Submitted:
02 May 2024
Posted:
02 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
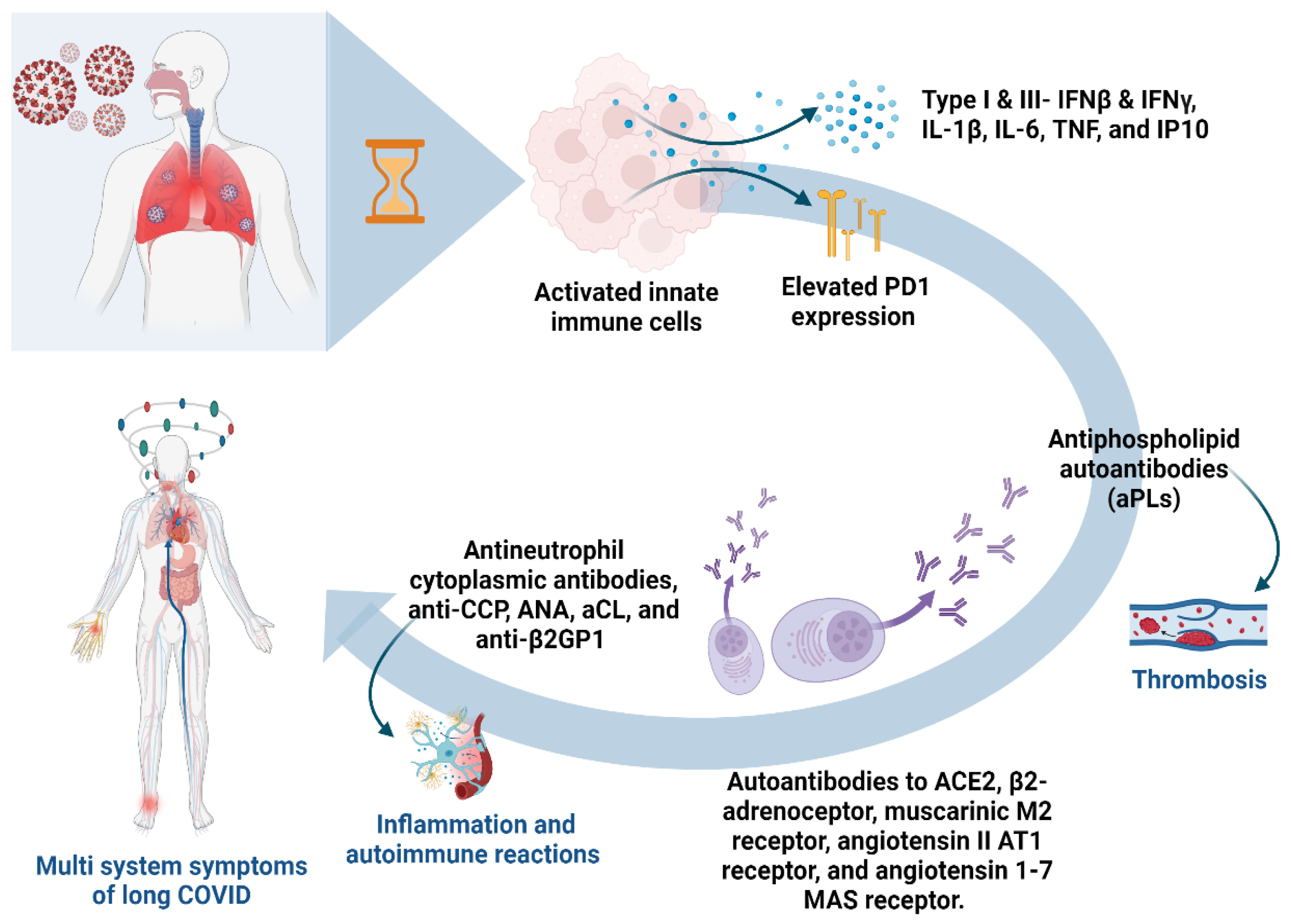
2. Long COVID and Autoantibody
2.1. Unraveling Persistent Symptoms and Immune Responses
2.2. Molecular Roots and Immune Dysregulation in Long COVID: Insights from Studies
2.3. Immune Complexes as Potential Markers for Critical COVID-19 Disease Progression
2.4. Autoantibodies in COVID-19-Associated Thrombosis
2.5. Autoimmune Reactions and Connective Tissue Diseases
3. Cardiovascular Complications
3.1. In-Depth Examination of Cardiovascular Issues Associated with Long COVID
3.2. Discussion of the Potential Mechanisms Involved
3.3. Addressing Potential Interactions between different Comorbidities and Their Impact on Long COVID
4. Respiratory Complications
4.1. Analysis of Persistent Respiratory Symptoms
4.2. Autoimmune Antibodies and Lung Involvement
4.3. Lung Function Abnormalities in Long COVID
5. Neurological Complications
5.1. Long COVID's Diverse and Systemic Neurological Impact
5.2. Long COVID's Potential Neurological Mechanisms
5.3. Neurological Symptoms in Long COVID
6. Psychosocial Impact
6.1. Psychological and Social Consequences of Long COVID
6.2. Challenges Faced by Individuals with Prolonged Symptoms
7. Obesity and Its Impact
7.1. Influence of Obesity on Inflammatory Mediators and Long COVID Consequences
7.2. Effects of Metabolic Syndrome on Health in the Context of Long COVID-19
8. Diagnostic Challenges
8.1. Analysis of Difficulties in Diagnosing and Characterizing Long COVID
8.2. Discussion on the Evolving Diagnostic Criteria and Methodologies
9. Management and Treatment Strategies
9.1. Overview of Current Approaches to Managing Long COVID
10. Conclusion
11. Challenges and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: major findings, mechanisms and recommendations. Nature Reviews Microbiology. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, A.; Norouzi, S.; Dehdarirad, H.; Aghlmand, S.; Yusefzadeh, H.; Javan-Noughabi, J. The global economic burden of COVID-19 disease: a comprehensive systematic review and meta-analysis. Syst Rev. 2024, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Silva Andrade, B.; Siqueira, S.; de Assis Soares, W.R.; de Souza Rangel, F.; Santos, N.O.; Dos Santos Freitas, A.; et al. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses. 2021, 13, 700. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H. How common is long COVID? Why studies give different answers. Nature. 2022, 606, 852–853. [Google Scholar] [PubMed]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. Condition WHOCCDWGoP-C-. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Center of disease control and prevention (CDC). Long COVID or Post-COVID Conditions. 2023 [Available from: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html.
