1. Introduction
Contamination of soils with cadmium (Cd) disrupts plant growth and endanger human health [
1,
2]. Ca
2+ signalling and Ca
2+-dependent protein kinase (CPK) have been shown to be crucial for the adaptation of plants to Cd environments [
3,
4]. AtCPK21 and AtCPK23 interact with NRAMP6 and limit Cd transport in Arabidopsis [
3]. Recently, the calcium sensor PeCPK21 from
Populus euphratica was found to interact with heavy metal transport proteins (PDF2.2, COPT5, OPT3 and annexin) and subunits of vacuolar ATPases (AVA-P2, VHA-B1, and VHA-C) to control Cd homeostasis [
4]. Our previous studies have shown that
P. euphratica attenuates Cd toxicity by limiting Cd absorption and increasing Cd compartmentalization [
5,
6]. It is noteworthy that
P. euphratica decreases the expression of
ANN1 to limit Cd accumulation, as ANN1 promotes Cd entry through Ca
2+-permeable channels (CaPCs) [
7,
8]. The addition of ABA leads to the activation of antioxidant enzymesthat effectively scavenge H
2O
2 in the Cd-exposed
P. euphratica cells and thus contributing to the limitation of Cd entry through CaPCs [
9]. The molecule H
2S promotes Cd efflux and facilitates vacuolar Cd sequestration in
P. euphratica cells [
5]. In addition,
P. euphratica upregulates transcription of xyloglucan endotransglucosylase/hydrolase (
XTH) and promotes xyloglucan degradation, which leading to a reduction in binding sites and thus reduces Cd accumulation in the roots [
6]. To mitigate the damage caused by Cd stress, plants can also use non-enzymatic and enzymatic antioxidant defense systems to scavenge the Cd-triggered reactive oxygen species (ROS) [
5,
9]. Catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX) and superoxide dismutase (SOD) are dominant enzymes in the plant defense strategies [
10,
11,
12,
13]. PeCPK21 has been shown to interact with CDSP32, GPX3, APX1, APX2, TAPX, TRXM4 and PRXQ to maintain ROS homeostasis under Cd stress [
4]. PeCPK21 regulates water status by interacting with intrinsic proteins in the plasma membrane (PIP2A, PIP1–1 and PIP2–7) [
4], as water transport is severely restricted in Cd-stressed roots [
14]. Although PeCPK21 attenuates Cd stress by interacting with various heavy metal stress-associated proteins (HMAPs) in transgenic Arabidopsis [
4], it is unknown whether PeCPK21 interacts with transcription factors to control Cd and ROS homeostasis in stressed plants.
The transcription factor (TF), nuclear factor Y (NF-Y) or heme-activated protein (HAP), consists of three different subunits, NF-YA, NF-YB and NF-YC [
15]. NF-Y regulates crucial aspects of growth, development and environmental stress responses [
16,
17,
18,
19]. For example,
AsNF-YC8 from garlic positively regulates plant tolerance to hyperosmotic stress in tobacco [
20]. AtNF-YA5 is critical for the induction of drought-responsive genes in Arabidopsis [
21]. AtNF-YB1 and ZmNF-YB2 improved drought resistance by regulating stomatal conductance [
22]. NF-YC from
Amaranthus hypochondriacus increases ABA sensitivity and confers resistance to water deficit in Arabidopsis [
23]. Soybean GmNF-YC14 activates the GmPYR1-mediated ABA signalling pathway to regulate drought tolerance [
24].
Physcomitrella patens PpNF-YC1 activates the
PpLEA1 promoter to enhance drought/desiccation tolerance [
25]. The
NF-Y genes,
PgNF-YB09,
PgNF-YC02 and
PgNF-YC07-04, were induced by salinity in
Panax ginseng [
26]. The
OsNF-YC13 gene increases salt tolerance in rice plants [
27].
MsNF-YC2 overexpression confers alkali tolerance in transgenic alfalfa cultivars [
28]. Bermudagrass Cdt-NF-YC1 improves the ability of transgenic rice to tolerate drought and salt stress [
29]. AtHAP5A has been shown to modulate freezing tolerance in Arabidopsis [
30]. However, the function of NF-Y transcription factors under Cd stress is still unclear and remains to be investigated.
In this study, we confirmed the interaction of PeCPK21 with AtNF-YC3 by HaloTag pull-down, Y2H and BiFC experiments. We found that Cd induced AtNF-YC3 expression in PeCPK21-transformed Arabidopsis. AtNF-YC3 was transferred into Arabidopsis to further determine whether the PeCPK21-interacting TF could enhance Cd tolerance. Overexpression of AtNF-YC3 in Arabidopsis resulted in decreased Cd uptake and activated antioxidant enzymes, which reduced H2O2 accumulation and improved Cd stress tolerance. Thus, in Arabidopsis overexpressed with PeCPK21, PeCPK21 interacts with AtNF-YC3 to decrease Cd accumulation and strengthen the antioxidant system to reduce the Cd-triggered ROS. This discovery of the interaction between PeCPK21 and AtNF-YC3 can be utilized to improve Cd resistance in higher plants.
2. Results
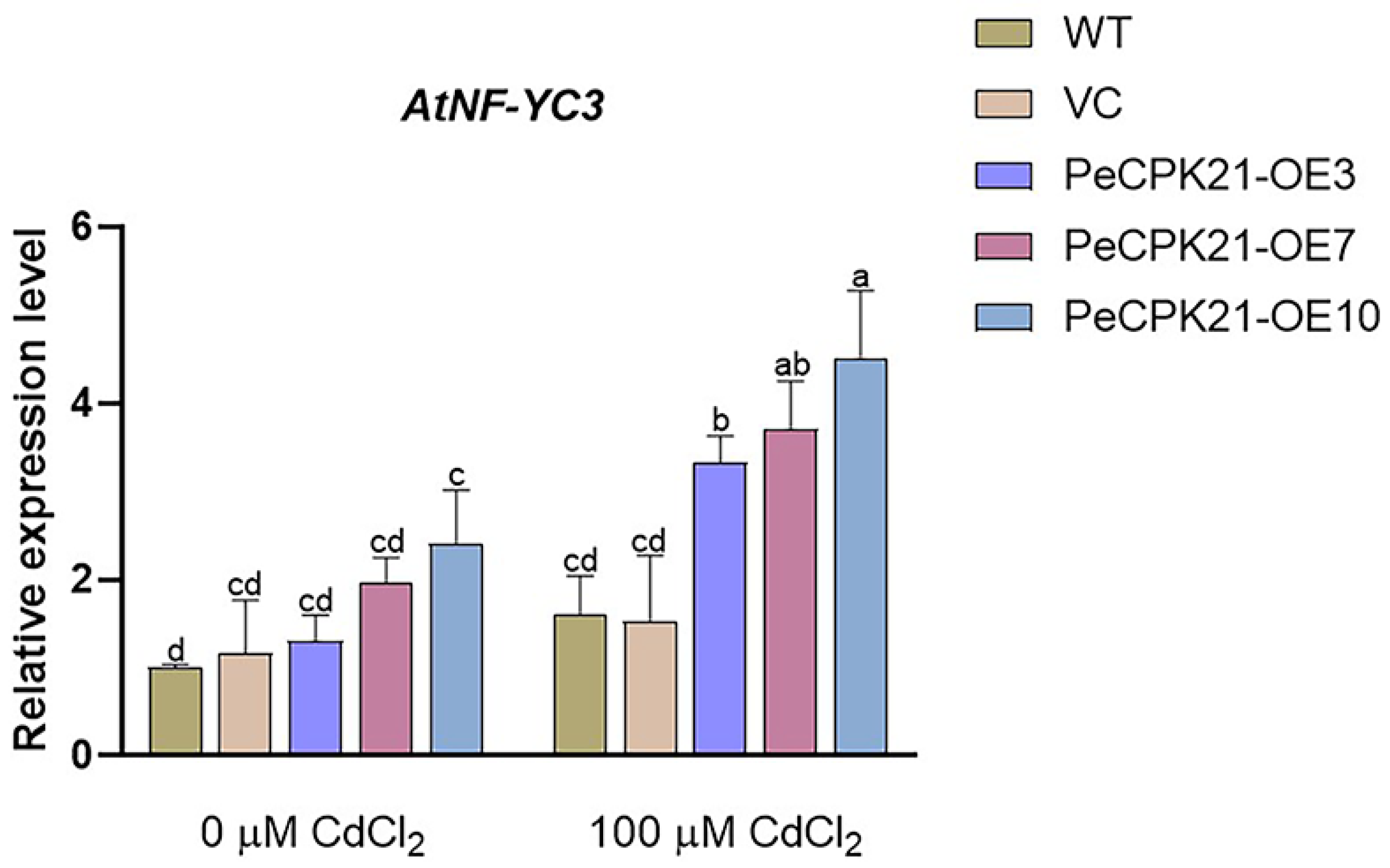
2.1. Cd-Induced AtNF-YC3 Expression in PeCPK21-Transgenic Arabidopsis
We have previously shown that
P. euphratica PeCPK21 enhances Cd tolerance in Arabidopsis, and the PeCPK21-interacting proteins were identified in
PeCPK21-transgenic plants [
4]. Expression profiles of PeCPK21-interacting proteins indicate that various HMAPs were upregulated by Cd stress in transgenic Arabidopsis. In this work, we observed that the expression of the transcription factor AtNF-YC3 was upregulated by Cd exposure in
PeCPK21-overexpressed lines. The
AtNF-YC3 transcript increased significantly by 60-155% upon Cd exposure in the
PeCPK21 transgenic lines, OE3, OE7 and OE10, which was 2.50-fold higher than in WT and VC. The result suggests that PeCPK21 may interact with AtNF-YC3 to increase Cd tolerance, as overexpression of
Picea wilsonii NF-YB3 in Arabidopsis increases
CDPK1 expression and confers salt and drought tolerance [
31]. Therefore, the interaction between PeCPK21 and AtNF-YC3 and the role of AtNF-YC3 in Cd tolerance were investigated in the present study.
Figure 1.
Cd-induced transcription of AtNF-YC3 in PeCPK21-transgenic Arabidopsis. Seedlings of wild-type (WT), vector control (VC) and PeCPK21-overexpressed lines, OE3, OE7, and OE10 (T3 generation) were grown on 1/2 MS medium supplemented with 0 or 100 μM CdCl2. RT-qPCR analysis of AtNF-YC3 was performed after 7 days of Cd treatment. Data are mean values of three biological samples, and bars with different letters (a–d) indicate significant differences (p < 0.05).
Figure 1.
Cd-induced transcription of AtNF-YC3 in PeCPK21-transgenic Arabidopsis. Seedlings of wild-type (WT), vector control (VC) and PeCPK21-overexpressed lines, OE3, OE7, and OE10 (T3 generation) were grown on 1/2 MS medium supplemented with 0 or 100 μM CdCl2. RT-qPCR analysis of AtNF-YC3 was performed after 7 days of Cd treatment. Data are mean values of three biological samples, and bars with different letters (a–d) indicate significant differences (p < 0.05).
