1. Introduction
Acemetacin (ACM) is a new non-steroidal anti-inflammatory drug (NSAID) with anti-inflammatory, analgesic, and antipyretic effects. It is a biopharmaceutics classification system class II drug, and its poor water solubility hinders oral bioavailability [
1]. However, ACM may cause gastrointestinal damage [
2] despite of the increased gastrointestinal tolerance [
3], because it is a prodrug of indomethacin [
4]. Therefore, how to improve the solubility and eliminate the negative effects of ACM has become a key issue for clinical application.
Co-amorphous (CAM) is a promising approach to improve the solubility and bioavailability of poorly water-soluble drugs. CAM is a homogeneous amorphous single-phase system composed of two or more low molecular weight components [
5]. Co-formers (citric acid, lactose, mannitol, amino acids, alginate, etc.) were usually used to stabilize the CAM by the formation of hydrogen bonds or charges with the drug. Among these co-formers, amino acids are the most widely used [
6]. Especially, basic amino acids such as lysine (Lys) [
7], arginine (Arg) [
8,
9], and histidine (His) [
10] have been successfully used as co-formers for acidic drugs. Recently, relevant reports showed that these basic amino acids have protective effects on the gastrointestinal tract [
11,
12,
13,
14,
15,
16]. For example, Lys forms aldehyde lysine catalyzed by lysyl oxidase and participates in the formation of connective tissues, and also contributes to the protection of the gastric mucosa barriers [
12,
13]. Arg can generate nitric oxide under the synthase for vasodilatory, thus the integrity of the gastric mucosal barrier was maintained and mucosal damage caused by gastric ulcers were reduced [
14,
15]. His can inhibit peptic ulcers induced by phytanergic nervousness, and it has a preventive and mitigating effect on the damage of intestinal mucosa, because it is an effective scavenger of free radicals [
11,
16]. Therefore, these basic amino acids are promising co-formers for CAMs to mitigate the gastrointestinal side effects of ACM.
In this study, to improve the solubility and mitigate the gastrointestinal side effects of ACM, ACM-basic amino acids co-amorphous formulations (ACM CAMs) were formulated by a cryo-milling method. The physicochemical properties of ACM CAMs as well as their in vitro dissolution were evaluated. Moreover, the gastro-protective effects and histology studies of ACM CAMs were also performed in rat gastric ulcer models to test the mitigating of gastrointestinal side effects. This study improved the compliance of ACM in clinical use and provided a proising method for mitigating drug-induced gastrointestinal damage.
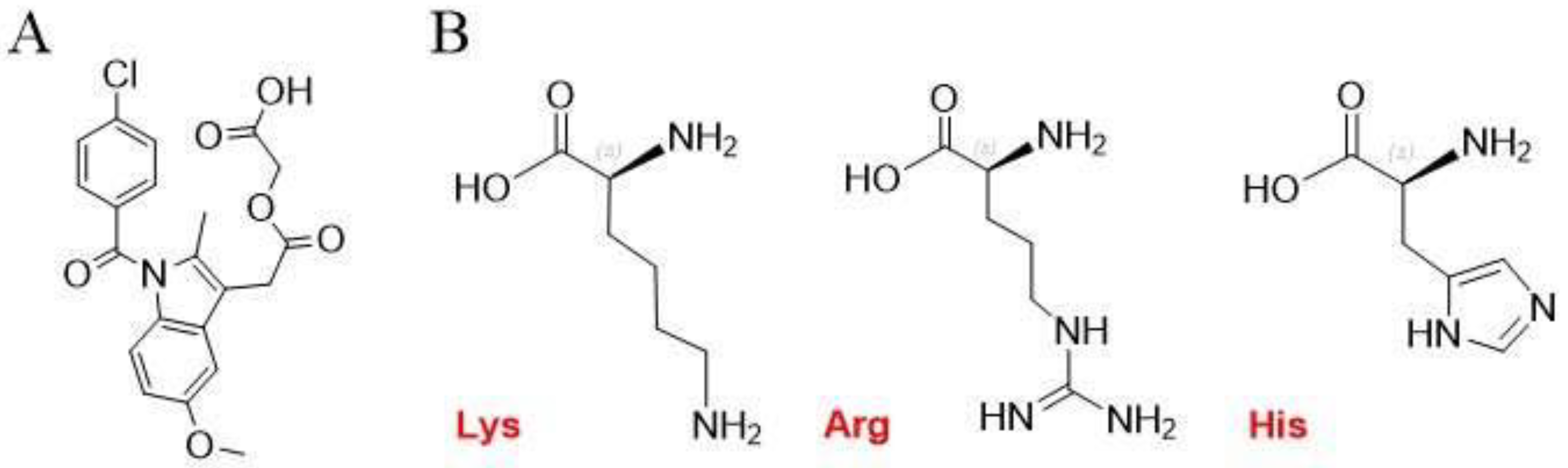
Figure 1.
Molecular structures of (A) ACM and (B) Lys, Arg, and His.
Figure 1.
Molecular structures of (A) ACM and (B) Lys, Arg, and His.
3. Results and Discussion
3.1. Polarizing Microscope
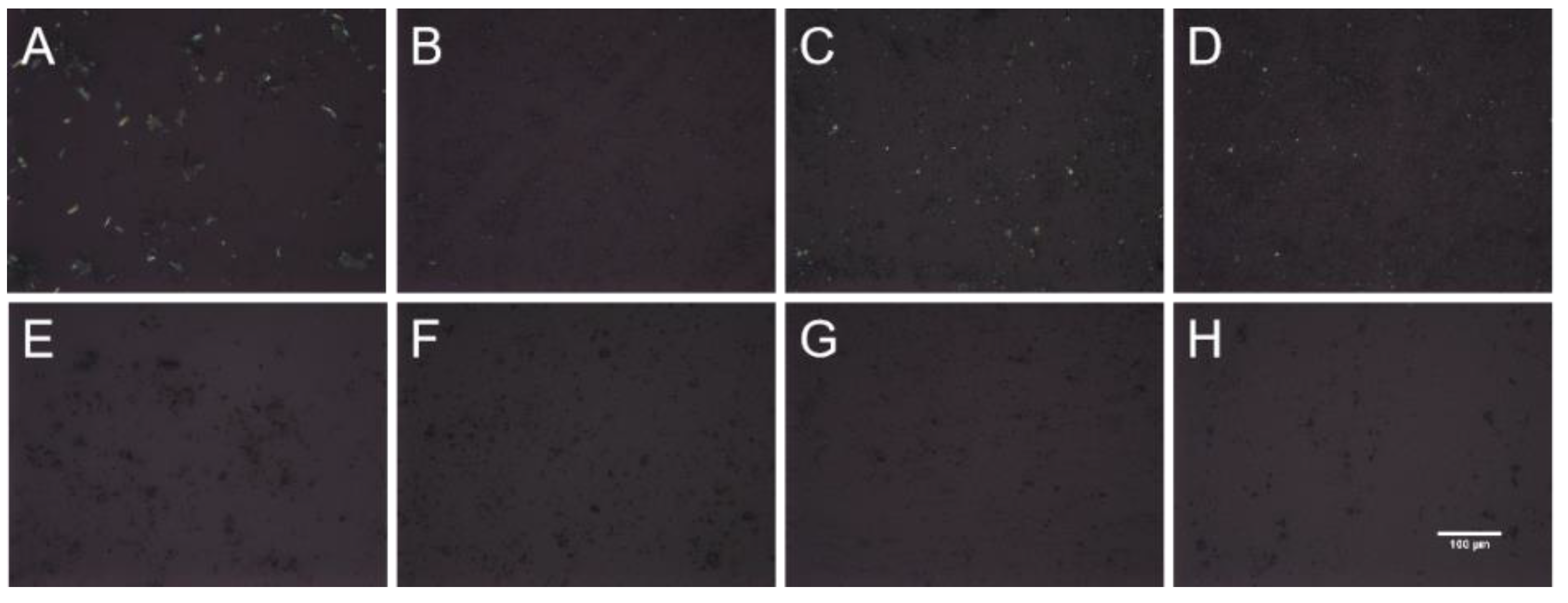
As shown in
Figure 2, the three PMs displayed a birefringence phenomenon, indicating that the substances existed in crystalline state. In contrast, ACM-AM and three CAMs did not show birefringence, suggesting the formation of amorphous form.
