1. Introduction
Chlorophyll, the green pigment found in plant leaves, plays a fundamental role in photosynthesis, the process by which plants convert light energy into chemical energy, ultimately sustaining life on Earth. It serves as a crucial indicator of a plant’s photosynthetic vitality and overall health. Measuring chlorophyll concentration in plants is crucial in assessing a plant’s photosynthetic efficiency, overall health, and response to environmental stressors. In terms of its structure, chlorophyll consists of a tetra pyrrole ring housing a central magnesium ion, accompanied by an elongated hydrophobic phytol chain [
1]. Its presence extends across a wide spectrum of organisms, encompassing higher plants, ferns, mosses, green algae, and specific prokaryotic entities like Prochloron varieties inhabiting plants and algae [
2,
3].
In higher plants, chlorophyll manifests primarily as chlorophyll a (Chla), serving as the principal pigment, and chlorophyll b (Chlb), operating as a supplementary pigment. These chlorophyll variants coexist in a typical chlorophyll ratio (Chla/b) of roughly 3 to 1, although this proportion can be subject to fluctuations influenced by growth conditions and environmental factors [
3]. The primary differentiation between these two chlorophyll types is the presence of a methyl group in Chla, replaced by a formyl group in Chlb [
3,
4]. Chlorophyll predominantly absorbs light within the red (650–700 nm) and blue-violet (400–500 nm) regions of the visible spectrum and interestingly, it exhibits a unique property of reflecting green light (~550 nm), which imparts the characteristic green coloration to chlorophyll [
1,
3,
4,
5].
Sesame, a valuable oilseed crop, is highly regarded for its oil-rich seeds, finding applications in cooking, cosmetics, and traditional medicine [
6,
7]. This crop is esteemed for its resilience to varying climates, impressive oil content, and exceptional antioxidant properties [
8]. Sesame serves as a vital source of premium-quality edible oil and protein-rich food. Sesame seeds typically contain oil in the range of 50-60%, and this oil boasts a significant proportion of natural antioxidants such as sesamolin, sesamin, and sesamol in which hese antioxidants contribute to the extended shelf life and stability of sesame oil [
6,
9]. Studies have revealed that sesame seeds are rich in protein, comprising approximately 19-25% of their composition [
6]. Moreover, sesame seeds are abundant in essential minerals like iron, magnesium, copper, and calcium, along with vitamins B1 and E, as well as phytosterols at which these nutritional components were reported haveing a role in reducing blood cholesterol levels [
10]. Additionally, sesame seeds encompass all the essential amino acids and fatty acids, further enhancing their nutritional value [
11]. Sesame, therefore, emerges as a versatile and nutritious crop with immense potential for various applications, from culinary to health-enhancing products. In this case, understanding and optimizing chlorophyll extraction from sesame leaves are of particular importance because chlorophyll content can provide insights into the crop’s health, growth, and potential yield.
Accurately quantifying chlorophyll concentration demands the utilization of effective extraction techniques. Traditionally, wet chemical methods have entailed dissolving chlorophyll in a solvent, followed by assessing the absorbance of the chlorophyll solution via spectrophotometry. Concentration values are then derived using well-established equations. Previous investigations have explored a range of methods to evaluate chlorophyll content in higher plant leaves, encompassing non-destructive chlorophyll meters [
12], fluorometry [
13], photo-acoustic spectroscopy [
14], chromatographic approaches [
15], and spectrophotometry [
4,
16,
17,
18,
19]. Numerous studies have demonstrated that chlorophylls, which are lipid-soluble compounds present in plant tissues, can be extracted using water-miscible organic solvents. These solvents include acetone, pyridine, methanol, ethanol, diethyl ether, DMF, and DMSO, all of which have the ability to absorb water [
4,
16,
20,
21,
22,
23,
24,
25,
26,
27,
28].
Despite extensive experimentation dedicated to chlorophyll extraction and quantification, no single method has been identified that simultaneously offers simplicity, widespread applicability, ease of reproducibility, and high sensitivity [
26]. The presence of numerous variable conditions affecting chlorophyll pigments, their extractability, and their reactions complicates the task of selecting a single extraction procedure that can accurately estimate all the green components present in various plants and plant-derived products. Different researchers have reported varying degrees of effectiveness with different extraction procedures. The acetone method [
4], has been widely accepted for chlorophyll determination due to its simplicity, sensitivity, and safety compared to the DMSO and DMF methods [
29,
30]. However, the acetone method involves grinding plant tissue in acetone, followed by centrifugation, which can be time-consuming for large sample numbers [
25]. In contrast, the DMSO method is simpler and faster as it eliminates the need for grinding and centrifugation [
12], offering similar efficiency to acetone with superior chlorophyll stability [
25]. Nevertheless, DMSO and DMF are associated with toxicity and unpleasant odors during extraction [
30], and DMSO extraction is sensitive to temperature fluctuations [
31]. When comparing acetone and ethanol, both considered less harmful solvents, 96% ethanol has been reported as an efficient extraction solvent compared to 90% acetone [
32].
Given the economic significance of sesame and the importance of chlorophyll as an indicator of plant health and productivity, there is a pressing need to optimize chlorophyll extraction techniques to ensure accurate and efficient measurements. This research aims to address these challenges by exploring innovative methods and leaf disk positions to enhance the precision and reliability of chlorophyll extraction from sesame leaves. The findings of this study can have broader implications for agriculture and plant biology. By improving chlorophyll measurement techniques, researchers, agronomists, and farmers can better monitor and manage the health and growth of sesame crops, leading to increased yields and sustainable agricultural practices.
2. Results
2.1. SPAD Value Index (SVI) Variation within Sesame Genotypes
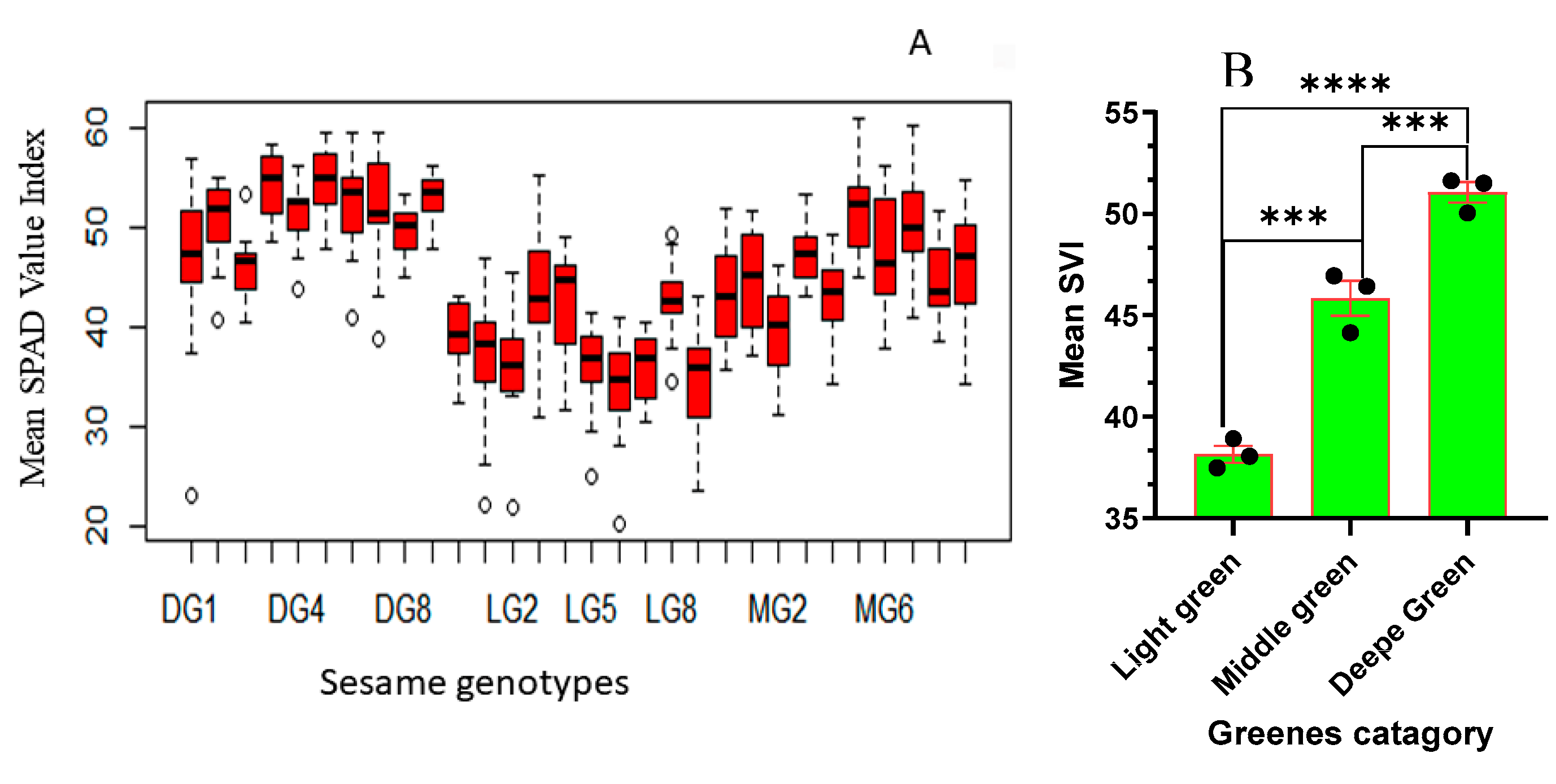
The ANOVA analysis for the SPAD Value Index (SVI) reveals significant effects attributed to the genotype variation (F29, 420 = 25.41; P < 0.001) on the relative chlorophyll concentration of sesame leaves. The results presented in Figure 2A underscore the significant variation in chlorophyll content among sesame genotypes. Among the evaluated sesame genotypes, a wide range of chlorophyll content was observed. Notably, DG5 and DG3 exhibit the highest levels of chlorophyll content, signifying their exceptional chlorophyll production capabilities. DG5, in particular, stands out with remarkably high chlorophyll content. Moving to the medium green category, we continue to observe variability in chlorophyll content. MG5 and MG7 fall within the intermediate range, indicating moderate chlorophyll content. MG6 and MG3 are also categorized here, with slightly lower chlorophyll content compared to MG5 and MG7. Conversely, LG6, LG9, and LG7 are the genotypes that exhibit the lowest chlorophyll content.
