Submitted:
02 May 2024
Posted:
06 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
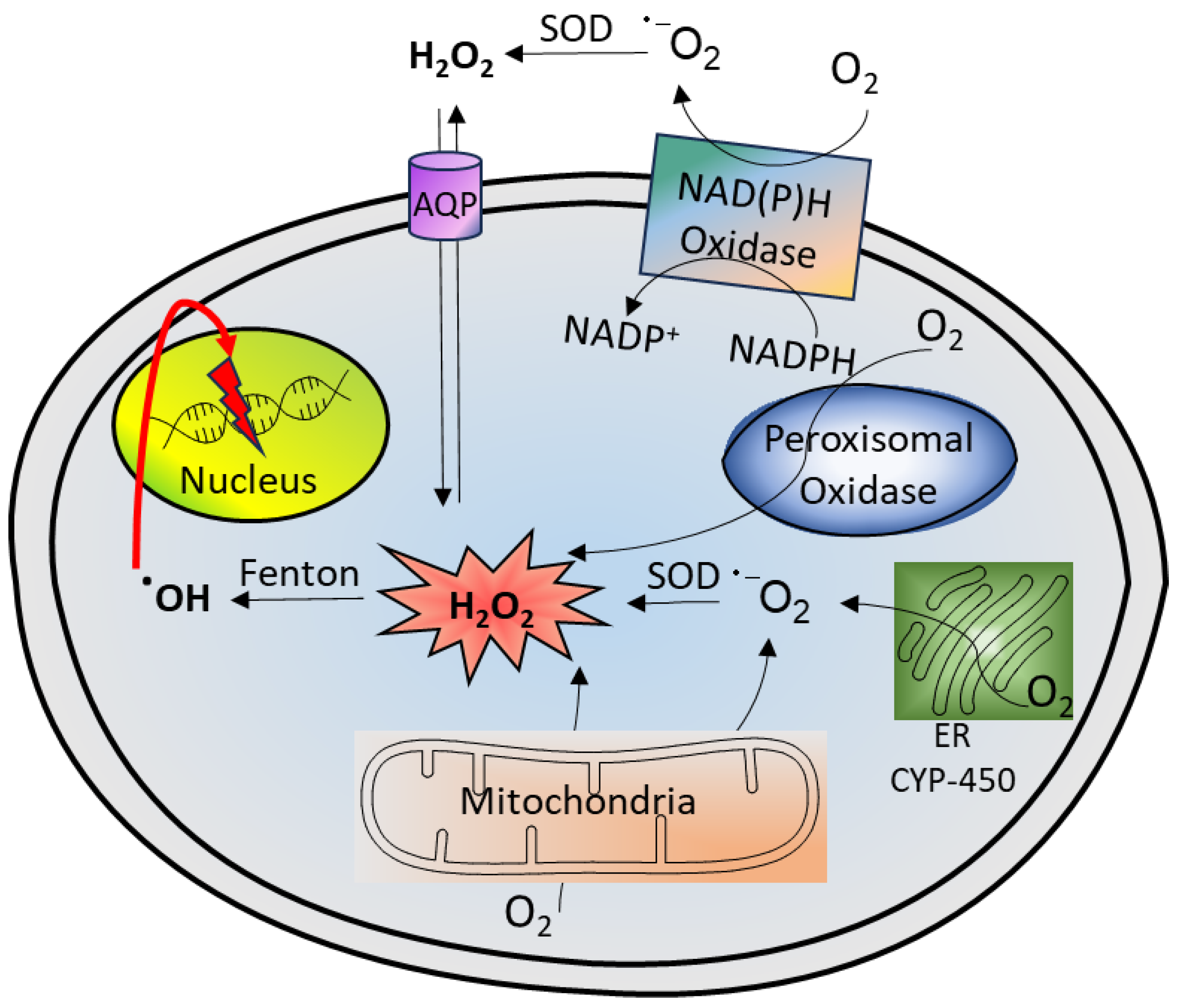
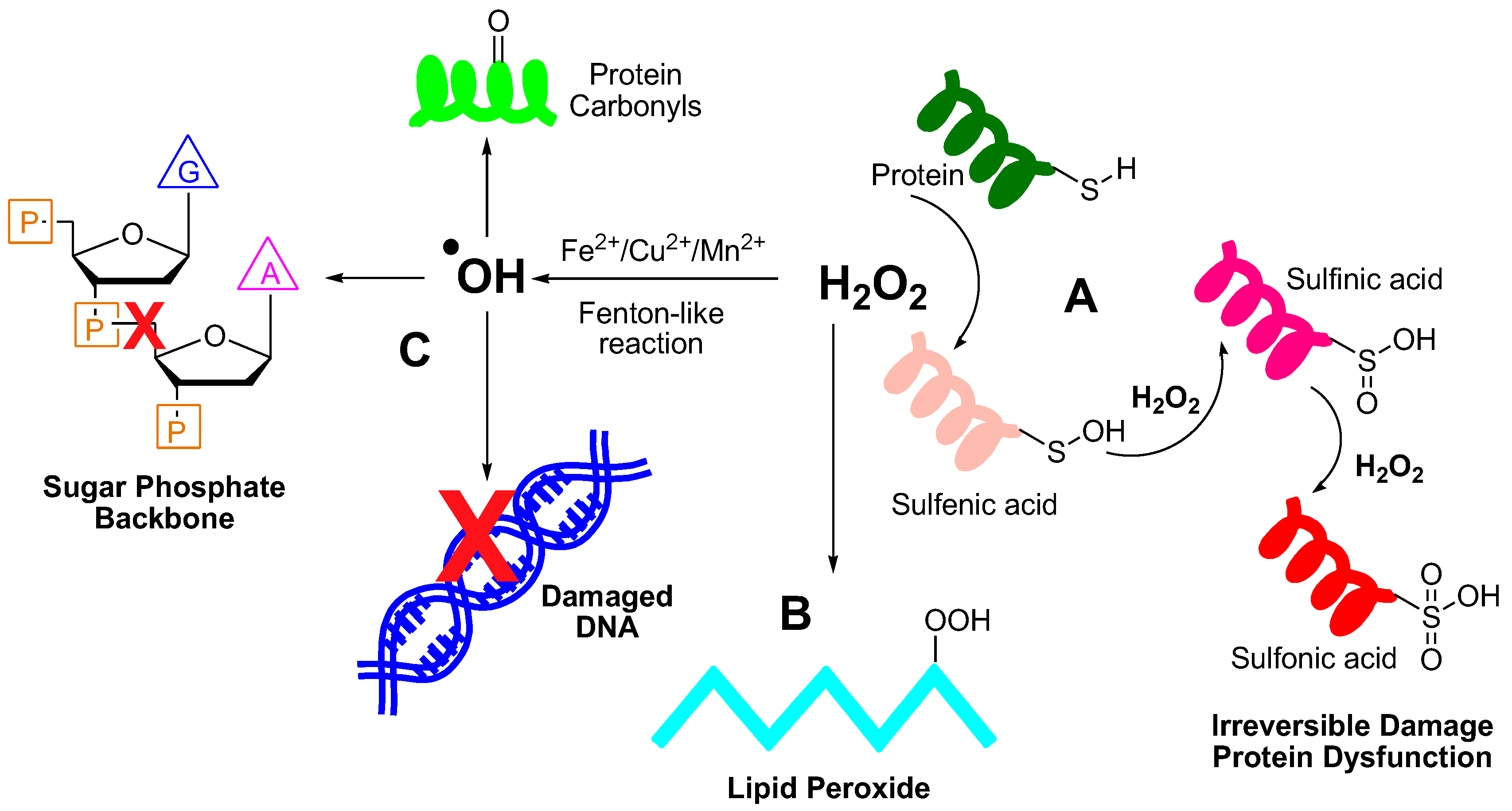
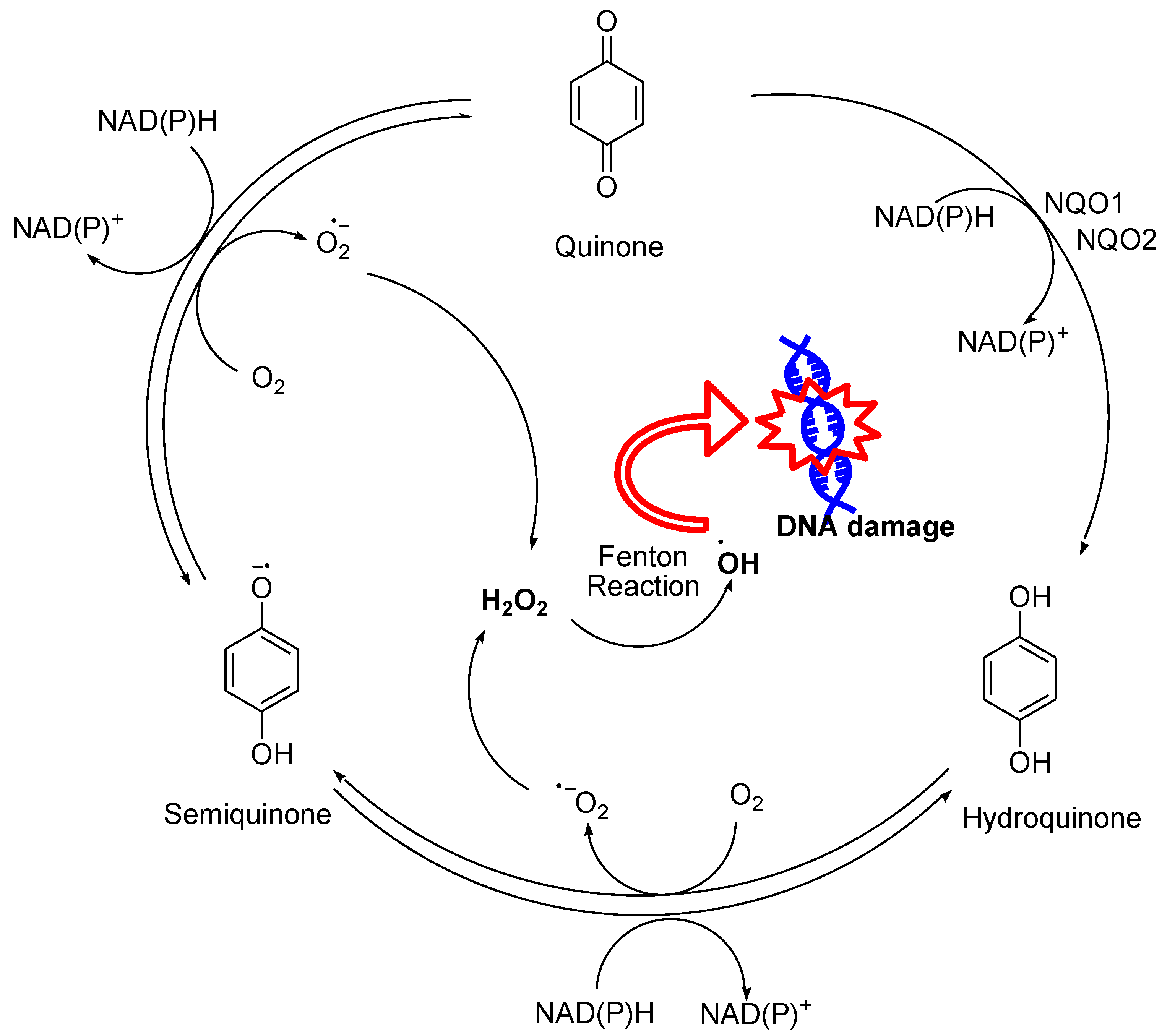
2. Hydrogen Peroxide Generation and Its Reactivity with Biomolecules
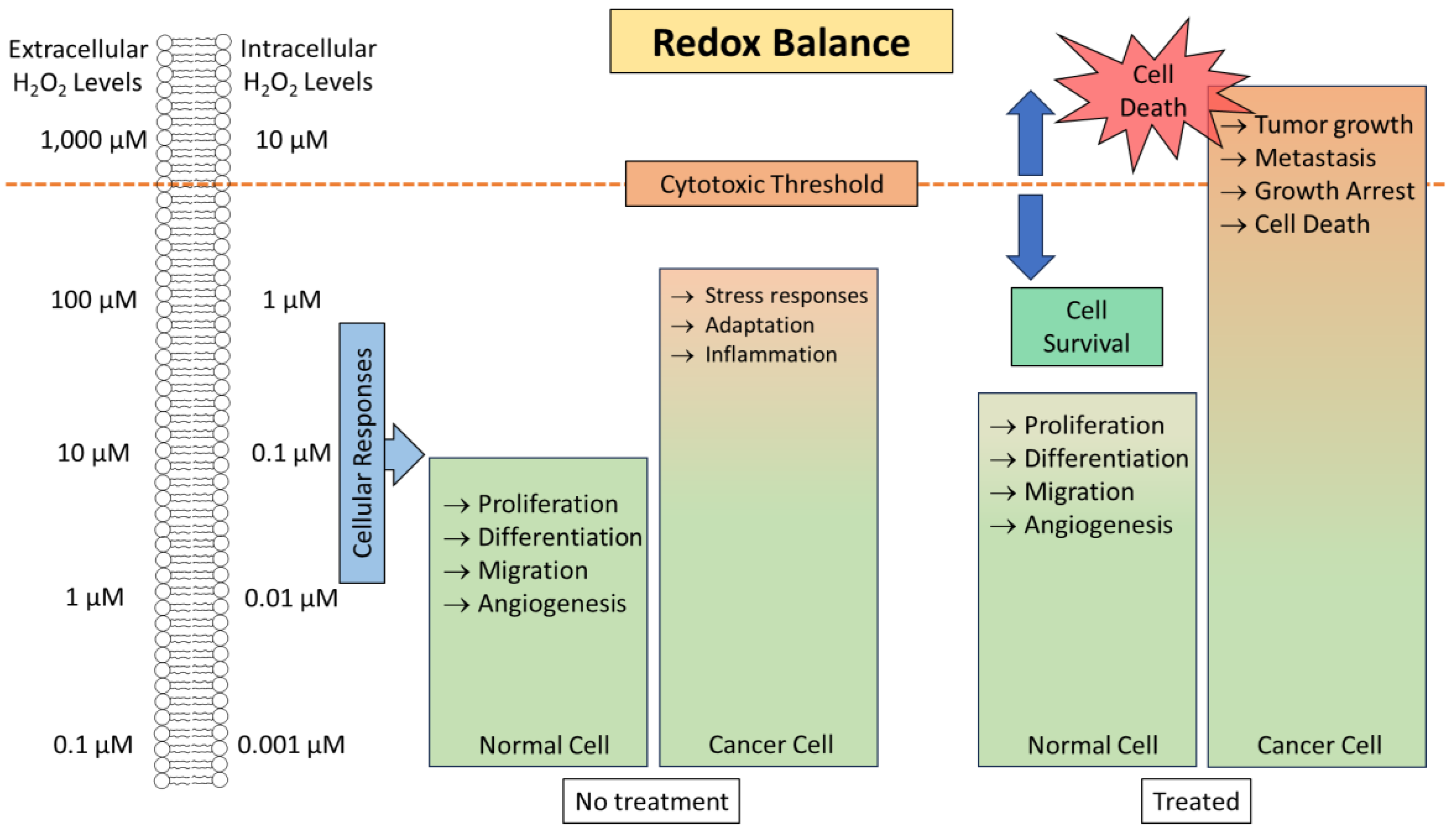
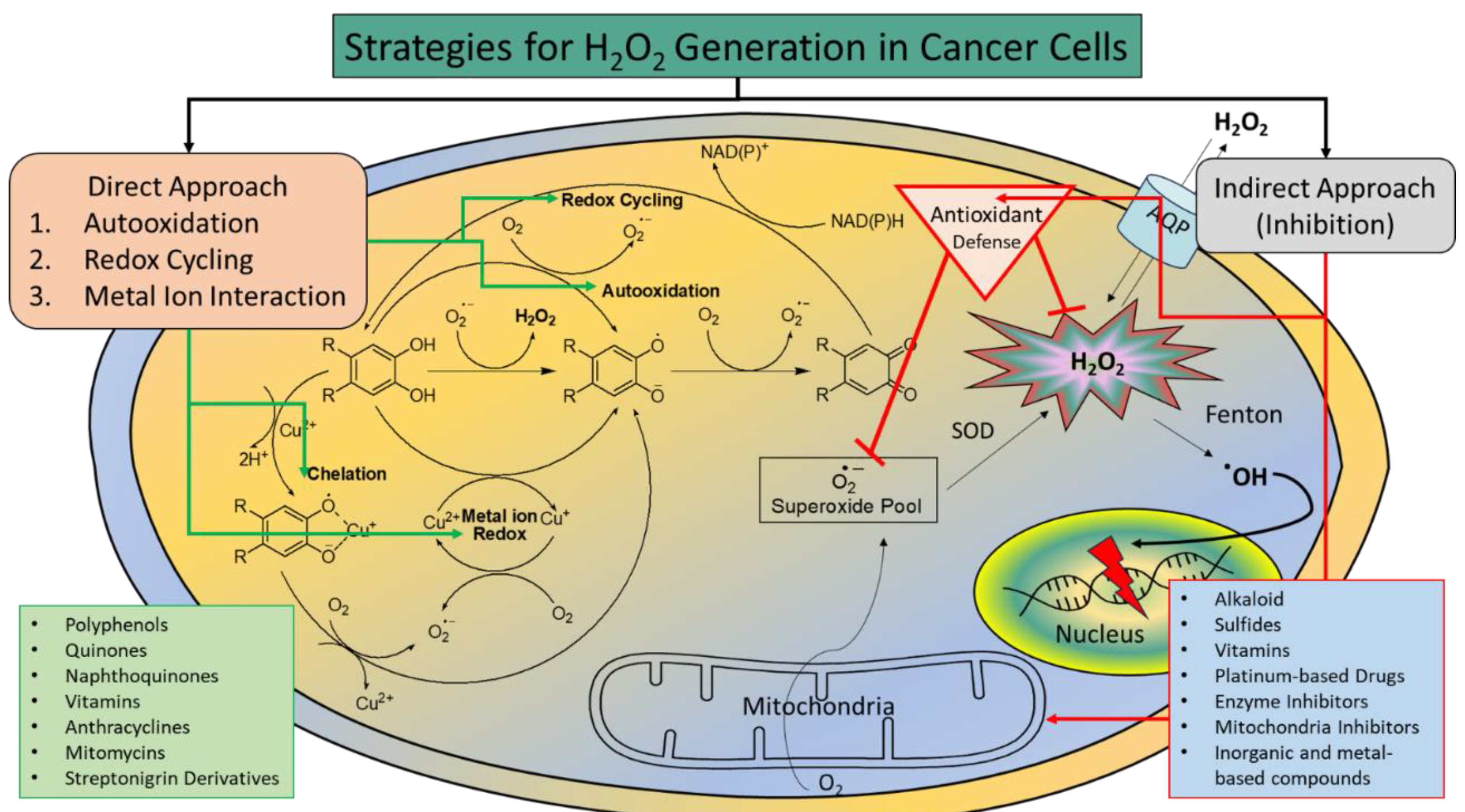
3. Increasing H2O2 Level as an Anticancer Therapy
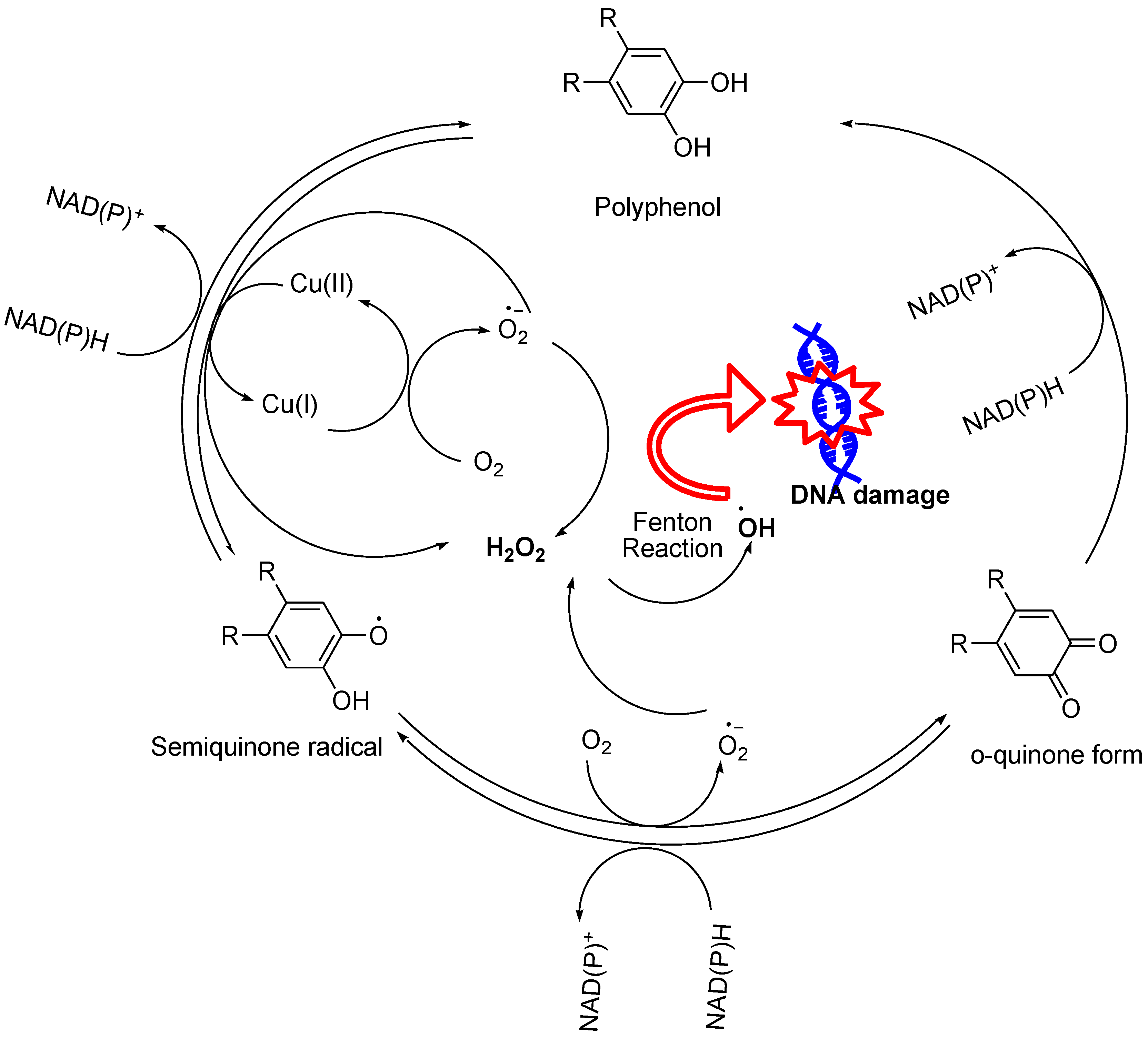
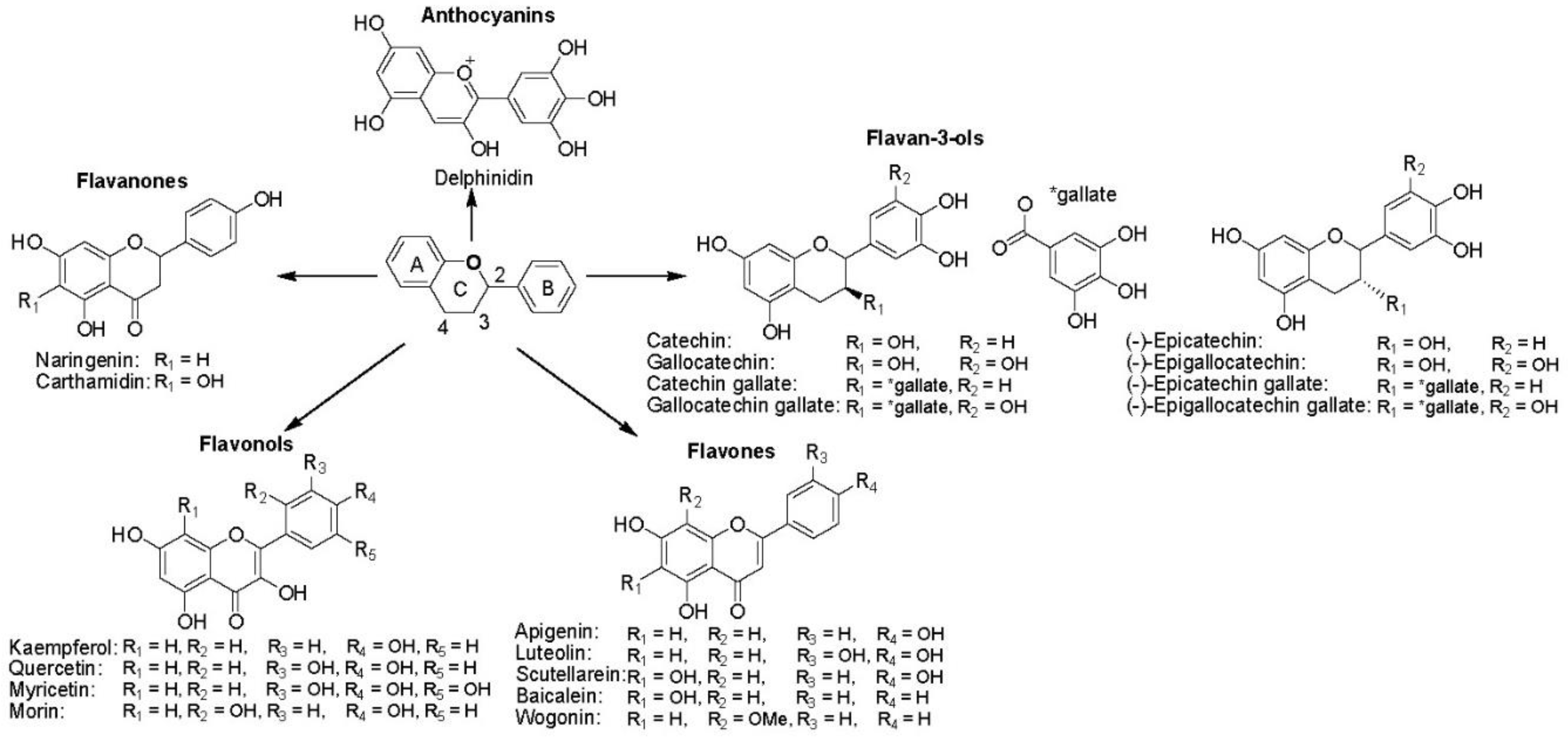
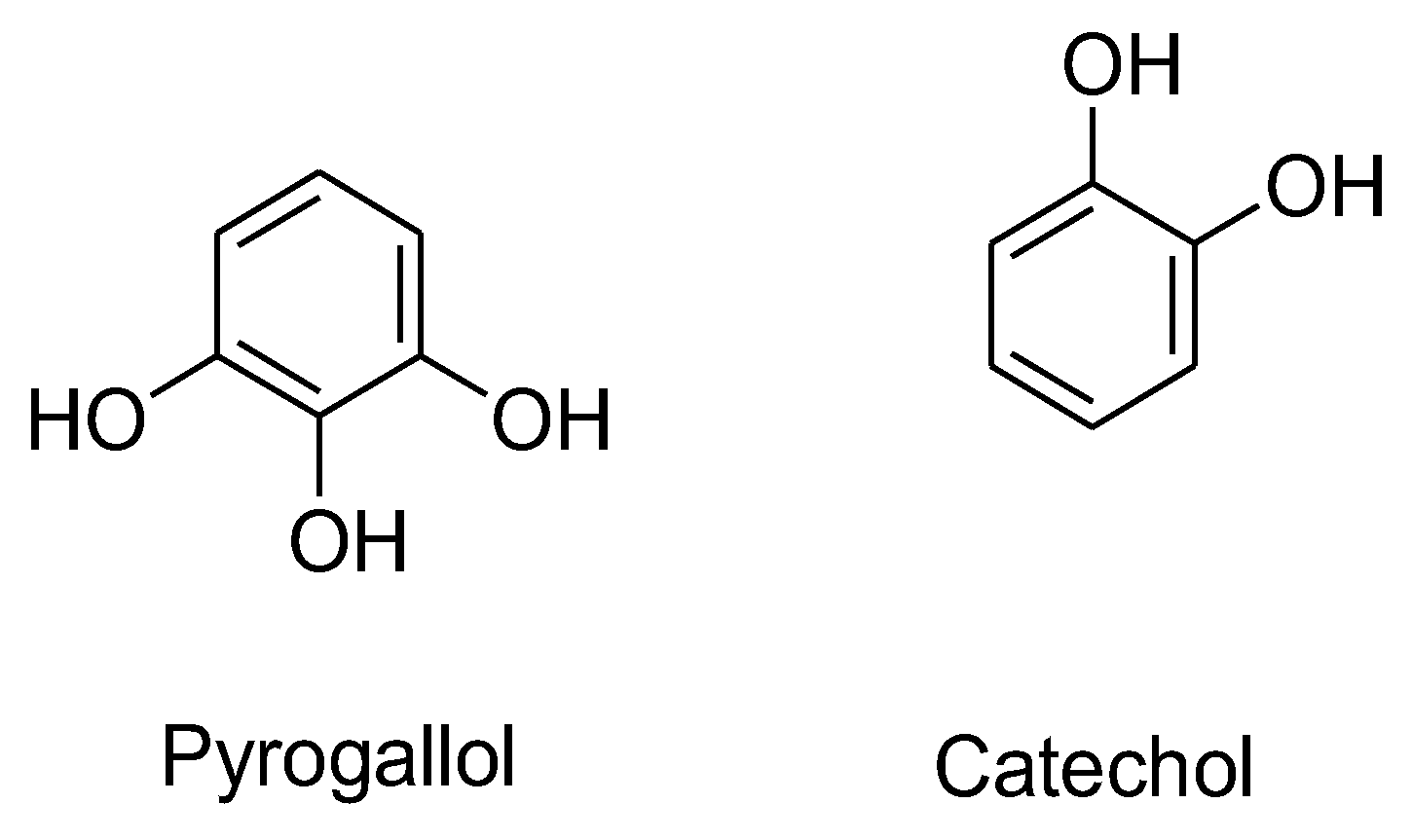
3.1. Phenol and Polyphenol Analogues
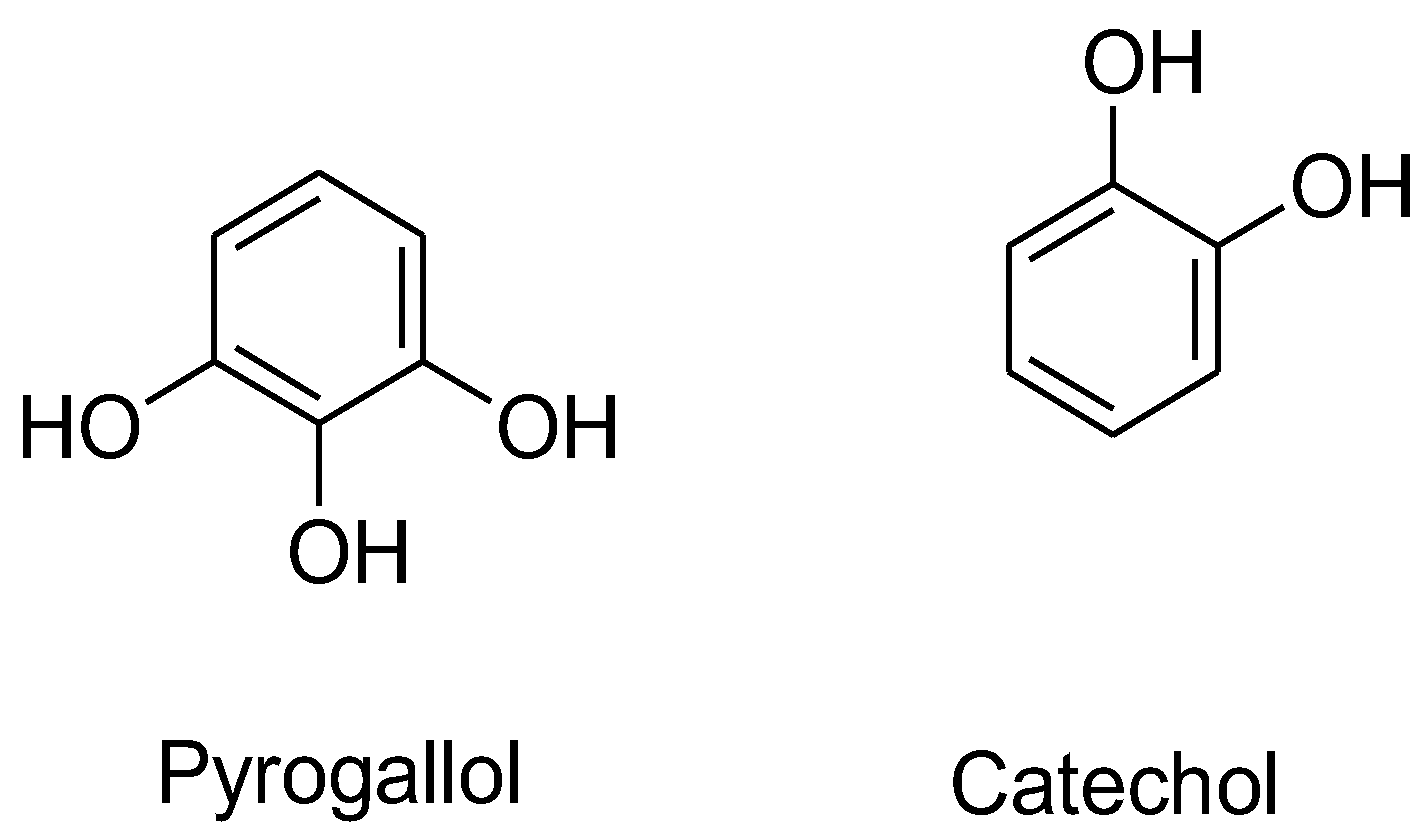
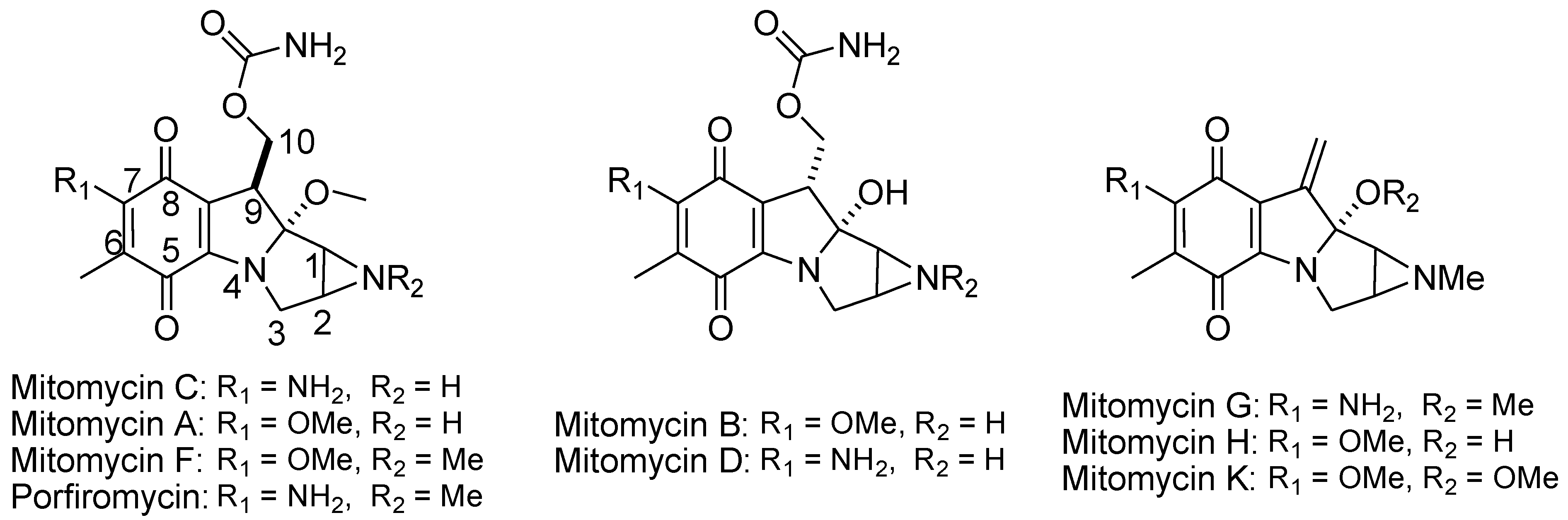
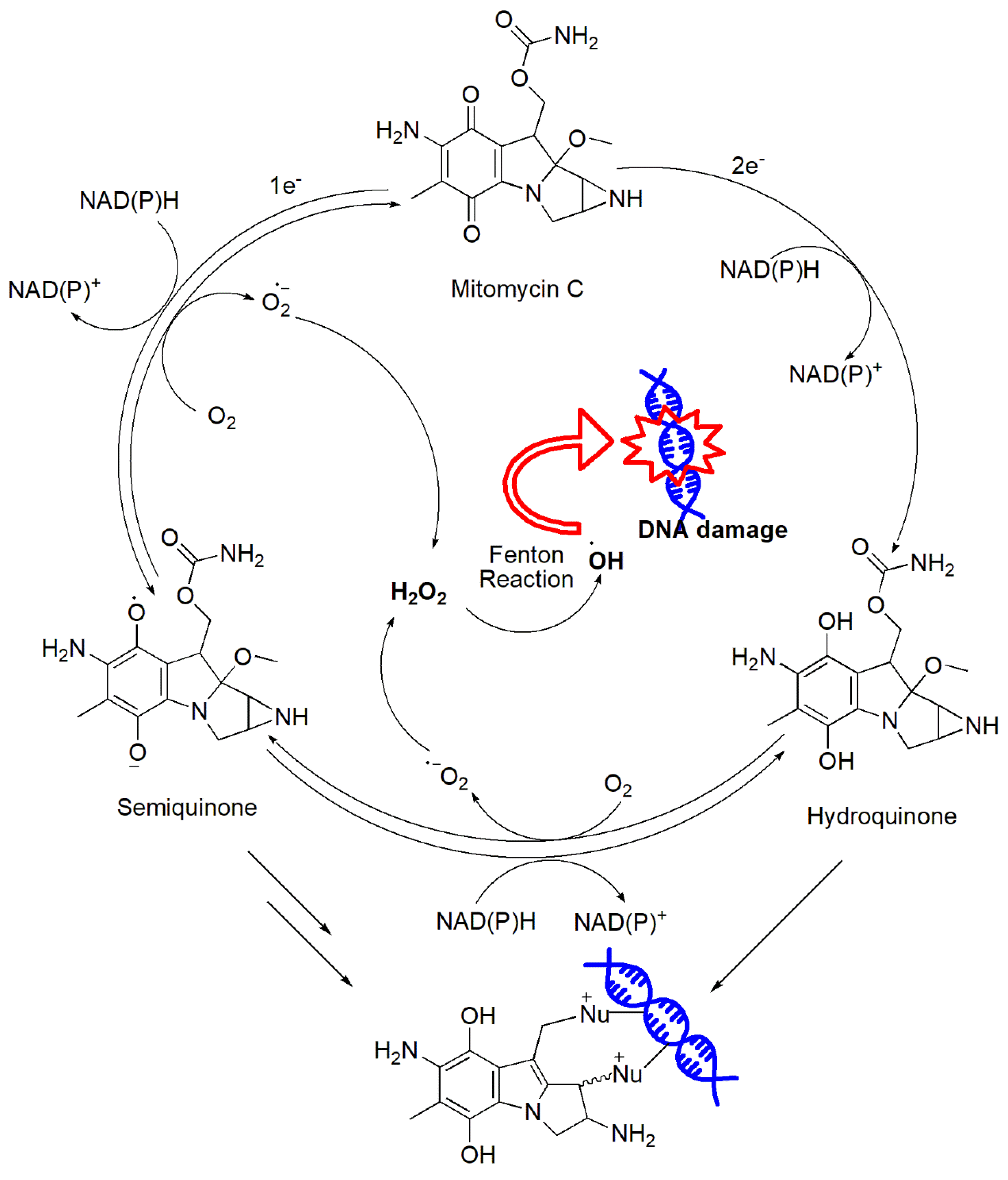
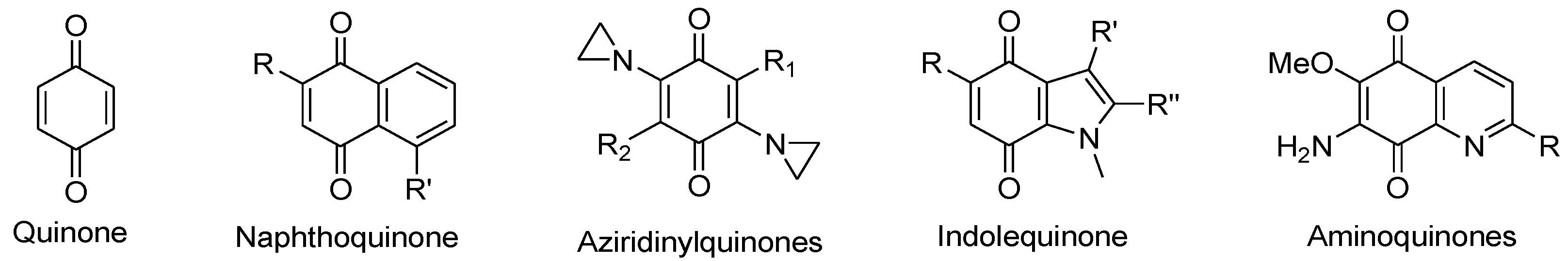
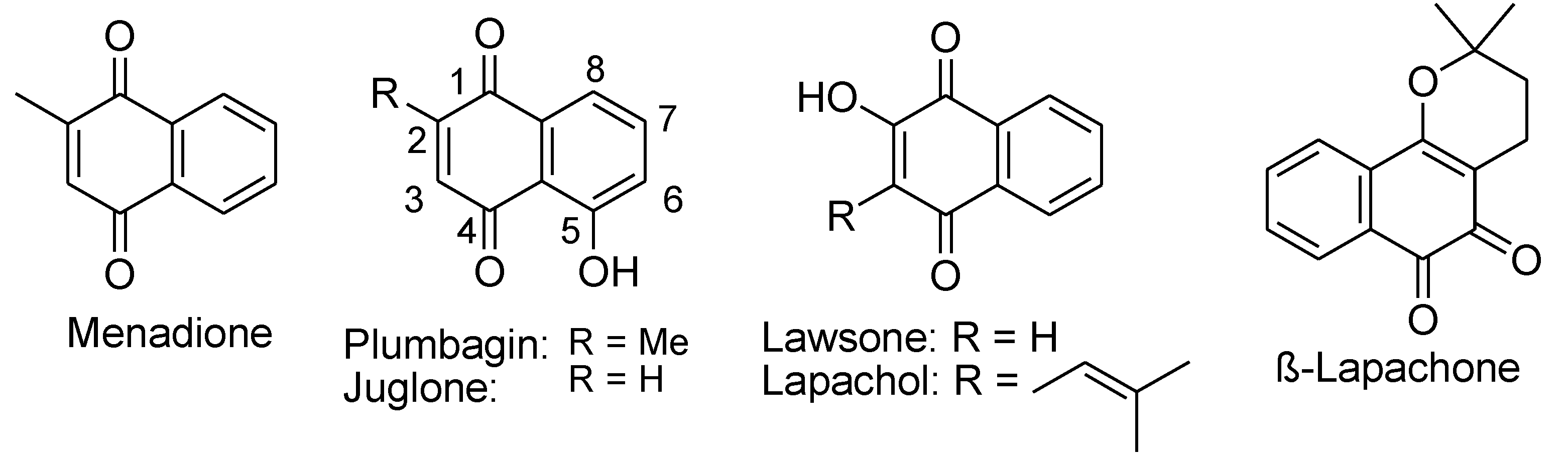
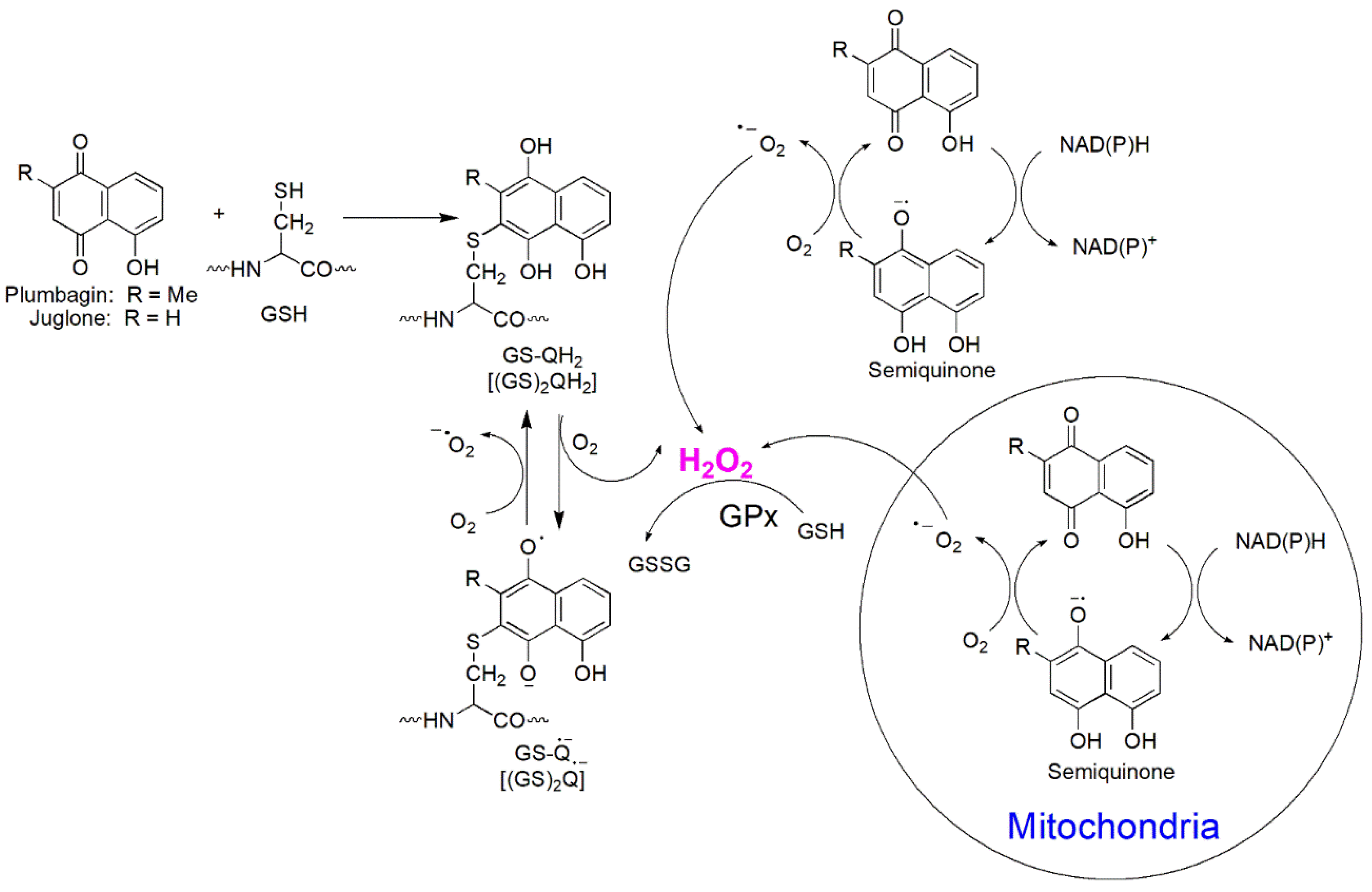
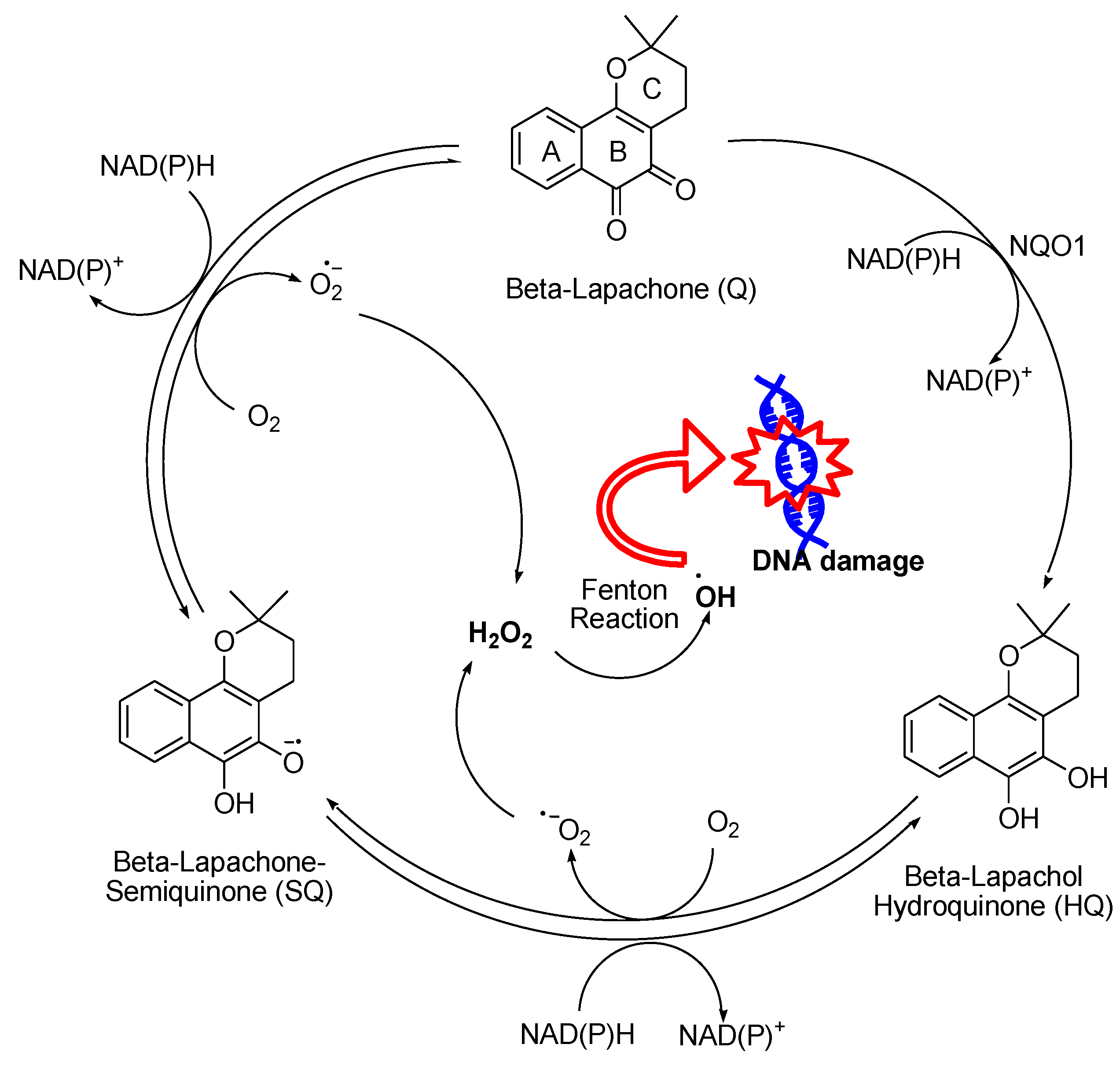
3.2. Compounds with Quinone Moieties
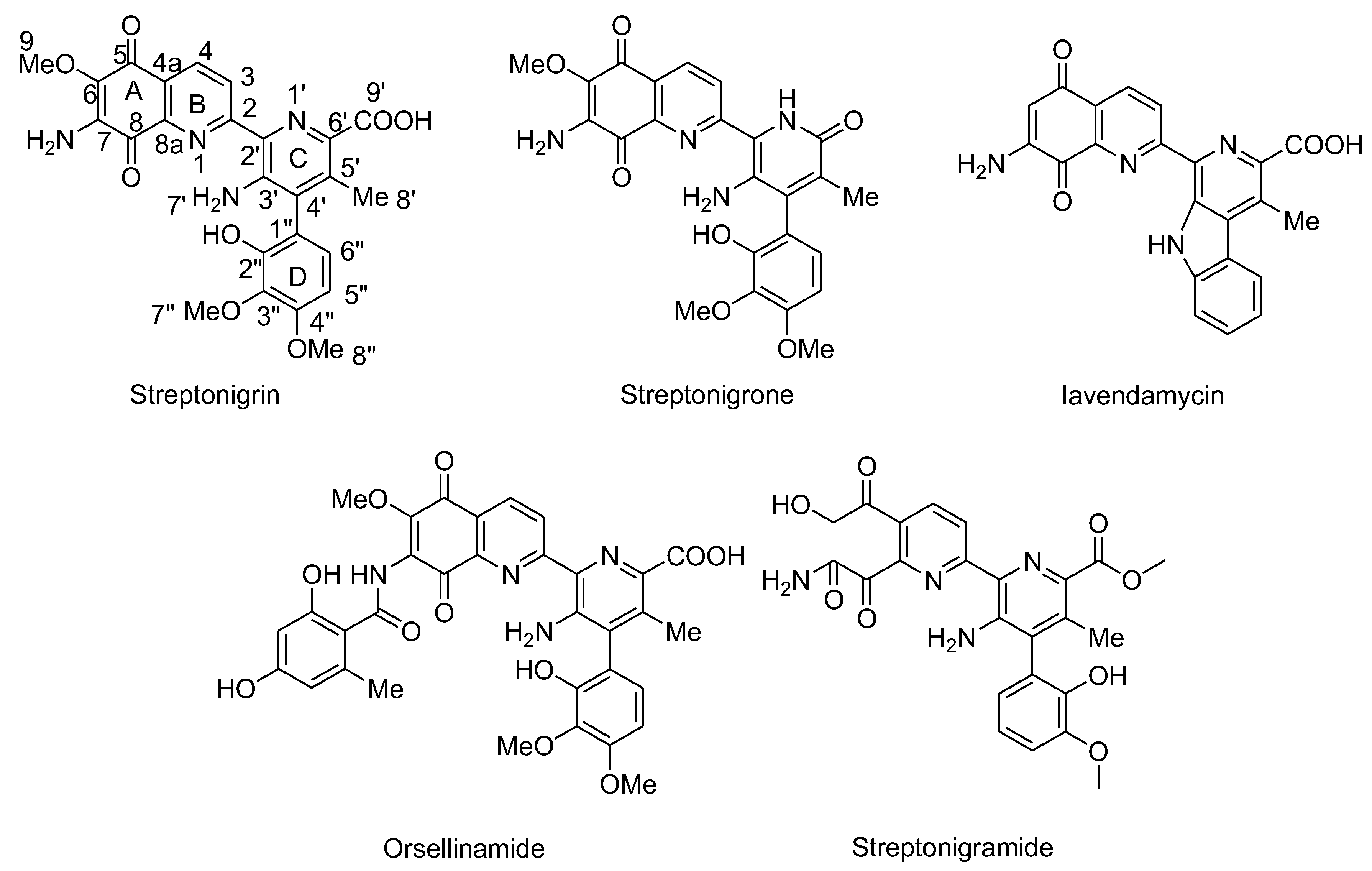
3.3. Vitamin C
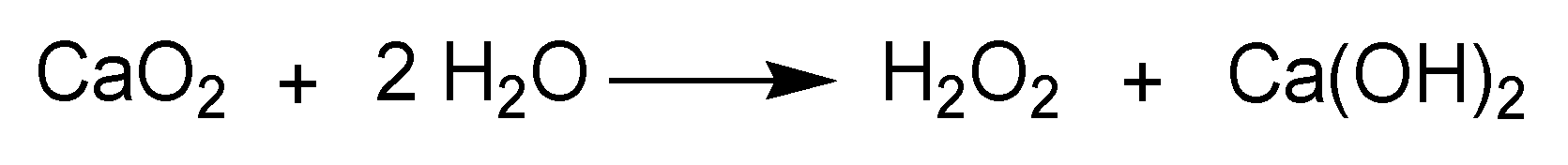
3.4. Metal, Metal Oxides, and Metal Peroxides
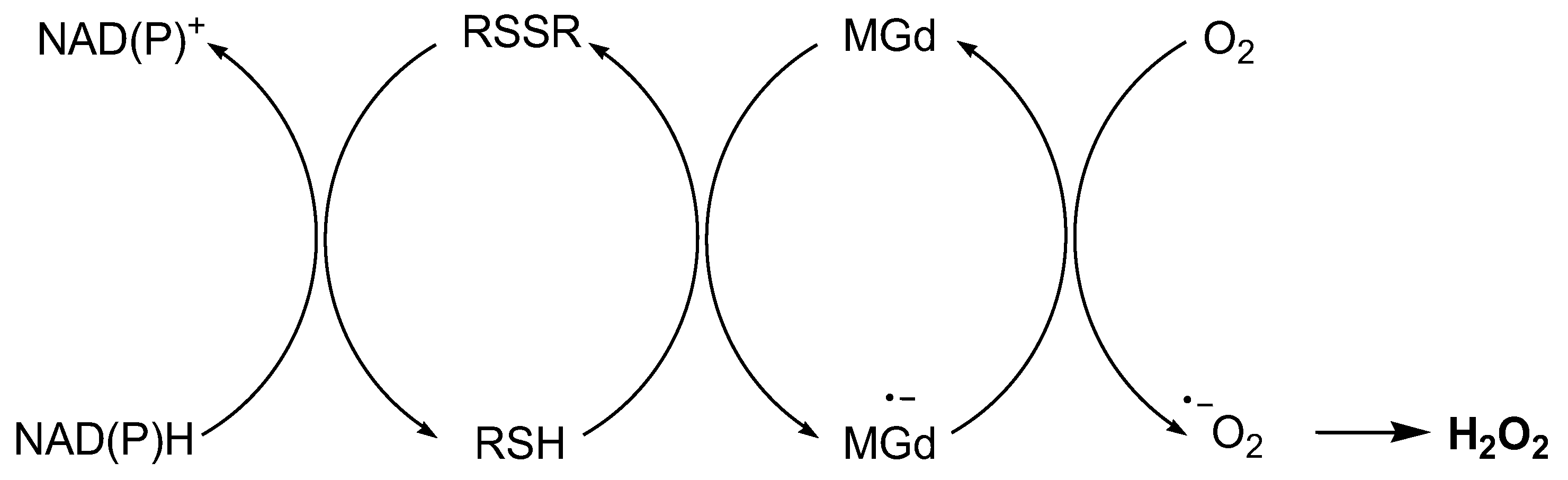
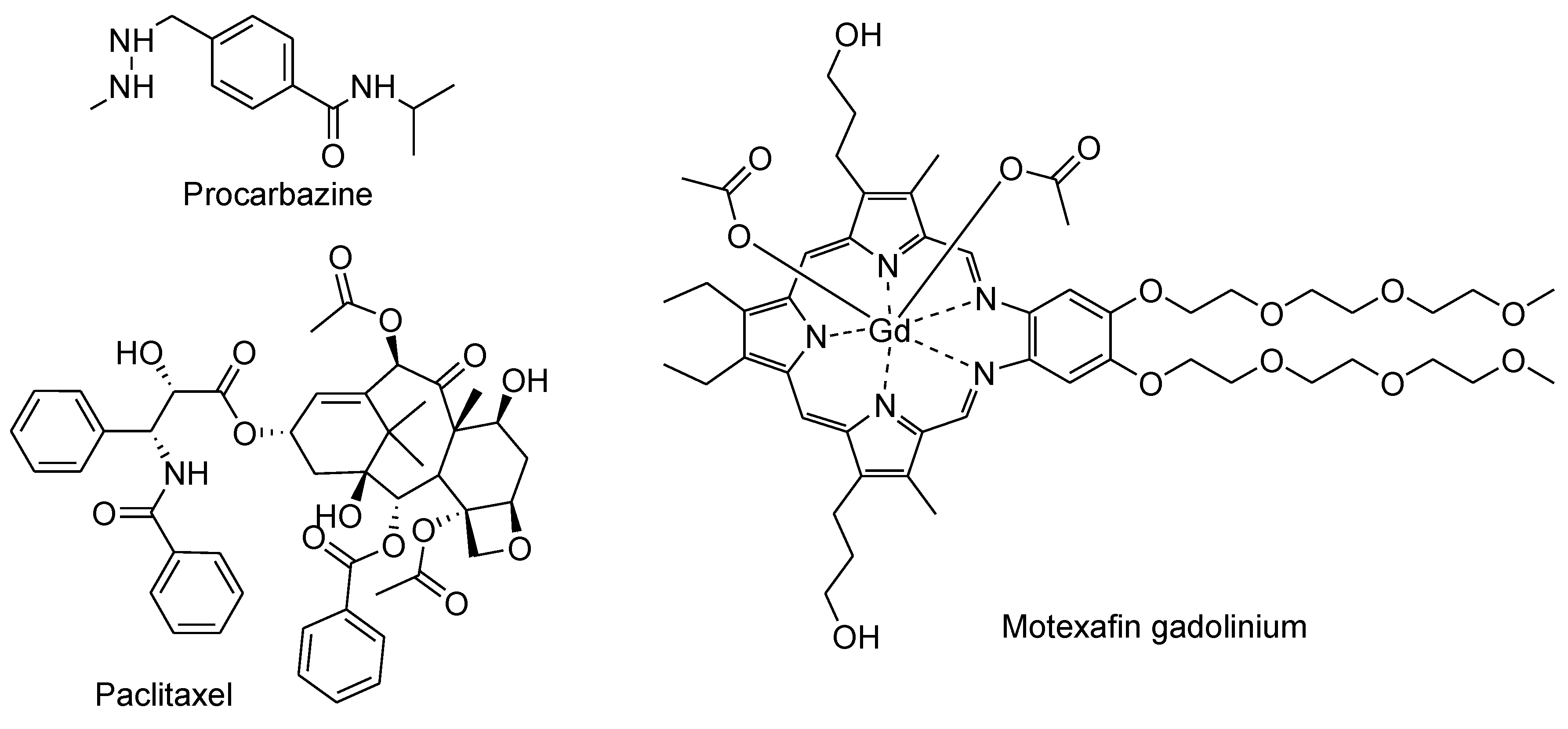
3.5. FDA-Approved Drugs
Conclusion
References
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic Res 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat Rev Drug Discov 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, V.; Hay, N. Molecular pathways: Reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef] [PubMed]
- Schumacker, P.T. Reactive oxygen species in cancer cells: Live by the sword, die by the sword. Cancer Cell 2006, 10, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Hileman, E.O.; Liu, J.; Albitar, M.; Keating, M.J.; Huang, P. Intrinsic oxidative stress in cancer cells: A biochemical basis for therapeutic selectivity. Cancer Chemother. Pharmacol. 2004, 53, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.Y.J.; Zheng, W.; Sun, J.; Wang, W.; Qian, H. Recent Advances on Modulation of H2O2 in Tumor Microenvironment for Enhanced Cancer Therapeutic Efficacy. Coord. Chem. Rev. 2023, 481, 215049. [Google Scholar] [CrossRef]
- Dharmaraja, A.T. Role of Reactive Oxygen Species (ROS) in Therapeutics and Drug Resistance in Cancer and Bacteria. J Med Chem 2017, 60, 3221–3240. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxid Med Cell Longev 2019, 2019, 5381692. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M. Dual role of hydrogen peroxide in cancer: Possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007, 252, 1–8. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M.M. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Celia María Curieses Andrés, J.M.P.d.l.L. , Celia Andrés Juan, Francisco J. Plou; Pérez-Lebeña, a. E., Chemistry of Hydrogen Peroxide Formation and Elimination in Mammalian Cells, and Its Role in Various Pathologies. Stresses 2022, 2, 256–274. [Google Scholar] [CrossRef]
