Submitted:
02 May 2024
Posted:
03 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. General
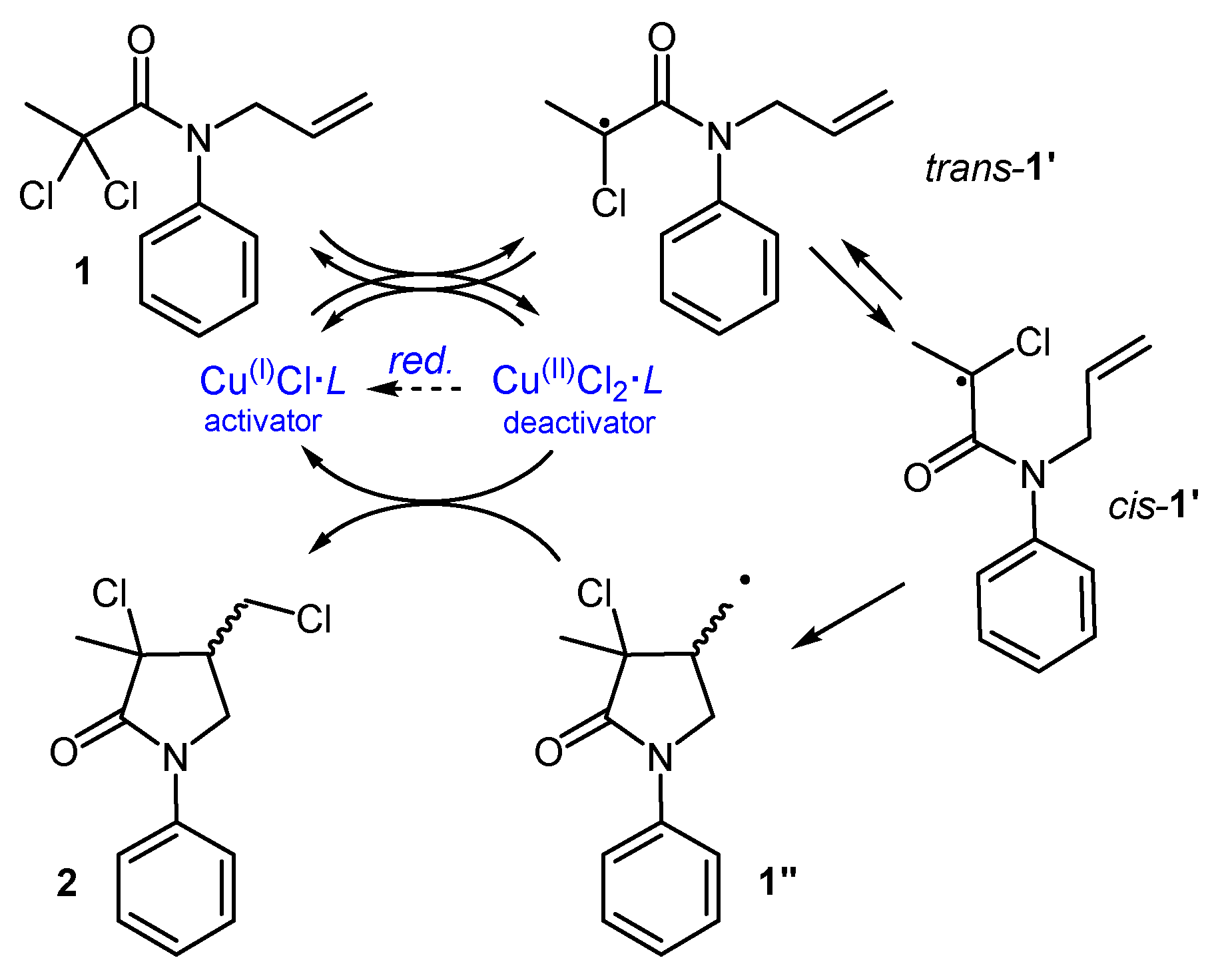
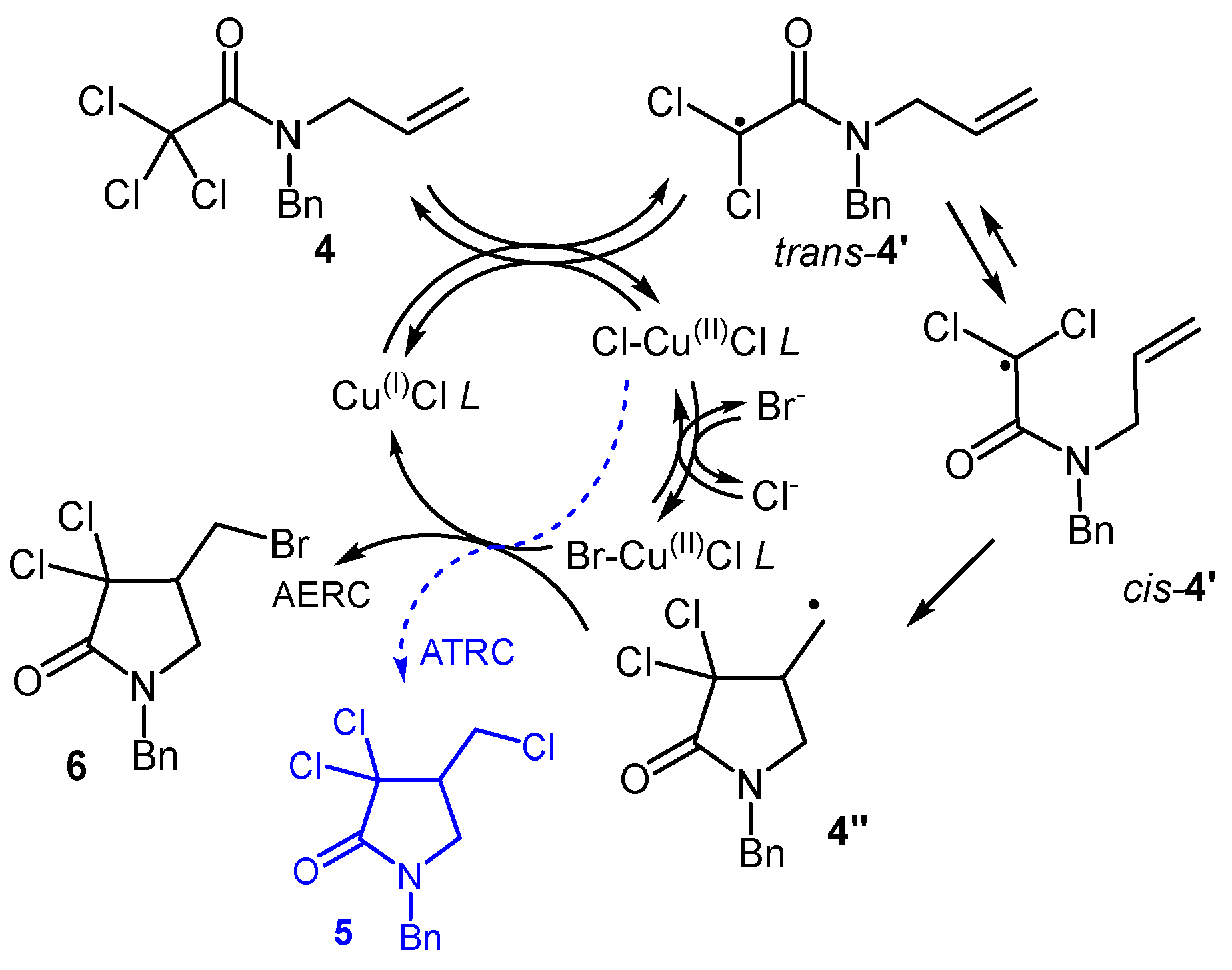
2.1. ARGET-ATRC towards 2
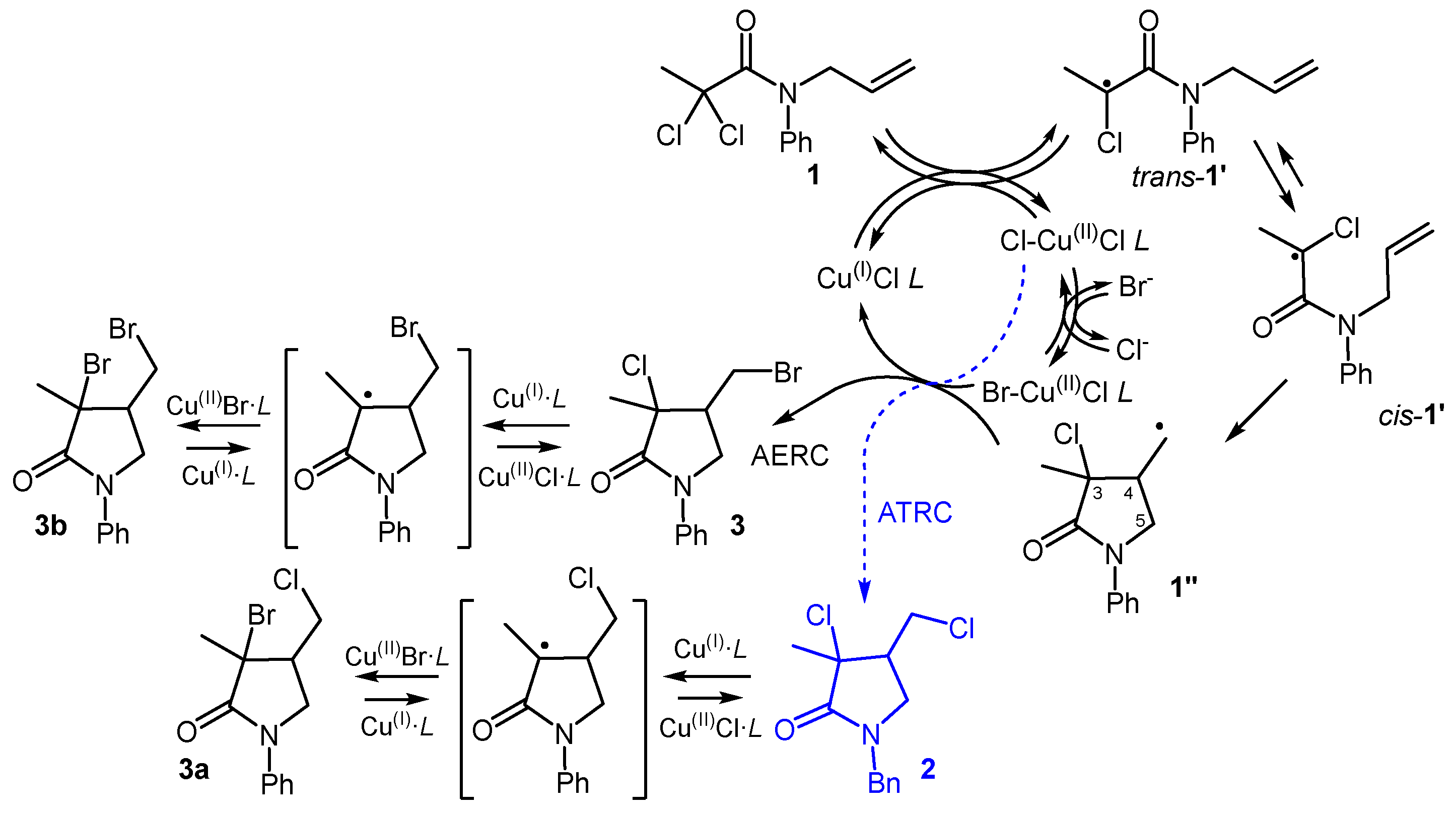
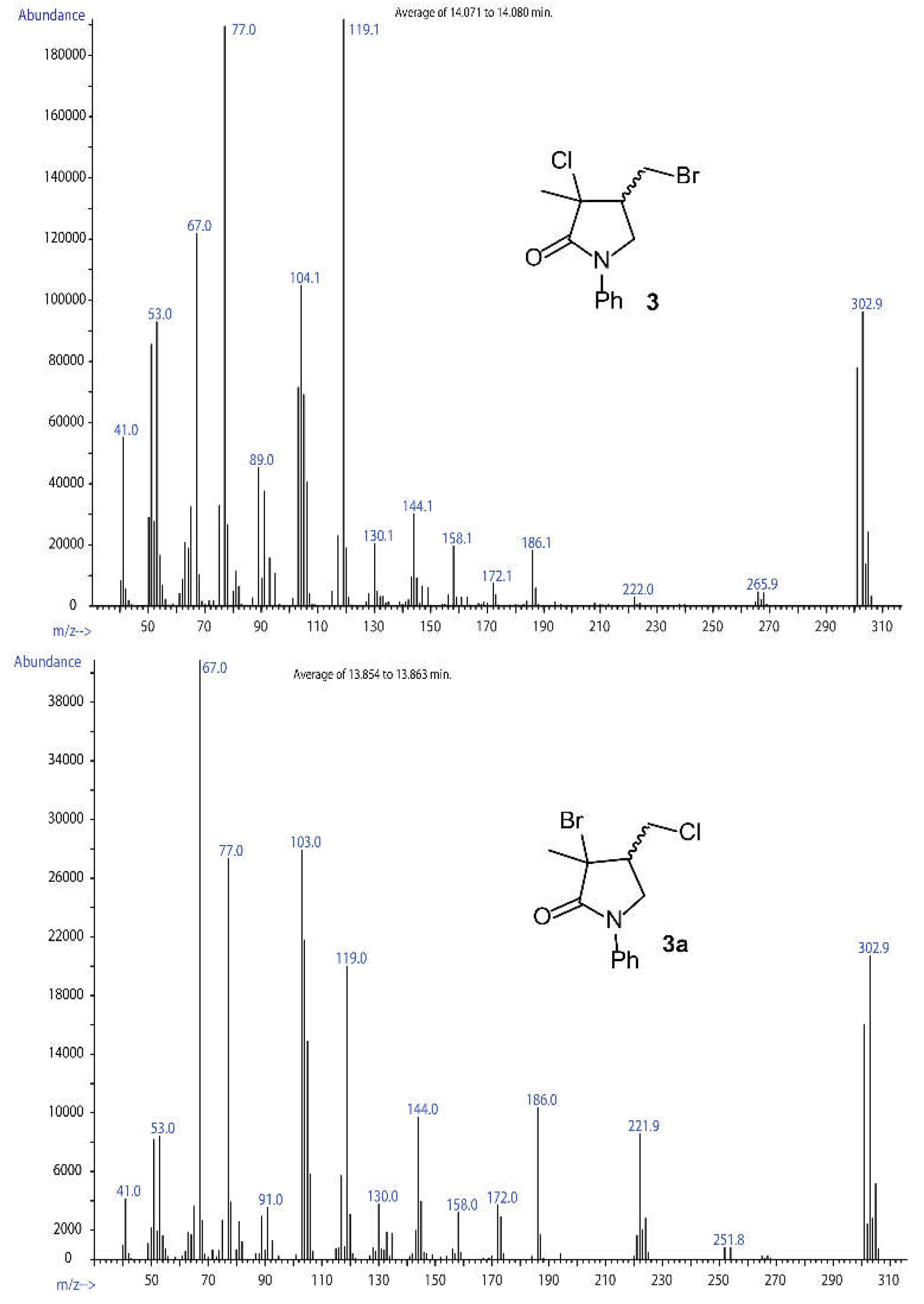
2.2. SARA-AERC towards 3 or 6
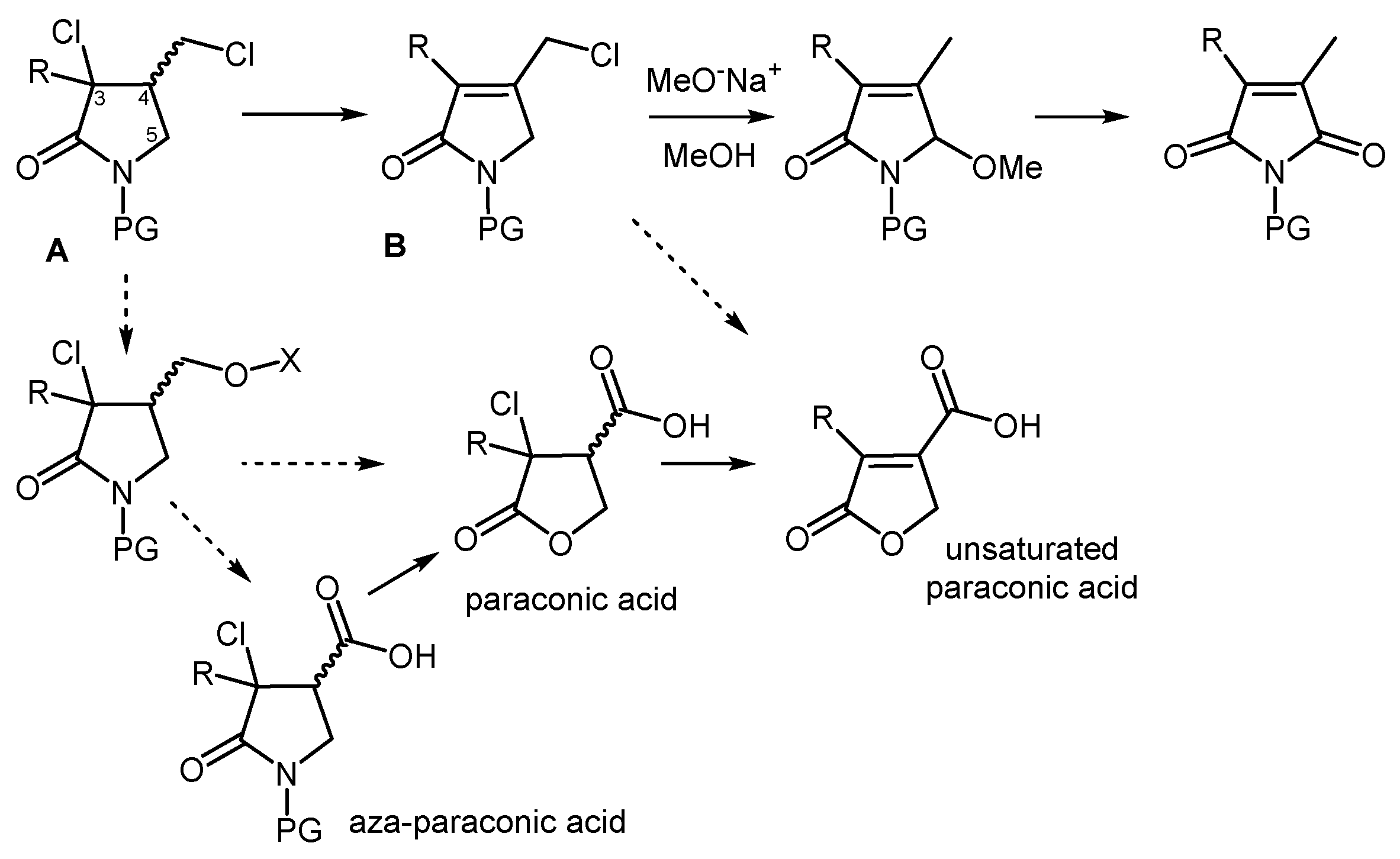
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Clark, A.J. Copper Catalyzed Atom Transfer Radical Cyclization Reactions. Eur J Org Chem 2016, 2016, 2231–2243. [Google Scholar] [CrossRef]
- Clark, A.J.; Cornia, A.; Felluga, F.; Gennaro, A.; Ghelfi, F.; Isse, A.A.; Menziani, M.C.; Muniz-Miranda, F.; Roncaglia, F.; Spinelli, D. Arylsulfonyl Groups: The Best Cyclization Auxiliaries for the Preparation of ATRC Γ-Lactams Can Be Acidolytically Removed. Eur J Org Chem 2014, 2014, 6734–6745. [Google Scholar] [CrossRef]
- Fischer, H. The Persistent Radical Effect: A Principle for Selective Radical Reactions and Living Radical Polymerizations. Chem Rev 2001, 101, 3581–3610. [Google Scholar] [CrossRef] [PubMed]
