Introduction
Universality of ultrasound examination and the availability of devices, as well as the popularization of the point of care ultrasonography (POCUS) examination lung ultrasound (LUS) is rapidly evolving diagnostic modality available for the diagnosis of the most common pathologies in the lungs. In veterinary medicine LUS is not well studied, and there are only a few studies describing the utility of LUS for the diagnosis of parenchymal lung diseases associated with lung consolidations in small animal patient [
1,
2,
3,
4]. There is no information in veterinary medicine about color doppler analysis of the consolidation and the impact of doppler findings to clinical decision except one case report [
5]. This imaging modality has been thoroughly studied in human medicine and is considered an important tool for increasing the diagnostic accuracy in dyspneic patients [
6]. According to newly published guidelines, LUS it’s the first diagnostic approach in patients with acute dyspnea, should include thoracic ultrasound [
7,
8,
9]. It allows differential diagnosis between potentially life-threatening conditions such as pulmonary edema and bronchopneumonia, including moderate, severe, and critical lung injury[
6,
10,
11]. LUS plays an important role in many fields, from emergency and intensive care through cardiology, internal medicine, pediatrics, and neonatology [
12,
13]. Its sensitivity and specificity are comparable with computed tomography (CT) of the chest and exceed that of thoracic radiography (TXR) [
13]. Guidelines and publication about adults and children suggest that color doppler analysis can be beneficial if it’s possible to perform [
8,
14,
15,
16,
17]. In veterinary medicine LUS is used as a POCUS and it’s a part of an emergency protocols for cats and dogs like VETBLUE
®, TFAST
® or CALGARY PLUS and rodents RATTUS [
18,
19,
20,
21]. The emergency protocols are based on the shape of the consolidation and presence or absence of vertical artefacts without Doppler analysis of the consolidation [
18]. In this retrospective study consolidation was analyzed using color Doppler, the so-called vascular criterion and the parenchymal criteria [
13]. The analysis of mentioned criteria had an impact on subsequent diagnostic and therapeutic decisions [
15].
Materials and Methods.
Between May 2018-2023 in “Vetcardia” veterinary cardiology clinic in Warsaw 45148 echocardiography and non-cardiac thoracic ultrasound was performed. In those examination lung consolidations were find 2157 cases of dogs, cats, guinea pigs, ferrets, and rabbits. From all cases 1066 with the best quality descriptions of consolidation and confirmed diagnosis was chosen. We exclude rabbits, ferrets and guinea pigs to have more homogeneous group and analysis was made on 981 animals, 347 cats and 634 dogs different breeds presented in
Table 1. Echocardiography and LUS were performed by 6 veterinarians, 3 investigators with >15 years of experience in echocardiography (including KK, MG) and trained in LUS by human specialist (NB) 2 investigators >5 years in echocardiography and 1 investigator >2 years also trained by human specialist (NB).
All examinations we performed on machine GE VIVID IQ. Consolidations were examine using multifrequency linear probe. Two presets were used during the examination, first special “lung preset”. This preset has harmonics turned off, uses the lowest frequency of the probe (6 MHz) with persistence turned to zero, has a focal position set at the level of the pleural line, and uses an increased time gain compensation (TGC) at the distal (far) field of the screen [
22,
23,
24]. Such settings create a “coarser” picture color doppler analysis of the consolidations was performed on this preset, with the Nyquist limit set at the level of 0.7m/s. For better visualization of consolidations, the preset was switched to a default “thyroid” preset. “Thyroid” preset has harmonics turned on, uses a higher dynamic range, and has spatial compound imaging turned on, resulting in a “smoother” image for a better depiction of the consolidation; the focus was set at the center of the consolidation. When using color doppler, the sector size was adjusted to the size of consolidation or its selected fragment to eliminate noise resulting from respiratory artifacts.
The lung ultrasound examination followed VetLus protocol [
5]. It utilizes a horizontal sliding technique with the transducer placed at three different vertical locations on each side of the thorax at a level in line with: the middle of the scapula below the rib heads and epaxial muscles (dorsal line); the shoulder joint (at the level of the heart base, middle line); and just dorsal to sternebrae (ventral line). In this protocol the probe was rotated 90 degrees into a transverse plane to give an image of a continuous pleural line without rib shadows, which were visible in the frontal plane [
25]. That can be beneficial if part of the consolidation hides in the area where the ultrasonic beam is attenuated by the shadows of the ribs [
5,
25,
26].