- Bull-Otterson, L.; Baca, S.; Saydah, S.; Boehmer, T.K.; Adjei, S.; Gray, S.; et al. Post–COVID Conditions Among Adult COVID-19 Survivors Aged 18–64 and ≥65 Years — United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep. 2022, 71, 713–717. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Bosworth, M.L.; King, S.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; et al. Risk of long COVID in people infected with severe acute respiratory syndrome coronavirus 2 after 2 doses of a coronavirus disease 2019 vaccine: community-based, matched cohort study. Open Forum Infectious Diseases; 2022: Oxford University Press US.
- Altmann, D.M.; Whettlock, E.M.; Liu, S.; Arachchillage, D.J.; Boyton, R.J. The immunology of long COVID. Nat Rev Immunol. 2023, 23, 618–634. [Google Scholar] [CrossRef]
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Shaar, B.A.; Almamlouk, R.; Riaz, M.; et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin Microbiol Infect. 2022, 28, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J Infect Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef] [PubMed]
- Appelman, B.; Charlton, B.T.; Goulding, R.P.; Kerkhoff, T.J.; Breedveld, E.A.; Noort, W.; et al. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nature communications. 2024, 15, 17. [Google Scholar] [CrossRef]
- Xie, Y.; Al-Aly, Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022, 10, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022, 28, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Spudich, S.; Nath, A. Nervous system consequences of COVID-19. Science. 2022, 375, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Zawilska, J.B.; Kuczynska, K. Psychiatric and neurological complications of long COVID. J Psychiatr Res. 2022, 156, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022, 185, 881–895. [Google Scholar] [CrossRef]
- Turner, S.; Khan, M.A.; Putrino, D.; Woodcock, A.; Kell, D.B.; Pretorius, E. Long COVID: pathophysiological factors and abnormalities of coagulation. Trends Endocrinol Metab. 2023, 34, 321–344. [Google Scholar] [CrossRef]
- Takakura, K.; Suka, M.; Kajihara, M.; Koido, S. Clinical features, therapeutic outcomes, and recovery period of long COVID. J Med Virol. 2023, 95, e28316. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Sivan, M.; Delaney, B.; Evans, R.; Milne, R. Long covid-an update for primary care. BMJ. 2022, 378, e072117. [Google Scholar] [CrossRef]
- El-Maradny, Y.A.; Rubio-Casillas, A.; Uversky, V.N.; Redwan, E.M. Intrinsic factors behind long-COVID: I. Prevalence of the extracellular vesicles. J Cell Biochem. 2023, 124, 656–673. [Google Scholar] [CrossRef]
- El-Maradny, Y.A.; Rubio-Casillas, A.; Mohamed, K.I.; Uversky, V.N.; Redwan, E.M. Intrinsic factors behind long-COVID: II. SARS-CoV-2, extracellular vesicles, and neurological disorders. J Cell Biochem. 2023, 124, 1466–1485. [Google Scholar] [CrossRef]
- El-Baky, N.A.; Amara, A.A.; Uversky, V.N.; Redwan, E.M. Intrinsic factors behind long COVID: III. Persistence of SARS-CoV-2 and its components. J Cell Biochem. 2024, 125, 22–44. [Google Scholar] [CrossRef] [PubMed]
- Eltayeb, A.; Al-Sarraj, F.; Alharbi, M.; Albiheyri, R.; Mattar, E.H.; Abu Zeid, I.M.; et al. Intrinsic factors behind long COVID: IV. Hypothetical roles of the SARS-CoV-2 nucleocapsid protein and its liquid–liquid phase separation. Journal of Cellular Biochemistry. 2024. [CrossRef] [PubMed]
- Sharma, C.; Bayry, J. High risk of autoimmune diseases after COVID-19. Nat Rev Rheumatol. 2023, 19, 399–400. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Rodriguez, Y.; Acosta-Ampudia, Y.; Monsalve, D.M.; Zhu, C.; Li, Q.Z.; et al. Autoimmunity is a hallmark of post-COVID syndrome. J Transl Med. 2022, 20, 129. [Google Scholar] [CrossRef]
- Glynne, P.; Tahmasebi, N.; Gant, V.; Gupta, R. Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J Investig Med. 2022, 70, 61–67. [Google Scholar] [CrossRef]
- Arthur, J.M.; Forrest, J.C.; Boehme, K.W.; Kennedy, J.L.; Owens, S.; Herzog, C.; et al. Development of ACE2 autoantibodies after SARS-CoV-2 infection. PLoS One. 2021, 16, e0257016. [Google Scholar] [CrossRef]
- Wallukat, G.; Hohberger, B.; Wenzel, K.; Furst, J.; Schulze-Rothe, S.; Wallukat, A.; et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J Transl Autoimmun. 2021, 4, 100100. [Google Scholar] [CrossRef]
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L.; Gruell, H.; et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur. 2021, 6, 100122. [Google Scholar] [CrossRef]
- García-Abellán, J.; Padilla, S.; Fernández-González, M.; García, J.A.; Agulló, V.; Andreo, M.; et al. Antibody response to SARS-CoV-2 is associated with long-term clinical outcome in patients with COVID-19: a longitudinal study. Journal of clinical immunology. 2021, 41, 1490–1501. [Google Scholar] [CrossRef]
- Fedorchenko, Y.; Zimba, O. Long COVID in autoimmune rheumatic diseases. Rheumatol Int. 2023, 43, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Quinones-Moya, H.; Ocampo-Del Valle, A.; Camargo-Coronel, A.; Jiménez-Balderas, F.J.; Bernal-Enriquez, M.B.; Madinabeitia-Rodríguez, P.; et al. Long COVID in patients with rheumatologic disease: a single center observational study. Indian Journal of Rheumatology. 2023, 18, 212–217. [Google Scholar] [CrossRef]
- Lopes, L.A.; Agrawal, D.K. Thromboembolism in the Complications of Long COVID-19. Cardiology and cardiovascular medicine. 2023, 7, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Ahmad, M.N.; Khalid, M.; Minhas, A.; Ali, R.; Sarfraz, Z.; et al. Long COVID and Wavering Incidence of Pulmonary Embolism: A Systematic Review. Journal of community hospital internal medicine perspectives. 2023, 13, 23–31. [Google Scholar] [CrossRef]
- Manzo, G. COVID-19 as an immune complex hypersensitivity in antigen excess conditions: theoretical pathogenetic process and suggestions for potential therapeutic interventions. Frontiers in Immunology. 2020, 11, 566000. [Google Scholar] [CrossRef]
- Kim, D.M.; Kim, Y.; Seo, J.W.; Lee, J.; Park, U.; Ha, N.Y.; et al. Enhanced eosinophil-mediated inflammation associated with antibody and complement-dependent pneumonic insults in critical COVID-19. Cell Rep. 2021, 37, 109798. [Google Scholar] [CrossRef]