- Bellesia, F.; Clark, A.J.; Felluga, F.; Gennaro, A.; Isse, A.A.; Roncaglia, F.; Ghelfi, F. Efficient and Green Route to Γ-Lactams by Copper-Catalysed Reversed Atom Transfer Radical Cyclisation of A-Polychloro- N -allylamides, Using a Low Load of Metal (0.5 Mol%). Adv Synth Catal 2013, 355, 1649–1660. [Google Scholar] [CrossRef]
- Bergmann, R.; Gericke, R. Synthesis and Antihypertensive Activity of 4-(1,2-Dihydro-2-Oxo-1-Pyridyl)-2H-1-Benzopyrans and Related Compounds, New Potassium Channel Activators. J Med Chem 1990, 33, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Moody, C.M.; Young, D.W. Stereospecific Synthesis of 4-Alkylglutamates and 4-Alkylprolines. Tetrahedron Lett 1994, 35, 7277–7280. [Google Scholar] [CrossRef]
- Corey, E.J.; Li, W.-D.Z. Total Synthesis and Biological Activity of Lactacystin, Omuralide and Analogs. Chem Pharm Bull (Tokyo) 1999, 47, 1–10. [Google Scholar] [CrossRef]
- Nilsson, B.M.; Hacksell, U. Base-Catalyzed Cyclization of N -propargylamides to Oxazoles. J Heterocycl Chem 1989, 26, 269–275. [Google Scholar] [CrossRef]