3.2. PXRD
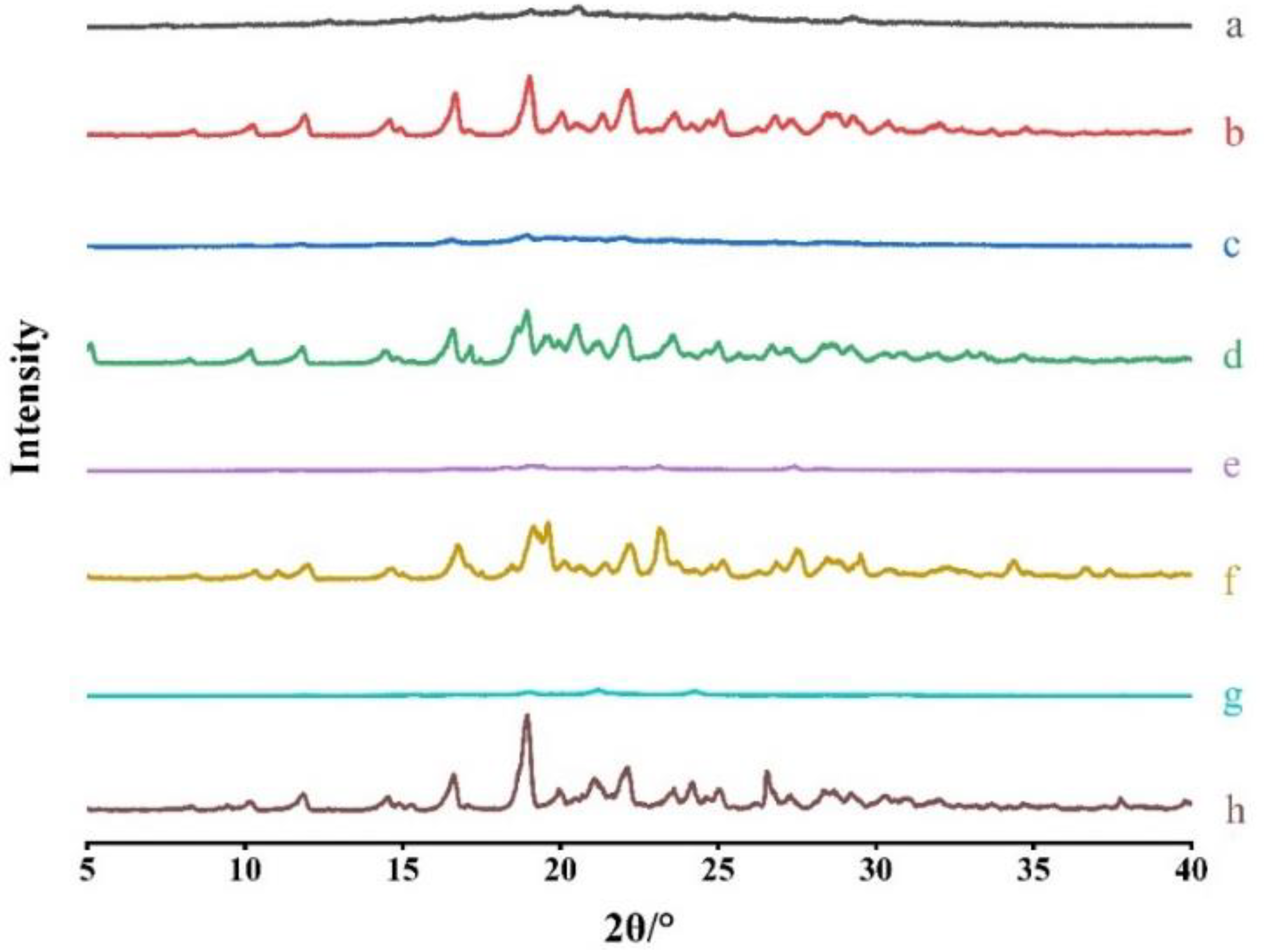
To confirm the formation of CAMs, PXRD was performed. The patterns of ACM-AM, ACM, ACM-CAMs and PMs are shown in
Figure 3. ACM exhibited Bragg’s diffraction peaks at 2θ of 8.34°, 10.20°, 11.90°, 14.60°, 16.68°, 19.02°, 20.04°, 21.34°, 23.62°, 25.08°, which was consistent with a previous report [
18]. ACM-AM showed a broad halo. As it was shown, PMs displayed characteristic peaks of both ACM and amino acids [
19] , however, no characteristic peaks could be observed in the patterns of ACM-CAMs, suggesting the successful preparation.
