Submitted:
02 May 2024
Posted:
03 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Setup
2.1. Research Engine and Equipment
2.2. Tested Fuels
2.3. Test Conditions and Procedure
3. Results and Discussion
3.1. Variable-Speed Tests at Full Load
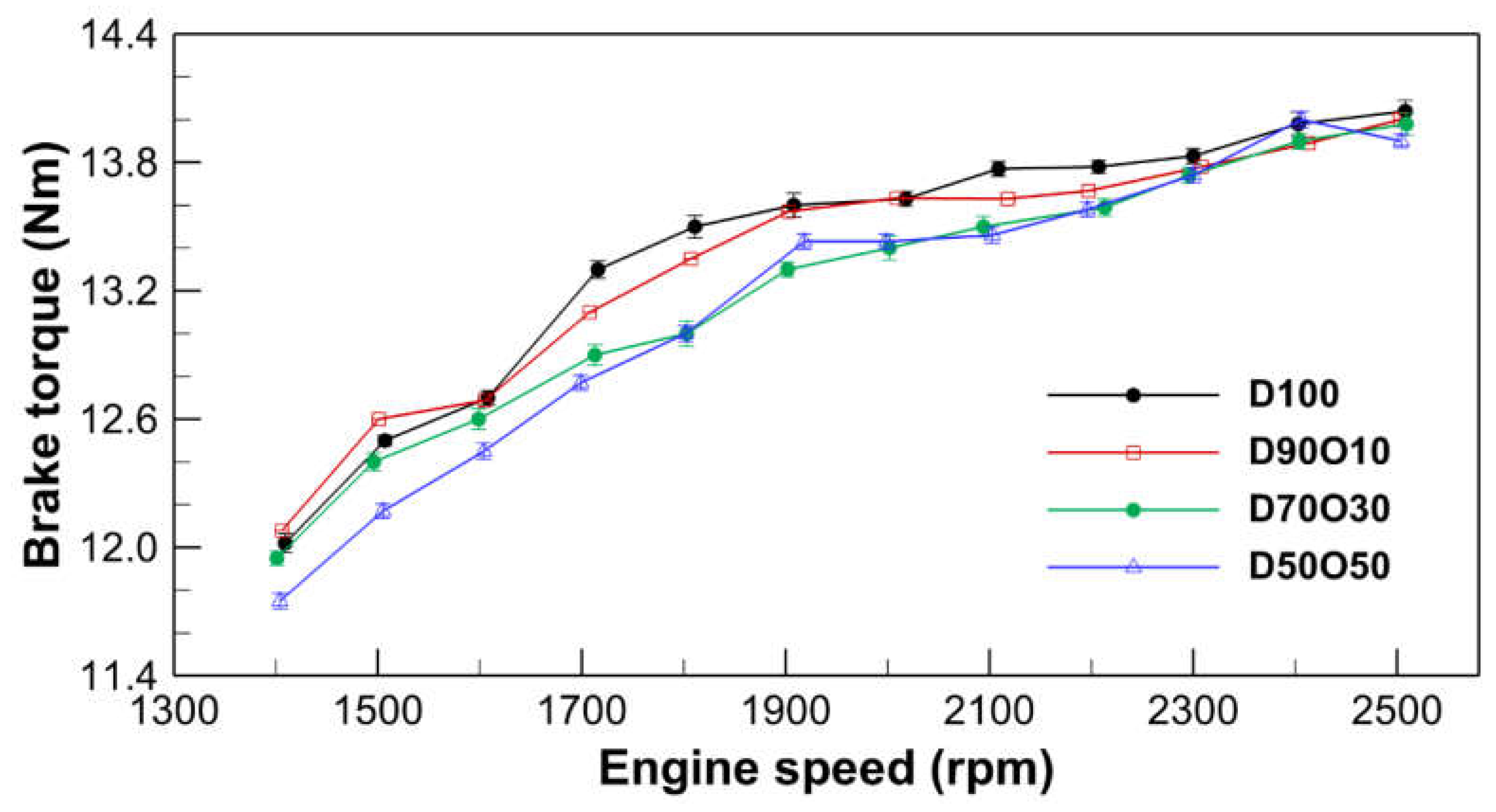
3.1.1. Brake Torque
3.1.2. Brake Power
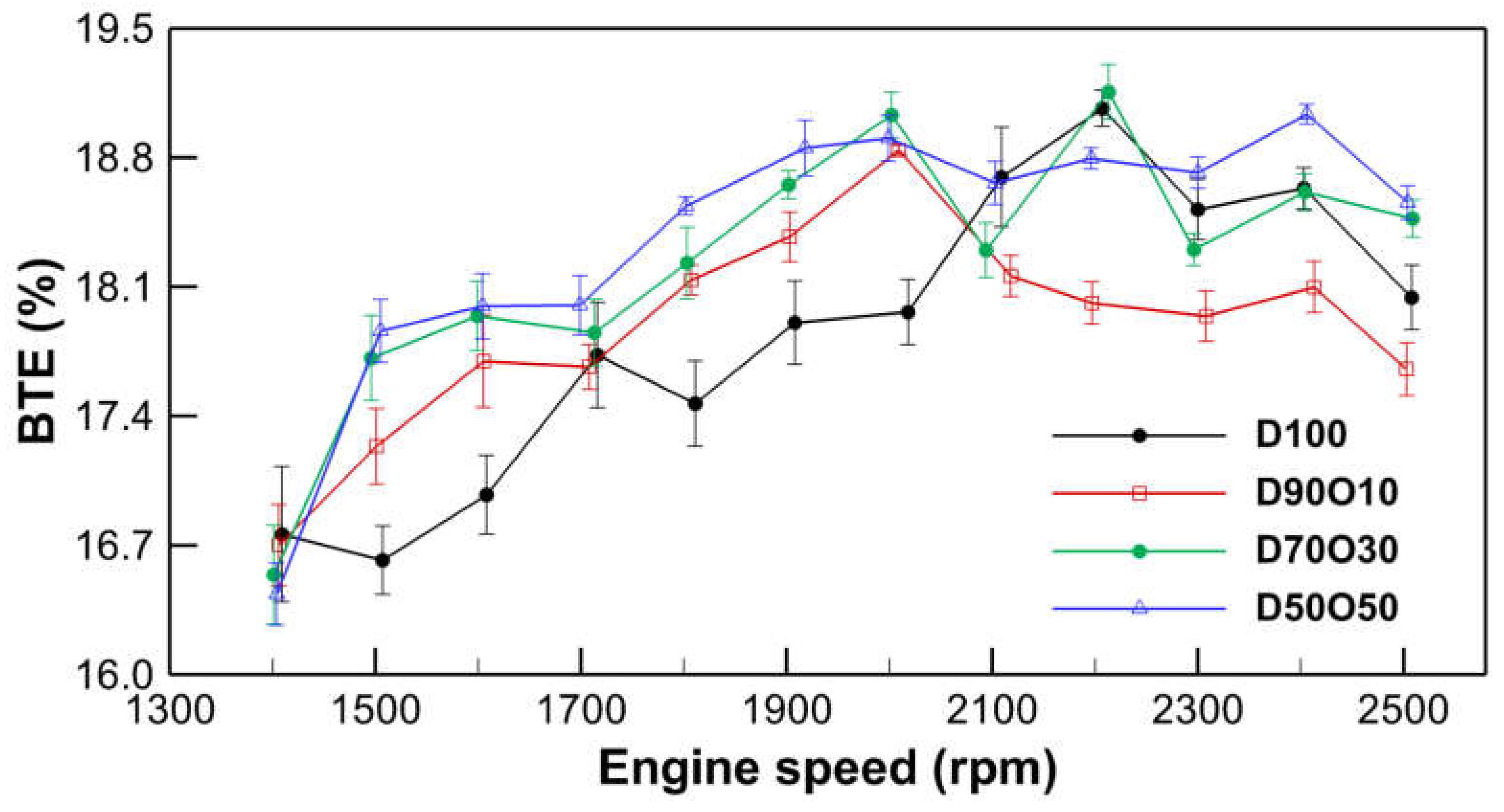
