Keywords adhesive capsulitis; breast cancer; intra-articular injection; post-mastectomy; shoulder pain
Introduction
Upper extremity dysfunction is a common issue among the breast cancer survivors. With increasing breast cancer survival rates, upper extremity pain and dysfunction have emerged as significant complications after breast cancer surgery [1, 2]. Post-mastectomy pain syndrome affects 25-60% of patients after breast cancer surgery [
3]. Post-breast cancer surgery patients often experience soft tissue fibrosis, deficits in muscle flexibility, glenohumeral joint stiffness, and adhesive capsulitis of the shoulder [
4]. Adhesive capsulitis of the shoulder is classified as either idiopathic or secondary. Idiopathic or primary adhesive capsulitis diagnosed when patient history or physical examination cannot explain the onset of the condition. Secondary adhesive capsulitis diagnosed when the condition develops from known causes such as pre-existing shoulder disorders, trauma, or surgery [5, 6]. Secondary adhesive capsulitis after breast cancer surgery causes pain and limited motion in the affected shoulder joint, disturbing daily activities and reducing quality of life [
7].
Post-breast cancer surgery patients have a greater risk of adhesive capsulitis of the shoulder compared to the general population. A single-center study showed a cumulative prevalence of 10.3% of adhesive capsulitis in breast cancer patients over a postoperative period between 13 and 18 months [
8]. A cross-sectional observational study that recruited 135 Asian women who underwent breast cancer surgery determined the prevalence of adhesive capsulitis to be 22.2% [
9]. Despite the high prevalence of adhesive capsulitis of the shoulder after breast cancer surgery, the risk factors for the condition are largely unknown. Presence of lymphedema, age 50-59 years, and mastectomy are possible risk factors for secondary adhesive capsulitis after breast cancer surgery [8-10]. Considering the high prevalence and the long-term sequelae of secondary adhesive capsulitis after breast cancer surgery, early detection and proper management are essential.
A variety of treatment options is available for adhesive capsulitis, including physical therapy, pharmacologic therapy, intra-articular steroid injection, and surgical management. Idiopathic adhesive capsulitis caused by thickening and contraction of the joint capsule due to inflammation and fibrosis, so a combination of intra-articular steroid injections and physical therapy is effective in management [11, 12]. However, a consensus on the treatment of secondary adhesive capsulitis after breast cancer surgery has not been established. Intra-articular steroid injections are commonly used to treat idiopathic adhesive capsulitis, yet its effectiveness in patients with secondary adhesive capsulitis after breast cancer surgery remains insufficiently studied. Furthermore, comparisons of the clinical efficacy of intra-articular steroid injection between idiopathic and secondary adhesive capsulitis are lacking.
Patients with adhesive capsulitis after breast cancer surgery have chronic pain and decreased quality of life and require further treatment. Limited evidence regarding the treatment options for this patient population is an obstacle to the implementation of more active physical therapy and injections due to side effect concerns. Therefore, investigation of the therapeutic effect and safety of intra-articular steroid injection in breast cancer patients may be helpful in the prevention of long-term morbidities and establishment of a treatment option for patients after breast cancer surgery.
We hypothesized that intra-articular triamcinolone injection is an effective treatment for adhesive capsulitis after breast cancer surgery. To prove our hypothesis, we evaluated short-term and long-term clinical and functional outcomes of intra-articular triamcinolone injection in adhesive capsulitis after breast cancer surgery. We focused on the therapeutic effects on pain, range of motion (ROM), and functional disability of the shoulder after breast cancer surgery and compared these effects with those of idiopathic adhesive capsulitis patients.
Materials and Methods
Study Design and Participant
This study was a single-center, prospective, two-arm clinical trial involving two patient groups with adhesive capsulitis of the shoulder: the breast cancer surgery group and the idiopathic group. Enrollment took place from July 2019 to November 2021 at a tertiary hospital rehabilitation center in South Korea. The inclusion criteria for the breast cancer surgery group were: (1) age of 19 years or older; (2) clinical diagnosis of adhesive capsulitis; and (3) affected shoulder joint restriction of at least 30˚ compared to the contralateral side. Participants met this criterion if the joint restriction was present in two or more of forward flexion, abduction, or external rotation (with 90˚ shoulder abduction). ROMs were measured by a goniometer in the supine position. Additional inclusion criteria were (4) ability to receive in-hospital physical therapy and (5) surgery for breast cancer. The exclusion criteria were: (1) bilateral adhesive capsulitis; (2) secondary adhesive capsulitis caused by trauma (shoulder fracture, dislocation) and/or systemic inflammatory disease (rheumatoid arthritis); (3) other mimicking disorders such as glenohumeral arthritis, bursitis, rotator cuff disease, or calcific tendinitis of shoulder; (4) unable to perform exercise due to general deconditioning; and (5) communication difficulties. For the idiopathic group, the diagnosis of breast cancer was an exclusion criterion rather than an inclusion criterion. Plain shoulder X-ray and ultrasonography were performed to exclude mimicking disorders. This clinical study was performed in accordance with principles of the Declaration of Helsinki. Written informed consent was obtained from all participants, and the study protocol was approved by the Institutional Review Board of Samsung Medical Center (approval number: SMC-2019-05-021). Trial registration: This trial was retrospectively registered at the Clinicaltrials.gov website on 20 March 2020, with the identifier NCT04316130.