Figure 2.
Assessment of SPAD Value Indices for: (A) Different Sesame Genotypes (Genotypes are arranged in ascending order from left to right: DG1, DG10, DG2–DG9; LG1, LG10, LG2–LG9; MG1, MG10, MG2–MG9), (B) Categories of Sesame Greenness groups. The bars represent the mean values, and error bars depict one standard error (SE) pair comparison of t-test. The experiment involved 10 genotypes for each catagory, each replicated three times. Significant level represents, “***” ≤ 0.0001, “****” ≤ 0.00001.
Figure 2.
Assessment of SPAD Value Indices for: (A) Different Sesame Genotypes (Genotypes are arranged in ascending order from left to right: DG1, DG10, DG2–DG9; LG1, LG10, LG2–LG9; MG1, MG10, MG2–MG9), (B) Categories of Sesame Greenness groups. The bars represent the mean values, and error bars depict one standard error (SE) pair comparison of t-test. The experiment involved 10 genotypes for each catagory, each replicated three times. Significant level represents, “***” ≤ 0.0001, “****” ≤ 0.00001.
2.2. SPAD Value Index (SVI) Variation among Different Sesame Greenness Categories
The SPAD Value Index (SVI) ANOVA analysis reveals significant effects of the greenness category (F2, 447 = 191.53; P < 0.001) on the relative chlorophyll concentration in sesame leaves. Categorizing sesame genotypes into DG, MG, and LG groups elucidates the diverse chlorophyll content among genotypes, emphasizing substantial variations irrespective of genotype differences. In Figure 2B, the distinct chlorophyll difference among DG, MG, and LG categories is evident. DG plants exhibit the highest chlorophyll content, with a mean SVI of 51.052 and a range from 29.00 to 59.70 (Figure 2B), significantly differing from MG and LG. MG, with a mean chlorophyll content of 45.839 and a range from 31.100 to 61.000 (Figure 2B), shows lower chlorophyll content than DG but significantly higher than LG. LG, with the lowest mean chlorophyll content at 38.15 and a range from 20.30 to 55.40 (Figure 2B), indicates significantly lower chlorophyll content compared to DG and MG. These findings emphasize the substantial impact of greenness categories on chlorophyll content, providing insights into the relative chlorophyll concentrations of sesame genotypes. The categorization based on greenness proves informative, offering a clearer understanding of the inherent chlorophyll variations among sesame plants.
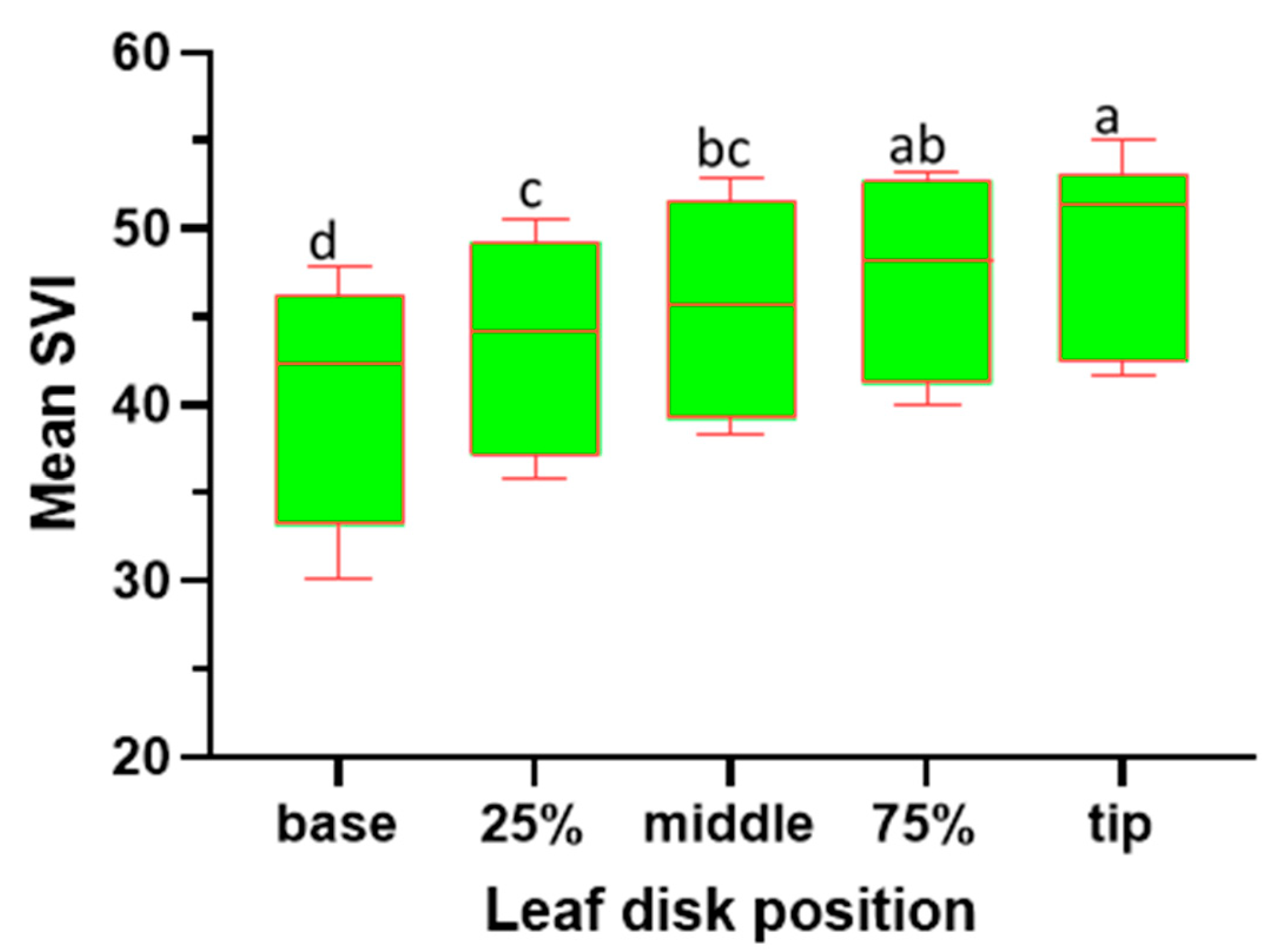
2.2. Variation in SPAD Value Index (SVI) Across Different Leaf Disk Positions along the Leaf Blade
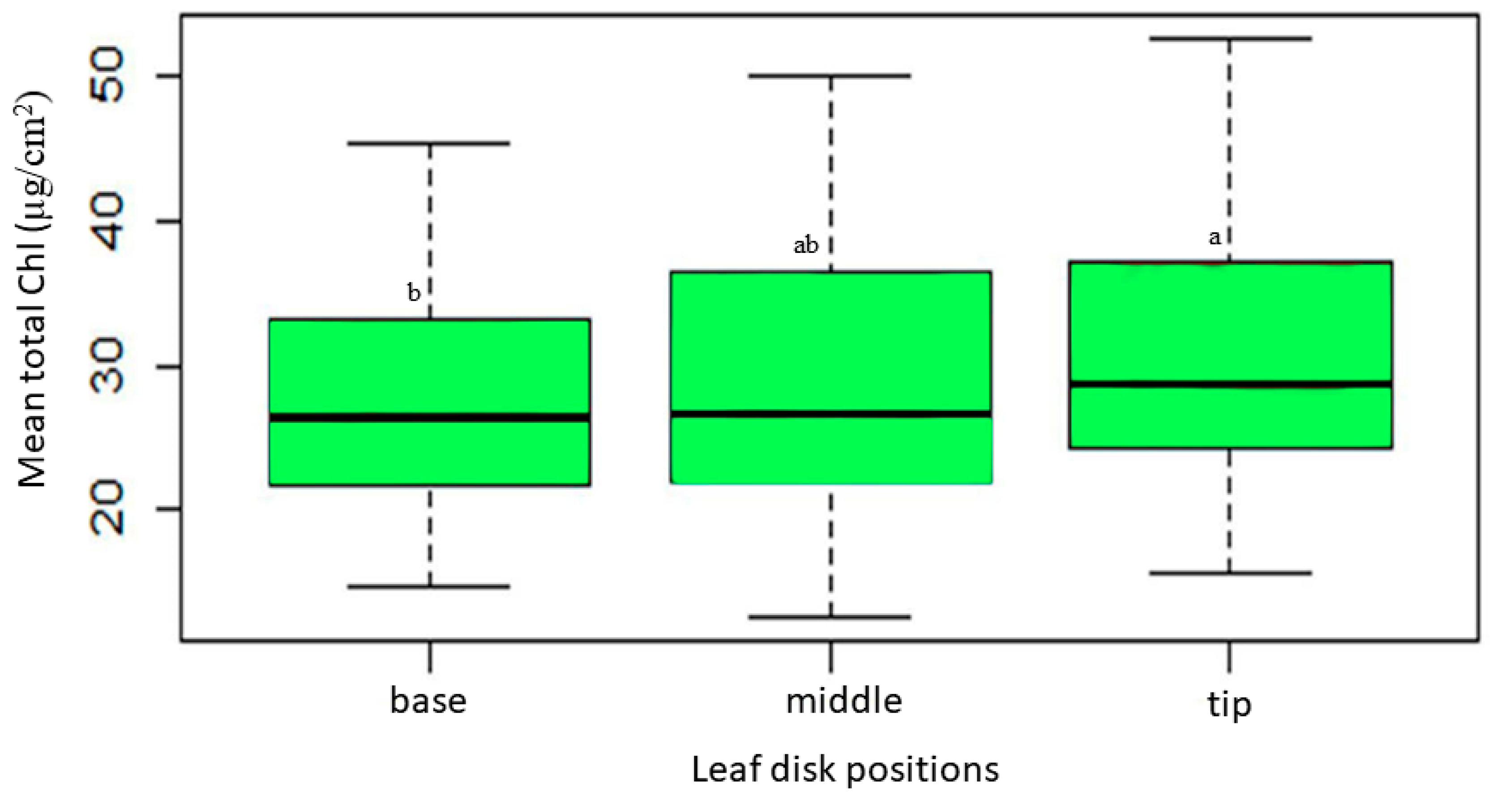
The ANOVA analysis for the SPAD Value Index (SVI) reveals significant effects attributed to the leaf disk position (F4, 445 = 19.46; P < 0.001) on the relative chlorophyll concentration of sesame leaves. The study reveals notable variations in chlorophyll concentration across different positions of sesame leaves (Figure 3). The leaf tip exhibits the highest chlorophyll content, with a mean SVI of 49.00 and a range of 34.40 to 61.00, significantly differing from other positions. At the 75.00% position, the leaf disk shows an intermediate chlorophyll level (47.00), not significantly different from the tip but distinct from the base. The middle position, with a mean SVI of 45.45, displays moderate chlorophyll