Submitted:
27 July 2024
Posted:
30 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Maternal Respiratory Changes Related to Pregnancy
2.1. Hormonal Changes
2.2. Anatomical Changes
3. Effect of Intermittent Hypoxia in the Pregnant Women
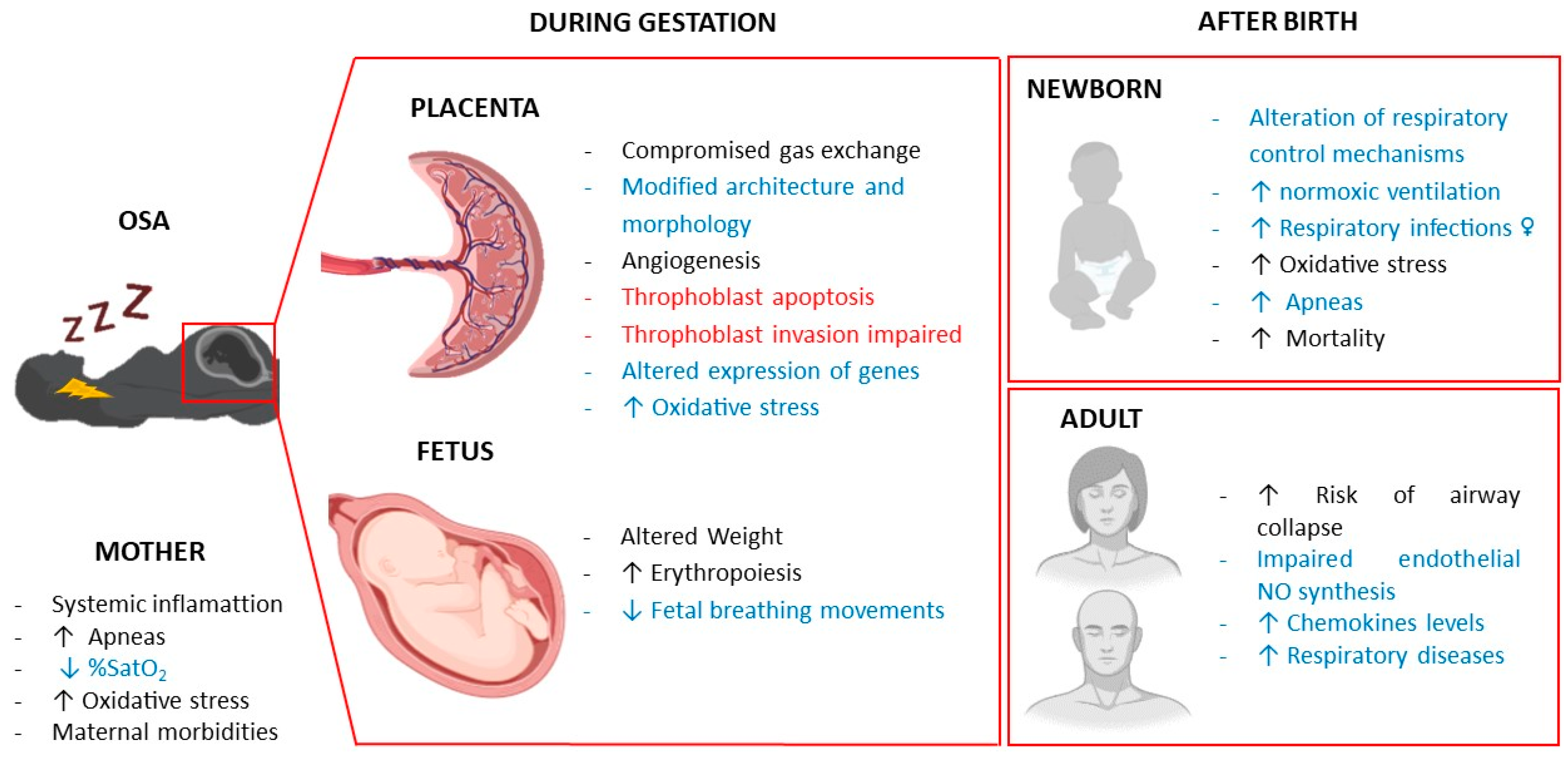
4. Effects of Intermittent Hypoxia in the Placenta
5. Fetal Respiratory Distress in Gestational Intermittent Hypoxia
6. Offspring Respiratory Disorders following Gestational Intermittent Hypoxia
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Canever, J.B.; Zurman, G.; Vogel, F.; Sutil, D.V.; Diz, J.B.M.; Danielewicz, A.L.; Moreira, B.d.S.; Cimarosti, H.I.; de Avelar, N.C.P.; Canever, J.B.; et al. Worldwide prevalence of sleep problems in community-dwelling older adults: A systematic review and meta-analysis. Sleep Med. 2024, 119, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Mediano, O.; González Mangado, N.; Montserrat, J.M.; Alonso-Álvarez, M.L.; Almendros, I.; Alonso-Fernández, A.; Barbé, F.; Borsini, E.; Caballero-Eraso, C.; Cano-Pumarega, I.; et al. Spanish Sleep Network. International Consensus Document on Obstructive Sleep Apnea. Arch. Broconeumol. 2022, 58, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, B.; Dasti, S.; Park, Y.; Brown, T.; Davis, H.; Akhtar, B. Hour-to-Hour Variability of Oxygen Saturation in Sleep Apnea. Chest 1998, 113, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Almaraz, L.; Obeso, A.; Rigual, R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol. Rev. 1994, 74, 829–898. [Google Scholar] [CrossRef]
- Song, R.; Baker, T.L.; Watters, J.J.; Kumar, S. Obstructive Sleep Apnea-Associated Intermittent Hypoxia-Induced Immune Responses in Males, Pregnancies, and Offspring. Int. J. Mol. Sci. 2024, 25, 1852. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Peng, Y.; Nanduri, J. Carotid body hypersensitivity in intermittent hypoxia and obtructive sleep apnoea. J. Physiol. 2023, 601, 5481–5494. [Google Scholar] [CrossRef]
- Young, T. Rationale, Design, and Findings from the Wisconsin Sleep Cohort Study: Toward Understanding the Total Societal Burden of Sleep-Disordered Breathing. Sleep Med. Clin. 2009, 4, 37–46. [Google Scholar] [CrossRef]