Baseline Characteristics and Clinical Assessments
Participant medical records were reviewed for demographic data, weight, height, calculated body mass index, and medical history. For all participants, the scores of the Shoulder Pain and Disability Index (SPADI), the passive range of motion (PROM) of the affected shoulder joint, and the pain intensity on the Numeric Rating Scale (NRS) were measured for the assessment of pain and disability of the affected shoulder joint. SPADI is a self-reported questionnaire consisting of two subscales with a total of 13 items, five items for pain and eight items for disability [
13]. Participants report a level of difficulty performing activities of daily living (ADLs) due to pain and limited motion for the previous week. The final score ranges from 0 to 130 with a percentage score of 0 indicating no shoulder disability and 100 indicating complete shoulder dysfunction. The PROM of the affected shoulder joint was obtained with an electric goniometer in units of 1˚ with the participant in a supine position. Shoulder forward elevation (FE), abduction, internal rotation (IR) with 90˚ shoulder abduction, and external rotation (ER) with 90˚ shoulder abduction were measured. The pain intensity at rest and at activity were measured by an 11-point NRS for the previous week, with 0 and 10 representing “no pain” and “the worst possible pain”, respectively. The clinical outcomes were assessed at baseline and at 1, 3, and 6 months after the glenohumeral joint triamcinolone injection.
The primary outcome of this study was the difference of the total SPADI score from baseline to 6 months after the intervention. The secondary outcomes were the difference of the total SPADI score from baseline to 1 month and 3 months after the intervention, and the difference of the pain subscale of the SPADI, the disability subscale of the SPADI, FE of the PROM, abduction of the PROM, IR of the PROM, ER of the PROM. And the pain intensity on the NRS from baseline to 1 month, 3 months, and 6 months after the intervention. Throughout the study period, all side effects possibly related to the intervention recorded including worsening pain, skin lesion, lymphedema aggravation, flushing, infection, and hyperglycemia. All clinical parameters assessed by blinded physiotherapists and occupational therapists during the visits.
Intervention Procedure and Physical Therapy
All participants received physical therapy once or twice a week for 12 weeks at our rehabilitation center and were instructed to exercise at home at least once. Physical therapy was consisted of warm-up, scapular stabilization, glenohumeral joint stretching, strengthening exercise, and cool down exercises. All participants received intra-articular glenohumeral joint injection in the affected shoulder joint under ultrasonographic guidance with 20 mg of triamcinolone and 6 ml of normal saline through a posterior approach at the initial visit. In the posterior approach, the patient lies in a lateral decubitus position facing the physician with the affected side upward. The patient’s ipsilateral arm is internally rotated and adducted. After visualizing the infraspinatus, humeral head, posterior labrum, and the joint capsule, the needle inserted toward the joint capsule via an in-plane technique. Repeated injections performed if the participant had any of these features 1 month after the initial intervention: a high intensity of pain (> 7 in the NRS scale), persistent night or resting pain, or severe level of self-reported shoulder disability.
Statistical Analysis
Descriptive statistics were used to characterize the demographic and clinical parameters of the participants. Continuous variables were presented as means and standard deviations. To compare the baseline demographic and clinical properties, Shapiro-Wilk test was used to determine the normal distribution of the continuous variables. Independent t-test was used for normally distributed continuous variables, and Wilcoxon rank sum test was used for non-normal continuous variables. For categorical variables, chi-square test or Fisher’s exact test were used. To evaluate the effects of the interventions over time in each group, paired T-test or Wilcoxon-signed rank test was used for normal and non-normal values, respectively. To conduct inter-group analysis of the effects of the interventions over time, generalized estimating equations (GEEs) were performed to confirm statistical differences, and the results were corrected for age, sex, and baseline clinical values. The Bonferroni’s correction was used to adjust the p-values of the inter-group and the intra-group analyses. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA). Statistical significance was defined as a p-value < 0.05.