- Ghelfi, F.; Pattarozzi, M.; Roncaglia, F.; Parsons, A.; Felluga, F.; Pagnoni, U.; Valentin, E.; Mucci, A.; Bellesia, F. Preparation of the Maleic Anhydride Nucleus from Dichloro γ-Lactams: Focus on the Role of the N-Substituent in the Functional Rearrangement and in the Hydrolytic Steps. Synthesis (Stuttg) 2008, 2008, 3131–3141. [Google Scholar] [CrossRef]
- Slough, G.A. (Ph3P)3RuCl2 Catalyzed Equilibration and Elimination of α-Chloro-N-Tosyl-2-Pyrrolidinones: A Unique Route to Unsaturated 2-Pyrrolidinones. Tetrahedron Lett 1993, 34, 6825–6828. [Google Scholar] [CrossRef]
- Kornblum, N.; Jones, W.J.; Anderson, G.J. A New and Selective Method of Oxidation. The Conversion of Alkyl Halides and Alkyl Tosylates to Aldehydes. J Am Chem Soc 1959, 81, 4113–4114. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, J.; Shen, Z.; Zhang, Y. Efficient and Convenient Oxidation of Organic Halides to Carbonyl Compounds by H2O2 in Ethanol. Tetrahedron Lett 2007, 48, 1919–1921. [Google Scholar] [CrossRef]
