although DWLSs might increase access to obesity treatment, the concern that the treatment in such modalities compromises integral features of obesity management is yet to be refuted.
At present, peer-reviewed research on the quality and safety of real-world DWLSs is scarce. Most studies in this field have investigated DWLSs providing stand-alone behavioural therapy [30,31], which while important in its own ways, does not come close to the scale of potential benefits, risks, and present-day uptake of GLP-1 RA-supported DWLSs. To our knowledge, less than a handful of peer-reviewed studies have been published on a service of the latter description, all of which pertain to the Eucalyptus (Juniper) DWLS. A 2024 study found that the Eucalyptus weight-loss program can deliver meaningful weight-loss outcomes to UK-based patients who adhere to it for 5 months, with a reasonable degree of MDT contact [32]. However, the study’s authors emphasised that the cohort’s high drop-out rate (77.3%) “was the most salient conclusion that could be drawn about the effectiveness of the Juniper UK service”. An analysis of Australian cohort of Eucalyptus DWLS patients found that program adherers achieved comparable weight-loss outcomes to the UK cohort but reported an even higher attrition rate (>95%) [33]. To address concerns about DWLS quality, GLP-1 RA-supported services like Eucalyptus need to demonstrate a lot more than meaningful weight-loss outcomes among engaged or ideal patients. If the average patient discontinues a program before it has any meaningful impact, one could not reasonably claim that the program adheres to WHO and NICE recommendations around care continuity, regardless of its design.
This study aims to conduct a focussed analysis of patient adherence to the Eucalyptus Australia weight-loss program. It is believed that an assessment of when and why Australian Eucalyptus weight-loss patients tend to discontinue their subscriptions will complement the earlier effectiveness studies and enable a more rounded foundational evaluation of the degree to which GLP-1 RA-supported DWLSs can follow international advice in delivering comprehensive and continuous care to good effect.
2. Materials and Methods
2.1. Study Design
The study adopted a mixed-methods design that combined observational quantitative analysis with survey-based research. Bellberry Limited approved the study’s ethics on 22 November 2022.
2.2. Program Overview
This investigation used data from the Eucalyptus digital health company. Eucalyptus has delivered a comprehensive DWLS (under the brand Juniper for women and Pilot for men) to over 70,000 patients across Australia, Germany, Japan, and the UK since 2021. The Eucalyptus DWLS is delivered asynchronously through a mobile application and identical platform that can be accessed via personal computers. The service is accredited by the Australian Council on Healthcare Standards (ACHS) and the UK Digital Technology Assessment Criteria (DTAC).
All Eucalyptus weight-loss patients are allocated a coordinated MDT, consisting of a doctor, a university-qualified dietitian, a pharmacist and a registered nurse, to guide them through personalised health coaching and GLP-1 RA therapy. Health coaching is informed by patient health data which is collected from pre-consultation questionnaires and every subsequent interaction between patients and their MDT. Pre-consultation questionnaires contain up to over 100 questions, including requests for test results and photos, and are used by doctors to determine patient eligibility for the Eucalyptus DWLS. Once MDTs develop personalised diet and exercise plans in consultation with patients, they send through a series of multimodal educational materials to assist them with their care journey. Dietitians message patients at fortnightly intervals to encourage them to upload data to the program progress tracker, and nurses send automated messages to patients every month to assess general health and wellbeing. Patients are free to solicit advice from any member of their MDT as often as they wish, which MDTs typically respond to within less than 24 hours. Patients are also free to request changes to their diet and exercise programs in consultation with their dietitian.
To supplement their behavioural treatment, Eucalyptus DWLS patients are sent a box of GLP-1 RA medications every month. Patients are sent three reminder messages in the lead-up to each order informing them that payment will be taken from their account. Transactions continue to be made unless a patient indicates to their MDT that they wish to cancel their subscription, or if they fail to attend a review or follow-up consult without communicating a reason within 50 days. Follow-up consults are held every three months by registered nurses to renew medication scripts. If patients report side effects, program dissatisfaction or less-than-desirable weight-loss outcomes, nurses will refer them to their prescribing doctor. Review consults are ad hoc and created by MDTs in response to adverse or substandard outcomes reported at any stage of a patient’s care journey. Monthly subscription to the Eucalyptus Australia DWLS costs $285 AUD, which covers every aspect of the service, including medication, app access, and all MDT consults.