content, differing significantly from the base. The 25.00% position presents intermediate chlorophyll levels (43.40), significant compared to other positions except the base. The leaf base has the lowest mean SVI (40.25) and significantly differs from all other positions, indicating markedly lower chlorophyll content. These findings emphasize a clear chlorophyll gradient along the leaf blade, with the tip demonstrating superior content compared to other positions, particularly the base.
Figure 3.
SVI variability across various leaf disk positions alongside of the sesame leaf blades. Box plots with the same letter(s) indicate no significant difference as per the SNK test at a 5% significance level. (Each box represent the Interquartile Range (IQR); the line inside the box (Median Line) represents the median value; the Whiskers extended from the box represents the Minimum and Maximum Values within 1.5 times IQR).
Figure 3.
SVI variability across various leaf disk positions alongside of the sesame leaf blades. Box plots with the same letter(s) indicate no significant difference as per the SNK test at a 5% significance level. (Each box represent the Interquartile Range (IQR); the line inside the box (Median Line) represents the median value; the Whiskers extended from the box represents the Minimum and Maximum Values within 1.5 times IQR).
2.2. Quantification of Chlorophyll Concentration Variation in Sesame Leaves
The overall ANOVA results of an analysis for chlorophyll concentration in sesame genotypes based on both genotype variations and leaf disk positions demonstrated that both genotype (F29, 180 = 15.65, P < 0.001) and leaf disk position (F2, 180 = 11.65, P < 0.001) have significant effects on chlorophyll concentration in sesame genotypes using 96% ethanol incubated at 85oC for 10 minutes . The interaction between these factors (F58, 180 = 0.99, P = 0.50) however, does not appear to contribute significantly to the variation in chlorophyll concentration. The variation in genotype has significant effect on chlorophyll concentration, which suggested that there are significant differences in chlorophyll concentration among the different sesame genotypes being studied. Similarly, the chlorophyll concentration varies significantly depending on the position from which leaf disks are taken within the leaf blade.
2.2.1. Variation in Chlorophyll Concentration across Sesame Genotypes and Leaf Greenness Categories
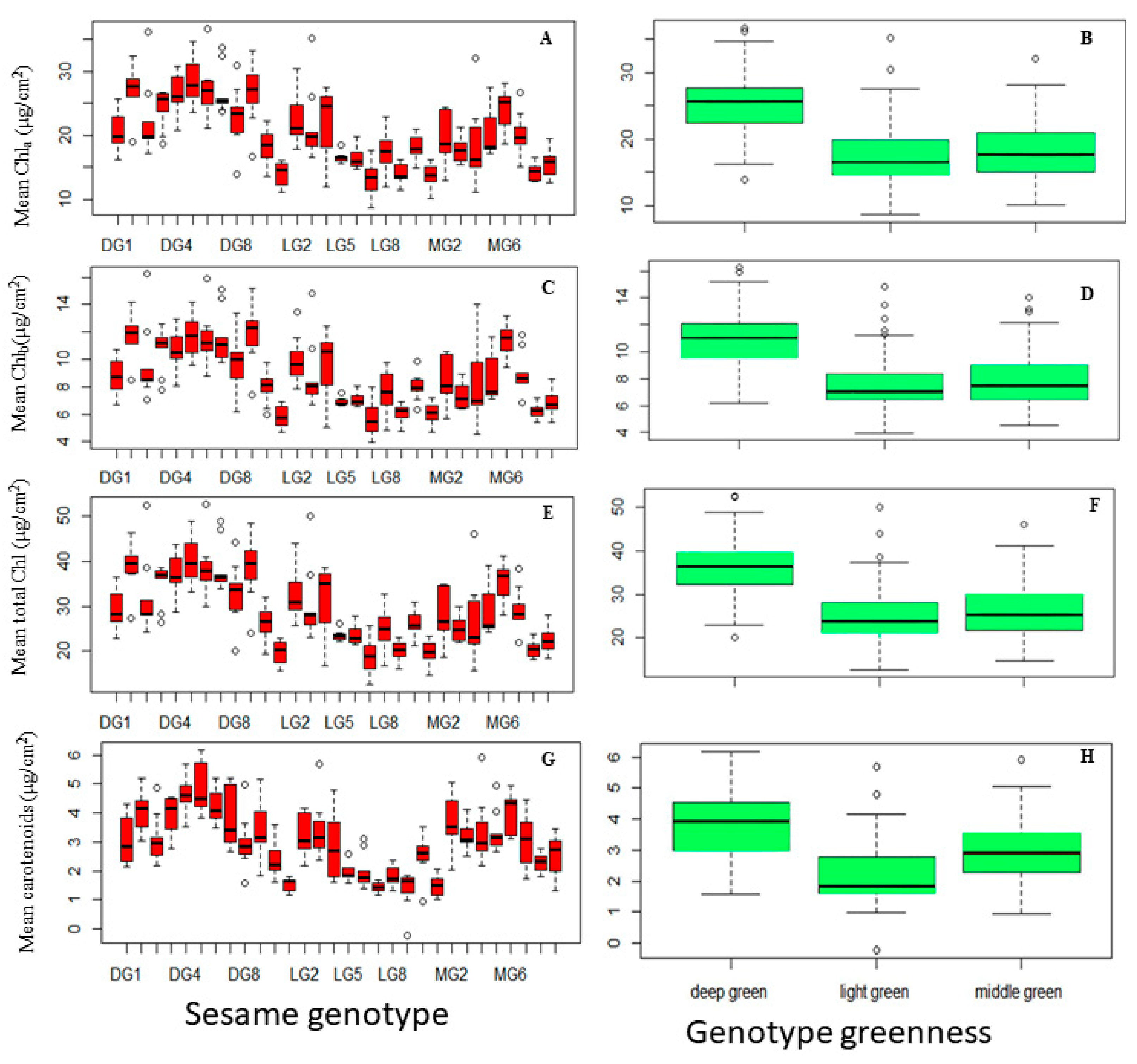
The ANOVA analysis in this study unveiled significant diversity in Chla concentration among various sesame genotypes (F29, 240 = 14.4, P < 0.001) and different greenness categories (F2, 267 = 80.34). DG5 (mean = 28.55) and DG10 (mean = 27.42) stood out as exceptional genotypes with the highest Chla content, surpassing other genotypes. MG6 (24.22) and DG2 (22.19) displayed intermediate Chla concentrations, while LG7 (13.32), LG10 (13.860), and MG10 (13.45) exhibited the lowest Chla levels. Further insights from Figure 5B emphasized the link between Chla concentration and greenness categories. DG genotypes demonstrated significantly higher mean Chla concentration (25.30) compared to MG (18.279) and LG (17.529) categories, with no statistically significant differences between MG and LG.