- Pien, G.W.; I Pack, A.; Jackson, N.; Maislin, G.; A Macones, G.; Schwab, R.J. Risk factors for sleep-disordered breathing in pregnancy. Thorax 2013, 69, 371–377. [Google Scholar] [CrossRef]
- Zhu, B.; Bronas, U.G.; Carley, D.W.; Lee, K.; Steffen, A.; Kapella, M.C.; Izci-Balserak, B. Relationships between objective sleep parameters and inflammatory biomarkers in pregnancy. Ann. New York Acad. Sci. 2020, 1473, 62–73. [Google Scholar] [CrossRef]
- Louis, J.M.; Koch, M.A.; Reddy, U.M.; Silver, R.M.; Parker, C.B.; Facco, F.L.; Redline, S.; Nhan-Chang, C.-L.; Chung, J.H.; Pien, G.W.; et al. Predictors of sleep-disordered breathing in pregnancy. Am. J. Obstet. Gynecol. 2018, 218, 521–e1. [Google Scholar] [CrossRef]
- Eleftheriou, D.; Athanasiadou, K.I.; Sifnaios, E.; Vagiakis, E.; Katsaounou, P.; Psaltopoulou, T.; Paschou, S.A.; Trakada, G. Sleep disorders during pregnancy: an underestimated risk factor for gestational diabetes mellitus. Endocrine 2023, 83, 41–50. [Google Scholar] [CrossRef]
- Louis, J.M.; Mogos, M.F.; Salemi, J.L.; Redline, S.; Salihu, H.M. Obstructive Sleep Apnea and Severe Maternal-Infant Morbidity/Mortality in the United States, 1998-2009. Sleep 2014, 37, 843–849. [Google Scholar] [CrossRef]
- Khalyfa, A.; Cortese, R.; Qiao, Z.; Ye, H.; Bao, R.; Andrade, J.; Gozal, D. Late gestational intermittent hypoxia induces metabolic and epigenetic changes in male adult offspring mice. J. Physiol. 2017, 595, 2551–2568. [Google Scholar] [CrossRef]
- Dahlgren, J.; Samuelsson, A.-M.; Jansson, T.; Holmäng, A. Interleukin-6 in the Maternal Circulation Reaches the Rat Fetus in Mid-gestation. Pediatr. Res. 2006, 60, 147–151. [Google Scholar] [CrossRef]
- Contreras, G.; Gutiérrez, M.; Beroíza, T.; Fantín, A.; Oddó, H.; Villarroel, L.; Cruz, E.; Lisboa, C. Ventilatory Drive and Respiratory Muscle Function in Pregnancy. Am. Rev. Respir. Dis. 1991, 144, 837–841. [Google Scholar] [CrossRef]
- Lyons HA, Antonio R. The sensitivity of the respiratory center in pregnancy and after administration of progesterone. Trans Assoc Am Physicians 1959, 72, 173–180.