All patient-MDT communication is stored on Eucalyptus’ central data repository on Metabase, including all questionnaire responses. MDTs have complete access to all communications concerning their patients through Metabase patient profiles to facilitate care coordination. All data on the Eucalyptus central data repository is encrypted and can only be accessed by MDTs, the Eucalyptus data analytics team, and the Eucalyptus clinical auditing team. When patients report side effects, the message not only goes directly to their MDT, but it also triggers an alarm in the Eucalyptus clinical auditing system. This allows the clinical auditing team to escalate the matter appropriately and ensure a timely intervention is made.
2.3. Participants
The study’s cohort consisted of all Juniper and Pilot weight-loss patients who started the services between 1 January and 29 June 2022. Patients who were already using the service at the start of the study period were excluded.
2.4. Measures
2.4.1. Demographics
Patients entered their baseline demographic data in their pre-consultation questionnaire, including their age, ethnicity, height, weight and gender. Patient Body Mass Index (BMI) is calculated by dividing their weight (in kilograms) by the square of their height (in metres).
2.4.2. Adherence Period
Patient adherence data was retrieved from Eucalyptus’ central data repository on Metabase. Data will be summarised by the 4 possible discontinuation categories. 1/ Patient notified discontinuation – patient notifies their MCT that they are pausing or stopping their subscription; 2/ Consult drop-out – patient fails to attend review or follow-up consult and does not communicate the reason within 50 days (a period long enough for patient to sustain GLP-1 RA order if they stretch dosing schedule); 3/ Doctor decision – patient’s prescribing doctor decides to terminate patient subscription for medical reasons; 4/ Still active – patient has not paused their subscription at any point and is still an active user. For categories 1 and 3, the day the decision was communicated will be taken as the discontinuation date. For consult drop-outs, a patient’s last scheduled consult (the one they failed to attend) was considered their discontinuation date, and for still active patients, the date of analysis was used (5 March, 2024).
2.4.3. Discontinuation Reason
All patients in the cohort were emailed a link on 22 Feb 2024 to a one-question survey hosted by Typeform to establish their primary reason for discontinuing the program. The question was framed as follows: ‘We noticed you paused or stopped your Juniper/Pilot weight-loss program recently. What was the main reason for this decision?’ The order in which the list of responses appeared was randomised and patients could only select one response option. Free-text responses that were added to the ‘other (please specify)’ option were analysed and recoded using the Braun and Clarke thematic analysis method [34]. This method consists of a six-phase analytical procedure grounded in reflexivity. Phase one required the study’s investigators to familiarise themselves with the data, by reading and re-reading all free-text responses. Initial codes, effectively sub-codes in the context of our investigation, were generated in phase two, which were then reviewed and analysed in phase three to allow initial themes (codes) to be identified. The two investigators’ themes were reviewed and compared in phase four and then determined and named in phase five. For the sixth and final phase of the analysis, we completed a final inspection of the analysis and established a percentage-based order of the final codes to ensure all responses had been accounted for.
2.4.4. Endpoints
The coprimary endpoints were mean program adherence days (total days from program initiation to discontinuation) and the percentage distribution of discontinuation reason. Exploratory endpoints included a sub-group analysis of the coprimary endpoints, including gender, age, ethnicity, BMI and discontinuation categories.
2.5. Statistical Analyses
We ran an analysis of variance (ANOVA) to measure the effect of discontinuation category, a categorical variable with four levels, on adherence period, a continuous variable. Post hoc Tukey tests were then conducted to determine the precise levels across which significant differences were observed. To assess the relationship between adherence period and binary categorical predictor variables, such as gender and ethnicity (Caucasian/Non-Caucasian), two-sample T-tests were used. We ran Pearson correlation tests to measure the association between adherence period and continuous predictor variables, including age and BMI.