- Nicco, C.; Laurent, A.; Chereau, C.; Weill, B.; Batteux, F. Differential modulation of normal and tumor cell proliferation by reactive oxygen species. Biomed. Pharmacother. 2005, 59, 169–174. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol 2011, 7, 504–511. [Google Scholar] [CrossRef]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat Rev Microbiol 2013, 11, 443–454. [Google Scholar] [CrossRef]
- Kong, Q.; Lillehei, K.O. Antioxidant inhibitors for cancer therapy. Med Hypotheses 1998, 51, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Conklin, K.A. Chemotherapy-associated oxidative stress: Impact on chemotherapeutic effectiveness. Integr Cancer Ther 2004, 3, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Buytaert, E.; Dewaele, M.; Agostinis, P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim. Biophys. Acta 2007, 1776, 86–107. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.; Seo, D.; Kim, D.; Kim, K.; Kim, E.; Kang, J.; Seong, K.M.; Youn, H.; Youn, B. Cellular Stress Responses in Radiotherapy. Cells, 2019; 8. [Google Scholar]
- Wang, C.Y. J.; Dong, C.; Shi, S. Glucose Oxidase-Related Cancer Therapies. Adv.Ther. 2020; 3, No.2000110. [Google Scholar]
- Firczuk, M.; Bajor, M.; Graczyk-Jarzynka, A.; Fidyt, K.; Goral, A.; Zagozdzon, R. Harnessing altered oxidative metabolism in cancer by augmented prooxidant therapy. Cancer Lett. 2020, 471, 1–11. [Google Scholar] [CrossRef]
- Foglietta, F.; Serpe, L.; Canaparo, R. ROS-generating nanoplatforms as selective and tunable therapeutic weapons against cancer. Discov Nano 2023, 18, 151. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Tian, X.; Ai, H.W. Molecular Tools to Generate Reactive Oxygen Species in Biological Systems. Bioconjug. Chem. 2019, 30, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Peng, L.; Zhou, L.; Huang, Z.; Zhou, C.; Huang, C. Oxidative Stress in Cancer Immunotherapy: Molecular Mechanisms and Potential Applications. Antioxidants (Basel), 2022; 11. [Google Scholar]
- Giansanti, M.; Karimi, T.; Faraoni, I.; Graziani, G. High-Dose Vitamin C: Preclinical Evidence for Tailoring Treatment in Cancer Patients. Cancers (Basel), 2021; 13. [Google Scholar]