- Weinberger, S.E.; Weiss, S.T.; Cohen, W.R.; Weiss, J.W.; Johnson, T.S. Pregnancy and The Lung1,2. Am. Rev. Respir. Dis. 1980, 121, 559–581. [Google Scholar] [CrossRef]
- McAuliffe, F.; Kametas, N.; Costello, J.; Rafferty, G.F.; Greenough, A.; Nicolaides, K. Respiratory function in singleton and twin pregnancy. BJOG: Int. J. Obstet. Gynaecol. 2002, 109, 765–769. [Google Scholar] [CrossRef]
- Izci, B.; Vennelle, M.; Liston, W.A.; Dundas, K.C.; Calder, A.A.; Douglas, N.J. Sleep-disordered breathing and upper airway size in pregnancy and post-partum. Eur. Respir. J. 2006, 27, 321–327. [Google Scholar] [CrossRef]
- Izci, B.; Riha, R.L.; Martin, S.E.; Vennelle, M.; Liston, W.A.; Dundas, K.C.; Calder, A.A.; Douglas, N.J. The Upper Airway in Pregnancy and Pre-Eclampsia. Am. J. Respir. Crit. Care Med. 2003, 167, 137–140. [Google Scholar] [CrossRef]
- Bende, M.; Gredmark, T. Nasal Stuffiness During Pregnancy. Laryngoscope 1999, 109, 1108–1110. [Google Scholar] [CrossRef]
- Williams, P.; Goldspink, G. CHANGES IN SARCOMERE LENGTH AND PHYSIOLOGICAL PROPERTIES IN IMMOBILIZED MUSCLE. 1978, 127, 459–468.
- Johns, E.C.; Denison, F.C.; Reynolds, R.M. Sleep disordered breathing in pregnancy: A review of the pathophysiology of adverse pregnancy outcomes. Acta Physiol. 2020, 229, e13458. [Google Scholar] [CrossRef]
- Bourne, T.; Ogilvy, A.J.; Vickers, R.; Williamson, K. Nocturnal hypoxaemia in late pregnancy. Br. J. Anaesth. 1995, 75, 678–682. [Google Scholar] [CrossRef]
- Guilleminault, C.; Kreutzer, M.; Chang, J.L. Pregnancy, sleep disordered breathing and treatment with nasal continuous positive airway pressure. Sleep Med. 2004, 5, 43–51. [Google Scholar] [CrossRef]
- Garvey, J.F.; Taylor, C.T.; McNicholas, W.T. Cardiovascular disease in obstructive sleep apnoea syndrome: the role of intermittent hypoxia and inflammation. Eur. Respir. J. 2009, 33, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Rath, G.; Aggarwal, R.; Jawanjal, P.; Tripathi, R.; Batra, A. HIF-1 Alpha and Placental Growth Factor in Pregnancies Complicated With Preeclampsia: A Qualitative and Quantitative Analysis. J. Clin. Lab. Anal. 2014, 30, 75–83. [Google Scholar] [CrossRef]
- Lu, D.; Li, N.; Yao, X.; Zhou, L. Potential inflammatory markers in obstructive sleep apnea-hypopnea syndrome. Bosn. J. Basic Med. Sci. 2017, 17, 47–53. [Google Scholar] [CrossRef]
- Lavie, L. Oxidative stress inflammation and endothelial dysfunction in obstructive sleep apnea. Front. Biosci. 2012, E4, 1391–403. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Kumar, G.K.; Nanduri, J.; Semenza, G.L. ROS Signaling in Systemic and Cellular Responses to Chronic Intermittent Hypoxia. Antioxidants Redox Signal. 2007, 9, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Mahmoudi, S.; Hattar, K.; Sibelius, U.; Olschewski, H.; Mayer, K.; Seeger, W.; Grimminger, F. Enhanced Release of Superoxide from Polymorphonuclear Neutrophils in Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2000, 162, 566–570. [Google Scholar] [CrossRef]
- Okun, M.L.; Coussons-Read, M.E. Sleep disruption during pregnancy: How does it influence serum cytokines? J. Reprod. Immunol. 2007, 73, 158–165. [Google Scholar] [CrossRef]
