1. Introduction
Mood disorders as well as drug addiction are major neuropsychiatric conditions with staggering medical, social, and financial costs. Depression accounts for one of the highest work force disabilities worldwide [
1]. Similarly, the burden attributed to alcohol and illicit drug use, excluding smoking, is estimated to result in loss of nearly 5 percent of total life span [
2]. Moreover, substance use disorders (SUDs) are co-morbid with other psychiatric disorders such as anxiety, major depressive disorder, bipolar disorder, conduct disorder, and schizophrenia, resulting in unemployment, domestic violence, loss of productivity, and even suicide [3-6].
Although modest treatments for both disorders are available, incidences of treatment-resistant depression and relapse to drug abuse are major medical challenges. Thus, more effective interventions are urgently needed. However, without a thorough understanding of the neurobiological substrates of these disorders novel therapies would be hard to come by. Tremendous effort is being expended in this regard. Indeed, discoveries in mid 1950’s heralded a new era of intervention in depression based on the monoamine hypothesis of mood disorders. This hypothesis posited that decreases in the levels of monoamines such as dopamine (DA) and norepinephrine (NE) in the brain were the cause of depression. Hence, several monoamine-based drugs such as iproniazid, a monoamine-oxidase inhibitor (MAOI) that had been used in the treatment of tuberculosis, and imipramine, the first drug in the tricyclic antidepressant family, offered substantial help to many patients suffering from depression. Later, in 1980’s, selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine (Prozac) were introduced [
7]. More recently (March 2019), ketamine, an antagonist of glutamate N-methyl-D-aspartate (NMDA)-receptor was approved for use in treatment-resistant depression [
8,
9]. It is now believed that in addition to NMDA antagonism, ketamine modulation of metabotropic mGluR5 receptors also contributes to its antidepressant effects [
10]. Nonetheless, considering the relatively high rate of depressive mood relapse (more than 40%), novel interventions are urgently needed.
Similarly, limited options in combatting drug addiction in general and opioid use in particular, evidenced by recent surge in deaths due to opioid overdose, underscore the need for fuller understanding of the brain circuitry controlling this behavior. Recent advances in unraveling some of the complexities of the central nervous system (CNS) including elucidation of the role of glial cells in neuronal functions, especially in relation to neuroinflammation have been encouraging. This is because neuroinflammation is now considered a main culprit in almost all neuropsychiatric/neurodegenerative disorders and targeting it may be of therapeutic value. Thus, in this review, we provide an update on glial-neuron interactions visa-a-vis major depressive disorder and drug addiction. We specifically concentrate on the role of different glial cells in neuroinflammation and down-stream consequences relevant to depression and drug addiction. Moreover, potential exploitation of this knowledge for novel interventions is touched upon.
2. Major Depressive Disorder (MDD)
As of 2017, major depressive disorder, a chronic, recurring, and debilitating mental illness, was ranked as the leading cause of disability around the world [
11]. The advent of antidepressants in mid 1950’s followed by more effective medications in the following decades provided remarkable pharmaceutical interventions in this devastating illness. However, the lack of universal response, delay in onset of action as well as the side effects associated with all available drugs, necessitate the need for the development of more efficacious medications. Recent introduction of ketamine for the treatment-resistant depression, has been a step in the right direction. Nevertheless, it is imperative to enhance our understanding of the neuronal dynamics and circuitries involved in mood regulation to be able to provide better treatments. The complexity of the system regulating this behavior is further compounded by diversity and intensity of the symptoms and variations from patient to patient.
Whereas genetics may provide the foundational platform for any behavioral function, the manifestation of the phenotype may be equally dependent on the triggering environmental factors which can exert a direct or indirect influence via epigenetic changes. Fortunately, the availability of animal models of MDD with construct, predictive and face validity allows in-depth investigation of not only the neurocircuitry but also the molecular intricacy. This, in addition to advances in neuroimaging can facilitate translation of the preclinical findings to human studies. Below, we start with the discussion of several notable achievements in identification of major players in MDD such as: 1. Neurotrophic factors 2. Neuroinflammation, and 3. Gut microbiota.
2.1. Neurotrophic Factors and MDD
Contrary to the original conviction that after brain maturation no more neurons are generated, it was discovered in 1970s that neurogenesis does occur in select brain areas. Neurogenesis refers to synaptogenesis, formation and pruning of spines and dendrites and strengthening of the synaptic connections. However, for this process to be fully expressed, actions of neurotrophic factors are required [
12,
13]. One of the most important and extensively studied neurotrophins is brain-derived neurotrophic factor (BDNF), which is crucial in modulating neuronal differentiation, growth, survival, repairment and plasticity [
12,14-16]. Neuroplasticity is an umbrella term that refers to the ability of the brain to change and adapt following insults. This adaptation may include functional, structural as well as molecular reorganization, which are essential not only for normal function but also play a critical role in pathological conditions including neuropsychiatric diseases [
17].
The crucial role of hippocampal BDNF in mood regulation has been amply documented [18- 22]. Thus, it has been confirmed that dysregulation or impairment in BDNF could precipitate MDD [
23,
24]. Conversely, BDNF injection directly into the hippocampus imparts an antidepressant effect, and up-regulation of BDNF is often associated with an anti-depressant response [
25]. More recently, it was proposed that circulating levels of BDNF may provide a biomarker for MDD [
24].
It is of relevance to note that the two major areas in the brain that contain neural stem cells (NSCs), consist of subventricular zone of the lateral wall of the lateral ventricle and the subgranular zone (SGZ) of the dentate gyrus (DG) of the hippocampus which are referred to as “neurogenic niches” [
26]. The neurogenesis process is believed to occur in four stages. At stage 1, a pool of NSCs is formed which can subsequently differentiate into neuroblasts and immature neurons. At stage 2, the neuroblasts and immature neurons migrate to the granular cell layer. At stage 3, the immature neurons differentiate to mature neurons and establish synaptic connections with other neurons. At stage 4, the mature neurons establish synaptic connections via their dendrites and axons, with other neurons and glial cells, ensuring normal CNS functioning [
27]. Indeed, newly born neurons in the DG of the adult brain contribute to the cognitive and emotional functions of the hippocampus [
23,
24,
28].
Interestingly, a reciprocal relationship between BDNF and DA has been noted. Thus, early on using genetically modified mice, it was shown that BDNF is responsible for maintaining the expression of DA D
3 receptors [
29,
30]. This, in addition to the studies showing that BDNF enhances the survival of DAergic neurons and improves DAergic neurotransmission and motor performance, led to the suggesting that BDNF may play an important role in pathophysiology of PD, mood disorders, and drug addiction [31-33]. More recently, it was reported that deep brain stimulation of the nucleus accumbens improves depressive-like behaviors via BDNF-mediated alterations of the DAergic pathway [
34].
Conversely, it was demonstrated that in cultured embryonic mouse striatal cells as well as in rat brain tissue, DA regulates BDNF expression [
35,
36]. BDNF action is mediated primarily via its high affinity TrkB (tyrosine kinase B) receptor, which leads to activation of the signal transduction cascades (IRS1/2, PI3K, Akt) [
37,
38]. A recent in-vivo study demonstrated that DA modulates TrkB turnover and BDNF sensitivity in the striatal medium spiny neurons [
39]. Curiously, a reciprocal interaction between BDNF and serotonin with implications in mood regulation has also been reported [
40].
Taken together, it may be concluded that BDNF plays a central role in mood regulation via its interaction with the neurotransmitters implicated in this behavior.
2.2. Neuroinflammation and MDD
In addition to the roles of neurotransmitters and neurotrophic factors in MDD, involvement of neuroinflammation is becoming a central theme in this disorder. Indeed, early on, an “inflammatory hypothesis” of depression was postulated based on the findings that elevated levels of circulating pro-inflammatory cytokines can lead to depressive symptoms [41-43]. Thus, it was observed that the concentrations of interleukin (IL)-6 and tumor necrosis factor (TNF)-α, were higher in individuals with depression compared to non-depressed individuals. Conversely, plasma levels of IL-10, an anti-inflammatory cytokine were negatively correlated with depressive symptoms [
43,
44]. Furthermore, it was proposed that mRNA of the pro-inflammatory cytokine IL-1-β may serve as markers for depression [
45].
Persistent peripheral inflammation triggered by infection or immune disruption may lead to MDD. Thus, MDD is often co-morbid with systemic inflammatory diseases or conditions in which pro-inflammatory cytokines are overexpressed. These may include inflammatory bowel disease, rheumatoid arthritis, allergies of different types, multiple sclerosis, cardiovascular disease, diabetes, chronic liver disease, cancer, and even periodontitis, which is one of the most common chronic inflammatory disorders [
46]. In these scenarios, during systemic inflammation, macrophages and monocytes get activated and release pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α, which in turn, lead to microglial activation and an increase of central pro-inflammatory cytokines [
46], which can precipitate MDD [
25,
46,
47]. In addition, high level of blood C-Reactive Protein (CRP), a specific inflammatory biomarker, was associated with greater MDD severity and a worse treatment response [
48].
Interestingly, postpartum depression (PPD), a serious psychiatric disorder that dramatically affects the women’s physical and mental health post-delivery, has been tied to inflammatory reactions [
49]. PPD, in addition to affecting the emotion and cognition of the mother, it can also damage the mother-infant relationship, and delay the growth and mental health of the child [
50]. Similarly, association between stress and depressive mood may be mediated through inflammatory processes. Thus, it was revealed recently that following stress, the expression of claudin-5, a protein regulating the BBB is reduced leading to penetration of IL-6 and induction of depression [
51]. Chronic stress can also lead to increased inflammatory cytokines which can also cause impairment in BDNF function and neurogenesis leading to neurodegenerative and/or neuropsychiatric disorders including MDD [
42,
52].
Finally, regarding the fast-acting antidepressant, ketamine, it was shown that in addition to its role in glutamatergic modulation, being an N-methyl-d-Aspartate (NMDA) receptor antagonist, it also promotes BDNF [
53,
54] and possesses anti-neuroinflammatory properties. The latter is manifested through its interaction with the complement system, which is an integral component of the innate immune system and has been implicated in the pathophysiology of depression [
55].
2.3. Gut Microbiota and MDD
The gut microbiota (GM) and its connection to the brain referred to as gut-brain axis (GBA) have become an area of intensive research in the last few years. Although from the earliest centuries, gastrointestinal system was suspected as the root cause of physical and mental illnesses, only recently the GBA is being touted as a potential therapeutic target [
56]. This approach has been validated by the observation that GBA closely interacts with the immune system and can exert profound influence on inflammatory processes. Thus, the role of GBA in maintaining mental health in general and MDD in particular, has been verified by numerous studies. It is of relevance to note that the GM, in the human harbors a range of 2000 bacterial species where its genomic content (microbiome) is slightly higher than the entire human genome [57-59].
Dysbiosis or perturbation of the GM, can alter not only the neurotransmitters and neurotrophic factors involved in mood regulation, but can also affect the immune system leading to neuroinflammation, all of which can precipitate MDD [
47,60-63]. Indeed, blockade of peripheral IL-6 receptor was shown to impart a rapid and sustained antidepressant effect in a mouse of model of depression generated by social defeat. This effect was attributed to normalization of the GM [
64]. Moreover, pre-treatment with pre- or pro-biotics or even fecal microbiota transplantation, have been advocated in MDD therapy [
65]. Additionally, it has been proposed that examination of the gut microbiota may predict both MDD and cognitive dysfunction, suggesting that the readout of the microbiome may present a noninvasive prognostic tool for MDD and cognitive functions [
66].
3. Drug Addiction
Drug addiction, defined as compulsive drug seeking despite adverse consequences is a brain disorder of universal medical and social concern. With approximately 300 million people using illicit drugs, and nearly 40 million people affected by substance use disorders (SUDs), resulting in over 600,000 deaths annually worldwide, the challenge for public health and economic burden is staggering [
67]. The challenge is exacerbated by the fact that there is a high relapse rate of 50 to 70%, despite the advances in our understanding of the neurobiological substrates and availability of newer treatment modalities [
68]. Thus, the urgent need for more effective therapies.
Drug addiction involves functional changes to brain circuits controlling reward or pleasure and extends to pathways dealing with stress response and self-control [
69]. In particular, the mesocorticolimbic DAergic pathway, originating in the ventral tegmental area (VTA) and projecting to the nucleus accumbens (NACC), central amygdala, and medial prefrontal cortex (mFCX) has been referred to as the “reward pathway” due to its intimate association with natural rewards as well as euphoria induced by substances of abuse. Modifications in this circuitry has also been linked to reward dysfunction, drug tolerance and escalation of drug intake and eventual compulsive use of the drug [70-74].
Although a very strong influence of genetics, where genes may account for about half of a person's risk of addiction is evident, experience with drugs especially at younger or adolescent age stems from several factors. For example, the use of stimulants such as cocaine is attributed to the initial feeling of euphoria, followed by power, self-confidence, and increased energy, whereas the euphoria caused by opioids is usually followed by feelings of relaxation and satisfaction. In other instances, an addictive substance (e.g. alcohol or nicotine via smoking) may be used to
mitigate stress, anxiety, or depression. In addition, curiosity, and peer-pressure may be key reasons for trying drugs during developmental period [75-78]. In all, negative affects “Hyperkatifeia” may be a major player in continuing drug use as well as in relapse [
79]. In this context it should be noted that because of development of tolerance to the drug, more and more would be needed to produce the same effect [
70,
80].