Chlb concentration exhibited significant variation among genotypes (F24, 240 = 13.47, P < 0.001) and greenness categories (F2. 267 = 67.28, P < 0.001). Several genotypes, including DG9, DG10, DG5, DG7, DG6, and MG6, showcased the highest mean Chlb concentrations (11.49 to 11.94), indicating a robust capacity for Chlb production. Genotypes DG4 and DG3 displayed intermediate Chlb concentrations (10.75 to 10.77), demonstrating noteworthy Chlb content. Another group of genotypes exhibited lower mean Chlb concentrations (5.71 to 10.00), indicating relatively lower Chlb content. Similar to Chla trends, the DG category (Mean = 10.85) displayed a significantly higher mean Chlb concentration compared to MG (Mean = 8.03) and LG (Mean = 7.55) categories, reaffirming the association between deep green characteristics and higher Chb content in DG genotypes. These findings elucidate the intricate relationship between genotype, greenness category, and chlorophyll composition in sesame plants, offering valuable insights into the factors influencing chlorophyll variability.
Regarding the total chlorophyll content, the ANOVA analyses underscore substantial variations in total chlorophyll content among sesame genotypes (F29, 240 = 14.21, P < 0.001) and greenness categories (F2, 267 = 76.95, P < 0.001), revealing diversity in chlorophyll production. Figure 5E depicts distinct total chlorophyll levels among genotypes, with DG5 leading at 40.35, followed by DG10, DG6, DG9, DG7, and DG4 with elevated content. Genotypes MG6 and DG3 fall within an intermediate range, while others show lower chlorophyll content. Greenness categories mirror the patterns seen in Chla and Chlb (Figure 5F).
Figure 5G illustrates significant variability in carotenoid content among genotypes (F29, 240 = 14.93, P < 0.001). DG5, DG4, DG6, DG10, MG6, and DG3 exhibit noteworthy carotenoid levels, contrasting with LG9, LG7, MG10, and LG10 displaying the lowest. Intermediate levels are observed in genotypes like DG4, MG2, and LG3. Significant variation (F2, 267 = 61.47, P < 0.001) also exists among deep green, middle green, and light green groups (Figure 5H). These findings highlight the diverse carotenoid profiles within sesame genotypes, offering insights into their photosynthetic pigment composition.
Figure 5.
Variation in Pigment Concentrations in Sesame Leaves Across Different Genotypes and greenness categories: (A) Chlorophyll a (Chla), (C) Chlorophyll b (Chlb), (C) Total Chlorophyll (Chla+b), and(D) Carotenoid Concentration; Greenness Categories: (E) Chlorophyll a (Chla), (F) Chlorophyll b (Chlb), (G) Total Chlorophyll (Chla+b), (H) Carotenoid Concentration. Sesame Genotypes (arranged from left to right in ascending order): DG1, DG10, DG2–DG9; LG1, LG10, LG2–LG9; MG1, MG10, MG2–MG90. (Each box represent the Interquartile Range (IQR); the line inside the box (Median Line) represents the median value; the Whiskers extended from the box represents the Minimum and Maximum Values within 1.5 times IQR; Individual data points beyond 1.5 times IQR represents the Outliers.
Figure 5.
Variation in Pigment Concentrations in Sesame Leaves Across Different Genotypes and greenness categories: (A) Chlorophyll a (Chla), (C) Chlorophyll b (Chlb), (C) Total Chlorophyll (Chla+b), and(D) Carotenoid Concentration; Greenness Categories: (E) Chlorophyll a (Chla), (F) Chlorophyll b (Chlb), (G) Total Chlorophyll (Chla+b), (H) Carotenoid Concentration. Sesame Genotypes (arranged from left to right in ascending order): DG1, DG10, DG2–DG9; LG1, LG10, LG2–LG9; MG1, MG10, MG2–MG90. (Each box represent the Interquartile Range (IQR); the line inside the box (Median Line) represents the median value; the Whiskers extended from the box represents the Minimum and Maximum Values within 1.5 times IQR; Individual data points beyond 1.5 times IQR represents the Outliers.
2.2.1. Variation in Chlorophyll Concentration across the Different Leaf Disk Positions along the Leaf Blade
Regarding the leaf disk positions, the results of ANOVA analyses highlight the substantial variations in Chla+b among leaf disk positions (F2, 267 = 4.71, P < 0.001), providing valuable insights into the diversity of chlorophyll production within the plant specimens studied. Figure 6 presented the outcomes of the mean comparisons for leaf disk positions: tip, middle, and base, along the leaf blade of sesame genotypes. Specifically, the “tip” and “middle” leaf disk positions (Mean = 31.05 and 29.14, respectively) share the same letter, indicating that their chlorophyll content does not significantly differ. Conversely, both the “tip” and “middle” positions exhibit significantly higher chlorophyll content compared to the “base” leaf disk position (Mean: 27.35).
Figure 6.
Total chlorophyll (Chla+b) content variation among Leaf Disk Position across Sesame Genotypes’ Leaf Blades. (Each box represent the Interquartile Range (IQR); the line inside the box (Median Line) represents the median value; the Whiskers extended from the box represents the minimum and maximum values within 1.5 times IQR).
Figure 6.
Total chlorophyll (Chla+b) content variation among Leaf Disk Position across Sesame Genotypes’ Leaf Blades. (Each box represent the Interquartile Range (IQR); the line inside the box (Median Line) represents the median value; the Whiskers extended from the box represents the minimum and maximum values within 1.5 times IQR).