3.3. Temperature Differential Scanning Calorimetry
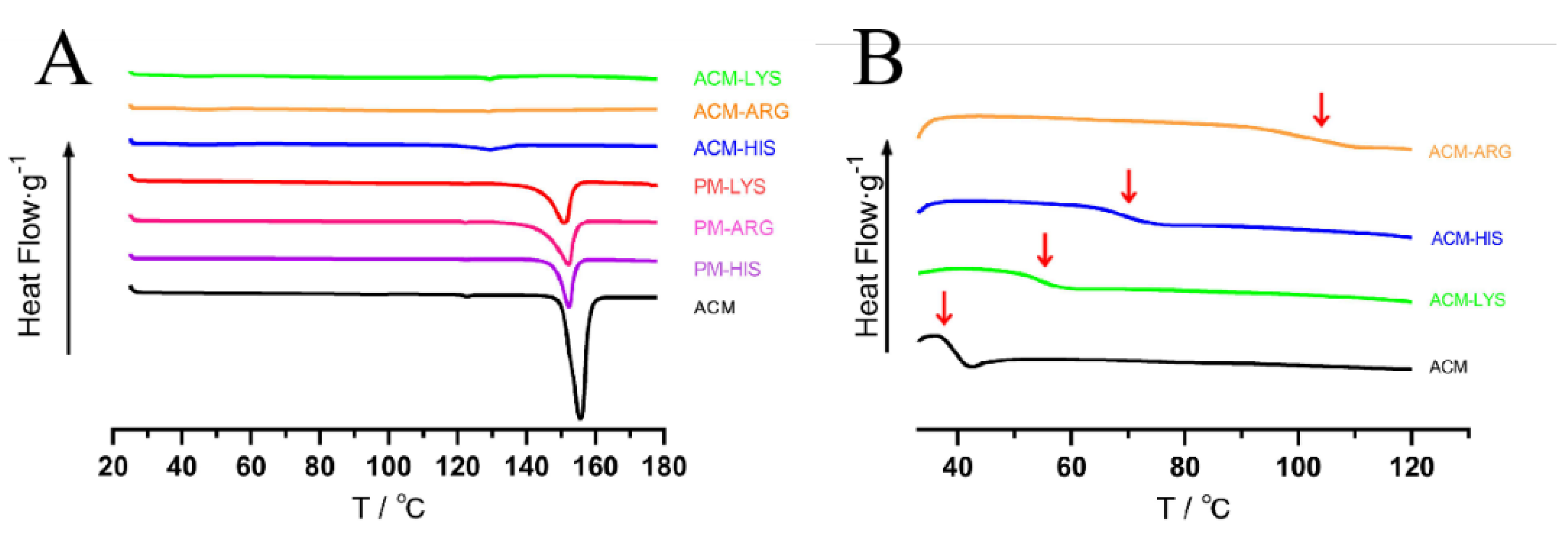
As shown in
Figure 4A, ACM had a significant melting peak at 153°C. The melting peak of ACM in PMs was moved to 150°C, due to the interaction with amino acids. While no peaks were observed in ACM-Lys, ACM-Arg or ACM-His, indicating the successful preparation of CAM systems.
Tg is one of the characteristic parameters of amorphous systems. As shown in
Figure 4B, all the systems only have one
Tg, indicating the formation of a single homogeneous amorphous phase. Concretely, ACM-AM has a
Tg at 37°C, and the Tg of ACM-Arg, ACM-His and ACM-Lys increased to 105°C, 70°C, and 55°C, respectively. The increased
Tg indicated the intermolecular interaction. In addition, the theoretical
Tg(mix) of ACM-Lys, ACM-Arg and ACM-His were then calculated using Gordon-Taylor equation, and the
Tg(mix) were 40.69°C, 42.79°C, and 37.00°C, respectively. Because of the intermolecular interactions between ACM and amino acids, they were much lower than the actual
Tg values of the ACM CAMs systems. According to previous reports, high difference between
Tg(mix) and actual
Tg tends to imply intermolecular interactions [
20]. Therefore, we can conclude that ACM-Arg has the strongest intermolecular forces among the ACM-CAMs, followed by ACM-His and ACM-Lys.
3.4. Investigation of Molecular Interaction
3.4.1. FTIR
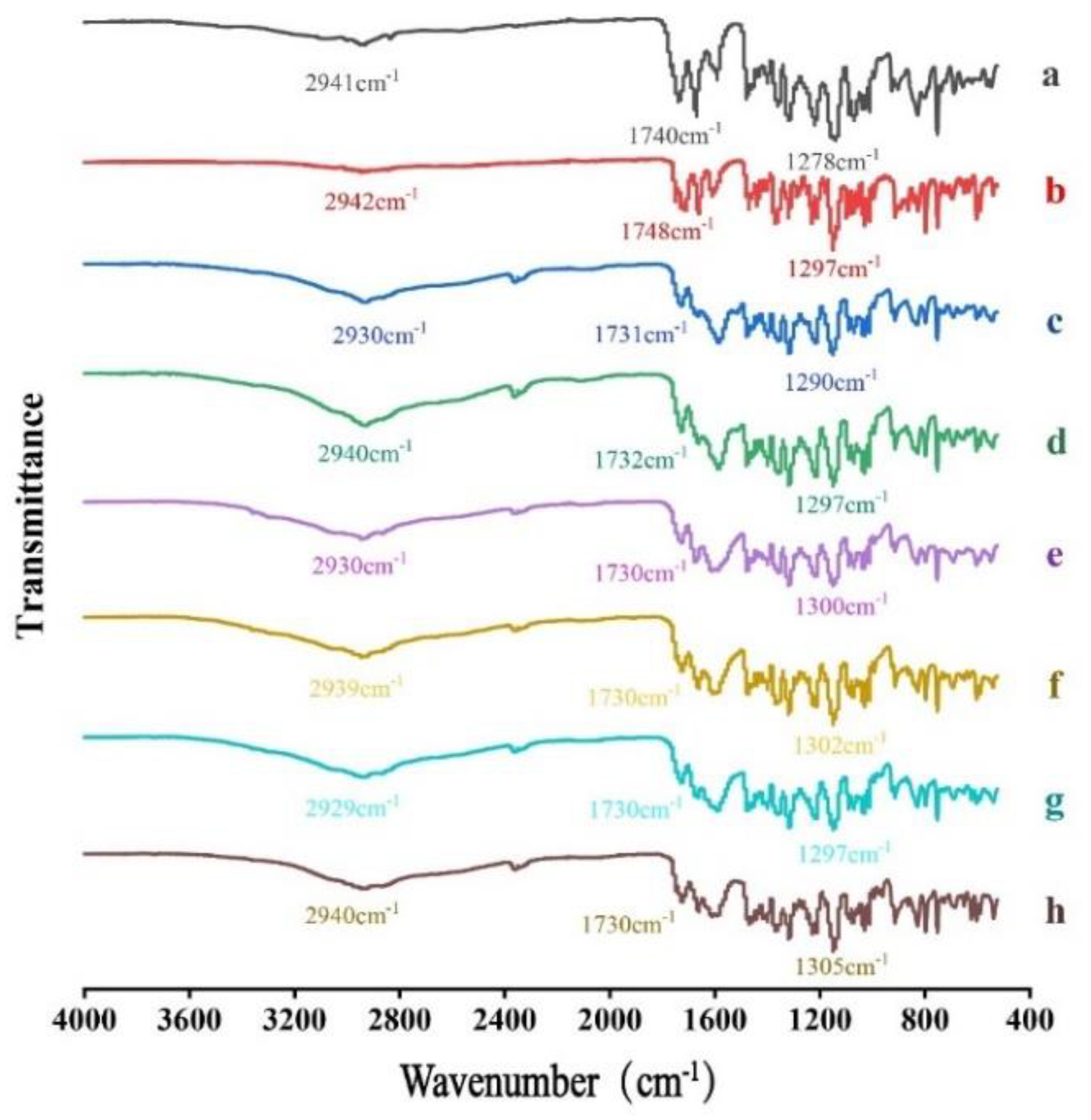
FTIR was performed to test whether molecular interactions exist between ACM and basic amino acids. As shown in
Figure 5, the FTIR spectrum for ACM showed its characteristic peaks at 2942 cm
−1, 1748cm
−1 and 1297 cm
−1, which correspond to the stretching vibration of O−H, C=O and C−O in carboxylic, respectively. In the spectrum for ACM-AM, the characteristic peaks were shifted to 2942 cm
−1, 1748 cm
−1 and 1278 cm
−1, which may be due to the disruption of crystal structure and reconfiguration of ACM molecular [
21]. Compared with PMs, the stretching vibration peaks of O−H and C−O in ACM CAMs showed slight red shift, indicating hydrogen bonding interactions exist between ACM and amino acids.
