Submitted:
01 May 2024
Posted:
02 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
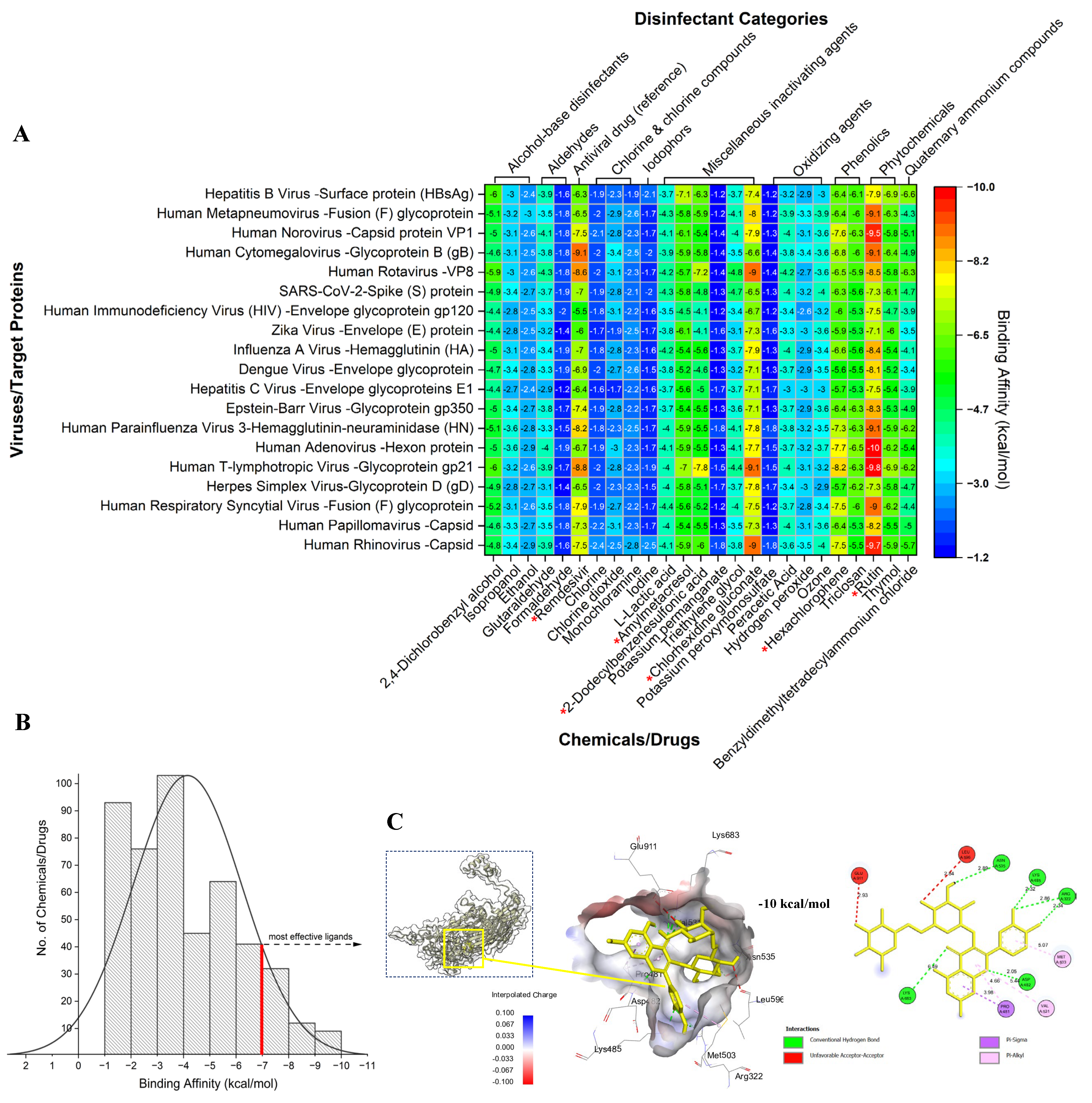
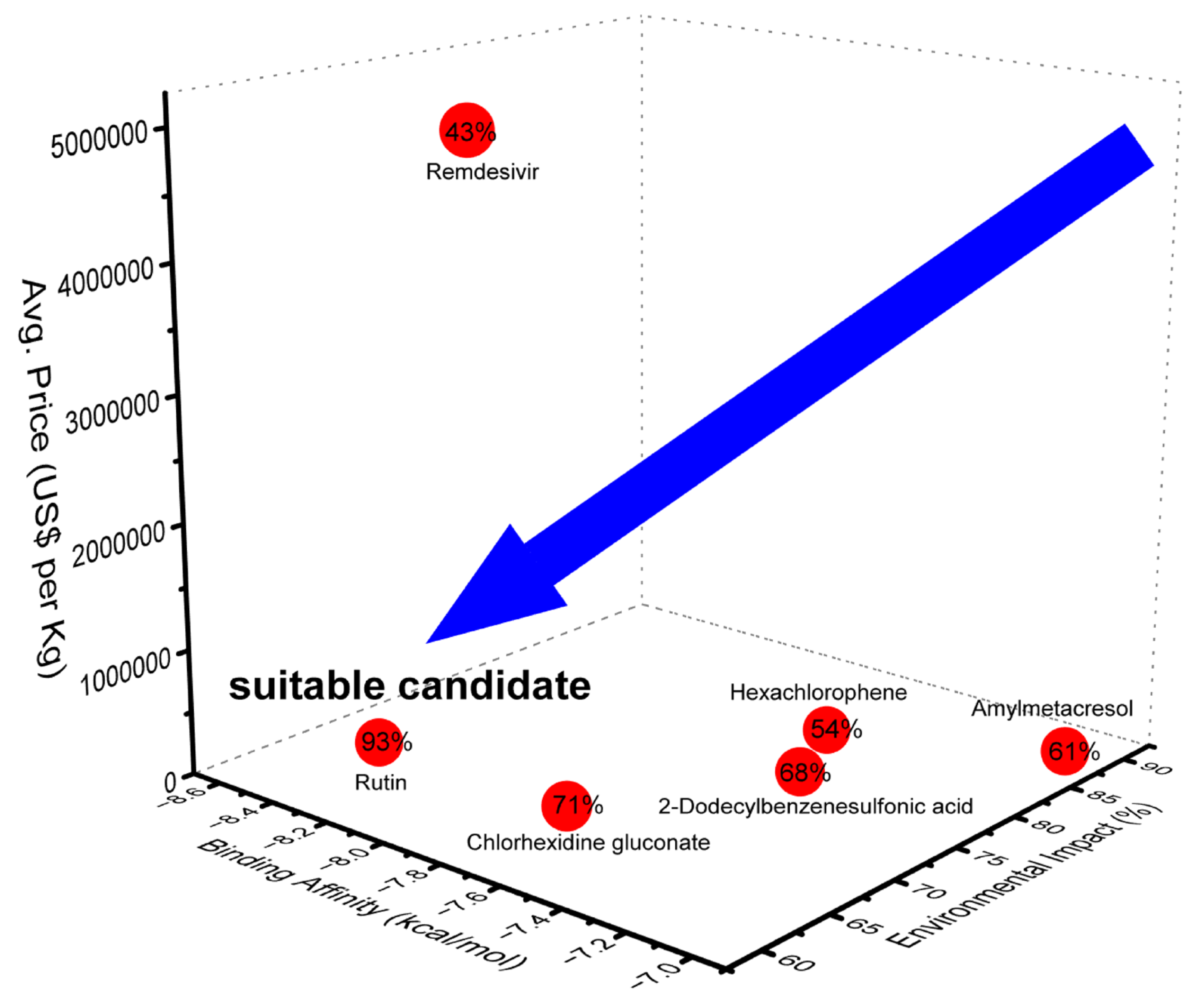
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, J.; Ma, X.; Xu, X.; Guo, Y.; Li, B.; Wang, M.; Wang, Y. Zika Virus Infection and Development of Drug Therapeutics. Appl Microbiol 2022, 2, 782–799. [Google Scholar] [CrossRef]
- Ouyang, Y.; Chen, Y.; Shang, J.; Sun, S.; Wang, X.; Huan, S.; Xiong, B.; Zhang, X.-B. Virus-like Plasmonic Nanoprobes for Quick Analysis of Antiviral Efficacy and Mutation-Induced Drug Resistance. Anal Chem 2023, 95, 5009–5017. [Google Scholar] [CrossRef] [PubMed]
- Sangkham, S. A Review on Detection of SARS-CoV-2 RNA in Wastewater in Light of the Current Knowledge of Treatment Process for Removal of Viral Fragments. J Environ Manage 2021, 299, 113563. [Google Scholar] [CrossRef] [PubMed]
- Adwani, C. Antimalarial and Antiviral Drugs: A Review of Trials and Effectiveness in Treating COVID-19. Biosci Biotechnol Res Commun 2021, 14, 231–236. [Google Scholar] [CrossRef]