Results
Baseline Demographic and Clinical Characteristics
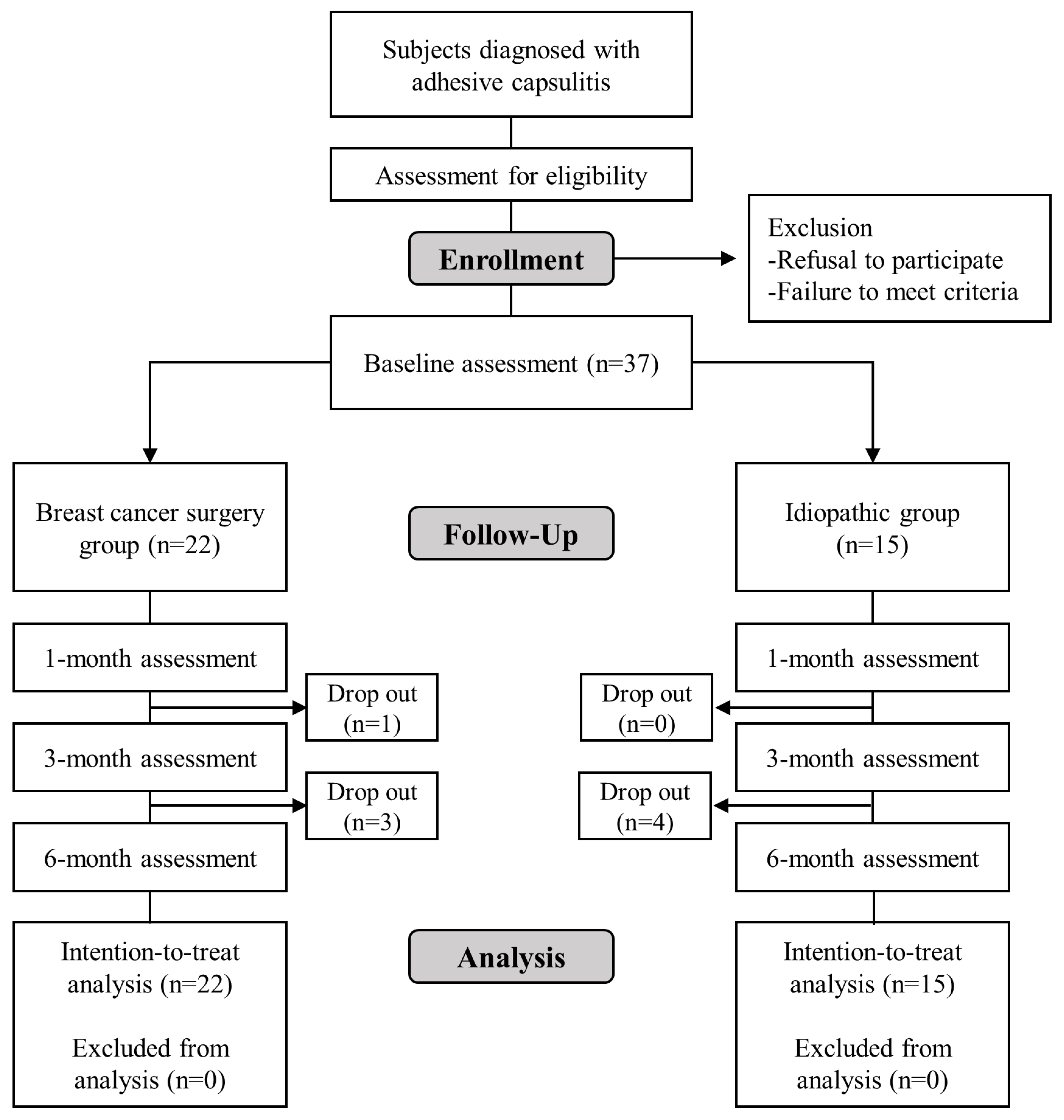
This study prospectively enrolled 37 participants, 22 of the breast cancer surgery group and 15 of the idiopathic group. Only one participant in the idiopathic group was male. The mean age of the 22 participants in the breast cancer surgery group was 52.1 ± 7.3 (range: 44-69) years, and the mean age of the 15 participants in the idiopathic group was 55.7 ± 6.7 (range: 45-67) years. The mean body mass index was 22.1 ± 3.4 in the breast cancer surgery group and 23.4 ± 3.6 in the idiopathic group. The dominant upper extremity was affected in 13 (59.1%) patients in the breast cancer surgery group and 8 (53.3%) patients in the idiopathic group. In the breast cancer surgery group, 11 (50.0%) patients had the right side affected, compared to 8 (53.3%) in the idiopathic group. Four patients (18.2%) in the breast cancer surgery group and no patient in the idiopathic group had diabetes mellitus. There was no significant difference in the demographic or clinical properties between the two groups (
Table 1). Of the 8 participants who discontinued the study, 4 were from the breast cancer surgery group and 4 from the idiopathic group. Five withdrew voluntarily, 2 due to deteriorating health conditions, and 1 because of contralateral adhesive capsulitis (
Figure 1).
Baseline Primary and Secondary Outcome Measures
The baseline scores of the total SPADI (%) was 44.5 ± 18.3 in the breast cancer surgery group and 50.1 ± 16.9 in the idiopathic group. There was no significant difference in the baseline scores of the total SPADI between the two groups. The baseline scores of the pain subscale of the SPADI (%) was 44.5 ± 16.9 in the breast cancer surgery group and 50.1 ± 16.9 in the idiopathic group, and there was a statistically significant difference between two groups (
p = 0.042). There was no significant difference in the baseline scores of the disability subscale of the SPADI, FE of the PROM, abduction of the PROM, IR of the PROM, ER of the PROM, the NRS at rest, and the NRS at activity between two groups (
Table 2).
