1. Introduction
Nearly 1% of the general population are afflicted with rheumatoid arthritis (RA), a chronic autoimmune disease characterized by articular inflammation as well as joint and tissue damage. While a variety of innate and adaptive immune cells play a role in the pathogenesis of RA, macrophages are central to initiating and driving the disease [
1]. These cells act as primary sources of cytokines (e.g., TNF and IL-1β), chemokines, and degradative enzymes, contributing to joint inflammation and ultimately leading to the degradation of cartilage and bone. Importantly, the number of macrophages in synovial tissue is of clinical importance, serving as a reliable marker for assessing disease severity and therapy response [
1].
Genetic polymorphisms can influence the incidence and severity of RA. Genome-wide association studies have strongly linked a region on chromosome 9 in the TRAF1/C5 locus tagged by several single nucleotide polymorphisms (SNPs) in linkage disequilibrium, including rs3761847, with an elevated risk of rheumatoid arthritis [
2,
3]. Moreover, RA patients harboring these SNPs exhibit higher mortality from sepsis [
4]. A recent study has identified rs7034653 in TRAF1 as the functional variant linked to increased susceptibility to RA [
5]. TRAF1 is an important signaling adapter protein that plays disparate roles in various immune cells, where it can promote proliferation and survival of T and B lymphocytes downstream of TNFR signaling (e.g., 4-1BB and CD40) but inhibit inflammatory cytokine production from monocytes and macrophages downstream of innate immune signaling [
6,
7]. A key study showed that healthy individuals with the disease-associated SNP exhibit decreased TRAF1 expression in their peripheral blood monocytes and T lymphocytes [
8]. Consistent with TRAF1′s pro-survival role in lymphocytes, T cells from individuals with the disease associated SNP produced fewer cytokines following activation. On the other hand, monocytes from those same individuals produced higher levels of proinflammatory cytokines (TNF, IL-6) following lipopolysaccharide (LPS) stimulation compared to those from the lower risk group. Importantly, the study showed that the increase in pro-inflammatory cytokines from monocytes due to reduced TRAF1 levels outweighed the reduction in cytokines produced from T cells leading to an overall pro-inflammatory state. The study went on to demonstrate that TRAF1 interferes with a critical step in the toll-like receptor (TLR)-induced NF-κB activation. TRAF1 directly binds and lowers the formation of the linear ubiquitin chain assembly complex (LUBAC), an important ubiquitin ligase that catalyzes the linear (M1-linked) poly-ubiquitination of NEMO (a.k.a. IKK-γ), the regulatory subunit of the IKK complex. Using TRAF1 knockout mice, the study showed that macrophages lacking TRAF1 displayed increased NF-κB activation and cytokine production after TLR stimulation, and that TRAF1 deficient mice were more susceptible to mortality from sepsis [
8].
Crucially, this study did not investigate whether the lower levels of TRAF1 in macrophages directly contribute to rheumatoid arthritis pathogenesis. In fact using using TRAF1 knickout mice to determine if and how it affects a complex autoimmune disease, like rheumatoid arthritis, will be complicated by the opposing role that TRAF1 plays in monocytes/macrophages versus T/B lymphocytes. Consistent with the multifaceted role of TRAF1, a study by Cheng et al. employed the KRN/I-A(g7) (KxB/N) model of RA and demonstrated that TRAF1 knockout mice developed equally progressive and severe arthritis to that observed in wildtype mice [
9]. Interestingly, TRAF1 knockout mice had markedly lower levels of anti-GPI antibodies, which is consistent with a positive role for TRAF1 in promoting lymphocyte activation [
9]. Therefore, a model that studies the role of TRAF1 in macrophages without interference from lymphocytes is needed to properly evaluate how TRAF1 contributes to disease pathogenesis.
In a more recent study, we employed a monosodium urate (MSU) crystal-induced model of gout in mice and reported that TRAF1 deficient mice exhibited a significant increase in joint swelling and inflammatory cell infiltration into the synovium compared to wildtype littermates [
10]. Mechanistically, TRAF1 lowered IL-1β secretion and inhibited NLRP3 inflammasome assembly in macrophages by limiting the linear ubiquitination of the adapter protein, ASC. In this report, we sought to determine for the first time whether TRAF1′s ability to limit inflammatory cytokine production in macrophages contributes to its role in RA pathogenesis. We show that intraarticular injection of bone marrow-derived macrophages prepared from TRAF1 knockout mice exacerbated joint inflammation, bone erosion and inflammatory cell recruitment in the collagen antibody induced arthritis (CAIA) model of RA. To our knowledge, this is the first report that implicates TRAF1 in modulating disease in a model of RA.
