Submitted:
30 April 2024
Posted:
02 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Plant Extracts
2.1.2. Reference Substance
2.1.3. Polysaccharides and Lipids
2.1.4. Light Source
2.1.5. Chemicals
2.1.6. Cell Lines
2.1.7. Virus
2.2. Methods
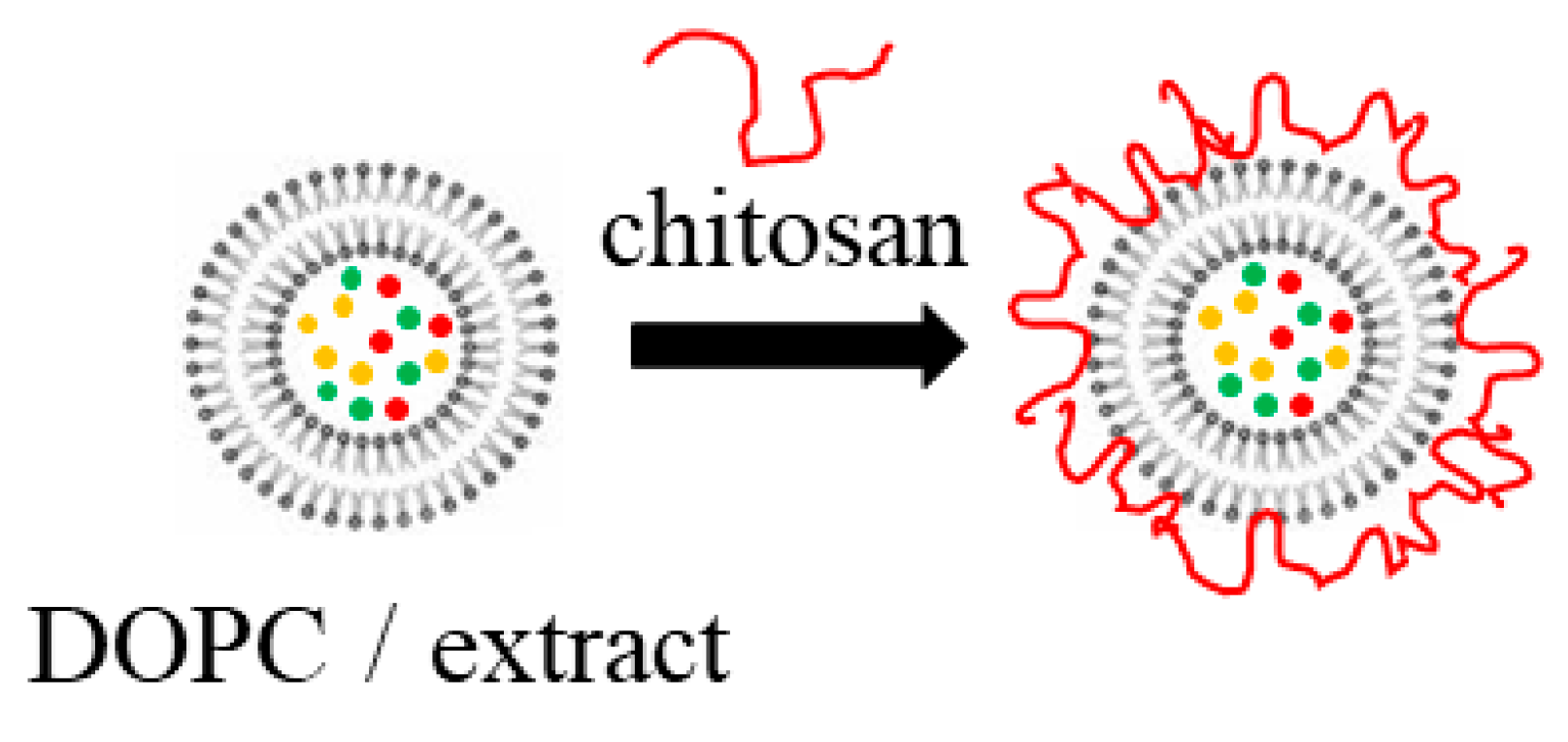
2.2.1. Formation of the Liposomes
2.2.2. Determination of Encapsulated Amount of Extracts
2.2.3. Determination of the Electrokinetic Charge and the Size of the Liposomes
2.2.4. Release of TFC or TPC from the Liposomes
2.2.5. Safety Test
2.2.6. Cytotoxicity Assay
2.2.7. Determination of Infectious Viral Titers
2.2.8. Antiviral Activity Assay
2.2.9. Virucidal Assay
