Among the organic electrically conductive materials, polyacetylene (i.e., -[CH=CH]
n-) has been the first intrinsically conductive polymer to be developed. Initially, only the cis- and trans-isomers with undoped conductivity values of respectively 10
-11 and 10
-6 ohm
-1·cm
-1 were studied. In the most famous synthesis scheme, polyacetylene (PA) has been obtained by Zielgler-Natta polymerization of acetylene [
1]. In particular, a polymeric film resulted on the surface of the glass reactor by exposing the acetylene gas to a titanium salt catalyst. Subsequently, several complex chain and step polymerization methods for the high-regular PA synthesis have been developed (e.g., ring-opening metathesis polymerization from precursor, reverse Diels-Alder reaction processes, modified-Ziegler homogeneous, radiation polymerization) [
1]. Other less used approaches for the PA synthesis are the dehydroalogenation of polymers [
2] and polyvinyl alcohol (PVOH) thermal dehydration [
3,
4,
5]. The dehydrochlorination of poly(vinyl chloride) by treatment with a very strong base (e.g., t-BuO
-K
+) in a polar medium has been the first example of methods based on reducing a plastic precursor, however this elimination reaction never reaches completion and hence it is unsuitable for producing a highly electrically-conductive material. Differently, the PVOH thermal-dehydration for PA films synthesis [
3,
4] has shown great technological potentialities because it leads to a high conversion yield. Before the discovery of the PA Ziegler-Natta synthesis, it was found that extended polyenic groups, (-CH=CH-CH=CH-)
n, known also as polyvinylenes, were generated in polyvinyl alcohol (PVOH) molecules simply by exposing the polymer to a very common chemical-dehydrating agent, known as fuming sulfuric acid (H
2SO
4) [
6]. Operating under H
2SO
4 refluxing conditions, this chemical treatment lasts for a few days and leads to whole PA molecules. A great potentiality of this very simple chemical approach is represented by the possibility to control the heterogeneous reaction conversion degree. For example, the process of solid PVOH reduction to PA can be end at a desired conversion degree by slightly diluting the sulfuric acid or by acting at low temperatures (e.g., room temperature) for different time periods. The whole PVOH conversion to PA results only under very drastic dehydration conditions (i.e., hot/boiling fuming sulfuric acid), while polyenes-PVOH copolymers (i.e., -(CH=CH)
n-(CH
2-CH(OH))
m-, with n>m) are achieved by polymer dehydration treatments based on H
2SO
4 aqueous solutions. Thus, depending on the H
2SO
4 concentration and reaction temperature, different PA/PVOH ratios are possible in the final linear macromolecular product. The physical properties (color, fluorescence, isomeric composition, etc.) of these PA-PVOH copolymers significantly differ and consequently the obtained polymeric product is suitable for diversified technological applications (color filters, fluorescent plastics, molecular memories, etc.).
In particular, at quite high concentrations (fuming conditions), sulfuric acid contains a small percentage of sulfuric anhydride (SO
3) and this
in situ generated chemical specie results an extremely strong dehydrating agent. As well known, fuming sulfuric acid is capable to act on alcohols and polyols at 140-180°C for generating olefins and polyenes [
7]; also carbohydrates are readily converted to carbon materials by dehydration with fuming sulfuric acid at room temperature [
8]. Similarly, sulfuric acid can act on the hydroxyl groups present in the linear PVOH molecules for generating conjugated polyenic groups of different extensions in the molecular chain up to the limit case of a whole PA molecule. In this case, an hydrocarbon (C
nH
n) is obtained instead of a carbon material because PVOH has an hydrogen content slightly higher than carbohydrates; indeed, one H
2O molecule per each carbon atom is contained in carbohydrates (the carbohydrate formula is: C
m(H
2O)
n, with m=n or very close) and therefore pure elemental carbon results by their dehydration. Concerning the exact type of chemical specie acting as dehydrating agent under such drastic reduction conditions (i.e., highly concentrated sulfuric acid), a compound named pyrosulfuric acid (H
2SO
4.xSO
3) and also known as disulfuric acid (ySO
3.H
2O) should be present. However, when PVOH dehydration takes place under milder conditions like, for example, by treatment with H
2SO
4 aqueous solutions (e.g., 50% by volume of H
2SO
4) at room temperature, sulfuric anhydride (SO
3) is not present and only protons/hydrogenions (H
+, H
3O
+) are involved in the dehydration process. Consequently, acid solutions lead to a mild PVOH reduction to polyene-PVOH copolymers and during this reaction also a chemical cross-linking process with condensated sulfate bridge formation could take place [
9].