- Akagawa, M.; Shigemitsu, T.; Suyama, K. Production of hydrogen peroxide by polyphenols and polyphenol-rich beverages under quasi-physiological conditions. Biosci. Biotechnol. Biochem. 2003, 67, 2632–2640. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Yamazaki, S.; Kano, K.; Ikeda, T. Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochim. Biophys. Acta, 2002; 1569, 35–44. [Google Scholar]
- Zhang, Z.; Shi, J.; Nice, E.C.; Huang, C.; Shi, Z. The Multifaceted Role of Flavonoids in Cancer Therapy: Leveraging Autophagy with a Double-Edged Sword. Antioxidants (Basel), 2021; 10. [Google Scholar]
- Oikawa, S.; Furukawaa, A.; Asada, H.; Hirakawa, K.; Kawanishi, S. Catechins induce oxidative damage to cellular and isolated DNA through the generation of reactive oxygen species. Free Radic Res 2003, 37, 881–890. [Google Scholar] [CrossRef]
- Furukawa, A.; Oikawa, S.; Murata, M.; Hiraku, Y.; Kawanishi, S. (-)-Epigallocatechin gallate causes oxidative damage to isolated and cellular DNA. Biochem. Pharmacol. 2003, 66, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Hasumi, K.; Woo, J.T.; Nagai, K.; Wachi, M. Generation of hydrogen peroxide primarily contributes to the induction of Fe(II)-dependent apoptosis in Jurkat cells by (-)-epigallocatechin gallate. Carcinogenesis 2004, 25, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Arakawa, H.; Maeda, M.; Satoh, K.; Kadofuku, T.; Fukuchi, K.; Gomi, K. Production of hydrogen peroxide and methionine sulfoxide by epigallocatechin gallate and antioxidants. Anticancer Res. 2001, 21 (4A), 2633–2641. [Google Scholar]
- Yen, G.C.; Duh, P.D.; Tsai, H.L.; Huang, S.L. Pro-oxidative properties of flavonoids in human lymphocytes. Biosci. Biotechnol. Biochem. 2003, 67, 1215–1222. [Google Scholar] [CrossRef]
- Canada, A.T.; Giannella, E.; Nguyen, T.D.; Mason, R.P. The production of reactive oxygen species by dietary flavonols. Free Radic Biol Med 1990, 9, 441–449. [Google Scholar] [CrossRef]
- Miura, Y.H.; Tomita, I.; Watanabe, T.; Hirayama, T.; Fukui, S. Active oxygens generation by flavonoids. Biol. Pharm. Bull. 1998, 21, 93–96. [Google Scholar] [CrossRef]
- Baumann, S.; Fas, S.C.; Giaisi, M.; Muller, W.W.; Merling, A.; Gulow, K.; Edler, L.; Krammer, P.H.; Li-Weber, M. Wogonin preferentially kills malignant lymphocytes and suppresses T-cell tumor growth by inducing PLCgamma1- and Ca2+-dependent apoptosis. Blood 2008, 111, 2354–2363. [Google Scholar] [CrossRef]