3.1. Neurotrophic Factors and Drug Addiction
The widespread effects of the drugs of abuse on the structure and function of neurons and glia are the basis of the long-lasting behavioral changes associated with drug addiction. In this regard, BDNF, via regulating phosphatidylinositol 3′-kinase (PI3K), mitogen-activated protein kinase (MAPK), phospholipase Cγ (PLCγ) and nuclear factor kappa B (NFκB) signaling pathways, plays a key role. Indeed, BDNF via alteration of the reward circuitry can modulate the motivation to take drugs [
70,
81]. Interestingly, administration of both BDNF and glial cell line-derived neurotrophic factor (GDNF), discussed in more detail below, increase craving when administered into the mesolimbic reward pathway [
71].
DAergic neurons contain both BDNF and GDNF receptors, however, their therapeutic use is still unclear [
71]. It is noteworthy that age, sex and even age of first drug use influence BDNF levels in patients suffering from SUD. The lower level of serum BDNF levels in SUD subjects has prompted the suggestion for using BDNF as a marker in SUD [
82,
83].
3.2. Neuroinflammation and Drug Addiction
Physiological homeostasis of the body including that of the brain is critically dependent on proper function of the immune system. Thus, hyperactivation of the immune signaling can induce peripheral inflammatory responses as well as central neuroinflammation. The latter, leads to dysregulated neuronal processes which can contribute significantly to SUD [
4,
84]. In this regard, substances of abuse trigger the release of cytokines such as interleukins (ILs), interferons (IFNs), tumor necrosis factors (TNF), chemokines, and lymphokines from both the immune cells like macrophages, and lymphocytes (e.g., T-cells, B-cells, and natural killer cells), or non-immune cells such as fibroblasts and endothelial cells [
4]. The cytokines in turn stimulate their specific receptors and enhance the immune response leading to neuroinflammation. Some cytokines (e.g. IL-10) may impart anti-inflammatory effects [
85].
In the brain, neuroinflammation is underscored by gliosis and microglial activation, which also cause the release of pro-inflammatory factors and in this case can compromise the integrity of the BBB and lead to further complications [
4,
86][ . Thus, when neuroinflammation is ensued, it may further exacerbate SUD or induce MDD [
4,
86]. For example, it has been shown that psychostimulants or endocannabinoids activate microglia causing the release of different pro-inflammatory cytokines and leading to neuroinflammation, which can further contribute to drug abuse [
84]. Similarly, mu-opioid receptors (MORs) that are well-known for their role in analgesia and drug addiction, have been related to neuroinflammation where together with toll-like receptors (TLRs) enhance the release of pro-inflammatory cytokines [
87].
On the other hand, some addictive substances such as alcohol may suppress the immune system in some individuals rendering them susceptible to infection and other diseases [
88,
89]. Taken together, it is suggested that neuroinflammation promotes addiction-related brain and behavioral deficits and that strategies to reduce neuroinflammation may be viable novel interventions in SUD [
4,
90].
3.3. Gut Microbiota and Drug Addiction
That SUD has a reciprocal relation with GBA and is associated with dysbiosis [91-93]. GBA communication is manifested through different mechanisms including the immune response, where the bacterial products may alter the intestinal barrier allowing such products to enter the bloodstream and induce inflammatory response. Other bacterial products such as SCFAs may interact directly with the brain. Finally, some bacterial products may affect the hypothalamic-pituitary-adrenal (HPA) axis, which in turn can activate microglia [
91,
93]. Among substances of abuse, alcohol causes dysbiosis and peripheral inflammation, and its withdrawal has been shown to cause neuroinflammation [
91]. Similarly, chronic morphine or cocaine result in dysbiosis, increased intestinal permeability and neuroinflammation, which can in turn influence the drug response [
91,
92].
Brain’s modulation of the gut microbiota occurs primarily through the autonomic nervous system and several neurotransmitters that act directly on bacterial gene expression. In sum, substances of abuse, by causing dysbiosis and oxidative stress, part of which may be due to alteration of antioxidant capacity, induce neuroinflammation which can lead to neuropsychiatric/neurodegenerative diseases [
93]. For this reason, it has been proposed that the use of "psychobiotics" may be a novel approach in dealing with these devastating diseases [
93].
4. Glial Cells
Glial cells were first identified in mid 19th century by the leading neuroscientists of the time including, Santiago Ramón y Cajal and Pío del Río-Hortega. The term
neuro-glia (neuro-glue) was coined by the pathologist Rudolf Virchow to signify their role in maintaining the integrity of the neurons. Camillo Golgi described astrocytes and oligodendrocytes in his book in 1871. The term astrocyte was introduced by Michael von Lenhossek in 1893 to reflect their star shape [
94,
95]. Until relatively recently, glial cells were considered as “passive” cell populations to merely provide structural support and sustain neuronal cells. However, glial cells are now considered as one of the most versatile cells in the body due to their varied functions including, axonal guidance, proliferation, trophic effects, and maintaining neural function and development. Indeed, with 10 times more cells than neurons, glial cells represent the biggest cellular population in the brain [96-98]. These cells play a critical role not only as energetic support for neurons [
54,
99,
100], but also control of metabolism [
101,
102], myelination [
103,
104], formation of the blood-brain barrier (BBB) [
105,
106], development and remodeling of synapses [107-109], regulation of neurotransmitters [110-112], control of the fluid/electrolyte homeostasis [
113], neuroendocrine function [
114] innate immunity response [
115,
116], and detoxification [
117,
118]. It is not surprising, therefore, that their disruption or dysregulation can lead to neuropsychiatric and neurodegenerative diseases [
50,
104,119-123]. Indeed, recent developments suggest potential novel interventions in neurodegenerative diseases by targeting glial receptors and enzymes [
124]. We have recently proposed that glial nAChRs may be a suitable target for intervention in Parkinson’s disease (PD) [
123]. It would be of interest to determine if this hypothesis can extend to MDD and SUD.
To date, four main subsets of glial cells (microglia, astrocytes, oligodendrocytes and synantocytes or NG2 cells) have been identified. Here, following a brief description of each, we specifically concentrate on their potential roles of in MDD and SUD as the glial cells have been implicated in both these disorders [
125,
126].
4.1. Microglia
Although microglia are an important component of the brain’s glia, unlike all other glial cells, they do not originate from the ectodermal tissue but from yolk-sac progenitors that are abundant during brain development. Microglia have been shown to cover a huge volume of the adult brain parenchyma amounting to 10%–15% of all CNS cells. They constantly survey the environment through rapid movements of their fine filopodia, allowing them to react quickly to any kind of insult. Lately, it was shown that the action of filopodia is C-AMP dependent [
127]. Microglia are considered the immunocompetent and phagocytic cells of the nervous system and share the same origin and express many common cellular markers with peripheral macrophages. Because of their function in innate immune response, they play a key role in neuroinflammation, which as alluded to above may be responsible for manifestation of MDD and SUD. Microglia, play a vital role in maintaining brain homeostasis by eliminating cell residues as well as pathogens. For this reason, they are referred to as resident macrophages in the CNS [
97,
128]. Microglia also regulate neurogenesis, formation and elimination of neuronal synapse, control the number of neuronal precursor cells and mediate T-cell infiltration into the brain [
129].
Activity of microglia consists of three states termed resting, activated and phagocytic stages. At the resting state, they are highly ramified but not active. When activated in response to injury or insult, they contract, assume an enlarged cell body, and proliferate, culminating into full-blown phagocytic microglia. This serves the purpose of eliminating debris which is essential for repair and recovery. Overactivation of microglia, however, leads to neuroinflammatory and neurological conditions including MDD [130-133].
Initially, two distinct profiles were ascribed to microglia. M1, or a pro-inflammatory state where production of chemokines, cytokines, and metabolites lead to neuroinflammation, and M2 an opposite anti-inflammatory state involved in damage repair and neuroprotection [
129,
134,
135]. However, emerging evidence suggests that differences in microglia functions are not driven exclusively by their milieu, but rather by the unique properties they possess. Therefore, it is suggested that microglial subtype categorization be based on their function [
124,
136,
137].
Microglial polarization into various stages occurs due to perturbation in the microglial micro-environment. In the resting state or under physiological conditions, microglia assume a small cell body and very fine, highly ramified processes, allowing them to survey their local environment for cellular damage of pathogens. This stage is now referred to as a “surveilling” stage, rather than resting state [138-140]. Upon activation, the cell body enlarges, and microglia processes assume a shorter or amoebic shape, allowing them to quickly migrate to the site of injury and initiate phagocytic activity. The amoeboid microglia have completely retracted processes with a swollen cell soma to facilitate phagocytosis. These morphological transformations may specify disease-specific stages. In mice, a fourth morphology of microglia referred to as rod-like microglial cells, that exhibit fewer secondary branches and narrowing of the cell soma have been observed [140-142]. Microglia express various receptors including low-density lipoprotein receptor-related protein 1 (LRP1), triggering receptor expressed on myeloid cells-2 (TREM2), calcium-sensing receptor (CASR), nicotinic cholinergic receptors (nAChRs), and toll-like receptors 2 and 4 (TLR2 and TLR4) [
123]. TLRs, expressed by both microglia and astrocytes (discussed below), are a well-characterized family of pattern recognition receptors (PRRs), that sense endogenous debris or pathogens and initiate the innate immune response. TLRs contribute significantly to CNS pathology and are under intense investigation for potential therapeutic targets [
129,143-145].
4.1.1. Microglia-Depression
Glial cells in general and microglia in particular exert a critical role in MDD. Indeed, this involvement is so much so that it was proposed to designate depression as a microglial disease [
146,
147]. This is because microglia regulate synaptic plasticity, formation of neural networks, and neuroinflammation, all of which affect depression [
146,
147]. In the last three decades it has become evident that patients with chronic inflammation manifest increased levels of circulating cytokines, microglia overactivation, and depressive symptoms. Moreover, microglia as well as astrocytes and OLs are responsible for transfer of exosomes or secreted extracellular vesicles (EVs) to neurons [
148]. EVs are key players in intercellular signaling as they carry mRNAs, microRNAs (miRNAs), and specific proteins to the neuron. Interestingly, depressed patients manifest changes in miRNAs, which are known to affect neurotrophic factors, immune cells, synaptic plasticity, cognition, and mood [
148]. More recently, using transcriptional profiling, it was revealed that MDD was associated with microglia inhibition in the cortical gray matter [
149].
It is also a well-established fact that chronic stress may lead to depression. Stress does this by disruption of the homeostasis via its effects on microglia [
150,
151]. Homeostasis imbalance may include GBA dysregulation, unbalanced pro- vs anti-inflammatory cytokines and neurotransmitters [
150].
It is noteworthy that GM maturation, which is critical for neuronal maturation, appears to parallel the temporal course of brain development [
152,
153]. Although further elucidation of microglia’s role in the etiology of depression is warranted, it can be summed up that microglial dysfunction can lead to a variety of neuropsychiatric diseases including MDD. Indeed, the term "microgliopathy" has been coined to refer to such diseases. Accordingly, growing studies are suggesting that targeting microglia (inhibition or stimulation depending on the microglial status) could be a novel personalized medical approach in these devastating diseases [
146,
154].
4.1.2. Microglia - Drug Addiction
The critical function of microglia and astroglia in synaptic formation and refinement is well-recognized. Drugs of abuse, on the other hand, cause persistence alterations in synaptic and neuronal function. Thus, growing evidence suggest that disruptions in glial function may be associated with SUD [
155,
156]. Specifically, glial and neuroimmune mechanisms are believed to contribute to opioids, alcohol, and psychostimulants abuse. It is postulated that microglia and astrocytes activation by drugs of abuse occurs via stimulation of innate immune receptors, which result in secretion of chemokines and cytokines, which in turn influence neuronal function [
155,
156]. Stimulation of the innate immune receptors may also lead directly to synaptic remodeling [
155,
156].
More recently, the xenobiotic hypothesis has been advanced which posits that substances of abuse are exogenous molecules and are therefore considered as foreign “invaders” which trigger the protective immune response. Microglia, as the primary resident immune cells in the brain, provide the initial defense mechanism. However, with persistent and repeat administration of the drug, overactivation of microglia and a neuroinflammatory condition ensues that in turn can further contribute to drug addiction via modulation of neuronal function [
157].
Thus, further probe of glial-neural interactions not only enhances our understanding of SUDs but may also provide novel therapies.
4.2. Astroglia (Astrocytes)
The term astrocyte or astroglia was coined by the Hungarian anatomist and histologist Michael von Lenhossék in 1895 due to the star-like shape of these cells [
158]. Since then, based on morphology, function and spatiotemporal distribution, various subpopulations have been identified. Depending on the brain area, astrocytes may constitute anywhere between 17 to 61% of the total cells. Like microglia, in response to various insults, they exhibit heterogeneous phenotypes, commonly referred to as astrocyte reactivity [
159]. Astrocytes play a crucial role in maintaining neuronal integrity and function as they form synapses with neurons and at least for the glutamatergic neurons are critical in reuptake of this excitatory neurotransmitter [
158,
160]. In addition to providing structural integrity to the extracellular matrix, astrocytes monitor and regulate pH homeostasis, provide nutrients, and remove waste and are a key component and regulator of the BBB [
158,
160].