When the PVOH semi-crystalline powder (Aldrich, MW=89,000-98,000, 99+% hydrolyzed) was dispersed into pure H2SO4 (J.T. Baker, 95-97%) at room temperature, the dehydration reaction took place immediately, according to the observed white powder color change to reddish-brown/black. However, in order to simplify the product (reduced polymer) isolation from the very aggressive (acid and hygroscopic) liquid medium at dehydration process end, this heterogeneous reaction was preferentially performed by reacting PVOH in form of films. Indeed, in this case, the reduced PVOH film can be mechanically separated from the liquid phase simply by using a plastic (nylon) sieve. In particular, PVOH films with the desired thickness (ca. 0.5mm) were prepared by solution-casting technique. The PVOH semi-crystalline powder was dissolved in hot distilled water (3g of PVOH in 30ml of H2O at 70°C) and the obtained stable aqueous solution was cast into a Petri dish. After water evaporation, the achieved films were cut in small pieces and successively dried in oven under vacuum (3h at 60°C) in order to achieve a completely dried reactant. During the heterogeneous reaction, with polymeric films dipped in H2SO4 (without stirring), the formation of extended polyenic groups in PVOH caused a slow darkening of the initially transparent and colorless films (a copolymer: -(CH=CH)n-(CH2-CH(OH))m- with n>>m resulted). In particular, the films were swollen by sulfuric acid and became gradually reddish-brown and then reddish-black colored. Such darkening process can be attributed to the formation of chromophoric groups in the PVOH films with nonselective absorption in visible spectral region. These chromophores consisted of both conjugated carbon-carbon double bonds and some isolated olefinic groups in the macromolecular backbone. When the darkened residual films were removed from the reaction medium, they had a gelatinous consistency because of the swelling phenomenon due to the adsorbed sulfuric acid.
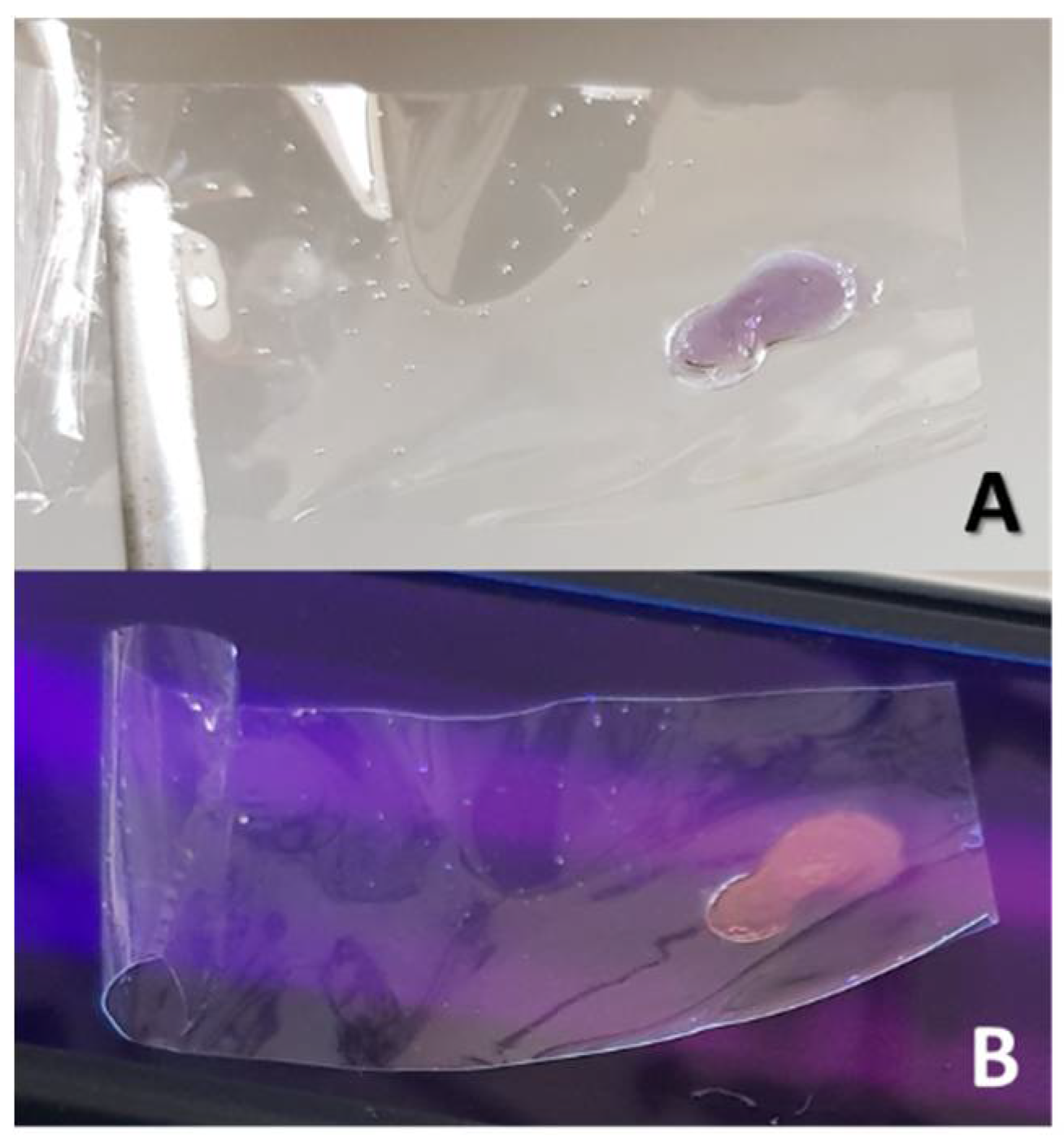
At beginning of the chemical dehydration process, the PVOH films resulted purple colored and also slightly fluorescent, with a white emission, when observed under the UV-light of a mercury-vapor lamp (TLC lamp, short wave: 254nm) (see
Figure 1 A,B). Such visible coloration/fluorescence phenomenon, that reduced and then disappeared with progress of PVOH conversion to PA, has been already described in the literature for thermally-dehydrated PVOH films [
10]. The visible coloration/fluorescence of partially reduced PVOH could be ascribed to the initial formation of short chains of conjugated carbon-carbon double bonds (i.e., dienes, trienes, etc.).