2. Materials and Methods
2.1. Cell Culture and Reagents
Primary bone marrow derived macrophages (BMDMs) were prepared from C57/Bl6 wildtype (WT) and TRAF1-/- mice and cultured, as described previously [
10,
11]. Briefly, femur and tibia of mice were flushed and cultured in RPMI 1640 (Sigma) supplemented with 10% fetal bovine serum (Wisent), 2-Mercapthoethanol (Gibco), L-Glutamine (Sigma), Pyruvate (Sigma), penicillin (Sigma), streptomycin (Sigma), non-essential amino acids (Gibco) and 25% L929-conditioned media.
2.2. Histological Examinations
Knee joints were dissected and fixed in 10% formalin neutral buffer solution (Thermo Fisher Scientific) overnight and decalcified with Rapid Decalcifier Solution (RDO; Apex Engineering Products) for 2 hours, washed and then transferred to 70 % ethanol and embedded in paraffin (Fisher Scientific). Paraffin blocks were then sectioned (5 μm) and deparaffinized in xylene (Fisher Scientific) then stained with hematoxylin (Sigma) and eosin (Thermo Fisher Scientific), as previously described [
10]. The slides were scanned (EVOS FL-Auto, Thermo Fisher Scientific), and quantification of inflammatory cells was performed in a blinded fashion.
2.3. Mice and CAIA Model
7-8 weeks old C57/Bl6 wild type male mice (18-20 g; Charles River) were injected interperitoneally (i.p.) with either 2.5 or 5 mg monoclonal antibody cocktail against type II collagen (Arthrogen-CIA
® Arthritogenic Monoclonal Antibody, Chondrex) on day 0. This was followed by intraarticular (i.a.) injection with PBS in the left knee or with 2.5 x 10
5 BMDMs from WT mice or TRAF1-/- mice in 10ul PBS in the right knee. Some mice received i.a. injection of WT BMDMs in the left knee and TRAF1-/- BMDMs in the right knee. N = 3 mice per group. i.a. injection was performed using a 30-gauge Hamilton syringe (Hamilton Company) into contralateral sides [
10]. Animals were daily and arthritis score, body weight and grimace scale were evaluated. Knee and ankle measurements was performed on day 0, 3 and 6 using 3-Mode Digital Fractional Caliper (Husky).
2.4. Statistical Analyses
All statistical analysis was done using GraphPad software (Prism) using 2-way analysis of variance (ANOVA) for comparison of multiple groups with multiple comparisons with Sidak correction. p-values are indicated in the figure legends.