2.2.10. Effect on the Viral Adsorption
2.2.11. Statistical Analysis
3. Results
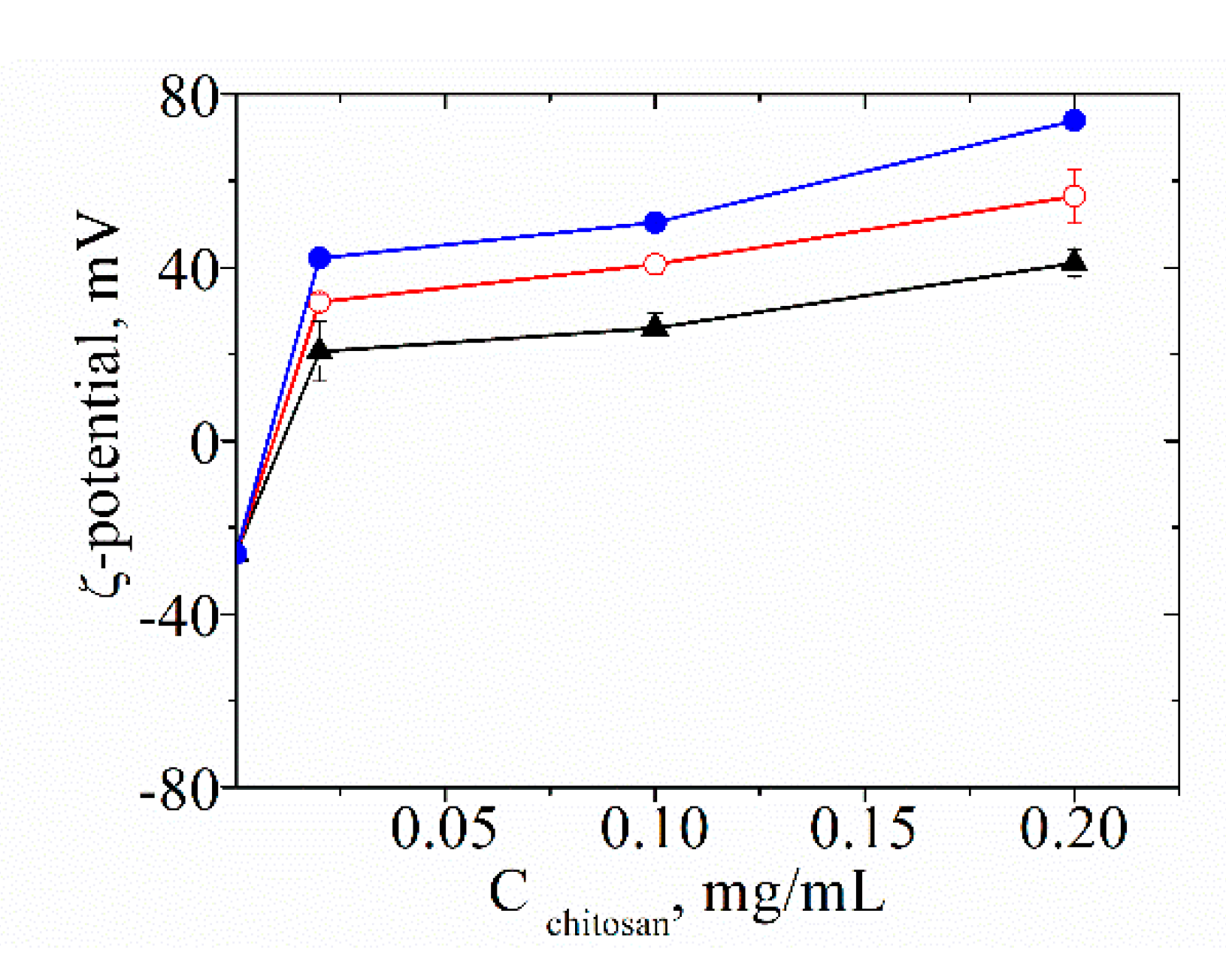
3.1. Characterization of the Physicochemical Properties of the Produced Liposomes
3.2. Determination of the Encapsulation Amount of Extracts
| Plant extract | µg/mL | EE (quercetin) % | EE (gallic acid) % | |
|---|---|---|---|---|
| LSN | 0.1700 | 99 | 1.53 | ≈ 100 |
| LAS | 0.0014 | 70 | 0.57 | 98 |
| LPR | 10.7959 | ≈ 100 | 2.54 | ≈ 100 |
| LAH | 14.7531 | ≈ 100 | 2.10 | ≈ 99.5 |
| LGGL. | 8.2132 | ≈ 100 | 3.40 | ≈ 100 |
3.3. Release of TFC and TPC from the Liposomes
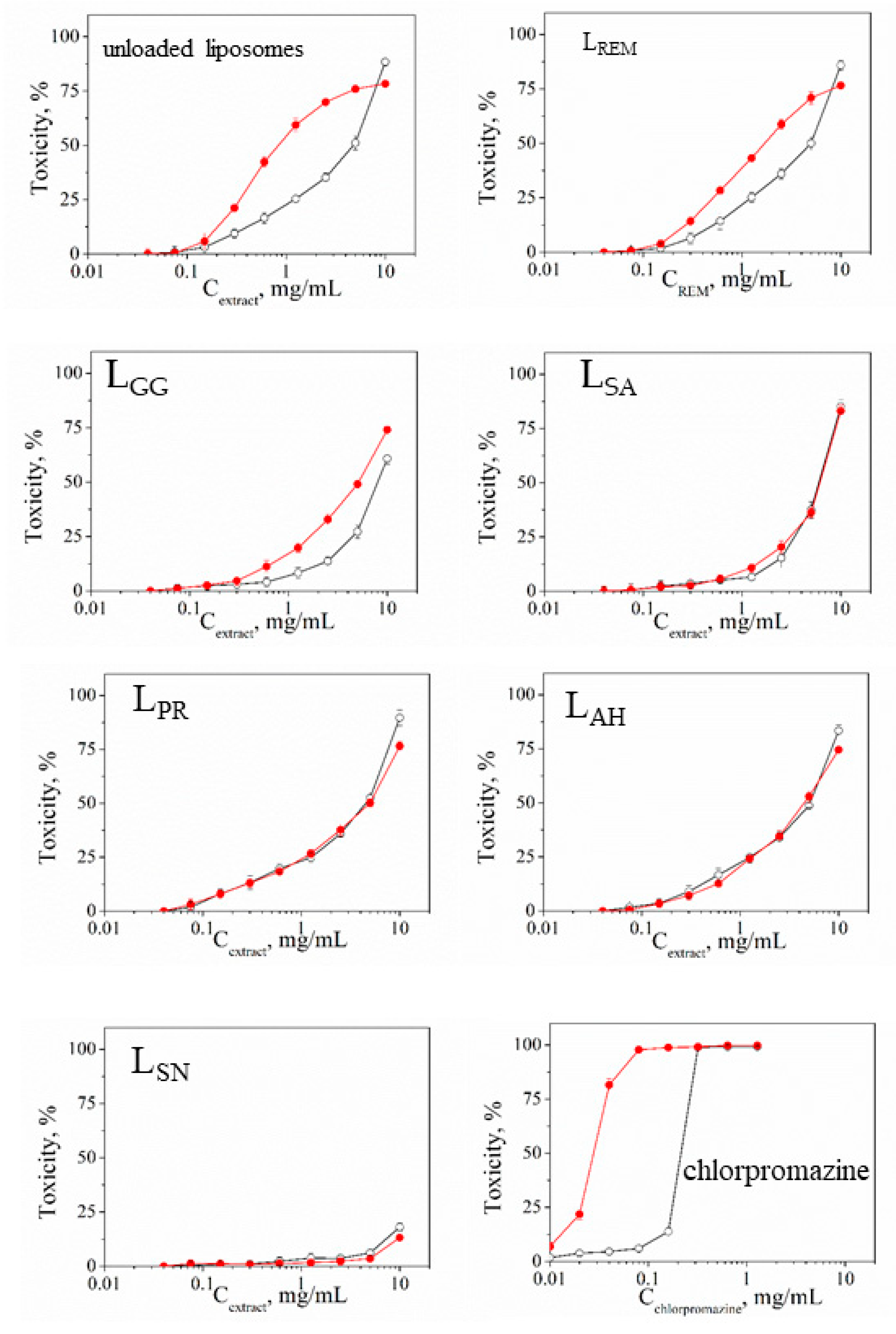
3.4. Safety Testing
4. Discussion
4.1. Unloaded and Extract-Loaded Liposomes
4.2. Antiviral Activity of Extract-Loaded Liposomes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Armendáriz-Barragán, B.; Zafar, N.; Badri, W.; Arturo, S.; Rodríguez, G.; Kabbaj, D.; Fessi, H.; Elaissari, A. Plant extracts: from encapsulation to application. Expert Opinion on Drug Delivery 2016, 13, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.K., Agarwal, R.K., Shah, M.A., Suriya, M. Chapter 4 - Encapsulation techniques for plant extracts. In Plant Extracts: Applications in the Food Industry; Mir, S.A., Manickavasagan, A., Shah, M.A; Academic Press, UK, 2022, 75-88.