3. Results
5604 patients initiated a Eucalyptus weight-loss program within the study period, including 3339 women and 2266 men (all baseline data in Table 1). The mean adherence period observed for the entire cohort was 171.2 (±158.2) days. 2088 of these patients responded to the one-question survey on their discontinuation reason. Inadequate GLP-1 RA supply was the most common response (43.7%), followed by program cost (26.2%), dissatisfaction with results (9.9%) and dissatisfaction with the service (7.2%). All primary endpoint data are presented in Table 2. Four response categories were re-coded from open text responses in the ‘other (please specify)’ option, using the Braun and Clarke thematic analysis method, including ‘Inadequate GLP-1 RA supply’, ‘Dissatisfaction with Eucalyptus service’, ‘MDT encouraged me to discontinue’, and ‘Concerns about the long-term effect of GLP-1 RAs’. The ‘Inadequate GLP-1 RA supply’ category captured all responses that emphasised either the unavailability or shortage of a certain GLP-1 RA, or an unwillingness to change GLP-1 RA type (as necessitated by an inadequate supply). Examples of such responses include “Medication no longer available and alternative wasn’t for me”; “You ran out of product”; “I wanted to wait until you got stock of the original Ozempic brand”. In the few cases where open text responses referred to GLP-1 RA supply and another reason, responses were re-coded as the other reason, as the authors felt they were likely to have been more impactful e.g., the response “Poor supply and customer service” was re-coded as ‘Dissatisfaction with the service.”
The bulk of the cohort fell into one of two discontinuation categories. 49.9% paused or ended their subscription at a mean of 141.7(±136) days after initiation. 48.1% stopped following up with their MDT at a mean of 180.4 (±144) days post program commencement. 113 (2%) patients were still active users of their respective programs at the time of analysis, for whom the mean adherence period was 678 (±50.5) days. 1 patient was discontinued from the Juniper program by their doctor on day 166 due to medical reasons (Figure 1). The distribution of reasons given for discontinuation was similar across Juniper and Pilot programs. Inadequate Semaglutide supply was the most common reason (Juniper 43.2%; Pilot 45.1%), followed by program cost (Juniper 25.2%; Pilot 23.5%), dissatisfaction with results (Juniper 10.2%; Pilot 8.8%), and dissatisfaction with service (Juniper 6.6%; Pilot 6.9%).
An analysis of variance (ANOVA) revealed significant variation among the discontinuation categories, F(2, 5601) = [817.8], p < 0.01), and a post hoc Tukey test showed that all three groups (excluding doctor cancellations) differed significantly from one another at p <0.01. Although a slightly higher mean adherence period was observed among Juniper patients (M =173.0, SD = 160) compared to Pilot patients (M = 168.5, SD = 155), a two-sample t-test revealed that this difference was not statistically significant, t(5603) =1.04, p=0.3. A Pearson correlation test showed that patient adherence was positively associated with age (r (5601) = .09, p <0.01) to a small, yet statistically significant degree; and that BMI had no significant effect on adherence (r (5602) = 0.01, p >0.4. Due to the sample’s low representation across non-Caucasian ethnic groups, we created a Caucasian/non-Caucasian binary variable for ethnicity. A two-sample t-test revealed that Caucasian patients (M=175.8, SD = 160) tended to adhere to the Eucalyptus program for longer than non-Caucasian patients (M = 148.7, SD = 149) and that this difference was statistically significant, t(5603) = 4.84, p <0.01.
4. Discussion
To our knowledge, this study represents the first focussed investigation of patient adherence to a real-world digital GLP-1 RA-supported weight-loss program. A mixed-methods approach was used to discover why and after how long Australian patients tend to discontinue the Eucalyptus (Juniper and Pilot) DWLS. The quantitative analysis found that mean adherence to the Eucalyptus weight-loss program (Juniper and Pilot) was 171.2 (±158.2) days for patients who commenced their subscription between January and June 2022. However, data were widely dispersed and therefore the median adherence result of 115 days may be more informative. We also found that over two-thirds of patients who completed the survey, discontinued for reasons concerning an inadequate supply of their desired GLP-1 RA medication or program cost. A significant portion of patients dropped out of the program due to their dissatisfaction with their weight loss outcomes or the Eucalyptus DWLS in general. Ethnicity and age were both observed to have had a statistically significant effect on patient adherence. These findings suggest that DWLSs have the potential to mitigate a significant factor in global overweight and obesity levels, but that care models need to improve upon the one observed in the Eucalyptus Australia DWLS.