- Clarridge, K.E.; Blazkova, J.; Einkauf, K.; Petrone, M.; Refsland, E.W.; Justement, J.S.; Shi, V.; Huiting, E.D.; Seamon, C.A.; Lee, G.Q.; et al. Effect of Analytical Treatment Interruption and Reinitiation of Antiretroviral Therapy on HIV Reservoirs and Immunologic Parameters in Infected Individuals. PLoS Pathog 2018, 14, e1006792. [Google Scholar] [CrossRef]
- Fischer, W.A.; Uyeki, T.M.; Tauxe, R.V. Ebola Virus Disease: What Clinicians in the United States Need to Know. Am J Infect Control 2015, 43, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Comunale, B.A.; Larson, R.J.; Jackson-Ward, E.; Singh, A.; Koback, F.L.; Engineer, L.D. The Functional Implications of Broad Spectrum Bioactive Compounds Targeting RNA-Dependent RNA Polymerase (RdRp) in the Context of the COVID-19 Pandemic. Viruses 2023, 15, 2316. [Google Scholar] [CrossRef]
- Wang, R.; Luo, J.; Li, C.; Chen, J.; Zhu, N. Antiviral Drugs in Wastewater Are on the Rise as Emerging Contaminants: A Comprehensive Review of Spatiotemporal Characteristics, Removal Technologies and Environmental Risks. J Hazard Mater 2023, 457, 131694. [Google Scholar] [CrossRef]
- Ashokkumar, S.; Kaushik, N.K.; Han, I.; Uhm, H.S.; Park, J.S.; Cho, G.S.; Oh, Y.-J.; Shin, Y.O.; Choi, E.H. Persistence of Coronavirus on Surface Materials and Its Control Measures Using Nonthermal Plasma and Other Agents. Int J Mol Sci 2023, 24, 14106. [Google Scholar] [CrossRef]
- Zure, D.; David Kuo, H.-W.; Drizo, A. Insights of Phytoremediation Mechanisms for Viruses Based on In-Vitro, in-Vivo and in-Silico Assessments of Selected Herbal Plants. Chemosphere 2024, 351, 141101. [Google Scholar] [CrossRef]
- Kuo, H.-W.D.; Zure, D.; Lin, C.-R. Occurrences of Similar Viral Diversity in Campus Wastewater and Reclaimed Water of a University Dormitory. Chemosphere 2023, 330, 138713. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Estimation Programs Interface SuiteTM for Microsoft® Windows, v 4.11. United States Environmental Protection Agency: Washington, DC 2024. https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface.