2.2. Chloroplast Anatomical Variation between Deep Green and Light Green Sesame Leaves
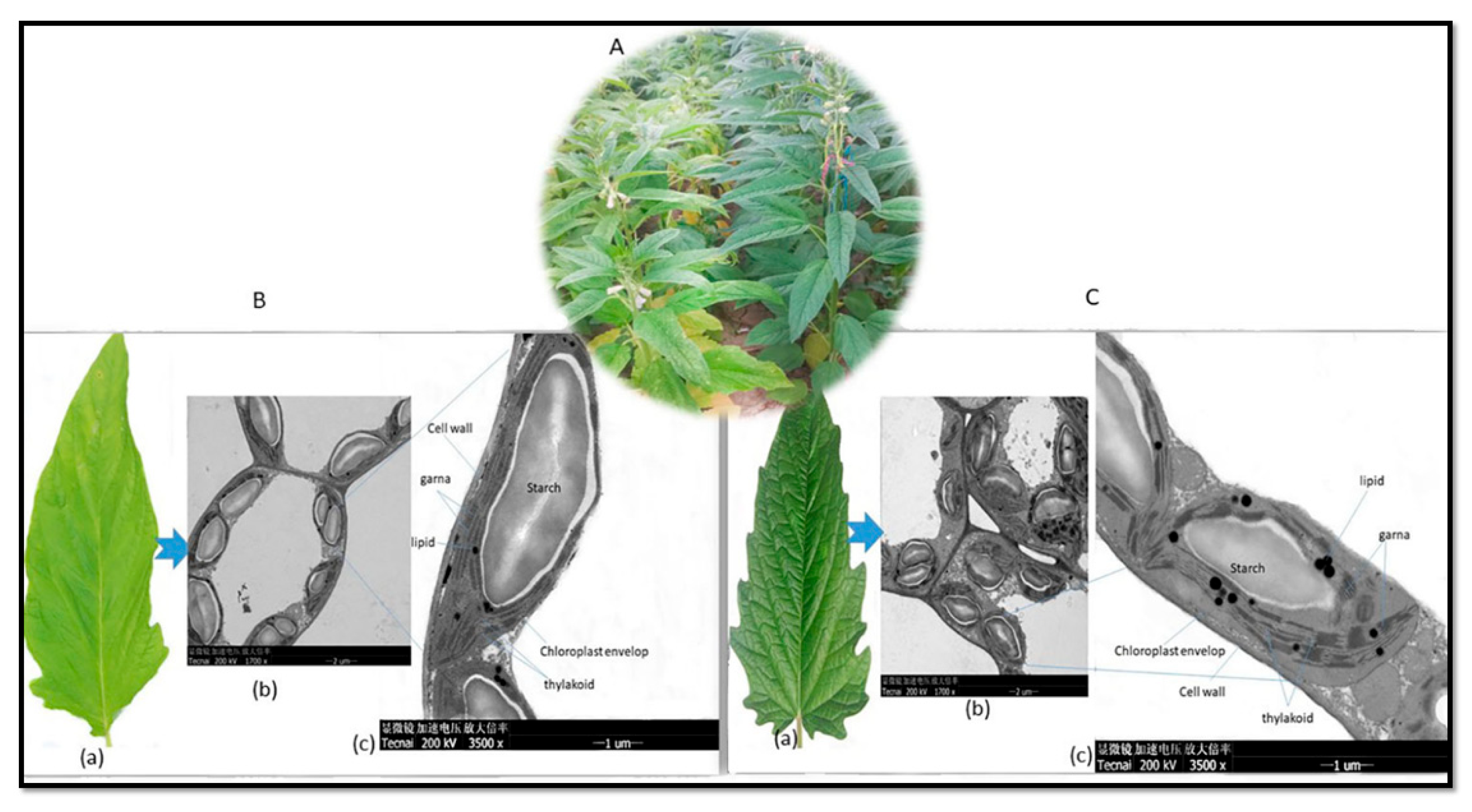
The electron micrographs of chloroplasts obtained from the leaves of both deep green and light green sesame genotypes (Figure 7A), reveal notable difference in their chloroplast structures, encompassing grana, thylakoids, and lipid droplets. The deep green leaf exhibit a higher density of chloroplasts within their mesophyll cells, as presented in Figure 7B(b), which typically translates to greater efficiency in capturing and converting light energy into chemical energy. In contrast, the light green leaf display reduced chloroplast densities, (Figure 7C (b)). Likewise, in the deep green leaf, there was greater density and more well defined stacking of grana (Figure 7C (c)), enabling more effective light capture and energy transfer during photosynthesis. Conversely, the light green leaf featured fewer and less tightly stacked grana (Figure 7B (c)), potentially resulting in less photosynthetic efficiency. Furthermore, the deep green leaf manifest a higher abundance of thylakoids, while light green leaf exhibit fewer thylakoids. Additionally, the deep green leaf were characterized by larger and more numerous lipid droplets, indicative of their enhanced energy storage capacity while, in contrast, the light green leaf have smaller or fewer lipid droplets (Figure 7C (c)), suggesting a reduced capacity for energy storage.
Figure 7.
Electron Micrographs of Chloroplasts: (A) Chloroplasts from light green (left) and deep green (right) sesame genotypes in the field. (B) (a) A single leaf from the light green sesame genotype, (b) multiple chloroplasts, (c) a thin section of a single chloroplast revealing the cell wall, chloroplast envelope, starch granules, lipid (fat) droplets, grana (stack of thylakoids), and thylakoids. (C) (a) A single leaf from the deep green sesame genotype, (b) multiple chloroplasts, (c) a thin section of a single chloroplast showing the cell wall, chloroplast envelope, starch granules, lipid (fat) droplets, grana (stack of thylakoids), and thylakoids.
Figure 7.
Electron Micrographs of Chloroplasts: (A) Chloroplasts from light green (left) and deep green (right) sesame genotypes in the field. (B) (a) A single leaf from the light green sesame genotype, (b) multiple chloroplasts, (c) a thin section of a single chloroplast revealing the cell wall, chloroplast envelope, starch granules, lipid (fat) droplets, grana (stack of thylakoids), and thylakoids. (C) (a) A single leaf from the deep green sesame genotype, (b) multiple chloroplasts, (c) a thin section of a single chloroplast showing the cell wall, chloroplast envelope, starch granules, lipid (fat) droplets, grana (stack of thylakoids), and thylakoids.
3. Discussion
This study identified significant variations in chlorophyll content, assessed through the SVI, and chlorophyll/carotenoid concentrations per leaf area, across diverse factors such as genotype diversity, greenness categories, and leaf disk positions. The findings revealed strong associations between SVI, chlorophyll ratios, total chlorophyll, carotenoid concentrations, and genotype variation, indicating distinct sesame genotypes with varying chlorophyll levels. These variations likely stem from genetic differences influencing chlorophyll production and synthesis pathways. Understanding genotype-specific chlorophyll variations is crucial for selecting genotypes aligned with specific objectives, such as higher chlorophyll extraction or other desired traits. Furthermore, the categorization of sesame genotypes into DG, MG, and LG groups based on their greenness showed the substantial influence of visual leaf appearance on chlorophyll content measured as SVI, chlorophyll and carotenoid concentration. DG plants showed the highest chlorophyll content, MD plants exhibit intermediate chlorophyll content, and LG plants showed the lowest chlorophyll content. This significance variation suggesting that DG plants have significantly higher chlorophyll content on average followed by the MG category while the LG category has the lowest mean chlorophyll content indicating that plants in this category exhibit significantly lower chlorophyll content values compared to the other two groups. This variation has practical implications that a rapid estimates of chlorophyll content can be made by visually assessing leaf greenness. This can be particularly advantageous for applications in large-scale agriculture and plant breeding initiatives, eliminating the need for sophisticated equipment. Additionally, the study indicated further variance within the DG, MG, and LG categories. For example, some genotypes within the deep green category exhibited higher chlorophyll content than others. This variation may be attributed to genetic factors [
33] , environmental conditions [
3,
34], or specific growth-related elements associated with each genotype [
35].
Interestingly, the study exhibited a similar pattern between the concentration of carotenoids and chlorophyll across all factors, including genotypes, greenness categories, and leaf disk positions. This alignment with previous research, which established a positive correlation between total carotenoids and chlorophyll in cauliflower [
36], suggests a consistent relationship between these pigments. In the context of sesame genotypes which is grown in warm and tropical regions with high temperatures, the higher levels of carotenoids in deep green sesame genotypes compared to light ones can be attributed to the plant’s adaptation to its environment which are characterized by often receive abundant, high-intensity sunlight [
37]. Studies indicated that carotenoids serve as a photo-protective mechanism, dissipating excess light energy as heat, thus safeguarding the photosynthetic apparatus [
37,
38]. Consequently, deep green sesame genotypes, which were characterized by higher chlorophyll concentrations in this study, may have evolved to excel in these conditions by producing more carotenoids. Moreover, high temperatures can lead to oxidative stress in plants due to increased production of reactive oxygen species (ROS) and carotenoids were reported possessing antioxidant properties, enabling them to effectively mitigate this stress [
38,
39]. This suggests that deep green sesame genotypes, with their higher carotenoid levels, could be better equipped to cope with temperature-related stress. The observed correlation between carotenoid and chlorophyll concentrations in sesame leaves in general, suggested that sesame plants in warm and tropical regions could be adapted to their environment by producing greater amounts of carotenoids. These carotenoids serve dual purposes, acting as both photo-protective agents and antioxidants, ultimately contributing to the plant’s resilience in high-temperature and high-light conditions.