3.4.2. Molecular Docking
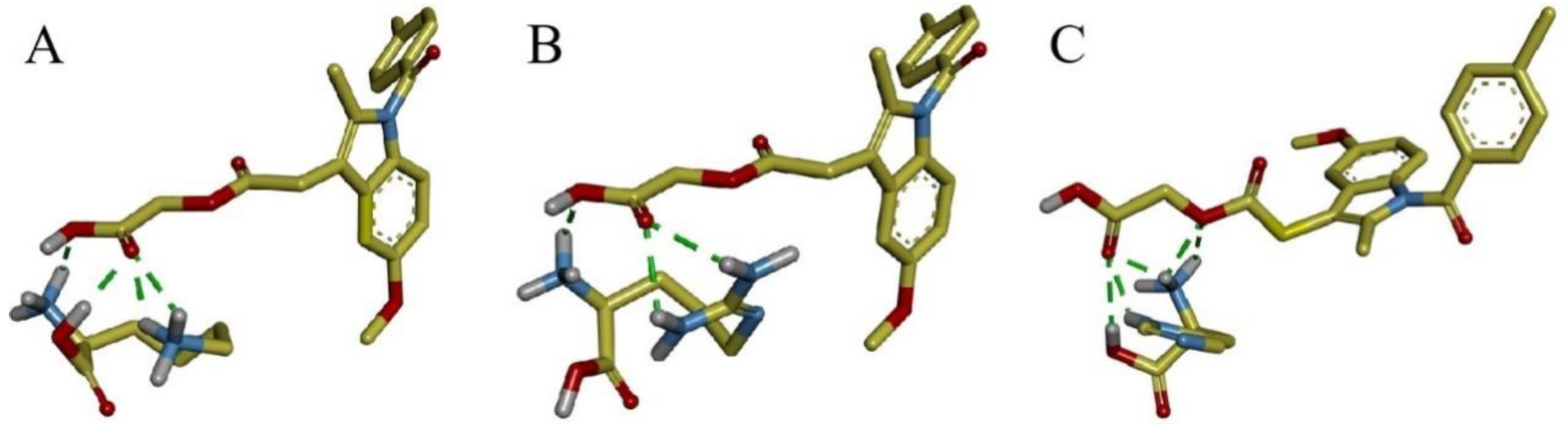
Molecular docking was carried out to further investigate the molecular interactions between ACM and basic amino acids. The results are shown in
Figure 6. The carboxyl group of ACM forms hydrogen bonds with the amino groups in Lys and Arg, as well as with the amino and hydroxyl groups in His. The intermolecular forces was consistent with FTIR. The magnitude of the binding energy reflects the strength of the intermolecular forces, and the binding energies for ACM-Lys, ACM-Arg and ACM-His are -2.08 kcal/mol, -2.46 kcal/mol and -2.13 kcal/mol, respectively. The prediction of the strength of the intermolecular forces was consistent with the
Tg results described in section 3.3.
3.5. Dissolution Testing
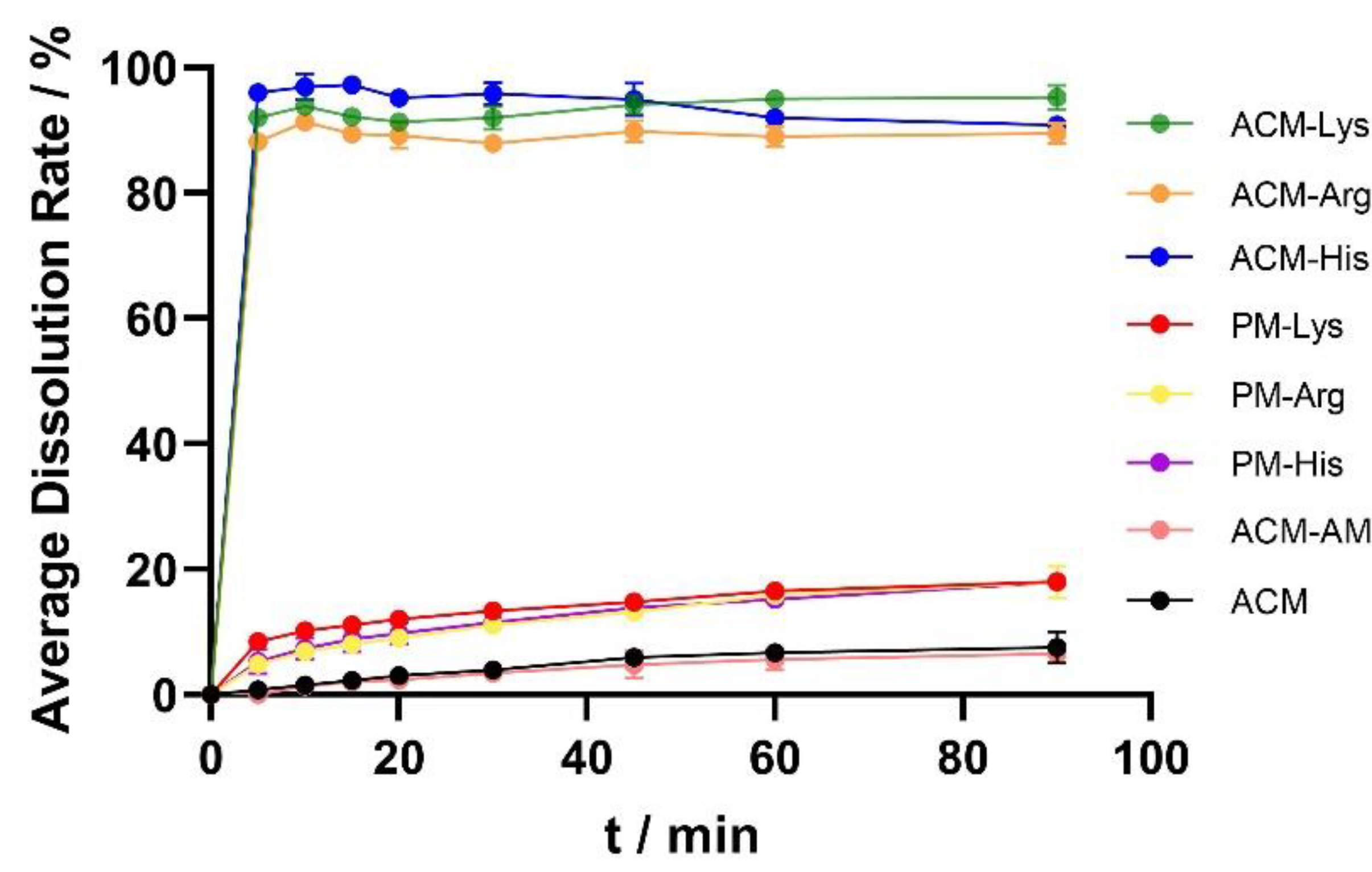
The dissolution curves of ACM, ACM-AM, PMs and ACM CAMs are shown in
Figure 7. The cumulative release of ACM was 7.53% at 60 min, and the dissolution did not increase after the formulation of ACM-AM (6.57% at 60 min), indicating that neat amorphous could not improve the dissolution of ACM. In addition, the dissolution of PMs was slightly improved compared with that of ACM, which released 18% of the total drug. However, the ACM CAMs showed complete dissolution within 5 min. The results showed that ACM CAMs could improve the dissolution of ACM, probably due to the strong interaction between ACM and amino acids [
20].