3. Results
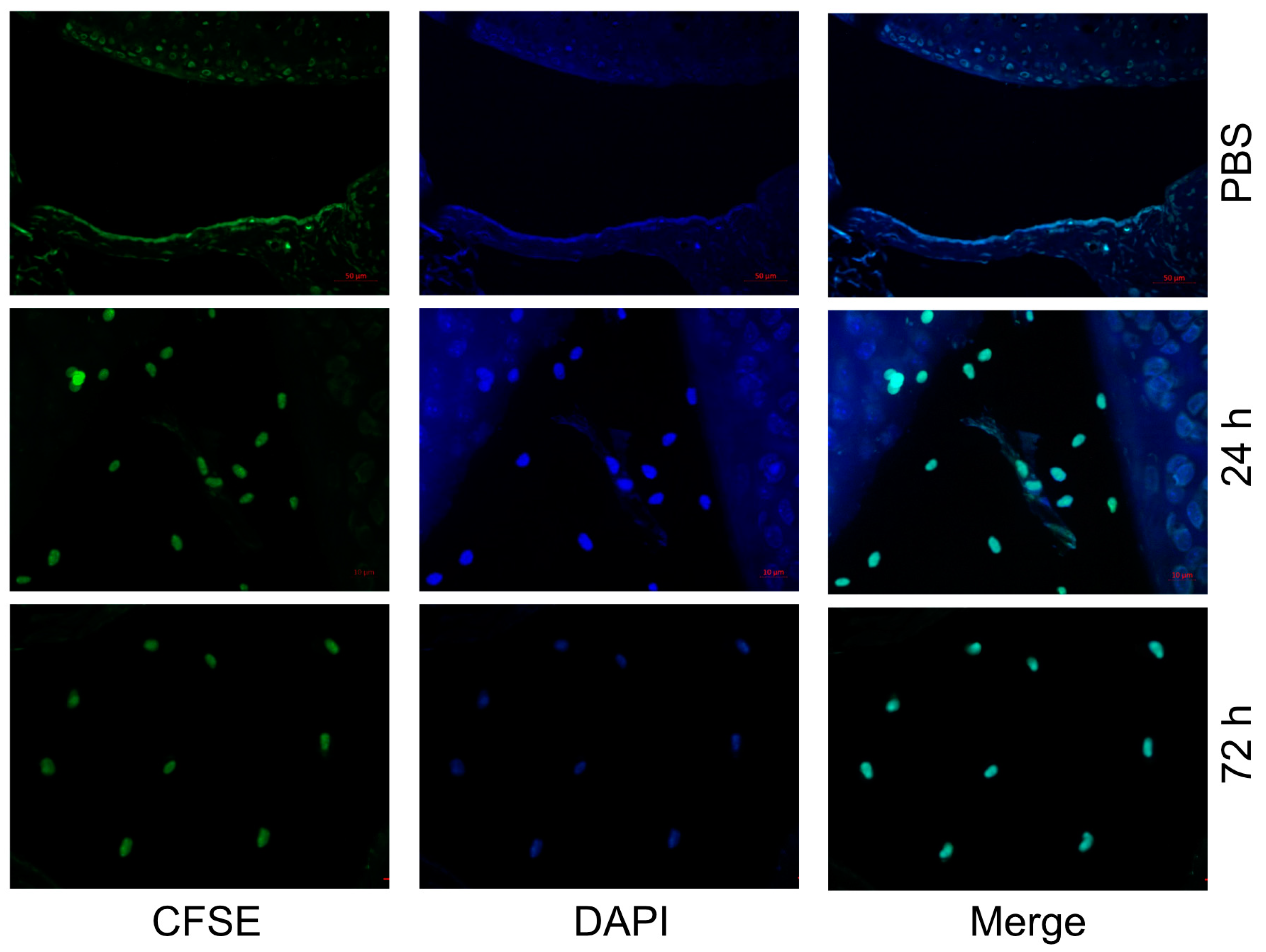
3.1. Macrophages Injected Intraarticularly into the Knee Joint Become Resident in the Synovial Tissue
Since TRAF1 plays opposing roles in innate signaling in inflammatory cells compared to TNFR signaling in adaptive lymphocytes, we wanted to design a model where we can test its effects in macrophages without the contribution from other immune cells. Therefore, we prepared macrophages from the bone marrow of wildtype (WT) mice and injected them intraarticularly into the knee joints of WT C57/Bl6 mice. We first examined whether the injected macrophages reside in the synovial space and for how long. Indeed, we observed that CFSE-labeled macrophages remained resident in the synovial tissues for at least 72 h post injection (
Figure 1).