Regarding the leaf disk position, the SVI and the pigment concentration measurements reveal substantial variations across different leaf disk positions within the sesame leaves. These results indicate a clear and distinct chlorophyll gradient along the leaf blade. These findings underscore the critical importance of considering leaf disk positioning when assessing chlorophyll content in sesame genotypes, as it profoundly affects the recorded measurements. Our results is consistent with previous studies in various crops including wheat [
40], maize [
41,
42] and rice [
42,
43] that indicated chlorophyll was not evenly distributed along the leaf blade. In this study, specifically, the leaf tip exhibits the highest chlorophyll content, followed by the 75.00% position and the middle position, while the 50% and 25.00% position displays intermediate chlorophyll content, and the leaf base has the lowest chlorophyll content. The observed variations in chlorophyll content can be attributed to several factors, including differences in light exposure, nutrient distribution, leaf age, and physiological factors [
35,
44,
45,
46]. The leaf tip may received more direct sunlight, which stimulates greater chlorophyll production for photosynthesis compared to other positions while the relatively older portion nearer to the base tend to have lower chlorophyll content due to reduced chlorophyll production with leaf maturity [
47]. Variations may also arise from differences in nutrient distribution within the leaf, with the base receiving fewer nutrients, impacting chlorophyll production [
35]. Furthermore, the leaf’s physiological characteristics, such as its ability to retain chlorophyll or respond to environmental stress, can vary along the blade, contributing to differences in chlorophyll content [
35,
47]. The study’s key conclusion in this regard is therefore, the existence of a significant chlorophyll gradient along the sesame leaf blade, with the leaf tip to 75% portion of the leaf blade having the highest chlorophyll content and the 25% to the base having the lowest. This gradient has implications for various applications, including understanding the leaf’s photosynthetic efficiency and identifying optimal positions for chlorophyll extraction. To ensure accurate and consistent results in chlorophyll content analysis, it is vital to consider leaf disk position and establish standardized protocols for positioning when conducting measurements, minimizing variations caused by leaf position. For applications requiring higher chlorophyll extraction, focusing on leaf tips and 75% position is recommended due to their consistently higher chlorophyll content.
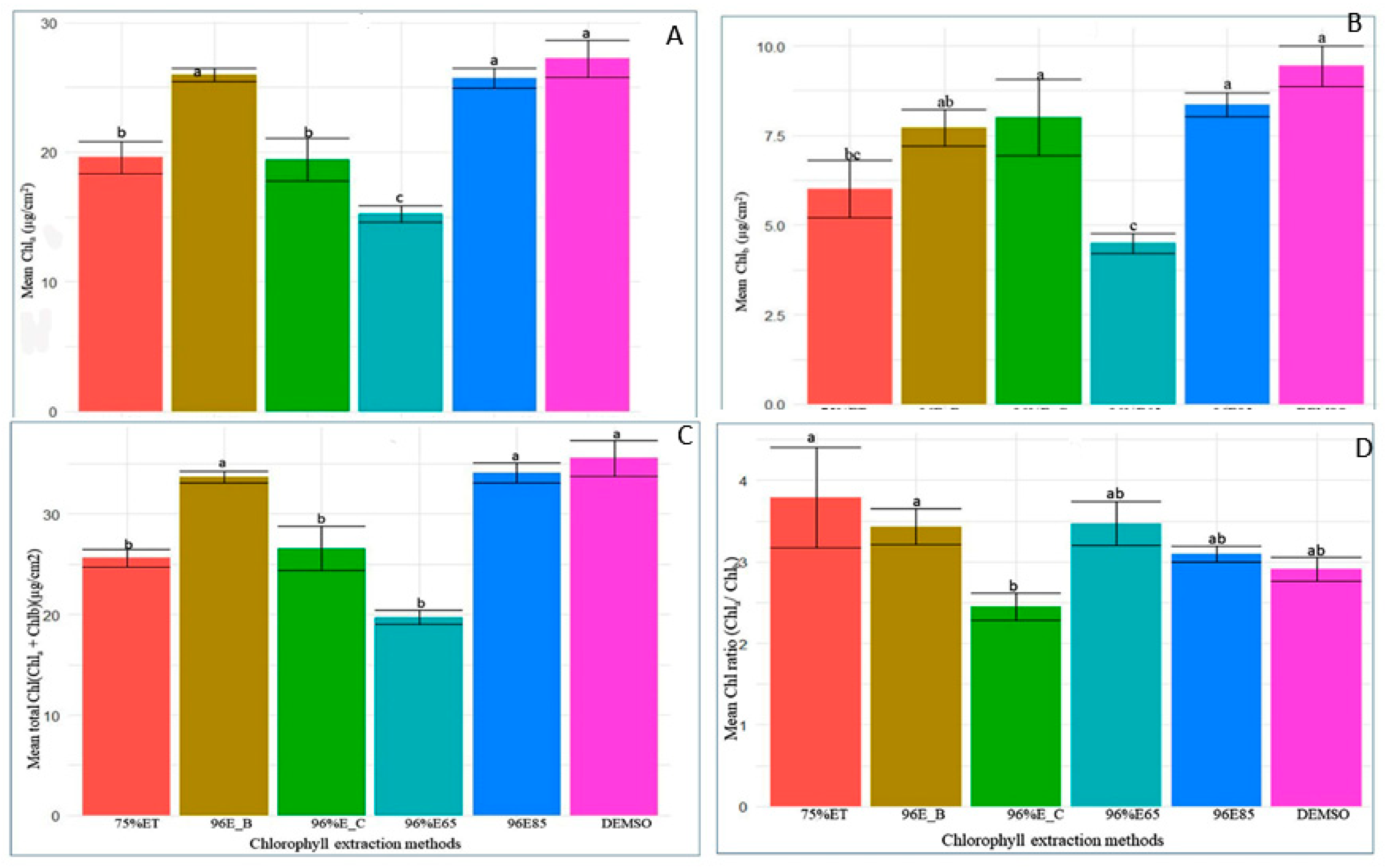
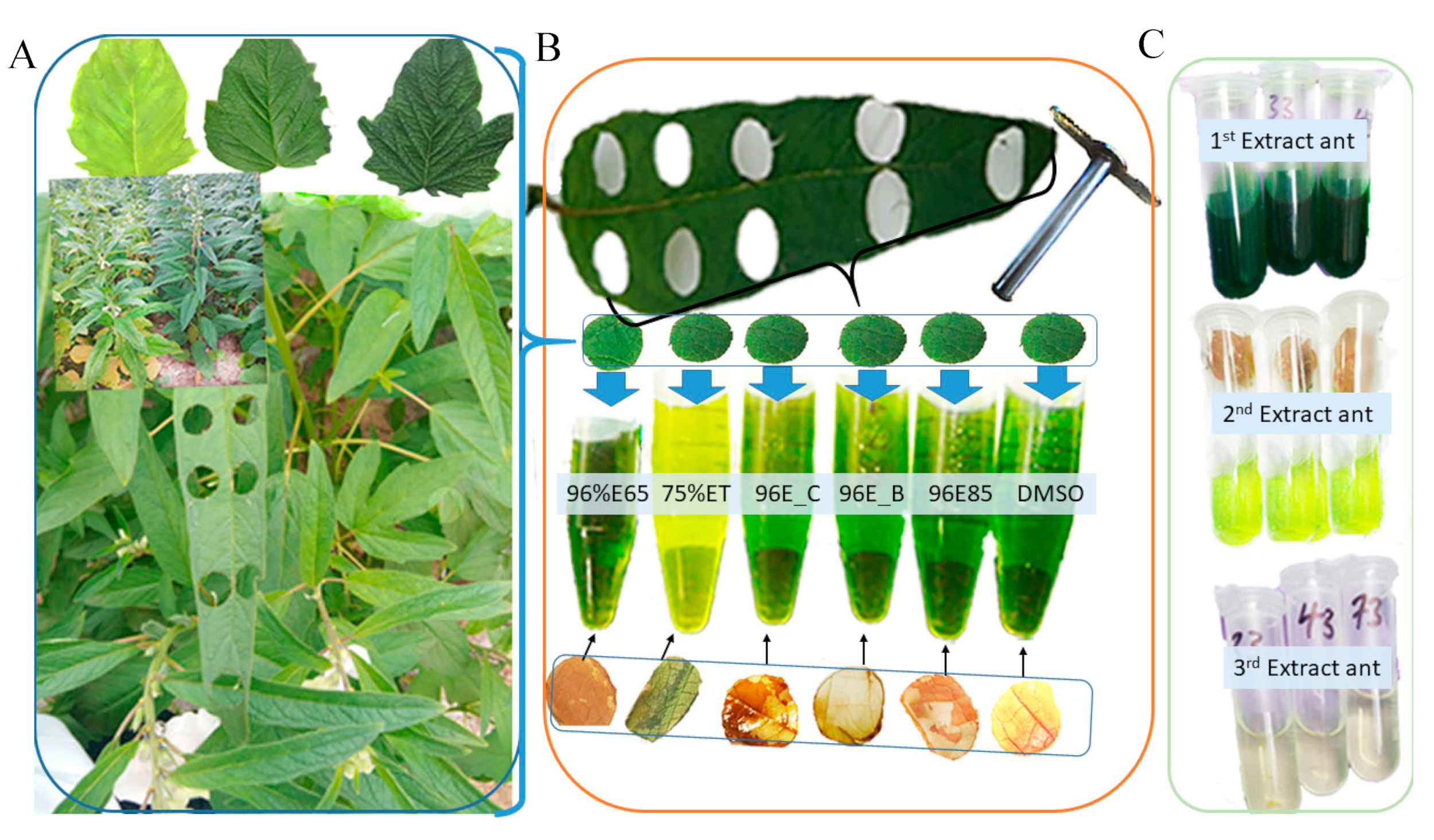
The results obtained from the chlorophyll extraction experiment, which involved the comparison of various solvents and procedures (Figure 8A–C); showed significant variations in Chla, Chlb, and total chlorophyll content. These distinctions underscore the substantial impact of both solvent choice and extraction methodology on the efficiency of chlorophyll extraction (Figure 8B). Specifically, the most remarkable outcomes is achieved with DMSO incubated for 25 minutes at 65°C (DMSO) and 96% ethanol incubated for 10 minutes at 85°C (96%E85), which extracted significantly higher levels of Chla, Chlb, and total chlorophyll content. It is worth noting that the first extraction in both solvents proved to be the most effective, making a significant contribution to the total chlorophyll content (Figure 8C). These findings are in accordance with previous research, which has consistently demonstrated that DMSO and 96% ethanol are highly efficient solvents for chlorophyll extraction [
12,
25,
32]. Previous investigations have explored several methods for evaluating chlorophyll content in higher plant leaves, and several studies have substantiated that chlorophylls, which are lipid-soluble compounds found in plant tissues, can be effectively extracted using water-miscible organic solvents [
4,
16,
20,
21,
22,
23,
24,
25,
26,
27,
28].