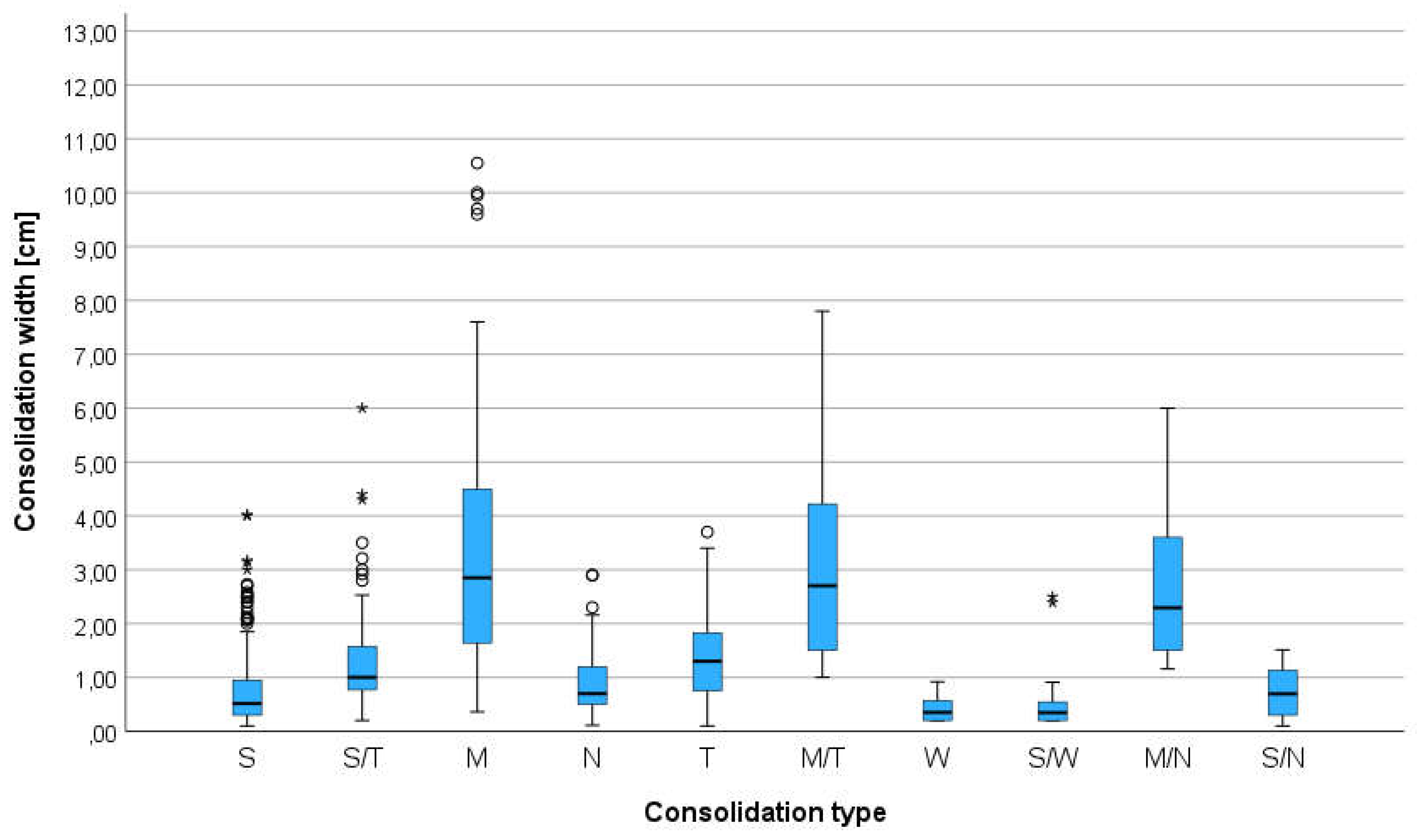
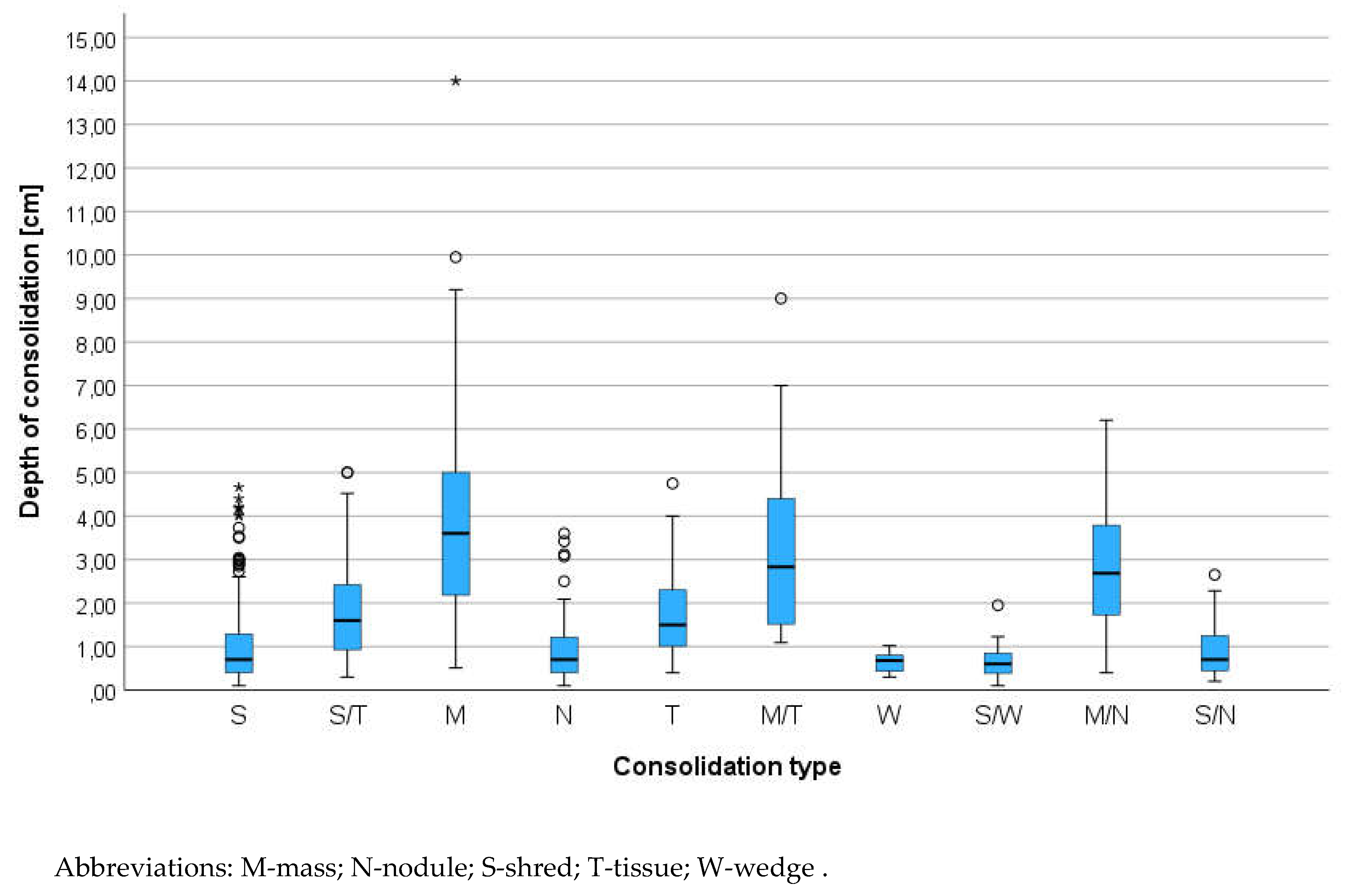
The animals were examined in the sternal or side lying position, standing or sitting, depending on the severity of dyspnea. The alcohol and gel were used as a coupling agent. All consolidation were classified using two criteria: parenchymal and vascular [
5,
27,
28]. The consolidation was classified by size - width and depth. Width was the diameter parallel to the pleura line and depth was the distance between the pleura line and the far border of consolidation. The consolidations were classified by operators to different category of shape relay on previous publication from the human and veterinary medicine: shred (S), tissue (T), wedge (W), nodule (N) and additional category mass (M)[
18,
29]. Nodule we described as round hypoechogenic consolidation in contrast to mass which was also the same shape consolidation but with heterogeneous echogenicity. In some cases, where few types of consolidation were found and they were classified as complex findings for example: tissue and mass (T/M), shred and nodule (S/N) and other combinations presented in
Table 2. Consolidations were described with presence and type of bronchograms. Types of bronchogram include dynamic air bronchogram (Db), static air bronchogram (Sb) and fluid bronchogram (Fb) additional type was absence of bronchogram (Ab). Dynamic air bronchogram is visible in inspiration and disappears on expiration, static air bronchogram represents the presence of air in the bronchial tree and is visible though all breathing phase, fluid bronchogram presence the fluid in bronchial tree [