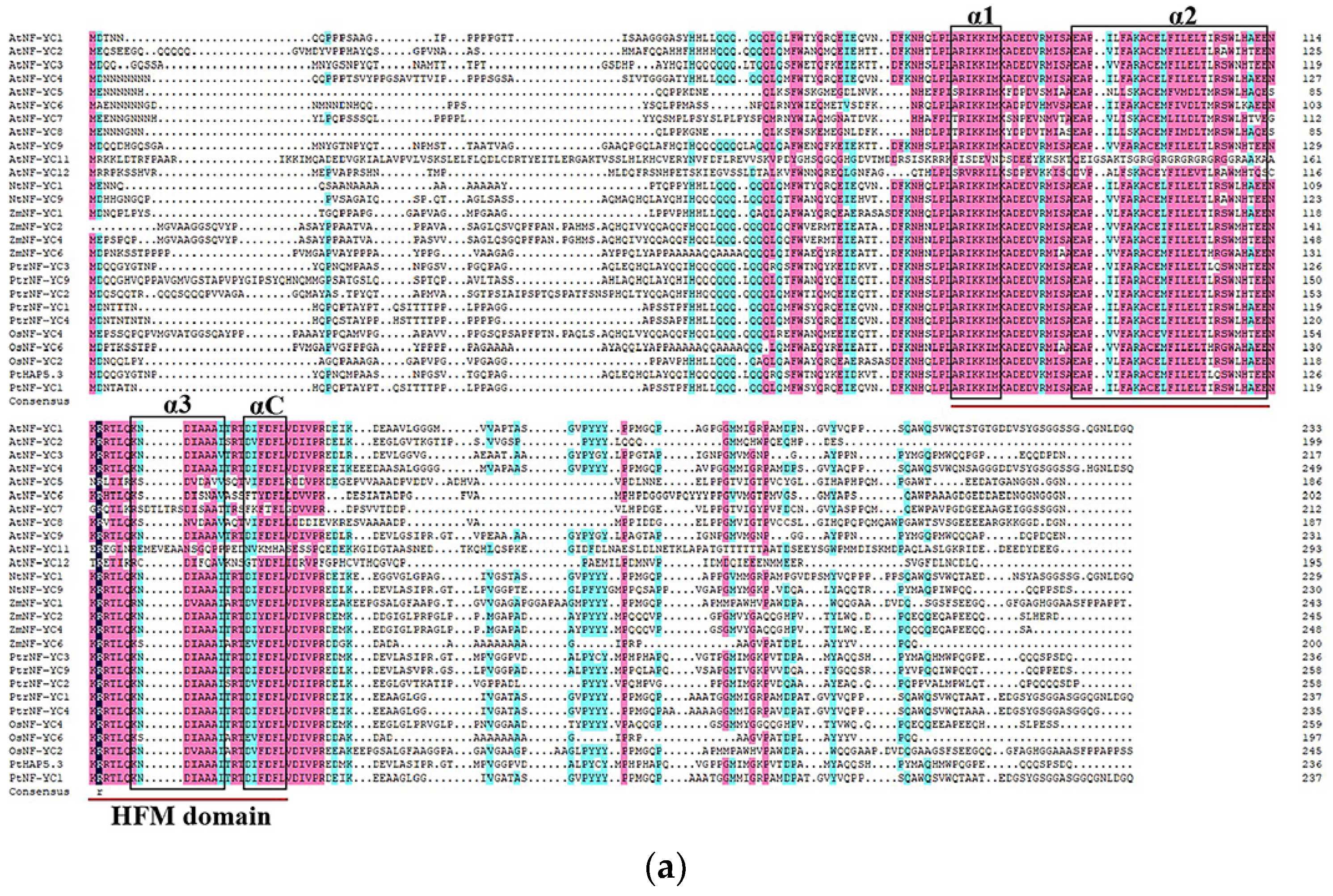
2.2. AtNF-YC3 Sequence Analysis
The coding sequence (CDS) of
AtNF-YC3 (654 bp) was isolated from
Arabidopsis thaliana. AtNF-YC3 encodes 217 amino acids (24.32 kDa) with an isoelectric point of 4.75 (
Figure 2a). The phylogenetic tree shows that AtNF-YC3 in
A. thaliana has a close evolutionary relation to AtNF-YC9 (
Figure 2b). The NF-YC conserved domain contains an HFM domain that plays an important role in protein-DNA and protein-protein interactions (
Figure 2a). The domain consists of three α-helices (α1, α2 and α3) separated by two β-chain ring domains. Outside the HFM folding region is a fourth α-helix with a length of 7 amino acids, called αC (
Figure 2a).
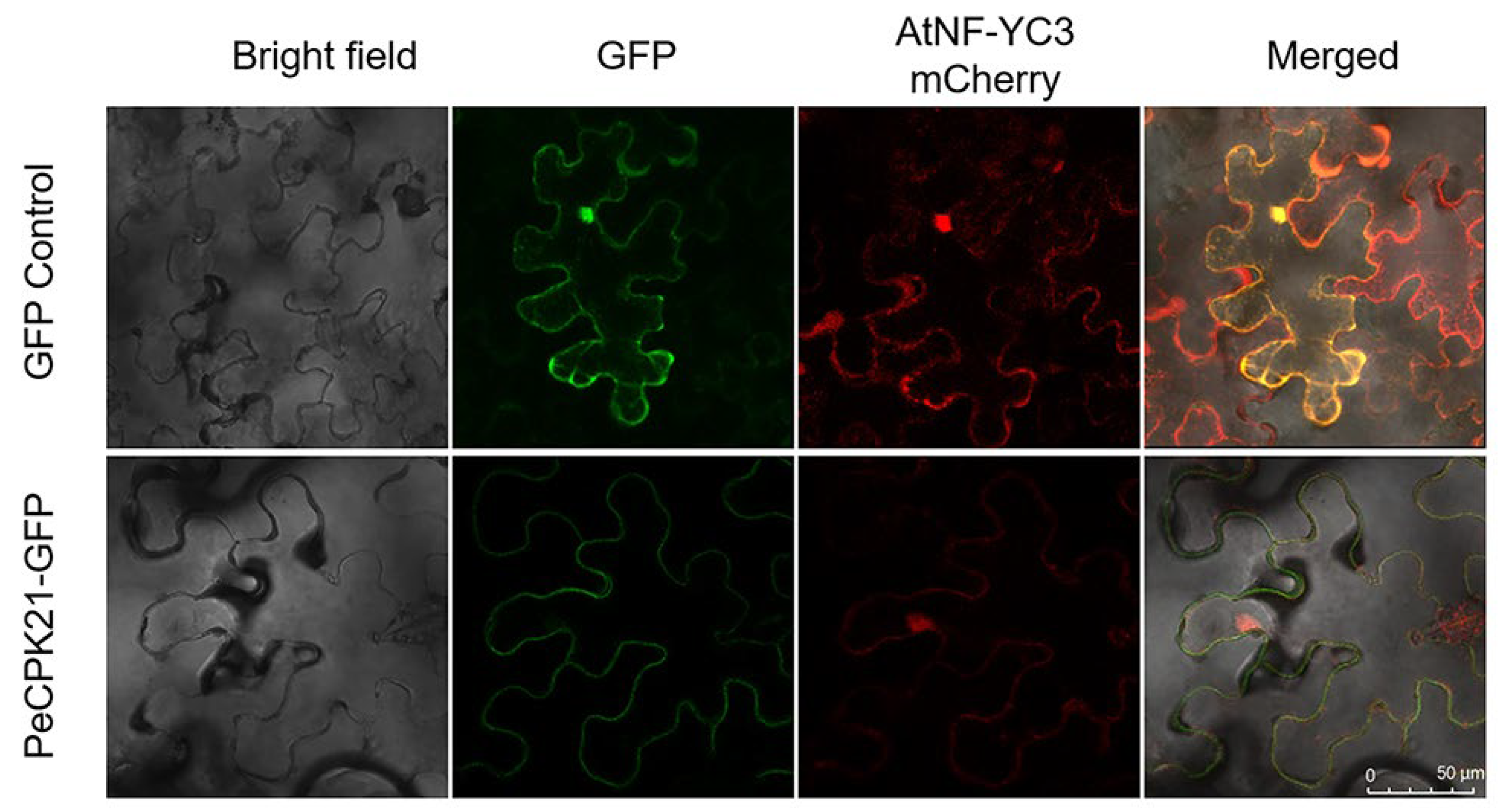
2.3. Subcellular Localization of PeCPK21and AtNF-YC3
We determined the subcellular co-localization of PeCPK21 and AtNF-YC3 in leaves of
Nicotiana benthamiana. GFP-tagged PeCPK21 (PeCPK21-GFP), which was localized in the cytoplasm, was expressed together with mCherry-tagged AtNF-YC3 (AtNF-YC3-mCherry) in tobacco leaves, which was localized in the nucleus and cytoplasm (
Figure 3). The colocalization assay showed that PeCPK21 and AtNF-YC3 exhibited overlapping fluorescence in the cytoplasm.
2.4. PeCPK21 Interacts with AtNF-YC3 In Vitro and In Vivo
In this study, yeast two-hybrid (Y2H), HaloTag pull-down and bimolecular fluorescence complementation (BiFC) assays were performed to verify the interaction between PeCPK21 and AtNF-YC3.