Comparison of Total SPADI Scores and Subscales (Pain and Disability) Over Time in Breast Cancer Surgery and Idiopathic Groups
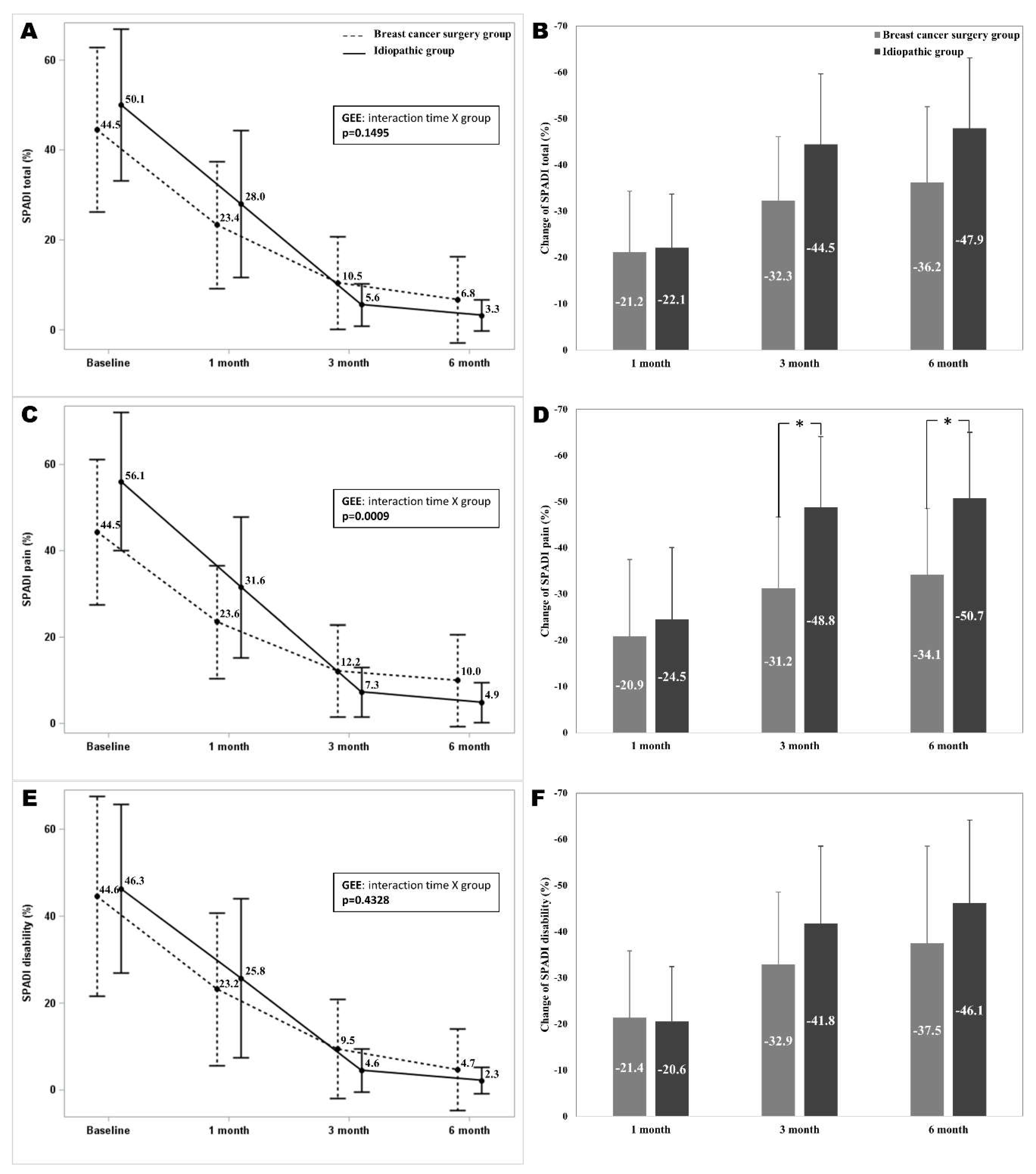
The total SPADI scores at 1, 3, and 6-month follow-ups were significantly different from the baseline in both groups (p<0.05). The total SPADI scores did not show significant interaction between time and group. The mean differences of the total SPADI scores from baseline to 6 months after the intervention were 36.2 ± 16.4 and 47.9 ± 15.2 in the breast cancer surgery group and the idiopathic group. The mean differences of the total SPADI scores from baseline to 1, 3, and 6 months after the intervention were not show significant difference between two groups. The pain subscale of the SPADI at 1, 3, and 6-month follow-ups were significantly different from the baseline in both groups. The pain subscale of the SPADI scores showed a significant interaction between time and group (
p = 0.0009). The mean differences of the pain subscale of the SPADI from baseline to 3 and 6 months after the intervention showed significant differences between two groups (
p = 0.0042,
p = 0.0006). The disability subscale of the SPADI at 1, 3, and 6-month follow-ups were significantly different from the baseline in both groups. The disability subscale of the SPADI scores were not show significant interaction between time and group. The mean differences in SPADI disability from baseline to 1, 3, and 6 months after the intervention were not significantly different between two groups (
Figure 2).
Comparison of PROM Measurements Over Time in Breast Cancer Surgery and Idiopathic Groups
The PROM measurements at 1, 3, and 6-month follow-ups showed significant differences from baseline in both groups. Abduction of the PROM showed a significant interaction between time and group (
p = 0.0058), while FE, IR, and ER of the PROM did not show significant interaction between time and group. The mean differences of abduction of the PROM from baseline to 3 months after the intervention showed a significant difference between two groups (
p = 0.0072). The mean differences of FE, IR, and ER of the PROM from baseline to 1, 3, and 6 months after the intervention were not show significant difference between two groups (
Figure 3).
