Submitted:
02 May 2024
Posted:
03 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Collection of Predators and Prey Items
2.2. Predation Experiments
2.3. Fisheries Trends
2.4. Data Analysis
3. Results
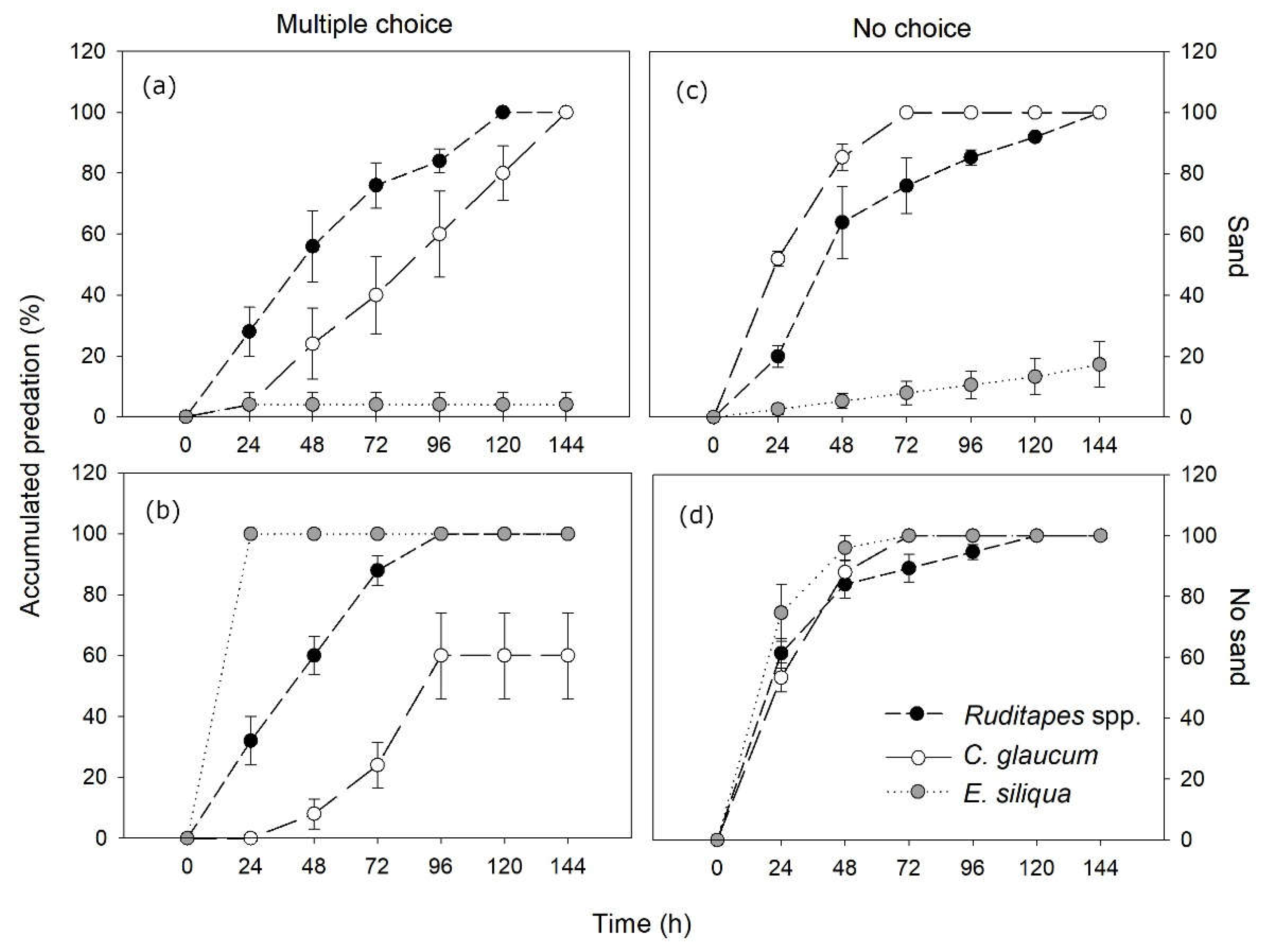
3.1. Multiple Choice Experiments
3.2. No Choice Experiments
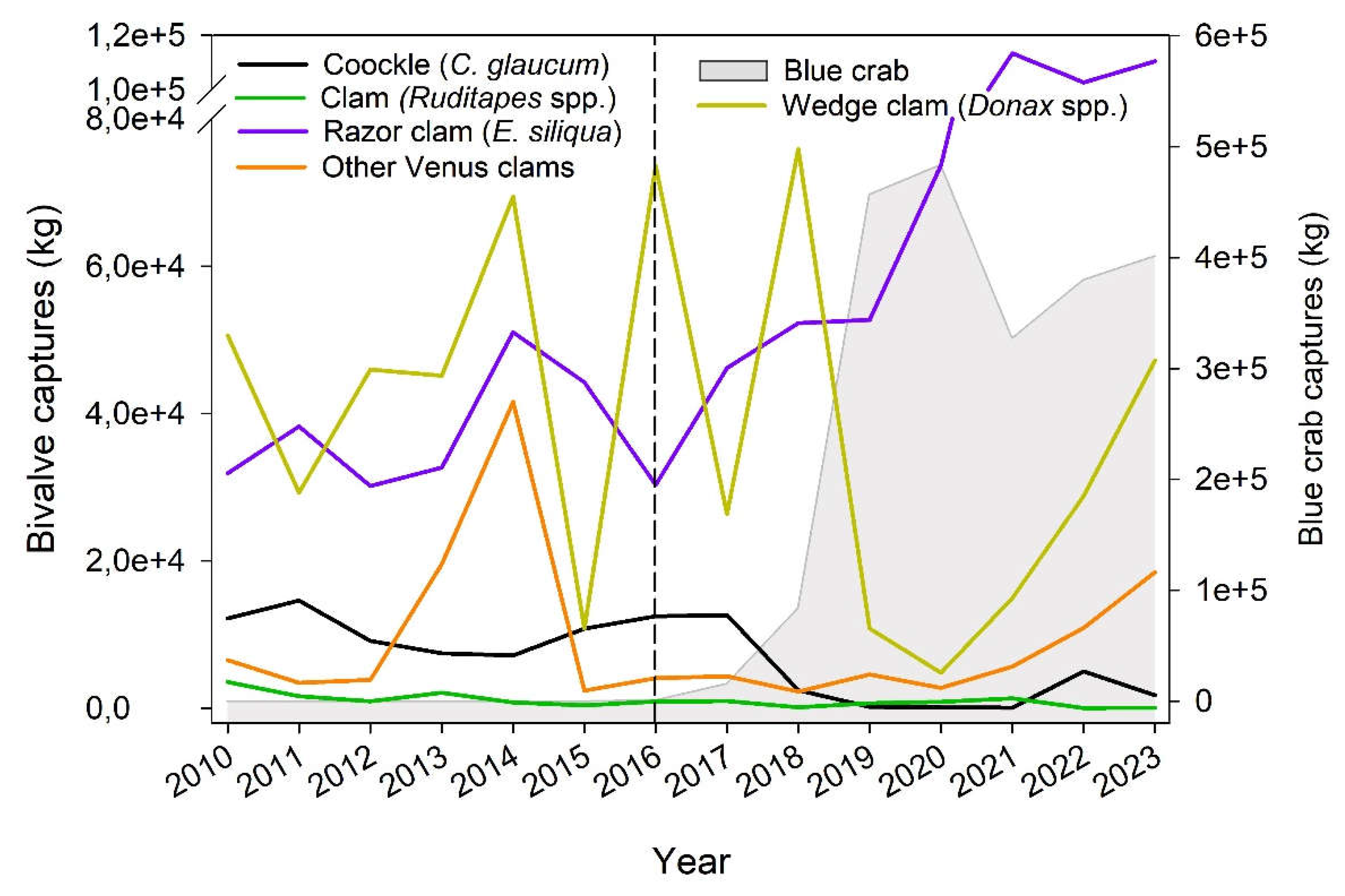
3.3. Fisheries Trends
4. Discussion
4.1. Patterns of Experimental Predation
4.2. Bivalves’ Fisheries Patterns
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, J.; Fowler, D.L.; Avyle, M.V. Species profiles: Life histories and environmental requirements of coastal fishes and invertebrates (Mid-Atlantic) – Blue crab. U.S. Fish and Widlife Service Biological Report. 1989, 82, 18 pp. [Google Scholar]
- Hines, A.H. Ecology of juvenile and adult blue crabs: summary of discussion of research themes and directions. Bull. Mar. Sci. 2003, 72, 423–433. [Google Scholar]
- Mancinelli, G.; Carrozzo, L.; Marini, G.; Costantini, M.L.; Rossi, L.; Pinna, M. Occurrence of the Atlantic blue crab Callinectes sapidus (Decapoda, Brachyura, Portunidae) in two Mediterranean coastal habitats: temporary visitor or permanent resident? Estuar. Coast. Shelf Sci. 2013, 135, 46–56. [Google Scholar] [CrossRef]
- Nehring, S. Invasion history and success of the American blue crab Callinectes sapidus in European and adjacent waters. In Galil, B.S., Clark, P.F., Carlton, J.T. (Eds.), In the wrong place–alien marine crustaceans: distribution, biology and impacts; Invading Nature-Springer Series 6: Berlin, Germany, 2011; pp. 607–624. [Google Scholar]
- Mancinelli, G.; Chainho, P.; Cilenti, L.; Falco, S.; Kapiris, K.; Katselis, G.; Ribeiro, F. The Atlantic blue crab Callinectes sapidus in southern European coastal waters: distribution, impact and prospective invasion management strategies. Mar. Poll. Bull. 2017, 119, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Clavero, M.; Franch, N.; Bernardo-Madrid, R.; López, V.; Abelló, P.; Queral, J. M.; Mancinelli, G. Severe, rapid and widespread impacts of an Atlantic blue crab invasion. Mar. Poll. Bull. 2022, 176, 113479. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Weissburg, M.J. Habitat complexity and predator size mediate interactions between intraguild blue crab predators and mud crab prey in oyster reefs. Mar. Ecol. Progr. Ser. 2013, 488, 209–219. [Google Scholar] [CrossRef]