Interestingly, glial cells contain their own neurotrophic factor, referred to as glial cell line derived neurotrophic factor (GDNF), a class of proteins that also provide trophic support to neuronal cells including DAergic neurons [
161]. Indeed, it has been proposed that lacrimal GDNF may serve as a marker in MDD [
162]. Astrocytes also express high level of glial fibrillary astrocytic protein (GFAP) which are commonly used as a marker for their identification. More recently it was reported that astrocytes are the necessary source of TNF-α for mediation of homeostatic synaptic plasticity [
163]. Similarly, taurine, considered the most abundant free amino acid in the brain required for optimal postnatal brain development occurs predominantly in astrocytes. Taurine has antioxidative, and anti-inflammatory functions, hence cytoprotective properties [
164].
Although compared to microglia, astrocytes are not well equipped with receptors recognizing pathogens, they become reactive when activated by polarized microglia and release inflammatory mediators and modulate inflammation [
129].
Thus, it may be suggested that astrocytes work together with microglia to provide the first line of defense against insults. However, overstimulation of the proinflammatory signals may synergistically contribute to neuronal dysregulation and ensuing neuropsychiatric/neurodegenerative diseases [165-167].
In addition to the intimate interaction between astrocytes and microglia, an interaction between astrocytes and neurons referred to as crosstalk has been identified [
159,
161,
168,
169]. It is anticipated that as our knowledge of such cross talks expands, novel intervention in neuropsychiatric/neurodegenerative diseases, including MDD and possibly SUD may be realized [
159,
169,
170].
4.2.1. Astrocytes – Depression
Numerous studies including analysis of postmortem brain tissue have confirmed a central role for astrocytes in MDD pathology. Thus, human postmortem studies demonstrate significant reduction in GFAP in gray matters of the dorsolateral prefrontal cortex and the orbitofrontal cortex, the white matters of the anterior cingulate cortex and the orbitofrontal cortex and CA1 and CA2 subregions of the hippocampus of depressed individuals [
171]. It is of relevance to note that GFAP which is used as a primary marker of astrocytes, helps maintain the shape, mechanical strength, cell movement and astrocyte-neuron communication [
171]. In animal models of stress-induced depression also significant reductions in the cortical and hippocampal astrocytes were noted. Interestingly, the effects of chronic stress on astrocytes could be reversed by antidepressant treatment. Specifically, astrocyte’s intracellular signaling pathways, receptor expressions, release of various trophic factors, and gene expression were restored by antidepressants, suggesting that the efficacy of the treatment with the antidepressants also involves astrocyte modifications [
172].
That reduction in astrocytes is a key feature in MDD pathology has also been confirmed in other animal models of depression. It is argued that astrocyte dysfunction by disrupting astrocyte-neuron interaction, affects the neurotrophic function, monoaminergic transmission, and the excitatory–inhibitory balance of local networks, leading to mood disorders [
173,
174]. Interestingly, the reduction in astrocytes in MDD have been partially attributed to potassium imbalance and animal studies suggest that the antidepressant effects of ketamine might also be due to correction of this imbalance [
175]. The reduced astrocyte densities in MDD were correlated with elevated S100 beta (S100B) in cerebrospinal fluid levels. S100B is a multifunctional protein that is expressed in large amount in protoplasmic astrocytes [
176], and in myelinating oligodendrocytes [
177]. It is a Ca
2+-binding protein necessary for neurotrophic functions and may therefore be involved in neurodegenerative diseases and/or MDD [
175].
Recent studies indicate that dysregulation of astrocytic purinergic system contribute to the pathophysiology of MDD. Thus, overactivation of adenosine A2A receptors (Rs), P2X7, P2Y1, and P2Y11Rs, result in neuroinflammation, disruption of the neuro-glia communication and synaptic plasticity in depression-relevant areas of the brain such as hippocampus, medial prefrontal cortex, and amygdala. Curiously, astrocytic A1Rs may impart neuroprotective and immunosuppressive effects, which could present novel intervention targets in MDD [
23,
178].
More recently, revelation of the importance of astrocyte Ca
2+ signaling in homeostatic control of brain circuits and behavior has led to the suggestion of potential exploitation of this system in MDD treatment [
179,
180]. Further understanding of astrocyte-neuron involvement in MDD pathology including the alterations of mid-spiny neurons and pyramidal neurons, as well as the alterations of gliotransmitters between astrocytes and neurons could provide novel targets in MDD [
181].
4.2.2. Astrocytes – Drug Addiction
The role of astrocytes in synaptic functions and glutamate clearance are well established. It is also known that substances of abuse compromise this capacity and facilitate relapse. For example, astrocytes in the NACC undergo rapid and transient plasticity in response to drug-associated cues [
182,
183]. Several key astrocytic signaling pathways that are involved in cocaine-induced synaptic and circuit adaptations have been identified [
184]. Overall, it is postulated that NACC astrocytes play a critical role in modulating glutamate transmission during relapse [
182,
183]. Moreover, therapeutic potential of targeting astrocytic substrates to tackle drug addiction has been proposed [182-184].
More recently, observations of changes in gene expression mediated by transcriptional and epigenetic regulations brought about by drugs have provided fresh venues for potential therapeutic interventions. In this regard, evidence of robust transcriptional response of astrocytes to several substances of abuse, whereby astrocytes are directly implicated in drug seeking behaviors has been provided [
185].
4.3. Oligodendrocytes
Another major glial cell consists of oligodendrocytes (OLs), which are now well recognized as the primary source of myelination in the CNS [
186]. In the peripheral nervous system, however, neuroglia that provide myelination are called Schwann cells [
187]. OLs represent 75% of all glial cells in the adult CNS. In addition to axonal myelination, OLs control extracellular potassium concentration, provide metabolic and trophic supply to myelin, secrete GDNF and BDNF, and modulate the axonal growth [
186,
188] all of which highlight their importance in functioning of CNS. Like microglia and astrocytes OLs also express TLRs, considered of significant importance in myelin formation [
103,
189,
190]. Importantly, and of direct relevance to our discussion, dysregulation of these glial cells, can contribute to pathogenesis of MDD and affect SUD as detailed below.
4.3.1. Oligodendrocytes - Depression
As far back as two decades ago, involvement of OLs in depression was verified by the finding that glial reduction in amygdala in MDD was due to oligodendrocytes loss [
191]. Later, reductions in the density and ultrastructure of OLs were also detected in the PFCX and amygdala of MDD patients [
192]. It was hypothesized that pathological changes in OLs were due to the disruption of white matter tracts and that factors such as stress, altered gene expression of neurotrophic factors and glial transporters, modify glial cell number, and affect the neurophysiology of depression [
192]. Further evidence was provided by demonstrating that genetic alterations in these cells alone can result in major behavioral changes including MDD [
193].
More recently, some clues to the cellular and molecular bases of white matter pathology have been revealed. Thus, it was shown that oxidative damage was a major culprit in MDD and that interference with this pathway could be of potential benefit [
194]. Furthermore, in a repeat-defeat mouse model of depression, long-lasting losses, and transient proliferation of oligodendrocyte-precursor cells (OPCs), aberrant differentiation into oligodendrocytes, and severe hypomyelination in the prefrontal cortex were observed [
195]. In addition, morphological impairments in OPCs, excessive oxidative stress, OLs apoptosis were noted in these mice [
195]. Markers of oxidative stress were also observed in OLs obtained from the brainstem and occipital cortex of MDD patients [
196]. Analysis of single-nucleus transcriptomic data from MDD patients revealed OLs dysregulation and the presence of novel OLs expressing immune properties [
63,
195]. It is expected that further elucidation of interaction between OLs and neurons will provide novel insights into the pathology of MDD and suggest novel targets for intervention [
197].
4.3.2. Oligodendrocytes – Drug Addiction
Although the effects of drugs on myelination and white matter integrity have been reported, specific effects on OLs are yet to be investigated. Nonetheless, applying a transcriptome analysis, it was shown that heroin self-administration in rats upregulates markers of OLs maturation and differentiation [
144,
198]. Moreover, opioids may directly or indirectly regulate OLs development and myelin generation in PFCX of rats during prenatal, adolescence, and young adulthood [
199]. Interestingly, detrimental effects of prenatal alcohol exposure on OPCs and OLs lineage were recently detected in human fetal autopsies [
200].
4.4. Synantocytes (NG2 Cells)
Synantocytes also referred to as neuron glial 2, or nerve/glial antigen 2 (NG2) cells, are the fourth subset of major glial cells in CNS. These cells are OLs-precursor cells and are usually identified by the presence of two key markers: the chondroitin sulfate proteoglycan NG2 and the platelet-derived growth factor receptor alpha (PDGFRα) [
186,
201]. NG2 cells are almost uniformly distributed in both white and grey matter areas, exhibit a complex stellate morphology, tend to closely associate with neuronal cell bodies and dendrites, and maintain the ability to keep proliferating in the adult brain [
186,
201,
202].
Although initially NG2 cells were thought to be solely OLs progenitors, later their capacity to give rise to astrocytes and neurons were verified [
186,
201,
202]. Additionally, their potential influence in variety of conditions including neurodegenerative diseases such as multiple sclerosis and AD, traumatic brain injury, glioma, epilepsy, and mood disorders were investigated [
203,
204]. A role in experimental autoimmune encephalomyelitis (EAE), a disease associated with increased BBB permeability, and neuroinflammation was attributed to NG2 cells [
205]. The mechanism of their action was postulated to be via stimulation of reactive T cells and controlling IL-12 expression [
205]. NG2 cells may also play a critical role in modulation of neuroinflammation [
206] and neurovascular unit formation during development [
207]. Their importance in generation of OLs and angiogenesis following acute ischemic stroke were also recently reported [
207]. Importantly, their communication with neurons and their influence on neuronal plasticity and various behavior makes them a potential target for therapeutic interventions in a variety of diseases [207-209].
4.4.1. NG2 Cells - Depression
NG2-glia are heterogeneous glial cells with distinct roles in neuronal plasticity, dysfunction of which can lead to neurological and behavioral consequences [
209]. Indeed, the influence of NG2 cells in stress-induced mental disorders including depressive-like behavior, was recently highlighted [
210]. It was concluded that further probe of a causal role of NG2 cells in stress response and stress-related psychopathologies would be required before novel interventions in such behavioral disorders can be recommended [
210]. NG2 cells, however, have been proposed as a potential target in prevention of epilepsy following viral infection [
211]. Moreover, very recently it was revealed that NG2 cells may also act as neural progenitor cells in the cortex of adult mouse [
212]. If verified in human neuronal system, substantive exploitation of NG2 cells in neurodegenerative and/or neuropsychiatric diseases may become feasible [
212].
NG2 cells exert substantial influence in modulation of neuroinflammation [
206]. This, together with the observation that ablation of NG2 cells exacerbates dopaminergic neuronal cell loss suggest that NG2 cells may act as negative regulators of neuroinflammation [
206]. Given the causal relationship between neuroinflammation and MDD, a role of NG2 cells in this disorder is anticipated. Interestingly, it was recently reported that exosomes derived from dental pulp stem cells may promote NG2 cells proliferation and differentiation [
207]. Exosomes are small extracellular vesicles secreted by various stem cells and are potent mediators of intercellular communication and tissue repair [
213,
214]. Indeed, clinical applications of exosomes in general surgery, neurosurgery, orthopedic surgery, head and neck surgery, cardiothoracic surgery, plastic surgery, acute skin wound healing, urology, ophthalmology, and obstetrics and gynecology, and other diseases induced by cancer, ischemia, or inflammation have been suggested [
215,
216]. Whether their application in MDD may also be viable awaits further investigation.
4.4.2. NG2 Cells – Drug Adiction
Although very limited literature on direct involvement of NG2 cells in SUD is available, almost a decade ago the hint of their potential influence in drug addiction was reviewed [
217]. It was argued that further investigation of gliogenesis or new glia generation by NG2 cells in the cortex would be of relevance to SUD as NG2 cells may have a role in negative reinforcement and relapse [
217].
More recently, studies in human fetal brain have confirmed animal findings relevant to fetal alcohol syndrome (FAS), where dysregulated cytokine expression, apoptosis of NG2 cells and altered OLs differentiation were observed following prenatal alcohol exposure [
200,
218]. Thus, interactions of drugs of abuse with NG2 cells are verified. It is anticipated that further investigation of the mechanisms involved in these interactions would yield novel therapeutic targets [
200].