- Alonso-Fernández, A.; Quetglas, C.R.; Mochales, A.H.; De Larrinaga, A. .R.; Barón, A.S.; Rodríguez, P.R.; Gil Gómez, A.V.; Martínez, C.P.; Marín, J.P.C.; Nicolau, M.B.; et al. Influence of Obstructive Sleep Apnea on Systemic Inflammation in Pregnancy. Front. Med. 2021, 8. [Google Scholar] [CrossRef]
- Serednytskyy, O.; Alonso-Fernández, A.; Ribot, C.; Herranz, A.; Álvarez, A.; Sánchez, A.; Rodríguez, P.; Gil, A.V.; Pía, C.; Cubero, J.P.; et al. Systemic inflammation and sympathetic activation in gestational diabetes mellitus with obstructive sleep apnea. BMC Pulm. Med. 2022, 22, 1–11. [Google Scholar] [CrossRef]
- Schulz, R. The vascular micromilieu in obstructive sleep apnoea. Eur. Respir. J. 2005, 25, 780–782. [Google Scholar] [CrossRef]
- Yinon, D.; Lowenstein, L.; Suraya, S.; Beloosesky, R.; Zmora, O.; Malhotra, A.; Pillar, G. Pre-eclampsia is associated with sleep-disordered breathing and endothelial dysfunction. Eur. Respir. J. 2006, 27, 328–333. [Google Scholar] [CrossRef]
- Dharmashankar, K.; Widlansky, M.E. Vascular Endothelial Function and Hypertension: Insights and Directions. Curr. Hypertens. Rep. 2010, 12, 448–455. [Google Scholar] [CrossRef]
- Hadi, H.A.R.; Carr, C.S.; Al Suwaidi, J. Endothelial Dysfunction: Cardiovascular Risk Factors, Therapy, and Outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar]
- Lui, B.; Burey, L.; Ma, X.; Kjaer, K.; Abramovitz, S.; White, R. Obstructive sleep apnea is associated with adverse maternal outcomes using a United States multistate database cohort, 2007–2014. Int. J. Obstet. Anesthesia 2020, 45, 74–82. [Google Scholar] [CrossRef]
- Sağ. ; Cakmak, B.; Üstünyurt, E. Obstructive sleep apnea syndrome is associated with maternal complications in pregnant women. Ginekol. Polska 2021, 92, 571–574. [Google Scholar] [CrossRef]
- Sun, J.; Lin, C.; Wu, F.; Chung, C.; Sun, C.; Chien, W. The association between obstructive sleep apnea and the risk of poor delivery events in women: A population-based nested case–control study. J. Nurs. Sch. 2021, 54, 31–37. [Google Scholar] [CrossRef]
- Maltepe, E.; Fisher, S.J. Placenta: The Forgotten Organ. Annu. Rev. Cell Dev. Biol. 2015, 31, 523–552. [Google Scholar] [CrossRef]
- Zamudio, S. The Placenta at High Altitude. High Alt. Med. Biol. 2003, 4, 171–191. [Google Scholar] [CrossRef]
- Hutter, D.; Kingdom, J.; Jaeggi, E. Causes and Mechanisms of Intrauterine Hypoxia and Its Impact on the Fetal Cardiovascular System: A Review. Int. J. Pediatr. 2010, 2010, 1–9. [Google Scholar] [CrossRef]
- Sferruzzi-Perri, A.N.; Camm, E.J. The Programming Power of the Placenta. Front. Physiol. 2016, 7, 33–33. [Google Scholar] [CrossRef]
- Kalisch-Smith, J.; Simmons, D.; Dickinson, H.; Moritz, K. Review: Sexual dimorphism in the formation, function and adaptation of the placenta. Placenta 2017, 54, 10–16. [Google Scholar] [CrossRef]
- Caruso, M.; Evangelista, M.; Parolini, O. Human Term Placental Cells: Phenotype, Properties and New Avenues in Regenerative Medicine. 2012, 1, 64–74.
- Goplerud, J.; Delivoriapapadopoulos, M. PHYSIOLOGY OF THE PLACENTA - GAS-EXCHANGE. 1985, 15, 270–278.