- Prado, P.; Ibáñez, C.; Chen, L.; Caiola, N. Feeding habits and short-term mobility patterns of blue crab, Callinectes sapidus, across invaded habitats of the Ebro Delta subjected to contrasting salinity. Estuar. Coast. 2022, 45, 839–855. [Google Scholar] [CrossRef]
- Miller, R.E.; Sulkin, S.D.; Lippson, R.L. Composition and seasonal abundance of the blue crab, Callinectes sapidus Rathbun, in the Chesapeake and Delaware Canal and adjacent waters. Chesapeake Sci. 1975, 16, 27–31. [Google Scholar] [CrossRef]
- Laughlin, R.A. Feeding habits of the blue crab, Callinectes sapidus Rathbun, in the Apalachicola estuary, Florida. Bull. Mar. Sci. 1982, 32, 807–822. [Google Scholar]
- Arnold, W.S. The effects of prey size, predator size, and sediment composition on the rate of predation of the blue crab, Callinectes Sapidus Rathbun, on the hard clam, Mercenaria mercenaria (Linné). J. Exp. Mar. Biol. Ecol. 1984, 80, 207–219. [Google Scholar] [CrossRef]
- Eggleston, D.B. Foraging behavior of the blue crab, Callinectes sapidus, on juvenile oysters, Crassostrea virginica: effects of prey density and size. Bull. Mar. Sci. 1990, 46, 62–82. [Google Scholar]
- Lin, J. Predator-prey interactions between blue crabs and ribbed mussels living in clumps. Estuar. Coast Shelf Sci. 1991, 2, 61–69. [Google Scholar] [CrossRef]
- Prado, P.; Peñas, A.; Ibáñez, C.; Cabanes, P.; Jornet, L.; Álvarez, N.; Caiola, N. Prey size and species preferences in the invasive blue crab, Callinectes sapidus: Potential effects in marine and freshwater ecosystems. Estuar. Coast. Shelf Sci. 2020, 245, 106997. [Google Scholar] [CrossRef]
- Haddon, M.; Wear, R.G.; Packer, H.A. Depth and density of burial by the bivalve Paphies ventricosa as refuges from predation by the crab Ovalipes catharus. Mar. Biol 1987, 94, 25–30. [Google Scholar] [CrossRef]