3.1.3. Brake Thermal Efficiency
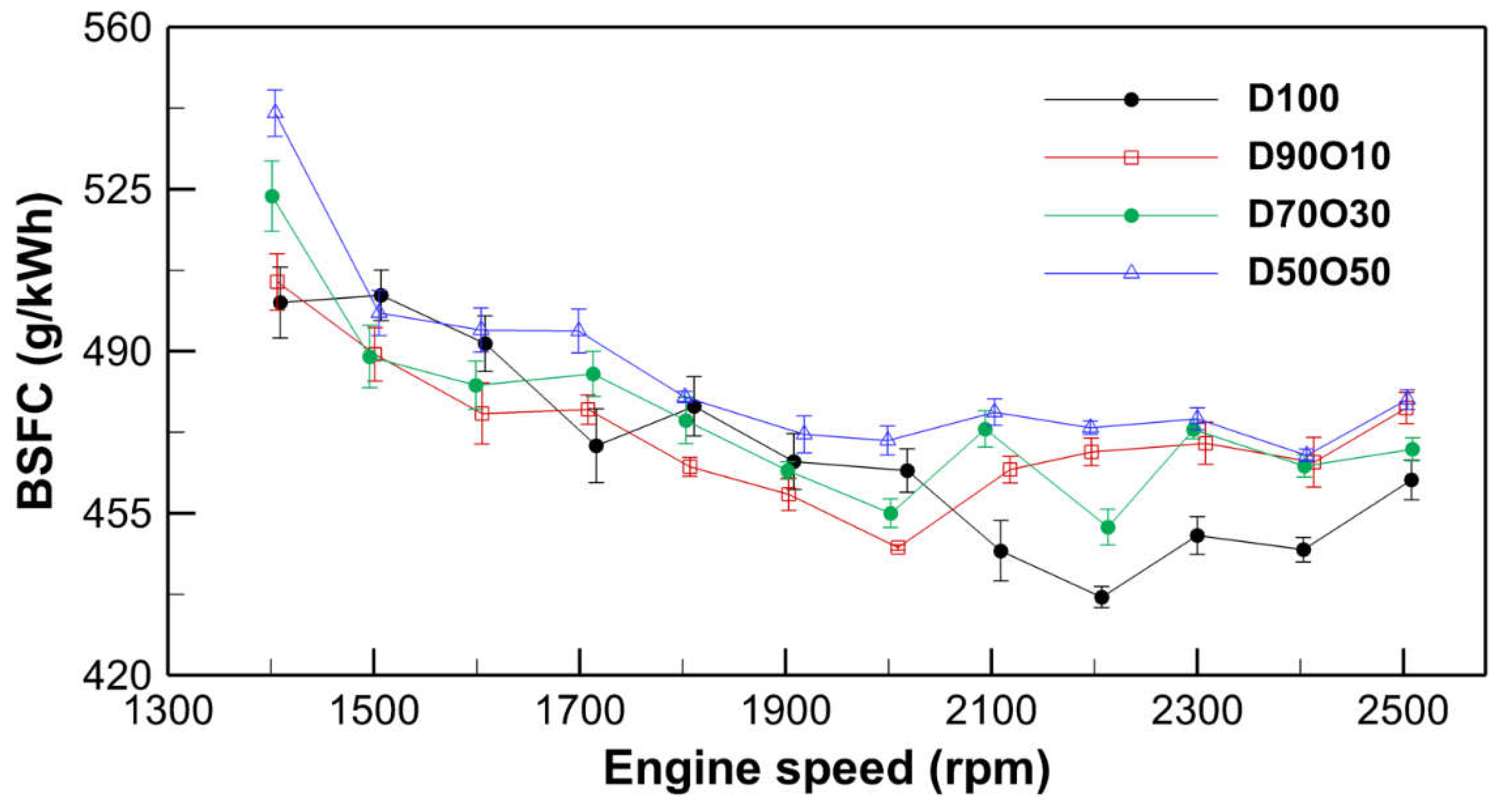
3.1.4. Brake Specific Fuel Consumption
3.2. Fixed-Speed Tests at Part Load
3.2.1. Brake Thermal Efficiency
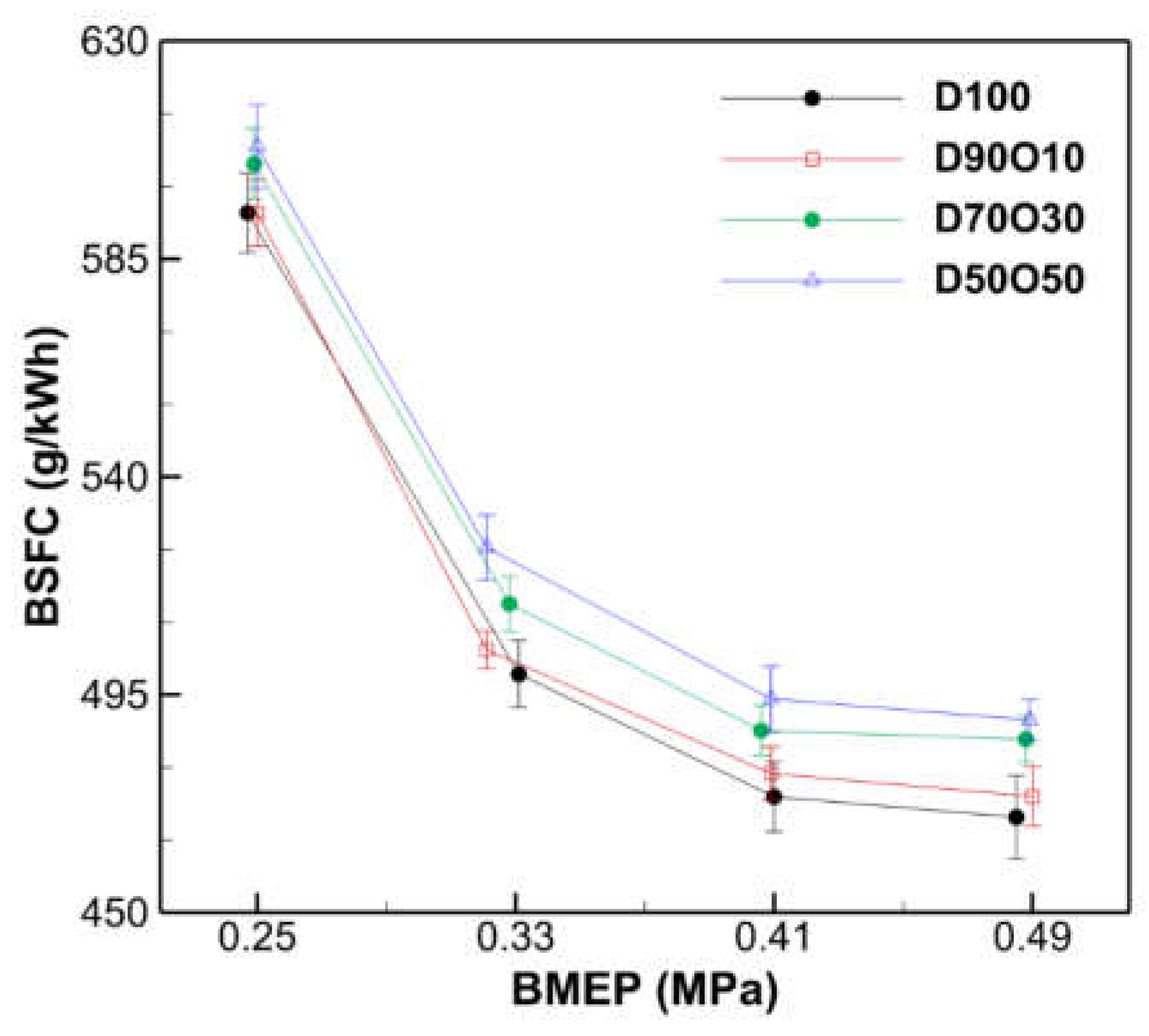
3.2.2. Brake Specific Fuel Consumption
3.2.3. NOx Emissions
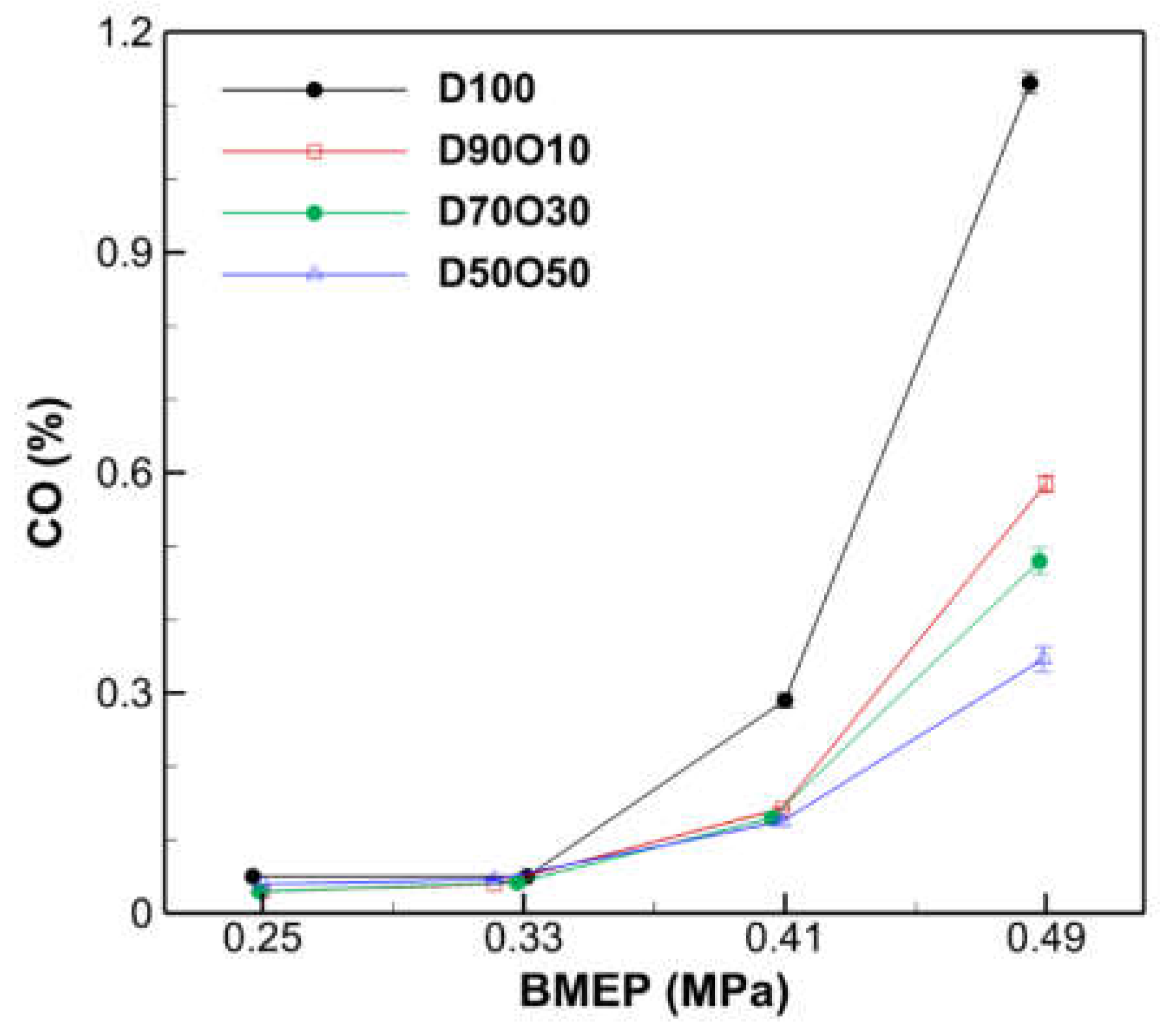
3.2.4. CO Emissions
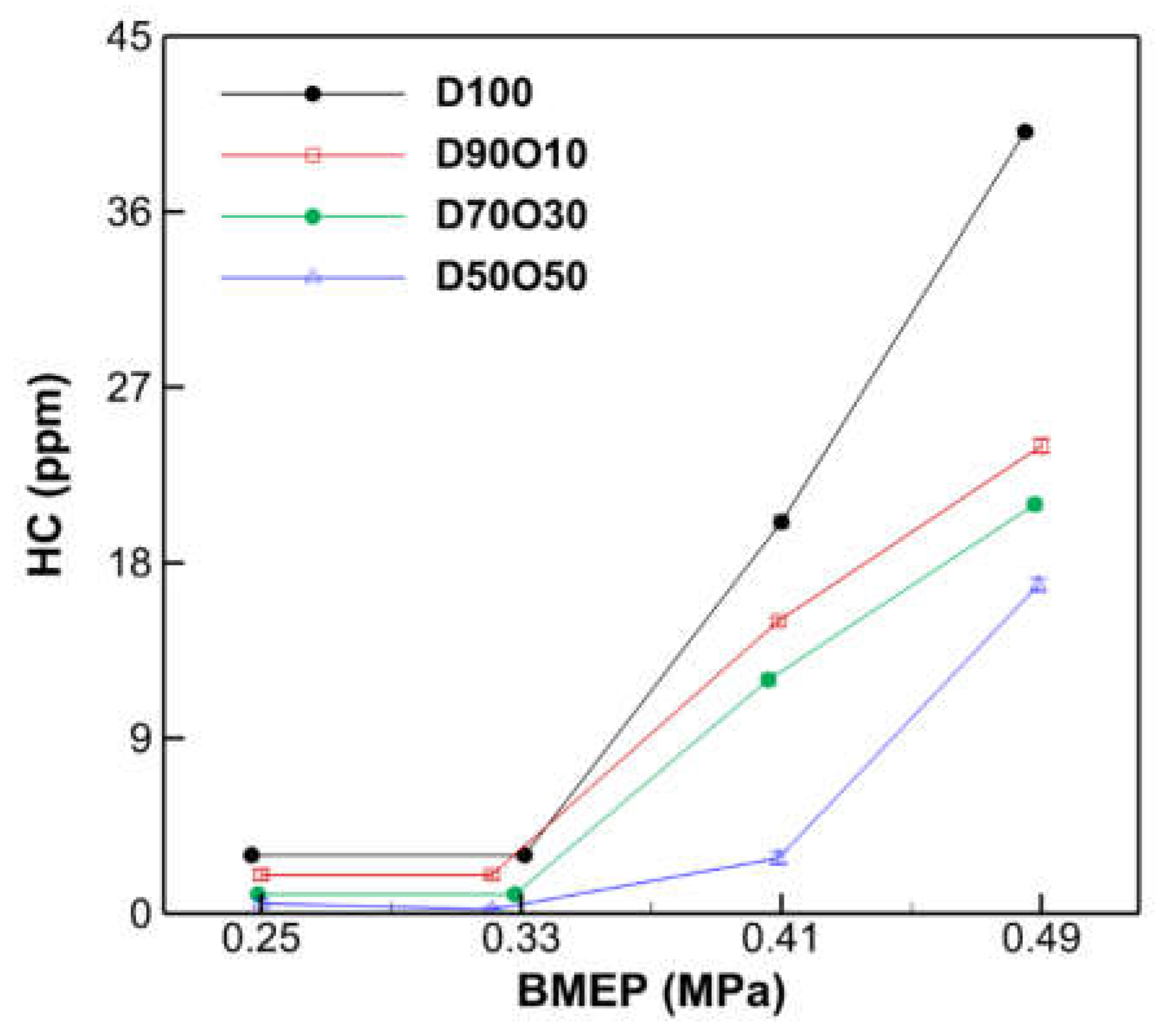
3.2.5. HC Emissions
3.2.6. Smoke Opacity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silverman, D.T.; Samanic, C.M.; Lubin, J.H.; Blair, A.E.; Stewart, P.A.; Vermeulen, R.; Coble, J.B.; Rothman, N.; Schleiff, P.L.; Travis, W.D.; Ziegler, R.G.; Wacholder, S.; Attfield, M.D. The diesel exhaust in miners study: a nested case–control study of lung cancer and diesel exhaust. J. Natl. Cancer. Inst. 2012, 104, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Grahame, T. J.; Schlesinger, R. B. Cardiovascular health and particulate vehicular emissions: a critical evaluation of the evidence. Air. Qual. Atmos. Health 2010, 3, 3–27. [Google Scholar] [CrossRef]
- Mohamed, L.; Al-Thukair, A.A. Environmental Assessments in the Oil and Gas Industry. Water Air Soil Pollut: Focus 2009, 9, 99–105. [Google Scholar] [CrossRef]
- Singh, A.P.; Agarwal, A.K. Low-temperature combustion: an advanced technology for internal combustion engines. In Advances in internal combustion engine research; Srivastava, D.K., Agarwal, A.K., Datta, A., Maurya, RK; Springer, Singapore, 2018, pp. 9-41.