- Paul, S., Bhattacharyya. Anticancer Potentials of Root Extract of Polygala senega and Its PLGA Nanoparticles-Encapsulated Form. Evid. Based Complement Alternat. Med. 2011, 2011, 517204. [Google Scholar] [CrossRef] [PubMed]
- Niksic, H., Becic. Cytotoxicity screening of Thymus vulgaris L. essential oil in brine shrimp nauplii and cancer cell lines. Sci. Reports 2021, 11, 13178. [Google Scholar] [CrossRef] [PubMed]
- Aygül, A., Şerbetçi. The antibacterial and antivirulent potential of Hypericum lydium against Staphylococcus aureus: Inhibition of growth, biofilm formation, and hemolytic activity. European Journal of Integrative Medicine 2020, 35, 101061. [Google Scholar] [CrossRef]
- Khalil, A.T., Khan. Synergistic antibacterial effect of honey and Herba Ocimi Basilici against some bacterial pathogens. J. Tradit. Chin. Med. 2013, 33, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Rouf, R., Uddin. Antiviral potential of garlic (Allium sativum) and its organosulfur compounds: A systematic update of pre-clinical and clinical data. Trends Food Sci Technol. 2020, 104, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Torabian, G., Valtchev, P. Qayyum Adil, Fariba Dehghani, Anti-influenza activity of elderberry (Sambucus nigra). Journal of Functional Foods 2019, 54, 353-360.
- Yuandani, Y., Rohani. Immunomodulatory Effects and Mechanisms of Curcuma Species and Their Bioactive Compounds: A Review. Frontiers in Pharmacology 2021, 12, 643119. [Google Scholar] [CrossRef] [PubMed]
- Samadder, A., Das. Ameliorative effects of syzygium jambolanum extract and its poly (lactic-Co-Glycolic) acid nanoencapsulated form on arsenic-induced hyperglycemic stress: a multi-parametric evaluation. J. Acupunct. Meridian Stud. 2012, 5, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, J., Tedesco. Anticholesterolemic Activity of Three Vegetal Extracts (Artichoke, Caigua, and Fenugreek) and Their Unique Blend. Frontiers Pharmacol. 2021, 12, 726199. [Google Scholar] [CrossRef] [PubMed]
- Strasser, M.P.N. Antiulcerogenic potential activity of free and nanoencapsulated Passiflora serratodigitata L. extracts. BioMed Res Int. 2014, 2014, 434067. [Google Scholar] [CrossRef] [PubMed]
- Bonaterra, G.A., Bronischewski. Anti-inflammatory and Anti-oxidative Effects of Phytohustil® and Root Extract of Althaea officinalis L. on Macrophages in vitro. Front Pharmacol. 2020, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Al-Dabbagh, B., Elhaty. Antioxidant and anticancer activities of chamomile (Matricaria recutita L.). BMC Res Notes. 2019, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.S., Huang. Artemisia annua L. extracts inhibit the in vitro replication of SARS-CoV-2 and two of its variants. Journal of Ethnopharmacology 2021, 274, 114016. [Google Scholar] [CrossRef] [PubMed]
- Das, S., Singh. Naturally occurring anthraquinones as potential inhibitors of SARS-CoV-2 main protease: an integrated computational study. Biologia (Bratisl) 2022, 77, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Vilhelmova-Ilieva, N., Petrova. Anti-Coronavirus Efficiency and Redox-Modulating Capacity of Polyphenol-Rich Extracts from Traditional Bulgarian Medicinal Plants. Life 2022, 12, 1088. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.S., Hanumanram, G., Suthakaran, P.K., Mohanan, J., Nair, L.D.V., Rajendran, K. Extracellular Oxidative Stress Markers in COVID-19 Patients with Diabetes as Co-Morbidity. Clin Pract. 2022, 12, 168-176.
- Zakay-Rones, Z., Thom. Randomized Study of the Efficacy and Safety of Oral Elderberry Extract in the Treatment of Influenza A and B Virus Infections. Journal of International Medical Research 2024, 32, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Wieland, L.S., Piechotta. Elderberry for prevention and treatment of viral respiratory illnesses: a systematic review. BMC Complement Med Ther 2021, 21, 112. [Google Scholar] [CrossRef]
- Mahboubi, M. Sambucus nigra (black elder) as alternative treatment for cold and flu. Adv Tradit Med (ADTM) 2021, 21, 405–414. [Google Scholar] [CrossRef]
- Chen, Ch., Zuckerman. Sambucus nigra extracts inhibit infectious bronchitis virus at an early point during replication. BMC Vet Res 2014, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Ahangar, N., Bakhshi Jouybari. Phytochemical Screening and Antinociceptive Activity of the Hydroalcoholic Extract of Potentilla reptans L. Pharm. Biomed. Res. 2021, 7, 271–278. [Google Scholar] [CrossRef]