3.6. IDR
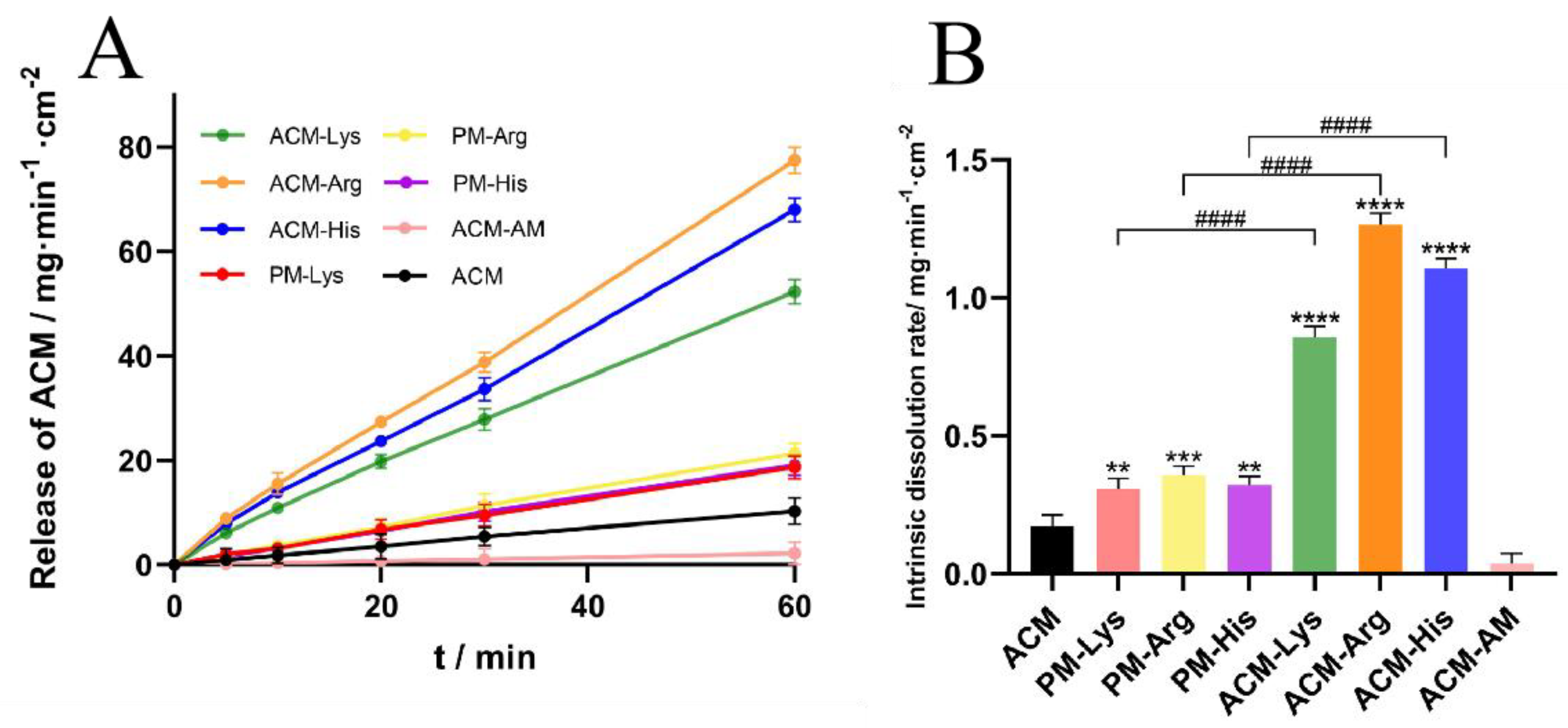
IDR can predict oral bioavailability to a certain extent [
22]. Thus, for poorly water-soluble drugs, it is significant to conduct the IDR tests. The IDR curves and the normalized dissolution rates are shown in
Figure 8. The IDRs of ACM and ACM-AM were 0.1712 mg·min
-1·cm
-2 and 0.0373 mg·min
-1·cm
-2, respectively. For the PMs (PM-Lys, PM-Arg, and ACM-His), the IDRs were increased to 0.3087 mg·min
-1·cm
-2, 0.3565 mg·min
-1·cm
-2, and 0.3200 mg·min
-1·cm
-2, respectively. In addition, the IDRs of ACM CAMs (ACM-Lys, ACM-Arg, and ACM-His) were further increased, which were 3.43, 7.39, and 6.46 times higher than those of ACM, respectively. The results indicated that basic amino acids as co-formers could improve the IDR of ACM.
From the results, it can be seen that ACM-Arg shows the highest IDR, followed by ACM-His and ACM-Lys. This may be attributed to the different strength of intermolecular interaction between amino acids and ACM. According to a previous study [
23], strong intermolecular interactions can prevent recrystallization of components during dissolution. Therefore, Arg has the highest IDR because it has the strongest intermolecular forces.