- Ayodhya, A.S.; Narayanappa, K.G. An overview of after-treatment systems for diesel engines. Environ. Sci. Pollut. Res. 2018, 25, 35034–35047. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zheng, Z.; Wang, H.; Wang, X.; Sun, H.; Li, J.; Yao, M. Thermal Efficiency Enhancement of a Turbocharged Diesel Engine Dedicated for Hybrid Commercial Vehicle Application. SAE Tech. Paper 2022, No. 2022-01-7053. [Google Scholar]
- Li, J.; Ge, Y.; Wang, H.; Yu, C.; Yan, X.; Hao, L.; Tan, J. Effects of different diesel particulate filter on emission characteristics of in-use diesel vehicles. Energy Sources Part A-Recovery Util. Environ. Eff. 2019, 41, 2989–3000. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Cheung, C.S.; Chan, T.L.; Yao, C.D. Emission reduction from diesel engine using fumigation methanol and diesel oxidation catalyst. Sci. Total Environ. 2009, 407, 4497–4505. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jo, S.; Kwon, S.; Lee, J.T.; Park, S. NOX emission analysis according to after-treatment devices (SCR, LNT+ SCR, SDPF), and control strategies in Euro-6 light-duty diesel vehicles. Fuel 2022, 310, 122297. [Google Scholar] [CrossRef]
- Wu, G.; Feng, G.; Li, Y.; Ling, T.; Peng, X.; Su, Z.; Zhao, X. A Review of Thermal Energy Management of Diesel Exhaust after-Treatment Systems Technology and Efficiency Enhancement Approaches. Energies 2024, 17, 584. [Google Scholar] [CrossRef]
- Xue, J.; Grift, T.E.; Hansen, A.C. Effect of biodiesel on engine performances and emissions. Renew. Sustain. Energy Rev. 2011, 15, 1098–1116. [Google Scholar] [CrossRef]
- Ghazali, W.N.M.W.; Mamat, R.; Masjuki, H.H.; Najafi, G. Effects of biodiesel from different feedstocks on engine performance and emissions: A review. Renew. Sustain. Energy Rev. 2015, 51, 585–602. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, C.S. Applicability of dimethyl ether (DME) in a compression ignition engine as an alternative fuel. Energy Convers. Manag. 2014, 86, 848–863. [Google Scholar] [CrossRef]
- Hänggi, S.; Elbert, P.; Bütler, T.; Cabalzar, U.; Teske, S.; Bach, C.; Onder, C. A review of synthetic fuels for passenger vehicles. Energy Reports 2019, 5, 555–569. [Google Scholar] [CrossRef]
- Verma, S.; Suman, A.; Das, L.M.; Kaushik, S.C.; Tyagi, S.K. A renewable pathway towards increased utilization of hydrogen in diesel engines. Int. J. Hydrogen Energy 2020, 45, 5577–5587. [Google Scholar] [CrossRef]
- Karagöz, Y.; Sandalcı, T.; Yüksek, L.; Dalkılıç, A.S.; Wongwises, S. Effect of hydrogen–diesel dual-fuel usage on performance, emissions and diesel combustion in diesel engines. Adv. Mech. Eng. 2016, 8, 1–13. [Google Scholar] [CrossRef]
- Shamsul, N.S.; Kamarudin, S.K.; Rahman, N.A.; Kofli, N.T. An overview on the production of bio-methanol as potential renewable energy. Renew. Sustain. Energy Rev. 2014, 33, 578–588. [Google Scholar] [CrossRef]
- Mielenz, J. R. Ethanol production from biomass: technology and commercialization status. Curr. Opin. Microbiol. 2001, 4, 324–329. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Saka, S. Chemical conversion of cellulose as treated in supercritical methanol. Cellulose 2001, 8, 189–195. [Google Scholar] [CrossRef]
- Güllü, D.; Demirbaş, A. Biomass to methanol via pyrolysis process. Energy Convers. Manag. 2001, 42, 1349–1356. [Google Scholar] [CrossRef]
- Speth, R.L.; Chow, E.W.; Malina, R.; Barrett, S.R.; Heywood, J.B.; Green, W.H. Economic and environmental benefits of higher-octane gasoline. Environ. Sci. Technol. 2014, 48, 6561–6568. [Google Scholar] [CrossRef]
- French, R.; Malone, P. Phase equilibria of ethanol fuel blends. Fluid Phase Equilib. 2005, 228, 27–40. [Google Scholar] [CrossRef]
- Amine, M.; Awad, E.N.; Ibrahim, V.; Barakat, Y. Effect of ethyl acetate addition on phase stability, octane number and volatility criteria of ethanol-gasoline blends. Egypt. J. Pet. 2018, 27, 567–572. [Google Scholar] [CrossRef]
- Tephly, T.R. The toxicity of methanol. Life Sci. 1991, 48, 1031–1041. [Google Scholar] [CrossRef]
- Moriarty, K. Handbook for Handling, Storing, and Dispensing E85 and Other Ethanol-Gasoline Blends; National Renewable Energy Lab. (NREL), Golden, CO, United States, 2013.