Figure 8.
Comparative Analysis of Extraction Procedures:(A) Sesame leaves of different genotypes, (B) Various extraction techniques with leaf disk positions, perforations, and leaf disk colors before and after extraction for each method. Extraction methods include DMSO (DMSO), 96%E85 (96% ethanol, 10 minutes at 85°C), 96%E_B (boiled leaf in 96% ethanol, 3 minutes at 85°C), 96%E_C (96% ethanol, 24 hours at 40°C, the cold method), 75%ET (75% ethanol, 25 minutes at 65°C), and 96%E65 (96% ethanol, 25 minutes at 65°C), (C) Extractant yield from 1st, 2nd, and 3rd extraction cycles using 96% ethanol incubated at 85°C for 10 minutes.
Figure 8.
Comparative Analysis of Extraction Procedures:(A) Sesame leaves of different genotypes, (B) Various extraction techniques with leaf disk positions, perforations, and leaf disk colors before and after extraction for each method. Extraction methods include DMSO (DMSO), 96%E85 (96% ethanol, 10 minutes at 85°C), 96%E_B (boiled leaf in 96% ethanol, 3 minutes at 85°C), 96%E_C (96% ethanol, 24 hours at 40°C, the cold method), 75%ET (75% ethanol, 25 minutes at 65°C), and 96%E65 (96% ethanol, 25 minutes at 65°C), (C) Extractant yield from 1st, 2nd, and 3rd extraction cycles using 96% ethanol incubated at 85°C for 10 minutes.
However, consideration of various factors including, cost effectiveness, time, safety and environmental regulations in the laboratory or research setting is also crucial when choosing an appropriate solvent. In this context, ethanol emerges as a practical and efficient choice for routine chlorophyll extraction, owing to its safety, cost-effectiveness, availability, and user-friendliness [
27,
48,
49,
50]. This conclusion is reinforced by prior research that has highlighted the toxicity and noxious odors associated with the DMSO method [
30] and the sensitivity of DMSO extraction to temperature fluctuations [
31]. Additionally, ethanol has been reported to be a less harmful yet efficient extraction solvent [
32]. It is important to note that that while both solvents resulted in comparable results, the use of 96% ethanol incubated for 10 minutes at 85°C (96%E85) simplifies the process and ensures consistent results across experiments. Nevertheless, researchers should always evaluate the specific requirements of their experiments and adhere to their lab safety protocols when making a final decision.
The examination of leaf anatomy, specifically the internal structure of chloroplasts within sesame leaves, gives valuable insights into their functions and adaptations. In this study, we investigate into the anatomical variations between deep green and light green sesame leaves, providing insight on how these differences impact chlorophyll content and distribution. Our examination revealed significant anatomical variations in the internal structure of the leaves between deep green and light green sesame plants, primarily linked to chloroplast density and pigment-producing structures. The deep green sesame leaves exhibit a high chloroplast density, well-organized grana, abundant thylakoids, and numerous lipid droplets, all of which point to efficient light capture and energy transfer during photosynthesis. Conversely, the light green leaves display fewer and less densely stacked grana, reduced thylakoids, and smaller lipid droplets, indicating lower photosynthetic efficiency. These findings supports earlier research, underlining that deep green leaves excel in converting light energy into chemical energy, primarily due to their high chloroplast density [
51,
52,
53,
54]. On the other hand, the light green leaves which were characterized by lower chloroplast density, may be adapted to high-light environments as a strategy to reduce the risk of photo-damage. They feature a loosely arranged mesophyll cell structure that enhances air circulation, reducing the likelihood of overheating (Ren
et al., 2019). This adaptation regulates the amount of absorbed light. As a result, the variations in leaf anatomy [
55] and tissue structure [
56] can influence pigment extraction from plants. This underscores the importance of using appropriate extraction procedures and solvents for achieving optimal chlorophyll extraction in plants. Therefore, having this knowledge is crucial in chlorophyll extraction studies, facilitating the optimization of techniques for accurate chlorophyll quantification.
4. Materials and Methods
4.1. Selection of Sesame Genotypes and Pigment Groups
For this study, a total of 30 sesame (Sesamum indicum L) genotypes were precisely chosen, representing three distinct pigment groups based on leaf coloration: light green (LG), middle green (MG), and deep green (DG). Each pigment group comprised 10 genotypes, carefully selected from a larger pool of sesame accessions cultivated in field experiments planted in a randomized complete block design (RCBD) with three replications. These selected genotypes formed the core of our investigation, which aimed to optimize SPAD value index measurements, quantify pigment concentrations, explore different extraction solvents, and determine the most suitable leaf disk positions for efficient chlorophyll extraction and quantification.
4.2. Measurement of SPAD Value Index (SVI)
To assess the SVI, we identified five plants situated in the middle of the second row for each genotype which were planted in the RCBD field layouts. These selected plants were labeled and subjected to SPAD value assessment using a SPAD meter (SPAD-502, Konica-Minolta, Japan). To ensure the precision of our readings, we calculated the average of five measurements taken along each leaf blade as marked in Figure 1. It is important to note that we specifically focused on fully extended young leaves of similar size, all originating from the main stem of the five selected plants at their 50% flowering stage, in order to obtain accurate measurements of photosynthetic pigments.
4.3. Comparative Chlorophyll Extraction Procedures
After measuring the SPAD value indices of the leaves, we conducted a comprehensive comparative analysis that encompassed six distinct chlorophyll extraction methods. These methods included both heated and cold assay techniques.
The heated assay procedures comprised the following five extraction techniques:
1. 75% Ethanol (75%ETHO) at 65°C for 25 minutes: Leaves were immersed in 75% ethanol and incubated at a temperature of 65°C for 25 minutes.
2. Boiling in hot water followed by extraction with 96% Ethanol (96%ETHO) at 85°C for 3 minutes: Initially, leaves were boiled in hot water, after which they were extracted using 96% ethanol at a temperature of 85°C for 3 minutes.
3. 96% Ethanol (96%ETHO) at 65°C for 25 minutes: Leaves were directly subjected to extraction using 96% ethanol at a temperature of 65°C for 25 minutes.
4. 96% Ethanol (96%ETHO) at 85°C for 10 minutes: Leaves were directly immersed in 96% ethanol and incubated at a temperature of 85°C for a duration of 10 minutes.
5. Dimethyl Sulfoxide (DMSO) at 65°C for 25 minutes: We employed a method utilizing Dimethyl Sulfoxide (DMSO) for extraction, with incubation at a temperature of 65°C for 25 minutes.
Conversely, the cold assay approach involved the utilization of 96% Ethanol (96%ETHO) extraction at a temperature of 4°C, with an extended incubation period of 24 hours.
To separately determine the content of Ch
a and Ch
b, fully extended young leaves of three plants in the middle from the second row of each genotype were detached from the plant to extract chlorophylls using a modified method described by [
4]. The content of chlorophyll a and chlorophyll b which are expressed as microgram (µg) of chlorophyll per leaf area (cm
2). For each extraction method used in this study, five circular leaf disks, each 15 mm in diameter, were punched from the leaf portion for which the SPAD value indices had been measured, using a cork borer. Subsequently, the leaves were rapidly frozen in liquid nitrogen and stored at −80°C in a deep freezer until the extraction procedure commenced. During the heated assay techniques, the leaf disks were removed from the deep freezer and promptly placed into 8 mL tubes. To each tube, 5 mL of the respective organic solvent was added immediately. The tubes were then incubated in a hot water bath at the specified temperature for the designated duration. In the boiling method, leaves were briefly dipped into boiling water for 10 seconds and being placed on filter paper to remove excess water, after which the organic solvents were added. For the cold assay techniques, following the immediate addition of 5 mL of the organic solvent (96%Etanol), each tube was wrapped in aluminum foil to shield it from light and then incubated at 4°C in the dark for 24 hours.