- Chakraborty, S.; Gonzalez, J.; Edwards, K.; Mallajosyula, V.; Buzzanco, A.S.; Sherwood, R.; et al. Proinflammatory IgG Fc structures in patients with severe COVID-19. Nat Immunol. 2021, 22, 67–73. [Google Scholar] [CrossRef]
- Kolb, P.; Giese, S.; Voll, R.E.; Hengel, H.; Falcone, V. Immune complexes as culprits of immunopathology in severe COVID-19. Medical microbiology and immunology. 2023, 212, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, H.; Soegiarto, G. Risk of Thrombosis during and after a SARS-CoV-2 Infection: Pathogenesis, Diagnostic Approach, and Management. Hematol Rep. 2023, 15, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, C.; Jing, H.; Wu, X.; Novakovic, V.A.; Xie, R.; et al. Long COVID: The Nature of Thrombotic Sequelae Determines the Necessity of Early Anticoagulation. Front Cell Infect Microbiol. 2022, 12, 861703. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.S.; Caricchio, R.; Casanova, J.L.; Combes, A.J.; Diamond, B.; Fox, S.E.; et al. The intersection of COVID-19 and autoimmunity. J Clin Invest. 2021, 131. [Google Scholar] [CrossRef]
- Zuo, Y.; Zuo, M.; Yalavarthi, S.; Gockman, K.; Madison, J.A.; Shi, H.; et al. Neutrophil extracellular traps and thrombosis in COVID-19. J Thromb Thrombolysis. 2021, 51, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Hønge, B.L.; Hermansen, M.-L.F.; Storgaard, M. Reactive arthritis after COVID-19. BMJ Case Reports CP. 2021, 14, e241375. [Google Scholar] [CrossRef] [PubMed]
- Batur, E.B.; Korez, M.K.; Gezer, I.A.; Levendoglu, F.; Ural, O. Musculoskeletal symptoms and relationship with laboratory findings in patients with COVID-19. International journal of clinical practice. 2021, 75, e14135. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, H.R.; Nune, A. Long COVID from rheumatology perspective - a narrative review. Clinical rheumatology. 2022, 41, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Gyongyosi, M.; Alcaide, P.; Asselbergs, F.W.; Brundel, B.; Camici, G.G.; Martins, P.D.C.; et al. Long COVID and the cardiovascular system-elucidating causes and cellular mechanisms in order to develop targeted diagnostic and therapeutic strategies: a joint Scientific Statement of the ESC Working Groups on Cellular Biology of the Heart and Myocardial and Pericardial Diseases. Cardiovasc Res. 2023, 119, 336–356. [Google Scholar]
- Lerma, L.A.; Chaudhary, A.; Bryan, A.; Morishima, C.; Wener, M.H.; Fink, S.L. Prevalence of autoantibody responses in acute coronavirus disease 2019 (COVID-19). J Transl Autoimmun. 2020, 3, 100073. [Google Scholar] [CrossRef]
- Kouranloo, K.; Dey, M.; Elwell, H.; Nune, A. A systematic review of the incidence, management and prognosis of new-onset autoimmune connective tissue diseases after COVID-19. Rheumatol Int. 2023, 43, 1221–1243. [Google Scholar] [CrossRef]
- Lorente-Ros, M.; Das, S.; Elias, J.; Frishman, W.H.; Aronow, W.S. Cardiovascular Manifestations of the Long COVID Syndrome. Cardiology in review. 2023. [CrossRef]
- Goerlich, E.; Chung, T.H.; Hong, G.H.; Metkus, T.S.; Gilotra, N.A.; Post, W.S.; et al. Cardiovascular effects of the post-COVID-19 condition. Nature Cardiovascular Research. 2024, 3, 118–129. [Google Scholar] [CrossRef]
- Tolu-Akinnawo, O.; Adusei Poku, F.; Elimihele, T.; League, M.; Adkins, C.F.; Okafor, H. Acute Cardiovascular Complications of COVID-19: A Systematic Review. Cureus. 2023, 15, e38576. [Google Scholar] [CrossRef]
- Terzic, C.M.; Medina-Inojosa, B.J. Cardiovascular Complications of Coronavirus Disease-2019. Phys Med Rehabil Clin N Am. 2023, 34, 551–561. [Google Scholar] [CrossRef]
- Cremonesi, M.; Felicetta, A.; Cannata, F.; Serio, S.; van Beek, J.J.P.; Bombace, S.; et al. Long COVID-19 Cardiac Complications Are Associated With Autoimmunity to Cardiac Self-Antigens Sufficient to Cause Cardiac Dysfunction. Circulation. 2023, 148, 504–507. [Google Scholar] [CrossRef]
- Sander, L.E.; Garaude, J. The mitochondrial respiratory chain: A metabolic rheostat of innate immune cell-mediated antibacterial responses. Mitochondrion. 2018, 41, 28–36. [Google Scholar] [CrossRef]
- Adebayo, A.; Varzideh, F.; Wilson, S.; Gambardella, J.; Eacobacci, M.; Jankauskas, S.S.; et al. l-Arginine and COVID-19: An Update. Nutrients. 2021, 13, 3951. [Google Scholar] [CrossRef]
- DePace, N.L.; Colombo, J. Long-COVID Syndrome and the Cardiovascular System: A Review of Neurocardiologic Effects on Multiple Systems. Current cardiology reports. 2022, 24, 1711–1726. [Google Scholar] [CrossRef]
- Czeisler, M.E.; Ibrahim, S.A. Cardiovascular Risks in Patients With Post-COVID-19 Condition. JAMA Health Forum. 2023, 4, e224664. [Google Scholar] [CrossRef]
- Golchin Vafa, R.; Heydarzadeh, R.; Rahmani, M.; Tavan, A.; Khoshnoud Mansorkhani, S.; Zamiri, B.; et al. The long-term effects of the Covid-19 infection on cardiac symptoms. BMC cardiovascular disorders. 2023, 23, 286. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Khunti, K.; Nafilyan, V.; Maddox, T.; Humberstone, B.; Diamond, I.; et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021, 372, n693. [Google Scholar] [CrossRef]
- Xie, Y.; Bowe, B.; Al-Aly, Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nature communications. 2021, 12, 6571. [Google Scholar] [CrossRef]
- Kanne, J.P.; Little, B.P.; Schulte, J.J.; Haramati, A.; Haramati, L.B. Long-term Lung Abnormalities Associated with COVID-19 Pneumonia. Radiology. 2023, 306, e221806. [Google Scholar] [CrossRef]
- Törnberg, A.; Svensson-Raskh, A.; Rydwik, E.; Björnsson, M.; Runold, M.; Bruchfeld, J.; et al. Twenty months follow-up in non-hospitalised adults with post COVID-19 condition: a preliminary longitudinal cohort study. European Respiratory Journal. 2022, 60 (suppl 66), 4637. [Google Scholar]
- Børvik, T.; Evensen, L.H.; Morelli, V.M.; Melbye, H.; Brækkan, S.K.; Hansen, J.B. Impact of respiratory symptoms and oxygen saturation on the risk of incident venous thromboembolism—the Tromsø study. Research and Practice in Thrombosis and Haemostasis. 2020, 4, 255–262. [Google Scholar] [CrossRef]
- Chaaya, G.; Vishnubhotla, P. Pulmonary Vein Thrombosis: A Recent Systematic Review. Cureus. 2017, 9, e993. [Google Scholar] [CrossRef]
- Vyas, V.; Goyal, A. Acute Pulmonary Embolism. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Amandeep Goyal declares no relevant financial relationships with ineligible companies.: StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.; 2024.