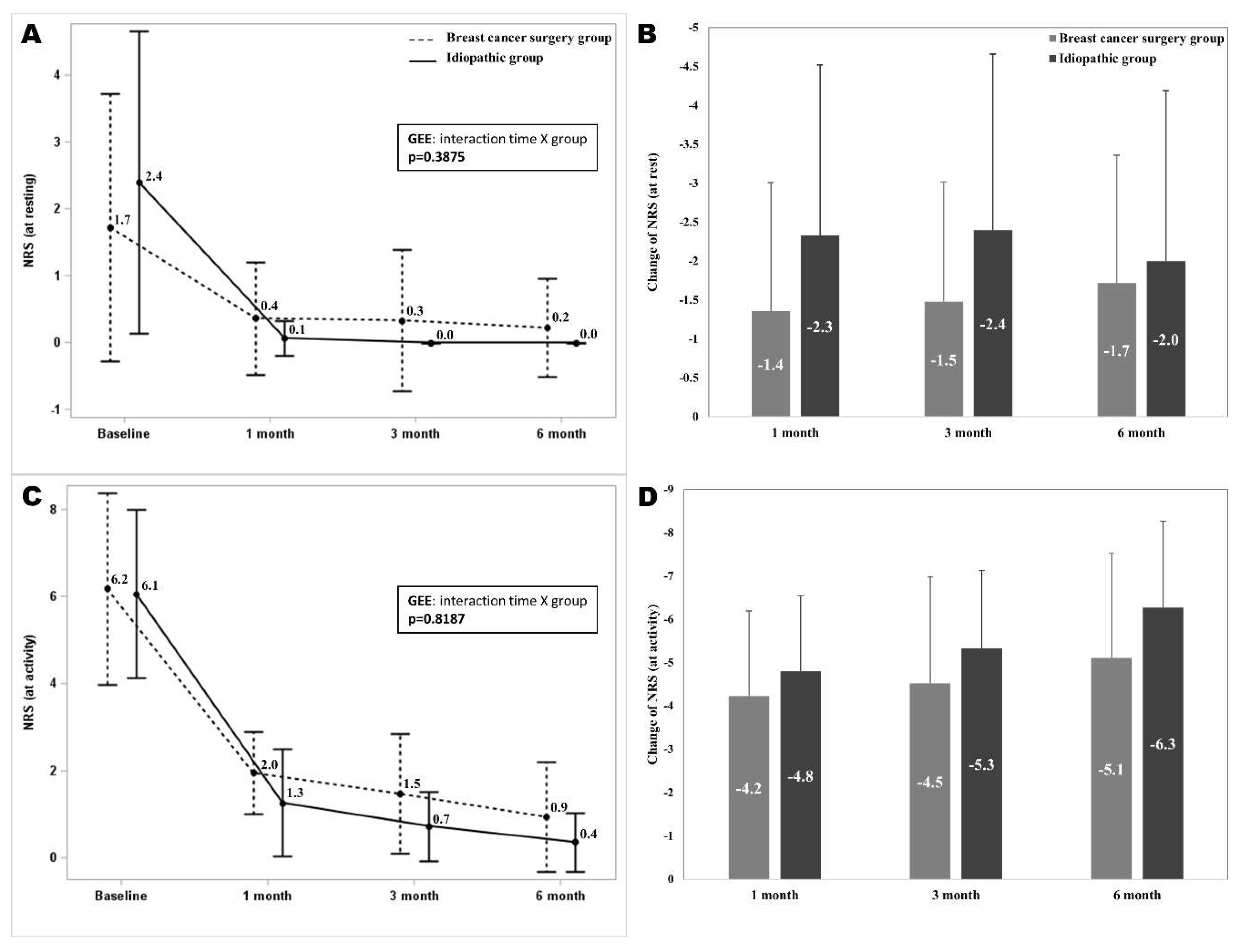
Comparison of Pain Intensity as Measured by NRS Scores Over Time in Breast Cancer Surgery and Idiopathic Groups
The pain intensity on the NRS scores at 1, 3, and 6-month follow-ups were significantly different from the baseline in both groups. The NRS at rest and at did not show significant interaction between time and group (
p = 0.3875,
p = 0.8187). The mean differences of the NRS at rest and at activity from baseline to 1, 3, and 6 months after the intervention did not show significant difference between two groups (
Figure 4).
Number of Injections and Safety Measures
One patient in the breast cancer surgery group and 1 patient in the idiopathic group received second injections during the follow-up period, and there was no significant difference in the proportion of patients who needed second injections between the both groups. There was no adverse event including facial flushing, pain flare, skin color change, joint hematoma, and septic arthritis of the shoulder after intra-articular injection in either group.