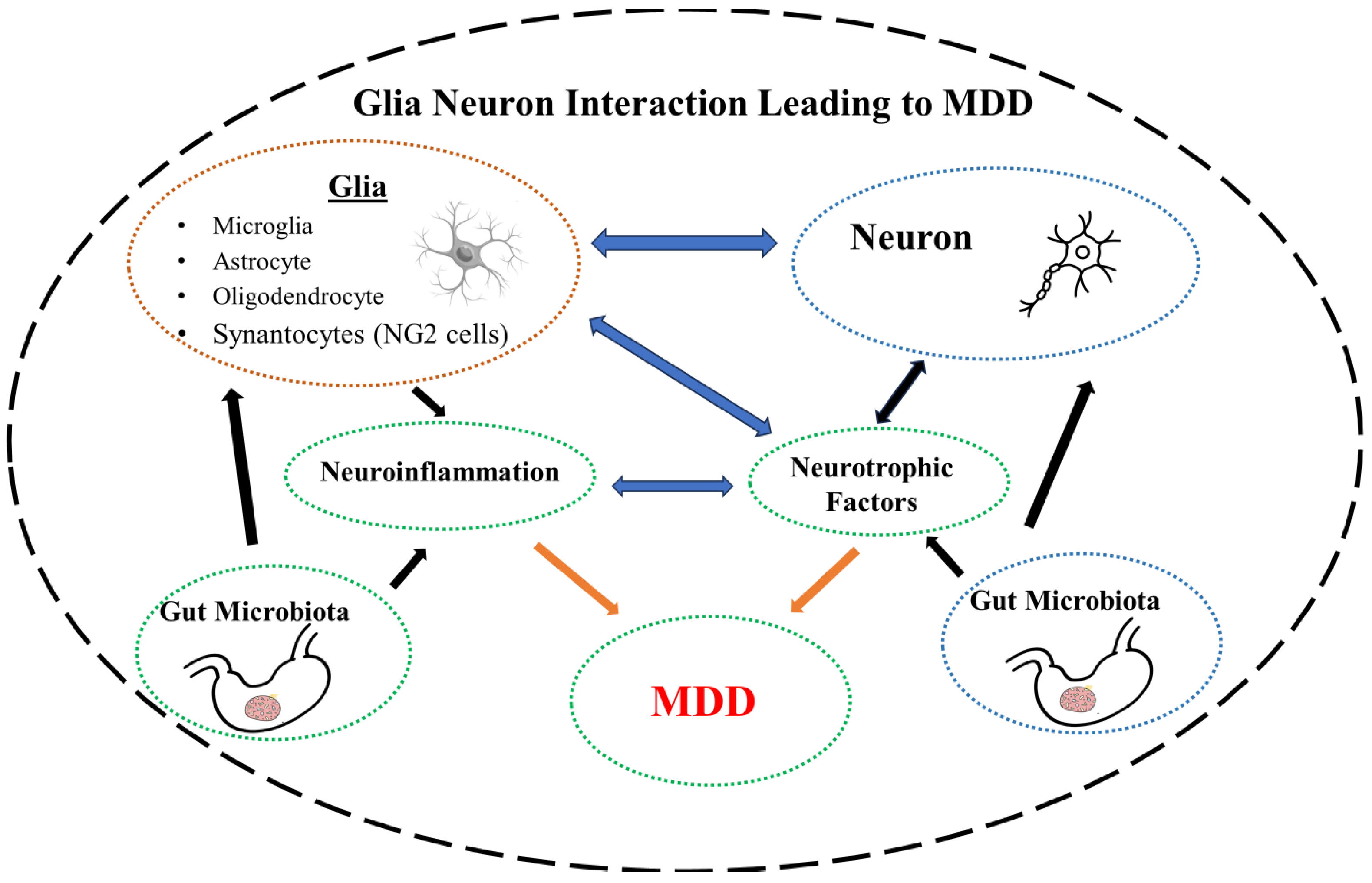
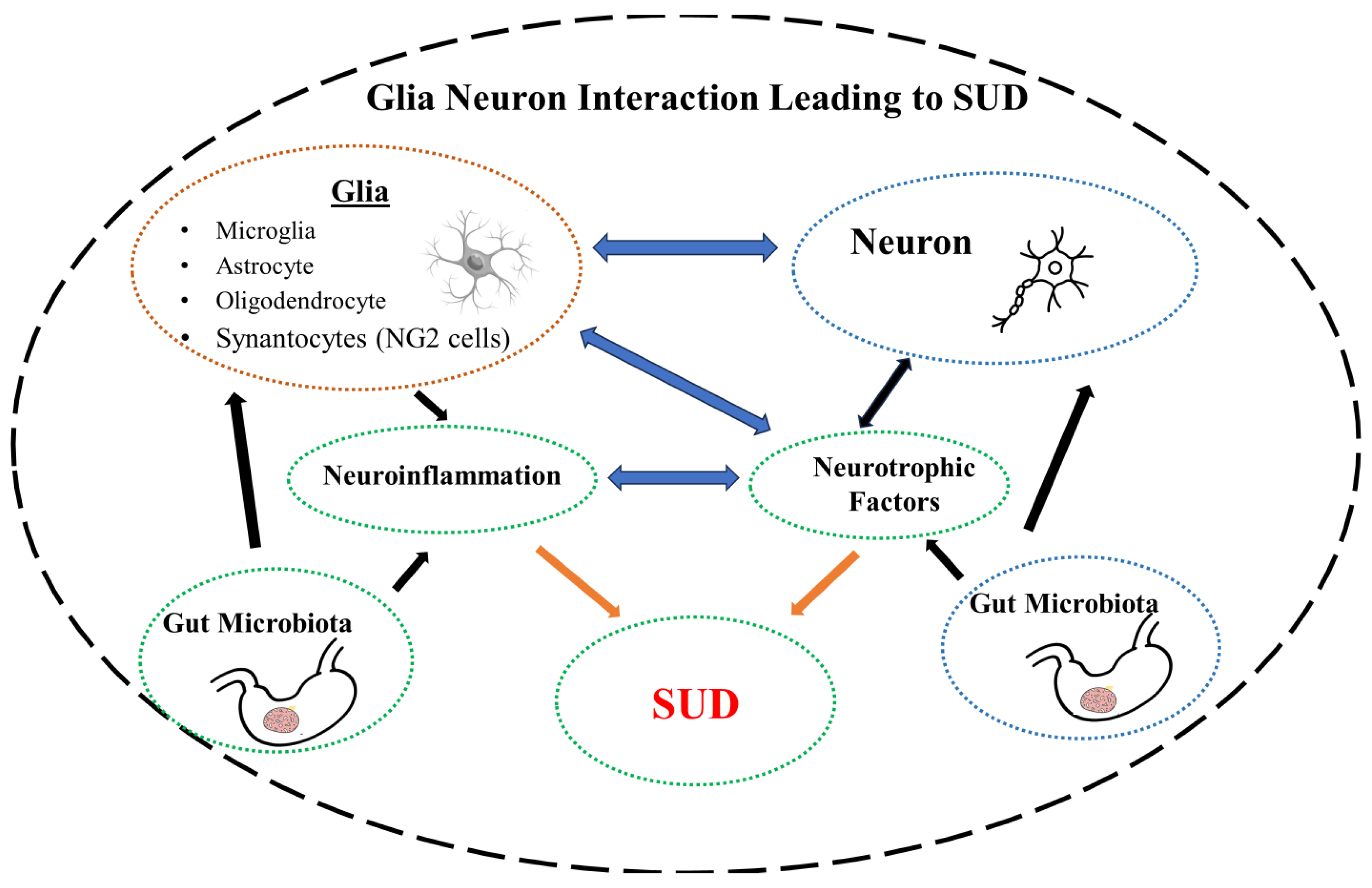
In summary glial-neuron interaction is influenced by the NTFs as welk as the GM. Disruption of this intricate balance due to changes in the NTFs or the GM, may lead to neuroinflammation and MDD (
Figure 1) or SUD (
Figure 2).
5. Microbiota-Neurotrophic Factors-Neuroinflammation
It is noteworthy that there exists an intimate interaction between the major factors that affect MDD and SUD. This interaction, while adding to the complexity of the overall picture may also suggest novel interventions. For instance, butyrate, a short-chain fatty acid (SCFA) metabolite of the GM, is critical in maintaining proper gastrointestinal and GBA integrity. Disruption of its function can lead to MDD [
219,
220]. Conversely, sodium butyrate, can exert antidepressant effects. This property is attributed to its ability to both inhibit HDAC [
221,
222], and increase central levels of BDNF [
223]. Antidepressant effectiveness of butyrate was also shown in two animal models of depression. One induced by maternal deprivation, and the other via chronic mild stress. Interestingly, in both cases butyrate increased the central levels of both BDNF and GDNF [
224,
225].
Neurotrophic factors (NTFs), as alluded to earlier are essential in maintaining homeostasis of the CNS as they regulate the differentiation, maturation, development, and survival of neurons. They also may control glial cells function by directing them toward an anti-inflammatory and neuroprotective phenotype [
226]. Cytokines, which are key mediators of the immune response, are also involved in communication between the immunes system and the CNS. It is postulated that damage produced by over-expression of the cytokines, dysregulates neuroplasticity by disrupting BDNF and leads to a variety of neurological and/or neuropsychiatric conditions including MDD [
227]. This hypothesis was verified recently by the findings that neuroinflammation leads to dysregulated neurogenesis, particularly in the DG of the hippocampus [
228].
It is anticipated that as our knowledge of the relationship between GM, GBA and the role of SCFAs in MDD expands, more therapeutic interventions by manipulating the GM would become available [229-232].
6. Concluding Remarks
MDD and SUD are complex brain disorders involving intricate neuronal circuitries that may be influenced by a plethora of factors including genetics, environment - either directly or indirectly via epigenetics, immune system, metabolic products, neurotrophins, gut microbiota, non-neuronal glial cells, etc. The complexity gets compounded by the complicated interactions between the factors.