- Wei, L.; Lu, N.; Dai, Q.; Rong, J.; Chen, Y.; Li, Z.; You, Q.; Guo, Q. Different apoptotic effects of wogonin via induction of H(2)O(2) generation and Ca(2+) overload in malignant hepatoma and normal hepatic cells. J Cell Biochem 2010, 111, 1629–1641. [Google Scholar] [CrossRef]
- Tsai, C.F.; Yeh, W.L.; Huang, S.M.; Tan, T.W.; Lu, D.Y. Wogonin induces reactive oxygen species production and cell apoptosis in human glioma cancer cells. Int J Mol Sci 2012, 13, 9877–9892. [Google Scholar] [CrossRef]
- Dzah, C.S.Z. H.; Gobe, V.; Asante-Donyinah, D.A.; Duan, Y. Anti- and pro-oxidant properties of polyphenols and their role in modulating glutathione synthesis, activity and cellular redox potential: Potential synergies for disease management. Adv. Red. Res. 2024, 11, 100099. [Google Scholar] [CrossRef]
- Braicu, C.; Ladomery, M.R.; Chedea, V.S.; Irimie, A.; Berindan-Neagoe, I. The relationship between the structure and biological actions of green tea catechins. Food Chem 2013, 141, 3282–3289. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhu, K.; Liu, Z.; Huang, J. Prooxidant Effects of Epigallocatechin-3-Gallate in Health Benefits and Potential Adverse Effect. Oxid Med Cell Longev 2020, 2020, 9723686. [Google Scholar] [CrossRef]
- Saeki, K.; Hayakawa, S.; Isemura, M.; Miyase, T. Importance of a pyrogallol-type structure in catechin compounds for apoptosis-inducing activity. Phytochemistry 2000, 53, 391–394. [Google Scholar] [CrossRef]
- Hong, J.; Lu, H.; Meng, X.; Ryu, J.H.; Hara, Y.; Yang, C.S. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (-)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002, 62, 7241–7246. [Google Scholar]
- Park, W.H.; Han, Y.W.; Kim, S.H.; Kim, S.Z. A superoxide anion generator, pyrogallol induces apoptosis in As4.1 cells through the depletion of intracellular GSH content. Mutat Res, 2007; 619, 81–92. [Google Scholar]
- Han, Y.H.; Kim, S.Z.; Kim, S.H.; Park, W.H. Pyrogallol as a glutathione depletor induces apoptosis in HeLa cells. Int J Mol Med 2008, 21, 721–730. [Google Scholar]
- Liu, T.P. P.; Shi, H.; Feng, J.; Zhang, X. Assembled polyphenol-based systems as advanced therapeutics. J. Poly. Sci. 62(2).
- Awad, H.M.; Boersma, M.G.; Boeren, S.; van Bladeren, P.J.; Vervoort, J.; Rietjens, I.M. The regioselectivity of glutathione adduct formation with flavonoid quinone/quinone methides is pH-dependent. Chem. Res. Toxicol. 2002, 15, 343–351. [Google Scholar] [CrossRef]
- Galati, G.; Sabzevari, O.; Wilson, J.X.; O'Brien, P.J. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology 2002, 177, 91–104. [Google Scholar] [CrossRef]