- Hariri, L.P.; North, C.M.; Shih, A.R.; Israel, R.A.; Maley, J.H.; Villalba, J.A.; et al. Lung histopathology in coronavirus disease 2019 as compared with severe acute respiratory sydrome and H1N1 influenza: a systematic review. Chest. 2021, 159, 73–84. [Google Scholar] [CrossRef]
- Weerahandi, H.; Hochman, K.A.; Simon, E.; Blaum, C.; Chodosh, J.; Duan, E.; et al. Post-Discharge Health Status and Symptoms in Patients with Severe COVID-19. Journal of general internal medicine. 2021, 36, 738–745. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent symptoms in patients after acute COVID-19. Jama. 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Ceccarini, M.R.; Bonetti, G.; Medori, M.C.; Dhuli, K.; Tezzele, S.; Micheletti, C.; et al. Autoantibodies in patients with post-COVID syndrome: a possible link with severity? Eur Rev Med Pharmacol Sci. 2023, 27, 48–56. [Google Scholar]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N Engl J Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Han, X.; Fan, Y.; Alwalid, O.; Li, N.; Jia, X.; Yuan, M.; et al. Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia. Radiology. 2021, 299, E177–E86. [Google Scholar] [CrossRef]
- Mandal, S.; Barnett, J.; Brill, S.E.; Brown, J.S.; Denneny, E.K.; Hare, S.S.; et al. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021, 76, 396–398. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; et al. Post-acute COVID-19 syndrome. Nat Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Hodgson, C.L.; Broadley, T. Long COVID—unravelling a complex condition. The Lancet Respiratory Medicine. 2023, 11, 667–668. [Google Scholar] [CrossRef]
- Shehata, G.A.; Lord, K.C.; Grudzinski, M.C.; Elsayed, M.; Abdelnaby, R.; Elshabrawy, H.A. Neurological Complications of COVID-19: Underlying Mechanisms and Management. International journal of molecular sciences. 2021, 22. [Google Scholar] [CrossRef]
- Maury, A.; Lyoubi, A.; Peiffer-Smadja, N.; de Broucker, T.; Meppiel, E. Neurological manifestations associated with SARS-CoV-2 and other coronaviruses: A narrative review for clinicians. Rev Neurol (Paris). 2021, 177, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.J.; Movassaghi, M.; Gordy, D.; Olson, M.G.; Zhang, T.; Khurana, M.S.; et al. Neuropathology of COVID-19 (neuro-COVID): clinicopathological update. Free Neuropathol. 2021, 2. [Google Scholar]
- Balachandran, H.; Phetsouphanh, C.; Agapiou, D.; Adhikari, A.; Rodrigo, C.; Hammoud, M.; et al. Maintenance of broad neutralizing antibodies and memory B cells 1 year post-infection is predicted by SARS-CoV-2-specific CD4+ T cell responses. Cell reports. 2022, 38. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, J.; Pei, S.; Lu, Y.; Li, C.; Zhu, J.; et al. An updated review of epidemiological characteristics, immune escape, and therapeutic advances of SARS-CoV-2 Omicron XBB. 1.5 and other mutants. Front Cell Infect Microbiol. 2023, 13, 1297078. [Google Scholar] [CrossRef] [PubMed]
- Varatharaj, A.; Thomas, N.; Ellul, M.A.; Davies, N.W.S.; Pollak, T.A.; Tenorio, E.L.; et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. The lancet Psychiatry. 2020, 7, 875–882. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health (NIH). NIH study identifies features of Long COVID neurological symptoms 2023 [Available from: https://www.nih.gov/news-events/news-releases/nih-study-identifies-features-long-covid-neurological-symptoms#:~:text=People%20with%20post%2Dacute%20sequelae,fog%2C%E2%80%9D%20or%20cognitive%20impairment.