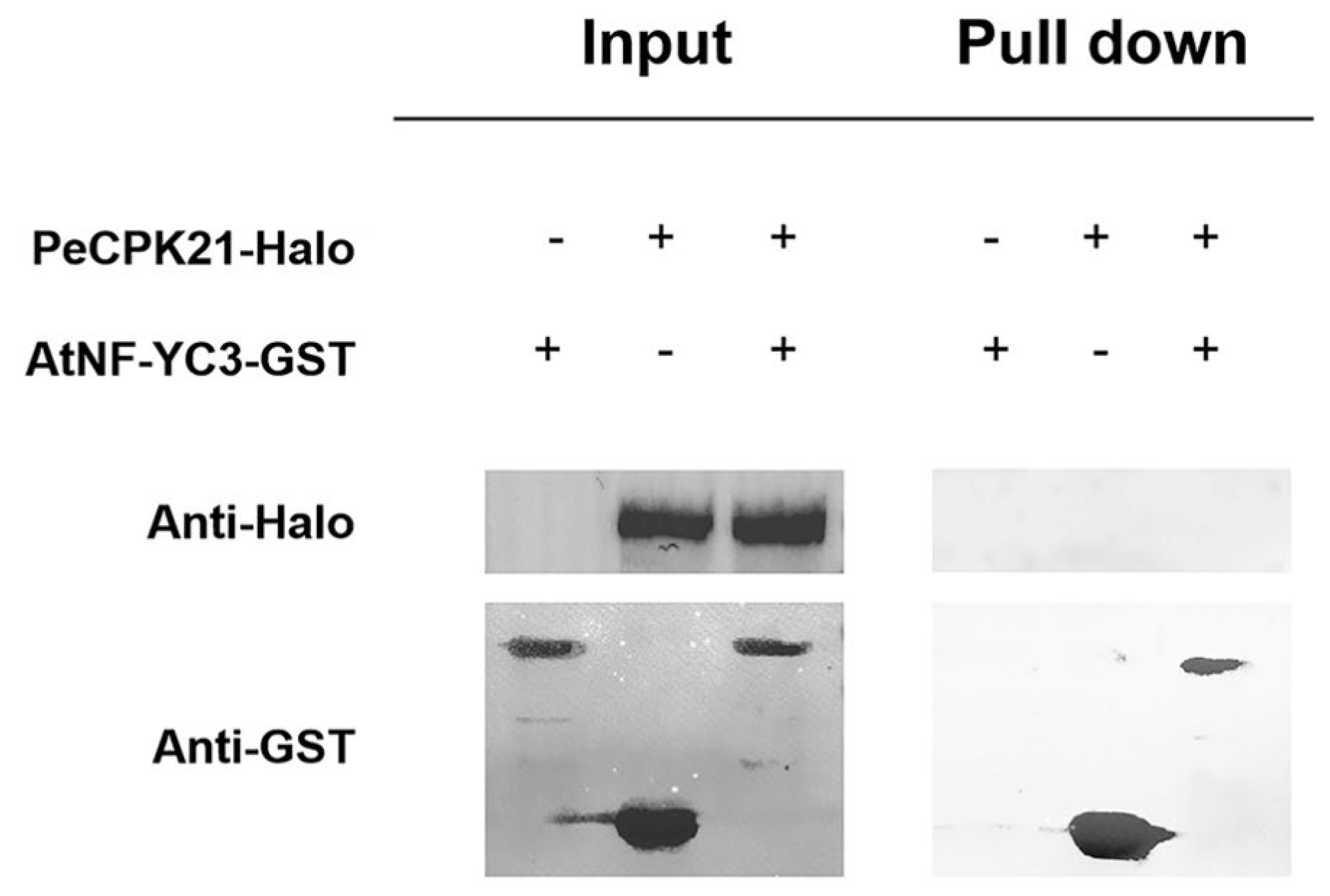
In the HaloTag pull-down assay, PeCPK21 served as the bait protein and AtNF-YC3 as the prey protein. The combinations of PeCPK21-Halo were expressed using a cell-free protein expression system (the Wheat Germ Protein Expression System) and AtNF-YC3-GST using the myTXTL Sigma 70 Master Mix Kit. The AtNF-YC3-GST fusion protein was shown to be pulled down by the PeCPK21-Halo fusion protein (
Figure 4). The Halo Tag pull-down assay shows a direct physical interaction between PeCPK21 and AtNF-YC3.
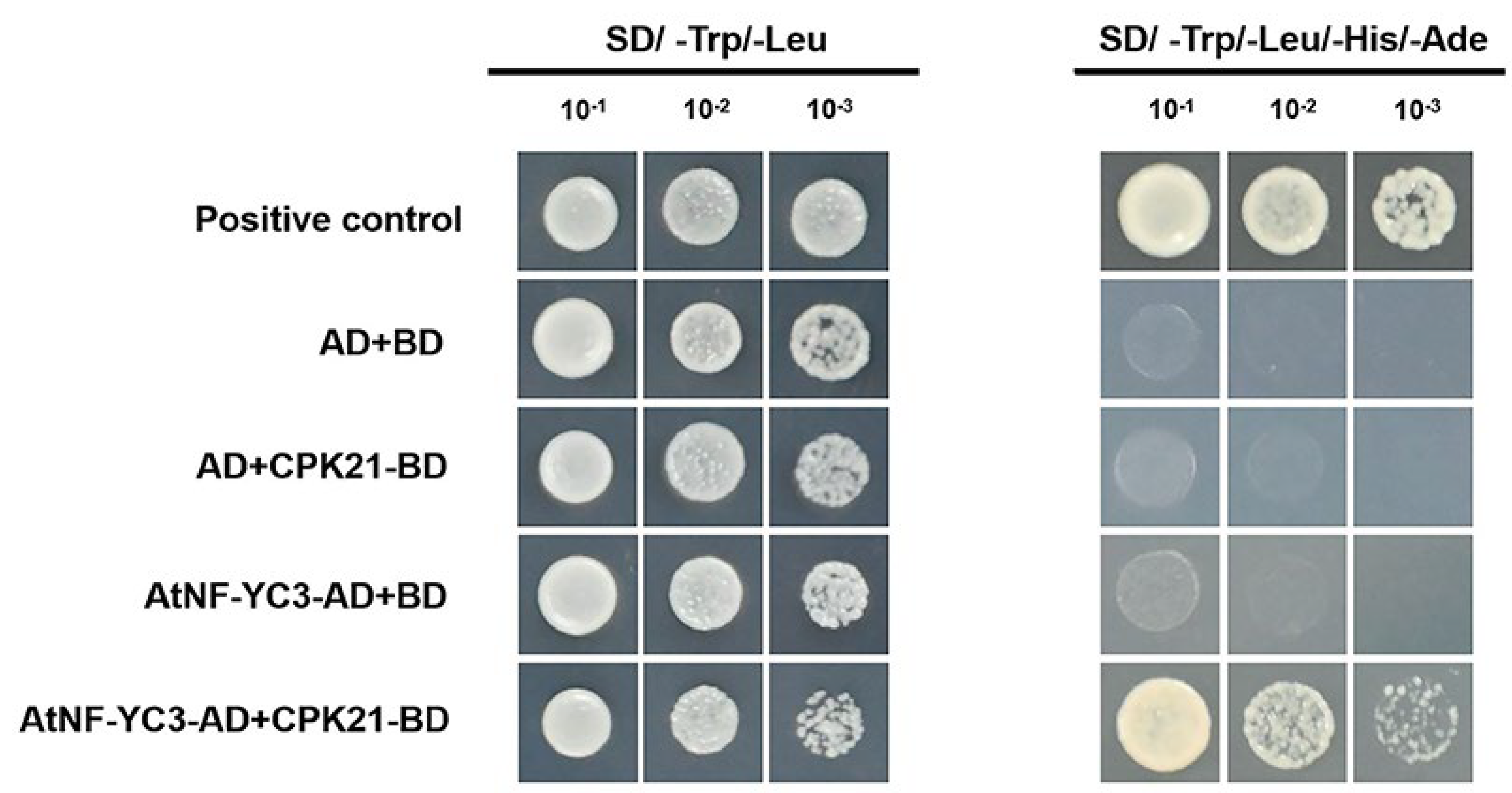
The Y2H was used to investigate whether PeCPK21 interacts with AtNF-YC3 in vitro. Prior to transformation into yeast cells, AtNF-YC3 was linked to the GAL4 activation domain (AtNF-YC3-AD), and PeCPK21 was fused to the GAL4 DNA binding domain (PeCPK21-BD). The transformation combinations showed that only the AH109 yeast cells carrying AtNF-YC3-AD and PeCPK21-BD could grow on the selection medium (SD/ -Trp/-Leu/-His/-Ade). Therefore, Y2H assays showed that PeCPK21 could interact with AtNF-YC3 in yeast cells (
Figure 5).
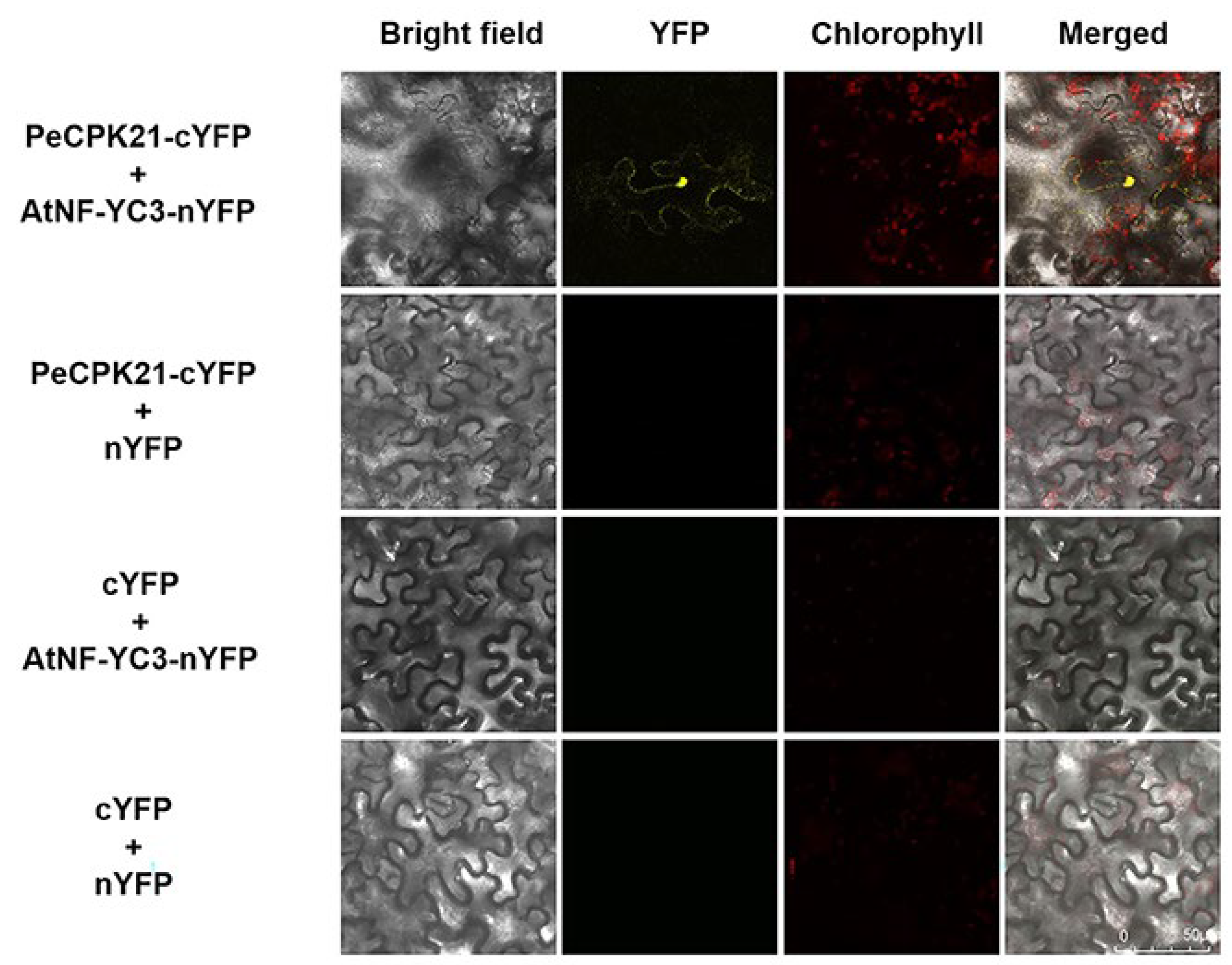
The interaction between PeCPK21 and AtNF-YC3 was further confirmed by BiFC assays in the leaves of
N. benthamiana. BiFC assays showed that co-expression of PeCPK21-cYFP and AtNF-YC3-nYFP in tobacco leaves resulted in YFP signalling in the nucleus and cytoplasm, which was not observed with other transforming combinations, such as PeCPK21-cYFP + nYFP, AtNF-YC3-nYFP + CFP, cYFP + nYFP (
Figure 6). BiFC assays show that PeCPK21 was able to interact specifically with AtNF-YC3, and the protein-protein interaction probably occurred mainly in the nucleus and cytoplasm.