8]. Absence of bronchogram was described when there was no visible anatomical structure of the bronchus in the consolidation. In some cases, were few types of bronchogram were described in one consolidation then in the statistical analysis they were described like complex findings for example: dynamic and static air bronchogram (Db/Sb), fluid and absence of bronchogram (Fb/Ab) and other combinations presented in
Table 3.
The color doppler analysis based on the presence or absence of the blood flow in consolidation and the shape of the vascularization [
30,
31]. In consolidations five types of vascularization was found: anatomical tree (Tv) like vascularization - Figure 1, Movie 1, residual vascularization (Rv) -
Figure 2, Movie 2, chaotic (Hv) vascularization –
Figure 3, vascularization penetrating (Pv) inside the consolidation from the chest wall – Movie 3 and amputation of the blood flow so called “
vascular sign” (Vs) – Movie 4 [
5,
15,
17,
27,
28,
31]. The “
vascular sign” is believed to result from the occlusion of a vessel by embolic material [
5,
31,
32]. Abrupt cessation of blood flow at the “tip” of the consolidation can be seen in color doppler. For differentiation the residual from the chaotic vascularization in some cases the pulsed wave doppler was performed [
17]. Chaotic vascularization in pulsed wave doppler in human medicine is low resistant continuous flow (
Figure 4), pulmonary artery or bronchial artery flow is high resistant and coordinated with the heart rate [
16,
28,
30] (
Figure 5).