In this review, we have highlighted the influence of some of the major players such as neurotrophic factors, neuroinflammation (immune system dysregulation), dysbiosis and glial cells (all four major subtypes) in these devastating disorders. Although we covered in some detail the contribution of the glial cells and their primary influence on neuroinflammation, interactions of these cells with other major players such as the neurotrophins or gut microbiota cannot be ignored. The grand jigsaw puzzle is perhaps beyond comprehension now. However, placing even few pieces in their right place helps towards completing the final picture.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
YT was supported in part by NIH/NIAAA R03AA022479 and NIH/NIGMS (2 SO6 GM08016-39). SRH was supported by RO1AA029788 and NIH/NIDA R03DA054335.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Greenberg, P.E.; Fournier, A.A.; Sisitsky, T.; Simes, M.; Berman, R.; Koenigsberg, S.H.; Kessler, R.C. The Economic Burden of Adults with Major Depressive Disorder in the United States (2010 and 2018). Pharmacoeconomics 2021, 39, 653–665. [CrossRef]
- Formánek, T.; Krupchanka, D.; Mladá, K.; Winkler, P.; Jones, P.B. Mortality and life-years lost following subsequent physical comorbidity in people with pre-existing substance use disorders: a national registry-based retrospective cohort study of hospitalised individuals in Czechia. The Lancet Psychiatry 2022, 9, 957–968. [CrossRef]
- Peterson, C.; Li, M.; Xu, L.; Mikosz, C.A.; Luo, F. Assessment of Annual Cost of Substance Use Disorder in US Hospitals. JAMA Netw. open 2021, 4. [CrossRef]
- Agarwal, K.; Manza, P.; Chapman, M.; Nawal, N.; Biesecker, E.; McPherson, K.; Dennis, E.; Johnson, A.; Volkow, N.D.; Joseph, P. V. Inflammatory Markers in Substance Use and Mood Disorders: A Neuroimaging Perspective. Front. Psychiatry 2022, 13, 863734. [CrossRef]
- Davis, M.E. Exosomes What Do We Love So Much About Them? Circ. Res. 2016, 119, 1280–1282. [CrossRef]
- Leza, L.; Haro, B.; López-Goñi, J.J.; Fernández-Montalvo, J. Substance use disorder and lifetime suicidal behaviour: A scoping review. Psychiatry Res. 2024, 334. [CrossRef]
- Hillhouse, T.M.; Porter, J.H. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp. Clin. Psychopharmacol. 2015, 23, 1–21. [CrossRef]
- Matveychuk, D.; Thomas, R.K.; Swainson, J.; Khullar, A.; MacKay, M.A.; Baker, G.B.; Dursun, S.M. Ketamine as an antidepressant: overview of its mechanisms of action and potential predictive biomarkers. Ther. Adv. Psychopharmacol. 2020, 10. [CrossRef]
- Krystal, J.H.; Kaye, A.P.; Jefferson, S.; Girgenti, M.J.; Wilkinson, S.T.; Sanacora, G.; Esterlis, I. Ketamine and the neurobiology of depression: Toward next-generation rapid-acting antidepressant treatments. Proc. Natl. Acad. Sci. U. S. A. 2023, 120, e2305772120. [CrossRef]
- Elmeseiny, O.S.A.; Müller, H.K. A molecular perspective on mGluR5 regulation in the antidepressant effect of ketamine. Pharmacol. Res. 2024, 200. [CrossRef]
- Friedrich, M.J. Depression Is the Leading Cause of Disability Around the World. JAMA 2017, 317, 1517–1517. [CrossRef]
- Lee, J.; Duan, W.; Mattson, M.P. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2002, 82, 1367–1375. [CrossRef]
- Hagg, T. From neurotransmitters to neurotrophic factors to neurogenesis. Neuroscientist 2009, 15, 20–27. [CrossRef]
- Manji, H.K.; Drevets, W.C.; Charney, D.S. The cellular neurobiology of depression. Nat. Med. 2001 75 2001, 7, 541–547. [CrossRef]
- Wang, T.Y.; Lee, S.Y.; Hu, M.C.; Chen, S.L.; Chang, Y.H.; Chu, C.H.; Lin, S.H.; Li, C.L.; Wang, L.J.; Chen, P.S.; et al. More inflammation but less brain-derived neurotrophic factor in antisocial personality disorder. Psychoneuroendocrinology 2017, 85, 42–48. [CrossRef]
- Poo, M. Ming. Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2001 21 2001, 2, 24–32. [CrossRef]
- Dremencov, E.; Jezova, D.; Barak, S.; Gaburjakova, J.; Gaburjakova, M.; Kutna, V.; Ovsepian, S. V. Trophic factors as potential therapies for treatment of major mental disorders. Neurosci. Lett. 2021, 764. [CrossRef]
- Berton, O.; McClung, C.A.; DiLeone, R.J.; Krishnan, V.; Renthal, W.; Russo, S.J.; Graham, D.; Tsankova, N.M.; Bolanos, C.A.; Rios, M.; et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 2006, 311, 864–868. [CrossRef]
- Duman, R.S.; Deyama, S.; Fogaça, M.V. Role of BDNF in the pathophysiology and treatment of depression: Activity-dependent effects distinguish rapid-acting antidepressants. Eur. J. Neurosci. 2021, 53, 126–139. [CrossRef]
- Amidfar, M.; Réus, G.Z.; de Moura, A.B.; Quevedo, J.; Kim, Y.K. The Role of Neurotrophic Factors in Pathophysiology of Major Depressive Disorder. Adv. Exp. Med. Biol. 2021, 1305, 257–272. [CrossRef]
- Numakawa, T.; Kajihara, R. Involvement of brain-derived neurotrophic factor signaling in the pathogenesis of stress-related brain diseases. Front. Mol. Neurosci. 2023, 16, 1247422. [CrossRef]
- Zelada, M.I.; Garrido, V.; Liberona, A.; Jones, N.; Zúñiga, K.; Silva, H.; Nieto, R.R. Brain-Derived Neurotrophic Factor (BDNF) as a Predictor of Treatment Response in Major Depressive Disorder (MDD): A Systematic Review. Int. J. Mol. Sci. 2023, Vol. 24, Page 14810 2023, 24, 14810. [CrossRef]
- Fang, S.; Wu, Z.; Guo, Y.; Zhu, W.; Wan, C.; Yuan, N.; Chen, J.; Hao, W.; Mo, X.; Guo, X.; et al. Roles of microglia in adult hippocampal neurogenesis in depression and their therapeutics. Front. Immunol. 2023, 14. [CrossRef]
- Zou, Y.; Zhang, Y.; Tu, M.; Ye, Y.; Li, M.; Ran, R.; Zou, Z. Brain-derived neurotrophic factor levels across psychiatric disorders: A systemic review and network meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 131. [CrossRef]
- Porter, G.A.; O’Connor, J.C. Brain-derived neurotrophic factor and inflammation in depression: Pathogenic partners in crime? World J. psychiatry 2022, 12, 77–97. [CrossRef]
- Gage, F.H. Mammalian neural stem cells. Science 2000, 287, 1433–1438. [CrossRef]
- Burgess, N.; Maguire, E.A.; O’Keefe, J. The human hippocampus and spatial and episodic memory. Neuron 2002, 35, 625–641. [CrossRef]
- Kang, E.; Wen, Z.; Song, H.; Christian, K.M.; Ming, G.L. Adult Neurogenesis and Psychiatric Disorders. Cold Spring Harb. Perspect. Biol. 2016, 8. [CrossRef]
- Sokoloff, P.; Giros, B.; Martres, M.P.; Bouthenet, M.L.; Schwartz, J.C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 1990, 347, 146–151. [CrossRef]
- Guillin, O.; Diaz, J.; Carroll, P.; Griffon, N.; Schwartz, J.C.; Sokoloff, P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nat. 2001 4116833 2001, 411, 86–89. [CrossRef]
- Staley, J.K.; Mash, D.C. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J. Neurosci. 1996, 16, 6100–6106. [CrossRef]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, Vol. 21, Page 1170 2020, 21, 1170. [CrossRef]
- Lindholm, P.; Saarma, M. Cerebral dopamine neurotrophic factor protects and repairs dopamine neurons by novel mechanism. Mol. Psychiatry 2021 273 2021, 27, 1310–1321. [CrossRef]
- Li, R.; Zhang, P.; Zhang, M.; Yao, Z. The roles of neuron-NG2 glia synapses in promoting oligodendrocyte development and remyelination. Cell Tissue Res. 2020, 381, 43–53. [CrossRef]
- Küppers, E.; Beyer, C. Dopamine regulates brain-derived neurotrophic factor (BDNF) expression in cultured embryonic mouse striatal cells. Neuroreport 2001, 12, 1175–1179. [CrossRef]
- Williams, S.N.; Undieh, A.S. Dopamine D1-like receptor activation induces brain-derived neurotrophic factor protein expression. Neuroreport 2009, 20, 606–610. [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [CrossRef]
- Colucci-D’amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 1–29. [CrossRef]
- Andreska, T.; Lüningschrör, P.; Wolf, D.; McFleder, R.L.; Ayon-Olivas, M.; Rattka, M.; Drechsler, C.; Perschin, V.; Blum, R.; Aufmkolk, S.; et al. DRD1 signaling modulates TrkB turnover and BDNF sensitivity in direct pathway striatal medium spiny neurons. Cell Rep. 2023, 42. [CrossRef]
- Martinowich, K.; Lu, B. Interaction between BDNF and Serotonin: Role in Mood Disorders. Neuropsychopharmacol. 2008 331 2007, 33, 73–83. [CrossRef]
- Maes, M. The cytokine hypothesis of depression: inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuro Endocrinol. Lett. 2008, 29, 287–291.
- Hurley, L.L.; Tizabi, Y. Neuroinflammation, Neurodegeneration, and Depression. Neurotox. Res. 2012 232 2012, 23, 131–144. [CrossRef]
- Audet, M.C.; McQuaid, R.J.; Merali, Z.; Anisman, H. Cytokine variations and mood disorders: Influence of social stressors and social support. Front. Neurosci. 2014, 8, 119363. [CrossRef]
- Dhabhar, F.S.; Burke, H.M.; Epel, E.S.; Mellon, S.H.; Rosser, R.; Reus, V.I.; Wolkowitz, O.M. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. J. Psychiatr. Res. 2009, 43, 962–969. [CrossRef]
- Cattaneo, A.; Ferrari, C.; Uher, R.; Bocchio-Chiavetto, L.; Riva, M.A.; Pariante, C.M. Absolute Measurements of Macrophage Migration Inhibitory Factor and Interleukin-1-β mRNA Levels Accurately Predict Treatment Response in Depressed Patients. Int. J. Neuropsychopharmacol. 2016, 19, 1–10. [CrossRef]
- Hashioka, S.; Inoue, K.; Hayashida, M.; Wake, R.; Oh-Nishi, A.; Miyaoka, T. Implications of systemic inflammation and periodontitis for major depression. Front. Neurosci. 2018, 12, 380928. [CrossRef]
- Kouba, B.R.; de Araujo Borba, L.; Borges de Souza, P.; Gil-Mohapel, J.; Rodrigues, A.L.S. Role of Inflammatory Mechanisms in Major Depressive Disorder: From Etiology to Potential Pharmacological Targets. Cells 2024, Vol. 13, Page 423 2024, 13, 423. [CrossRef]
- Orsolini, L.; Pompili, S.; Valenta, S.T.; Salvi, V.; Volpe, U. C-Reactive Protein as a Biomarker for Major Depressive Disorder? Int. J. Mol. Sci. 2022, 23. [CrossRef]
- Zhu, J.; Jin, J.; Tang, J. Inflammatory pathophysiological mechanisms implicated in postpartum depression. Front. Pharmacol. 2022, 13. [CrossRef]
- Zhu, H.; Guan, A.; Liu, J.; Peng, L.; Zhang, Z.; Wang, S. Noteworthy perspectives on microglia in neuropsychiatric disorders. J. Neuroinflammation 2023, 20, 1–23. [CrossRef]
- Gal, Z.; Torok, D.; Gonda, X.; Eszlari, N.; Anderson, I.M.; Deakin, B.; Juhasz, G.; Bagdy, G.; Petschner, P. Inflammation and Blood-Brain Barrier in Depression: Interaction of CLDN5 and IL6 Gene Variants in Stress-Induced Depression. Int. J. Neuropsychopharmacol. 2023, 26, 189–197. [CrossRef]
- Dadkhah, M.; Baziar, M.; Rezaei, N. The regulatory role of BDNF in neuroimmune axis function and neuroinflammation induced by chronic stress: A new therapeutic strategies for neurodegenerative disorders. Cytokine 2024, 174. [CrossRef]
- Akinfiresoye, L.; Tizabi, Y. Antidepressant effects of AMPA and ketamine combination: role of hippocampal BDNF, synapsin, and mTOR. Psychopharmacology (Berl). 2013, 230, 291–298. [CrossRef]
- Kim, J.; He, M.J.; Widmann, A.K.; Lee, F.S. The role of neurotrophic factors in novel, rapid psychiatric treatments. Neuropsychopharmacol. 2023 491 2023, 49, 227–245. [CrossRef]
- Quintanilla, B.; Zarate, C.A.; Pillai, A. Ketamine’s mechanism of action with an emphasis on neuroimmune regulation: can the complement system complement ketamine’s antidepressant effects? Mol. Psychiatry 2024. [CrossRef]
- Lewandowska-Pietruszka, Z.; Figlerowicz, M.; Mazur-Melewska, K. The History of the Intestinal Microbiota and the Gut-Brain Axis. Pathog. (Basel, Switzerland) 2022, 11. [CrossRef]
- Shoemaker, W.R.; Chen, D.; Garud, N.R. Comparative Population Genetics in the Human Gut Microbiome. Genome Biol. Evol. 2022, 14. [CrossRef]
- Chatterjee, G.; Negi, S.; Basu, S.; Faintuch, J.; O’Donovan, A.; Shukla, P. Microbiome systems biology advancements for natural well-being. Sci. Total Environ. 2022, 838. [CrossRef]
- VanEvery, H.; Franzosa, E.A.; Nguyen, L.H.; Huttenhower, C. Microbiome epidemiology and association studies in human health. Nat. Rev. Genet. 2022 242 2022, 24, 109–124. [CrossRef]
- Radjabzadeh, D.; Bosch, J.A.; Uitterlinden, A.G.; Zwinderman, A.H.; Ikram, M.A.; van Meurs, J.B.J.; Luik, A.I.; Nieuwdorp, M.; Lok, A.; van Duijn, C.M.; et al. Gut microbiome-wide association study of depressive symptoms. Nat. Commun. 2022 131 2022, 13, 1–10. [CrossRef]
- Tizabi, Y.; Bennani, S.; El Kouhen, N.; Getachew, B.; Aschner, M. Interaction of Heavy Metal Lead with Gut Microbiota: Implications for Autism Spectrum Disorder. Biomol. 2023, Vol. 13, Page 1549 2023, 13, 1549. [CrossRef]
- Niemela, L.; Lamoury, G.; Carroll, S.; Morgia, M.; Yeung, A.; Oh, B. Exploring gender differences in the relationship between gut microbiome and depression - a scoping review. Front. Psychiatry 2024, 15, 1361145. [CrossRef]
- Xie, Z.; Huang, J.; Sun, G.; He, S.; Luo, Z.; Zhang, L.; Li, L.; Yao, M.; Du, C.; Yu, W.; et al. Integrated multi-omics analysis reveals gut microbiota dysbiosis and systemic disturbance in major depressive disorder. Psychiatry Res. 2024, 334. [CrossRef]
- Zhang, J.C.; Yao, W.; Dong, C.; Yang, C.; Ren, Q.; Ma, M.; Hashimoto, K. Blockade of interleukin-6 receptor in the periphery promotes rapid and sustained antidepressant actions: a possible role of gut-microbiota-brain axis. Transl. Psychiatry 2017, 7. [CrossRef]
- Bahmani, M.; Mehrtabar, S.; Jafarizadeh, A.; Zoghi, S.; Heravi, F.S.; Abbasi, A.; Sanaie, S.; Rahnemayan, S.; Leylabadlo, H.E. The Gut Microbiota and Major Depressive Disorder: Current Understanding and Novel Therapeutic Strategies. Curr. Pharm. Biotechnol. 2024, 25. [CrossRef]
- Kolobaric, A.; Andreescu, C.; Jašarević, E.; Hong, C.H.; Roh, H.W.; Cheong, J.Y.; Kim, Y.K.; Shin, T.S.; Kang, C.S.; Kwon, C.O.; et al. Gut microbiome predicts cognitive function and depressive symptoms in late life. Mol. Psychiatry 2024 2024, 1–12. [CrossRef]
- United Nations Office on Drugs and Crime Executive summary, World Drug Report; 2023; Vol. 2012;.