- Uysal, S., Zengin. Chemical profile, antioxidant properties and enzyme inhibitory effects of the root extracts of selected Potentilla species. S. Afr. J. Bot. 2019, 120, 124–128. [Google Scholar] [CrossRef]
- El-Saber Batiha, G., Beshbishy. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Redondo, G.L.M., Loria. General aspects about Allium sativum – A review. Ars Pharm. 2021, 62, 471–481. [Google Scholar] [CrossRef]
- Ianovici, N., Latis. Foliar traits of Juglans regia, Aesculus hippocastanum and Tilia platyphyllos in urban habitat. Rom. Biotechnol. Lett. 2017, 22, 12400–12408. [Google Scholar]
- Dudek-Makuch, M., Studzińska-Sroka. Horse chestnut–efficacy and safety in chronic venous insufficiency: An overview. Rev. Bras. Farmacogn. 2015, 25, 533–541. [Google Scholar] [CrossRef]
- Parvaiz, M., Hussain. A review: Medicinal importance of Glycyrrhiza glabra L. (Fabaceae family). Glob. J. Pharm. 2014, 8, 8–13. [Google Scholar]
- Wahab, S., Ahmad. Pharmacological Efficacy and Safety of Glycyrrhiza glabra in the treatment of respiratory tract infections. Mini Rev. Med. Chem. 2021, 22, 1476–1494. [Google Scholar]
- Pandey, D.N., Rastogi. Influenza-like illness-related clinical trial on AYUSH-64 requires cautious interpretation. J. Ayurveda Integr. Med. 2022, 13, 100346. [Google Scholar] [CrossRef] [PubMed]
- Emami, S., Azadmard-Damirchi. Liposomes as carrier vehicles for functional compounds in food sector. Journal of Experimental Nanoscience 2016, 11, 737–759. [Google Scholar] [CrossRef]
- Bilia, A.R., Piazzini. Plants Extracts Loaded in Nanocarriers: an Emergent Formulating Approach. Natural Product Communications 2018, 13, 1157–1160. [Google Scholar] [CrossRef]
- Ðordevic, V., Balanc. Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds. Food Eng. Rev. 2015, 7, 452–490. [Google Scholar] [CrossRef]
- Fanga, Z., Bhandari. Encapsulation of polyphenols – a review. Trends in Food Science & Technology 2010, 21, 510–523. [Google Scholar]
- Tarone, A.G., Cazarin. Anthocyanins: New techniques and challenges in microencapsulation, review. Food Research International 2020, 133, 109092. [Google Scholar] [CrossRef] [PubMed]
- Hupfeld, S., Holsæter. Liposome Size Analysis by Dynamic/Static Light Scattering upon Size Exclusion-/Field Flow-Fractionation. Journal of Nanoscience and Nanotechnology 2006, 6, 3025–3031. [Google Scholar] [CrossRef] [PubMed]
- Rashidinejad, A., Birch, E.J., Sun-Waterhouse, D., Everett, D.W. Delivery of green tea catechin and epigallocatechin gallate in liposomes incorporated into low-fat hard cheese. Food Chemistry 2014, 156, 176–183.
- Gibis, M., Vogta. Encapsulation of polyphenolic grape seed extract in polymer-coated liposomes. Food & Function 2012, 3, 246–254. [Google Scholar]
- Akgün, D., Gültekin-Ozgüven. Stirred-type yoghurt incorporated with sour cherry extract in chitosan-coated liposomes. Food Hydrocolloids 2020, 101, 105532. [Google Scholar] [CrossRef]
- Aranaz, I., Alcántara. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Shoueir, K.R., El-Desouky. Chitosan based-nanoparticles and nanocapsules: Overview, physicochemical features, applications of a nanofibrous scaffold, and bioprinting. Int. J. Biol. Macromol. 2021, 167, 1176–1197. [Google Scholar] [CrossRef]
- Scheidt, H.A., Pampel. Investigation of the membrane localization and distribution of flavonoids by high-resolution magic angle spinning NMR spectroscopy. Biochim. Biophys. Acta 2004, 1663, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Karonen, M. Insights into Polyphenol–Lipid Interactions: Chemical Methods, Molecular Aspects and Their Effects on Membrane Structures- review. Plants 2022, 11, 1809. [Google Scholar] [CrossRef] [PubMed]
- Sanver, D., Murray. Experimental Modelling of Flavonoid-Biomembrane Interactions. Langmuir 2016, 32, 13234–13243. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, K., Kumazawa. Effects of external factors on the interaction of tea catechins with lipid bilayers. Biosci. Biotechnol. Biochem. 2002, 66, 2330–2335. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, I., Paunova-Krasteva, T., Petrova, Z., Grozdanov, P., Nikolova, N., Tsonev, G., Triantafyllidis, A., Andreev, S., Trepechova, M., Milkova, V., Vilhelmova-Ilieva, N. Bulgarian Medicinal Extracts as Natural Inhibitors with Antiviral and Antibacterial Activity. Plants 2022, 11, 1666.