- Chan, T.; Galati, G.; O'Brien, P.J. Oxygen activation during peroxidase catalysed metabolism of flavones or flavanones. Chem. Biol. Interact. 1999, 122, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Hider, R.C.; Liu, Z.D.; Khodr, H.H. Metal chelation of polyphenols. Methods Enzymol 2001, 335, 190–203. [Google Scholar]
- Pan, Y.Q. R.; Hou, M.; Xue, J.; Zhou, M.; Xu, L.; Zhang, Y. The interactions of polyphenols with Fe and their application in Fenton/Fenton-like reactions. Sep. Purif. Technol. 2022, 300, 121831. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Spencer, J.P.; Rossi, R.; Aeschbach, R.; Khan, A.; Mahmood, N.; Munoz, A.; Murcia, A.; Butler, J.; Halliwell, B. An evaluation of the antioxidant and antiviral action of extracts of rosemary and Provencal herbs. Food Chem Toxicol 1996, 34, 449–456. [Google Scholar] [CrossRef] [PubMed]
- al-Sereiti, M.R.; Abu-Amer, K.M.; Sen, P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Indian J Exp Biol 1999, 37, 124–130. [Google Scholar] [PubMed]
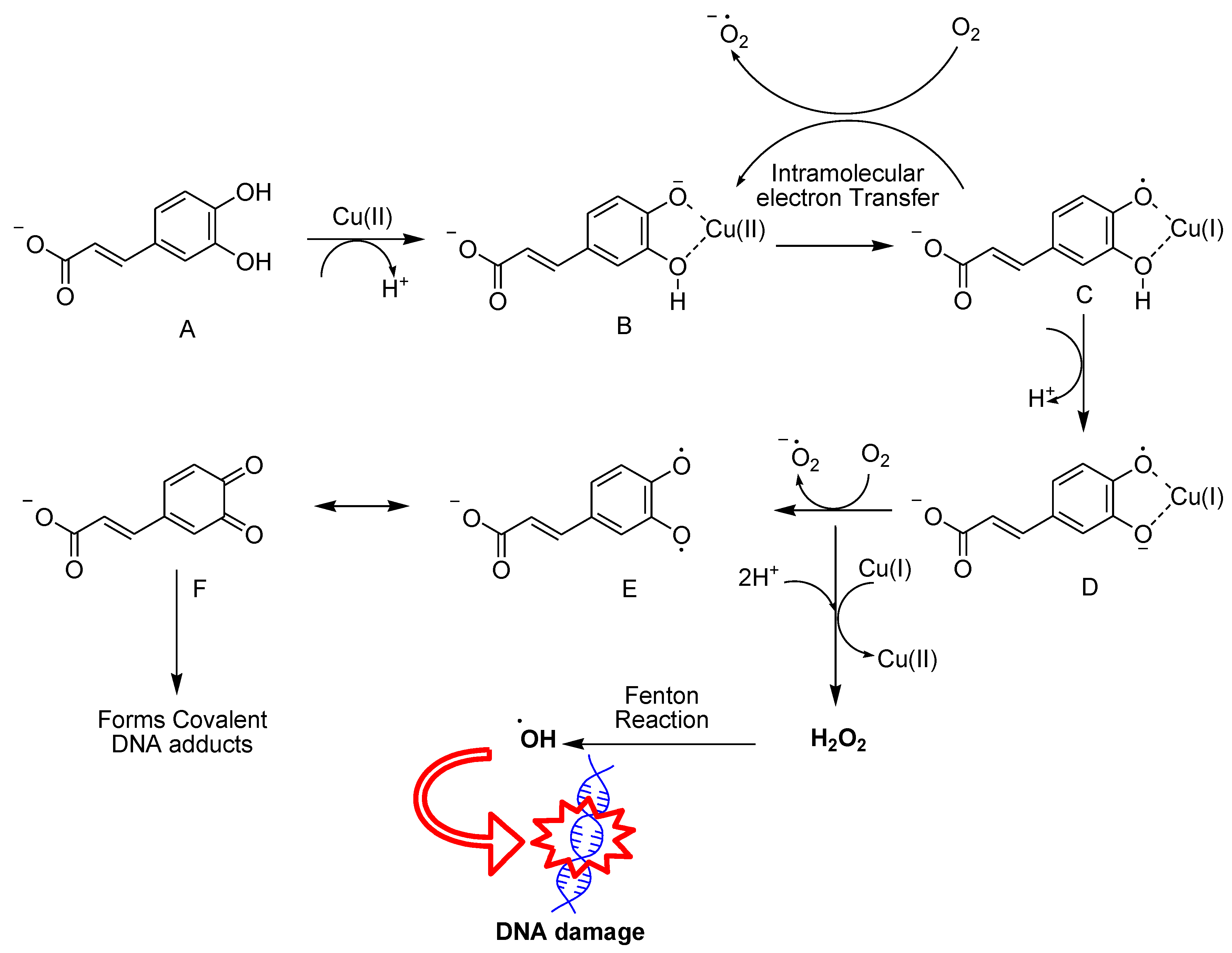
- Zheng, L.F.; Dai, F.; Zhou, B.; Yang, L.; Liu, Z.L. Prooxidant activity of hydroxycinnamic acids on DNA damage in the presence of Cu(II) ions: Mechanism and structure-activity relationship. Food Chem Toxicol 2008, 46, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, S.S.; Dantas, B.B.; Ribeiro-Filho, J.; Antonio, M.A.D.; Galberto, M. d. C. J. Chemical Properties of Caffeic and Ferulic Acids in Biological System: Implications in Cancer Therapy. A Review. Curr Pharm Des 2017, 23, 3015–3023. [Google Scholar] [CrossRef]
- Li, Y.; Trush, M.A. Reactive oxygen-dependent DNA damage resulting from the oxidation of phenolic compounds by a copper-redox cycle mechanism. Cancer Res. 1994, 54 (7 Suppl), 1895s–1898s. [Google Scholar]
- Pirker, K.F.; Kay, C.W.; Stolze, K.; Tunega, D.; Reichenauer, T.G.; Goodman, B.A. Free radical generation in rosmarinic acid investigated by electron paramagnetic resonance spectroscopy. Free Radic Res 2009, 43, 47–57. [Google Scholar] [CrossRef]
- Murakami, K.; Haneda, M.; Qiao, S.; Naruse, M.; Yoshino, M. Prooxidant action of rosmarinic acid: Transition metal-dependent generation of reactive oxygen species. Toxicol In Vitro 2007, 21, 613–617. [Google Scholar] [CrossRef]
- Muñoz-Muñoz, J. l. G.-M. F.; Ros, E.; Tudela, J.; García-Canovas, F.; Rodriguez-Lopez, J.N. , Prooxidant and Antioxidant Activities of Rosmarinic Acid. Journal of Food Biochemistry,. 2013,, 37 (4).