3.7. Gastro-Protective Effects
3.7.1. UIR
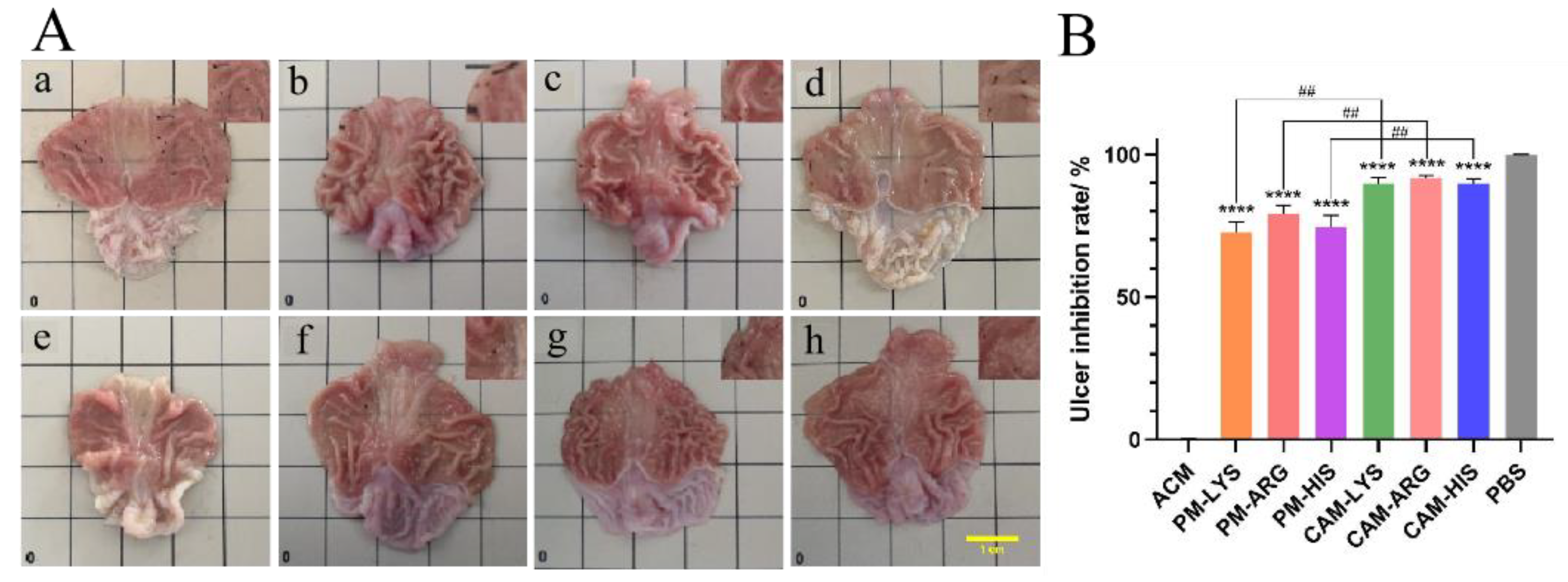
To test the potential of ACM CAMs in protection of the stomach from gastric ulcers, UIR was evaluated. The representative images of the stomachs and the UIR of each group are shown in
Figure 9. The gastric mucosa of the blank control group was thick with more folds, light pink in color, and no ulcers or bleeding spots were observed. In contrast, the model group showed looser and thinner gastric mucosa with fewer folds, and a large number of bleeding spots were spread over the whole gastric tissues. Moreover, the total area of ulcerative lesions in the model group is 10.171 mm
-2, indicating that the gastric ulcer model was successfully established. PM treated groups showed reduced gastric ulcers and visible bleeding spots. The areas of ulcerative lesions for PM-Lys, PM-Arg and PM-His treated groups have reduced to 3.166 mm
-2, 2.361 mm
-2 and 2.841 mm
-2, and the ulcer inhibition rate of those three groups was 72.7%, 79.3%, and 74.7%, respectively. As for ACM CAMs treated groups, ulcers and bleeding spots cannot be observed. These groups (ACM-Lys, ACM-Arg and ACM-His) had the smallest gastric ulcer areas of 1.108 mm
-2, 0.983 mm
-2, and 1.141 mm
-2, with ulcer inhibition rates of 89.7%, 91.7%, and 89.9%, respectively. The ulcer inhibition effects may attribute to the gastric protecting efficacy of basic amino acids. Lys could convert to aldehyde lysine under the catalyzed of lysine oxidase, which helps to protect the gastric mucosal barrier [
12,
13]. Arg generates nitric oxide under the action of vasodilatory synthase, thus maintaining the integrity of the gastric mucosal barrier and reducing mucosal damage [
14,
15]. His is an effective free radical scavenger and has preventive and mitigating effects on mucosal damage [
11,
16]. Above all, basic amino acids have the ability to maintain the integrity of the gastric mucosal barrier.
The results above demonstrated that the gastro-protective effects of the ACM CAMs treated groups are significantly better than that of the PMs treated groups (p<0.05). This may be due to the synchronized release of ACM and basic amino acids. According to the investigation of molecular interaction, ACM forms hydrogen bonds with amino acids. Thus, it could be speculated that ACM and basic amino acids were released and absorbed simultaneously, resulting in superior gastric mucosal protection and higher UIRs.
3.7.2. Histopathological Observation of the Stomach
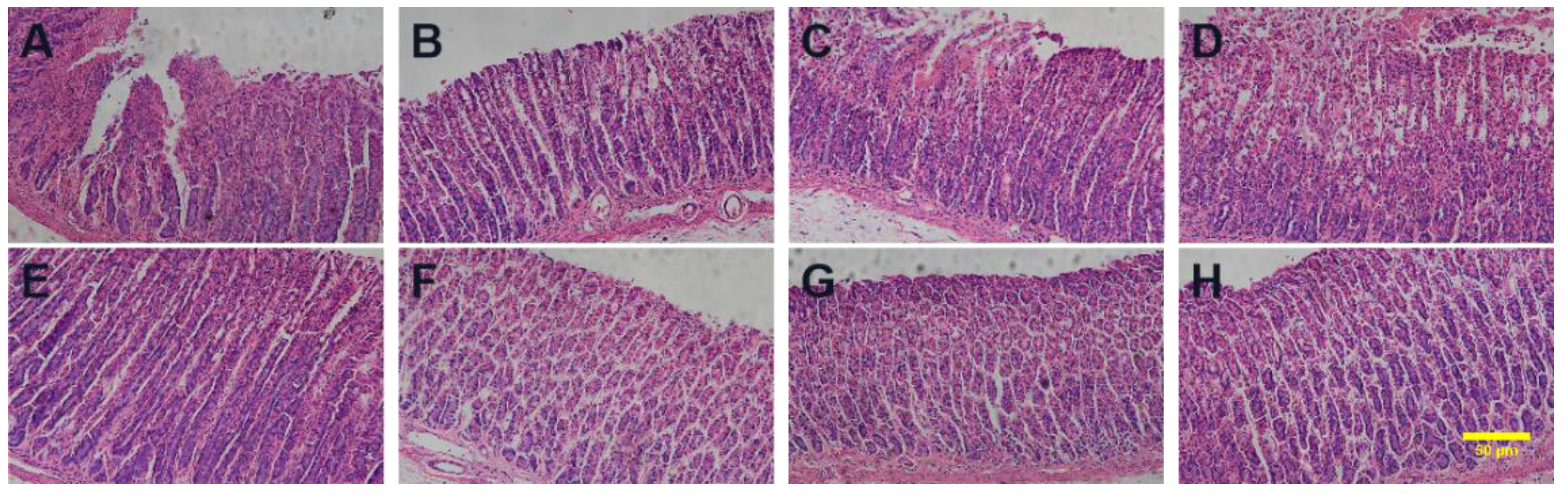
Gastric mucosal damage is a typical symptom of gastric ulcer. The histopathological observation of the gastric tissues is shown in
Figure 10. Normal gastric tissue has intact gastric mucosa but did not show edema in the submucosa, or epithelial cell detachment, congestion, or inflammatory cell infiltration. In ACM treated group, the gastric mucosa was damaged, with edema in the submucosa, mesenchymal congestion, and inflammatory cells infiltrated. In PMs treated groups, the intact gastric mucosa structure showed less congestion and inflammatory infiltration compared to ACM treated group. As for ACM CAM treated groups, the gastric tissue was intact, no damage was observed, and less congestion or inflammatory infiltration were shown, which is similar to that of the blank control group. The results showed that the ACM CAM treated group could almost eliminate the damage of ACM on the gastric tissue.