In a typical preparation, the film was reacted for one week at room temperature, the reduced solid material (swollen by H
2SO
4) was mechanically separated from the polyene/H
2SO
4 liquid solution and, in order to eliminate residual sulfuric acid, it was repeatedly washed first by distilled water and then by ethanol in presence of ultrasounds. During the reduced film drying in air, a significant volume decrease occurred.
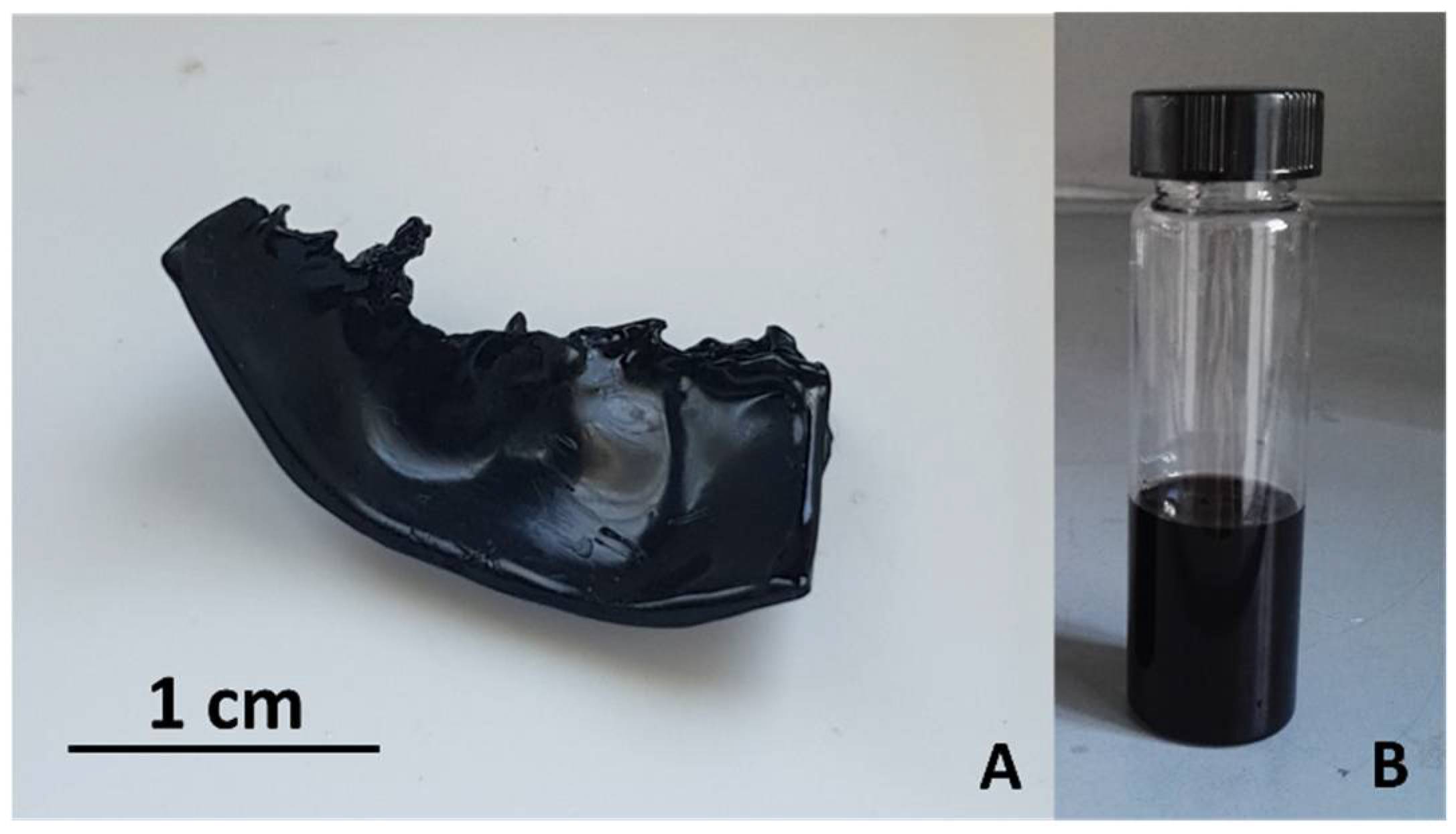
Figure 2A shows a piece of dehydrated PVOH film after such washing/drying treatment. In addition, overtime a little amount of sulfuric acid was segregated at surface of the solid phase probably because of a progressive PA crystallization process taking place in the solid material. The dry reaction product resulted electrically conductive and behaved like a highly resistive material (ca. 10 MΩ/square at 10kHz), as tested by a LCR-meter (UT612, Uni-Trend, China), and had a quite brittle consistency. Such observed brittleness would suggest the presence of the trans-PA isomeric form in the product.