4.1. The Significance of Patient Adherence to DWLSs
The difficulty of accessing continuous coordinated multidisciplinary obesity care has contributed to the steady rise in the disease’s incidence over the past 3 decades. Many care providers have responded to this access barrier by launching DWLSs. Yet while the logic of digital modalities improving initial access to obesity care is broadly accepted, prior to this study, no one had attempted to investigate whether DWLSs improve access to the type of care obesity requires. In other words, no study had investigated whether patients can adhere to DWLSs for long enough for them to have a meaningful effect. Previous studies had indicated that a high proportion of patients drop out of the Eucalyptus DWLS before 5- and 7-month weight-loss measurements [35,36]. However, those were effectiveness studies whose discontinuation rates were significantly impacted by strict inclusion criteria. This study, on the other hand, was a focussed adherence analysis that included every single patient who started the Juniper DWLS within the study window.
Although the observed mean adherence period [171.2 (±158.2) days] for the Eucalyptus DWLS appears reasonable, it is difficult to assess without any comparable data available. WHO emphasises Obesity's chronicity and the importance of ongoing comprehensive care. While some DWLSs might be tempted to argue that many patients who supplement their lifestyle treatment with GLP-1 RA therapy can achieve enough weight loss to move into a healthy BMI range within 171 days, until there is more knowledge around the sustainability of GLP-1 RA-induced weight loss, DWLSs should be underpinned by long-term lifestyle interventions. It is unlikely WHO or other major health institutions would consider 171 days long enough for an individual to adopt sustainable behavioural changes. Nevertheless, researchers now have an initial benchmark for ongoing research in patient adherence to GLP-1 RA-supported DWLSs.
Discoveries of the cohort’s skewed adherence distribution and the distribution of discontinuation reasons were arguably more informative than the mean adherence rate. Given the relatively large sample (5604), the asymmetrical adherence distribution can be interpreted as a reliable reflection of the varied experiences among Eucalyptus DWLS patients rather than random noise. The broad range of discontinuation reason responses enriches this data by illuminating the many factors that impact adherence to real-world GLP-1 RA supported DWLSs. In addition to highlighting the importance of program cost, service quality, and side effect management, the survey responses indicate that a high percentage of patients will drop out of a DWLS if their desired GLP-1 RA dose becomes unavailable, even if the service is supported by a behavioural intervention. This problem may be specific to care models such as Eucalyptus’ that only offer GLP-1-supported programs, rather than services with standalone behavioural options for which a program transfer could be available. It is understandable that many Eucalyptus patients were not willing to tolerate anything less than their most desired GLP-1 RA medication given the service’s high monthly subscription fee of $285 AUD. The direct and indirect impact of cost on DWLS adherence is likely to be significant in any GLP-1 RA-supported program that is not subsidised.
4.2. Future Research
The ultimate goal of this study was to assess average patient adherence to the Eucalyptus DWLS and the service’s key discontinuation factors. This research question represents one half of the question as to whether DWLSs have the potential to increase access to the type of care people with obesity need, and one part of the broader series of questions that need to be addressed around DWLS quality and safety. While this study generated some findings around average adherence to the Eucalyptus Australia program and patient discontinuation reasons, similar research on several other DWLSs and F2F obesity services is needed before strong conclusions around adherence standards and safeguards can be made. Regarding the other half of the obesity care access question, future studies should seek to conduct deep analyses of real-world DWLS care models. Although this study assumed that the Eucalyptus DWLS delivered holistic multidisciplinary care underpinned by behavioural therapy to the investigated cohort (based on the service’s claims and its ACHS and DTAC accreditations), it did not attempt to systematically verify this. Future research might, for example, endeavour to quantify the number of behaviour-related messages a DWLS provides in a certain period or assess the precise behavioural changes patients adopt over the course of a program. Researchers should also consider prospective analyses of real-world DWLSs that measure the association between adherence, engagement, and effectiveness outcomes across segmented adherence periods.
4.3. Strengths and Limitations
The study used a large sample of real-world patients and did not exclude anyone who initiated the Eucalyptus DWLS within the specified analysis period. Data were collected from a commercially available, ACHS-accredited data repository, and the collection and analysis of these data did not interfere with Eucalyptus patient experiences in any way. To our knowledge, the research question is the first of its kind and thus the findings set an initial benchmark for ongoing research into real-world DWLS adherence.