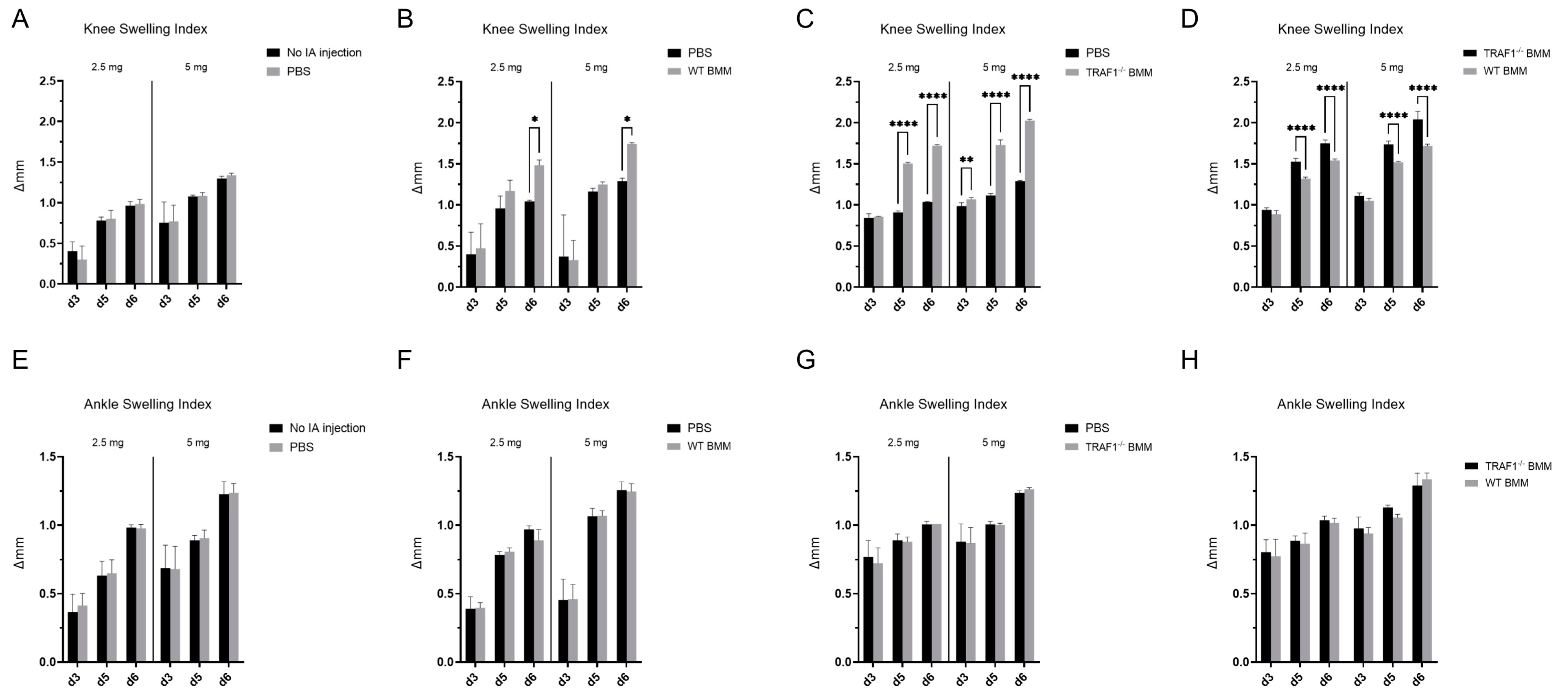
3.2. Intraarticular Injection of TRAF1 Deficient Macrophages Exacerbates Joint Inflammation in CAIA
Next, to determine whether TRAF1 plays a role in the contribution of macrophages to RA pathogenesis, we injected WT and TRAF1 knockout (TRAF1
-/-) macrophages into the knee joint intraarticular space of WT C57/Bl6 mice. Intraarticular injection of PBS served as the vehicle control. This was followed by intraperitoneal (i.p.) injection of a low dose (2.5 mg) or a high dose (5 mg) of Arthrogen-CIA
® arthritogenic monoclonal antibody cocktail to trigger the collagen antibody induced arthritis (CAIA) model of RA. Intraarticular injection of PBS in one knee joint did not induce additional knee joint swelling compared to knees with no intraarticular injections (
Figure 2A). Contrastingly, mice injected with WT macrophages exhibited more swelling in their knee joints than those injected with PBS in the contralateral side (
Figure 2B). Remarkably, intraarticular injection of TRAF1
-/- macrophages led to a significant increase in knee joint inflammation compared to the contralateral side injected with PBS (
Figure 2C) and WT macrophages (
Figure 2D). The increase in joint inflammation driven by macrophages did not extend to nearby ankle joints that received no injections (
Figure 2 E-F).