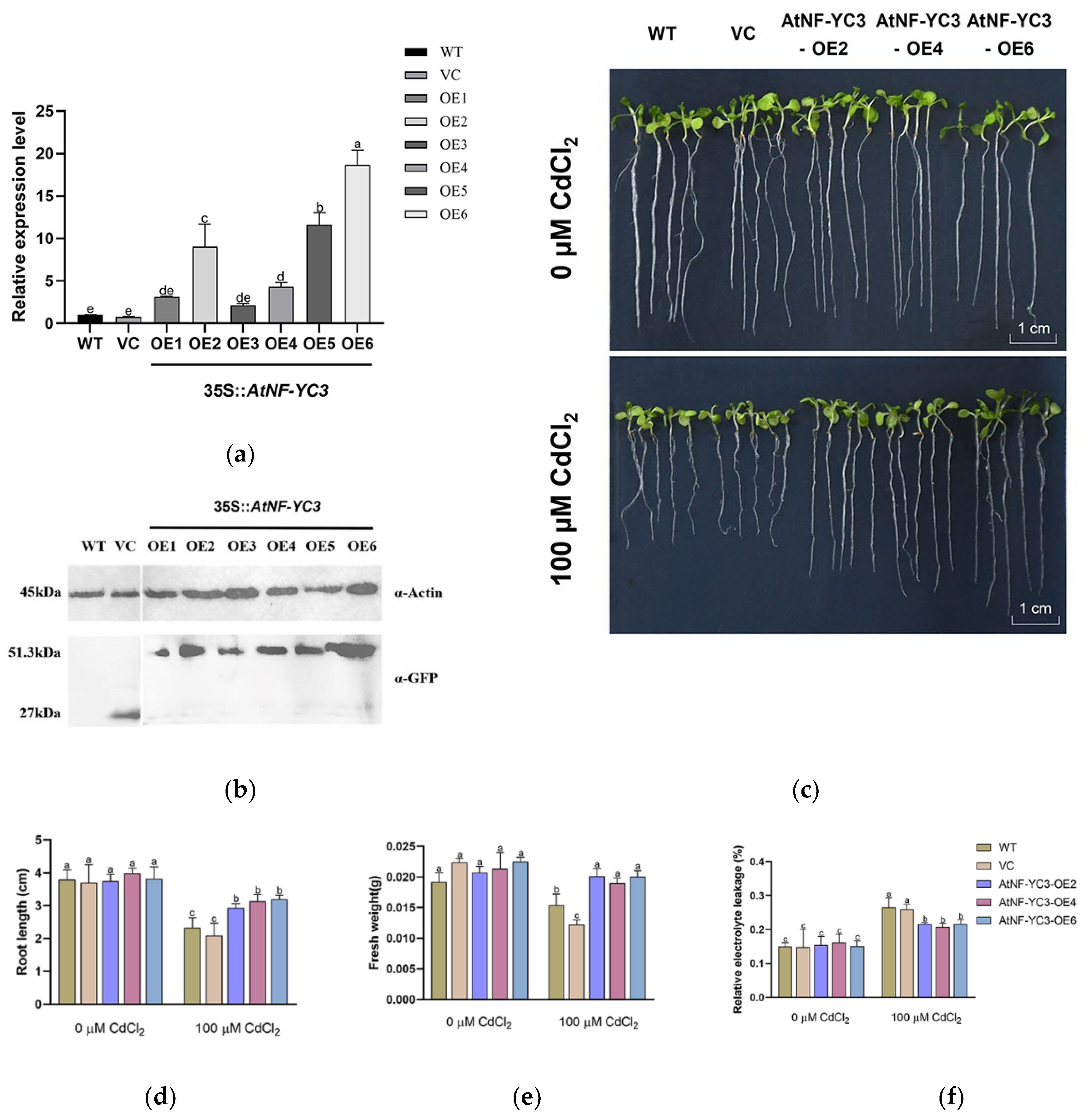
2.5. Cd Tolerance in AtNF-YC3-Transgenic Arabidopsis
To further determine whether the PeCPK21-interacting TF, AtNF-YC3, could improve Cd tolerance, the A
tNF-YC3 gene was transformed into Arabidopsis. In this study six transgenic Arabidopsis lines, OE1–OE6, were generated. Among the six transgenic lines, RT-qPCR showed that
AtNF-YC3 transcription was highest in OE6 and lowest in OE3 (
Figure 7a). Western blotting confirmed that the AtNF-YC3-GFP protein was expressed in all transgenic lines, with protein abundance being highest in OE6 and lowest in OE1 (
Figure 7b). Here, the transgenic lines OE2, OE4 and OE6 (T3 generation) were used for cadmium tests. WT, VC and three
AtNF-YC3-OE lines were treated with CdCl
2 (0 or 100 μM) for 7 days.
The growth of Arabidopsis seedlings was reduced by 100 μM CdCl
2 (7 days,
Figure 7c). It is noticeable that the root length of the three
AtNF-YC3-OE lines under Cd stress was 26-53% higher than that of WT and VC (
Figure 7d). Similarly, the transgenic lines showed 23–64% greater fresh weight (per 15 plants) than WT and VC (
Figure 7e). Under control conditions no difference in root and plant growth was observed between the tested genotypes (
Figure 7c-e). CdCl
2 treatment caused a significant increase in relative electrolyte leakage (EL) in all tested lines (
Figure 7f). However, the EL in the
AtNF-YC3-OE lines was 17–22% lower than in the WT and VC lines, indicating that membrane integrity was less affected by CdCl
2 in the transgenic lines (
Figure 7f). Collectively, AtNF-YC3 positively regulates Cd tolerance in Arabidopsis in terms of improved root length, fresh weight and membrane stability.
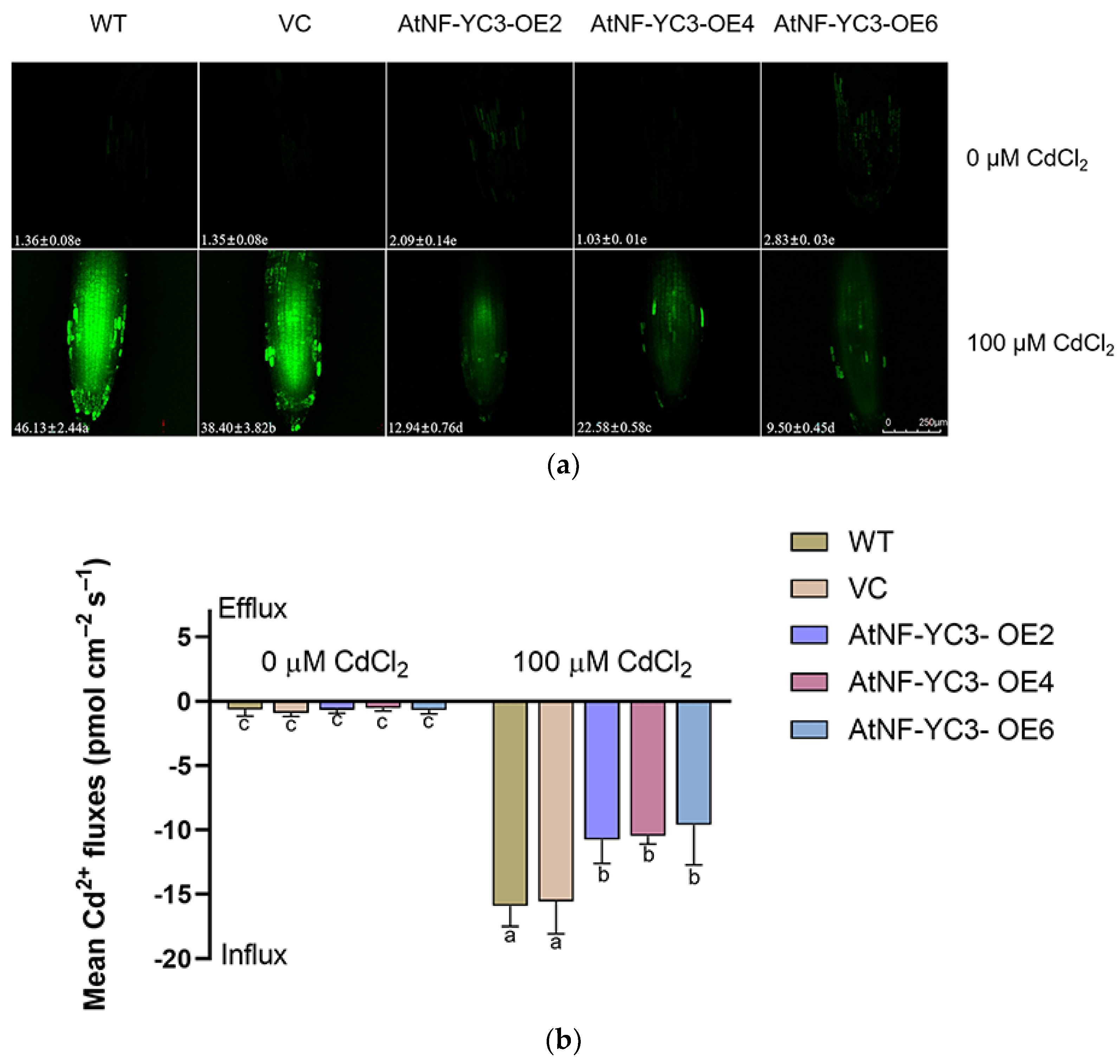
2.6. Root Cd Flux and Concentration
The Cd content in the root cells was detected with a fluorescent probe, Leadmium™ Green AM [
5]. CdCl
2 led to a marked increase in fluorescence intensity in the root cells, but the fluorescence in the
AtNF-YC3-OE lines was only 16–45 % of that in WT and VC (
Figure 8a). In contrast, the fluorescence in control plants was extremely low or undetectable (
Figure 8a). The steady-state Cd flux in the root tips was monitored using non-invasive micro-test technology (NMT). The Cd flux was almost undetectable in the roots of the control plants, while a remarkable influx was recorded in the roots exposed to 100 μM CdCl
2 (
Figure 8b). In particular, the transgenic
AtNF-YC3-OE lines OE2, OE4 and OE6 showed a significantly lower Cd influx, and the flux rate was 58–67% of the values in the WT and VC plants (
Figure 8b).