- Patil, R.D.; Adimurthy, S. Direct and Selective Conversion of Benzyl Bromides to Benzaldehydes with Aqueous H 2 O 2 Without Catalyst. Synth Commun 2011, 41, 2712–2718. [Google Scholar] [CrossRef]
- Lei, Z.; Wang, R. Oxidation of Alcohols Using H2O2 as Oxidant Catalyzed by AlCl3. Catal Commun 2008, 9, 740–742. [Google Scholar] [CrossRef]
- Zheng, P.; Yan, L.; Ji, X.; Duan, X. A Green Procedure for the Oxidation of Benzyl Halides to Aromatic Aldehydes or Ketones in Aqueous Media. Synth Commun 2010, 41, 16–19. [Google Scholar] [CrossRef]
- Smith, M.B. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. Ed. 8th; John Wiley & Sons.; 2020; Section 10.45; pp. 534.
- Boivin, J.; Yousfi, M.; Zard, S.Z. A Versatile Radical Based Synthesis of γ—Lactams Using Nickel Powder / Acetic Acid. Tetrahedron Lett 1994, 35, 5629–5632. [Google Scholar] [CrossRef]
- Peng, C.-H.; Kong, J.; Seeliger, F.; Matyjaszewski, K. Mechanism of Halogen Exchange in ATRP. Macromolecules 2011, 44, 7546–7557. [Google Scholar] [CrossRef]