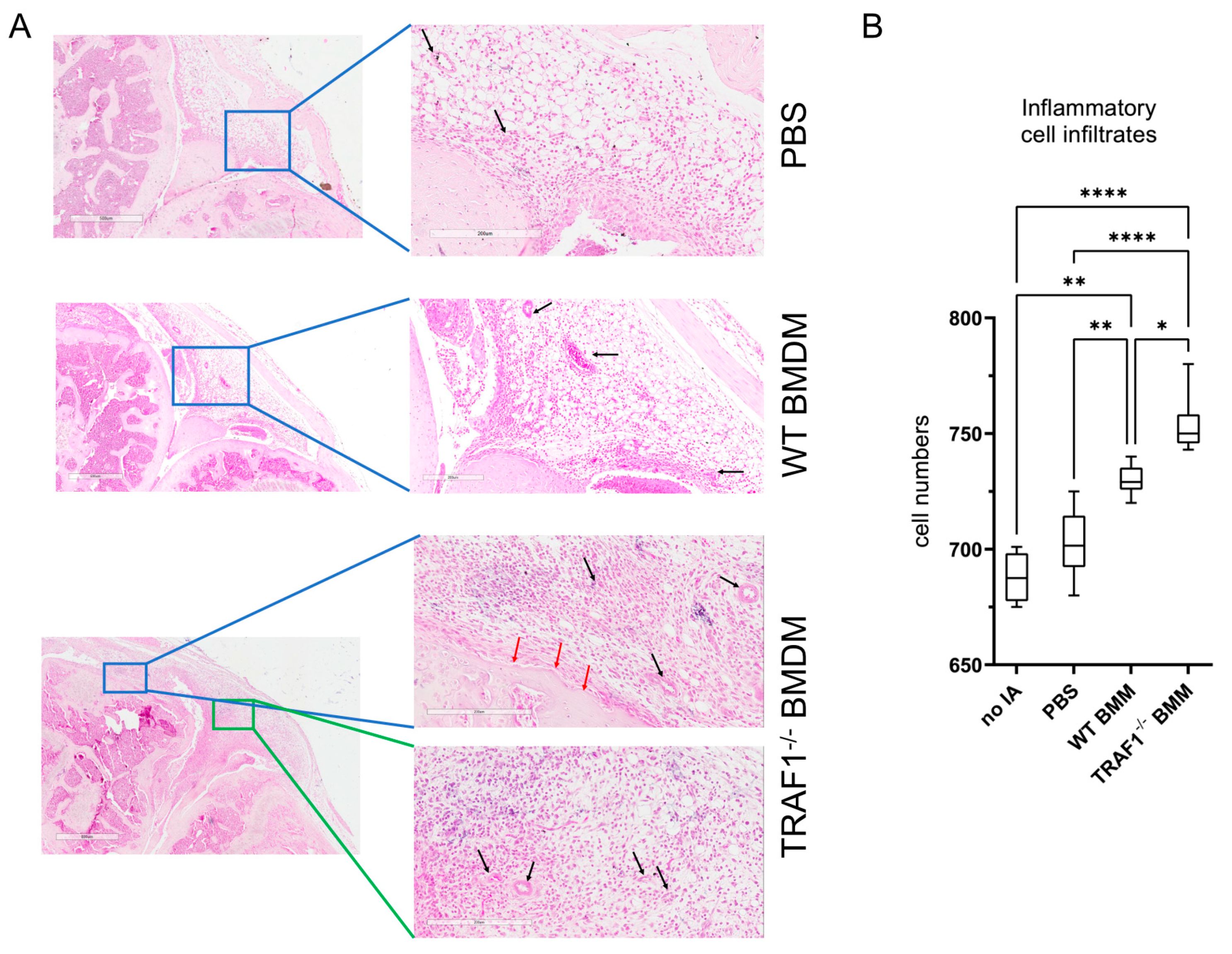
3.3. Increased Cellular Infiltration, Angiogenesis and Bone Erosion in Knee Joints of CAIA Mice Injected with TRAF1 Deficient Macrophages
We then examined whether the increase in knee joint swelling following intraarticular injection of macrophages was accompanied with an increase in cellular infiltration and tissue damage. Indeed, we observed that there was a significant increase in inflammatory cell infiltrates in the synovia of knee joints injected with TRAF1
-/- macrophages compared to those injected with WT macrophages and PBS (
Figure 3A). This was accompanied by an increase in angiogenesis and bone erosion indicating that the presence of TRAF1
-/- macrophages exacerbated tissue damage in the knee joints when compared to WT macrophages and PBS controls (
Figure 3B).