- Jamrozik, A.; Tutak, W.; Grab-Rogaliński, K. Combustion stability, performance and emission characteristics of a CI engine fueled with diesel/n-butanol blends. Energies 2021, 14, 2817. [Google Scholar] [CrossRef]
- Yilmaz, N.; Vigil, F.M.; Atmanli, A.; Donaldson, B. Detailed analysis of PAH formation, toxicity and regulated pollutants in a diesel engine running on diesel blends with n-propanol, n-butanol and n-pentanol. Energies 2022, 15, 6487. [Google Scholar] [CrossRef]
- Wyman, C.E. Biomass ethanol: technical progress, opportunities, and commercial challenges. Annu. Rev. Energy Environ. 1999, 24, 189–226. [Google Scholar] [CrossRef]
- Yilmaz, N.; Ileri, E.; Atmanli, A. Performance of biodiesel/higher alcohols blends in a diesel engine. Int. J. Energy Res. 2016, 40, 1134–1143. [Google Scholar] [CrossRef]
- Algayyim, S.J.M.; Wandel, A.P.; Yusaf, T.; Hamawand, I. The impact of n-butanol and iso-butanol as components of butanol-acetone (BA) mixture-diesel blend on spray, combustion characteristics, engine performance and emission in direct injection diesel engine. Energy 2017, 140, 1074–1086. [Google Scholar] [CrossRef]
- Heufer, K.A.; Sarathy, S.M.; Curran, H.J.; Davis, A.C.; Westbrook, C.K.; Pitz, W. J. Detailed kinetic modeling study of n-pentanol oxidation. Energy Fuels 2012, 26, 6678–6685. [Google Scholar] [CrossRef]
- Devarajan, Y.; Munuswamy, D.B.; Nagappan, B. ; Pandian, AK Performance, combustion and emission analysis of mustard oil biodiesel and octanol blends in diesel engine. Heat Mass Transf. 2018, 54, 1803–1811. [Google Scholar] [CrossRef]
- Kumar, B.R.; Saravanan, S.; Rana, D.; Anish, V.; Nagendran, A. Effect of a sustainable biofuel–n-octanol–on the combustion, performance and emissions of a DI diesel engine under naturally aspirated and exhaust gas recirculation (EGR) modes. Energy Convers. Manag. 2016, 118, 275–286. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Wang, Z.; Liu, H. Combustion and emissions of compression ignition in a direct injection diesel engine fueled with pentanol. Energy 2015, 80, 575–581. [Google Scholar] [CrossRef]
- Sidharth; Kumar, N. Performance and emission studies of ternary fuel blends of diesel, biodiesel and octanol. Energy Sources Part A-Recovery Util. Environ. Eff. 2020, 42, 2277–2296. [Google Scholar]