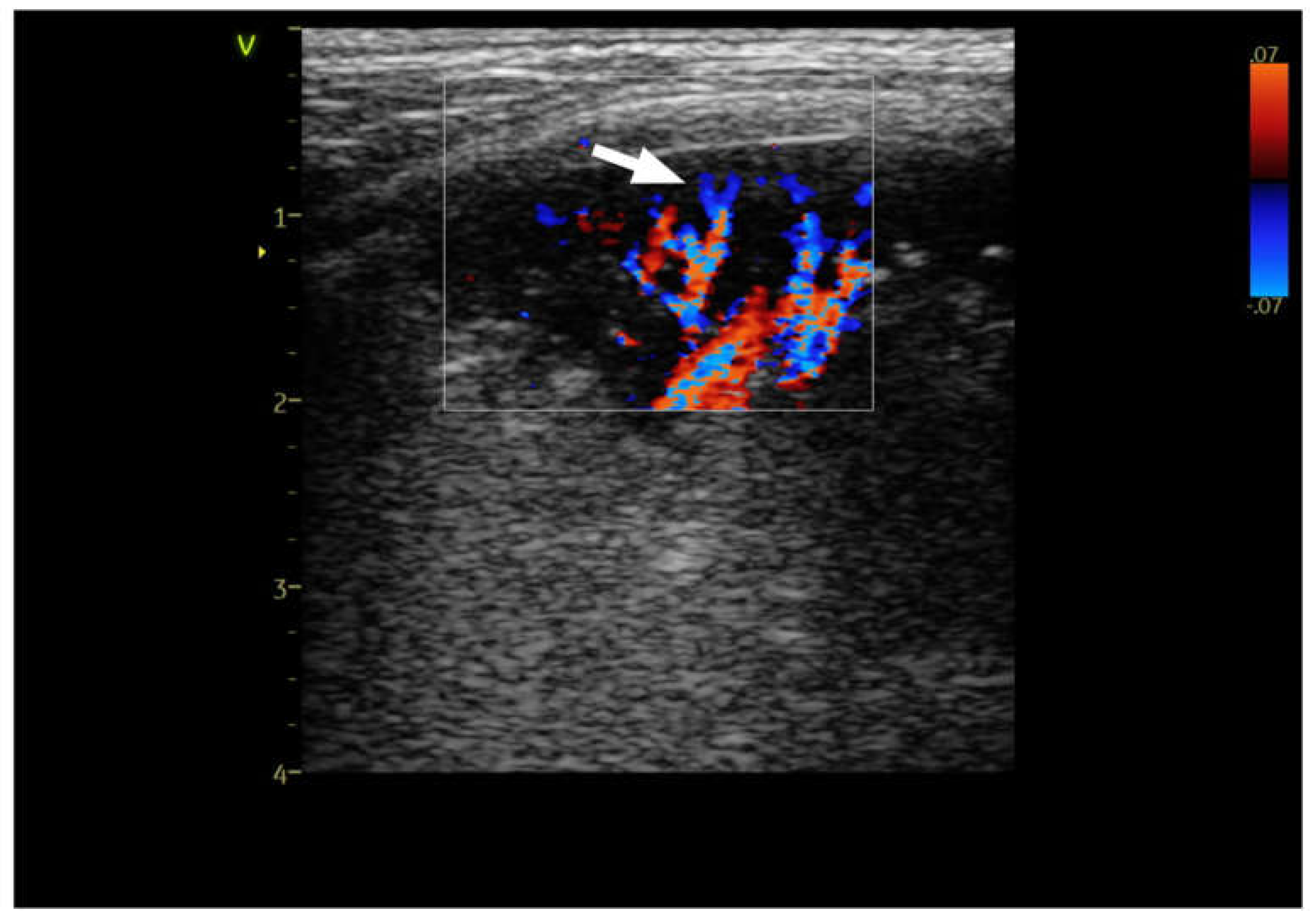
Figure 1. Linear probe, lung preset. Still image, color doppler imaging of the anatomical tree like vascularisation (→) in dog with pneumonia.