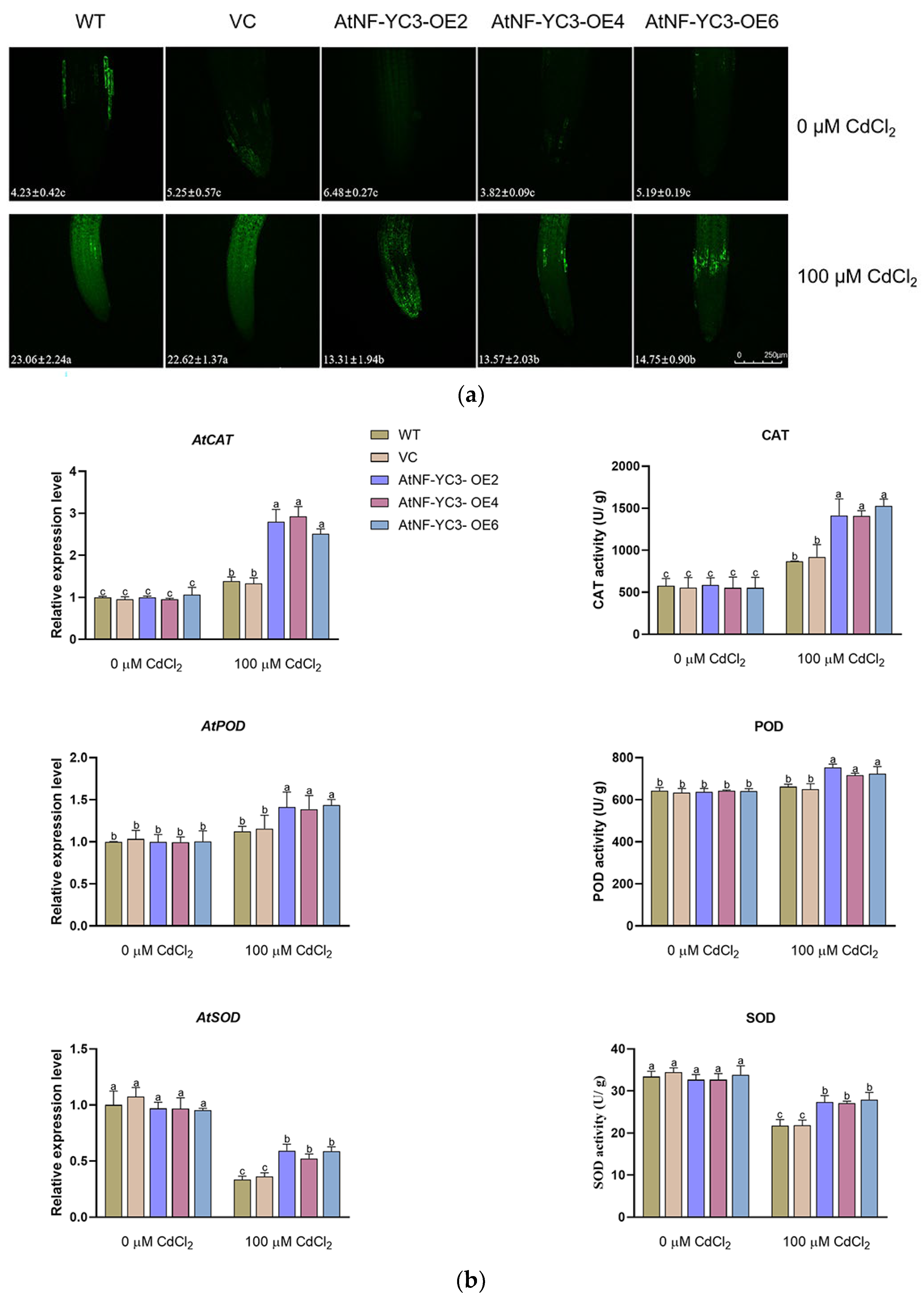
2.6. H2O2 Concentration, Activities and Transcription of Antioxidant Enzyme
Cadmium in general stimulates ROS accumulation in stressed plants [
4,
5,
8]. The H
2O
2 content in root cells was detected with a fluorescent probe, H2DCFDA. CdCl
2 exposure (100 μM) resulted in a significant rise in DCF fluorescence in root cells (
Figure 9a). However, the
AtNF-YC3-OE lines showed a significantly lower H
2O
2 level compared to WT and VC. Control plants exhibit very low DCF fluorescence in all tested lines (
Figure 9a).
The transcription levels of
AtCAT,
AtPOD and
AtSOD as well as CAT, POD and SOD activities were analyzed to determine the ability of Cd-treated plants to scavenge ROS. CdCl
2 exposure (100 μM) increased the transcription of
AtCAT and
AtPOD but inhibited the expression of
AtSOD in all lines tested. Remarkably, transcription of antioxidant enzymes was significantly higher in the
AtNF-YC3-OE lines OE2, OE4 and OE6 regardless of Cd up-regulation of
AtCAT and
AtPOD and Cd down-regulation of
AtSOD. Compared to WT and VC, the
AtNF-YC3 transgenic lines maintained higher activities of CAT, POD and SOD under Cd stress, which is consistent with the transcription of the coding genes (
Figure 9b).
3. Discussion
3.1. PeCPK21 Interacts with AtNF-YC3 to Increase Cd Tolerance in Arabidopsis
We have previously shown that PeCPK21 increases Cd tolerance by interacting with various heavy metal stress-associated proteins (HMAPs) in transgenic Arabidopsis [
4]. Here, we found that PeCPK21 also interacts with the transcription factor AtNF-YC3 by performing in vitro and in vivo assays, including Y2H, HaloTag pull-down, and BiFC. Transcription of
AtNF-YC3 was strongly increased in transgenic seedlings when treated with CdCl
2 (
Figure 1), suggesting that AtNF-YC3 is responsible for Cd stress. Similarly, in rice, the
NFY-A6 gene is up-regulated by Cd treatment [
32].
AtNF-YC3 was overexpressed in Arabidopsis to determine whether the transcription factor interacting with PeCPK21 confers Cd tolerance. As shown in
Figure 7, overexpression of
AtNF-YC3 enhanced Cd tolerance in terms of improved root length, fresh weight and membrane stability in Arabidopsis. Here we confirmed for the first time that AtNF-YC3 improves Cd tolerance, although NF-YC transcription factors are required for plant response to ABA (e.g., NF-YC1 in
Physcomitrella patens [
25]; Cdt-NF-YC1 in bermudagrass [
29]) and abiotic stress, such as salt (e.g.,
NF-YC13 in indica rice [
27];
PgNF-YB02,
PgNF-YC09, and
PgNF-YC07-04 in
Panax ginseng [
26]), drought (e.g.,
NF-YC in
Amaranthus hypochondriacus [
23]), and alkali stress (e.g.,
NF-YC2 in
Medicago sativa [
28]). Our data show that PeCPK21 interacts with the transcription factor AtNF-YC3 to limit Cd uptake and enhance ROS degration in transgenic plants.
3.2. PeCPK21 Interacts with AtNF-YC3 to Restrict Cd Uptake in Arabidopsis Roots
The confocal results showed that the
AtNF-YC3 transgenic lines effectively limited the buildup of Cd in Cd-exposed roots compared to WT and VC (
Figure 8). This was resulted from the lower Cd influx into the root tips (
Figure 8). We have shown that PeCPK21 interacts with heavy metal transport proteins and channels, PDF2.2, OPT3, COPT5 and annexin, to effectively limit Cd accumulation in the roots of
PeCPK21 transgenic lines [
4]. Accordingly, we hypothesize that PeCPK21 also interacts with the transcription factor AtNF-YC3 to limit Cd uptake and thereby increase Cd tolerance. Therefore, PeCPK21 interacts with both the transcription factor AtNF-YC3 and heavy metal transport proteins to limit Cd uptake and accumulation under cadmium stress.
3.3. PeCPK21 Interacts with AtNF-YC3 to Improve Activities of Antioxidant Enzymes
Cd treatment resulted in a lower H
2O
2 levels in
AtNF-YC3-overexpressed plants compared to WT and VC (
Figure 9). The transcription of
AtPOD,
AtCAT and
AtSOD was higher in the transgenic lines than in the WT and VC lines (
Figure 9). In agreement with gene expression, the enzymatic activities were higher in the
AtNF-YC3-overexpressed plants than in WT and VC, regardless of Cd-stimulated CAT and POD and Cd-restricted SOD (
Figure 9). The results suggest that AtNF-YC3 increases Cd tolerance by enhancing the activities of antioxidant enzymes in Arabidopsis. Similarly, the NF-YC transcription factor MsNF-YC2 positively regulates the activities of SOD and POD, such that the increased antioxidant enzymes reduce oxidative damage of H
2O
2 to the cell membrane in transgenic alfalfa [
28]. Overexpression of garlic
AsNF-YC8 enabled tobacco plants to control ROS levels by activating antioxidant enzymes [
20]. We have shown that PeCPK21 interacts with a variety of antioxidant enzymes, especially CDSP32, APX1, APX2, GPX3, PRXQ, TAPX and TRXM4, to maintain ROS homeostasis in
PeCPK21 transgenic lines under Cd stress [
4]. Here, we hypothesize that PeCPK21 also interacts with AtNF-YC3 to scavenge the Cd-triggered ROS and increase cadmium tolerance. Therefore, PeCPK21 interacts directly with antioxidant enzymes or interacts with the transcription factor AtNF-YC3 to activate antioxidant enzymes under Cd stress.
4. Materials and Methods
4.1. Culture of Plant Materials
Arabidopsis thaliana wild type, control vector,
PeCPK21-tansgenic lines, OE3, OE7 and OE10, were surface sterilized, germinated and grown in 1/2 MS medium (0.8 % agar and 1 % sucrose, w/v) containing 0 or 100 μM CdCl
2. The seedlings were used for quantitative real-time PCR analyses of
AtNF-YC3 expression [
4]. The primers used for RT-qPCR are shown in
Supplementary Table S1.
4.2. AtNF-YC3 Cloning and Bioinformatic Analysis
Total RNA was isolated from Arabidopsis using Trizol reagent (Invitrogen, Carlsbad, CA, USA). The reverse transcriptase kit HiFiScript RT MasterMix (Cowin Bio, Jiangsu, China) was used for first-strand cDNA synthesis.
AtNF-YC3 was cloned by PCR amplification, and the 50 μl reaction mixture contained the cDNA product (2 μl), forward and reverse primers (10 μM, 1 μl), and KOD OneTM PCR Master Mix (TOYOBO, OSAKA, JAPAN, 25 μl). The primer sequences for gene cloning are listed in
Supplementary Table S2. The PCR product was gel purified and sequenced for multiple sequence alignments and phylogenetic analyses [
33]. The GenBank accession numbers of the NF-YC proteins are shown in
Supplementary Table S3.