- Ravishankar, S.; Bourjeily, G.; Lambert-Messerlian, G.; He, M.; De Paepe, M.E.; Gündoğan, F. Evidence of Placental Hypoxia in Maternal Sleep Disordered Breathing. Pediatr. Dev. Pathol. 2015, 18, 380–386. [Google Scholar] [CrossRef]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef]
- Hung, T.-H.; Skepper, J.N.; Burton, G.J. In Vitro Ischemia-Reperfusion Injury in Term Human Placenta as a Model for Oxidative Stress in Pathological Pregnancies. Am. J. Pathol. 2001, 159, 1031–1043. [Google Scholar] [CrossRef]
- Lavie, L. Obstructive sleep apnoea syndrome – an oxidative stress disorder. Sleep Med. Rev. 2003, 7, 35–51. [Google Scholar] [CrossRef]
- Cuffe, J.S.M.; Walton, S.L.; Singh, R.R.; Spiers, J.G.; Bielefeldt-Ohmann, H.; Wilkinson, L.; Little, M.H.; Moritz, K.M. Mid- to late term hypoxia in the mouse alters placental morphology, glucocorticoid regulatory pathways and nutrient transporters in a sex-specific manner. J. Physiol. 2014, 592, 3127–3141. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.S.; Vaughan, O.R.; de Liger, E.F.; Fowden, A.L.; Sferruzzi-Perri, A.N. Placental phenotype and resource allocation to fetal growth are modified by the timing and degree of hypoxia during mouse pregnancy. J. Physiol. 2015, 594, 1341–1356. [Google Scholar] [CrossRef]
- Rosario, G.X.; Konno, T.; Soares, M.J. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev. Biol. 2007, 314, 362–375. [Google Scholar] [CrossRef]
- Lum, D.H.; Kuwabara, P.E.; Zarkower, D.; Spence, A.M. Direct protein–protein interaction between the intracellular domain of TRA-2 and the transcription factor TRA-1A modulates feminizing activity in C. elegans. Genes Dev. 2000, 14, 3153–3165. [Google Scholar] [CrossRef]
- Badran, M.; Abuyassin, B.; Ayas, N.; Laher, I. Intermittent hypoxia impairs uterine artery function in pregnant mice. J. Physiol. 2019, 597, 2639–2650. [Google Scholar] [CrossRef] [PubMed]
- Arterial Chemoreceptors; Springer Nature: Dordrecht, GX, Netherlands, 2023; ISBN:.
- Genbacev, O.; Zhou, Y.; Ludlow, J.W.; Fisher, S.J. Regulation of Human Placental Development by Oxygen Tension. Science 1997, 277, 1669–1672. [Google Scholar] [CrossRef]
- Jauniaux, E.; Poston, L.; Burton, G.J. Placental-related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Hum. Reprod. Updat. 2006, 12, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, M.; Herr, F.; Münstedt, K.; Lang, U.; Liang, O.D. Angiogenesis and vasculogenesis in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110, S10–S18. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Anand, V.; Roy, S. Vascular Endothelial Growth Factor Signaling in Hypoxia and Inflammation. J. Neuroimmune Pharmacol. 2014, 9, 142–160. [Google Scholar] [CrossRef]
- Dailey, L.; Ambrosetti, D.; Mansukhani, A.; Basilico, C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005, 16, 233–247. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, Y.-Z.; Chen, D.-B.; Zheng, J. Hypoxia Enhances FGF2- and VEGF-Stimulated Human Placental Artery Endothelial Cell Proliferation: Roles of MEK1/2/ERK1/2 and PI3K/AKT1 Pathways☆. Placenta 2009, 30, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.; Huang, L.; Feng, H.; He, Q.; Lin, X.; Jiang, T.; Lin, J.; Wang, X.; Liu, Q. Gestational chronic intermittent hypoxia induces hypertension, proteinuria, and fetal growth restriction in mice. Sleep Breath. 2021, 26, 1661–1669. [Google Scholar] [CrossRef]
- Song, W.; Chang, W.-L.; Shan, D.; Gu, Y.; Gao, L.; Liang, S.; Guo, H.; Yu, J.; Liu, X. Intermittent Hypoxia Impairs Trophoblast Cell Viability by Triggering the Endoplasmic Reticulum Stress Pathway. Reprod. Sci. 2020, 27, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Giannubilo, S.R.; Cecati, M.; Marzioni, D.; Ciavattini, A. Circulating miRNAs and Preeclampsia: From Implantation to Epigenetics. Int. J. Mol. Sci. 2024, 25, 1418. [Google Scholar] [CrossRef]
- Vanderplow, A.M.; Kermath, B.A.; Bernhardt, C.R.; Gums, K.T.; Seablom, E.N.; Radcliff, A.B.; Ewald, A.C.; Jones, M.V.; Baker, T.L.; Watters, J.J.; et al. A feature of maternal sleep apnea during gestation causes autism-relevant neuronal and behavioral phenotypes in offspring. PLOS Biol. 2022, 20, e3001502. [Google Scholar] [CrossRef]