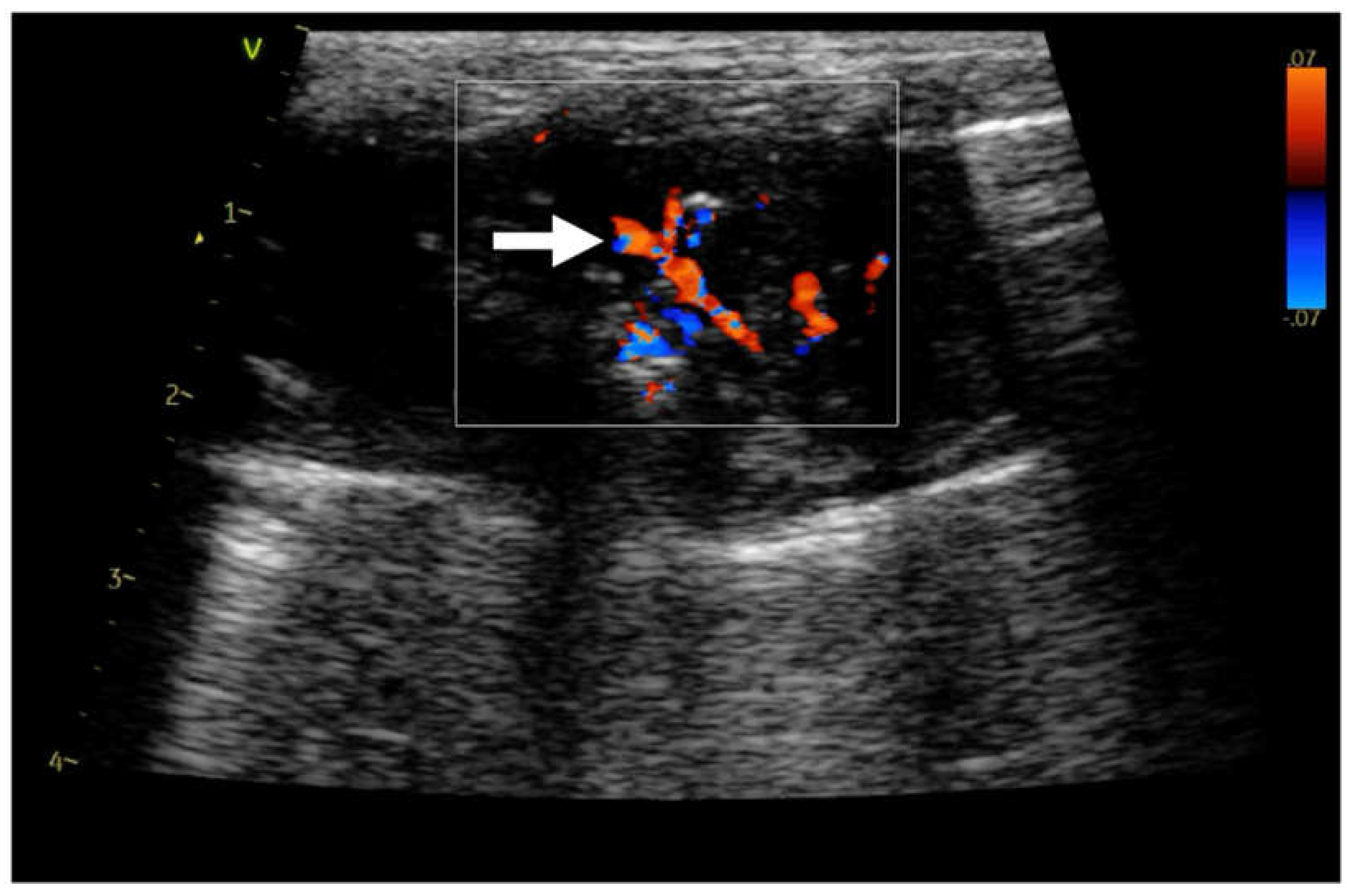
Figure 2.
Linear probe, lung preset. Still image, color doppler imaging of the residual vascularisation (→) in consolidation.
Figure 2.
Linear probe, lung preset. Still image, color doppler imaging of the residual vascularisation (→) in consolidation.