4.3. Subcellular Localisation of PeCPK21 and AtNF-YC3
For the subcellular localisation test, the PeCPK21 and AtNF-YC3 sequences were inserted into the pCAMBIA-1300 GFP vector and the PBI121-mCherry vector, respectively. The recombinant plasmid pCAMBIA-1300 GFP-PeCPK21 and PBI121-mCherry-AtNF-YC3 was then transformed into A. tumefaciens (strain GV3101) and subsequently co-infiltrated into tobacco leaves. The fluorescence of mCherry and GFP was analyzed using a Leica confocal microscope (TCS SP8, Leica Microsystem GmbH, Wetzlar, Germany).
4.4. HaloTag Pull-Down
The CDS of
PeCPK21 was ligated to the pFN19K (Halo Tag) T7 SP6 Flexi expression vector (Promega, Madison, WI 53711, USA). The High-Yield Wheat Germ Protein Expression System TNT SP6 (Promega, USA) was used to produce the bait protein, PeCPK21-Halo [
4]. The produced bait protein was determined by Western blotting analysis.
The HaloTag pull-down protocol was performed based on the material provided by the manufacturer (Magne HaloTag Beads Technical Manual, Promega, USA). In brief, the bait protein was mixed with the Magne HaloTag beads, which were previously equilibrated with Tris buffer saline (TBS, 100 mM Tris-HCl/150 mM NaCl, pH 7.5). No bait protein was added to the mixture for the negative control. The complex of magnetic beads and bait protein was washed three times with pre-cooled TBS buffer. Then, the AtNF-YC3-GST protein prepared with the myTXTL Sigma 70 Master Mix Kit, was added to the magnetic bead-bait protein complex solution. After 4-5 hours of incubation (4 °C), a magnetic bead-bait protein-bead protein complex was formed. The complex was further washed with pre-cooled TBS and centrifuged at 1500 rpm for 5 min. The obtained proteins were separated by SDS-PAGE (Solarbio Life Science, China) and used for Western blotting analysis. The pull-down proteins were analyzed with anti-GST (ABclonal Technology, Wuhan, China) and anti-halo antibodies (Promega, USA). The primers used for the pull-down assay are shown in
Supplementary Table S2.
4.5. Yeast Two Hybrid
The Matchmaker Gal4-based Yeast two hybrid system (Clontech) was used to verify the interaction between PeCPK21 and AtNF-YC3. The CDS of
AtNF-YC3 and
PeCPK21 were ligated to pGADT7 and pGBKT7 vectors, respectively. The recombinant plasmids were co-transformed into yeast strain AH109, which was then cultured on SD/-Leu/-Trp medium and SD/-Leu/-Trp/-His/-Ade medium to test the possible protein-protein interactions. The primers used for the Y2H assay are shown in
Supplementary Table S2.
4.6. Bimolecular Fluorescence Complementation
BiFC assays were performed to determine whether PeCPK21 interacts with AtNF-YC3 in planta. The CDS of
PeCPK21 and
AtNF-YC3 were ligated to pSPY-CE and pSPY-NE vectors, respectively. Plasmids containing pSPY-
PeCPK21-CE and pSPY-
AtNF-YC3-NE were transferred into
A. tumefaciens strain GV3101 (pSoup19 GV3101). The transgenic strains containing pSPY-
PeCPK21-CE/pSPY-CE were thoroughly mixed with pSPY-
AtNF-YC3-NE/pSPY-NE at a volume ratio of 1:1 and then maintained at 28 °C for 2-4 hours. The
A. tumefaciens strains were infiltrated into tobacco leaves and kept in the dark for 48 hours. YFP fluorescence was finally detected using a confocal microscope.
Supplementary Table S2 lists the primers used for the BiFC assays.
4.7. Overexpression of AtNF-YC3 in Arabidopsis
The CDS of
AtNF-YC3 was cloned into the pCAMBIA-1300-GFP vector with the
KpnI and
SalI sites and driven by the CaMV35S promoter. The constructed
AtNF-YC3-GFP was transformed into
A. tumefaciens (strain GV3101), which was used for plant transformation [
4]. The hygromycin (25 mg/L)-resistant plants (T1 generation) were selected and used to produce the homozygous transgenic lines of the T2 and T3 generations. Six transgenic lines overexpressing
AtNF-YC3, i.e., OE1, OE2, OE3, OE4, OE5, OE6, were obtained and verified by RT-qPCR and Western blotting.
4.8. Real-Time Quantitative PCR Analysis
Total RNA was extracted from WT Arabidopsis, VC,
PeCPK21 transgenic lines (OE3, OE7 and OE10), and
AtNF-YC3 transgenic lines, OE2, OE4 and OE6, following the previously described method. The RNA samples were purified, quantified and utilized for RT-qPCR analysis with a LineGene 9600 Plus Real-Time Quantitative PCR System (FQD-96A, BIOER Technology, Hangzhou, China).
AtACT2 served as an internal reference gene for Arabidopsis [
33]. The transcription of
AtNF-YC3,
AtCAT,
AtPOD and
AtSOD in Arabidopsis was assessed in both control and Cd-stressed plants. The specific primers for the target and reference genes can be found in
Supplementary Table S1.
4.9. Extraction of Total Protein from Arabidopsis and Western Blotting
The leaves of 4-week-old
Arabidopsis thaliana were grounded in liquid nitrogen and the appropriate protein extract was added. The mixture was shaken for 1 minute and then placed on ice for 10 minutes. The samples were centrifuged at 1300 rpm (4 °C) for 15 min and the supernatant (total protein) was used for Western blotting. The supernatant was mixed with 5 × SDS loading buffer, completely denatured at 95 °C for 5 min, then cooled down on ice. The mixture was subjected to SDS-PAGE at 120 V for 2 hours and transferred to PVDF membranes. The immunoblots were probed with anti-GFP antibodies, and equal loading was confirmed by probing with anti-Actin antibodies (ABclonal Technology, Wuhan, China) [
34].
4.10. Phenotype Test under Cd Stress
Seeds of wild type Arabidopsis (Col-0), vector control (VC) and
AtNF-YC3 overexpressing lines, OE2, OE4 and OE6, were surface sterilised and germinated in 1/2 MS solid medium (0.8 % agar, 1 % sucrose, w/v) containing 0 or 100 μM CdCl
2. After vernalisation at 4 °C for 48 hours, the seeds were germinated and grown at 22 °C with an illumination of 60 μmol m
−2 s
−1. Fresh weight of plants, root length, and electrolyte leakage (EL) were examined after 7 days of CdCl
2 treatment [
33].
4.11. Cellular Cd and H2O2 Measurement
Cd and H
2O
2 concentrations in root cells were measured using the Leadmium
TM Green AM fluorescent probe (Invitrogen, Carlsbad, USA) and H2DCFDA (Molecular Probes, Biorigin, Beijing, China) as previously described [
5,
33]. Briefly, WT, VC and
AtNF-YC3 overexpressing lines (OE2, OE4 and OE6) treated with or without CdCl
2 (100 μM, 7 d) were incubated with 50 μM Leadmium
TM Green AM for 1 h or with 10 μM H2DCFDA for 0.5 hour in the dark. The roots were then sampled to measure the fluorescence intensities of Cd and H
2O
2 using a Leica confocal microscope (TCS SP8).
4.12. Recordings of the Cd Flux in the Roots
Net Cd fluxes in root tips were recorded with microelectrodes equipped with the non-invasive micro-test system (NMT-YG-100, Younger USA, LLC, Amherst, MA 01002, USA) [
5,
6,
35]. Roots were collected from control and CdCl
2-treated plants of WT, VC and
AtNF-YC3-overexpressing lines (OE2, OE4 and OE6), and immediately equilibrated in measuring solutions for 30 min. For each treatment five individual plants were used for flux recording. Cd flux rates were calculated using the programme JCal version V3.2.1 (
http://www.xuyue.net/).
4.13. Measurement of the Activities of the Antioxidant Enzymes
WT, VC and
AtNF-YC3-overexpressing lines (OE2, OE4 and OE6) were exposed to 0 or 100 μM CdCl
2 for 7 days. The control and stressed plants were collected to measure the activities of antioxidant enzymes using the assay kits for SOD, POD, and CAT (Njjcbio, Nanjing, China) [
36].
4.14. Statistical Analysis
All experimental data were statistically analyzed using SPSS version 19.0 (IBM Corporation, Armonk, NY, USA). Significant differences between mean values were determined using the Duncan Multiple Range Test (DMRT). For post-hoc multiple comparisons, the least significant difference (LSD) method was used. p < 0.05 was considered significant unless otherwise stated.
5. Conclusions
We conclude that PeCPK21 interacts with the transcription factor AtNF-YC3 to reduce Cd accumulation and strengthen the antioxidant system to reduce ROS triggered by Cd stress. This enables the transgenic plants to adapt to the Cd environment. This study highlights the regulatory role of PeCPK21 and AtNF-YC3 in Cd stress tolerance, which can be utilized to improve Cd tolerance in higher plants.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org., Table S1: Primers used for quantitative real-time PCR; Table S2: Primers used for gene cloning; Table S3: Accession numbers of NF-Y orthologs.
Author Contributions
K.Y.: investigation, data curation, validation, visualization, writing—original draft. Y.L.: investigation, validation, data curation, visualization. Z.L.: investigation, methodology, validation, visualization. R.Z.: conceptualization, investigation, methodology, software. Y.Z.: investigation, methodology, resources. C.Y.: investigation, methodology, visualization. Z.Z.: investigation, methodology. B.F.: investigation, methodology. X.Z.: investigation, methodology. K.A.: investigation, methodology. J.L. (Jing Li): investigation, methodology. J.L. (Jian Liu): investigation, methodology. K.D.: investigation, methodology. J.Y.: investigation, methodology. N.Z.: methodology, resources. X.Z.: resources. S.C.: conceptualization, supervision, funding acquisition, project administration, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported jointly by the National Natural Science Foundation of China (grant nos. 32371828, 32071730, and 31770643).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and supplemental materials.
Acknowledgments
We thank Bluescape Scientific Company Limited (Baoding, Hebei, China) for contributing to the expression and purification of HaloTag protein.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
ABA: abscisic acid; ACT2: Actin2; APX: Ascorbate peroxidase; APX1: ascorbate peroxidase 1; APX2: ascorbate peroxidase 2; AVA-P2: V-type proton ATPase proteolipid subunit; BiFC: Bimolecular fluorescence complementation; CAT: Catalase; CDS: Coding sequence; Cd: Cadmium; CDSP32: thioredoxin-like protein CDSP32; COPT5: copper transporter 5; CPK: Calcium-dependent protein kinase; DMSO: Dimethyl sulfoxide; EL: Relative electrolyte leakage; GPX3: glutathione peroxidase; HAP: heme-activated protein; HMAPs: heavy metal stress-associated proteins; NF-Y: Nuclear transcription factor Y; NMT: Noninvasive micro-test technology; OPT3: oligopeptide transporter 3; PDF2.2: plant defensin-like protein 2.2; PIP1–1: plasma membrane intrinsic protein 1-1; PIP2A: plasma membrane intrinsic protein 2A; PIP2–7: plasma membrane intrinsic protein 2-7; POD: Peroxidase; PRXQ: thioredoxin superfamily protein; ROS: Reactive oxygen species; RT-qPCR: Quantitative real-time polymerase chain reaction; SD: Synthetic dropout; SOD: Superoxide dismutase; TAPX: thylakoid ascorbate peroxidase; TBS: Tris buffer saline; TF: transcription factor; TRXM4: thioredoxin M4; VC: vector control; VHA-B1: V-type proton ATPase subunit B1; VHA-C: V-type proton ATPase subunit C; WT: wild-type; XTH: Xyloglucan endotransglucosylase/hydrolase; Y2H: Yeast two hybrid.