The study also contained several limitations. Firstly, survey responses were collected from less than half the cohort (37.3%), and were sent to most patients long after they had discontinued (22 Feb 2024) the Eucalyptus program. It is feasible that many non-responders did not feel as strongly about their previous discontinuation reason as those who could no longer receive their desired GLP-1 RA medication or afford the program. Consequently, the reported discontinuation reasons may not have been representative of the cohort. Secondly, survey data could not be linked to other discontinuation data due to privacy restrictions and so a deeper investigation of the observed impact of ethnicity and age on adherence could not be conducted. This issue may have been resolvable had patient income data been collected, but these data were also unavailable. A third limitation of the study was its predominantly Caucasian sample. Finally, investigators had to estimate the discontinuation date for patients who failed to attend or review follow-up consults. Although the method for estimating this date (50 days after the last attended consult) seemed logical given the standard coverage period of GLP-1 RA orders and historical consultation intervals of Eucalyptus weight-loss patients, it is possible that a high percentage of this category of discontinuers stopped their therapy earlier than the estimated date.
5. Conclusions
Increasingly large numbers of people are using DWLSs to treat overweight and obesity, yet stakeholders remain sceptical of these services’ capability to provide continuous and comprehensive care. To assess the validity of these concerns, scholars need to investigate patient adherence to real-world comprehensive DWLSs. This study adopted a mixed methods approach to measure why and after how long patients of the Eucalyptus Australia DWLS discontinue the service. Our findings set an important foundation in the emerging literature on modern weight-loss interventions. They indicate that patient adherence to digital GLP-1 RA-supported weight-loss programs vary significantly in real-world, non-subsidised settings, and that medication supply and program cost are two of the key determinants of this adherence. To deepen understanding of the potential of DWLSs in mitigating global obesity rates, similar adherence data needs to be obtained from numerous other DWLSs. Future research should also consider exploring the relationship between adherence, MDT engagement and weight-loss effectiveness across segmented adherence periods.
Author Contributions
Conceptualization, L.T., and M.V.; methodology, L.T; software, L.T.; validation, L.T and M.V.; formal analysis, L.T., and M.V.; investigation, L.T.; resources, L.T., and M.V.; data curation, L.T.; writing—original draft preparation, L.T.; writing—review and editing, L.T. and M.V.; visualization, L.T.; supervision, M.V.; project administration, L.T.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Bellberry Ethics Committee (# 2023-05-563-A-1, 22 November 2023).
Informed Consent Statement
This study received exemption status from the Bellberry Ethics Committee (# 2023-05-563-A-1, 22 November 2023) for retrospective analyses of de-identified data. Eucalyptus patients consent to the service’s privacy policy at subscription, which includes permission to use their de-identified data for research.
Data Availability Statement
The data presented in this study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to thank all patients and clinicians involved in the Eucalyptus weight-loss program over the study period.
Conflicts of Interest
LT and MV are paid a salary by Eucalyptus.
References
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217–155217. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, X., Astrup, A., Fujioka, K., et al. A randomized, controlled trial of 3.0mg of Liragltuide in weight management. N Engl J Med 2015, 373, 11–22. [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Irvin, L.; Madden, L.A.; Marshall, P.; Vince, R.V. Digital Health Solutions for Weight Loss and Obesity: A Narrative Review. Nutrients 2023, 15, 1858. [Google Scholar] [CrossRef]
- Hinchliffe, N.; Capehorn, M.S.; Bewick, M.; Feenie, J. The Potential Role of Digital Health in Obesity Care. Adv. Ther. 2022, 39, 4397–4412. [Google Scholar] [CrossRef] [PubMed]
- Irvin, L.; Madden, L.A.; Marshall, P.; Vince, R.V. Digital Health Solutions for Weight Loss and Obesity: A Narrative Review. Nutrients 2023, 15, 1858. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Health service delivery framework for prevention and management of obesity, 2023. Geneva: WHO.