- Fabiani, R.; De Bartolomeo, A.; Rosignoli, P.; Servili, M.; Montedoro, G.F.; Morozzi, G. Cancer chemoprevention by hydroxytyrosol isolated from virgin olive oil through G1 cell cycle arrest and apoptosis. Eur J Cancer Prev 2002, 11, 351–358. [Google Scholar] [CrossRef]
- Sun, L.; Luo, C.; Liu, J. Hydroxytyrosol induces apoptosis in human colon cancer cells through ROS generation. Food Funct 2014, 5, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Long, L.H.; Hoi, A.; Halliwell, B. Instability of, and generation of hydrogen peroxide by, phenolic compounds in cell culture media. Arch. Biochem. Biophys. 2010, 501, 162–169. [Google Scholar] [CrossRef]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Guichard, C.; Pedruzzi, E.; Fay, M.; Marie, J.C.; Braut-Boucher, F.; Daniel, F.; Grodet, A.; Gougerot-Pocidalo, M.A.; Chastre, E.; Kotelevets, L.; Lizard, G.; Vandewalle, A.; Driss, F.; Ogier-Denis, E. Dihydroxyphenylethanol induces apoptosis by activating serine/threonine protein phosphatase PP2A and promotes the endoplasmic reticulum stress response in human colon carcinoma cells. Carcinogenesis 2006, 27, 1812–1827. [Google Scholar] [CrossRef]
- Luo, C.; Li, Y.; Wang, H.; Cui, Y.; Feng, Z.; Li, H.; Li, Y.; Wang, Y.; Wurtz, K.; Weber, P.; Long, J.; Liu, J. Hydroxytyrosol promotes superoxide production and defects in autophagy leading to anti-proliferation and apoptosis on human prostate cancer cells. Curr. Cancer Drug Targets 2013, 13, 625–639. [Google Scholar] [CrossRef]
- Fabiani, R.; Fuccelli, R.; Pieravanti, F.; De Bartolomeo, A.; Morozzi, G. Production of hydrogen peroxide is responsible for the induction of apoptosis by hydroxytyrosol on HL60 cells. Mol Nutr Food Res 2009, 53, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; Sepporta, M.V.; Rosignoli, P.; De Bartolomeo, A.; Crescimanno, M.; Morozzi, G. Anti-proliferative and pro-apoptotic activities of hydroxytyrosol on different tumour cells: The role of extracellular production of hydrogen peroxide. Eur J Nutr 2012, 51, 455–464. [Google Scholar] [CrossRef]
- Shingai, Y.; Fujimoto, A.; Nakamura, M.; Masuda, T. Structure and function of the oxidation products of polyphenols and identification of potent lipoxygenase inhibitors from Fe-catalyzed oxidation of resveratrol. J Agric Food Chem 2011, 59, 8180–8186. [Google Scholar] [CrossRef]
- Chen, C.H.; Lin, W.C.; Kuo, C.N.; Lu, F.J. Role of redox signaling regulation in propyl gallate-induced apoptosis of human leukemia cells. Food Chem Toxicol 2011, 49, 494–501. [Google Scholar] [CrossRef] [PubMed]
- You, B.R.; Park, W.H. The enhancement of propyl gallate-induced apoptosis in HeLa cells by a proteasome inhibitor MG132. Oncol Rep 2011, 25, 871–877. [Google Scholar]
- Han, Y.H.; Park, W.H. Propyl gallate inhibits the growth of HeLa cells via regulating intracellular GSH level. Food Chem Toxicol 2009, 47, 2531–2538. [Google Scholar] [CrossRef]
- Jacobi, H.; Eicke, B.; Witte, I. DNA strand break induction and enhanced cytotoxicity of propyl gallate in the presence of copper(II). Free Radic Biol Med 1998, 24, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, M.; Bartosz, G.; Stefaniuk, I.; Pichla, M.; Namiesnik, J.; Sadowska-Bartosz, I. Dietary antioxidants as a source of hydrogen peroxide. Food Chem 2019, 278, 692–699. [Google Scholar] [CrossRef]
- Criddle, D.N.; Gillies, S.; Baumgartner-Wilson, H.K.; Jaffar, M.; Chinje, E.C.; Passmore, S.; Chvanov, M.; Barrow, S.; Gerasimenko, O.V.; Tepikin, A.V.; Sutton, R.; Petersen, O.H. Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. J Biol Chem 2006, 281, 40485–40492. [Google Scholar] [CrossRef]
- Mishra, P.K.; Park, I.; Sharma, N.; Yoo, C.M.; Lee, H.Y.; Rhee, H.W. Enzymatic Recording of Local Hydrogen Peroxide Generation Using Genetically Encodable Enzyme. Anal. Chem. 2022, 94, 14869–14877. [Google Scholar] [CrossRef]
- Loor, G.; Kondapalli, J.; Schriewer, J.M.; Chandel, N.S.; Vanden Hoek, T.L.; Schumacker, P.T. Menadione triggers cell death through ROS-dependent mechanisms involving PARP activation without requiring apoptosis. Free Radic Biol Med 2010, 49, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Lasalvia-Prisco, E.; Cucchi, S.; Vazquez, J.; Lasalvia-Galante, E.; Golomar, W.; Gordon, W. Serum markers variation consistent with autoschizis induced by ascorbic acid-menadione in patients with prostate cancer. Med Oncol 2003, 20, 45–52. [Google Scholar] [CrossRef]
- Checker, R.; Patwardhan, R.S.; Sharma, D.; Menon, J.; Thoh, M.; Sandur, S.K.; Sainis, K.B.; Poduval, T.B. Plumbagin, a vitamin K3 analogue, abrogates lipopolysaccharide-induced oxidative stress, inflammation and endotoxic shock via NF-kappaB suppression. Inflammation 2014, 37, 542–554. [Google Scholar] [CrossRef]
- Sand, J.M.; Bin Hafeez, B.; Jamal, M.S.; Witkowsky, O.; Siebers, E.M.; Fischer, J.; Verma, A.K. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone), isolated from Plumbago zeylanica, inhibits ultraviolet radiation-induced development of squamous cell carcinomas. Carcinogenesis 2012, 33, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Klaus, V.; Hartmann, T.; Gambini, J.; Graf, P.; Stahl, W.; Hartwig, A.; Klotz, L.O. 1,4-Naphthoquinones as inducers of oxidative damage and stress signaling in HaCaT human keratinocytes. Arch. Biochem. Biophys. 2010, 496, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kapur, A.; Beres, T.; Rathi, K.; Nayak, A.P.; Czarnecki, A.; Felder, M.; Gillette, A.; Ericksen, S.S.; Sampene, E.; Skala, M.C.; Barroilhet, L.; Patankar, M.S. Oxidative stress via inhibition of the mitochondrial electron transport and Nrf-2-mediated anti-oxidative response regulate the cytotoxic activity of plumbagin. Sci Rep 2018, 8, 1073. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, J.J.; Chignell, C.F. Cytotoxic action of juglone and plumbagin: A mechanistic study using HaCaT keratinocytes. Chem. Res. Toxicol. 2004, 17, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kappus, H. Overview of enzyme systems involved in bio-reduction of drugs and in redox cycling. Biochem. Pharmacol. 1986, 35, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ollinger, K.; Brunmark, A. Effect of hydroxy substituent position on 1,4-naphthoquinone toxicity to rat hepatocytes. J Biol Chem 1991, 266, 21496–21503. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Hu, J.; Wang, P.; Li, X.; Zhang, X. A comprehensive review on beta-lapachone: Mechanisms, structural modifications, and therapeutic potentials. Eur J Med Chem 2021, 210, 112962. [Google Scholar] [CrossRef] [PubMed]
- Pink, J.J.; Planchon, S.M.; Tagliarino, C.; Varnes, M.E.; Siegel, D.; Boothman, D.A. NAD(P)H:Quinone oxidoreductase activity is the principal determinant of beta-lapachone cytotoxicity. J Biol Chem 2000, 275, 5416–5424. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.P.; Shiah, S.G.; Don, M.J.; Kuo, M.L. Involvement of hydrogen peroxide in topoisomerase inhibitor beta-lapachone-induced apoptosis and differentiation in human leukemia cells. Free Radic Biol Med 1998, 24, 660–670. [Google Scholar] [CrossRef]