In addition to the solid-state PVOH dehydration, a small fraction of this polymeric reactant (probably low-molecular-weight PVOH chains) dissolved in the viscous liquid (fuming H
2SO
4), thus leading to a progressively increasing coloration of the liquid phase. Like the solid phase, the liquid medium coloration changed to reddish-brown and then to reddish-black for the presence of reduced oligomers dissolved in it (see
Figure 2B). This remaining part of produced PA can be separated from the reactive medium by diluting it with water. In particular, the reactive medium was added dropwise to distilled water under magnetic stirring (typically, 80ml of liquid medium was dissolved in 300ml of distilled water) and a PA flocculation process followed. The flocculated PA was separated by vacuum filtration on a paper filter (Whatman 2 filter papers, hydrophilic membrane). In order to completely remove residual sulfuric acid from this product, it was washed repeatedly by distilled water and ethanol, then recovered from the filter by a spatula and dried in air.
Since the synthesized PA is insoluble in all types of organic solvents (e.g., chloroform, acetone, ethanol), its characterization was carried out at solid state. In particular, the degree of PVOH conversion to polyene molecules was established by ATR analysis (PerkinElmer, Frontier, FT-IR Spectrometer) of the solid reaction product. Spectroscopic analysis is very sensible to the presence of adsorbed water molecules, which produces a strong and broad signal at 3400cm
-1 (OH stretching vibration) and a medium-intensity signal at 1650cm
-1 (HOH bending vibration) [
11] and these two bands could obscure important sample information within the mid-IR spectrum. Therefore, the reaction product was accurately dried before ATR-mode analysis by mildly heating the sample under vacuum first at 40°C for 8h and then at 60°C for 3h. The ATR spectrum of the pure dehydrated product is shown in
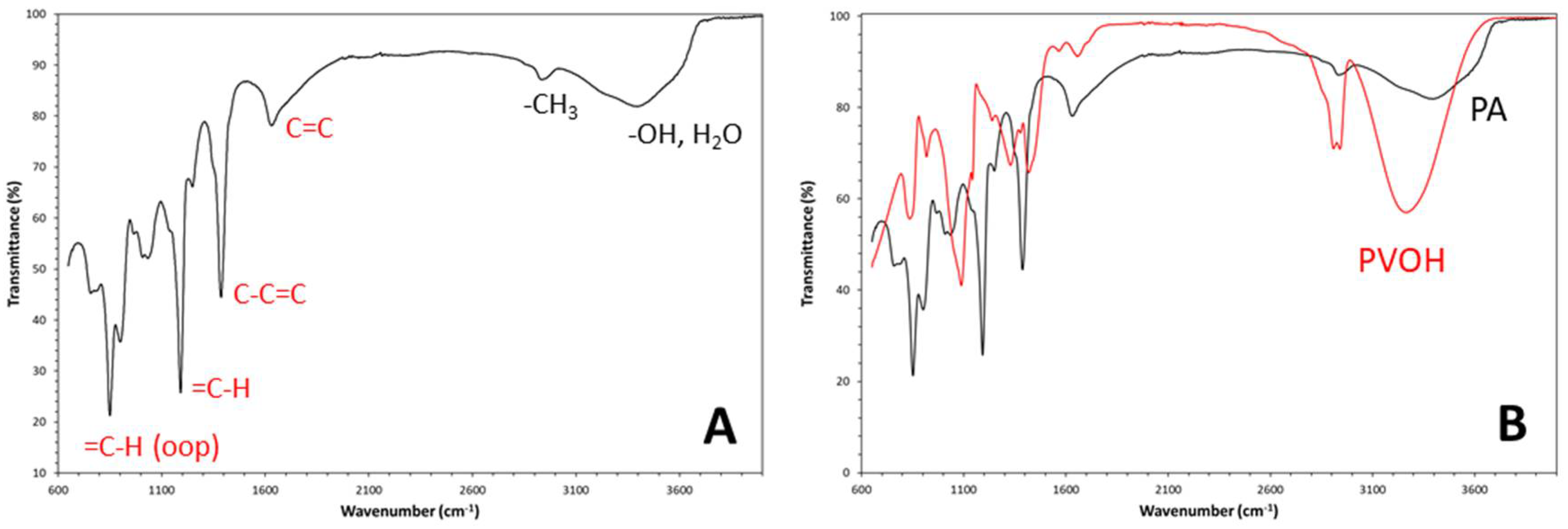
Figure 3A. The incomplete reduction of the polyalcohol to an unsaturated hydrocarbon is readily noticed for the presence of residual hydroxyl groups (OH) absorption in the spectrum. Indeed, these groups produce the characteristic intense and broad absorption band due to the O-H stretching vibration, which is centred at 3398cm
-1. Such absorption is accompanied by the small intensity absorption band due to C-O stretching vibration at 1041cm
-1 and a less intensive OH bending vibration band located at 1250cm
-1. The small IR resonance appearing at 2934cm
-1 is generated mainly by the terminal methyl groups, that are present also in the PA molecules but also the stretching vibration of methylene/methine groups in residual PVOH may contribute. Such aliphatic groups also generate bending vibrations appearing at 1039cm
-1. As for the case of -OH stretching, these signals have strongly reduced intensities compared to that in pristine PVOH spectrum (see spectral comparison in
Figure 2B). However, the IR spectrum of reduction product also shows clear evidences of conjugated olefinic groups formation. Indeed, the IR spectrum of the dehydration product contains the four main characteristic absorption bands generated by conjugated olefinic groups: conjugated carbon-carbon double bond (C=C) stretching vibration, conjugated C-C single bond stretching vibration, in-plane =C-H olefinic (vinyl) =C-H bending vibrations, and out-of-plane olefinic =C-H bending vibrations [
12,
13,
14,
15,
16,
17]. In particular, the C=C stretching vibration band appears at a wavenumber of ca. 1632cm
-1 and it is characterized by medium intensity absorption. This band extents over a wide spectral region (from ca. 1602cm
-1 to ca. 1705cm