- Ince, J.; Ross, T.M.; Shipman, M.; Slawin, A.M.Z.; Ennis, D.S. Observations on the Synthesis of Functionalised Methyleneaziridines. Tetrahedron 1996, 52, 7037–7044. [Google Scholar] [CrossRef]
- Bellesia, F.; D’Anna, F.; Felluga, F.; Frenna, V.; Ghelfi, F.; Parsons, A.; Reverberi, F.; Spinelli, D. Breakthrough in the α-Perchlorination of Acyl Chlorides. Synthesis (Stuttg) 2012, 2012, 605–609. [Google Scholar] [CrossRef]
- Benedetti, M.; Forti, L.; Ghelfi, F.; Pagnoni, U.M.; Ronzoni, R. Halogen Atom Transfer Radical Cyclization of N-Allyl-N-Benzyl-2,2-Dihaloamides to 2-Pyrrolidinones, Promoted by Fe0-FeCl3 or CuCl-TMEDA. Tetrahedron 1997, 53, 14031–14042. [Google Scholar] [CrossRef]
- McGivern, W.S.; Derecskei-Kovacs, A.; North, S.W.; Francisco, J.S. Computationally Efficient Methodology to Calculate C−H and C−X (X = F, Cl, and Br) Bond Dissociation Energies in Haloalkanes. J Phys Chem A 2000, 104, 436–442. [Google Scholar] [CrossRef]