- Gerber, D.E.; Beg, M.S.; Fattah, F.; Frankel, A.E.; Fatunde, O.; Arriaga, Y.; Dowell, J.E.; Bisen, A.; Leff, R.D.; Meek, C.C.; Putnam, W.C.; Kallem, R.R.; Subramaniyan, I.; Dong, Y.; Bolluyt, J.; Sarode, V.; Luo, X.; Xie, Y.; Schwartz, B.; Boothman, D.A. Phase 1 study of ARQ 761, a beta-lapachone analogue that promotes NQO1-mediated programmed cancer cell necrosis. Br. J. Cancer 2018, 119, 928–936. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Cho, J.Y.; Yoon, S.H.; Jang, I.J.; Yu, K.S. Pharmacokinetics and tolerability of MB12066, a beta-lapachone derivative targeting NAD(P)H: Quinone oxidoreductase 1: Two independent, double-blind, placebo-controlled, combined single and multiple ascending dose first-in-human clinical trials. Drug Des Devel Ther 2017, 11, 3187–3195. [Google Scholar] [CrossRef]
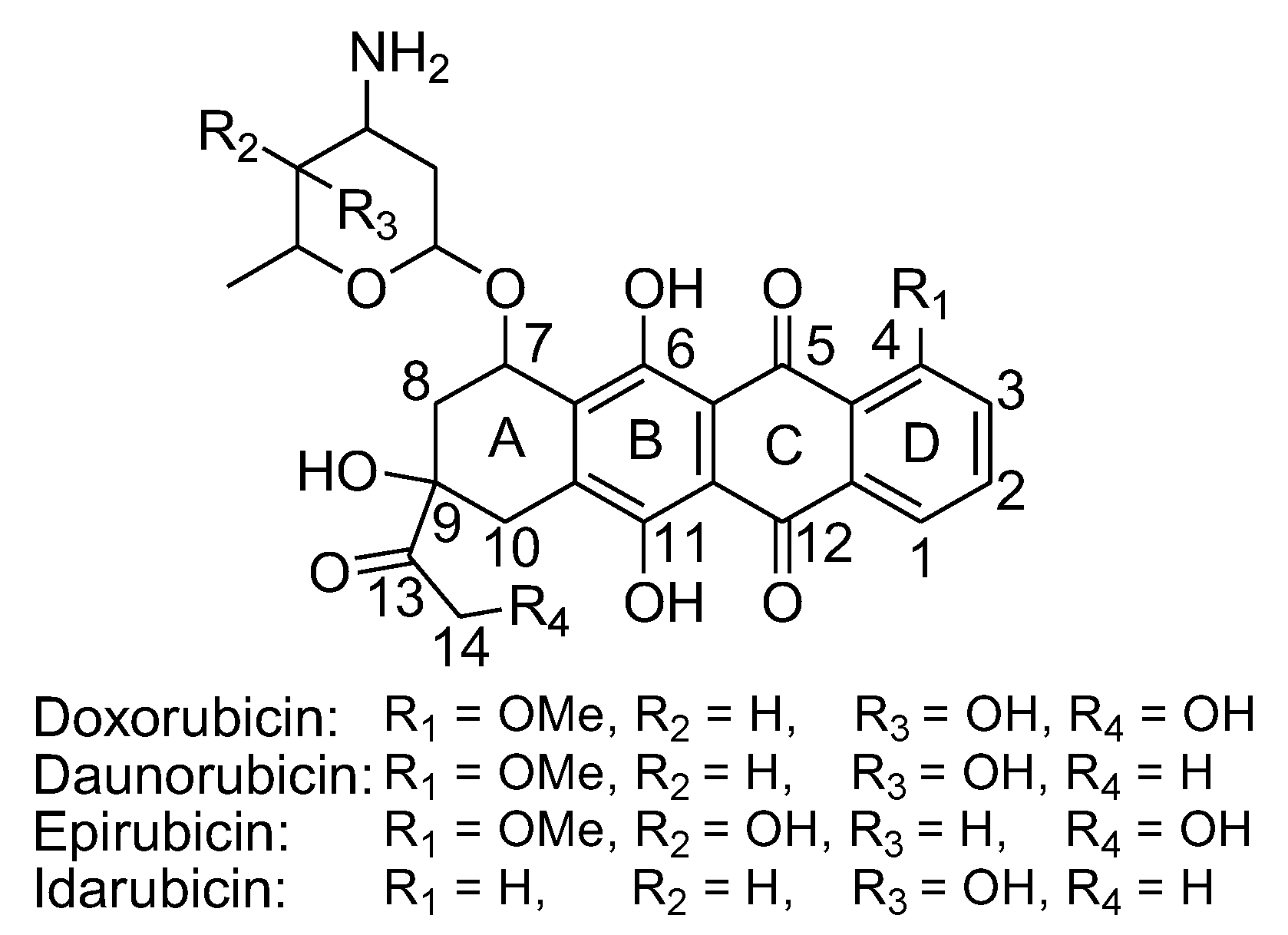
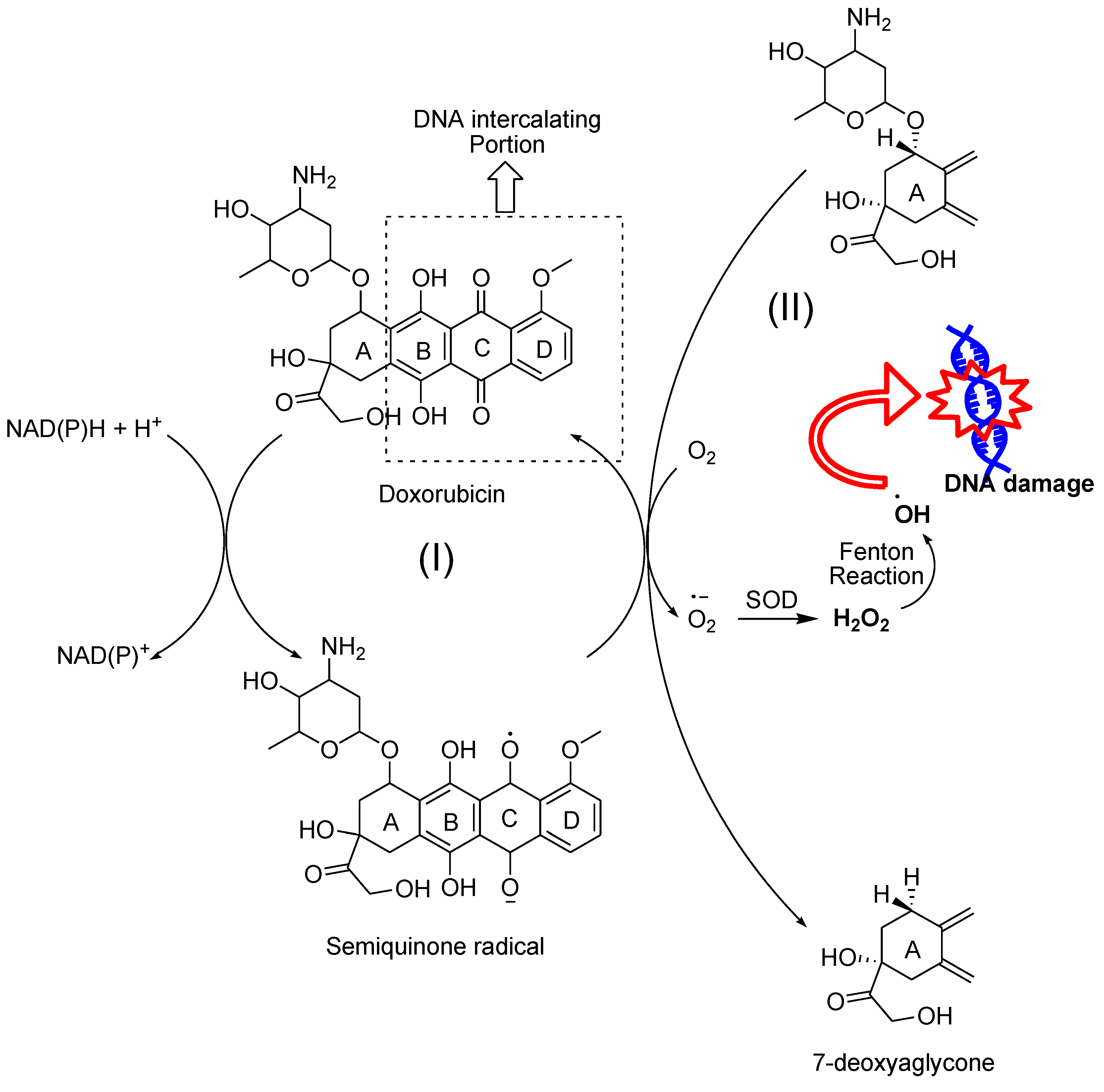
- Weiss, R.B. The anthracyclines: Will we ever find a better doxorubicin? Semin Oncol 1992, 19, 670–686. [Google Scholar] [PubMed]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.K.; Mimnaugh, E.G.; Rajagopalan, S.; Myers, C.E. Adriamycin activation and oxygen free radical formation in human breast tumor cells: Protective role of glutathione peroxidase in adriamycin resistance. Cancer Res. 1989, 49, 3844–3848. [Google Scholar] [PubMed]
- Ubezio, P.; Civoli, F. Flow cytometric detection of hydrogen peroxide production induced by doxorubicin in cancer cells. Free Radic Biol Med 1994, 16, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.A.; Evig, C.B.; Reszka, K.J.; Buettner, G.R.; Burns, C.P. Doxorubicin increases intracellular hydrogen peroxide in PC3 prostate cancer cells. Arch. Biochem. Biophys. 2005, 440, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Thayer, W.S. Adriamycin stimulated superoxide formation in submitochondrial particles. Chem. Biol. Interact. 1977, 19, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Binaschi, M.; Farinosi, R.; Borgnetto, M.E.; Capranico, G. In vivo site specificity and human isoenzyme selectivity of two topoisomerase II-poisoning anthracyclines. Cancer Res. 2000, 60, 3770–3776. [Google Scholar] [PubMed]
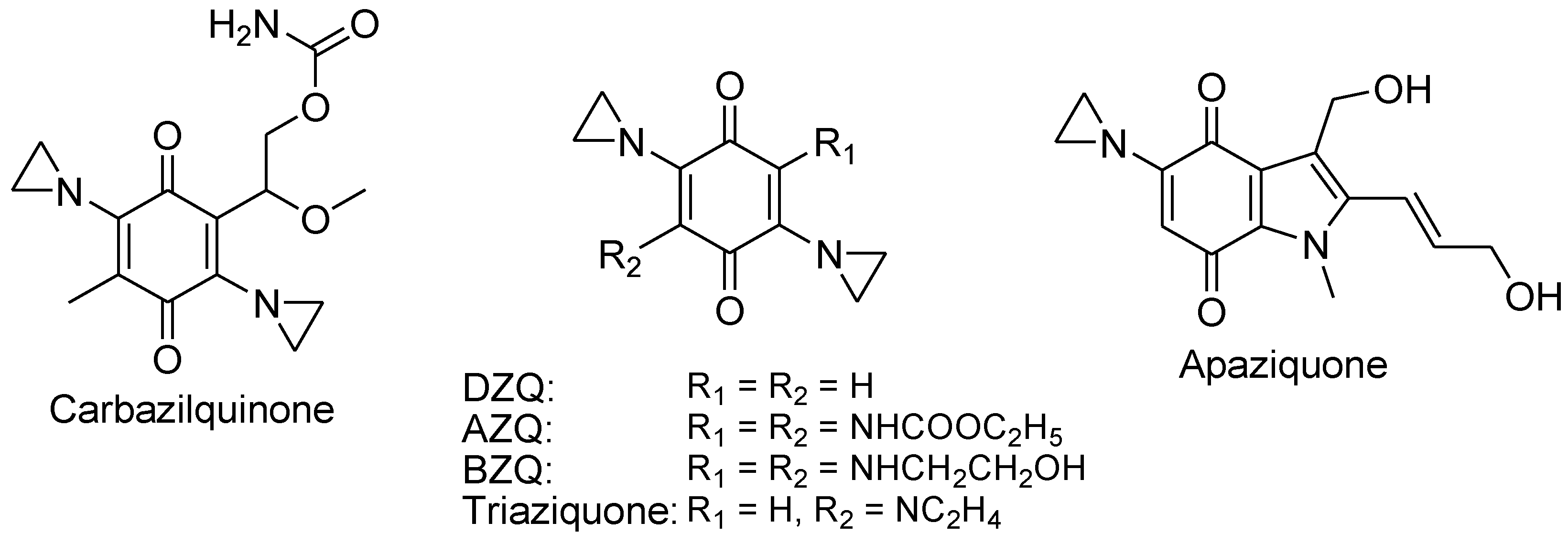
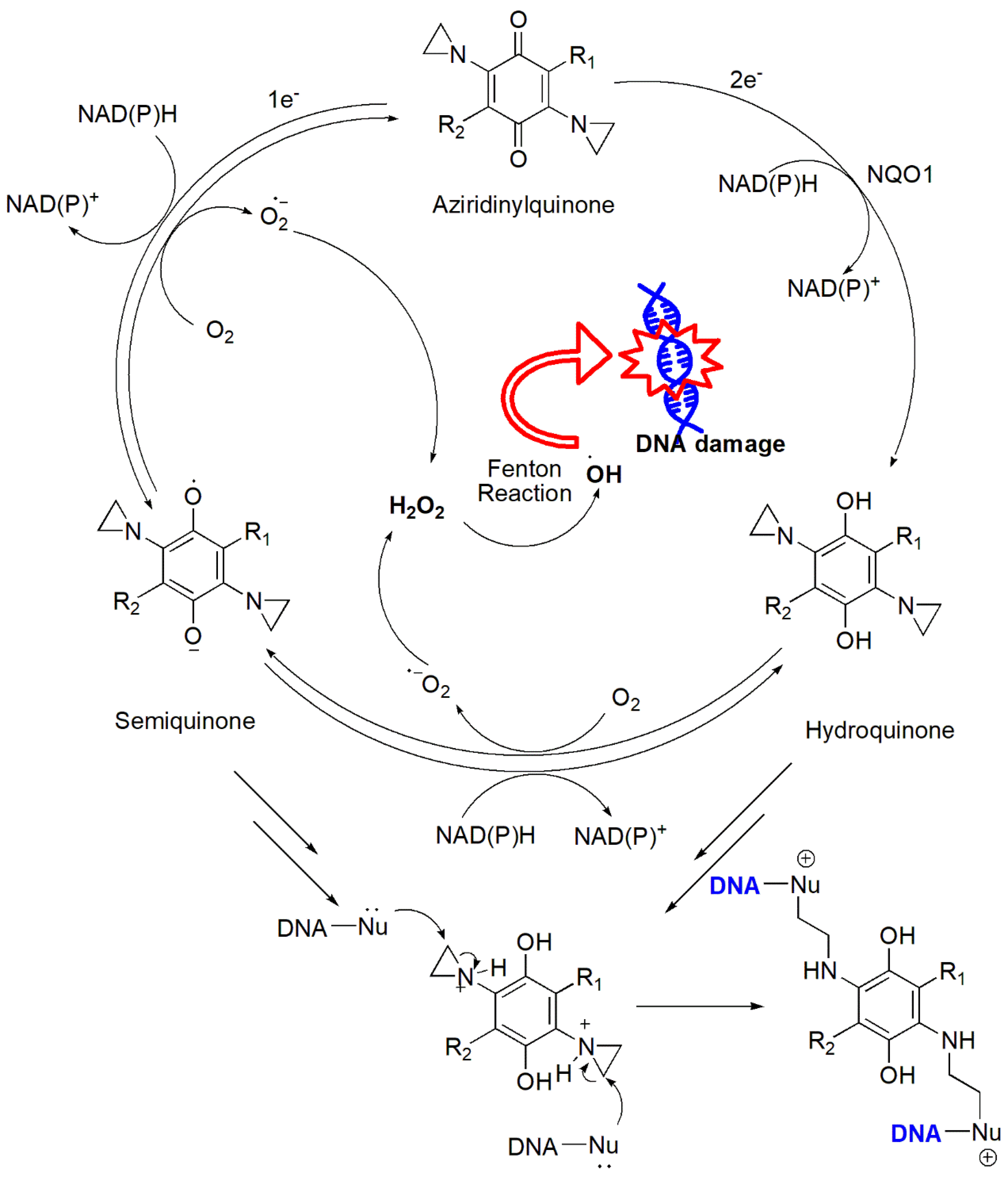
- Skibo, E.B.; Xing, C. Chemistry and DNA alkylation reactions of aziridinyl quinones: Development of an efficient alkylating agent of the phosphate backbone. Biochemistry 1998, 37, 15199–15213. [Google Scholar] [CrossRef]
- Rashid, M.H.; Babu, D.; Siraki, A.G. Interactions of the antioxidant enzymes NAD(P)H: Quinone oxidoreductase 1 (NQO1) and NRH: Quinone oxidoreductase 2 (NQO2) with pharmacological agents, endogenous biochemicals and environmental contaminants. Chem. Biol. Interact. 2021, 345, 109574. [Google Scholar] [CrossRef]
- Avendaño, C.M.J.C. Chapter 11 - Drug Targeting in Anticancer. In Medicinal Chemistry of Anticancer Drugs; Avendaño, C., Menéndez, J.C., Eds.; Elsevier: Amsterdam, 2008; pp. 351–385. [Google Scholar]
- Webb, J.S.C. D.B.; Mowat, J.H.; Patrick, J.B.; Broschard, R.W.; Meyer, W.E.; Williams, R.P.; Wolf, C.F.; Fulmor, William. ; Pidacks, Charles.; Lancaster, J.E., The Structures of Mitomycins A, B and C and Porfiromycin--Part I. J. Am. Chem. Soc. 1962, 84, 3185–3187. [Google Scholar] [CrossRef]
- Tomasz, M. H2O2 generation during the redox cycle of mitomycin C and dna-bound mitomycin C. Chem. Biol. Interact. 1976, 13, 89–97. [Google Scholar] [CrossRef]
- Andrez, J.C. Mitomycins syntheses: A recent update. Beilstein J. Org. Chem. 2009, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Remers, W.A.; Schepman, C.S. Structure-activity relationships of the mitomycins and certain synthetic analogs. J Med Chem 1974, 17, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Hornemann, U.H. M.J. , Stereochemical Relationship between Mitomycins A, B, and C. J. Org. Chem. 1985, 50, 1301–1302. [Google Scholar] [CrossRef]
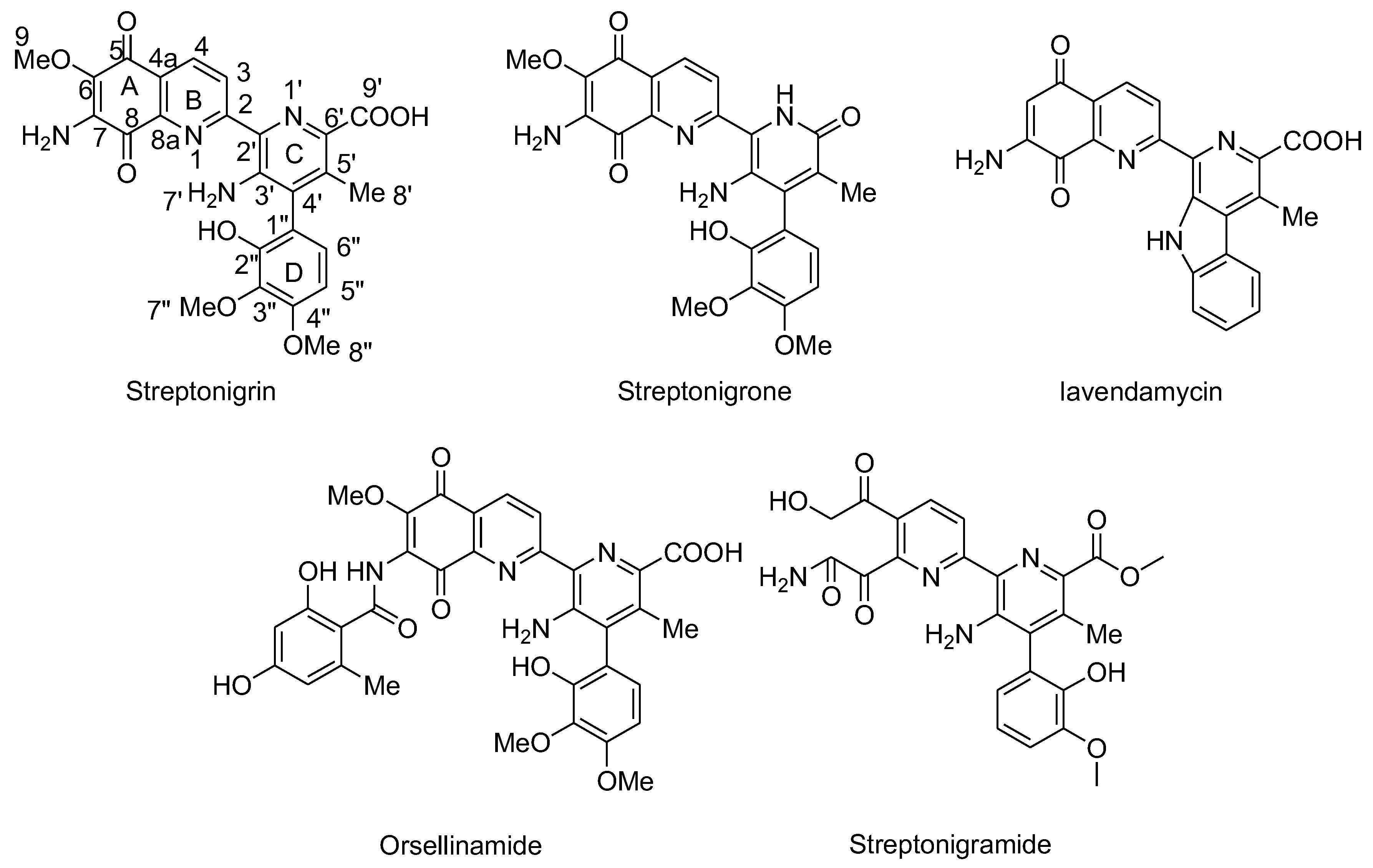
- Mizuno, N.S. Effects of streptonigrin on nucleic acid metabolism of tissue culture cells. Biochim. Biophys. Acta 1965, 108, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Cone, R.; Hasan, S.K.; Lown, J.W.; Morgan, A.R. The mechanism of the degradation of DNA by streptonigrin. Can. J. Biochem. 1976, 54, 219–223. [Google Scholar] [CrossRef]
- Bolzan, A.D.; Bianchi, M.S. Genotoxicity of streptonigrin: A review. Mutat Res 2001, 488, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Testoni, M.I.; Bolzan, A.D.; Bianchi, M.S.; Bianchi, N.O. Effects of antioxidants on streptonigrin-induced DNA damage and clastogenesis in CHO cells. Mutat Res 1997, 373, 201–206. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci U S A 2005, 102, 13604–13609. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Lee, J.H.; Krishna, M.C.; Shacter, E.; Choyke, P.L.; Pooput, C.; Kirk, K.L.; Buettner, G.R.; Levine, M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci U S A 2007, 104, 8749–8754. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Pooput, C.; Kirk, K.L.; Krishna, M.C.; Khosh, D.B.; Drisko, J.; Levine, M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci U S A 2008, 105, 11105–11109. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, N.; Creagan, E.; Witzig, T.; Levine, M. Ascorbic Acid in Cancer Treatment: Let the Phoenix Fly. Cancer Cell 2018, 34, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Martin, S.M.; Levine, M.; Wagner, B.A.; Buettner, G.R.; Wang, S.H.; Taghiyev, A.F.; Du, C.; Knudson, C.M.; Cullen, J.J. Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin. Cancer Res. 2010, 16, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chapman, J.; Levine, M.; Polireddy, K.; Drisko, J.; Chen, Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med 2014, 6, 222ra18. [Google Scholar] [CrossRef] [PubMed]
- Nauman, G.; Gray, J.C.; Parkinson, R.; Levine, M.; Paller, C.J. Systematic Review of Intravenous Ascorbate in Cancer Clinical Trials. Antioxidants (Basel), 2018; 7. [Google Scholar]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.R.; Vaze, O.; D'Souza, G.G.; Rockwell, K.; Torchilin, V.P. Palmitoyl ascorbate-loaded polymeric micelles: Cancer cell targeting and cytotoxicity. Pharm Res 2011, 28, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.R.; Vaze, O.S.; Wang, T.; D'Souza, G.G.; Rockwell, K.; Gada, K.; Khaw, B.A.; Torchilin, V.P. Palmitoyl ascorbate liposomes and free ascorbic acid: Comparison of anticancer therapeutic effects upon parenteral administration. Pharm Res 2012, 29, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, M.; Strafella, E.; Staffolani, S.; Santarelli, L.; Neuzil, J.; Guerrieri, R. alpha-Tocopheryl succinate promotes selective cell death induced by vitamin K3 in combination with ascorbate. Br. J. Cancer 2010, 102, 1224–1234. [Google Scholar] [CrossRef]
- Wang, L.; Luo, X.; Li, C.; Huang, Y.; Xu, P.; Lloyd-Davies, L.H.; Delplancke, T.; Peng, C.; Gao, R.; Qi, H.; Tong, C.; Baker, P. Triethylenetetramine Synergizes with Pharmacologic Ascorbic Acid in Hydrogen Peroxide Mediated Selective Toxicity to Breast Cancer Cell. Oxid Med Cell Longev 2017, 2017, 3481710. [Google Scholar] [CrossRef]
- Li, J.; Ke, W.; Wang, L.; Huang, M.; Yin, W.; Zhang, P.; Chen, Q.; Ge, Z. Self-sufficing H2O2-responsive nanocarriers through tumor-specific H2O2 production for synergistic oxidation-chemotherapy. J Control Release 2016, 225, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Orvig, C.; Abrams, M.J. Medicinal inorganic chemistry: Introduction. Chem. Rev. 1999, 99, 2201–2204. [Google Scholar] [CrossRef]