-1) because the conjugation phenomenon variously extends in the linear polymer chains and even isolated C=C could be present in these molecules. In particular, the wavenumber of the carbon-carbon double bond absorption band decreases with the extent of conjugation since the bond order (i.e., force) reduces. On the other hand, owing to the same conjugation phenomenon, the C-C single bond stretching absorption appears at higher wavenumber (i.e., 1387cm
-1), with an absorption band of quite high intensity. Very intensive absorption bands are also generated by the in-plane =C-H bending resonance, that is visible at 1192 cm
-1, and by the out-of-plane (oop) =C-H bending absorptions, that are located at 851cm
-1 for the cis-isomer and at 902cm
-1 for the trans-isomer. In particular, the comparison between the intensities of these =C-H oop bending vibration bands allows to easily distinguish the product between cis and trans isomers [
15]. In the present case, both isomers seems to be contained in the dehydrated product; however, according to the band intensities, conformation mostly results of the cis-type. The generated molecular structure should be mostly consisting of cis-PA, also because cis-alkenes have a non symmetric structure, and therefore they are capable to absorb more strongly than trans-alkenes at 1632cm
-1, as found for our sample. It must be pointed out that polyenes should show also vinyl =C-H bond stretching absorption, appearing at ca. 3080cm
-1, but in our PA sample this band is probably obscured by the broader residual hydroxyls absorption centered at ca. 3000cm
-1 (stretching vibration). Yet carbon-carbon double bond (C=C) bending vibration is located outside the explored infrared spectral region (i.e., below 400cm
-1 [
12]). Finally, comparison between the ATR spectra of pristine and dehydrated PVOH, shown in
Figure 3B, clearly evidences as the most intensive absorptions bands of the two compounds do not correspond. The fuming sulfuric acid treatment has caused variations in the intensity and position of bands and the appearance of completely new absorptions corresponding mostly to that of a cis-rich PA sample.
Similarly, the PVOH dehydration process produced a radical change in the polymeric material optical properties. Indeed, the perfectly transparent and colorless PVOH film, whose optical absorption spectrum measured by a double-beam UV-Vis spectrophotometer (VWR, UV-6300PC, China; distilled water was used as both sample solvent and reference) showed no absorption peaks above 400nm (see the red-curve in
Figure 4A), thus indicating the absence of conjugated double bonds as defects in the original sample, acquired a strong absorption band in the visible spectral region after dehydration. In particular, as visible in
Figure 4A, the electronic absorption spectrum of PA (fraction recovered from the liquid phase) was characterized by a very broad absorption band, which extended over the 250-600nm spectral range and was generated by the convolution of five main elementary absorptions with maxima located at 272, 324, 433, 489, and 589nm. These elementary bands correspond to the optical absorption of the generated linear polyenes, that are characterized by a variable number (≥10) of conjugated carbon-carbon double bonds. In particular, the positioning for some bands at a quite high wavelength values indicates the presence in the product of polyvinylenes with very extended conjugation. The observed UV-Vis absorption band corresponded exactly to the optical behavior usually ascribed to PA [
18] and it was obtained by using sulfuric acid as both dispersing medium for the PA molecules and reference for the optical spectrum. Polyenes obtained by reacting PVOH with H
2SO
4 aqueous solutions (e.g., 50% by volume of H
2SO
4) show a quite similar UV-Vis spectrum. According to the distribution of peak intensities in the two spectra shown in
Figure 4, highly conjugated carbon-carbon double bonds are much more abundant in PVOH films treated by fuming H
2SO
4 respect to the case of dehydration by H
2SO
4 aqueous solution (50% by volume of H
2SO
4).