4. Discussion
Numerous studies have established a connection between single nucleotide polymorphisms (SNPs) in the TRAF1 gene and an increased risk of developing rheumatoid arthritis [
2,
3,
4,
12,
13]. TRAF1 plays multifaceted roles in various immune cells and downstream of distinct immune signaling pathways [
6,
7]. We recently showed that TRAF1 is critical in restraining inflammasome activation and the production of IL-1β after macrophage exposure to MSU crystals, and thereby limiting gout flares in a mouse model of crystal-induced arthritis [
10]. Yet, to our knowledge, no studies have demonstrated a direct involvement of TRAF1 in driving pathogenesis of RA. In this study, we showed that macrophages deficient in TRAF1 can significantly exacerbate joint inflammation and tissue damage when injected into the intraarticular space of knee joints of mice subjected to the collagen antibody induced arthritis (CAIA) model of RA.
A multitude of immune cells play an important role in the pathogenesis of complex autoimmune diseases, like RA. However, macrophages and T lymphocytes are recognized as key drives of the disease [
1,
14]. Importantly, macrophages and their products are implicated in synovial angiogenesis, a process crucial to the development of RA [
15]. Investigating the role of TRAF1 in RA is complicated by TRAF1′s opposing roles in immune signaling, where it promotes T cell activation and proliferation downstream of 4-1BB, while inhibiting inflammation in monocytes and macrophages downstream of TLR signaling [
7]. Indeed, in one study, TRAF1 knockout mice were equally susceptible to wild type mice to a spontaneous model of RA [
9]. The researchers noted that TRAF1 deficient mice had lower anti-GPI antibody titers and that this was due to the decrease in activation of B and T cells. This is consistent with the positive role that TRAF1 plays in lymphocyte activation. However, the study failed to explain the comparable disease between WT and TRAF1 knockout mice. We believe that it was likely due to increased inflammation from macrophages in knockout mice that compensated for the decrease in reduced antibody titers. Therefore, to isolate the effects of TRAF1 in macrophages in RA, we deliberately injected WT or TRAF1 knockout macrophages into the knee joint of WT mice followed by induction of the CAIA model of RA. Remarkably, TRAF1 knockout macrophages increased knee joint but not ankle joint inflammation compared to WT macrophages. Knees injected with TRAF1 knockout macrophages also exhibited exacerbated tissue damage as indicated by increased bone erosion and angiogenesis. Investigating the cell specific role of TRAF1 in RA is hindered by the lack of conditional TRAF1 knockout mice and future studies should focus on generating these mice to confirm these observations.
While great progress has been made in understanding RA pathogenesis, a cure remains elusive. Further research into the molecular mechanisms driving RA inflammation is vital. This study points to TRAF1 in macrophages as a potential therapeutic target for reducing inflammation and joint damage in RA.
Author Contributions
AAS and AM conceived, designed, interpreted experiments and wrote the manuscript. AM conducted all experiments and acquired the data.
Funding
“This work was supported by the Canadian Institutes of Health Research (201809PJT) and the Arthritis Society (18-0276).