- Castaño, N.; Cordts, S.C.; Kurosu Jalil, M.; Zhang, K.S.; Koppaka, S.; Bick, A.D.; Paul, R.; Tang, S.K.Y. Fomite Transmission, Physicochemical Origin of Virus–Surface Interactions, and Disinfection Strategies for Enveloped Viruses with Applications to SARS-CoV-2. ACS Omega 2021, 6, 6509–6527. [Google Scholar] [CrossRef] [PubMed]
- Marquès, M.; Domingo, J.L. Contamination of Inert Surfaces by SARS-CoV-2: Persistence, Stability and Infectivity. A Review. Environ Res 2021, 193, 110559. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Fraser, K.; Kuo, L.; Chauhan, N.; Adrian, A.T.; Zhang, F.; Linhardt, R.J.; Kwon, P.S.; Wang, X. Designer DNA Nanostructures for Viral Inhibition. Nat Protoc 2022, 17, 282–326. [Google Scholar] [CrossRef] [PubMed]
- Borisevich, S.S.; Zarubaev, V.V.; Shcherbakov, D.N.; Yarovaya, O.I.; Salakhutdinov, N.F. Molecular Modeling of Viral Type I Fusion Proteins: Inhibitors of Influenza Virus Hemagglutinin and the Spike Protein of Coronavirus. Viruses 2023, 15, 902. [Google Scholar] [CrossRef]
- Dassanayake, M.K.; Khoo, T.-J.; Chong, C.H.; Di Martino, P. Molecular Docking and In-Silico Analysis of Natural Biomolecules against Dengue, Ebola, Zika, SARS-CoV-2 Variants of Concern and Monkeypox Virus. Int J Mol Sci 2022, 23, 11131. [Google Scholar] [CrossRef] [PubMed]
- Ghildiyal, R.; Prakash, V.; Chaudhary, V.K.; Gupta, V.; Gabrani, R. Phytochemicals as Antiviral Agents: Recent Updates. In Plant-derived Bioactives; Springer Singapore: Singapore, 2020; pp. 279–295. [Google Scholar] [CrossRef]
- Kim, E.-H.; Lee, B.W.; Ryu, B.; Cho, H.M.; Kim, S.-M.; Jang, S.-G.; Casel, M.A.B.; Rollon, R.; Yoo, J.-S.; Poo, H.; et al. Inhibition of a Broad Range of SARS-CoV-2 Variants by Antiviral Phytochemicals in HACE2 Mice. Antiviral Res 2022, 204, 105371. [Google Scholar] [CrossRef] [PubMed]
- Umar, Abd. K.; Zothantluanga, J.H.; Aswin, K.; Maulana, S.; Sulaiman Zubair, M.; Lalhlenmawia, H.; Rudrapal, M.; Chetia, D. Antiviral Phytocompounds “Ellagic Acid” and “(+)-Sesamin” of Bridelia Retusa Identified as Potential Inhibitors of SARS-CoV-2 3CL pro Using Extensive Molecular Docking, Molecular Dynamics Simulation Studies, Binding Free Energy Calculations, and Bioactivity Prediction. Struct Chem 2022, 33, 1445–1465. [Google Scholar] [CrossRef]
- Farooq, S.; Ngaini, Z. Natural and Synthetic Drugs as Potential Treatment for Coronavirus Disease 2019 (COVID-2019). Chemistry Africa 2021, 4, 1–13. [Google Scholar] [CrossRef]
- Spengler, J.R.; Welch, S.R.; Deval, J.; Gentry, B.G.; Brancale, A.; Carter, K.; Moffat, J.; Meier, C.; Seley-Radtke, K.L.; Schang, L.M. Meeting Report: 35th International Conference on Antiviral Research in Seattle, Washington, USA–March 21–25, 2022. Antiviral Res 2023, 211, 105521. [Google Scholar] [CrossRef]
- Eryildiz, B.; Ozgun, H.; Ersahin, M.E.; Koyuncu, I. Antiviral Drugs against Influenza: Treatment Methods, Environmental Risk Assessment and Analytical Determination. J Environ Manage 2022, 318, 115523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, M.; Wang, Q.; Yang, H. The Combined Use of Copper Sulfate and Trichlorfon Exerts Stronger Toxicity on the Liver of Zebrafish. Int J Mol Sci 2023, 24, 11203. [Google Scholar] [CrossRef] [PubMed]
- Zalazar-García, D.; Torres, E.; Rodriguez-Ortiz, L.; Deng, Y.; Soria, J.; Bucalá, V.; Rodriguez, R.; Mazza, G. Cleaner and Sustainable Processes for Extracting Phenolic Compounds from Bio-Waste. J Environ Manage 2020, 273, 111154. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