- Peng, C.-H.; Zhong, M.; Wang, Y.; Kwak, Y.; Zhang, Y.; Zhu, W.; Tonge, M.; Buback, J.; Park, S.; Krys, P.; et al. Reversible-Deactivation Radical Polymerization in the Presence of Metallic Copper. Activation of Alkyl Halides by Cu 0. Macromolecules 2013, 46, 3803–3815. [Google Scholar] [CrossRef]
- Clark, A.J.; Duckmanton, J.N.; Felluga, F.; Gennaro, A.; Ghelfi, F.; Hardiman, J.R.D.; Isse, A.A.; Manferdini, C.; Spinelli, D. Cu 0 -Promoted Cyclisation of Unsaturated A-Halogeno Amides To Give Β- and Γ-Lactams. Eur J Org Chem 2016, 2016, 2479–2491. [Google Scholar] [CrossRef]
- Braidi, N.; Buffagni, M.; Ghelfi, F.; Parenti, F.; Gennaro, A.; Isse, A.A.; Bedogni, E.; Bonifaci, L.; Cavalca, G.; Ferrando, A.; et al. ARGET ATRP of Styrene in EtOAc/EtOH Using Only Na 2 CO 3 to Promote the Copper Catalyst Regeneration. J Macromol Sci A 2021, 58, 376–386. [Google Scholar] [CrossRef]
- Pattarozzi, M.; Roncaglia, F.; Giangiordano, V.; Davoli, P.; Prati, F.; Ghelfi, F. ‘Ligand-Free-Like’ CuCl-Catalyzed Atom Transfer Radical Cyclization of N-Substituted N-Allyl Polychloroamides to γ-Lactams. Synthesis (Stuttg) 2010, 2010, e2–e2. [Google Scholar] [CrossRef]