References
- Khan, M.U.; Shahbaz, N.; Waheed, S.; Mahmood, A.; Shinwari, Z.K.; Malik, R.N. Comparative health risk surveillance of heavy metals via dietary foodstuff consumption in different land-use types of Pakistan. Hum. Eco. Risk Assess. 2015, 22, 168–186. [Google Scholar] [CrossRef]
- Kubier, A.; Pichler, T. Cadmium in groundwater−A synopsis based on a large hydrogeochemical data set. Sci. Total Environ. 2019, 689, 831–842. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Wang, Z.Q.; Liu, Y.S.; Zhang, T.Q.; Liu, J.M.; You, Z.; Huang, P.P.; Zhang, Z.Q.; Wang, C. Plasma membrane-associated calcium signaling modulates cadmium transport. New Phytol.
- Yin, K.X.; Zhao, R.; Liu, Z.; Qi, S.; Zhang, Y.; Liu, Y.; Yan, C.X.; Zhao, Z.Y.; Zhang, X.M.; Yao, J.; Zhang, Y.L.; Liu,J. ; Li, J.; Zhao, N.; Zhou, X.Y.; Chen, S.L. Populus euphratica CPK21 interacts with heavy metal stress-associated proteins to mediate Cd tolerance in Arabidopsis. Plant Stress 2024, 11, 100328. [Google Scholar]
- Sun, J.; Wang, R.G.; Zhang, X.; Yu, Y.C.; Zhao, R.; Li, Z.Y.; Chen, S.L. Hydrogen sulfide alleviates cadmium toxicity through regulations of cadmium transport across the plasma and vacuolar membranes in Populus euphratica cells. Plant Physiol. Biochem. 2013, 65, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Sa, G.; Sun, J.; Shen, Z.D.; Zhao, R.; Ding, M.Q.; Deng, S.R.; Lu, Y.J.; Zhang, Y.H.; Shen, X.; Chen, S.L. Overexpression of Populus euphratica xyloglucan endotransglucosylase/hydrolase gene confers enhanced cadmium tolerance by the restriction of root cadmium uptake in transgenic tobacco. Environ. Exp. Bot. 2014, 100, 74–83. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Sa, G.; Zhang, Y.; Hou, S.Y.; Wu, X.; Zhao, N.; Zhang, Y.H.; Deng, S.R.; Deng, C.; Deng, J.Y.; Zhang. H.L.; Yao, J.; Zhang, Y.L.; Zhao, R.; Chen, S.L. Populus euphratica annexin1 facilitates cadmium enrichment in transgenic Arabidopsis. J. Hazard. Mater. 2021, 405, 124063. [Google Scholar] [PubMed]
- Yan, C.X. , Feng, B., Zhao, Z.Y., Zhang, Y., Yin, K.X., Liu, Y., Zhang, X.M., Liu, J., Li, J., Zhao, R., Zhao, N., Zhou, X.Y., Chen, S.L. Populus euphratica R2R3-MYB transcription factor RAX2 binds ANN1 promoter to increase cadmium enrichment in Arabidopsis. Plant Sci. 2024, 344, 112082. [Google Scholar] [PubMed]
- Han, Y.S.; Wang, S.J.; Zhao, N.; Deng, S.R.; Zhao, C.J.; Li, N.F.; Sun, J.; Zhao, R.; Yi, H.L.; Shen, X.; Chen, S.L. Exogenous abscisic acid alleviates cadmium toxicity by restricting Cd2+ influx in Populus euphratica Cells. J. Plant Growth Regul. 2016, 35, 827–837. [Google Scholar] [CrossRef]
- Lee, M.Y.; Shin, H.W. Cadmium-induced changes in antioxidant enzymes from the marine alga Nannochloropsis oculate. J. Appl. Phycol. 2003, 15, 13–19. [Google Scholar] [CrossRef]
- Xu, W.F. , Shi, W.M., Liu, F., Ueda, A., Takabe, T. Enhanced zinc and cadmium tolerance and accumulation in transgenic Arabidopsis plants constitutively overexpressing a barley gene (HvAPX1) that encodes a peroxisomal ascorbate peroxidase. Botany 2008, 86, 567–575. [Google Scholar] [CrossRef]
- Bočová, B. , Huttova, J., Liptakova, Ľ., Mistrik, I., Olle, M., Tamas, L. Impact of short-term cadmium treatment on catalase and ascorbate peroxidase activities in barley root tips. Biol. Plant. 2012, 56, 724–728. [Google Scholar] [CrossRef]
- Hu, S.H.; Jinn, T.L. Impacts of Mn, Fe, and oxidative stressors on MnSOD activation by AtMTM1 and AtMTM2 in Arabidopsis. Plants 2022, 11, 619. [Google Scholar] [CrossRef] [PubMed]
- Perfus-Barbeoch, L.; Leonhardt, N.; Vavasseur, A.; Forestier, C. Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J. 2002, 32, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, R. The molecular biology of the CCAAT-binding factor NF-Y. Gene 1999, 239, 15–27. [Google Scholar] [CrossRef]
- Stephenson, T.J.; McIntyre, C.L.; Collet, C.; Xue, G.P. Genome-wide identification and expression analysis of the NF-Y family of transcription factors in Triticum aestivum. Plant Mol. Biol. 2007, 65, 77–92. [Google Scholar] [CrossRef]
- Siefers, N.; Dang, K.K.; Kumimoto, R.W.; Bynum, W.E.; Tayrose, G.; Holt, B.F. Tissue-specific expression patterns of Arabidopsis NF-Y transcription factors suggest potential for extensive combinatorial complexity. Plant Physiol. 2009, 149, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Laloum, T.; De Mita, S.; Gamas, P.; Baudin, M. .; Niebel, A. CCAATbox binding transcription factors in plants: Y so many? Trends Plant Sci. 2013, 18, 157–166. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, Z.; Wang, Y.; Li, S.H.; Liang, Z.C. Genome-wide identification and characterization of the NF-Y gene family in grape (vitis vinifera L.). BMC Genom. 2016, 17, 605. [Google Scholar] [CrossRef]
- Sun, X.D.; Lian, H.F.; Liu, X.C.; Zhou, S.M.; Liu, S.Q. The garlic NF-YC gene, AsNF-YC8, positively regulates non-ionic hyperosmotic stress tolerance in tobacco. Protoplasma 2016, 254, 1353–1366. [Google Scholar] [CrossRef]
- Li, W.X.; Oono, Y.; Zhu, J.H.; He, X.J.; Wu, J.M.; Iida, K.; Lu, X.Y.; Cui, X.P.; Jin, H.L.; Zhu, J.K. The Arabidopsis NFYA5 Transcription Factor Is Regulated Transcriptionally and Posttranscriptionally to Promote Drought Resistance. Plant Cell 2008, 20, 2238–2251. [Google Scholar] [CrossRef]
- Nelson, D.E.; Repetti, P.P.; Adams, T.R.; Creelman, R.A.; Wu, J.R.; Warner, D.C.; Anstrom, D.C.; Bensen, R.J.; Castiglioni, P.P.; Donnarummo, M.G.; Hinchey, B.S.; Kumimoto, R.W.; Maszle, D.R.; Canales, R.D.; Krolikowski, K.A.; Dotson, S.B.; Gutterson, N.; Ratcliffe, O.J.; Heard, J.E. Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 16450–16455. [Google Scholar] [CrossRef] [PubMed]
- Palmeros-Suarez, P.A.; Massange-Sanchez, J.A.; Martinez-Gallardo, N.A.; Montero-Vargas, J.M.; Gomez-Leyva, J.F.; Delano-Frier, J.P. The overexpression of an Amaranthus hypochondriacus NF-YC gene modifies growth and confers water deficit stress resistance in Arabidopsis. Plant Sci. 2015, 240, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.F.; Liu, Y.; Fu, J.D.; Ma, J.; Fang, Z.W.; Chen, J.; Zheng, L.; Lu, Z.W.; Zhou, Y.B.; Chen, M.; Xu, Z. S.; Ma, Y.Z. The NF-Y-PYR module integrates the abscisic acid signal pathway to regulate plant stress tolerance. Plant Biotechnol. J. 2021, 19, 2589–2605. [Google Scholar] [CrossRef] [PubMed]
- Yotsui, I.; Saruhashi, M.; Kawato, T.; Taji, T.; Hayashi, T.; Quatrano, R.S.; Sakata, Y. ABSCISIC ACID INSENSITIVE3 regulates abscisic acid-responsive gene expression with the nuclear factor Y complex through the ACTT-core element in Physcomitrella patens. New Phytol. 2013, 199, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.M.; Pan, Z.X.; Yu, J.; Zhu, L.; Zhao, M.Z.; Wang, Y.F.; Chen, P.; Liu, C.; Hu, J.; Liu, T.; Wang, K.Y.; Wang, Y.; Zhang, M.P. Transcriptome-wide characterization, evolutionary analysis, and expression pattern analysis of the NF-Y transcription factor gene family and salt stress response in Panax ginseng. BMC Plant Biol. 2022, 22, 320. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, P.; Reddy, S.V.; Moin, M.; Reddy, M.R.; Yugandhar, P.; Mohanraj, S.S.; Balachandran, S.M.; Kirti, P.B. Activation-tagging in indica rice identifies a novel transcription factor subunit, NF-YC13 associated with salt tolerance. Sci. Rep. 2017, 7, 9341. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Q.; Yuan, Y.Y.; Zhang, W.K.; Song, T.T.; Hou, X.Y.; Kong, L.Z.L.; Cui, G.W. Overexpression of an NF-YC2 gene confers alkali tolerance to transgenic alfalfa (Medicago sativa L.). Front. Plant Sci. 2022, 13, 960160. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, Y.J.; Zhuo, C.L.; Lu, S.Y.; Guo, Z.F. Overexpression of a NF-YC transcription factor from bermudagrass confers tolerance to drought and salinity in transgenic rice. Plant Biotechnol. J. 2015, 13, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ye, T.; Zhong, B.; Liu, X.; Jin, R.; Chan, Z. AtHAP5A modulates freezing stress resistance in Arabidopsis through binding to CCAAT motif of AtXTH21. New Phytol. 2014, 203, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, D.; Liu, Y.J.; Luo, C.B.; Zhou, Y.N.; Zhang, L.Y. Overexpression of a NF-YB3 transcription factor from Picea wilsonii confers tolerance to salinity and drought stress in transformed Arabidopsis thaliana. Plant Physiol. Biochem. 2015, 94, 153–164. [Google Scholar] [CrossRef]
- Zhong, M.; Huang, F.L.; Luo, R.J.; Lv, Y.S.; Ali, U.; Sheng, Z.H.; Tang, S.Q.; Wei, X.J.; Hu, P.S. The effect of cadmium on the microRNAome, degradome and transcriptome of rice seedlings. Plant Growth Regul. 2020, 90, 15–27. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, J.; Yin, K.X.; Liu, Z.; Zhang, Y.L.; Deng, C.; Liu, J.; Zhang, Y.N.; Hou, S.Y.; Zhang, H.L.; Yu, D.D.; Zhao, N.; Zhao, R.; Chen, S.L. Populus euphratica phospholipase Dδ increases salt tolerance by regulating K+/Na+ and ROS homeostasis in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 4911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Deng, C.; Wu, X.; Yao, J.; Zhang, Y.L.; Zhang, Y.N.; Deng, S.R.; Zhao, N.; Zhao, R.; Zhou, X.Y.; Lu, C.F.; Lin, S.Z.; Chen, S.L. Populus euphratica remorin 6.5 activates plasma membrane H+-ATPases to mediate salt tolerance. Tree Physiol. 2014, 203, 554–567. [Google Scholar] [CrossRef]
- Deng, C.; Zhu, Z.M.; Liu, J.; Zhang, Y.; Zhang, Y.N.; Yu, D.D.; Hou, S.Y.; Zhang, Y.L.; Yao, J.; Zhang, H.L.; Zhao, N.; Sa, G.; Zhang, Y.H.; Ma, X.J.; Zhao, R.; Polle, A.; Chen, S.L. Ectomycorrhizal fungal strains facilitate Cd2+ enrichment in a woody hyperaccumulator under co-existing stress of cadmium and salt. Int. J. Mol. Sci. 2021, 22, 11651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, K.X.; Yao, J.; Zhao, Z.Y.; Liu, Z.; Yan, C.X.; Zhang, Y.L.; Liu, J.; Li, J.; Zhao, N.; Zhao, R.; Zhou, X.Y.; Chen, S.L. Populus euphratica GLABRA3 binds PLDδ promoters to enhance salt tolerance. Int. J. Mol. Sci. 24, 8208. [CrossRef] [PubMed]
Figure 2.