- García-Cabrerizo, R.; Cryan, J.F. A gut (microbiome) feeling about addiction: Interactions with stress and social systems. Neurobiol. Stress 2024, 30. [CrossRef]
- Goldstein, R.Z.; Volkow, N.D. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011 1211 2011, 12, 652–669. [CrossRef]
- Russo, S.J.; Mazei-Robison, M.S.; Ables, J.L.; Nestler, E.J. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology 2009, 56 Suppl 1, 73–82. [CrossRef]
- Koskela, M.; Bäck, S.; Võikar, V.; Richie, C.T.; Domanskyi, A.; Harvey, B.K.; Airavaara, M. Update of neurotrophic factors in neurobiology of addiction and future directions. Neurobiol. Dis. 2017, 97, 189–200. [CrossRef]
- Chen, W.; Meng, S.; Han, Y.; Shi, J. Astrocytes: The neglected stars in the central nervous system and drug addiction. Med. Rev. 2022, 2, 417–426. [CrossRef]
- Mann, L.G.; Claassen, D.O. Mesial temporal dopamine: From biology to behaviour. Eur. J. Neurosci. 2024, 59, 1141–1152. [CrossRef]
- Salamone, J.D.; Correa, M. The Neurobiology of Activational Aspects of Motivation: Exertion of Effort, Effort-Based Decision Making, and the Role of Dopamine. Annu. Rev. Psychol. 2024, 75, 1–32. [CrossRef]
- Nestler, E.J.; Lüscher, C. The Molecular Basis of Drug Addiction: Linking Epigenetic to Synaptic and Circuit Mechanisms. Neuron 2019, 102, 48–59. [CrossRef]
- Volkow, N.D. Drugs, Brains, and Behavior: The Science of Addiction: Preface Available online: https://nida.nih.gov/research-topics/addiction-science/drugs-brain-behavior-science-of-addiction (accessed on Apr 27, 2024).
- Getachew, B.; Hauser, S.R.; Bennani, S.; El Kouhen, N.; Sari, Y.; Tizabi, Y. Adolescent alcohol drinking interaction with the gut microbiome: implications for adult alcohol use disorder. Adv. drug alcohol Res. 2024, 4. [CrossRef]
- Hatoum, A.S.; Colbert, S.M.C.; Johnson, E.C.; Huggett, S.B.; Deak, J.D.; Pathak, G.A.; Jennings, M. V.; Paul, S.E.; Karcher, N.R.; Hansen, I.; et al. Multivariate genome-wide association meta-analysis of over 1 million subjects identifies loci underlying multiple substance use disorders. Nat. Ment. Heal. 2023 13 2023, 1, 210–223. [CrossRef]
- Koob, G.F. Anhedonia, Hyperkatifeia, and Negative Reinforcement in Substance Use Disorders. Curr. Top. Behav. Neurosci. 2022, 58, 147–165. [CrossRef]
- Tijani, A.O.; Garg, J.; Frempong, D.; Verana, G.; Kaur, J.; Joga, R.; Sabanis, C.D.; Kumar, S.; Kumar, N.; Puri, A. Sustained drug delivery strategies for treatment of common substance use disorders: Promises and challenges. J. Control. Release 2022, 348, 970–1003. [CrossRef]
- Barker, J.M.; Taylor, J.R.; De Vries, T.J.; Peters, J. Brain-derived neurotrophic factor and addiction: Pathological versus therapeutic effects on drug seeking. Brain Res. 2015, 1628, 68–81. [CrossRef]
- Ornell, F.; Hansen, F.; Schuch, F.B.; Pezzini Rebelatto, F.; Tavares, A.L.; Scherer, J.N.; Valerio, A.G.; Pechansky, F.; Paim Kessler, F.H.; von Diemen, L. Brain-derived neurotrophic factor in substance use disorders: A systematic review and meta-analysis. Drug Alcohol Depend. 2018, 193, 91–103. [CrossRef]
- Peregud, D.I.; Baronets, V.Y.; Terebilina, N.N.; Gulyaeva, N. V. Role of BDNF in Neuroplasticity Associated with Alcohol Dependence. Biochemistry. (Mosc). 2023, 88, 404–416. [CrossRef]
- Rodrigues, L.C.M.; Gobira, P.H.; De Oliveira, A.C.; Pelição, R.; Teixeira, A.L.; Moreira, F.A.; Campos, A.C. Neuroinflammation as a possible link between cannabinoids and addiction. Acta Neuropsychiatr. 2014, 26, 334–346. [CrossRef]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2015 161 2015, 16, 22–34. [CrossRef]
- Cuitavi, J.; Torres-Pérez, J.V.; Lorente, J.D.; Campos-Jurado, Y.; Andrés-Herrera, P.; Polache, A.; Agustín-Pavón, C.; Hipólito, L. Crosstalk between Mu-Opioid receptors and neuroinflammation: Consequences for drug addiction and pain. Neurosci. Biobehav. Rev. 2023, 145. [CrossRef]
- Friedman, H.; Newton, C.; Klein, T.W. Microbial Infections, Immunomodulation, and Drugs of Abuse. Clin. Microbiol. Rev. 2003, 16, 209. [CrossRef]
- Sarkar, D.; Jung, M.K.; Wang, H.J. Alcohol and the Immune System. Alcohol Res. 2015, 37, 153.
- Kohno, M.; Link, J.; Dennis, L.E.; McCready, H.; Huckans, M.; Hoffman, W.F.; Loftis, J.M. Neuroinflammation in addiction: A review of neuroimaging studies and potential immunotherapies. Pharmacol. Biochem. Behav. 2019, 179, 34–42. [CrossRef]
- Salavrakos, M.; Leclercq, S.; De Timary, P.; Dom, G. Microbiome and substances of abuse. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 105. [CrossRef]
- Chivero, E.T.; Sil, S.; Kumar, M.; Buch, S. Substance use, microbiome and psychiatric disorders. Pharmacol. Biochem. Behav. 2022, 219. [CrossRef]
- Gervasi, T.; Mandalari, G. The Interplay Between Gut Microbiota and Central Nervous System. Curr. Pharm. Des. 2023, 29, 3274–3281. [CrossRef]
- Ransom, B.R.; Kettenmann, H. Studying Human Glial Cells: Where Are We Today? Available online: https://pubmed.ncbi.nlm.nih.gov/32057156/ (accessed on Apr 27, 2024).
- Ndubaku, U.; de Bellard, M.E. Glial cells: old cells with new twists. Acta Histochem. 2008, 110, 182–195. [CrossRef]
- Herculano-Houzel, S. The Human Brain in Numbers: A Linearly Scaled-up Primate Brain. Front. Hum. Neurosci. 2009, 3. [CrossRef]
- Jäkel, S.; Dimou, L. Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Front. Cell. Neurosci. 2017, 11. [CrossRef]
- Shi, J.; Huang, S. Comparative Insight into Microglia/Macrophages-Associated Pathways in Glioblastoma and Alzheimer’s Disease. Int. J. Mol. Sci. 2024, Vol. 25, Page 16 2023, 25, 16. [CrossRef]
- Souza, D.G.; Almeida, R.F.; Souza, D.O.; Zimmer, E.R. The astrocyte biochemistry. Semin. Cell Dev. Biol. 2019, 95, 142–150. [CrossRef]
- Bonvento, G.; Bolaños, J.P. Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab. 2021, 33, 1546–1564. [CrossRef]
- Ebling, F.J.P.; Lewis, J.E. Tanycytes and hypothalamic control of energy metabolism. Glia 2018, 66, 1176–1184. [CrossRef]
- Chamberlain, K.A.; Huang, N.; Xie, Y.; LiCausi, F.; Li, S.; Li, Y.; Sheng, Z.H. Oligodendrocytes enhance axonal energy metabolism by deacetylation of mitochondrial proteins through transcellular delivery of SIRT2. Neuron 2021, 109, 3456-3472.e8. [CrossRef]
- Sanchez-Petidier, M.; Guerri, C.; Moreno-Manzano, V. Toll-like receptors 2 and 4 differentially regulate the self-renewal and differentiation of spinal cord neural precursor cells. Stem Cell Res. Ther. 2022, 13, 1–16. [CrossRef]
- Wies Mancini, V.S.B.; Mattera, V.S.; Pasquini, J.M.; Pasquini, L.A.; Correale, J.D. Microglia-derived extracellular vesicles in homeostasis and demyelination/remyelination processes. J. Neurochem. 2024, 168, 3–25. [CrossRef]
- Manu, D.R.; Slevin, M.; Barcutean, L.; Forro, T.; Boghitoiu, T.; Balasa, R. Astrocyte Involvement in Blood–Brain Barrier Function: A Critical Update Highlighting Novel, Complex, Neurovascular Interactions. Int. J. Mol. Sci. 2023, Vol. 24, Page 17146 2023, 24, 17146. [CrossRef]
- Fernandes, V.M.; Auld, V.; Klämbt, C. Glia as Functional Barriers and Signaling Intermediaries. Cold Spring Harb. Perspect. Biol. 2024, 16. [CrossRef]
- Savtchouk, I.; Volterra, A. Gliotransmission: Beyond Black-and-White. J. Neurosci. 2018, 38, 14–25. [CrossRef]
- Lalo, U.; Koh, W.; Lee, C.J.; Pankratov, Y. The tripartite glutamatergic synapse. Neuropharmacology 2021, 199, 108758. [CrossRef]
- Rasia-Filho, A.A.; Calcagnotto, M.E.; von Bohlen und Halbach, O. Glial Cell Modulation of Dendritic Spine Structure and Synaptic Function. Adv. Neurobiol. 2023, 34, 255–310. [CrossRef]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009, 32, 421–431. [CrossRef]
- Allen, N.J.; Eroglu, C. Cell Biology of Astrocyte-Synapse Interactions. Neuron 2017, 96, 697–708. [CrossRef]
- Novikov, N.I.; Brazhnik, E.S.; Kitchigina, V.F. Pathological Correlates of Cognitive Decline in Parkinson’s Disease: From Molecules to Neural Networks. Biochemistry. (Mosc). 2023, 88, 1890–1904. [CrossRef]
- Reed, M.M.; Blazer-Yost, B. Channels and Transporters in Astrocyte Volume Regulation in Health and Disease. Cell. Physiol. Biochem. 2022, 56, 12–30. [CrossRef]
- Clayton, R.W.; Lovell-Badge, R.; Galichet, C. The Properties and Functions of Glial Cell Types of the Hypothalamic Median Eminence. Front. Endocrinol. (Lausanne). 2022, 13, 953995. [CrossRef]
- Kofler, J.; Wiley, C.A. Microglia: key innate immune cells of the brain. Toxicol. Pathol. 2011, 39, 103–114. [CrossRef]
- Chen, X.; Holtzman, D.M. Emerging roles of innate and adaptive immunity in Alzheimer’s disease. Immunity 2022, 55, 2236–2254. [CrossRef]
- Dringen, R.; Brandmann, M.; Hohnholt, M.C.; Blumrich, E.M. Glutathione-Dependent Detoxification Processes in Astrocytes. Neurochem. Res. 2014 4012 2014, 40, 2570–2582. [CrossRef]
- Ioannou, M.S.; Jackson, J.; Sheu, S.H.; Chang, C.L.; Weigel, A. V.; Liu, H.; Pasolli, H.A.; Xu, C.S.; Pang, S.; Matthies, D.; et al. Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell 2019, 177, 1522-1535.e14. [CrossRef]
- Rahman, S.; Alzarea, S. Glial mechanisms underlying major depressive disorder: Potential therapeutic opportunities. Prog. Mol. Biol. Transl. Sci. 2019, 167, 159–178. [CrossRef]
- Scuderi, C.; Verkhratsky, A.; Parpura, V.; Li, B. Neuroglia in Psychiatric Disorders. Adv. Neurobiol. 2021, 26, 3–19. [CrossRef]
- Hanslik, K.L.; Marino, K.M.; Ulland, T.K. Modulation of Glial Function in Health, Aging, and Neurodegenerative Disease. Front. Cell. Neurosci. 2021, 15, 718324. [CrossRef]
- Zhao, G. Shared and disease-specific glial gene expression changes in neurodegenerative diseases. Nat. aging 2023, 3, 246–247. [CrossRef]
- Soares, É.N.; Carla, A.; Costa, S.; De, G.; Ferrolho, J.; Ureshino, R.P.; Getachew, B.; Lima Costa, S.; Diogenes Amaral Da Silva, V.; Tizabi, Y. Nicotinic Acetylcholine Receptors in Glial Cells as Molecular Target for Parkinson’s Disease. Cells 2024, Vol. 13, Page 474 2024, 13, 474. [CrossRef]