4. Conclusion
In this study, the feasibility of three basic amino acids (Lys, Arg and His) as co-formers forming CAMs with ACM was investigated. The results showed that ACM CAMs were formed through hydrogen bonds between ACM and amino acids, which were confirmed by the analysis of Tg, FTIR and molecular docking. The ACM CAMs showed increased dissolution and IDR significantly. Furthermore, ACM CAMs performed a great mitigation effect on the ACM-induced gastric ulcer with an UIR of up to almost 90%, probably due to the protective effect of basic amino acids on the gastric mucosa. Above all, this study improves adherence to the clinical use of ACM and provides a promising strategy for mitigating drug-induced side effects.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, Qiang Fu., Xiwei Jiang and Peixu Zhao.; methodology, Jiayue Hou.; validation, Jiayue Hou, Peixu Zhao and Yanfei Wang.; formal analysis, Peixu Zhao. and Yanfei Wang.; investigation, Peixu Zhao. and Jiayue Hou.; resources, Qiang Fu. and Xiwei Jiang.; writing—original draft preparation, Jiayue Hou.; writing—review and editing, Jiayue Hou. and Peixu Zhao; supervision, Qiang Fu. and Xiwei Jiang; project administration, Qiang Fu.; funding acquisition, Qiang Fu. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This work was supported by the Key Research Projects of the Liaoning Provincial Department of Education (JYTZD2023142), the Liaoning Revitalization Talents Program (XLYC2203044), the Ningxia Key Research and Invention Program (2021BEG02039), and the Outstanding Youth Lifting Program in the Shenyang Pharmaceutical University (YQ202113).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang: X., H. Xing, Y. Zhao and Z. Ma. “Pharmaceutical dispersion techniques for dissolution and bioavailability enhancement of poorly water-soluble drugs.” Pharmaceutics 10 (2018): <Go to ISI>://WOS:000447518800003. [CrossRef]
- Chang, C.-H., H.-C. Chen, J.-W. Lin, C.-W. Kuo, W.-Y. Shau and M.-S. Lai. “Risk of hospitalization for upper gastrointestinal adverse events associated with nonsteroidal anti-inflammatory drugs: A nationwide case-crossover study in taiwan.” Pharmacoepidemiology and Drug Safety 20 (2011): 763-71. <Go to ISI>://WOS:000293374600012. [CrossRef]
- Bori Segura, G., A. Torres y Gutierrez Rubio, L. E. Herrera Gomez and J. Olguin Uribe. “Efficacy and tolerability of acemetacin, a non-steroidal anti-inflammatory drug, in mexican patients: Result of the etapam study.” Proceedings of the Western Pharmacology Society 45 (2002): 104-7. <Go to ISI>://MEDLINE:12434547.
- Boltze, K. H., O. Brendler, H. Jacobi, W. Opitz, S. Raddatz, P. R. Seidel and D. Vollbrecht. “Chemical structure and anti-inflammatory activity in the group of substituted indole-3-acetic acids (author’s transl).” Arzneimittel-Forschung 30 (1980): 1314-25. <Go to ISI>://MEDLINE:7191301.
- Laitinen, R., K. Lobmann, C. J. Strachan, H. Grohganz and T. Rades. “Emerging trends in the stabilization of amorphous drugs.” International Journal of Pharmaceutics 453 (2013): 65-79. <Go to ISI>://WOS:000321741100008. [CrossRef]
- Liu, J., H. Grohganz, K. Lobmann, T. Rades and N.-J. Hempel. “Co-amorphous drug formulations in numbers: Recent advances in co-amorphous drug formulations with focus on co-formability, molar ratio, preparation methods, physical stability, in vitro and in vivo performance, and new formulation strategies.” Pharmaceutics 13 (2021): <Go to ISI>://WOS:000634066900001. [CrossRef]
- Kasten, G., H. Grohganz, T. Rades and K. Lobmann. “Development of a screening method for co-amorphous formulations of drugs and amino acids.” European Journal of Pharmaceutical Sciences 95 (2016): 28-35. <Go to ISI>://WOS:000390505100004. [CrossRef]
- Löbmann, K., R. Laitinen, C. Strachan, T. Rades and H. Grohganz. “Amino acids as co-amorphous stabilizers for poorly water-soluble drugs – part 2: Molecular interactions.” European Journal of Pharmaceutics and Biopharmaceutics 85 (2013): 882-88. [CrossRef]
- Löbmann, K., H. Grohganz, R. Laitinen, C. Strachan and T. Rades. “Amino acids as co-amorphous stabilizers for poorly water soluble drugs – part 1: Preparation, stability and dissolution enhancement.” European Journal of Pharmaceutics and Biopharmaceutics 85 (2013): 873-81. [CrossRef]
- Chambers, L. I., H. Grohganz, H. Palmelund, K. Löbmann, T. Rades, O. M. Musa and J. W. Steed. “Predictive identification of co-formers in co-amorphous systems.” European Journal of Pharmaceutical Sciences 157 (2021). [CrossRef]
- Cherkas, A., K. Zarkovic, O. Yelisyeyeva, A. Cipak, M. Jaganjac, O. Abrahamovych, O. Yatskevych, G. Waeg and N. Zarkovic. “Amaranth oil reduces accumulation of 4-hydroxynonenal-histidine adducts in gastric mucosa and improves heart rate variability in duodenal peptic ulcer patients undergoing <i>helicobacter pylori</i> eradication.” Free Radical Biology and Medicine 108 (2017): S86-S86. <Go to ISI>://WOS:000403716100242. [CrossRef]
- Sohail, R., M. Mathew, K. K. Patel, S. A. Reddy, Z. Haider, M. Naria, A. Habib, Z. U. Abdin, W. Razzaq Chaudhry and A. Akbar. “Effects of non-steroidal anti-inflammatory drugs (nsaids) and gastroprotective nsaids on the gastrointestinal tract: A narrative review.” Cureus (2023). [CrossRef]
- El Eter, E., H. H. Hagar, A. Al-Tuwaijiri and M. Arafa. “Nuclear factor-κb inhibition by pyrrolidinedithiocarbamate attenuates gastric ischemia-reperfusion injury in rats.” Canadian Journal of Physiology and Pharmacology 83 (2005): 483-92. <Go to ISI>://WOS:000231060900006. [CrossRef]
- Hung, C. R. and P. C. Hung. “Protective effects of several amino acid-nutrients on gastric hemorrhagic erosions in acid-irrigated stomachs of septic rats.” The Chinese journal of physiology 42 (1999): 161-9. <Go to ISI>://MEDLINE:10707890.