Discussion
This study revealed that intra-articular triamcinolone injection significant improved pain, shoulder function, and PROM with adhesive capsulitis, regardless of the history of breast cancer surgery. Outcomes comparison between idiopathic and breast cancer surgery groups showed similar effectiveness in the SPADI, PROM, and NRS. However, the breast cancer surgery group showed less improvement than the idiopathic group in the SPADI pain subscale and PROM abduction at 3 and 6 months post-intervention. Thus, for breast cancer patients, continuous pain and ROM management with close monitoring is essential beyond 3 months post-injection. Notably, no adverse events were noted from the intra-articular steroid injections in this study.
A considerable number of patients suffer from reduced shoulder mobility and restricted upper limb function after breast cancer surgery. In a previous study of breast cancer patients who received axillary lymph node dissection, 57% of patients experienced impaired shoulder mobility and 69% of patients had impaired shoulder function for ADL 3 months after the surgery [
14]. A systematic review of cases of late morbidity after treatment of breast cancer reported that there is a significant relationship between restricted ROM and patient-reported functional impairments, and the presence of arm problems after breast cancer surgery was related to psychological distress and reduced quality of life [
15]. A single-center, cross-sectional, observational study reported adhesive capsulitis in 22.2% after breast cancer surgery [
9]. A cumulative prevalence of 10.3% and a current prevalence of 7.7% of adhesive capsulitis reported in breast cancer patients with a postoperative period between 13 and 18 months in a single-center study in Korea [
8]. The increasing incidence of breast cancer is a global phenomenon, and the prevalence of secondary adhesive capsulitis of patients with breast cancer will increase and become an increasing a burden for society [
1]. The evaluation and treatment for adhesive capsulitis after breast cancer surgery found to be insufficient. Various physical exercise programs that focus on strengthening shoulder and scapular stabilizers can help reduce pain and enhance shoulder ROM and function. However, early physical therapy with aggressive shoulder stretching and strengthening exercise are limited in patients with adhesive capsulitis after breast cancer surgery due to severe shoulder pain, prominent limited ROM of the shoulder, and structural change after breast surgery. Our study showed intra-articular steroid injection for adhesive capsulitis after breast cancer surgery to be effective to reduce pain and to improve ROM and function of the shoulder joint. Hence, alongside physical therapy, appropriate interventions are necessary to enhance functional outcomes and quality of life for adhesive capsulitis after breast cancer surgery.
Adhesive capsulitis of the shoulder causes chronic pain, and the pathophysiology of adhesive capsulitis after breast cancer surgery is complex. Previous studies reported that several factors contribute to occurrence of adhesive capsulitis after breast surgery. After breast surgery, adhesions may occur in the axillary and pectoral areas. Between the pectoral muscles, subcutaneous tissue, and skin, these adhesions may inhibit extension of the pectoralis, resulting in limitation of shoulder flexion and abduction [16-18]. Weakness of scapular stabilizers after mastectomy causes alteration of scapular motion and contributes to adhesive capsulitis [19, 20]. A history of mastectomy is a major risk factor for adhesive capsulitis. Breast cancer surgery patients require more attention for early diagnosis and appropriate treatment of adhesive capsulitis [
8]. For idiopathic adhesive capsulitis, several treatment options such as physical therapy, pharmacologic therapy, intra-articular steroid injections, intra-articular hyaluronate injections, hydrodilatation, and surgical management that includes manipulation under anesthesia have been reported to be successful [5, 11, 21]. Despite the uncertainty of the pathophysiologic mechanism of adhesive capsulitis, capsular fibrosis and reductions of capsular volume after synovial inflammation are considered the primary mechanism [
11]. Because of this, non-steroidal anti-inflammatory drugs and corticosteroids are commonly used. Among these treatments, intra-articular triamcinolone injection is effective with rapid improvement in pain and shoulder ROM. This results in shortening of the natural course of the disease [11, 21]. However, the effects of intra-articular steroid injections have not been well studied in adhesive capsulitis patients after breast cancer surgery. A previous study evaluated the effects of hydrodilatation in patients with adhesive capsulitis after breast cancer surgery, but the study did not compare the effects with a control group [
22]. This study found that intra-articular triamcinolone injections for adhesive capsulitis post-breast cancer surgery significantly improved pain, shoulder function, and PROM, both in the short and long term following the injection. And, the improvements of the SPADI, PROM, and NRS of the breast cancer surgery group were comparable to those of the idiopathic group.
In our study, the pain subscale of the SPADI at 3 and 6 months, and abduction of the PROM at 3 months in the breast cancer surgery group showed inferior results compared to the idiopathic group. There are possible explanations for these results. Patients with adhesive capsulitis after breast cancer surgery experience greater structural changes, including pectoralis tightness, lymphedema, post-mastectomy pain syndrome, and axillary web syndrome [23, 24]. These conditions can directly cause pain and limitation of joint motion and may also limit participation in active physical therapy. Adhesive capsulitis after breast cancer surgery is accompanied by structural changes in addition to inflammation, and our results show that active rehabilitation and close surveillance of pain and shoulder ROM are necessary in addition to the use of anti-inflammatory treatment, even after 3 months post-injection. Previously, there have been some efforts to apply physical therapy and exercise in patients who underwent breast cancer surgery. Physical therapy including passive mobilization, stretching, and exercise programs introduced as early as 7 days after surgery can be effective for improvement of shoulder ROM and postoperative pain [25, 26]. A randomized controlled trial showed that addition of scapular stabilization exercises and Thera-band strengthening exercises can be beneficial for shoulder function and quality of life in post-mastectomy patients with adhesive capsulitis [
16]. Proper pharmacologic intervention for inflammation and the prescription of proper rehabilitation programs are important to improve ROM in patients with adhesive capsulitis after breast cancer surgery, even after 3 months post-treatment.