- Magni, G.; Riboldi, B.; Ceruti, S. Human Glial Cells as Innovative Targets for the Therapy of Central Nervous System Pathologies. Cells 2024, Vol. 13, Page 606 2024, 13, 606. [CrossRef]
- Sanadgol, N.; Miraki Feriz, A.; Lisboa, S.F.; Joca, S.R.L. Putative role of glial cells in treatment resistance depression: An updated critical literation review and evaluation of single-nuclei transcriptomics data. Life Sci. 2023, 331. [CrossRef]
- Saba, W. Glial dysfunction in substance use disorders. New insights from PET and MR imaging. Addict. Neurosci. 2023, 9, 100135. [CrossRef]
- Bernier, L.P.; Bohlen, C.J.; York, E.M.; Choi, H.B.; Kamyabi, A.; Dissing-Olesen, L.; Hefendehl, J.K.; Collins, H.Y.; Stevens, B.; Barres, B.A.; et al. Nanoscale Surveillance of the Brain by Microglia via cAMP-Regulated Filopodia. Cell Rep. 2019, 27, 2895-2908.e4. [CrossRef]
- Nebeling, F.C.; Poll, S.; Justus, L.C.; Steffen, J.; Keppler, K.; Mittag, M.; Fuhrmann, M. Microglial motility is modulated by neuronal activity and correlates with dendritic spine plasticity in the hippocampus of awake mice. Elife 2023, 12. [CrossRef]
- Pathak, D.; Sriram, K. Molecular Mechanisms Underlying Neuroinflammation Elicited by Occupational Injuries and Toxicants. Int. J. Mol. Sci. 2023, 24. [CrossRef]
- Saitgareeva, A.R.; Bulygin, K. V.; Gareev, I.F.; Beylerli, O.A.; Akhmadeeva, L.R. The role of microglia in the development of neurodegeneration. Neurol. Sci. 2020, 41, 3609–3615. [CrossRef]
- Costa, T.; Fernandez-Villalba, E.; Izura, V.; Lucas-Ochoa, A.; Menezes-Filho, N.; Santana, R.; de Oliveira, M.; Araújo, F.; Estrada, C.; Silva, V.; et al. Combined 1-Deoxynojirimycin and Ibuprofen Treatment Decreases Microglial Activation, Phagocytosis and Dopaminergic Degeneration in MPTP-Treated Mice. J. Neuroimmune Pharmacol. 2021, 16, 390–402. [CrossRef]
- De Marchi, F.; Munitic, I.; Vidatic, L.; Papić, E.; Rački, V.; Nimac, J.; Jurak, I.; Novotni, G.; Rogelj, B.; Vuletic, V.; et al. Overlapping Neuroimmune Mechanisms and Therapeutic Targets in Neurodegenerative Disorders. Biomedicines 2023, 11. [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023 81 2023, 8, 1–37. [CrossRef]
- Darwish, S.F.; Elbadry, A.M.M.; Elbokhomy, A.S.; Salama, G.A.; Salama, R.M. The dual face of microglia (M1/M2) as a potential target in the protective effect of nutraceuticals against neurodegenerative diseases. Front. Aging 2023, 4, 1231706. [CrossRef]
- Qin, J.; Ma, Z.; Chen, X.; Shu, S. Microglia activation in central nervous system disorders: A review of recent mechanistic investigations and development efforts. Front. Neurol. 2023, 14, 1103416. [CrossRef]
- Stratoulias, V.; Venero, J.L.; Tremblay, M.; Joseph, B. Microglial subtypes: diversity within the microglial community. EMBO J. 2019, 38. [CrossRef]
- Brandebura, A.N.; Paumier, A.; Onur, T.S.; Allen, N.J. Astrocyte contribution to dysfunction, risk and progression in neurodegenerative disorders. Nat. Rev. Neurosci. 2022 241 2022, 24, 23–39. [CrossRef]
- Tremblay, M.È.; Stevens, B.; Sierra, A.; Wake, H.; Bessis, A.; Nimmerjahn, A. The Role of Microglia in the Healthy Brain. J. Neurosci. 2011, 31, 16064–16069. [CrossRef]
- Nimmerjahn, A. Two-Photon Imaging of Microglia in the Mouse Cortex In Vivo. Cold Spring Harb. Protoc. 2012, 2012, pdb.prot069294. [CrossRef]
- Leyh, J.; Paeschke, S.; Mages, B.; Michalski, D.; Nowicki, M.; Bechmann, I.; Winter, K. Classification of Microglial Morphological Phenotypes Using Machine Learning. Front. Cell. Neurosci. 2021, 15, 701673. [CrossRef]
- Ziebell, J.M.; Taylor, S.E.; Cao, T.; Harrison, J.L.; Lifshitz, J. Rod microglia: Elongation, alignment, and coupling to form trains across the somatosensory cortex after experimental diffuse brain injury. J. Neuroinflammation 2012, 9, 1–11. [CrossRef]
- Taylor, S.E.; Morganti-Kossmann, C.; Lifshitz, J.; Ziebell, J.M. Rod Microglia: A Morphological Definition. PLoS One 2014, 9, e97096. [CrossRef]
- Fatoba, O.; Itokazu, T.; Yamashita, T. Microglia as therapeutic target in central nervous system disorders. J. Pharmacol. Sci. 2020, 144, 102–118. [CrossRef]
- Liu, J.; Li, J.X.; Wu, R. Toll-Like Receptor 4: A Novel Target to Tackle Drug Addiction? Handb. Exp. Pharmacol. 2022, 276, 275–290. [CrossRef]
- Heidari, A.; Yazdanpanah, N.; Rezaei, N. The role of Toll-like receptors and neuroinflammation in Parkinson’s disease. J. Neuroinflammation 2022 191 2022, 19, 1–21. [CrossRef]
- Yirmiya, R.; Rimmerman, N.; Reshef, R. Depression as a microglial disease. Trends Neurosci. 2015, 38, 637–658. [CrossRef]
- Wang, H.; He, Y.; Sun, Z.; Ren, S.; Liu, M.; Wang, G.; Yang, J. Microglia in depression: an overview of microglia in the pathogenesis and treatment of depression. J. Neuroinflammation 2022 191 2022, 19, 1–26. [CrossRef]
- Brites, D.; Fernandes, A. Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Front. Cell. Neurosci. 2015, 9, 1–20. [CrossRef]
- Scheepstra, K.W.F.; Mizee, M.R.; van Scheppingen, J.; Adelia, A.; Wever, D.D.; Mason, M.R.J.; Dubbelaar, M.L.; Hsiao, C.C.; Eggen, B.J.L.; Hamann, J.; et al. Microglia Transcriptional Profiling in Major Depressive Disorder Shows Inhibition of Cortical Gray Matter Microglia. Biol. Psychiatry 2023, 94, 619–629. [CrossRef]
- Böttcher, C.; Fernández-Zapata, C.; Snijders, G.J.L.; Schlickeiser, S.; Sneeboer, M.A.M.; Kunkel, D.; De Witte, L.D.; Priller, J. Single-cell mass cytometry of microglia in major depressive disorder reveals a non-inflammatory phenotype with increased homeostatic marker expression. Transl. Psychiatry 2020, 10. [CrossRef]
- Li, H.; Watkins, L.R.; Wang, X. Microglia in neuroimmunopharmacology and drug addiction. Mol. Psychiatry 2024. [CrossRef]
- Kierdorf, K.; Prinz, M. Microglia in steady state. J. Clin. Invest. 2017, 127, 3201–3209. [CrossRef]
- Schmidt, K.; Engel, P. Mechanisms underlying gut microbiota-host interactions in insects. J. Exp. Biol. 2021, 224. [CrossRef]
- Deng, S. long; Chen, J. guo; Wang, F. Microglia: A Central Player in Depression. Curr. Med. Sci. 2020, 40, 391–400. [CrossRef]
- Cadet, J.L.; Bisagno, V. Glial-neuronal ensembles: Partners in drug addiction- associated synaptic plasticity. Front. Pharmacol. 2014, 5 AUG, 111326. [CrossRef]
- Lacagnina, M.J.; Rivera, P.D.; Bilbo, S.D. Glial and Neuroimmune Mechanisms as Critical Modulators of Drug Use and Abuse. Neuropsychopharmacol. 2017 421 2016, 42, 156–177. [CrossRef]
- Li, L.; Acioglu, C.; Heary, R.F.; Elkabes, S. Role of astroglial toll-like receptors (TLRs) in central nervous system infections, injury and neurodegenerative diseases. Brain. Behav. Immun. 2021, 91, 740–755. [CrossRef]
- Stoklund Dittlau, K.; Freude, K. Astrocytes: The Stars in Neurodegeneration? Biomolecules 2024, 14, 289. [CrossRef]
- Pathak, D.; Sriram, K. Neuron-astrocyte omnidirectional signaling in neurological health and disease. Front. Mol. Neurosci. 2023, 16, 1169320. [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [CrossRef]
- Kotliarova, A.; Sidorova, Y.A. Glial Cell Line-Derived Neurotrophic Factor Family Ligands, Players at the Interface of Neuroinflammation and Neuroprotection: Focus Onto the Glia. Front. Cell. Neurosci. 2021, 15. [CrossRef]
- Zinchuk, M.S.; Guekht, A.B.; Druzhkova, T.A.; Gulyaeva, N. V.; Shpak, A.A. Glial cell line-derived neurotrophic factor (GDNF) in blood serum and lacrimal fluid of patients with a current depressive episode. J. Affect. Disord. 2022, 318, 409–413. [CrossRef]
- Heir, R.; Abbasi, Z.; Komal, P.; Altimimi, H.F.; Franquin, M.; Moschou, D.; Chambon, J.; Stellwagen, D. Astrocytes Are the Source of TNF Mediating Homeostatic Synaptic Plasticity. J. Neurosci. 2024, 44, e2278222024. [CrossRef]
- Ramírez-Guerrero, S.; Guardo-Maya, S.; Medina-Rincón, G.J.; Orrego-González, E.E.; Cabezas-Pérez, R.; González-Reyes, R.E. Taurine and Astrocytes: A Homeostatic and Neuroprotective Relationship. Front. Mol. Neurosci. 2022, 15. [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934. [CrossRef]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [CrossRef]
- Patani, R.; Hardingham, G.E.; Liddelow, S.A. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat. Rev. Neurol. 2023 197 2023, 19, 395–409. [CrossRef]
- Vainchtein, I.D.; Molofsky, A. V. Astrocytes and Microglia: In Sickness and in Health. Trends Neurosci. 2020, 43, 144–154. [CrossRef]
- Garland, E.F.; Hartnell, I.J.; Boche, D. Microglia and Astrocyte Function and Communication: What Do We Know in Humans? Front. Neurosci. 2022, 16, 824888. [CrossRef]
- von Bartheld, C.S.; Bahney, J.; Herculano-Houzel, S. The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. J. Comp. Neurol. 2016, 524, 3865–3895. [CrossRef]
- Rajkowska, G.; Stockmeier, C. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr. Drug Targets 2013, 14, 1225–1236. [CrossRef]
- Czéh, B.; Di Benedetto, B. Antidepressants act directly on astrocytes: evidences and functional consequences. Eur. Neuropsychopharmacol. 2013, 23, 171–185. [CrossRef]
- Zhou, X.; Xiao, Q.; Xie, L.; Yang, F.; Wang, L.; Tu, J. Astrocyte, a Promising Target for Mood Disorder Interventions. Front. Mol. Neurosci. 2019, 12. [CrossRef]
- Tsai, S.F.; Hsu, P.L.; Chen, Y.W.; Hossain, M.S.; Chen, P.C.; Tzeng, S.F.; Chen, P.S.; Kuo, Y.M. High-fat diet induces depression-like phenotype via astrocyte-mediated hyperactivation of ventral hippocampal glutamatergic afferents to the nucleus accumbens. Mol. Psychiatry 2022, 27, 4372–4384. [CrossRef]
- O’Leary, L.A.; Mechawar, N. Implication of cerebral astrocytes in major depression: A review of fine neuroanatomical evidence in humans. Glia 2021, 69, 2077–2099. [CrossRef]
- Marshak, D. S100 beta as a neurotrophic factor - PubMed. Prog Brain Res 1990, 86, 169–81.