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. European Guidelines for Obesity Management in Adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef]
- Hall, K.D.; Heymsfield, S.B.; Kemnitz, J.W.; Klein, S.; Schoeller, D.A.; Speakman, J.R. Energy balance and its components: implications for body weight regulation. Am. J. Clin. Nutr. 2012, 95, 989–994. [Google Scholar] [CrossRef]
- Javed, Z.; Valero-Elizondo, J.; Maqsood, M.H.; Mahajan, S.; Taha, M.B.; Patel, K.V.; Sharma, G.; Hagan, K.; Blaha, M.J.; Blankstein, R.; et al. Social determinants of health and obesity: Findings from a national study of US adults. Obesity 2022, 30, 491–502. [Google Scholar] [CrossRef]
- Verde, L.; Frias-Toral, E.; Cardenas, D. Editorial: Environmental factors implicated in obesity. Front. Nutr. 2023, 10. [Google Scholar] [CrossRef]
- Hall, K.D.; Farooqi, I.S.; Friedman, J.M.; Klein, S.; Loos, R.J.F.; Mangelsdorf, D.J.; O’rahilly, S.; Ravussin, E.; Redman, L.M.; Ryan, D.H.; et al. The energy balance model of obesity: beyond calories in, calories out. Am. J. Clin. Nutr. 2022, 115, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Health service framework for prevention and management of obesity. Geneva, 2023.
- Scheer, J.; Areias, A.C.; Molinos, M.; Janela, D.; Moulder, R.; Lains, J.; Bento, V.; Yanamadala, V.; Correia, F.D.; Costa, F. Engagement and Utilization of a Complete Remote Digital Care Program for Musculoskeletal Pain Management in Urban and Rural Areas Across the United States: Longitudinal Cohort Study. JMIR mHealth uHealth 2023, 11, e44316. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, D., & Marciniuk, D. Rural and remote care: Overcoming the challenges of distance. Chronic respiratory disease 2016, 13, 192–203.
- Sweet, C.C.; Jasik, C.B.; Diebold, A.; DuPuis, A.; Jendretzke, B. Cost Savings and Reduced Health Care Utilization Associated with Participation in a Digital Diabetes Prevention Program in an Adult Workforce Population. J. Heal. Econ. Outcomes Res. 2023, 7, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Ekman, B. Cost Analysis of a Digital Health Care Model in Sweden. PharmacoEconomics - Open 2017, 2, 347–354. [Google Scholar] [CrossRef]
- Naslund, J.; Deng, D. Addressing mental health stigma in low-income and middle-income countries: A new frontier for digital mental health. Ethic- Med. Public Heal. 2021, 19. [Google Scholar] [CrossRef] [PubMed]
- Quisel, T., Foschini, L., Zbikowski, S., et al. The association between medication adherence for chronic conditions and digital health activity tracking: Retrospective analysis. J Med Internet Res 2019, 21, e11486. [CrossRef]
- Talay, L. , Alvi, O. Digital healthcare solutions to better achieve the weight loss outcomes expected by payors and patients. Diabetes, Obesity and Metabolism.
- Pi-Sunyer, X., Astrup, A., Fujioka, K., et al. A randomized, controlled trial of 3.0mg of Liragltuide in weight management. N Engl J Med 2015, 373, 11–22. [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Carls, G.S.; Tuttle, E.; Tan, R.-D.; Huynh, J.; Yee, J.; Edelman, S.V.; Polonsky, W.H. Understanding the Gap Between Efficacy in Randomized Controlled Trials and Effectiveness in Real-World Use of GLP-1 RA and DPP-4 Therapies in Patients With Type 2 Diabetes. Diabetes Care 2017, 40, 1469–1478. [Google Scholar] [CrossRef]
- Palanca, A.; Ampudia-Blasco, F.J.; Calderón, J.M.; Sauri, I.; Martinez-Hervás, S.; Trillo, J.L.; Redón, J.; Real, J.T. Real-World Evaluation of GLP-1 Receptor Agonist Therapy Persistence, Adherence and Therapeutic Inertia Among Obese Adults with Type 2 Diabetes. Diabetes Ther. 2023, 14, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Fallows, E.; Ells, L.; Anand, V. Semaglutide and the future of obesity care in the UK. Lancet 2023, 401, 2093–2096. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K. The role of commercial weight loss programmes. In: Haslam, D., Malhotra, A., Capehorn, S. (eds) Bariatric surgery in clinical practice. In clinical practice. Springer. 2022.