With the limited evidence available, physicians may be reluctant to perform intra-articular steroid injection in patients who underwent breast cancer surgery. Postoperative consequences including adhesion near the surgical site and ipsilateral upper limb lymphedema could also magnify the fear of injection-associated adverse events. In our study, the glenohumeral joint injection protocol was carried out with a sterile technique, and the injection site was identified before and during the procedure using ultrasonography. To exclude infection and to reduce the possibility of procedure-associated joint infection, complete blood count, erythrocyte sedimentation rate, and C-reactive protein level were measured before the injection. To reduce the side effects of the corticosteroid hormone, we used low-dose injection (20 mg of triamcinolone) in the treatment of adhesive capsulitis of the shoulder. Using this injection protocol, we achieved favorable short-term and long-term clinical results without side effects. For improved and consistent outcomes of the procedure, establishing a standardized protocol with necessary physical examinations and essential laboratory studies before the procedure is crucial.
This study has several limitations. First, this study is a single-center study with a small sample size. The relatively small sample size limits the validity of subgroup analyses. Factors like lymphedema, history of radiotherapy or chemotherapy, and types of breast cancer surgery, while potential subjects for such analyses, have an unclear impact on outcomes. Future research with a larger participant numbers and subgroup analyses is needed. Despite small sample size, this study strength is its six-month follow-up duration, enabling the assessment of long-term effects of intra-articular steroid injections in breast cancer surgery patients. Second, the participants were not controlled in pharmacological treatments. The use of varying doses and types of analgesic medications among participants could have impacted the outcomes. Third, ultrasound-guided injection is operator-dependent procedure, potentially leading to variations in procedure execution among physicians. The physicians who participated in this study were well-experienced physiatrists at the same institute.
Conclusion
This study indicates that intra-articular triamcinolone injections are effectively reduce pain, improve shoulder function, and enhance PROM in patients with adhesive capsulitis after breast cancer surgery, showing similar efficacy to idiopathic adhesive capsulitis. Notably, the breast cancer group showed a poor improvement in the SPADI pain subscale and PROM abduction at 3 and 6 months post-intervention, indicating that continuous monitoring and follow up are necessary even after this period. This study presents the efficacy and safety of intra-articular triamcinolone injections as a treatment for secondary adhesive capsulitis after breast cancer surgery.
Author Contributions
S.W.K*: study concept design, data acquisition data analysis, and manuscript writing and revising. S.W.K.: data acquisition, data analysis. J.G.D.: study concept design, data acquisition data analysis, manuscript writing and revising, and final approval. J.H.H.: study concept design, data acquisition, data analysis, and manuscript writing and revising and final approval. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health &Welfare, Republic of Korea (grant number: HI19C0781).
Institutional Review Board Statement
This clinical study was performed in accordance with principles of the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of Samsung Medical Center (approval number: SMC-2019-05-021).
Informed Consent Statement
All patients were informed about the aim and experimental procedures before enrollment; therefore, written informed consent was obtained from all of them.
Data Availability Statement
The datasets generated and analyzed during this study are not publicly available due to privacy protection and medical confidentiality but are available from the corresponding author for reasonable requests and with patient permission.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lei S, Zheng R, Zhang S, Wang S, Chen R, Sun K, Zeng H, Zhou J, Wei W (2021) Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun (Lond) 41:1183-1194. [CrossRef]
- De Groef A, Meeus M, De Vrieze T, Vos L, Van Kampen M, Christiaens MR, Neven P, Geraerts I, Devoogdt N (2017) Pain characteristics as important contributing factors to upper limb dysfunctions in breast cancer survivors at long term. Musculoskelet Sci Pract 29:52-59. [CrossRef]
- Andersen KG, Kehlet H (2011) Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain 12:725-746. [CrossRef]
- Kim JH, Kim SH, Kim HR, Lee SH, Yoon SY, Yang JH, Yoo YB, Park KS, Nam SE, Hong S, Min HK (2020) Ultrasonographic evaluation of chronic shoulder pain after breast cancer surgery: single center, cross-sectional study. Sci Rep 10:16792. [CrossRef]
- Hsu JE, Anakwenze OA, Warrender WJ, Abboud JA (2011) Current review of adhesive capsulitis. J Shoulder Elbow Surg 20:502-514. [CrossRef]
- Sarasua SM, Floyd S, Bridges WC, Pill SG (2021) The epidemiology and etiology of adhesive capsulitis in the U.S. Medicare population. BMC Musculoskelet Disord 22:828. [CrossRef]
- Nesvold IL, Fossa SD, Holm I, Naume B, Dahl AA (2010) Arm/shoulder problems in breast cancer survivors are associated with reduced health and poorer physical quality of life. Acta Oncol 49:347-353. [CrossRef]
- Yang S, Park DH, Ahn SH, Kim J, Lee JW, Han JY, Kim DK, Jeon JY, Choi KH, Kim W (2017) Prevalence and risk factors of adhesive capsulitis of the shoulder after breast cancer treatment. Support Care Cancer 25:1317-1322. [CrossRef]
- Wong CJ, Tay MRJ, Aw HZ (2021) Prevalence and Risk Factors of Adhesive Capsulitis in Asian Breast Cancer Patients Undergoing an Outpatient Community Cancer Rehabilitation Program. Arch Phys Med Rehabil 102:843-848. [CrossRef]
- Klein I, Kalichman L, Chen N, Susmallian S (2021) A comprehensive approach to risk factors for upper arm morbidities following breast cancer treatment: a prospective study. BMC Cancer 21:1251. [CrossRef]
- Lee NK, Park SE, Kwon SJ, Shim S, Byeon Y, Kim JH, Na DL, Chang JW (2017) Agouti Related Peptide Secreted Via Human Mesenchymal Stem Cells Upregulates Proteasome Activity in an Alzheimer’s Disease Model. Sci Rep 7:39340. [CrossRef]
- Gurbuz AF, Keven A, Emir Yetim E, Elasan S, Karaali K (2023) Evaluation of the Differences in the MRI Findings Related to Primary and Secondary Adhesive Capsulitis. Can Assoc Radiol J 74:78-86. [CrossRef]
- Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y (1991) Development of a shoulder pain and disability index. Arthritis Care Res 4:143-149.