- Leng, A.; Shah, M.; Ahmad, S.A.; Premraj, L.; Wildi, K.; Li Bassi, G.; et al. Pathogenesis Underlying Neurological Manifestations of Long COVID Syndrome and Potential Therapeutics. Cells. 2023, 12, 816. [Google Scholar] [CrossRef]
- Hawes, M.T.; Szenczy, A.K.; Klein, D.N.; Hajcak, G.; Nelson, B.D. Increases in depression and anxiety symptoms in adolescents and young adults during the COVID-19 pandemic. Psychol Med. 2022, 52, 3222–3230. [Google Scholar] [CrossRef]
- Dubey, S.; Biswas, P.; Ghosh, R.; Chatterjee, S.; Dubey, M.J.; Chatterjee, S.; et al. Psychosocial impact of COVID-19. Diabetes & metabolic syndrome. 2020, 14, 779–788. [Google Scholar]
- Aghaei, A.; Zhang, R.; Taylor, S.; Tam, C.-C.; Yang, C.-H.; Li, X.; et al. Social Life of Females with Persistent COVID-19 Symptoms: A Qualitative Study. International Journal of Environmental Research and Public Health. 2022, 19, 9076. [Google Scholar] [CrossRef] [PubMed]
- Taube, M. Depression and brain fog as long-COVID mental health consequences: Difficult, complex and partially successful treatment of a 72-year-old patient-A case report. Front Psychiatry. 2023, 14, 1153512. [Google Scholar] [CrossRef] [PubMed]
- Kolivand, P.; Hosseindoost, S.; Kolivand, Z.; Gharaylou, Z. Psychosocial impact of COVID-19 2 years after outbreak on mental health of medical workers in Iran. Middle East Current Psychiatry. 2023, 30, 4. [Google Scholar] [CrossRef]
- Hazumi, M.; Okazaki, E.; Usuda, K.; Kataoka, M.; Nishi, D. Relationship between attitudes toward COVID-19 infection, depression and anxiety: a cross-sectional survey in Japan. BMC psychiatry. 2022, 22, 798. [Google Scholar] [CrossRef]
- Zhang, E.; Su, S.; Gao, S.; Liu, R.; Ding, X.; Zhang, Y.; et al. Coronavirus Disease 2019 Pandemic-Related Long-Term Chronic Impacts on Psychological Health of Perinatal Women in China. American journal of perinatology. 2023. [CrossRef]
- Hazumi, M.; Usuda, K.; Okazaki, E.; Kataoka, M.; Nishi, D. Differences in the course of depression and anxiety after COVID-19 infection between recovered patients with and without a psychiatric history: a cross-sectional study. International Journal of Environmental Research and Public Health. 2022, 19, 11316. [Google Scholar] [CrossRef] [PubMed]
- Jeelani, A.; Dkhar, S.A.; Quansar, R.; Khan, S.S. Prevalence of depression and anxiety among school-going adolescents in Indian Kashmir valley during COVID-19 pandemic. Middle East current psychiatry. 2022, 29, 18. [Google Scholar] [CrossRef]
- Kibria, M.G.; Kabir, R.; Rahman, U.S.; Ahmed, S.; Amin, S.; Arafat, S. Prevalence and factors associated with depression and anxiety among COVID-19 survivors in Dhaka city. Frontiers in Psychiatry. 2024, 15, 1280245. [Google Scholar] [CrossRef] [PubMed]
- Kosovali, B.D.; Tezcan, B.; Aytac, I.; Tuncer Peker, T.; Soyal, O.B.; Mutlu, N.M. Anxiety and Depression in the Relatives of COVID-19 and Non-COVID-19 Intensive Care Patients During the Pandemic. Cureus. 2021, 13, e20559. [Google Scholar] [CrossRef] [PubMed]
- Qiao, T.; Gao, D.; Lu, G.; Yi, W.; Lv, Z. Association of gastrointestinal symptoms and skipping breakfast with anxiety and depressive symptoms in quarantined Chinese college students during the Shanghai 2022 lockdown: a cross sectional survey. BMC Psychiatry. 2023, 23, 889. [Google Scholar] [CrossRef] [PubMed]
- Okogbenin, E.O.; Seb-Akahomen, O.J.; Edeawe, O.; Ehimigbai, M.; Eboreime, H.; Odike, A.; et al. Psychiatric manifestations and associated risk factors among hospitalised patients with COVID-19 in Edo State, Nigeria: a cross-sectional study. BMJ open. 2022, 12, e058561. [Google Scholar] [CrossRef]
- Lindau, S.T.; Makelarski, J.A.; Boyd, K.; Doyle, K.E.; Haider, S.; Kumar, S.; et al. Change in Health-Related Socioeconomic Risk Factors and Mental Health During the Early Phase of the COVID-19 Pandemic: A National Survey of U.S. Women. J Womens Health (Larchmt). 2021, 30, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Martín-Guerrero, J.D.; Cancela-Cilleruelo, I.; Moro-López-Menchero, P.; Rodríguez-Jiménez, J.; Pellicer-Valero, O.J. Trajectory curves of post-COVID anxiety/depressive symptoms and sleep quality in previously hospitalized COVID-19 survivors: the LONG-COVID-EXP-CM multicenter study. Psychological medicine. 2023, 53, 4298–4299. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Rodríguez-Jiménez, J.; Palacios-Ceña, M.; de-la-Llave-Rincón, A.I.; Fuensalida-Novo, S.; Florencio, L.L.; et al. Psychometric properties of the Hospital Anxiety and Depression Scale (HADS) in previously hospitalized COVID-19 patients. International journal of environmental research and public health. 2022, 19, 9273. [Google Scholar] [CrossRef]
- Feldman, D.E.; Boudrias, M.H.; Mazer, B. Long COVID symptoms in a population-based sample of persons discharged home from hospital. Can J Public Health. 2022, 113, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.; Schulze, H.; James, J.C.; Siems, N.; Trampe, N.; Gold, R.; et al. Hypoechogenicity of brainstem raphe in long-COVID syndrome-less common but independently associated with depressive symptoms: a cross-sectional study. Journal of neurology. 2022, 269, 4604–4610. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, J.; Wang, Y.; Bai, M.; Zhang, Y.; Chen, H.; et al. A cross-sectional study on the mental health of patients with COVID-19 1 year after discharge in Huanggang, China. Eur Arch Psychiatry Clin Neurosci. 2023, 273, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.J.; Rabheru, K.; Peisah, C.; Reichman, W.; Ikeda, M. Loneliness and social isolation during the COVID-19 pandemic. Int Psychogeriatr. 2020, 32, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Ashkenazi-Hoffnung, L.; Greenberg, D.; Dalal, I.; Livni, G.; Chapnick, G.; et al. The Burden of COVID-19 in Children and Its Prevention by Vaccination: A Joint Statement of the Israeli Pediatric Association and the Israeli Society for Pediatric Infectious Diseases. Vaccines 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Kader, N.; Elhusein, B.; Al Abdulla, S.; Hamza, A.H.; Al Maslamani, M.; Chandra, P.; et al. Risk perception and psychological impact of COVID-19 pandemic among healthcare workers in primary and secondary healthcare settings in Qatar: A national study. Journal of Primary Care & Community Health. 2021, 12, 21501327211039714. [Google Scholar]
- Callard, F.; Perego, E. How and why patients made Long Covid. Social science & medicine. 2021, 268, 113426. [Google Scholar]
- Rando, H.M.; Bennett, T.D.; Byrd, J.B.; Bramante, C.; Callahan, T.J.; Chute, C.G.; et al. Challenges in defining Long COVID: Striking differences across literature, Electronic Health Records, and patient-reported information. medRxiv : the preprint server for health sciences 2021. [Google Scholar]
- Fiscella, K.; Sanders, M.; Yaeger, J., editors. Strategies to promote equity in COVID-19 antiviral treatment. JAMA Health Forum; 2022: American Medical Association.