- Salinas, F., Vázquez. Aesculus hippocastanum L. seed extract shows virucidal and antiviral activities against respiratory syncytial virus (RSV) and reduces lung inflammation in vivo. Antivir. Res. 2019, 164, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, A., Kolodziejczykczepas. Potential activity mechanisms of aesculus hippocastanum bark: Antioxidant effects in chemical and biological in vitro models. Antioxidants 2021, 10, 995. [Google Scholar] [CrossRef] [PubMed]
- Rouf, R., Uddin. Anti-viral potential of garlic (Allium sativum) and it0 s organosulfur compounds: A systematic update of pre-clinical and clinical data. Trends Food Sci. Technol. 2020, 104, 219–234. [Google Scholar] [CrossRef]
- Chen, C., Zuckerman. Sambucus nigra extracts inhibit infectious bronchitis virus at an early point during replication. BMC Vet. Res. 2014, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Torabian, G., Valtchev. Anti-influenza activity of elderberry (Sambucus nigra). J. Funct. Foods 2019, 54, 353–360. [Google Scholar] [CrossRef]
- Kaur, R., Kaur. Glycyrrhiza glabra: A phytopharmacological review. Int. J. Pharm. Sci. Res. 2013, 4, 2470–2477. [Google Scholar]
- Wang, L., Yang. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B 2015, 5, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Hamad, G., Elaziz. Chemical Composition, Antioxidant, Antimicrobial and Anticancer Activities of Licorice (Glycyrrhiza glabra L.) Root and Its Application in Functional Yoghurt. J. Food Nutr. Res. 2020, 8, 707–715. [Google Scholar] [CrossRef]
- Tomczyk, M., Latté. Potentilla-A review of its phytochemical and pharmacological profile. J. Ethnopharmacol. 2009, 122, 184–204. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J., Muench. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Gouveia, S., Castilho. Characterization of phenolic acid derivates and flavonoids from different morphological parts of Helichrysum obconicum by RP-HPLC-DAD-(-)ESI-MSn method. Food Chem. 2011, 129, 333–344. [Google Scholar] [CrossRef]
- McDonald, S., Prenzeler. Phenolic content and antioxidant activity of olive extracts. Foods Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Srinivas, K., King. Solubility and solution thermodynamic properties of quercetin and quercetin dihydrate in subcritical water. J. of Food Eng 2010, 100, 208–218. [Google Scholar] [CrossRef]
- Daneshfar, A., Ghaziaskar. Solubility of Gallic Acid in Methanol, Ethanol, Water, and Ethyl Acetate. Chem. Eng. Data 2008, 53, 776–778. [Google Scholar] [CrossRef]
- ESAC. Statement on the scientific validity of the 3T3 NRU PT test (an in vitro test for phototoxic potential), 9th meeting of ECVAM Scientific Advisory Committee 1998.
- Halle, W. The Registry of Cytotoxicity: toxicity testing in cell cultures to predict acute toxicity (LD50) and to reduce testing in animals. Altern Lab Anim 2003, 31, 89–198. [Google Scholar] [CrossRef] [PubMed]
- Milkova, V., Kamburova, K., Martinov, P., Vilhelmova-Ilieva, N., Rashev, V. Chitosan-Based Nanocarriers for Delivery of Remdesivir. Sci. Pharm 2023, 91, 3.
- Boroumand, H., Badie, F., Mazaheri, S., Seyedi, Z.S., Nahand, J.S., Nejati, M., Baghi, H.B., Abbasi-Kolli, M., Badehnoosh, B., Ghandali, M., Hamblin, M.R., Mirzaei, H. Chitosan-Based Nanoparticles Against Viral Infections. Front Cell Infect Microbiol. 2021, 17;11:643953.
- Funkhouser, J. D., Aronson, N. N. Chitinase family GH18: evolutionary insights from the genomic history of a diverse protein family. BMC Evol. Biol. 2007, 7, 96.
- Ngo, D.-H., Vo, T.-S., Ngo, D.-N., Kang, K.-H., Je, J.-Y., Pham, H. N.-D., Byun, H.-G., Kim, S.-K. Biological effects of chitosan and its derivatives. Food Hydrocolloids, 2015, 51, 200–216.
- Calderón, L., Harris, R., Cordoba-Diaz, M., Elorza, M., Elorza, B., Lenoir, J., Adriaens, E., Remon, J.P., Heras, A., Cordoba-Diaz, D. Nano and microparticulate chitosan-based systems for antiviral topical delivery. Eur. J. Pharm. Sci. 2013, 48, 216–222.
- Hasanovic, A., Zehl, M., Reznicek, G., Valenta, C. Chitosan-tripolyphosphate nanoparticles as a possible skin drug delivery system for aciclovir with enhanced stability. J. Pharm. Pharmacol. 2009, 61, 1609–1616.
- Jamali A, Mottaghitalab F, Abdoli A, Dinarvand M, Esmailie A, Kheiri MT, Atyabi F. Inhibiting influenza virus replication and inducing protection against lethal influenza virus challenge through chitosan nanoparticles loaded by siRNA. Drug Deliv Transl 2018, 8(1):12-20.