- Johns, E.C.; Halligan, D.L.; Tammsalu, T.; Hill, E.A.; Riha, R.L.; Denison, F.C.; Reynolds, R.M. Gene expression profiling of placentae from women with obesity and obstructive sleep apnoea. Placenta 2022, 121, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Almendros, I.; Martínez-Ros, P.; Farré, N.; Rubio-Zaragoza, M.; Torres, M.; Gutiérrez-Bautista. J.; Carrillo-Poveda, J.M.; Sopena-Juncosa, J.J.; Gozal, D.; Gonzalez-Bulnes, A.; et al. Placental oxygen transfer reduces hypoxia-reoxygenation swings in fetal blood in a sheep model of gestational sleep apnea. J. Appl. Physiol. 2019, 127, 745–752. [Google Scholar] [CrossRef]
- Almendros, I.; Farré, R.; Planas, A.M.; Torres, M.; Bonsignore, M.R.; Navajas, D.; Montserrat, J.M. Tissue Oxygenation in Brain, Muscle, and Fat in a Rat Model of Sleep Apnea: Differential Effect of Obstructive Apneas and Intermittent Hypoxia. Sleep 2011, 34, 1127–1133. [Google Scholar] [CrossRef]
- Cahill, L.S.; Zhou, Y.-Q.; Seed, M.; Macgowan, C.K.; Sled, J.G. Brain Sparing in Fetal Mice: BOLD MRI and Doppler Ultrasound Show Blood Redistribution During Hypoxia. J. Cereb. Blood Flow Metab. 2014, 34, 1082–1088. [Google Scholar] [CrossRef]
- Carter, AM. Placental gas exchange and the oxygen supply to the fetus. Compr Physiol 2015, 5, 1381–1403. [Google Scholar] [CrossRef] [PubMed]
- Giussani, D.A. The fetal brain sparing response to hypoxia: physiological mechanisms. J. Physiol. 2016, 594, 1215–1230. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, W.; Ciriello, J. Effect of maternal chronic intermittent hypoxia during gestation on offspring growth in the rat. Am. J. Obstet. Gynecol. 2013, 209, 564–e1. [Google Scholar] [CrossRef] [PubMed]
- Cortese, R.; Khalyfa, A.; Bao, R.; Andrade, J.; Gozal, D. Epigenomic profiling in visceral white adipose tissue of offspring of mice exposed to late gestational sleep fragmentation. Int. J. Obes. 2015, 39, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Cortese, R.; Gileles-Hillel, A.; Almendros, I.; Akbarpour, M.; Khalyfa, A.A.; Qiao, Z.; Garcia, T.; Andrade, J.; Gozal, D. Aorta macrophage inflammatory and epigenetic changes in a murine model of obstructive sleep apnea: Potential role of CD36. Sci. Rep. 2017, 7, 43648. [Google Scholar] [CrossRef] [PubMed]
- Koos, BJ. Breathing and sleep states in the fetus and at birth. In: Marcus CL, Carroll JL, Donnelly DF, Loughlin GM, editors. Sleep and breathing in children: developmental changes in breathing during sleep. 2nd ed. New York, NY: Informa Healthcare; 2008. p. 1–17.
- Koos BJ, Rajaee A. Fetal breathing movements and changes at birth. Adv Exp Med Biol. 2014, 814, 89–101. [CrossRef]
- Patrick, J.; Campbell, K.; Carmichael, L.; Natale, R.; Richardson, B. A definition of human fetal apnea and the distribution of fetal apneic intervals during the last ten weeks of pregnancy. Am. J. Obstet. Gynecol. 1980, 136, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Arduini, D.; Rizzo, G.; Giorlandino, C.; Valensise, H.; Dell'Acqua, S.; Romanini, C. The development of fetal behavioural states: A longitudinal study. Prenat. Diagn. 1986, 6, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Patrick, J.; Campbell, K.; Carmichael, L.; Natale, R.; Richardson, B. PATTERNS OF HUMAN-FETAL BREATHING DURING THE LAST 10 WEEKS OF PREGNANCY. 1980, 56, 24–30.
- Koos, B.J.; Matsuda, K.; Power, G.G. Fetal breathing and cardiovascular responses to graded methemoglobinemia in sheep. J. Appl. Physiol. 1990, 69, 136–140. [Google Scholar] [CrossRef]
- Platt, L.D.; Manning, F.A.; Lemay, M.; Sipos, L. Human fetal breathing: Relationship to fetal condition. Am. J. Obstet. Gynecol. 1978, 132, 514–518. [Google Scholar] [CrossRef]
- Tauman, R.; Many, A.; Deutsch, V.; Arvas, S.; Ascher-Landsberg, J.; Greenfeld, M.; Sivan, Y. Maternal snoring during pregnancy is associated with enhanced fetal erythropoiesis – a preliminary study. Sleep Med. 2011, 12, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Templeton A, Kelman GR. Maternal blood-gases, (PAo2-Pao2), physiological shunt and VD/VT in normal pregnancy. Br J Anaesth 1976, 48(10), 1001–4.