Sequence analysis of
Arabidopsis thaliana AtNF-YC3. (
a) Multiple sequence alignment of the NF-YC proteins. The blue and pink shadings indicate identical and conserved amino acid residues. The HFM domain is indicated by red lines, and the α-helices are shown by black boxes; (
b) Phylogenetic analysis. The phylogenetic tree was constructed with the neighbour-joining method. At,
Arabidopsis thaliana; Os,
Oryza sativa; Nt,
Nicotiana tabacum; Zm,
Zea mays; Pt,
Populus tomentosa; Ptr,
Populus trichocarpa. The
Supplementary Table S2 lists the accession numbers of the NF-YC3 orthologues.
Figure 2.
Sequence analysis of
Arabidopsis thaliana AtNF-YC3. (
a) Multiple sequence alignment of the NF-YC proteins. The blue and pink shadings indicate identical and conserved amino acid residues. The HFM domain is indicated by red lines, and the α-helices are shown by black boxes; (
b) Phylogenetic analysis. The phylogenetic tree was constructed with the neighbour-joining method. At,
Arabidopsis thaliana; Os,
Oryza sativa; Nt,
Nicotiana tabacum; Zm,
Zea mays; Pt,
Populus tomentosa; Ptr,
Populus trichocarpa. The
Supplementary Table S2 lists the accession numbers of the NF-YC3 orthologues.
Figure 3.
Subcellular localization of PeCPK21 and AtNF-YC3. Agrobacterium tumefaciens strains containing PeCPK21-GFP and AtNF-YC3-mCherry were injected together into tobacco leaves. GFP, YFP and mCherry fluorescence was observed under a confocal microscope (Leica SP8).
Figure 3.
Subcellular localization of PeCPK21 and AtNF-YC3. Agrobacterium tumefaciens strains containing PeCPK21-GFP and AtNF-YC3-mCherry were injected together into tobacco leaves. GFP, YFP and mCherry fluorescence was observed under a confocal microscope (Leica SP8).
Figure 4.
Halo Tag pull-down assay between PeCPK21 and AtNF-YC3. The interaction of PeCPK21 and AtNF-YC3 in vitro was detected using a HaloTag pull-down assay. Halo-PeCPK21 or Halo was used to pull down GST-AtNF-YC3. “+” and “-” indicate the presence or absence of the tested proteins. The immunoblot assays were analyzed with anti-Halo antibodies and anti-GST antibodies.
Figure 4.
Halo Tag pull-down assay between PeCPK21 and AtNF-YC3. The interaction of PeCPK21 and AtNF-YC3 in vitro was detected using a HaloTag pull-down assay. Halo-PeCPK21 or Halo was used to pull down GST-AtNF-YC3. “+” and “-” indicate the presence or absence of the tested proteins. The immunoblot assays were analyzed with anti-Halo antibodies and anti-GST antibodies.
Figure 5.
Y2H analysis between PeCPK21 and AtNF-YC3. The ratios 1:10, 1:100 and 1:1000 correspond to 10-, 100- and 1000-fold dilution, respectively. Yeast transformants were grown on SD (-Leu/ -Trp) control medium and SD(-Leu/ -Trp/ -His/ -Ade) selection medium. SD, synthetic dropout; AD, activating domain; BD, binding domain.
Figure 5.
Y2H analysis between PeCPK21 and AtNF-YC3. The ratios 1:10, 1:100 and 1:1000 correspond to 10-, 100- and 1000-fold dilution, respectively. Yeast transformants were grown on SD (-Leu/ -Trp) control medium and SD(-Leu/ -Trp/ -His/ -Ade) selection medium. SD, synthetic dropout; AD, activating domain; BD, binding domain.
Figure 6.
BiFC analysis between PeCPK21 and AtNF-YC3. Agrobacterium tumefaciens strains containing PeCPK21-cYFP or cYFP were mixed in an equal volume with the strains containing AtNF-YC3-nYFP or nYFP. The Agrobacterium suspensions were then injected into the abaxial surface of tobacco leaves (6 weeks old) using a needleless syringe. The leaves injected with A. tumefaciens- were kept in the dark for 48-60 hours. Tobacco leaves injected with empty vector controls, nYFP and cYFP served as negative controls.
Figure 6.
BiFC analysis between PeCPK21 and AtNF-YC3. Agrobacterium tumefaciens strains containing PeCPK21-cYFP or cYFP were mixed in an equal volume with the strains containing AtNF-YC3-nYFP or nYFP. The Agrobacterium suspensions were then injected into the abaxial surface of tobacco leaves (6 weeks old) using a needleless syringe. The leaves injected with A. tumefaciens- were kept in the dark for 48-60 hours. Tobacco leaves injected with empty vector controls, nYFP and cYFP served as negative controls.
Figure 7.
Cadmium tolerance testing of AtNF-YC3 transgenic lines. (a) RT-qPCR analysis of AtNF-YC3; (b) Western blotting of AtNF-YC3-GFP fusion protein in transgenic Arabidopsis; (c) Representative images of phenotype tests under CdCl2 stress; (d) Root length; (e) Fresh weight; (f) Relative electrolyte leakage. Seedlings of all tested lines, WT, VC, and AtNF-YC3-OE2, OE4, and OE6 (T3 generation) were grown for 7 days in 1/2 MS medium supplied with 0 or 100 μM CdCl2. Data in (a), (d), (e), (f) is the mean of three individual plants, and bars with different letters indicate significant differences (p < 0.05).
Figure 7.
Cadmium tolerance testing of AtNF-YC3 transgenic lines. (a) RT-qPCR analysis of AtNF-YC3; (b) Western blotting of AtNF-YC3-GFP fusion protein in transgenic Arabidopsis; (c) Representative images of phenotype tests under CdCl2 stress; (d) Root length; (e) Fresh weight; (f) Relative electrolyte leakage. Seedlings of all tested lines, WT, VC, and AtNF-YC3-OE2, OE4, and OE6 (T3 generation) were grown for 7 days in 1/2 MS medium supplied with 0 or 100 μM CdCl2. Data in (a), (d), (e), (f) is the mean of three individual plants, and bars with different letters indicate significant differences (p < 0.05).
Figure 8.
Root Cd concentration and flux in AtNF-YC3 transgenic lines. Seedlings of all tested lines, WT, VC, and AtNF-YC3-OE2, OE4, and OE6 (T3 generation) were grown for 7 days in 1/2 MS medium containing 0 or 100 μM CdCl2. (a) Cd concentration in the root cells. The green fluorescence of Leadmium™ Green AM was visualized with a confocal microscope (Leica SP8); (b) Cd flux in the root tips. Net Cd flux was recorded continuously for 6–8 min at the apical zone. Each value (a) or column (b) is the mean of three to four individual plants, and different letters indicate significant differences (p < 0.05).
Figure 8.
Root Cd concentration and flux in AtNF-YC3 transgenic lines. Seedlings of all tested lines, WT, VC, and AtNF-YC3-OE2, OE4, and OE6 (T3 generation) were grown for 7 days in 1/2 MS medium containing 0 or 100 μM CdCl2. (a) Cd concentration in the root cells. The green fluorescence of Leadmium™ Green AM was visualized with a confocal microscope (Leica SP8); (b) Cd flux in the root tips. Net Cd flux was recorded continuously for 6–8 min at the apical zone. Each value (a) or column (b) is the mean of three to four individual plants, and different letters indicate significant differences (p < 0.05).
Figure 9.
H2O2 content, transcription and activity of antioxidant enzymes in AtNF-YC3 transgenic lines. (a) H2O2 concentration in root cells. The green fluorescence of H2DCFDA was visualized with a confocal microscope (Leica SP8). Scale bar = 250 μm; (b) Transcription and activity of antioxidant enzymes. Seedlings of all tested lines, WT, VC, and AtNF-YC3-OE2, OE4, and OE6 (T3 generation) were grown for 7 days in 1/2 MS medium containing 0 or 100 μM CdCl2. Each value (a) or column (b) is the mean of three individual plants, and different letters indicate significant differences (p < 0.05).
Figure 9.
H2O2 content, transcription and activity of antioxidant enzymes in AtNF-YC3 transgenic lines. (a) H2O2 concentration in root cells. The green fluorescence of H2DCFDA was visualized with a confocal microscope (Leica SP8). Scale bar = 250 μm; (b) Transcription and activity of antioxidant enzymes. Seedlings of all tested lines, WT, VC, and AtNF-YC3-OE2, OE4, and OE6 (T3 generation) were grown for 7 days in 1/2 MS medium containing 0 or 100 μM CdCl2. Each value (a) or column (b) is the mean of three individual plants, and different letters indicate significant differences (p < 0.05).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).