4.4. Estimation of Chlorophyll Content
After the incubation period, spectrophotometer measurements were made by transferring 300 µL of sample into a 1-cm pathlength quartz cell and reading absorbance in a spectrophotometer with a resolution of 1 nm bandwidth (UV-2550, Shimadzu, Kyoto, Japan). Light absorbance measurement were made at 665 nm, 649 nm, and 470 nm, corresponding to the maximum absorption of chlorophyll a (Chl
a), chlorophyll b (Chl
b), Chlorophyll total (Chl
(a+b) and carotenoids (Chl
(x+c), respectively. We employed the updated determined extinction coefficients and equations from [
3,
5,
57] to calculate specific pigment content with respect to the type of solvent used. The represented pigment contents were expressed in units of μg (cm
2)⁻¹, representing the amount of chlorophyll per unit area of the leaf.
For the Ethanol 96% (v/v) solvent, the concentrations of chlorophyll a, chlorophyll b, total chlorophyll, as well as carotenoids, were calculated using the following equations:
Ethanol 96% solvent [
3,
5]. :
For DMSO (Dimethyl Sulphoxide) Solvent [
57]:
where A is the absorption at the referenced wavelength and chlorophylls a and b are summed to obtain the total chlorophyll concentration. These analyses allowed us to accurately determine the chlorophyll content in the leaves for each extraction method and solvent type used.
4.5. Leaf Disk Position Determination Procedures
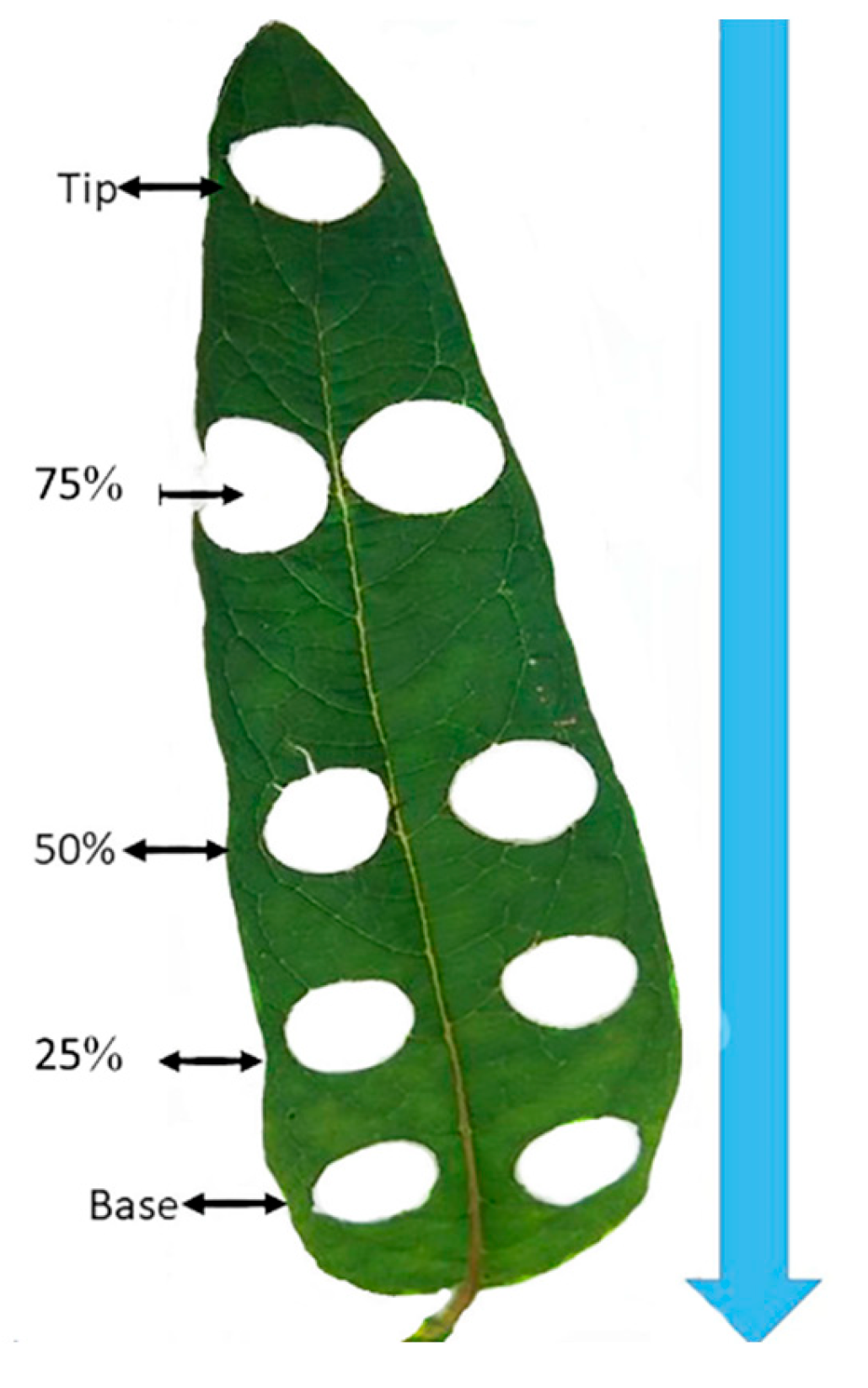
After selecting the appropriate solvent and optimizing the chlorophyll extraction methods, we proceeded to determine the positions of leaf disks. This was accomplished using a cork borer with a 15 mm diameter. We adhered to the criteria mentioned earlier, choosing young, fully extended leaves without any visible damage or signs of disease from the tagged plants with measured SPAD value indices. Each selected leaf was divided into five equal sections using a ruler, and the positions were marked as follows: tip, 25%, 50%, 75%, and base (refer to Figure 1). This marking was done before detaching the leaf from the plant. Beginning with one leaf, we carefully positioned the core borer over the marked point along the leaf blade, which was placed on a clean, flat cutting board surface. The cork borer was then pressed firmly and twisted slightly to create a clean circular leaf disk. Subsequently, the leaf disk was removed and placed into a labeled tube. It was then rapidly frozen using liquid nitrogen and stored at -80°C in a deep freezer until the extraction procedure was initiated. This leaf disk punching process was repeated for each marked position on every leaf from the tagged plants within the 30 genotypes. These genotypes were representative of three groups of sesame genotypes: deep green, middle green and light green. Once all the leaf disks had been punched, properly labeled, and stored in the deep freezer, we proceeded with the chlorophyll extraction procedures using the recommended solvent, (96% ethanol incubated at 85oC for 10 minutes, following the methods described in the previous sections.
Figure 1.
Leaf disk position marks.
Figure 1.
Leaf disk position marks.
4.6. Collection and Preparation of Sesame Samples for Electron Microscopy
Mature leaves from both deep green and light green sesame genotypes were carefully selected for sampling, employing clean and sterilized tools to minimize contamination risks. To ensure a representative sample set, multiple leaves were collected from various plants of each genotype. During sample collection, great care was taken to handle the leaves gently, minimizing any potential damage. Following collection, the leaves were rinsed with distilled water to remove any accumulated dust and debris. Subsequently, excess moisture was gently blotted dry from the leaves using clean paper towels. From each leaf, small sections measuring approximately 1 cm2 were then carefully excised using a sharp blade. These leaf sections were then placed into labeled sample containers and stored in a cool, dry environment, shielded from direct sunlight. The samples were maintained at a consistent temperature of 4°C to prevent any degradation of their structural integrity until they were ready to be sent for electron microscopy analysis. For the microscopic analysis of leaf morphology, Transmission Electron Microscopy (FEI Tecnai 20 Transmission Electron Microscopy) was utilized to provide high-resolution imaging of the cellular structures within the sesame leaf samples.
4.7. Statistical Analysis
In this study, we utilized ANOVA (Analysis of variance) to compare the mean chlorophyll values obtained using different extraction methods, assess differences in SPAD values among the 30 sesame genotypes selected for the study, examine variations in SPAD values among sesame genotypes grouped by leaf coloration, and evaluate disparities in chlorophyll content based on the position of the leaf disks sampled for analysis. Additionally, we employed the SNK (Student-Newman-Keuls) test for post-hoc analysis to conduct pairwise comparisons between group means. This allowed us to identify specific extraction methods, genotypes, greenness groups, or leaf disk positions that exhibited statistically significant differences in chlorophyll content.