- Huang, Y.; Lu, Y.; Huang, Y.M.; Wang, M.; Ling, W.; Sui, Y.; et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020, 113, 154378. [Google Scholar] [CrossRef]
- Xiang, M.; Wu, X.; Jing, H.; Novakovic, V.A.; Shi, J. The intersection of obesity and (long) COVID-19: Hypoxia, thrombotic inflammation, and vascular endothelial injury. Frontiers in cardiovascular medicine. 2023, 10, 1062491. [Google Scholar] [CrossRef]
- Bello-Chavolla, O.Y.; Bahena-Lopez, J.P.; Antonio-Villa, N.E.; Vargas-Vazquez, A.; Gonzalez-Diaz, A.; Marquez-Salinas, A.; et al. Predicting Mortality Due to SARS-CoV-2: A Mechanistic Score Relating Obesity and Diabetes to COVID-19 Outcomes in Mexico. J Clin Endocrinol Metab. 2020, 105, 2752–2761. [Google Scholar] [CrossRef] [PubMed]
- Landecho, M.F.; Marin-Oto, M.; Recalde-Zamacona, B.; Bilbao, I.; Fruhbeck, G. Obesity as an adipose tissue dysfunction disease and a risk factor for infections - Covid-19 as a case study. European journal of internal medicine. 2021, 91, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, C.E.; Phinney, S.D.; Fernandez, M.L.; Quann, E.E.; Wood, R.J.; Bibus, D.M.; et al. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. 2008, 43, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.M.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Emanuele, E.; Lucia, A.; Galvez, B.G. 'Adipaging': ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. The Journal of physiology. 2016, 594, 3187–3207. [Google Scholar] [CrossRef] [PubMed]
- Tibirica, E.; De Lorenzo, A. Increased Severity of COVID-19 in People with Obesity: Are We Overlooking Plausible Biological Mechanisms? Obesity (Silver Spring). 2020, 28, 1374. [Google Scholar] [CrossRef] [PubMed]
- Jose, R.J.; Manuel, A. Does coronavirus disease 2019 disprove the obesity paradox in acute respiratory distress syndrome? Obesity (Silver Spring, Md). 2020, 28, 1007. [Google Scholar] [CrossRef] [PubMed]
- de Lima, J.B.; Salazar, L.; Fernandes, A.; Teixeira, C.; Marques, L.; Afonso, C. Long COVID in children and adolescents: a retrospective study in a pediatric cohort. The Pediatric Infectious Disease Journal. 2023, 42, e109–e11. [Google Scholar] [CrossRef] [PubMed]
- Lacavalerie, M.R.; Pierre-Francois, S.; Agossou, M.; Inamo, J.; Cabie, A.; Barnay, J.L.; et al. Obese patients with long COVID-19 display abnormal hyperventilatory response and impaired gas exchange at peak exercise. Future Cardiology. 2022, 18, 577–584. [Google Scholar] [CrossRef]
- Lastra, G.; Manrique, C. Perivascular adipose tissue, inflammation and insulin resistance: link to vascular dysfunction and cardiovascular disease. Horm Mol Biol Clin Investig. 2015, 22, 19–26. [Google Scholar] [CrossRef]
- Denson, J.L.; Gillet, A.S.; Zu, Y.; Brown, M.; Pham, T.; Yoshida, Y.; et al. Metabolic Syndrome and Acute Respiratory Distress Syndrome in Hospitalized Patients With COVID-19. JAMA Netw Open. 2021, 4, e2140568. [Google Scholar] [CrossRef]
- Li, B.; Yang, J.; Zhao, F.; Zhi, L.; Wang, X.; Liu, L.; et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020, 109, 531–538. [Google Scholar] [CrossRef]
- Mahamid, M.; Nseir, W.; Khoury, T.; Mahamid, B.; Nubania, A.; Sub-Laban, K.; et al. Nonalcoholic fatty liver disease is associated with COVID-19 severity independently of metabolic syndrome: a retrospective case-control study. European Journal of Gastroenterology & Hepatology. 2021, 33, 1578–1581. [Google Scholar]
- Hariyanto, T.I.; Kurniawan, A. Dyslipidemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020, 14, 1463–1465. [Google Scholar]
- Xie, J.; Zu, Y.; Alkhatib, A.; Pham, T.T.; Gill, F.; Jang, A.; et al. Metabolic Syndrome and COVID-19 Mortality Among Adult Black Patients in New Orleans. Diabetes care. 2020, 44, 188–193. [Google Scholar] [CrossRef]
- Lohia, P.; Kapur, S.; Benjaram, S.; Pandey, A.; Mir, T.; Seyoum, B. Metabolic syndrome and clinical outcomes in patients infected with COVID-19: Does age, sex, and race of the patient with metabolic syndrome matter? Journal of diabetes. 2021, 13, 420–429. [Google Scholar] [CrossRef]
- Dalle Grave, R.; Calugi, S.; Centis, E.; Marzocchi, R.; El Ghoch, M.; Marchesini, G. Lifestyle modification in the management of the metabolic syndrome: achievements and challenges. Diabetes, metabolic syndrome and obesity : targets and therapy. 2010, 3, 373–385. [Google Scholar]
- Azizi, F.; Mirmiran, P.; Momenan, A.A.; Hadaegh, F.; Habibi Moeini, A.; Hosseini, F.; et al. The effect of community-based education for lifestyle intervention on the prevalence of metabolic syndrome and its components: tehran lipid and glucose study. Int J Endocrinol Metab. 2013, 11, 145–153. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Cozma, D.; Kamel, M.; Hamad, M.; Mohammad, M.G.; Khan, N.A.; et al. Long-COVID, Metabolic and Endocrine Disease. Horm Metab Res. 2022, 54, 562–566. [Google Scholar] [CrossRef]
- National Institute of Health. NIH’s Strategic Response 2023 [Available from: https://covid19.nih.gov/nih-strategic-response-covid-19#priority-2.