- World Health Organization. Health service framework for prevention and management of obesity. Geneva, 2023.
- National Institute for Health and Care Excellence. Semaglutide for managing overweight and obesity, Sep 2023.
- National Institute for Health and Care Excellence. Semaglutide for managing overweight and obesity, Sep 2023.
- Gemesi, K., Winkler, S., Schmidt-Tesch, S., et al. Efficacy of an app-based multimodal lifestyle intervention on bodyweight in persons with obesity: results from a randomised controlled trial. International Journal of Obesity, Nov 2023, 48, 118–126.
- upila, S., Joki, A., Suojanen, L, et al. The effectiveness of eHealth interventions for weight loss and weight loss maintenance in adults with overweight and obesity: A systematic review of systematic reviews. Curr Obes Rep;12, 371-394.
- Talay, L. , & Vickers, M. Effectiveness and care continuity in an app-based, glucagon-like peptide-1 receptor agonist-supported weight-loss service for women with overweight and obesity in the UK: A real-world retrospective cohort analysis. Diabetes, Obesity and Metabolism.
- Talay, L. , Alvi, O. Digital healthcare solutions to better achieve the weight loss outcomes expected by payors and patients. Diabetes, Obesity and Metabolism.
- Braun, V.; Clarke, V. Thematic Analysis: A Practical Guide. Qmip Bull. 2022, 1, 46–50. [Google Scholar] [CrossRef]
- Talay, L. , & Vickers, M. Effectiveness and care continuity in an app-based, glucagon-like peptide-1 receptor agonist-supported weight-loss service for women with overweight and obesity in the UK: A real-world retrospective cohort analysis. Diabetes, Obesity and Metabolism.
- Talay, L. , Alvi, O. Digital healthcare solutions to better achieve the weight loss outcomes expected by payors and patients. Diabetes, Obesity and Metabolism.
Figure 1.
Adherence distribution and mean by discontinuation category.

Table 1.
Patient characteristics.
| Demographic information | |
|---|---|
| Age – mean (SD) | 41.6 (10.12) |
| Gender – no. (%) | |
| Female (Juniper) | 3339 (59.6) |
| Male (Pilot) | 2266 (40.4) |
| Ethnicity – no. (%) | |
| Caucasian | 4645 (82.9) |
| Asian (Inc. subcontinent) | 162 (5.48) |
| Not listed | 298 (5.32) |
| Indigenous Australian | 137 (2.44) |
| Baseline clinical information – mean (SD) | |
| BMI | 35.17(8.8) |
Table 2.
Primary endpoint data.
| Adherence period – mean (SD); median | |
|---|---|
| Juniper (female) | 173.0 (160) days; 117 days |
| Pilot (male) | 168.5 (155) days; 113 days |
| Total cohort | 171.2 (158.2) days; 115 days |
| Discontinuation reasons – no. (%) | |
| Inadequate GLP-1 RA supply* | 912 (43.7) |
| “It was too expensive” | 547 (26.2) |
| “I was not seeing results” | 206 (9.9) |
| Dissatisfaction with the service | 150 (7.2) |
| “My side effects were intolerable” | 79 (3.8) |
| “Weight loss was no longer a priority for me” | 76 (3.6) |
| “I found a better digital weight-loss service” | 49 (2.3) |
| “I found a better in-person weight loss service” | 35 (1.7) |
| MDT encouraged me to discontinue | 19 (0.9) |
| Concerns about the long-term effect of GLP-1 RAs | 15 (0.7) |
* The four non-italicised response categories were re-coded from open text responses in the ‘other (please specify)’ category, using the Braun and Clarke thematic analysis method.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
A Process Evaluation of a Multi-Component Intervention in Dutch Dietetic Treatment to Improve Portion Control Behavior and Decrease Body Mass Index in Overweight and Obese Patients
Willemieke Kroeze
et al.
Nutrients,
2018
What Intervention Elements Drive Weight Loss in Blended-Care Behavior Change Interventions? A Real-World Data Analysis with 25,706 Patients
Felix Schirmann
et al.
Nutrients,
2022
Introducing zanadio—A Digitalized, Multimodal Program to Treat Obesity
Katarina Forkmann
et al.
Nutrients,
2022
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated