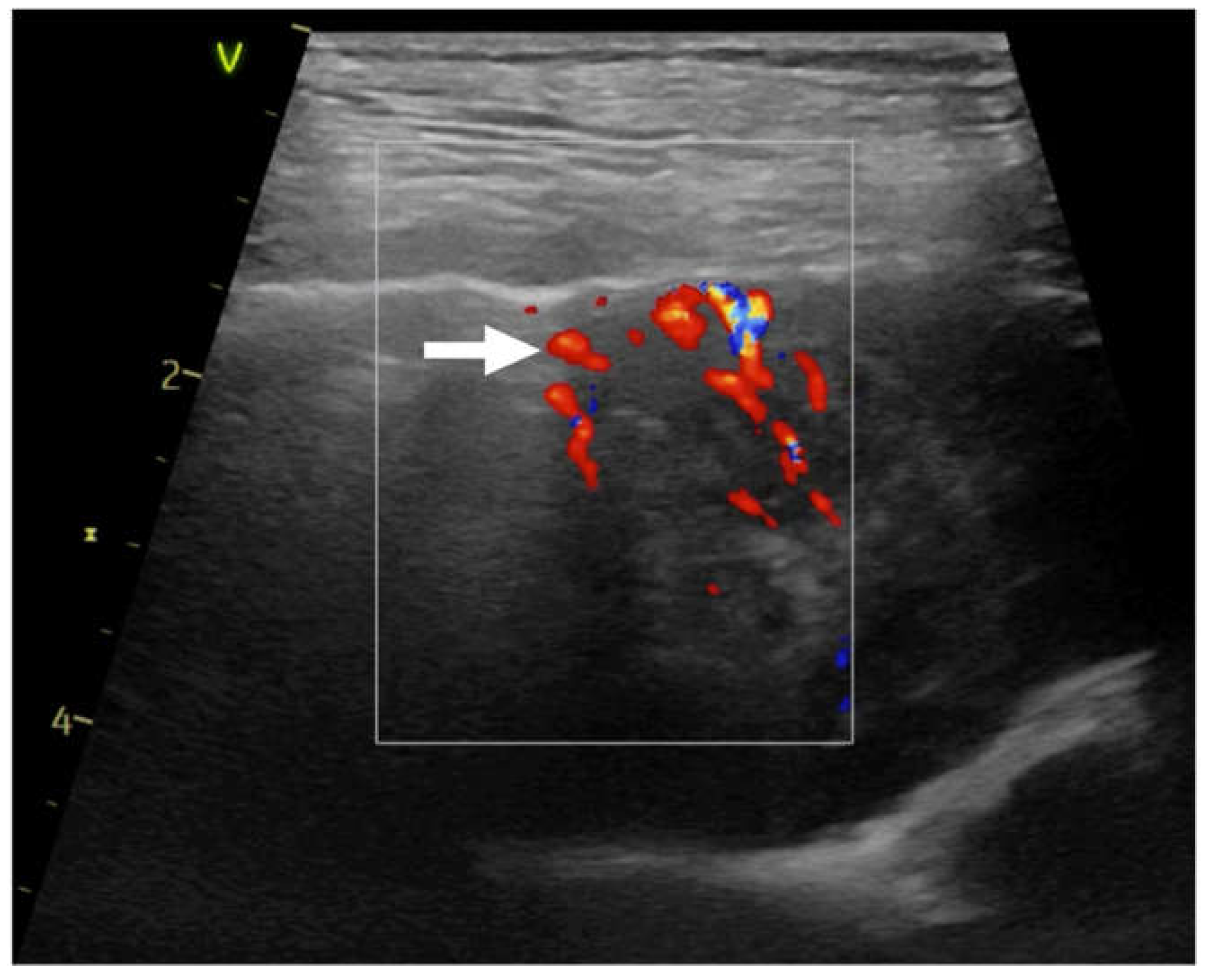
Figure 3.
Linear probe, thyroid preset. Still image, color doppler imaging of the chaotic vascularisation (→) in consolidation.
Figure 3.
Linear probe, thyroid preset. Still image, color doppler imaging of the chaotic vascularisation (→) in consolidation.
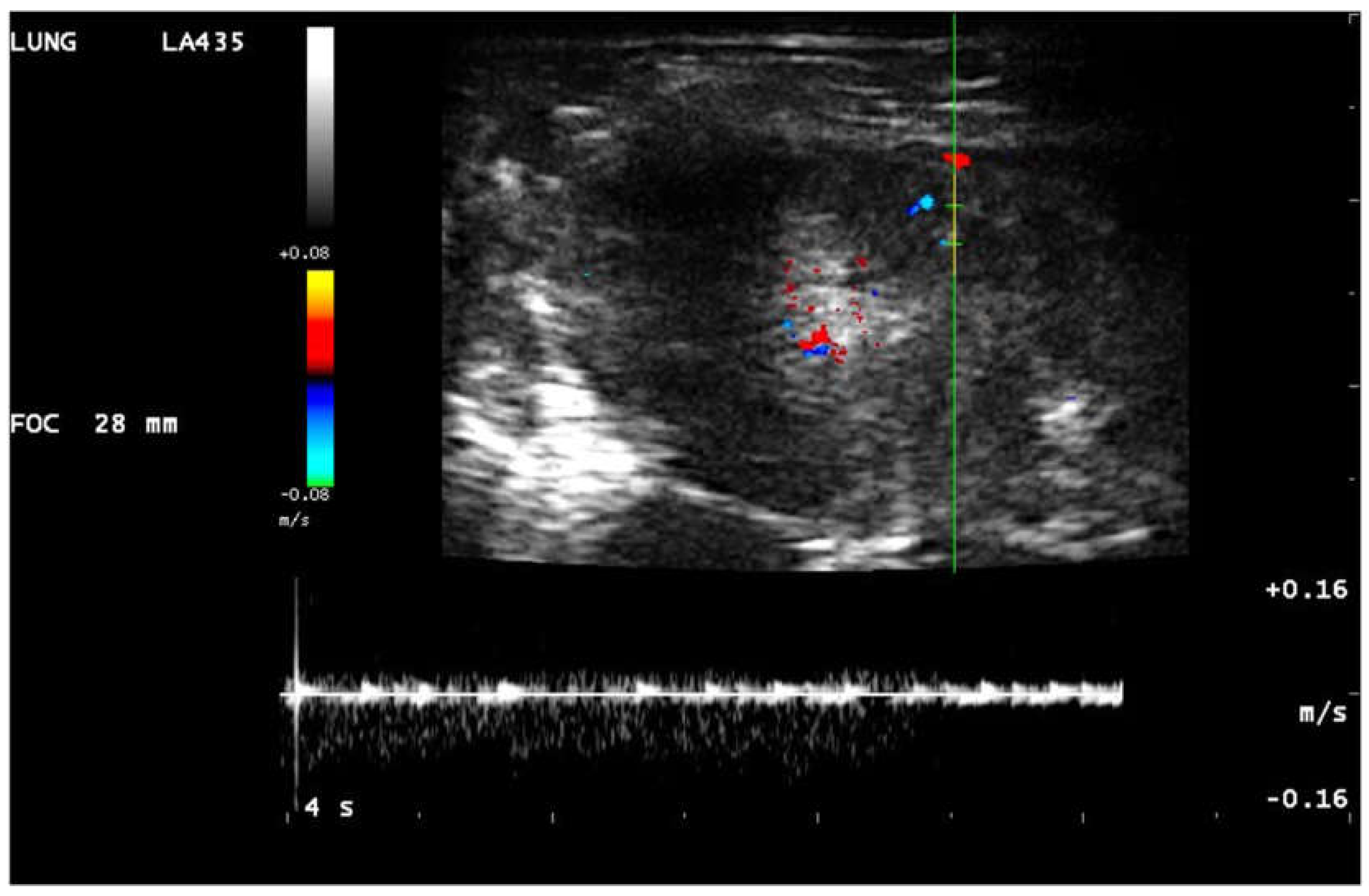
Figure 4.
Linear probe, lung preset. Still image, color doppler and pulsed wave doppler imaging of the flow in consolidation. Neovascularisation.
Figure 4.
Linear probe, lung preset. Still image, color doppler and pulsed wave doppler imaging of the flow in consolidation. Neovascularisation.
Figure 5.
Linear probe, lung preset. Still image, color doppler and pulsed wave doppler imaging of the flow in consolidation. Arterial type of vascularisation.
Figure 5.
Linear probe, lung preset. Still image, color doppler and pulsed wave doppler imaging of the flow in consolidation. Arterial type of vascularisation.
Movie 1.
Linear probe, lung preset. Color doppler imaging of the anatomical tree like vascularisation in dog with pneumonia.
Movie 1.
Linear probe, lung preset. Color doppler imaging of the anatomical tree like vascularisation in dog with pneumonia.
Movie 2. Linear probe, lung preset. Color doppler imaging of the residual vascularisation in consolidation.
Movie 3. Linear probe, thyroid preset. Color doppler imaging of the vascularisation penetrating from the chest wall.
Movie 4. Linear probe, Lung preset. Color doppler imaging abrupt cessation of blood flow at the “tip” of the consolidation “vascular sign” (→), C-line (←) hypoechoic triangular shape consolidation (↓).
The localization of the consolidations was also described by operators - left or right side of the chest and to the statistical analysis the lung area was divided in three zones: cranial, medial and caudal. The final diagnosis like pneumonia, parenchymal lung disease (PLD), tumor, metastasis, granuloma, abscess, acute respiratory distress syndrome (ARDS), pulmonary thromboembolic (PTE) congestive heart failure (CHF), atelectasis was confirmed by one or combination of the additional test or different diagnostic imaging method like: echocardiography, TXR, CT, bronchoscopy, biopsy, histopathology, bacteriology, complete blood count, C-reactive protein-dimer, positive reaction for antibiotic and autopsy.