- Srikanth, S.; Boulos, J.R.; Dover, T.; Boccuto, L.; Dean, D. Identification and diagnosis of long COVID-19: A scoping review. Progress in biophysics and molecular biology. 2023, 182, 1–7. [Google Scholar] [CrossRef]
- Areerob, Y.; Sagadevan, S.; Oh, W.-C. A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19. Nanotechnology Reviews. 2023, 12, 20220513. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.E.; Kim, T.; Yun, K.W.; Lee, S.H.; Lee, E.; et al. Preliminary Guidelines for the Clinical Evaluation Management of Long, C. O.V.I.D. Infect Chemother. 2022, 54, 566–597. [Google Scholar] [CrossRef]
- Munblit, D.; Nicholson, T.; Akrami, A.; Apfelbacher, C.; Chen, J.; De Groote, W.; et al. A core outcome set for post-COVID-19 condition in adults for use in clinical practice and research: an international Delphi consensus study. The Lancet Respiratory medicine. 2022, 10, 715–724. [Google Scholar] [CrossRef]
- Raveendran, A.V. Long COVID-19: Challenges in the diagnosis and proposed diagnostic criteria. Diabetes & metabolic syndrome. 2021, 15, 145–146. [Google Scholar]
- Shah, W.; Hillman, T.; Playford, E.D.; Hishmeh, L. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ. 2021, 372, n136. [Google Scholar] [CrossRef]
- Duerlund, L.S.; Shakar, S.; Nielsen, H.; Bodilsen, J. Positive Predictive Value of the ICD-10 Diagnosis Code for Long-COVID. Clin Epidemiol. 2022, 14, 141–148. [Google Scholar] [CrossRef]
- Froidure, A.; Mahsouli, A.; Liistro, G.; De Greef, J.; Belkhir, L.; Gerard, L.; et al. Integrative respiratory follow-up of severe COVID-19 reveals common functional and lung imaging sequelae. Respir Med. 2021, 181, 106383. [Google Scholar] [CrossRef]
- Mallia, P.; Meghji, J.; Wong, B.; Kumar, K.; Pilkington, V.; Chhabra, S.; et al. Symptomatic, biochemical and radiographic recovery in patients with COVID-19. BMJ Open Respir Res. 2021, 8, e000908. [Google Scholar] [CrossRef]
- Sun, B.; Tang, N.; Peluso, M.J.; Iyer, N.S.; Torres, L.; Donatelli, J.L.; et al. Characterization and Biomarker Analyses of Post-COVID-19 Complications and Neurological Manifestations. Cells. 2021, 10. [Google Scholar] [CrossRef]
- Tudoran, C.; Tudoran, M.; Pop, G.N.; Giurgi-Oncu, C.; Cut, T.G.; Lazureanu, V.E.; et al. Associations between the Severity of the Post-Acute COVID-19 Syndrome and Echocardiographic Abnormalities in Previously Healthy Outpatients Following Infection with SARS-CoV-2. Biology. 2021, 10. [Google Scholar] [CrossRef]
- Sabanoglu, C.; Inanc, I.H.; Polat, E.; Peker, S.A. Long-term predictive value of cardiac biomarkers in patients with COVID-19 infection. Eur Rev Med Pharmacol Sci. 2022, 26, 6396–6403. [Google Scholar] [PubMed]
- Motloch, L.J.; Jirak, P.; Gareeva, D.; Davtyan, P.; Gumerov, R.; Lakman, I.; et al. Cardiovascular Biomarkers for Prediction of in-hospital and 1-Year Post-discharge Mortality in Patients With COVID-19 Pneumonia. Frontiers in medicine. 2022, 9, 906665. [Google Scholar] [CrossRef]
- Alvarez, M.; Trent, E.; Goncalves, B.S.; Pereira, D.G.; Puri, R.; Frazier, N.A.; et al. Cognitive dysfunction associated with COVID-19: Prognostic role of circulating biomarkers and microRNAs. Front Aging Neurosci. 2022, 14, 1020092. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Sivan, M.; Taylor, S. NICE guideline on long covid. BMJ. 2020, 371, m4938. [Google Scholar] [CrossRef]
- Siso-Almirall, A.; Brito-Zeron, P.; Conangla Ferrin, L.; Kostov, B.; Moragas Moreno, A.; Mestres, J.; et al. Long Covid-19: Proposed Primary Care Clinical Guidelines for Diagnosis and Disease Management. Int J Environ Res Public Health 2021, 18. [Google Scholar] [CrossRef]
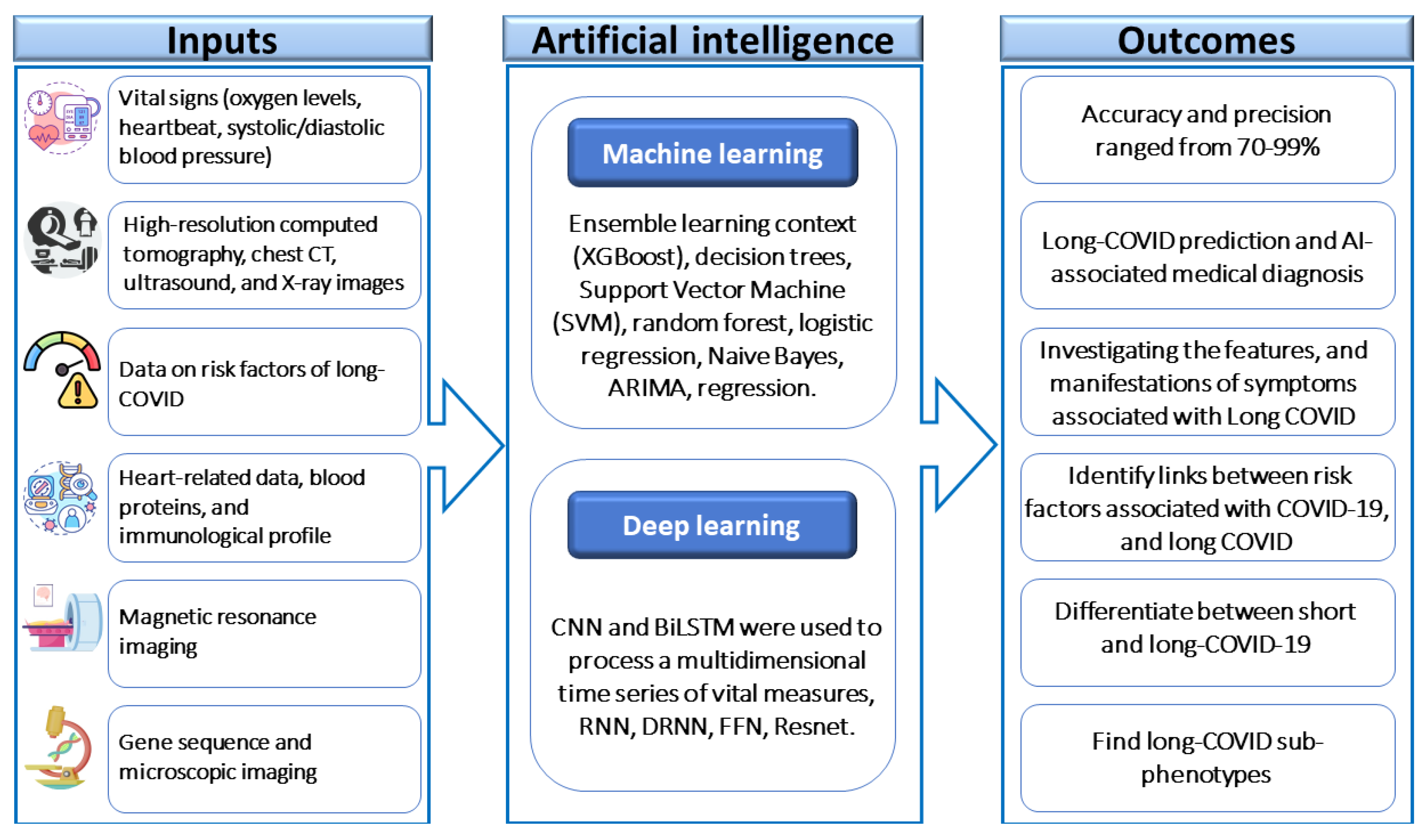
- Mondal, M.R.H.; Bharati, S.; Podder, P. Diagnosis of COVID-19 Using Machine Learning and Deep Learning: A Review. Current medical imaging. 2021, 17, 1403–1418. [Google Scholar]