- Sponaugle, S.; Lawton, P. Portunid crab predation on juvenile hard clams: effects of substrate type and prey density. Mar. Ecol. Progr. Ser. Oldendorf 1990, 67, 43–53. [Google Scholar]
- Seitz, R.D.; Lipcius, R.N.; Seebo, M.S. Food availability and growth of the blue crab in seagrass and unvegetated nurseries of Chesapeake Bay. J. Exp. Mar. Biol. Ecol 2005, 319, 57–68. [Google Scholar] [CrossRef]
- Gosling, E. Marine Bivalve Molluscs, 2nd edn. West Sussex UK Wiley Blackwell. 2015, West Sussex, UK. [Google Scholar]
- Fraser, S.; Shelmerdine, R.L.; Mouat, B. (2018). Razor clam biology, ecology, stock assessment, and exploitation: a review of Ensis spp. in Wales. UHI Research Database pdf download summary, 2018; Welsh Government, 2018; pp. 62. [Google Scholar]
- Castejón, D.; Guerao, G. (2013). A new record of the American blue crab, Callinectes sapidus Rathbun, 1896 (Decapoda: Brachyura: Portunidae), from the Mediterranean coast of the Iberian Peninsula. BioInvasions Rec. 2013, 2, 141–143. [Google Scholar] [CrossRef]
- López, V.; Rodon, J. , Diagnosi i situació actual del cranc blau (Callinectes sapidus) al Delta de l’Ebre. Informe Tècnic-Servei de Recursos Marins, Direcció General de Pesca i Afers Marítims, Generalitat de Catalunya, 2018; 86 pp.
- López, V. Seguiment del cranc blau (Callinectes sapidus) al Delta de l’Ebre. Informe Tècnic-Servei de Recursos Marins, Direcció General de Pesca i Afers Marítims, Monverte Estudis Ambientals, Amposta, 2020; 127 pp.
- Baeta, M.; Solís, M.A.; Frias-Vidal, S.; Claramonte, L.; Ballesteros, M. Management and ecology of the wedge clam (Donax trunculus) in the NW Mediterranean Sea: The case of Ebro Delta (NE Spain). Reg. Stud. Mar. Sci. 2023, 66, 103158. [Google Scholar] [CrossRef]
- Blundon, J.A.; Kennedy, V.S. (1982a). Refuges for infaunal bivalves from blue crab, Callinectes sapidus (Rathbun), predation in Chesapeake Bay. J. Exp. Mar. Biol. Ecol. 1982, 56, 67–81. [Google Scholar] [CrossRef]
- Muir, S.D. The biology of razor clams (Ensis spp.) and their emergent fishery on the West coast of Scotland. PhD thesis, University of London. 2003; pp. 280.