- Devoogdt N, Van Kampen M, Christiaens MR, Troosters T, Piot W, Beets N, Nys S, Gosselink R (2011) Short- and long-term recovery of upper limb function after axillary lymph node dissection. Eur J Cancer Care (Engl) 20:77-86. [CrossRef]
- Rietman JS, Dijkstra PU, Hoekstra HJ, Eisma WH, Szabo BG, Groothoff JW, Geertzen JH (2003) Late morbidity after treatment of breast cancer in relation to daily activities and quality of life: a systematic review. Eur J Surg Oncol 29:229-238. [CrossRef]
- Aboelnour NH, Kamel FH, Basha MA, Azab AR, Hewidy IM, Ezzat M, Kamel NM (2023) Combined effect of graded Thera-Band and scapular stabilization exercises on shoulder adhesive capsulitis post-mastectomy. Support Care Cancer 31:215. [CrossRef]
- Kim KH, Yeo SM, Cheong IY, Kim Y, Jeon BJ, Hwang JH (2019) Early Rehabilitation after Total Mastectomy and Immediate Reconstruction with Tissue Expander Insertion in Breast Cancer Patients: A Retrospective Case-control Study. J Breast Cancer 22:472-483. [CrossRef]
- Leonidou A, Woods DA (2014) A preliminary study of manipulation under anaesthesia for secondary frozen shoulder following breast cancer treatment. Ann R Coll Surg Engl 96:111-115. [CrossRef]
- Shamley D, Srinaganathan R, Oskrochi R, Lascurain-Aguirrebena I, Sugden E (2009) Three-dimensional scapulothoracic motion following treatment for breast cancer. Breast Cancer Res Treat 118:315-322. [CrossRef]
- Crosbie J, Kilbreath SL, Dylke E, Refshauge KM, Nicholson LL, Beith JM, Spillane AJ, White K (2010) Effects of mastectomy on shoulder and spinal kinematics during bilateral upper-limb movement. Phys Ther 90:679-692. [CrossRef]
- Redler LH, Dennis ER (2019) Treatment of Adhesive Capsulitis of the Shoulder. J Am Acad Orthop Surg 27:e544-e554. [CrossRef]
- Lee CW, Kim IS, Kim JG, Hwang H, Jung IY, Lee SU, Seo KS (2022) Effects of Hydrodilatation With Corticosteroid Injection and Biomechanical Properties in Patients With Adhesive Capsulitis After Breast Cancer Surgery. Ann Rehabil Med 46:192-201. [CrossRef]
- Cheville AL, Tchou J (2007) Barriers to rehabilitation following surgery for primary breast cancer. J Surg Oncol 95:409-418. [CrossRef]
- Yang EJ, Park WB, Seo KS, Kim SW, Heo CY, Lim JY (2010) Longitudinal change of treatment-related upper limb dysfunction and its impact on late dysfunction in breast cancer survivors: a prospective cohort study. J Surg Oncol 101:84-91. [CrossRef]
- De Groef A, Van Kampen M, Dieltjens E, Christiaens MR, Neven P, Geraerts I, Devoogdt N (2015) Effectiveness of postoperative physical therapy for upper-limb impairments after breast cancer treatment: a systematic review. Arch Phys Med Rehabil 96:1140-1153. [CrossRef]
- Rizzi S, Haddad CAS, Giron PS, Figueira PVG, Estevao A, Elias S, Nazario ACP, Facina G (2021) Exercise Protocol With Limited Shoulder Range of Motion for 15 or 30 Days After Conservative Surgery for Breast Cancer With Oncoplastic Technique: A Randomized Clinical Trial. Am J Clin Oncol 44:283-290. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).