- Paul, S.G.; Saha, A.; Biswas, A.A.; Zulfiker, M.S.; Arefin, M.S.; Rahman, M.M.; et al. Combating Covid-19 using machine learning and deep learning: Applications, challenges, and future perspectives. Array (New York, NY). 2023, 17, 100271. [Google Scholar] [CrossRef]
- Pfaff, E.R.; Girvin, A.T.; Bennett, T.D.; Bhatia, A.; Brooks, I.M.; Deer, R.R.; et al. Identifying who has long COVID in the USA: a machine learning approach using N3C data. The Lancet Digital health. 2022, 4, e532–e41. [Google Scholar] [CrossRef]
- Albaqer, H.A.; Al-Jibouri, K.J.; Martin, J.; Al-Amran, F.G.; Rawaf, S.; Yousif, M.G. Long-term Neurological Sequelae in Post-COVID-19 Patients: A Machine Learning Approach to Predict Outcomes. arXiv 2023, arXiv:230909993. [Google Scholar]
- Moore, S.; Hill, E.M.; Dyson, L.; Tildesley, M.J.; Keeling, M.J. Retrospectively modeling the effects of increased global vaccine sharing on the COVID-19 pandemic. Nat Med. 2022, 28, 2416–2423. [Google Scholar] [CrossRef]
- Azzolini, E.; Levi, R.; Sarti, R.; Pozzi, C.; Mollura, M.; Mantovani, A.; et al. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. Jama. 2022, 328, 676–678. [Google Scholar] [CrossRef]
- Gao, P.; Liu, J.; Liu, M. Effect of COVID-19 Vaccines on Reducing the Risk of Long COVID in the Real World: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2022, 19, 12422. [Google Scholar] [CrossRef]
- Su, S.; Zhao, Y.; Zeng, N.; Liu, X.; Zheng, Y.; Sun, J.; et al. Epidemiology, clinical presentation, pathophysiology, and management of long COVID: an update. Molecular psychiatry. 2023, 28, 4056–4069. [Google Scholar] [CrossRef]
- de Sire, A.; Moggio, L.; Marotta, N.; Agostini, F.; Tasselli, A.; Drago Ferrante, V.; et al. Impact of rehabilitation on fatigue in post-COVID-19 patients: a systematic review and meta-analysis. Applied Sciences. 2022, 12, 8593. [Google Scholar] [CrossRef]
- Joli, J.; Buck, P.; Zipfel, S.; Stengel, A. Post-COVID-19 fatigue: A systematic review. Front Psychiatry. 2022, 13, 947973. [Google Scholar] [CrossRef]
- Gibson, P.; Wang, G.; McGarvey, L.; Vertigan, A.E.; Altman, K.W.; Birring, S.S.; et al. Treatment of Unexplained Chronic Cough: CHEST Guideline and Expert Panel Report. Chest. 2016, 149, 27–44. [Google Scholar] [CrossRef]
- Mitchell, S.A.C.; Garrod, R.; Clark, L.; Douiri, A.; Parker, S.M.; Ellis, J.; et al. Physiotherapy, and speech and language therapy intervention for patients with refractory chronic cough: a multicentre randomised control trial. Thorax. 2017, 72, 129–136. [Google Scholar] [CrossRef]
- Parker, A.M.; Brigham, E.; Connolly, B.; McPeake, J.; Agranovich, A.V.; Kenes, M.T.; et al. Addressing the post-acute sequelae of SARS-CoV-2 infection: a multidisciplinary model of care. The Lancet Respiratory medicine. 2021, 9, 1328–1341. [Google Scholar] [CrossRef]
- Song, W.J.; Hui, C.K.M.; Hull, J.H.; Birring, S.S.; McGarvey, L.; Mazzone, S.B.; et al. Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses. The Lancet Respiratory medicine. 2021, 9, 533–544. [Google Scholar] [CrossRef]
- National Institute of Health. COVID-19 Treatment Guidelines 2 2024 [Available from: https://www.covid19treatmentguidelines.nih.gov/.
- Khani, E.; Khiali, S.; Beheshtirouy, S.; Entezari-Maleki, T. Potential pharmacologic treatments for COVID-19 smell and taste loss: A comprehensive review. Eur J Pharmacol. 2021, 912, 174582. [Google Scholar] [CrossRef]
- Vaniprabha, A.; Logeshwaran, J.; Kiruthiga, T.; Shah, K.B. Examination of the Effects of Long-term COVID-19 Impacts on Patients with Neurological Disabilities Using a Neuro machine Learning Model. BOHR International Journal of Neurology and Neuroscience. 2022, 1, 21–28. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