- Dawes, GS. Foetal and neonatal physiology; a comparative study of the changes at birth. Year Book Medical Publishers; 1968.
- Longo, L.D. The Rise of Fetal and Neonatal Physiology: Basic Science to Clinical Care, Perspectives in Physiology. Am Physiol Society 2013. [CrossRef]
- Herrington RT, Harned HS Jr, Ferreiro JI, Griffin CA 3rd. The role of the central nervous system in perinatal respiration: studies of chemoregulatory mechanisms in the term lamb. Pediatrics. 1971, 47(5), 857-64.
- Chou, P.J.; Ullrich, J.; Ackerman, B. Time of Onset of Effective Ventilation at Birth. Neonatology 1974, 24, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Katz-Salamon, M. Development of chemoreceptor responses in infants. Respir. Physiol. Neurobiol. 2005, 149, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Rigatto, H.; Verduzco, R.D.L.T.; Gates, D.B.; Dumont, F.S.; Kinkead, R.; Edwards, B.A.; Sands, S.A.; Skuza, E.M.; Brodecky, V.; Stockx, E.M.; et al. Effects of O2 on the ventilatory response to CO2 in preterm infants. J. Appl. Physiol. 1975, 39, 896–899. [Google Scholar] [CrossRef]
- Kumar P, Hanson MA. Re-setting of the hypoxic sensitivity of aortic chemoreceptors in the new-born lamb. J Dev Physiol. 1989, 11(4):199-206.
- Richardson, H.L.; Parslow, P.M.; Walker, A.M.; Harding, R.; Horne, R.S.C. Maturation of the initial ventilatory response to hypoxia in sleeping infants. J. Sleep Res. 2007, 16, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Fehring, C.; Lowry, T.F.; Wong-Riley, M.T.T.; Stryker, C.; Camperchioli, D.W.; Mayer, C.A.; Alilain, W.J.; Martin, R.J.; MacFarlane, P.M.; et al. Postnatal development of metabolic rate during normoxia and acute hypoxia in rats: implication for a sensitive period. J. Appl. Physiol. 2009, 106, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Trachsel, D.; Erb, T.O.; Hammer, J.; von Ungern-Sternberg, B.S. Developmental respiratory physiology. Pediatr. Anesthesia 2021, 32, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.M.; Rafiq, M.; Akhter, N.; AlGhamdi, F.S.; Tobias, J.D. Upper airway in infants—a computed tomography-based analysis. Pediatr. Anesthesia 2017, 27, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Praud, J.-P.; Reix, P. Upper airways and neonatal respiration. Respir. Physiol. Neurobiol. 2005, 149, 131–141. [Google Scholar] [CrossRef]
- Ramanathan, R.; Corwin, M.J.; Hunt, C.E.; Lister, G.; Tinsley, L.R.; Baird, T.; Silvestri, J.M.; Crowell, D.H.; Hufford, D.; Martin, R.J.; et al. Cardiorespiratory Events Recorded on Home Monitors. 2001, 285, 2199–2207. [CrossRef]
- Daftary, A.S.; Jalou, H.E.; Shively, L.; Slaven, J.E.; Davis, S.D. Polysomnography Reference Values in Healthy Newborns. J. Clin. Sleep Med. 2019, 15, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Hershenson, M.B.; Colin, A.A.; Wohl, M.E.B.; Stark, A.R. Changes in the Contribution of the Rib Cage to Tidal Breathing during Infancy. Am. Rev. Respir. Dis. 1990, 141, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Rosen, CL. Maturation of breathing during sleep. In: Marcus CL, Carroll JL, Donnelly DF, Loughlin GM, editors. Sleep and breathing in children: developmental changes in breathing during sleep. 2nd ed. New York, NY: Informa Healthcare; 2008. p. 117–30.