- Yaman, M.; Kaya, G.; Yekeler, H. Distribution of trace metal concentrations in paired cancerous and non-cancerous human stomach tissues. World J Gastroenterol 2007, 13, 612–618. [Google Scholar] [CrossRef]
- Lee, J.C.; Son, Y.O.; Pratheeshkumar, P.; Shi, X. Oxidative stress and metal carcinogenesis. Free Radic Biol Med 2012, 53, 742–757. [Google Scholar] [CrossRef]
- Frezza, M.; Hindo, S.; Chen, D.; Davenport, A.; Schmitt, S.; Tomco, D.; Dou, Q.P. Novel metals and metal complexes as platforms for cancer therapy. Curr Pharm Des 2010, 16, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Fu, L.H.; Qi, C.; Lin, J.; Huang, P. Metal peroxides for cancer treatment. Bioact Mater 2021, 6, 2698–2710. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Lv, G.; Bu, W. Redox dyshomeostasis strategy for tumor therapy based on nanomaterials chemistry. Chem. Sci. 2022, 13, 2202–2217. [Google Scholar] [CrossRef]
- Dugo, E.B.; Yedjou, C.G.; Stevens, J.J.; Tchounwou, P.B. Therapeutic Potential of Arsenic Trioxide (ATO) in Treatment of Hepatocellular Carcinoma: Role of Oxidative Stress in ATO-Induced Apoptosis. Ann Clin Pathol, 2017; 5. [Google Scholar]
- Chou, W.C.; Jie, C.; Kenedy, A.A.; Jones, R.J.; Trush, M.A.; Dang, C.V. Role of NADPH oxidase in arsenic-induced reactive oxygen species formation and cytotoxicity in myeloid leukemia cells. Proc Natl Acad Sci U S A 2004, 101, 4578–4583. [Google Scholar] [CrossRef]
- Jing, Y.; Dai, J.; Chalmers-Redman, R.M.; Tatton, W.G.; Waxman, S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood 1999, 94, 2102–2111. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials (Basel), 2020; 10. [Google Scholar]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photocatalytic production of hydrogen peroxides and organic peroxides in aqueous suspensions of titanium dioxide, zinc oxide, and desert sand. Environ Sci Technol 1988, 22, 798–806. [Google Scholar] [CrossRef]
- Murali, M.; Kalegowda, N.; Gowtham, H.G.; Ansari, M.A.; Alomary, M.N.; Alghamdi, S.; Shilpa, N.; Singh, S.B.; Thriveni, M.C.; Aiyaz, M.; Angaswamy, N.; Lakshmidevi, N.; Adil, S.F.; Hatshan, M.R.; Amruthesh, K.N. Plant-Mediated Zinc Oxide Nanoparticles: Advances in the New Millennium towards Understanding Their Therapeutic Role in Biomedical Applications. Pharmaceutics, 2021; 13. [Google Scholar]
- Singh, T.A.; Das, J.; Sil, P.C. Zinc oxide nanoparticles: A comprehensive review on its synthesis, anticancer and drug delivery applications as well as health risks. Adv. Colloid Interface Sci. 2020, 286, 102317. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.S.; Wang, J.F.; Song, J.; Liu, Y.; Zhu, G.; Dai, Y.; Shen, Z.; Tian, R.; Song, J.; Wang, Z.; Tang, W.; Yu, G.; Zhou, Z.; Yang, Z.; Huang, T.; Niu, G.; Yang, H.H.; Chen, Z.Y.; Chen, X. Cooperation of endogenous and exogenous reactive oxygen species induced by zinc peroxide nanoparticles to enhance oxidative stress-based cancer therapy. Theranostics 2019, 9, 7200–7209. [Google Scholar] [CrossRef] [PubMed]
- Mbugua, S.N. Targeting Tumor Microenvironment by Metal Peroxide Nanoparticles in Cancer Therapy. Bioinorg. Chem. Appl. 2022, 2022, 5041399. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Jin, Y.; Ge, K.; Li, Z.; Liu, H.; Dai, X.; Zhang, Y.; Chen, S.; Liang, X.; Zhang, J. Self-Supply of O(2) and H(2)O(2) by a Nanocatalytic Medicine to Enhance Combined Chemo/Chemodynamic Therapy. Adv Sci (Weinh) 2019, 6, 1902137. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-S.; Huang, T.; Song, J.; Ou, X.-Y.; Wang, Z.; Deng, H.; Tian, R.; Liu, Y.; Wang, J.-F.; Liu, Y.; Yu, G.; Zhou, Z.; Wang, S.; Niu, G.; Yang, H.-H.; Chen, X. Synthesis of Copper Peroxide Nanodots for H2O2 Self-Supplying Chemodynamic Therapy. Journal of the American Chemical Society 2019, 141, 9937–9945. [Google Scholar] [CrossRef] [PubMed]
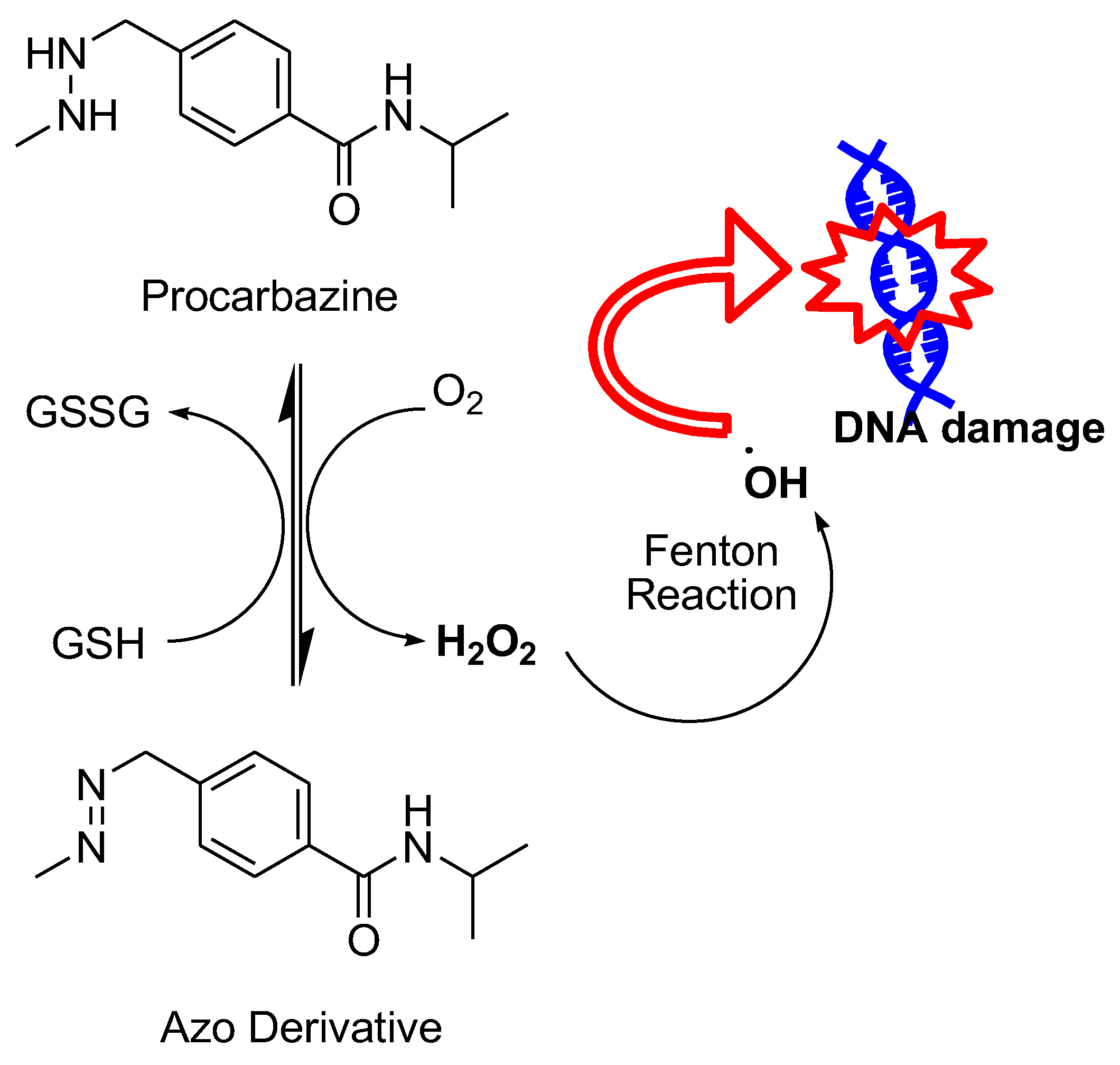
- Mathe, G.; Schweisguth, O.; Schneider, M.; Amiel, J.L.; Berumen, L.; Brule, G.; Cattan, A.; Schwarzenberg, L. Methyl-Hydrazine in Treatment of Hodgkin's Disease and Various Forms of Haematosarcoma and Leukaemia. Lancet 1963, 2, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Berneis, K.; Kofler, M.; Bollag, W.; Kaiser, A.; Langemann, A. The degradation of deoxyribonucleic acid by new tumour inhibiting compounds: The intermediate formation of hydrogen peroxide. Experientia 1963, 19, 132–133. [Google Scholar] [CrossRef]
- Berneis, K.; Kofler, M.; Bollag, W. The influence of chelating agents on the prooxidative effect of a hydrogen peroxide producing methylhydrazine compound. Experientia 1964, 20, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Devita, V.T. Jr.; Serpick, A.A.; Carbone, P.P. Combination chemotherapy in the treatment of advanced Hodgkin's disease. Ann. Intern. Med. 1970, 73, 881–895. [Google Scholar] [CrossRef]
- DeVita, V.T. Jr.; Simon, R.M.; Hubbard, S.M.; Young, R.C.; Berard, C.W.; Moxley, J.H. 3rd; Frei, E. 3rd; Carbone, P.P.; Canellos, G.P. Curability of advanced Hodgkin's disease with chemotherapy. Long-term follow-up of MOPP-treated patients at the National Cancer Institute. Ann. Intern. Med. 1980, 92, 587–595. [Google Scholar] [CrossRef]
- Singh, S.P.; Gao, Y.; Singh, L.D.; Kunapuli, S.P.; Ravindra, R. Role of microtubules in glucose uptake by C6 glioma cells. Pharmacol Toxicol 1998, 83, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.; Batteux, F.; Nicco, C.; Chereau, C.; Laurent, A.; Guillevin, L.; Weill, B.; Goldwasser, F. Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. Int J Cancer 2006, 119, 41–48. [Google Scholar] [CrossRef]
- Babior, B.M. NADPH oxidase. Curr Opin Immunol 2004, 16, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Diebold, B.A.; Fowler, B.; Lu, J.; Dinauer, M.C.; Bokoch, G.M. Antagonistic cross-talk between Rac and Cdc42 GTPases regulates generation of reactive oxygen species. J Biol Chem 2004, 279, 28136–28142. [Google Scholar] [CrossRef] [PubMed]
- Zang, M.; Waelde, C.A.; Xiang, X.; Rana, A.; Wen, R.; Luo, Z. Microtubule integrity regulates Pak leading to Ras-independent activation of Raf-1. insights into mechanisms of Raf-1 activation. J Biol Chem 2001, 276, 25157–25165. [Google Scholar] [CrossRef] [PubMed]
- Hadzic, T.; Aykin-Burns, N.; Zhu, Y.; Coleman, M.C.; Leick, K.; Jacobson, G.M.; Spitz, D.R. Paclitaxel combined with inhibitors of glucose and hydroperoxide metabolism enhances breast cancer cell killing via H2O2-mediated oxidative stress. Free Radic Biol Med 2010, 48, 1024–1033. [Google Scholar] [CrossRef]
- Magda, D.; Lepp, C.; Gerasimchuk, N.; Lee, I.; Sessler, J.L.; Lin, A.; Biaglow, J.E.; Miller, R.A. Redox cycling by motexafin gadolinium enhances cellular response to ionizing radiation by forming reactive oxygen species. Int J Radiat Oncol Biol Phys 2001, 51, 1025–1036. [Google Scholar] [CrossRef]
- Xu, S.; Zakian, K.; Thaler, H.; Matei, C.; Alfieri, A.; Chen, Y.; Koutcher, J.A. Effects of Motexafin gadolinium on tumor metabolism and radiation sensitivity. Int J Radiat Oncol Biol Phys 2001, 49, 1381–1390. [Google Scholar] [CrossRef]
- Peng, X. Ali, T., Combination therapy for cancer using ROS-activated prodrugs and ROS-amplifying therapeutics. Patent Application 2024, US. provisional patent No. 63/480 347; (Atty. File No. 020871-0011-WO01),
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