- Blundon, J.A.; Kennedy, V.S. Mechanical and behavioral aspects of blue crab, Callinectes sapidus (Rathbun), predation on Chesapeake Bay bivalves. J. Exp. Mar. Biol. Ecol. 1982, 65, 47–65. [Google Scholar] [CrossRef]
- Ebersole, E.L.; Kennedy, V.S. Prey preferences of blue crabs Callinectes sapidus feeding on three bivalve species. Mar. Ecol. Progr. Ser. 1995, 118, 167–177. [Google Scholar] [CrossRef]
- Kutluyer Kocabaş, F.; Kocabaş, M.; Çanakçi, A.; Karabacak, A.H. Mechanical property and structural-elemental analysis of marine bivalve mollusc shells: Cerastoderma edule, Chamelea gallina, Donax trunculus, Ruditapes decussatus. Internat. Aquat. Res. 2023, 15, 39–50. [Google Scholar]
- Bejaoui, S.; Rabeh, I.; Chetoui, I.; Telahigue, K.; Ghribi, F.; Fouzai, C.; El Cafsi, M. Examination of the nutritional value of four bivalves species from Bizerte lagoon. INSTM Bull. Mar. Freshw. Sci. 2019, 46, 71–79. [Google Scholar]
- Carrasco, N.; Hine, P.M.; Durfort, M.; Andree, K. B.; Malchus, N.; Lacuesta, B.; Gonzalez, M.; Roque, A.; Rodgers, R.; Furones, M.D. Marteilia cochillia sp. nov., a new Marteilia species affecting the edible cockle Cerastoderma edule in European waters. Aquaculture. 2013, 412, 223–230. [Google Scholar] [CrossRef]
- Baeta, M.; Rubio, C.; Breton, F. (2021). Impact of mechanized clam dredging on the discarded megabenthic fauna on the Catalan coast (NW Mediterranean). J. Mar. Biol. Assoc. UK. 2021, 101, 545–553. [Google Scholar] [CrossRef]
- Piersma, T.; Koolhaas, A.; Dekinga, A.; Beukema, J.J.; Dekker, R.; Essink, K. Long-term indirect effects of mechanical cockle-dredging on intertidal bivalve stocks in the Wadden Sea. J. Appl. Ecol. 2001, 38, 976–990. [Google Scholar] [CrossRef]
- Ramón, M.; Cano, J.; Peña, J.B.; Campos, M.J. Current status and perspectives of mollusc (bivalves and gastropods) culture in the Spanish Mediterranean. Bolet. Instit. Esp. Oceanograf 2005, 21, 361–373. [Google Scholar]
- Köck, M.; Farré, M.; Martínez, E.; Gajda-Schrantz, K.; Ginebreda, A.; Navarro, A.; López de Alda, M.; Barceló, D. Integrated ecotoxicological and chemical approach for the assessment of pesticide pollution in the Ebro River delta (Spain). J. Hydrol, 2010; 383, 73–82. [Google Scholar]