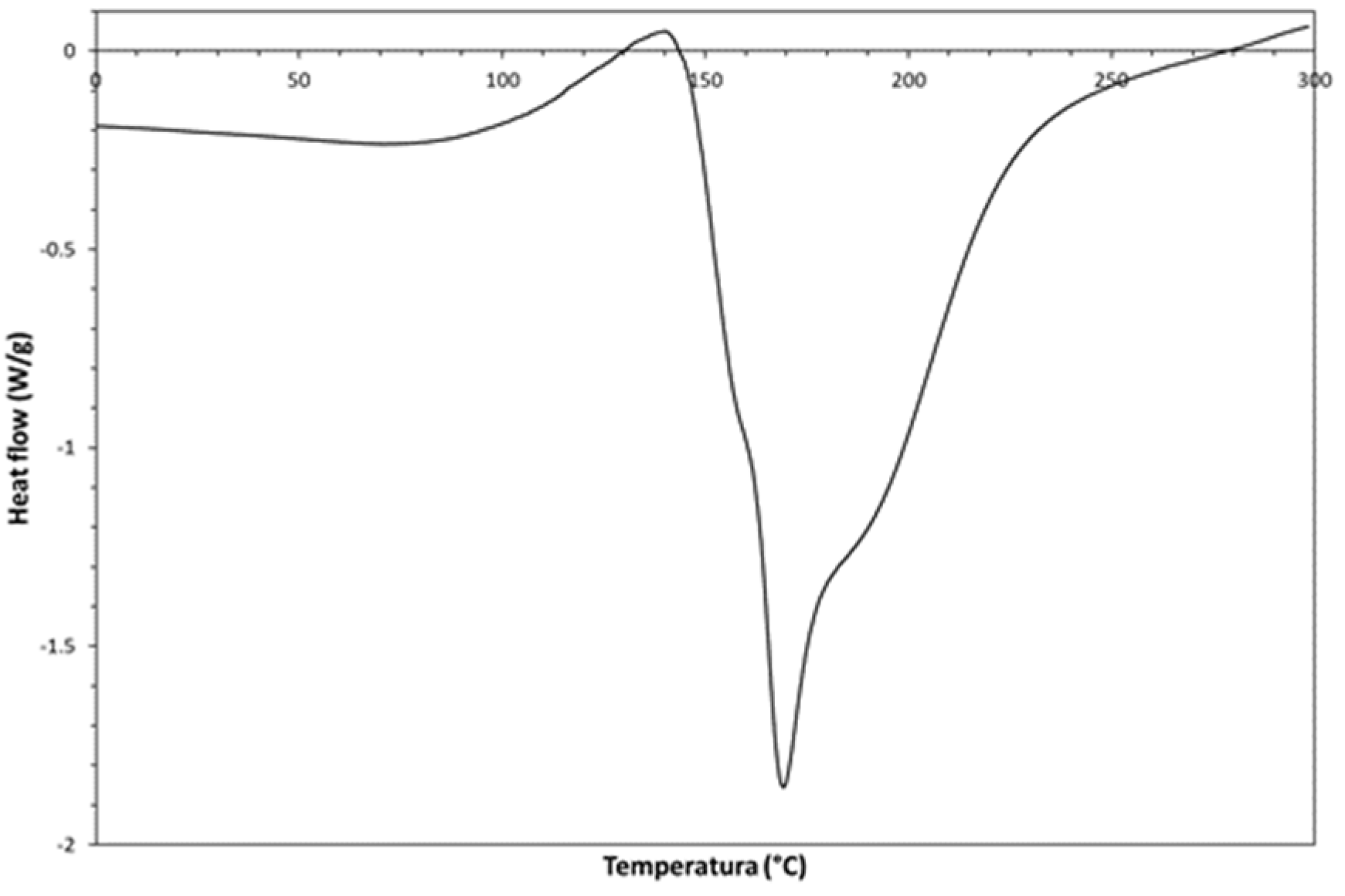
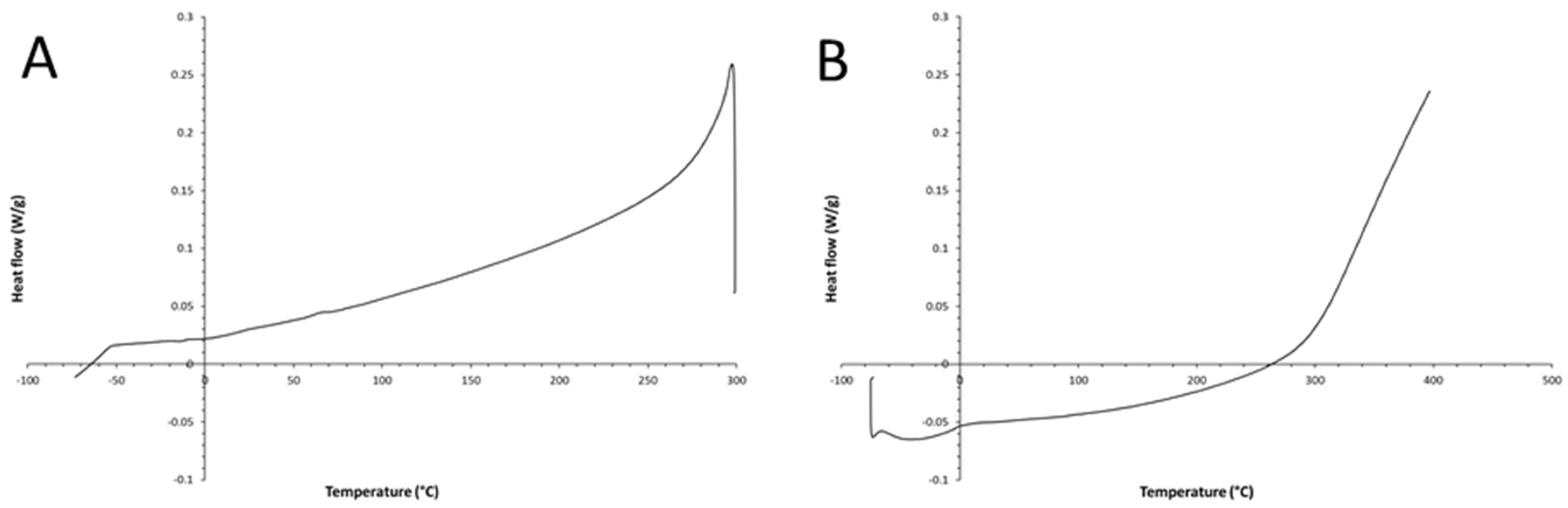
According to the differential scanning calorimetry (DSC) thermogram (1
st scan, 10°C/min, PerkinElmer, Discovery) shown in
Figure 5, the obtained chemical product undergoes a solid-state cis-trans isomerization, which starts at ca. 110°C. Such transition is due to the product conversion to the thermodynamically more stable trans-isomer. This thermally-activated solid-state transition shows a complex behavior (exotherm superimposed on an endotherm); indeed, it starts as an exothermic phenomenon, but it can be accomplished only by a simultaneous endothermic collapse of the previously formed cis-PA crystalline structure. The melting peak seems constituted by three different endothermic signals.
Such thermal behavior represents an irreversible phenomenon; indeed, as shown in
Figure 6 A and B, there are not thermal transitions in the subsequent DSC scans performed on the same DSC specimen. Owing to this unique thermal properties of the developed PA, the material can be technologically exploited for developing new types of thermally activated molecular memories [
19].
In conclusion, a substantial PVOH chemical transformation has occurred during the polymer room temperature treatment by fuming sulphuric acid. The IR spectral assignments have shown as the chemically synthesized compound mostly corresponded to a cis-rich PA sample with some residual alcoholic chain portions (PVOH) in its molecule. Such conformational analysis has been confirmed by DSC; and an UV-Vis investigation has shown the presence of variously extended conjugated carbon-carbon double bonds in the obtained product.
PA has been considered potentially useful as polymer principally for its very good electrical conductivity after doping; however, owing to the insolubility in all organic solvents, this polymer cannot be easily processed, for example, in form of ink to produce coatings, films or filaments to be used for technological applications like electrical wires, sensors, electrodes, etc. Nowadays, the polymer is being produced in form of nano-powder to make possible its processing in form of colloidal suspension [
20]. However, PA could have also a number of non-electrical applications [
21,
22,
23,
24], where it is used in form of powder or granular material. For example, owing to the presence of a very large number of carbon-carbon double bonds, PA can be exploited for drug delivery, as adsorbent for organic substances, oxygen scavenger, hydrogen storage medium (in a transition-metal decorated form), etc. The here described very simple chemical reaction scheme allows to produce high quality PA films or powders to be used for non-electrical applications. In addition, this approach could be used also for reusing a PVOH plastic waste; indeed, PVOH is an appealing target for recycling because it is a high-production-volume polymer.
References
- Olabisi, Olagoke, and Kolapo, Adewale, eds. Handbook of thermoplastics. Vol. 41. CRC press, 2016. Cap. 34.