| entry | EtOAc (mL) | EtOH (mL) |
Bromide | Carbonate | yield of 2 (%) | yield of 3 (%) |
|---|---|---|---|---|---|---|
| 1 | 1.5 | 0.5 | - | Na2CO3 | 100 | - |
| 2 | 1.5 | 0.5 | LiBr | Li2CO3 | 30 | 57 |
| 3 | 1.5 | 0.5 | NaBr | Na2CO3 | 13 | 71 |
| 4 | 1.5 | 0.5 | KBr | K2CO3 | 54 | 30 |
| 5 | 1.7 | 0.3 | NaBr | Na2CO3 | 35 | 32 |
| 6 | 1.0 | 1.0 | NaBr | Na2CO3 | 15 | 75 |
| 7 | 0.5 | 1.0 | NaBr | Na2CO3 | 15 | 75 |
| entry | Cosolvent (mL) |
Conversion of 1 (%) | yield of 2 (%) | yield of 3 (%) |
|---|---|---|---|---|
| 1 | MeCN (0.20) | 98 | 7 | 76 |
| 2 | DMSO (0.10) | 100 | 3 | 87 |
| 3 | DMSO (0.20) | 100 | 2 | 83 |
| 4 | DMSO (0.40) | 100 | 1 | 82 |
| 5 | H2O (0.02) | 100 | 2 | 97 |
| 6 | H2O (0.05) | 100 | 3 | 95 |
| entry | EtOAc (mL) | EtOH (mL) |
yield of 5 (%) | yield of 6 (%) |
|---|---|---|---|---|
| 1 | 1.75 | 0.25 | 76 | 20 |
| 2 | 1.50 | 0.50 | 13 | 81 |
| 3 | 1.00 | 1.00 | 12 | 82 |
| 4 | 0.50 | 1.50 | 22 | 73 |
| 5 | 0.75 | 0.752 | 5 | 96 |
| 6 | 0.50 | 0.502 | 13 | 86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