- Du, J.; Yi, M.; Zhou, F.; He, W.; Yang, A.; Qiu, M.; Huang, H. S100B is selectively expressed by gray matter protoplasmic astrocytes and myelinating oligodendrocytes in the developing CNS. Mol. Brain 2021, 14, 1–11. [CrossRef]
- Zhao, Y.F.; Verkhratsky, A.; Tang, Y.; Illes, P. Astrocytes and major depression: The purinergic avenue. Neuropharmacology 2022, 220. [CrossRef]
- González-Arias, C.; Sánchez-Ruiz, A.; Esparza, J.; Sánchez-Puelles, C.; Arancibia, L.; Ramírez-Franco, J.; Gobbo, D.; Kirchhoff, F.; Perea, G. Dysfunctional serotonergic neuron-astrocyte signaling in depressive-like states. Mol. Psychiatry 2023 289 2023, 28, 3856–3873. [CrossRef]
- Novakovic, M.M.; Korshunov, K.S.; Grant, R.A.; Martin, M.E.; Valencia, H.A.; Budinger, G.R.S.; Radulovic, J.; Prakriya, M. Astrocyte reactivity and inflammation-induced depression-like behaviors are regulated by Orai1 calcium channels. Nat. Commun. 2023 141 2023, 14, 1–20. [CrossRef]
- Yao, J.; Chen, C.; Guo, Y.; Yang, Y.; Liu, X.; Chu, S.; Ai, Q.; Zhang, Z.; Lin, M.; Yang, S.; et al. A Review of Research on the Association between Neuron–Astrocyte Signaling Processes and Depressive Symptoms. Int. J. Mol. Sci. 2023, Vol. 24, Page 6985 2023, 24, 6985. [CrossRef]
- Kruyer, A.; Kalivas, P.W. Astrocytes as cellular mediators of cue reactivity in addiction. Curr. Opin. Pharmacol. 2021, 56, 1–6. [CrossRef]
- Kruyer, A.; Scofield, M.D. Astrocytes in Addictive Disorders. Adv. Neurobiol. 2021, 26, 231–254. [CrossRef]
- Wang, J.; Holt, L.M.; Huang, H.H.; Sesack, S.R.; Nestler, E.J.; Dong, Y. Astrocytes in cocaine addiction and beyond. Mol. Psychiatry 2022, 27, 652–668. [CrossRef]
- Holt, L.M.; Nestler, E.J. Astrocytic transcriptional and epigenetic mechanisms of drug addiction. J. Neural Transm. 2023 1315 2023, 131, 409–424. [CrossRef]
- Michalski, J.P.; Kothary, R. Oligodendrocytes in a nutshell. Front. Cell. Neurosci. 2015, 9, 158759. [CrossRef]
- Salzer, J.L. Schwann cell myelination. Cold Spring Harb. Perspect. Biol. 2015, 7. [CrossRef]
- Zhou, Y.; Zhang, J. Neuronal activity and remyelination: new insights into the molecular mechanisms and therapeutic advancements. Front. Cell Dev. Biol. 2023, 11, 1221890. [CrossRef]
- Bsibsi, M.; Nomden, A.; van Noort, J.M.; Baron, W. Toll-like receptors 2 and 3 agonists differentially affect oligodendrocyte survival, differentiation, and myelin membrane formation. J. Neurosci. Res. 2012, 90, 388–398. [CrossRef]
- Kumar, V. Toll-like receptors in the pathogenesis of neuroinflammation. J. Neuroimmunol. 2019, 332, 16–30. [CrossRef]
- Hamidi, M.; Drevets, W.C.; Price, J.L. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol. Psychiatry 2004, 55, 563–569. [CrossRef]
- Rajkowska, G.; Miguel-Hidalgo, J. Gliogenesis and glial pathology in depression. CNS Neurol. Disord. Drug Targets 2007, 6, 219–233. [CrossRef]
- Zhou, B.; Zhu, Z.; Ransom, B.R.; Tong, X. Oligodendrocyte lineage cells and depression. Mol. Psychiatry 2020 261 2020, 26, 103–117. [CrossRef]
- Szebeni, A.; Szebeni, K.; DiPeri, T.P.; Johnson, L.A.; Stockmeier, C.A.; Crawford, J.D.; Chandley, M.J.; Hernandez, L.J.; Burgess, K.C.; Brown, R.W.; et al. Elevated DNA Oxidation and DNA Repair Enzyme Expression in Brain White Matter in Major Depressive Disorder. Int. J. Neuropsychopharmacol. 2017, 20, 363–373. [CrossRef]
- Kokkosis, A.G.; Madeira, M.M.; Mullahy, M.R.; Tsirka, S.E. Chronic stress disrupts the homeostasis and progeny progression of oligodendroglial lineage cells, associating immune oligodendrocytes with prefrontal cortex hypomyelination. Mol. Psychiatry 2022 276 2022, 27, 2833–2848. [CrossRef]
- Chandley, M.J.; Szebeni, A.; Szebeni, K.; Wang-Heaton, H.; Garst, J.; Stockmeier, C.A.; Lewis, N.H.; Ordway, G.A. Markers of elevated oxidative stress in oligodendrocytes captured from the brainstem and occipital cortex in major depressive disorder and suicide. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 117. [CrossRef]
- Liu, Y.; Yuan, J.; Dong, Y.; Jiang, S.; Zhang, M.; Zhao, X.; Ramos-Rodríguez, J.; Bermudez, B.; Liu, Y.; Yuan, J.; et al. Interaction between Oligodendrocytes and Interneurons in Brain Development and Related Neuropsychiatric Disorders. Int. J. Mol. Sci. 2024, Vol. 25, Page 3620 2024, 25, 3620. [CrossRef]
- Reissner, K.J.; Pletnikov, M. V. Contributions of nonneuronal brain cells in substance use disorders. Neuropsychopharmacology 2020, 45, 224–225. [CrossRef]
- Velasco, B.; Mohamed, E.; Sato-Bigbee, C. Endogenous and exogenous opioid effects on oligodendrocyte biology and developmental brain myelination. Neurotoxicol. Teratol. 2021, 86. [CrossRef]
- Marguet, F.; Brosolo, M.; Friocourt, G.; Sauvestre, F.; Marcorelles, P.; Lesueur, C.; Marret, S.; Gonzalez, B.J.; Laquerrière, A. Oligodendrocyte lineage is severely affected in human alcohol-exposed foetuses. Acta Neuropathol. Commun. 2022, 10, 1–14. [CrossRef]
- Hill, R.A.; Nishiyama, A. NG2 cells (polydendrocytes): Listeners to the neural network with diverse properties. Glia 2014, 62, 1195–1210. [CrossRef]
- Kirdajova, D.; Anderova, M. NG2 cells and their neurogenic potential. Curr. Opin. Pharmacol. 2020, 50, 53–60. [CrossRef]
- Xu, J.P.; Zhao, J.; Li, S. Roles of NG2 glial cells in diseases of the central nervous system. Neurosci. Bull. 2011, 27, 413–421. [CrossRef]
- Dimou, L.; Gallo, V. NG2-glia and their functions in the central nervous system. Glia 2015, 63, 1429–1451. [CrossRef]
- Ferrara, G.; Errede, M.; Girolamo, F.; Morando, S.; Ivaldi, F.; Panini, N.; Bendotti, C.; Perris, R.; Furlan, R.; Virgintino, D.; et al. NG2, a common denominator for neuroinflammation, blood–brain barrier alteration, and oligodendrocyte precursor response in EAE, plays a role in dendritic cell activation. Acta Neuropathol. 2016, 132, 23–42. [CrossRef]
- Zhang, S.Z.; Wang, Q.Q.; Yang, Q.Q.; Gu, H.Y.; Yin, Y.Q.; Li, Y.D.; Hou, J.C.; Chen, R.; Sun, Q.Q.; Sun, Y.F.; et al. NG2 glia regulate brain innate immunity via TGF-β2/TGFBR2 axis. BMC Med. 2019, 17, 1–22. [CrossRef]
- Hu, X.; Geng, P.; Zhao, X.; Wang, Q.; Liu, C.; Guo, C.; Dong, W.; Jin, X. The NG2-glia is a potential target to maintain the integrity of neurovascular unit after acute ischemic stroke. Neurobiol. Dis. 2023, 180. [CrossRef]
- Vélez-Fort, M.; Audinat, E.; Angulo, M.C. Functional α7-containing nicotinic receptors of NG2-expressing cells in the hippocampus. Glia 2009, 57, 1104–1114. [CrossRef]
- Timmermann, A.; Tascio, D.; Jabs, R.; Boehlen, A.; Domingos, C.; Skubal, M.; Huang, W.; Kirchhoff, F.; Henneberger, C.; Bilkei-Gorzo, A.; et al. Dysfunction of NG2 glial cells affects neuronal plasticity and behavior. Glia 2023, 71, 1481–1501. [CrossRef]
- Poggi, G.; Wennström, M.; Müller, M.B.; Treccani, G. NG2-glia: rising stars in stress-related mental disorders? Mol. Psychiatry 2022 282 2022, 28, 518–520. [CrossRef]
- Bell, L.A.; Wallis, G.J.; Wilcox, K.S. Reactivity and increased proliferation of NG2 cells following central nervous system infection with Theiler’s murine encephalomyelitis virus. J. Neuroinflammation 2020, 17, 1–14. [CrossRef]
- Janeckova, L.; Knotek, T.; Kriska, J.; Hermanova, Z.; Kirdajova, D.; Kubovciak, J.; Berkova, L.; Tureckova, J.; Camacho Garcia, S.; Galuskova, K.; et al. Astrocyte-like subpopulation of NG2 glia in the adult mouse cortex exhibits characteristics of neural progenitor cells. Glia 2024, 72, 245–273. [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology , function , and biomedical applications of exosomes. Science 2020, 367. [CrossRef]
- Wang, Y.; Xiao, T.; Zhao, C.; Li, G. The Regulation of Exosome Generation and Function in Physiological and Pathological Processes. Int. J. Mol. Sci. 2024, Vol. 25, Page 255 2023, 25, 255. [CrossRef]
- Sun, T.; Li, M.; Liu, Q.; Yu, A.; Cheng, K.; Ma, J.; Murphy, S.; McNutt, P.M.; Zhang, Y. Insights into optimizing exosome therapies for acute skin wound healing and other tissue repair. Front. Med. 2024. [CrossRef]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2023 91 2024, 9, 1–31. [CrossRef]
- Somkuwar, S.S.; Staples, M.C.; Galinato, M.H.; Fannon, M.J.; Mandyam, C.D. Role of NG2 expressing cells in addiction: a new approach for an old problem. Front. Pharmacol. 2014, 5. [CrossRef]
- Darbinian, N.; Darbinyan, A.; Merabova, N.; Bajwa, A.; Tatevosian, G.; Martirosyan, D.; Zhao, H.; Selzer, M.E.; Goetzl, L. Ethanol-mediated alterations in oligodendrocyte differentiation in the developing brain. Neurobiol. Dis. 2021, 148. [CrossRef]
- Nohesara, S.; Abdolmaleky, H.M.; Zhou, J.R.; Thiagalingam, S. Microbiota-Induced Epigenetic Alterations in Depressive Disorders Are Targets for Nutritional and Probiotic Therapies. Genes (Basel). 2023, 14. [CrossRef]
- Soret, R.; Chevalier, J.; De Coppet, P.; Poupeau, G.; Derkinderen, P.; Segain, J.P.; Neunlist, M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 2010, 138. [CrossRef]
- Candido, E.P.M.; Reeves, R.; Davie, J.R. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell 1978, 14, 105–113. [CrossRef]
- Sealy, L.; Chalkley, R. The effect of sodium butyrate on histone modification. Cell 1978, 14, 115–121. [CrossRef]
- Schroeder, F.A.; Lin, C.L.; Crusio, W.E.; Akbarian, S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol. Psychiatry 2007, 62, 55–64. [CrossRef]
- Valvassori, S.; Varela, R.; Arent, C.; Dal-Pont, G.; Bobsin, T.; Budni, J.; Reus, G.; Quevedo, J. Sodium butyrate functions as an antidepressant and improves cognition with enhanced neurotrophic expression in models of maternal deprivation and chronic mild stress. Curr. Neurovasc. Res. 2014, 11, 359–366. [CrossRef]
- Valvassori, S.S.; Varela, R.B.; Resende, W.R.; Della, T.P.; Borba, L.A.; Behenck, J.P.; Réus, G.Z.; Quevedo, J. Antidepressant Effect of Sodium Butyrate is Accompanied by Brain Epigenetic Modulation in Rats Subjected to Early or Late Life Stress. Curr. Neurovasc. Res. 2024, 21. [CrossRef]
- Palasz, E.; Wilkaniec, A.; Stanaszek, L.; Andrzejewska, A.; Adamczyk, A. Glia-Neurotrophic Factor Relationships: Possible Role in Pathobiology of Neuroinflammation-Related Brain Disorders. Int. J. Mol. Sci. 2023, 24. [CrossRef]
- Calabrese, F.; Rossetti, A.C.; Racagni, G.; Gass, P.; Riva, M.A.; Molteni, R. Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Front. Cell. Neurosci. 2014, 8. [CrossRef]
- Wu, A.; Zhang, J. Neuroinflammation, memory, and depression: new approaches to hippocampal neurogenesis. J. Neuroinflammation 2023, 20. [CrossRef]
- Liu, J.; Fang, Y.; Cui, L.; Wang, Z.; Luo, Y.; Gao, C.; Ge, W.; Huang, T.; Wen, J.; Zhou, T. Butyrate emerges as a crucial effector of Zhi-Zi-Chi decoctions to ameliorate depression via multiple pathways of brain-gut axis. Biomed. Pharmacother. 2022, 149. [CrossRef]
- Luqman, A.; He, M.; Hassan, A.; Ullah, M.; Zhang, L.; Rashid Khan, M.; Din, A.U.; Ullah, K.; Wang, W.; Wang, G. Mood and microbes: a comprehensive review of intestinal microbiota’s impact on depression. Front. psychiatry 2024, 15. [CrossRef]
- Wang, M.; Song, Z.; Lai, S.; Tang, F.; Dou, L.; Yang, F. Depression-associated gut microbes, metabolites and clinical trials. Front. Microbiol. 2024, 15. [CrossRef]
- Garg, K.; Mohajeri, M.H. Potential effects of the most prescribed drugs on the microbiota-gut-brain-axis: A review. Brain Res. Bull. 2024, 207. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).