- S.E. Eusyukov, Yu P. Kudryavtsev, Yu V. Korshak, “Chemical dehydroalogenation of halogen-containing polymers”, Russian Chemical Reviews 60(4)(1991)373-390. [CrossRef]
- I. Yu. Prosanov, N.F. Uvarov, “Electrical properties of dehydrated polyvinyl alcohol”, Physics of the Solid State 54(2)(2012)421-424. [CrossRef]
- I. Yu. Prosanov, A.A. Matvienko, “Study of PVA thermal destruction by means of IR and Raman spectroscopy”, Physics of the Solid State 52(10)(2010)2203-2206. [CrossRef]
- I. Yu. Prosanov, A.A. Matvienko, B.B. Bokhonov, “Influence of urea on polyvinyl alcohol molecular superstructure formation”, Physics of the Solid State 53(6)(2011)1302-1306. [CrossRef]
- S.B. Mainthia, P.L. Kronick, M.M. Labes, “Electrical measurements on polyvinylene and polyphenylene”, J. Chem. Phys. 37(1962)2509-2510. [CrossRef]
- D.J. Ward, D.J. Saccomando, G. Walker, S.M. Mansell, “Sustainable routes to alkenes: applications of homogeneous catalysis to the dehydration of alcohols to alkenes”, Catal. Sci. Technol. 13(9)(2023)2638-2647. [CrossRef]
- D.A. Dolson, R. Battino, T.M. Letcher, K.H. Pegel, N. Revaprasadu, “Carbohydrate dehydration demonstration”, J. Chem. Edu. 72(10)(1995)927. [CrossRef]
- B.S. Minhas, D.G. Peiffer, J.L. Soto, D.L. Stern, “Process for the recovery of sulfuric acid using polymeric membranes, PTC patent, WO 2004/074811 A2 (2 September 2004).
- J. Yang, P. Li, B. Zhao, K. Pan, J. Deng, “Electrospinning chiral fluorescent nanofibers from helical polyacetylene: preparation and enantioselective recognition ability”, Nanoscale Adv. 2(2020)1301-1308. [CrossRef]
- C. Hill, M. Altgen, P. Penttilä, L. Rautkari, “Review: interaction of water vapour with wood and other hygro-responsive materials”, J. Mater. Sci. 59(2024)7595-7635. [CrossRef]
- H. Shirakawa, S. Ikeda, “Infrared spectra of poly(acetylene)”, Polymer Journal 2(2)(1971)231-244. [CrossRef]
- F. Cataldo, “A spectroscopic study of polyacetylene prepared by using Rh(I) catalysts”, Polymer 35(24)(1994)5235-5240. [CrossRef]
- J.-Y. Kim, J.-T. Kim, M.-H. Kwon, D.-K. Han, S.-J. Kwon, “ATR-infrared spectroscopic study of n-doped polyacetylene films”, Macromolecular Research 15(1)(2007)5-9. [CrossRef]
- H.W. Gibson, S. Kaplan, R.A. Mosher, W.M. Prest, R.J. Weagley, “Isomerization of polyacetylene films of the Shirakawa type-spectroscopy and kinetics”, Journal of the American Chemical Society 108(22)(1986)6843-6851. [CrossRef]
- B. Alvarez, A. Sarmiento-Santos, E. Vera-Lopez, “Acetylene polymerization in plasma of direct current”, Journal of physics: Conference Series 1386(2019)012044. [CrossRef]
- Huan Li, G. Chen, P.N. Duchesne, P. Zhang, Y. Dai, H. Yang, B. Wu, S. Liu, C. Xu, N. Zheng, “A nanoparticulate polyacetylene-supported Pd(II) catalyst combining the advantages of homogeneous and heterogeneous catalysts”, Chinese Journal of Catalysis 36(2015)1560-1572. [CrossRef]
- W. Zhao, Y. Yamamoto, S. Seki, S. Tagawa, “Formation of conjugated double bonds in poly(vinyl alcohol) film under irradiation with γ-rays at elevated temperature”, Chemistry Letters (1997)183184. [CrossRef]
- H.-J. Yen, C. Sham, L. Wang, P. Xu, M. Zhou, H.-L. Wang, “Development of conjugated polymers for memory device applications”, Polymers 9(1)(2017)25. [CrossRef]
- V.M. Kobryanskii, “Nanopolyacetylene: optical properties and practical application”, Proceedings of SPIE, Volume 4277, Integrated Optics Devices V, Symposium on Integrated Optics, 2001, San Jose, CA, United States.
- H. Lee, W. Ih Choi, J. Jhm, “On hydrogen storage in metal-decorated trans-polyacetylene”, Journal of Alloys and Compounds 446-447(2007)373-375. [CrossRef]
- J. Liang, C. Song, J. Deng, “Optically active microspheres constructed by helical substituted polyacetylene and used for absorption of organic compounds in aqueous systems”, ACS Appl. Mater. Interfaces 6(21)(2014)19041-19049. [CrossRef]
- M. Chen, G. Hu, T. Shen, H. Zhang, J.Z. Sun, B.Z. Tang, “Applications of polyacetylene derivatives in gas and liquid separation”, Molecules 28(2023)2748. [CrossRef]
- H. Li, G. Chen, P.N. Duchesne, P. Zhang, Y. Dai, H. Yang, B. Wu, S. Liu, C. Xu, N. Zheng, “A nanoparticulate polyacetylene-supported Pd(II) catalyst combining the advantages of homogeneous and heterogeneous catalysts”, Chinese Journal of Catalysis 36(2015)1560-1572. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).