- Magierowski, M., K. Magierowska, S. Kwiecien and T. Brzozowski. “Gaseous mediators nitric oxide and hydrogen sulfide in the mechanism of gastrointestinal integrity, protection and ulcer healing.” Molecules 20 (2015): 9099-123. <Go to ISI>://WOS:000357157600097. [CrossRef]
- Thalacker-Mercer, A. E. and M. E. Gheller. “Benefits and adverse effects of histidine supplementation.” Journal of Nutrition 150 (2020): 2588S-92S. <Go to ISI>://WOS:000579421800014. [CrossRef]
- Baird, J. A. and L. S. Taylor. “Evaluation of amorphous solid dispersion properties using thermal analysis techniques.” Advanced Drug Delivery Reviews 64 (2012): 396-421. <Go to ISI>://WOS:000303032200003. [CrossRef]
- Sanphui, P., G. Bolla, A. Nangia and V. Chernyshev. “Acemetacin cocrystals and salts: Structure solution from powder x-ray data and form selection of the piperazine salt.” Iucrj 1 (2014): 136-50. <Go to ISI>://WOS:000356863800009. [CrossRef]
- Mishra, J., T. Rades, K. Löbmann and H. Grohganz. “Influence of solvent composition on the performance of spray-dried co-amorphous formulations.” Pharmaceutics 10 (2018). [CrossRef]
- Li, W., J. Song, J. Li, M. Li, B. Tian, Z. He, X. Liu and Q. Fu. “Co-amorphization of atorvastatin by lisinopril as a co-former for solubility improvement.” International Journal of Pharmaceutics 607 (2021). [CrossRef]
- Heinz, A., C. J. Strachan, K. C. Gordon and T. Rades. “Analysis of solid-state transformations of pharmaceutical compounds using vibrational spectroscopy.” Journal of Pharmacy and Pharmacology 61 (2009): 971-88. [CrossRef]
- Wei, Y., S. Zhou, T. Hao, J. Zhang, Y. Gao and S. Qian. “Further enhanced dissolution and oral bioavailability of docetaxel by coamorphization with a natural p-gp inhibitor myricetin.” European Journal of Pharmaceutical Sciences 129 (2019): 21-30. <Go to ISI>://WOS:000456591300003. [CrossRef]
- Jensen, K. T., L. I. Blaabjerg, E. Lenz, A. Bohr, H. Grohganz, P. Kleinebudde, T. Rades and K. Löbmann. “Preparation and characterization of spray-dried co-amorphous drug–amino acid salts.” Journal of Pharmacy and Pharmacology 68 (2016): 615-24. [CrossRef]
Figure 2.
The PLM images of (A) ACM, (B) PM-Lys, (C) PM-Arg, (D) PM-His, (E) AM, (F) CAM-Lys, (G) CAM-Arg, and (H) CAM-His. The scale bar represents 100 μm.
Figure 2.
The PLM images of (A) ACM, (B) PM-Lys, (C) PM-Arg, (D) PM-His, (E) AM, (F) CAM-Lys, (G) CAM-Arg, and (H) CAM-His. The scale bar represents 100 μm.
Figure 3.
PXRD Patterns of (a) ACM-AM, (b) ACM, (c) ACM-Lys, (d) PM-Lys, (e) ACM-Arg, (f) PM-Arg, (g) ACM-His, and (h) PM-His.
Figure 3.
PXRD Patterns of (a) ACM-AM, (b) ACM, (c) ACM-Lys, (d) PM-Lys, (e) ACM-Arg, (f) PM-Arg, (g) ACM-His, and (h) PM-His.
Figure 4.
DSC thermograms (A) and the second heating DSC curves (B) of the ACM-His, ACM-Lys, ACM-Arg, PM-His, PM-Lys, PM-Arg and ACM.
Figure 4.
DSC thermograms (A) and the second heating DSC curves (B) of the ACM-His, ACM-Lys, ACM-Arg, PM-His, PM-Lys, PM-Arg and ACM.
Figure 5.
FTIR spectra of (a) ACM-AM, (b) ACM, (c) ACM-Lys, (d) PM-Lys, (e) ACM-Arg, (f) PM-Arg, (g) ACM-His, and (h) PM-His.
Figure 5.
FTIR spectra of (a) ACM-AM, (b) ACM, (c) ACM-Lys, (d) PM-Lys, (e) ACM-Arg, (f) PM-Arg, (g) ACM-His, and (h) PM-His.
Figure 6.
Molecular docking of the interaction between ACM and (A) Lys, (B) Arg, and (C) His. Red represents oxygen atoms, blue for nitrogen atoms, grey for hydrogen atoms, and the green dotted lines indicate hydrogen bonds formed between atoms.
Figure 6.
Molecular docking of the interaction between ACM and (A) Lys, (B) Arg, and (C) His. Red represents oxygen atoms, blue for nitrogen atoms, grey for hydrogen atoms, and the green dotted lines indicate hydrogen bonds formed between atoms.
Figure 7.
Dissolution profiles of ACM, ACM-AM, PMs and ACM CAMs.
Figure 7.
Dissolution profiles of ACM, ACM-AM, PMs and ACM CAMs.
Figure 8.
IDR curves (A) and the normalized dissolution rates (B) of ACM, ACM-AM, PMs and ACM CAMs (n = 3, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs ACM and #### p < 0.0001 vs PMs groups).
Figure 8.
IDR curves (A) and the normalized dissolution rates (B) of ACM, ACM-AM, PMs and ACM CAMs (n = 3, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs ACM and #### p < 0.0001 vs PMs groups).
Figure 9.
Stomach anatomy images (A) and inhibition rate (B) of the (a) ACM, (b) PM-Lys, (c) PM-Arg, (d) PM-His, (e) PBS, (f) CAM-Lys, (g) CAM-Arg, and (h) CAM-His. The scale bar represents 1 cm (n = 4, ****p < 0.0001 vs ACM and ##p < 0.001 vs PMs groups).
Figure 9.
Stomach anatomy images (A) and inhibition rate (B) of the (a) ACM, (b) PM-Lys, (c) PM-Arg, (d) PM-His, (e) PBS, (f) CAM-Lys, (g) CAM-Arg, and (h) CAM-His. The scale bar represents 1 cm (n = 4, ****p < 0.0001 vs ACM and ##p < 0.001 vs PMs groups).
Figure 10.
Histopathological examinations of the (A) ACM, (B) PM-Lys, (C) PM-Arg, (D) PM-His, (E) PBS, (F) CAM-Lys, (G) CAM-Arg, and (H) CAM-His. The scale bar represents 50 μm.
Figure 10.
Histopathological examinations of the (A) ACM, (B) PM-Lys, (C) PM-Arg, (D) PM-His, (E) PBS, (F) CAM-Lys, (G) CAM-Arg, and (H) CAM-His. The scale bar represents 50 μm.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).