- De Juan, S.; Demestre, M.; Sanchez, P. Exploring the degree of trawling disturbance by the analysis of benthic communities ranging from a heavily exploited fishing ground to an undisturbed area in the NW Mediterranean. Sci. Mar. 2011, 75, 507–516. [Google Scholar] [CrossRef]
- Nerot, C.; Lorrain, A.; Grall, J.; Gillikin, D.P.; Munaron, J.M.; Le Bris, H.; Paulet, Y.M. Stable isotope variations in benthic filter feeders across a large depth gradient on the continental shelf. Estuar. Coast. Shelf Sci. 2012, 96, 228–235. [Google Scholar] [CrossRef]
- Prado, P.; Baeta, M.; Mestre, E.; Solis, M.A.; Sanhauja, I.; Gairin, I.; Camps-Castellà, J.; Falco, S.; Ballesteros, M. Trophic role and predatory interactions between the blue crab, Callinectes sapidus, and native species in open waters of the Ebro Delta. Estuar. Coast. Shelf Sci. 2024, 298, 108638. [Google Scholar] [CrossRef]

| A) Multiple choice experiments | df | MS | F | P | Eta square |
|---|---|---|---|---|---|
| Between subjects | |||||
| Time (Ti) | 4.33 | 32677.58 | 196.38 | 0.000 | 0.891 |
| Ti x S | 4.33 | 1568.55 | 9.42 | 0.000 | 0.282 |
| Ti x Sp | 8.66 | 4207.57 | 25.28 | 0.000 | 0.678 |
| Ti x S x Sp | 8.66 | 1915.41 | 11.51 | 0.000 | 0.490 |
| Error | 103.98 | 166.39 | |||
| Within subjects | |||||
| Substrate (S) | 1 | 31697.14 | 42.12 | 0.000 | 0.637 |
| Species (Sp) | 2 | 15716.19 | 20.88 | 0.000 | 0.635 |
| S x Sp | 2 | 45274.28 | 60.17 | 0.000 | 0.834 |
| Error | 24 | 752.38 | |||
| B) No choice experiments | df | MS | F | P | Eta square |
| Between subjects | |||||
| Time (Ti) | 3.57 | 50233.04 | 577.15 | 0.000 | 0.960 |
| Ti x S | 3.57 | 2235.88 | 25.68 | 0.000 | 0.517 |
| Ti x Sp | 7.13 | 1839.54 | 21.13 | 0.000 | 0.638 |
| Ti x S x Sp | 7.13 | 1607.84 | 18.47 | 0.000 | 0.606 |
| Error | 85.67 | 87.03 | |||
| Within subjects | |||||
| Substrate (S) | 1 | 44200.84 | 217.32 | 0.000 | 0.901 |
| Species (Sp) | 2 | 19663.06 | 96.67 | 0.000 | 0.890 |
| S x Sp | 2 | 26469.41 | 130.14 | 0.000 | 0.916 |
| Error | 24 | 203.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