Discussion
The etiologies of pulmonary consolidation are diverse, and a vascular morphological and hemodynamic changes had been investigated in human medicine. Pulmonary vascularization in animals and humans has organ-specific characteristic due to dual arterial supply of the lung [
30,
35]. Pulmonary arteries show a treelike pattern, their branches centrifugally course, and react to hypoxia by vasoconstriction induced by the Euler-Liljestrand reflex [
30] what can explain residual blood flow in atelectasis [
35,
36]. The TXR cannot demonstrate the vessel in consolidated lung and CT is still expensive and difficult-to-access method compared to common ultrasonography. In this retrospective study the different hemodynamic changes seen in different types of consolidations plus the bronchogram findings make it possible to distinguish pneumonia from atelectasis, tumor or PTE. From the clinical point of view information about vascularity, type of bronchogram or its absence in consolidation have clinical impact and often influence further diagnostic decisions.
Retrospective analysis of changes in the lungs show us that we can have more than one type of consolidations and some of them especially this one with bigger diameter can be not homogenic in their structure. This echo structure sometimes can be more helpful for clinician than TRX.
Anatomical tree like vascularization was found in animals with suspicion on pneumonia. The shape of the consolidation was irregular with presence of the C-line - comet-tail artifacts, extending from the far edge of these consolidations. The blood flow in color doppler appears and disappears with the breathing faze. Residual vascularization was found in atelectasis. The vascularization was reduced because of the mass above the atelectasis what compress the vessels, vasoconstriction in long term pneumonia or big amount of the fluid in chest. In consolidation the fragment of the anatomical tree was visible and disappears. In tumors the residual vascularization or chaotic neovascularization was present but there was no air or fluid bronchogram. The high oxygen blood is often used by tumors for better nutrition [
35]. In our findings some of the tumors had residual vascularization and high resistance flow but it was difficult to differentiate the origin from pulmonary artery or bronchial artery. Similar findings were published in contrast inhaled ultrasonography [
35,
36].The anatomical structures in tumors were destroyed in some individuals with presence of small hyperechoic round dots – calcifications. The fourth type is present when the consolidation in lung infiltrates the chest wall. The vessels from the chest wall penetrating to the consolidation as the intercostal arteries are high oxygenate blood [
37]. This type was observed in tumors infiltrate the chest wall. The last observe patten is a “
vascular sign.” The “
vascular sign” is believed to result from the occlusion of a vessel by embolic material. Abrupt cessation of blood flow at the “tip” of the consolidation can be seen using color doppler. The consolidation is usually triangular or basket shape and hypoechogenic in literature called “wedge sign”. The “
vascular sign” was present in dogs with infection of
Angiostrongylus vasorum [
5], cats with deferent types cardiomyopathy with spontaneous echocontrast in right atrium. In 1 case the thromboembolic lung disease was confirmed on postmortem examination. “Vascular sign” was present also in 7 Yorkshire terriers with severe dyspnea without final diagnosis as the dogs were euthanized because of no response for treatment. All the dogs were treated also for Cushing disease.
A general disadvantage of LUS is that lesions that fail to reach the lung periphery will not be detected, because lung pathology separated from the lung surface by air will be obscured and only reverberation artifact will be seen. Therefore, LUS cannot rule out some of the pathologies and examination must supportive clinical findings is always considered an indication to perform additional tests [
8,
38]. Otherwise for example metastatic changers usually are seen close to the pleura line. In human medicine finding even one wedge sign is an indication for further diagnosis of PTE.
A general drawback of color doppler sonography for lung ultrasound is that motion artifact as a result of the respirations may interfere with the quality of color doppler sonography. Good ultrasound settings, and doppler velocity scale adjustment are important to visualize small vessels within lung consolidations. For less experienced operators doppler analysis can be difficult to perform. Nevertheless, lung consolidation should be evaluated for bronchogram and vascularity whenever possible. If the patient is in severe dyspnea or panting, LUS can be performed after stabilization of the patient and sedation is administered. Sedatives should not directly influence the results of the examination, but LUS should be performed as soon as possible after achieving sedation to avoid atelectasis which may occur because of prolonged lateral recumbency.
As experienced with echocardiography clinicians we also could exclude the cardiogenic background of consolidated lung in progression of pulmonary edema what can be difficult for less experienced veterinarians or general practitioners and relay not only on left atrium size, but also on diastology parameters like mitral inflow or tissue doppler. In animals’ stage B2 according to ACVIM classification ARDS, pneumonia with atelectasis was significant more common probably because most of the animals were small breed dogs and in population were 22.6% of dogs with common disease of respiratory tract like Yorkshire Terriers (14.7%), WHWT (4.6%) and French Bulldog (3.3%). Consolidated lung was observed only in one case of PE in very server stadium in other cases the animals with CHF and consolidations in lung had also signs of inflammation confirmed by other diagnostic tests. In analysis W type of consolidation and diagnosis of PTE was significant mor often observed possibly because of cat’s patient with spontaneous echo contrast in right atrium as a risk factor of PTE. In animals’ stage ACVIM D more often atelectasis was observed as a result of pleural effusion in right sided heart failure. In animals with different types of heart tumor the atelectasis was significant more common as a consequence of pleural effusion in progression of oncology disease.