- Johnson, S.M.; Randhawa, K.S.; Epstein, J.J.; Gustafson, E.; Hocker, A.D.; Huxtable, A.G.; Baker, T.L.; Watters, J.J. Gestational intermittent hypoxia increases susceptibility to neuroinflammation and alters respiratory motor control in neonatal rats. Respir. Physiol. Neurobiol. 2018, 256, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Reeves, S.R.; Row, B.W.; Neville, J.J.; Guo, S.Z.; Lipton, A.J. Respiratory Effects of Gestational Intermittent Hypoxia in the Developing Rat. Am. J. Respir. Crit. Care Med. 2003, 167, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, J.M.; Martin, R.J.; Gauda, E.B. Apnea of prematurity – Perfect storm. Respir. Physiol. Neurobiol. 2013, 189, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Raffay, T.M.; Di Fiore, J.M.; Chen, Z.; Sánchez-Illana. ; Vento, M.; Piñeiro-Ramos, J.D.; Kuligowski, J.; Martin, R.J.; Tatsuoka, C.; Minich, N.M.; et al. Hypoxemia events in preterm neonates are associated with urine oxidative biomarkers. Pediatr. Res. 2023, 94, 1444–1450. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Adams-Chapman, I.; Fanaroff, A.A.; Hintz, S.R.; Vohr, B.; Higgins, R.D.; National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and Growth Impairment Among Extremely Low-Birth-Weight Infants with Neonatal Infection. JAMA 2004, 292, 2357–2365. [Google Scholar] [CrossRef]
- Alonso-Fernández, A.; Quetglas, C.R.; Mochales, A.H.; De Larrinaga, A. .R.; Barón, A.S.; Rodríguez, P.R.; Gil Gómez, A.V.; Martínez, C.P.; Marín, J.P.C.; Nicolau, M.B.; et al. Influence of Obstructive Sleep Apnea on Systemic Inflammation in Pregnancy. Front. Med. 2021, 8. [Google Scholar] [CrossRef]
- Ślusarczyk, J.; Trojan, E.; Głombik, K.; Budziszewska, B.; Kubera, M.; Lasoń, W.; Popiołek-Barczyk, K.; Mika, J.; Wędzony, K.; Basta-Kaim, A. Prenatal stress is a vulnerability factor for altered morphology and biological activity of microglia cells. Front. Cell. Neurosci. 2015, 9, 82–82. [Google Scholar] [CrossRef]
- Fournier, S.; Steele, S.; Julien, C.; Fournier, S.; Gulemetova, R.; Caravagna, C.; Soliz, J.; Bairam, A.; Kinkead, R. Gestational Stress Promotes Pathological Apneas and Sex-Specific Disruption of Respiratory Control Development in Newborn Rat. J. Neurosci. 2013, 33, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Lei, J.; Deng, F.; Zhao, C.; Xu, T.; Ji, B.; Fu, M.; Wang, X.; Sun, M.; Zhang, M.; et al. Gestational Hypoxia Impaired Endothelial Nitric Oxide Synthesis Via miR-155-5p/NADPH Oxidase/Reactive Oxygen Species Axis in Male Offspring Vessels. J. Am. Hear. Assoc. 2024, 13, e032079. [Google Scholar] [CrossRef] [PubMed]
- Morrison, N.R.; Johnson, S.M.; Hocker, A.D.; Kimyon, R.S.; Watters, J.J.; Huxtable, A.G. Time and dose-dependent impairment of neonatal respiratory motor activity after systemic inflammation. Respir. Physiol. Neurobiol. 2020, 272, 103314–103314. [Google Scholar] [CrossRef] [PubMed]
- Master, Z.R.; Porzionato, A.; Kesavan, K.; Mason, A.; Chavez-Valdez, R.; Shirahata, M.; Gauda, E.B. Lipopolysaccharide exposure during the early postnatal period adversely affects the structure and function of the developing rat carotid body. J. Appl. Physiol. 2016, 121, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Beyeler, S.A.; Hodges, M.R.; Huxtable, A.G. Impact of inflammation on developing respiratory control networks: rhythm generation, chemoreception and plasticity. Respir. Physiol. Neurobiol. 2019, 274, 103357–103357. [Google Scholar] [CrossRef] [PubMed]
- McDonald, F.B.; Dempsey, E.M.; O'Halloran, K.D. Effects of Gestational and Postnatal Exposure to Chronic Intermittent Hypoxia on Diaphragm Muscle Contractile Function in the Rat. Front. Physiol. 2016, 7. [Google Scholar] [CrossRef]
- McDonald, F.B.; Dempsey, E.M.; O'Halloran, K.D. Early Life Exposure to Chronic Intermittent Hypoxia Primes Increased Susceptibility to Hypoxia-Induced Weakness in Rat Sternohyoid Muscle during Adulthood. Front. Physiol. 2016, 7, 69. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