- Gautam, M.; Martin, D.W. Combustion characteristics of higher-alcohol/gasoline blends. Proc. Instn. Mech. Engrs. Part A 2000, 214, 497–511. [Google Scholar] [CrossRef]
- Yesilyurt, M.K. A detailed investigation on the performance, combustion, and exhaust emission characteristics of a diesel engine running on the blend of diesel fuel, biodiesel and 1-heptanol (C7 alcohol) as a next-generation higher alcohol. Fuel 2020, 275, 117893. [Google Scholar] [CrossRef]
- Gautam, M.; Martin, D.W.; Carder, D. Emissions characteristics of higher alcohol/gasoline blends. Proc. Instn. Mech. Engrs. Part A 2000, 214, 165–182. [Google Scholar] [CrossRef]
- Kumar, B.R.; Saravanan, S. Use of higher alcohol biofuels in diesel engines: A review. Renew. Sustain. Energy Rev. 2016, 60, 84–115. [Google Scholar] [CrossRef]
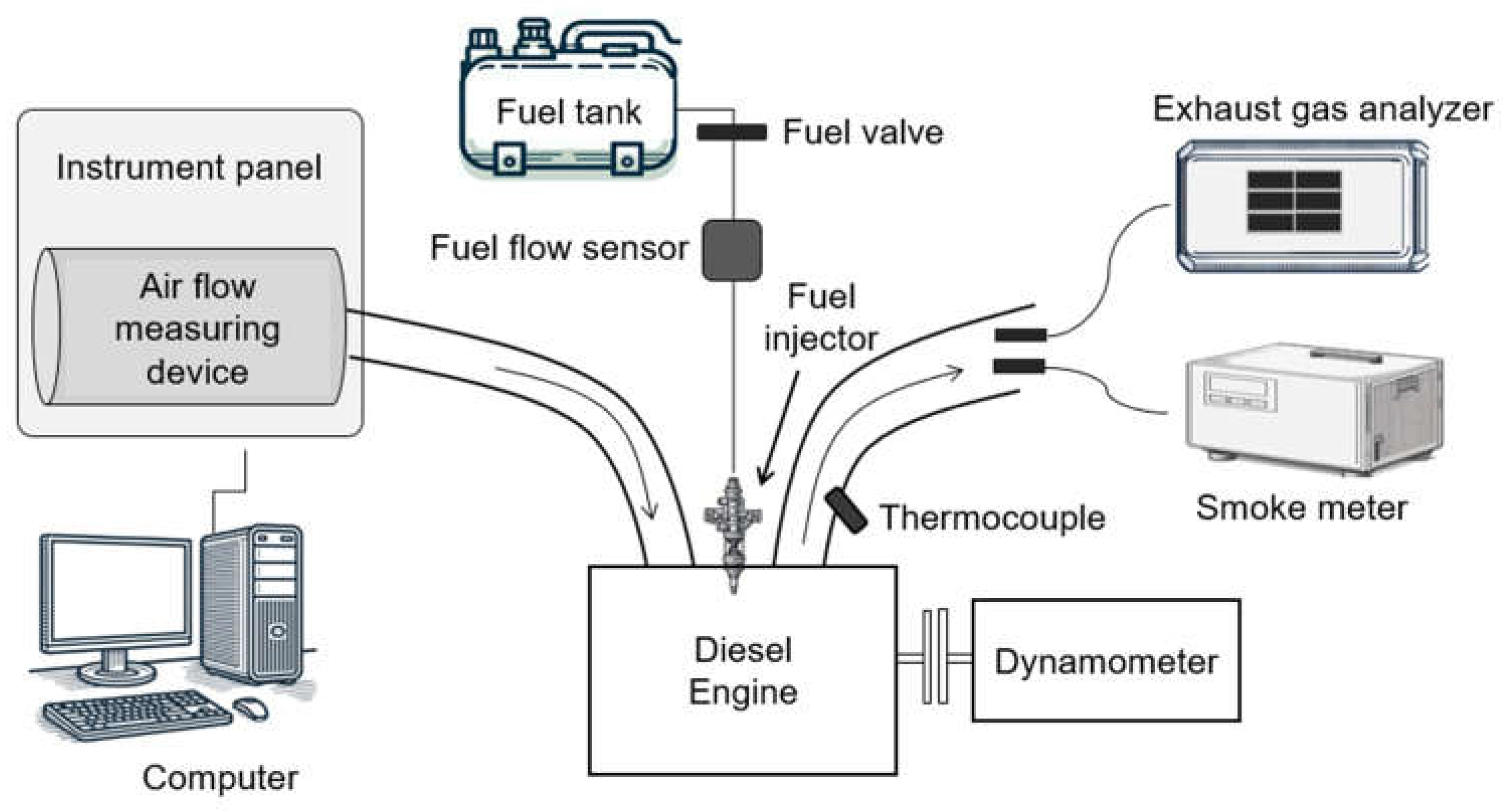




| Parameter | Specifications |
|---|---|
| Model | MITSUKI MIT-178F |
| Number of cylinders | 1 |
| Ignition | Compression ignition |
| Injection type | Direct injection |
| Injector nozzle | Hole type |
| Cooling system | Air-cooled |
| Rated power | 5.22 kW @ 3000 rpm |
| Swept volume | 298.6 cm3 |
| Bore | 78 mm |
| Stroke | 62.5 mm |
| Compression ratio | 21 |
| Lubrication oil | SAE 5W-30 API CF |
| Emissions | Range | Accuracy | Resolution |
|---|---|---|---|
| NOx | 0-5000 ppm | ±15 ppm | 1 ppm |
| CO | 0-10% | ±0.02% | 0.01% |
| HC | 0-9999 ppm | ±20 ppm | 1 ppm |
| Smoke | 0-100% | ±1% | 0.1% |
| Properties | Diesel | n-octanol |
|---|---|---|
| Lower heating value (MJ/kg) | 42.9 | 37.6 |
| Latent heat of vaporization (MJ/kg) | 0.27 | 0.55 |
| Cetane number | >52 | 37 |
| Self-ignition temperature (℃) | 260 | 253 |
| Density (kg/m3) | 840 | 820 |
| Kinematic viscosity at 20℃ (mm2/s) | 3.4 | 10.2 |
| Oxygen (wt.%) | 0 | 12.3 |
| Stoichiometric air-fuel ratio (AFR) | 14.9 | 12.7 |
| BMEP | AFRD100 | AFRD90O10 | AFRD70O30 | AFRD50O50 |
|---|---|---|---|---|
| 0.247 | 23.0 | 21.6 | 21.4 | 21.6 |
| 0.330 | 20.3 | 19.4 | 19.0 | 19.6 |
| 0.412 | 17.2 | 16.3 | 15.8 | 15.9 |
| 0.494 | 14.5 | 13.7 | 13.6 | 13.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