- Russo, E., Gaglianone, N., Baldassari, S., Parodi, B., Cafaggi, S., Zibana, C., Donalisio, M., Cagno, V., Lembo, D., Caviglioli, G. Preparation, characterization and in vitro antiviral activity evaluation of foscarnet-chitosan nanoparticles, Colloids and Surfaces B: Biointerfaces, 2014, 118, 117-125.
- Alcantara, K.P., Nalinratana, N., Chutiwitoonchai, N., Castillo, A.L., Banlunara, W., Vajragupta, O., Rojsitthisak, P., Rojsitthisak, P. Enhanced Nasal Deposition and Anti-Coronavirus Effect of Favipiravir-Loaded Mucoadhesive Chitosan–Alginate Nanoparticles. Pharmaceutics 2022, 14, 2680.
- Ng, S.W., Selvarajah, G.T., Hussein, M.Z., Yeap, S.K., Omar, A.R. In Vitro Evaluation of Curcumin-Encapsulated Chitosan Nanoparticles against Feline Infectious Peritonitis Virus and Pharmacokinetics Study in Cats. Biomed Res Int. 2020, 31;2020:3012198.
- Hadian, M., Fathi, M., Mohammadi, A., Eskandari, M.H., Asadsangabi, M., Pouraghajan, K., Shohrati, M., Mohammadpour, M., Samadi, M. Characterization of chitosan/Persian gum nanoparticles for encapsulation of Nigella sativa extract as an antiviral agent against avian coronavirus. Int J Biol Macromol. 2024, 11;265(Pt 1):130749.
- Elnosary, M.E., Aboelmagd, H.A., Habaka, M.A., Salem, S.R., El-Naggar, M.E. Synthesis of bee venom loaded chitosan nanoparticles for anti-MERS-COV and multi-drug resistance bacteria. Int J Biol Macromol. 2023, 1;224:871-880.
- Loutfy, S.A., Abdel-Salam, A.I., Moatasim, Y., Gomaa, M.R., Abdel Fattah, N.F., Emam, M.H., Ali, F., ElShehaby, H.A., Ragab, E.A., Alam El-Din, H.M., Mostafa, A., Ali, M.A., Kasry, A. Antiviral activity of chitosan nanoparticles encapsulating silymarin (Sil–CNPs) against SARSCoV-2 (in silico and in vitro study). : RSC Adv. 2022, 12, 15775.
| Plant Species | Area of the Collected Material | Biological Activities | References | |
|---|---|---|---|---|
| Aesculus hippocastanum (horse chestnut) |
 |
Seed | Anti-inflammatory, vascular supporting, immunomodulatory, antioxidant, virucidal, antiviral (against RSV, HSV-1, VSV, RSV, Dengue virus) activity. | [48,49] |
| Allium sativum (garlic) |
 |
Bulb | Immunomodulatory activity; prevention of infectious diseases; pronounced antiviral activity through various mechanisms of action: inhibition of virus entry into the cell, inhibition of viral RNA polymerase, reverse transcriptase, DNA synthesis. | [50] |
| Sambucus nigra (elderberry) |
 |
Fruit | Anti-inflammatory, immunomodulatory, antiviral activity. | [51,52] |
| Glycyrrhiza glabra L. (licorice) |
 |
Root | Positive effect in gastrointestinal problems (gastritis, peptic ulcer), in respiratory infections, arthritis and tremors. Pronounced anti-inflammatory, antispasmodic, antioxidant, antidiabetic, antimalarial, antifungal, antibacterial, antiviral effect. | [53,54,55] |
| Potentilla reptans (creeping cinquefoil) |
 |
Stem | Well manifested antidiarrheal, antidiabetic, hepatoprotective, antioxidant, antispasmodic, anti-inflammatory, antitumor, antifungal, antibacterial, antiviral action. | [56] |
| Liposomes | D*, nm | ζ-potential, mV |
|---|---|---|
| LSN | 197.6 ± 85.1 | -44.2 ± 1.0 |
| LAS | 50.8 ± 22.9 | -49.1 ± 1.5 |