Institutional Review Board Statement
All animals were housed under specific pathogen-free conditions, and animal-related procedures were approved by York University animal care committee in accordance with Canadian Council on Animal Care regulations (protocol 2019-12).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Edilova, M.I.; Akram, A.; Abdul-Sater, A.A. Innate Immunity Drives Pathogenesis of Rheumatoid Arthritis. Biomed. J. 2021, 44, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Plenge, R.M.; Seielstad, M.; Padyukov, L.; Lee, A.T.; Remmers, E.F.; Ding, B.; Liew, A.; Khalili, H.; Chandrasekaran, A.; Davies, L.R.; et al. TRAF1-C5 as a Risk Locus for Rheumatoid Arthritis--a Genomewide Study. N Engl J Med 2007, 357, 1199–1209. [Google Scholar] [CrossRef]
- Kurreeman, F.A.; Padyukov, L.; Marques, R.B.; Schrodi, S.J.; Seddighzadeh, M.; Stoeken-Rijsbergen, G.; van der Helm-van Mil, A.H.; Allaart, C.F.; Verduyn, W.; Houwing-Duistermaat, J.; et al. A Candidate Gene Approach Identifies the TRAF1/C5 Region as a Risk Factor for Rheumatoid Arthritis. PLoS Med 2007, 4, e278. [Google Scholar] [CrossRef]
- Panoulas, V.F.; Smith, J.P.; Nightingale, P.; Kitas, G.D. Association of the TRAF1/C5 Locus with Increased Mortality, Particularly from Malignancy or Sepsis, in Patients with Rheumatoid Arthritis. Arthritis Rheum 2009, 60, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Martínez-Bonet, M.; Kim, T.; Sparks, J.A.; Ishigaki, K.; Chen, X.; Sudman, M.; Aguiar, V.; Sim, S.; Hernandez, M.C.; et al. Identification of a Regulatory Pathway Governing TRAF1 via an Arthritis-Associated Non-Coding Variant. Cell Genomics 2023, 3, 100420. [Google Scholar] [CrossRef]
- Dhillon, B.; Aleithan, F.; Abdul-Sater, Z.; Abdul-Sater, A.A. The Evolving Role of TRAFs in Mediating Inflammatory Responses. Front. Immunol. 2019, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Edilova, M.I.; Abdul-Sater, A.A.; Watts, T.H. TRAF1 Signaling in Human Health and Disease. Front. Immunol. 2018, 9, 2969. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Sater, A.A.; Edilova, M.I.; Clouthier, D.L.; Mbanwi, A.; Kremmer, E.; Watts, T.H. The Signaling Adaptor TRAF1 Negatively Regulates Toll-like Receptor Signaling and This Underlies Its Role in Rheumatic Disease. Nat Immunol 2017, 18, 26–35. [Google Scholar] [CrossRef]
- Cheng, T.; Choi, Y.; Finkel, T.H.; Tsao, P.Y.; Ji, M.Q.; Eisenberg, R.A. Tumor Necrosis Factor Receptor-Associated Factor 1 Influences KRN/I-Ag7 Mouse Arthritis Autoantibody Production. J Clin Immunol 2013, 33, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Mirzaesmaeili, A.; Zangiabadi, S.; Raspanti, J.; Akram, A.; Inman, R.D.; Abdul-Sater, A.A. Cutting Edge: Negative Regulation of Inflammasome Activation by TRAF1 Can Limit Gout. J. Immunol. Baltim. Md 1950 2023, 210, 531–535. [Google Scholar] [CrossRef]
- Murugathasan, M.; Jafari, A.; Amandeep, A.; Hassan, S.A.; Chihata, M.; Abdul-Sater, A.A. Moderate Exercise Induces Trained Immunity in Macrophages. Am. J. Physiol. Cell Physiol. 2023, 325, C429–C442. [Google Scholar] [CrossRef] [PubMed]
- Albers, H.M.; Kurreeman, F. a. S.; Houwing-Duistermaat, J.J.; Brinkman, D.M.C.; Kamphuis, S.S.M.; Girschick, H.J.; Wouters, C.; Van Rossum, M. a. J.; Verduijn, W.; Toes, R.E.M.; et al. The TRAF1/C5 Region Is a Risk Factor for Polyarthritis in Juvenile Idiopathic Arthritis. Ann. Rheum. Dis. 2008, 67, 1578–1580. [Google Scholar] [CrossRef]
- Song, G.G.; Bae, S.C.; Kim, J.H.; Lee, Y.H. Associations between TRAF1-C5 Gene Polymorphisms and Rheumatoid Arthritis: A Meta-Analysis. Immunol Invest 2014, 43, 97–112. [Google Scholar] [CrossRef]
- Chen, Z.; Bozec, A.; Ramming, A.; Schett, G. Anti-Inflammatory and Immune-Regulatory Cytokines in Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2019, 15, 9–17. [Google Scholar] [CrossRef]
- Elshabrawy, H.A.; Chen, Z.; Volin, M.V.; Ravella, S.; Virupannavar, S.; Shahrara, S. The Pathogenic Role of Angiogenesis in Rheumatoid Arthritis. Angiogenesis 2015, 18, 433–448. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).