| LPR | 229.5 ± 14.6 | -36.5 ± 1.7 |
| LAH | 221.2 ± 15.2 | -27.9 ± 2.4 |
| LGGL. | 261.2 ± 9.8 | -26.1 ± 1.8 |
| L unloaded | 267.6 ± 16.2 | -50.2 ± 2.1 |
| Liposomes | D*, nm | ||
|---|---|---|---|
| COS | CS-L | CS-H | |
| LSN | 361.3 ± 110.5 | 568.3 ± 150.7 | 146.1 ± 78.7 |
| LAS | 229.8 ± 80.5 | 103.1 ± 32.4 | 488.7 ± 108. |
| LPR | 361.3 ± 86.5 | 229.8 ± 57.5 | 169.9 ± 50.1 |
| LAH | 187.5 ± 4.5 | 187.6 ± 2.3 | 222.8 ± 5.1 |
| LGGL. | 191.1 ± 6.6 | 187.5 ± 4.5 | 232.5 ± 22.2 |
| Sample | Mean CC50 ± SD (mg/mL) | PIF* | |
|---|---|---|---|
| - Irr | + Irr | ||
| unloaded liposomes | 2.364 ± 0.2434 | 0.411 ± 4.03 | 5.75 |
| LREM ** | 2.419 ± 0.2306 | 0.831 ± 2.57 | 2.91 |
| LGG | 4.002 ± 0.2005 | 2.543 ± 16.76 | 1.57 |
| LAS | 2.993 ± 0.1001 | 3.071 ± 5.1 | 0.97 |
| LPT | 2.269 ± 0.2151 | 2.459 ± 13.8 | 0.92 |
| LAH | 2.562 ± 0.0513 | 2.245 ± 23.73 | 1.14 |
| LSN | > 10 | > 10 | - |
| Chlorpromazine*** | 0.022 ± 0.003 | 0.003 ± 0.0006 | 7.33 |
| Sample | HCT-8 cell line | |
|---|---|---|
| CC50 * Mean ± SD ** [µg/mL] | MTC *** [µg/mL] | |
| unloaded liposomes | ≥ 1000 | ≥ 1000 |
| REM | 2500.00 ± 4.3# | 1000.0# |
| LREM | 2358.0 ± 25.2 | 1195.0 |
| Extract (A. hippocastani) | 1420.0 ± 46.2## | 800.0## |
| LAH | 1839.6 ± 28.7 | 900.0 |
| Extract (A. sativum) | 1880.0 ± 55.7## | 1200.0## |
| LAS | 2055.3 ± 37.2 | 1300.0 |
| Extract (S. nigra) | 1900.0 ± 48.3## | 1000.0## |
| LSN | 2350.0 ± 38.7 | 1150.0 |
| Extract (G. glabra) | 1820.0 ± 24.5## | 1000.0## |
| LGG | 1817.0 ± 27.3 | 1100.0 |
| Extract (P. reptans) | 1880.0 ± 37.1## | 200.0## |
| LRP | 1528.6 ± 26.8 | 350.0 |
| Compounds | HCoV-OC43 | |
|---|---|---|
| IC50 * Mean ± SD ** (µg/mL) | SI *** | |
| unloaded liposomes | - | - |
| REM | 12.5 ± 0.9# | 200.0# |
| LREM | 4.3 ± 0.8 | 548.3 |
| Extract (A. hippocastani) | 380.0 ± 9.5## | 3.7## |
| LAH | 31.2 ± 2.4 | 58.96 |
| Extract (A. sativum) | 900.0 ± 18.5## | 2.1## |
| LAS | 151.5 ± 8.2 | 13.56 |
| Extract (S. nigra) | 950.0 ± 32.7## | 2.0## |
| LSN | 215.0 ± 7.3 | 10.93 |
| Extract (G. glabra) | 400.0 ± 12.5## | 4.5## |
| LGG | 46.5 ± 3.9 | 39.1 |
| Extract (P. reptans) | 890.0 ± 17.3## | 2.1## |
| LPR | 47.5 ± 3.3 | 32.2 |
| Compounds | Δlg | |||||
|---|---|---|---|---|---|---|
| 15 min | 30 min | 60 min | 90 min | 120 min | ||
| unloaded liposomes | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | |
| LAH | 0.25 | 0.25 | 0.50 | 0.50 | 0.50 | |
| LAS | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | |
| LSN | 0.15 | 0.15 | 0.25 | 0.25 | 0.25 | |
| LGG | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | |
| LPR | 0.15 | 0.15 | 0.15 | 0.25 | 0.25 | |
| 70% etanol | 5.00 | 5.00 | 5.00 | 5.00 | 4.75 | |
| Compounds | Δlg | |||||
|---|---|---|---|---|---|---|
| 15 min | 30 min | 60 min | 90 min | 120 min | ||
| unloaded liposomes | 0.00 | 0.15 | 0.25 | 0.25 | 0.25 | |
| LAH | 0.00 | 0.00 | 0.25 | 0.25 | 0.25 | |
| LAS | 0.00 | 0.25 | 0.25 | 0.25 | 0.25 | |
| LSN | 0.00 | 0.15 | 0.15 | 0.15 | 0.15 | |
| LGG | 0.15 | 0.15 | 0.15 | 0.25 | 0.25 | |
| LPR | 0.00 | 0.15 | 0.15 | 0.15 | 0.15 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





