Submitted:
02 May 2024
Posted:
06 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
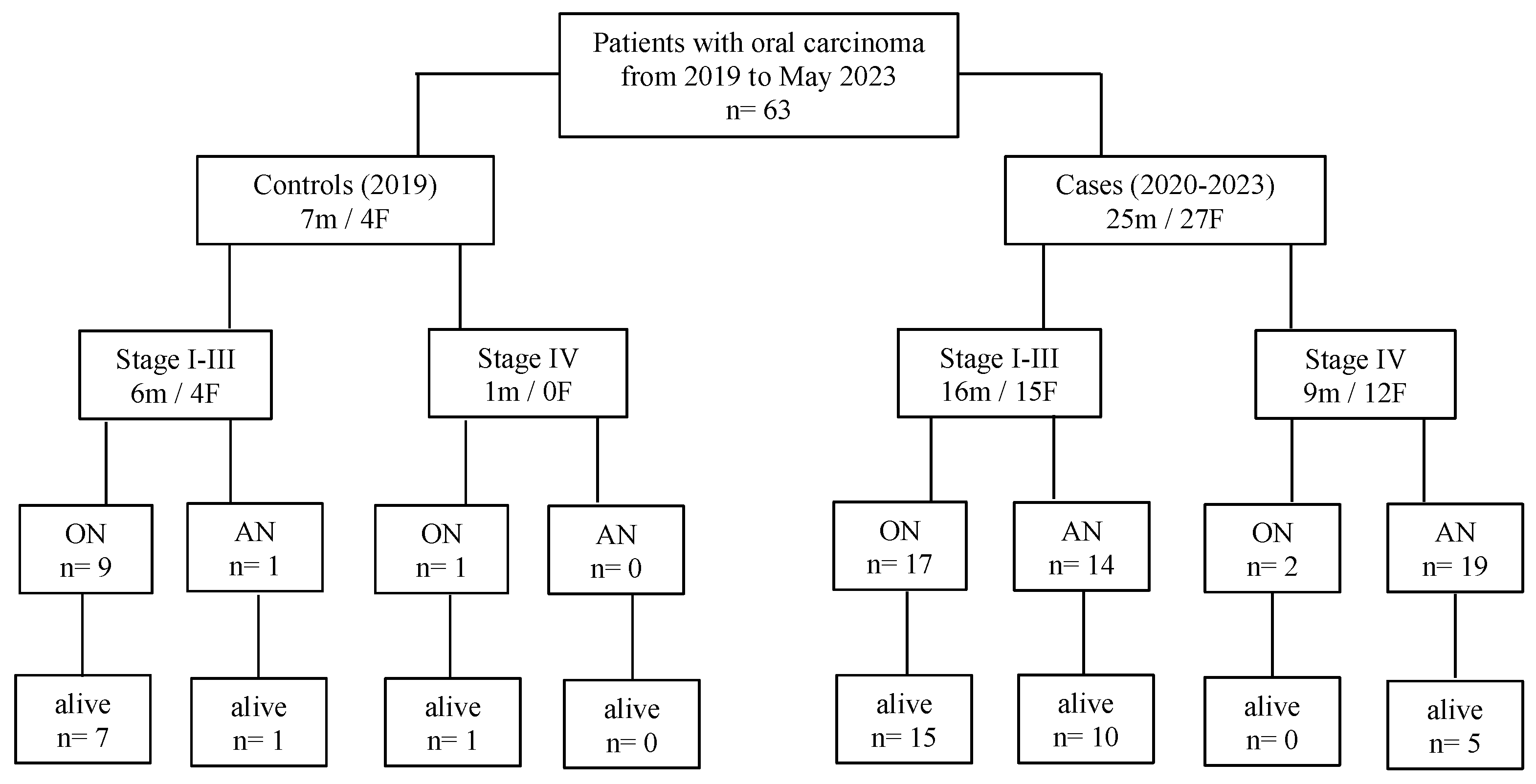
2.1. Selection of Patients
2.2. Measurements
2.3. Dietary Assessment and Support
2.4. Statistical Analysis
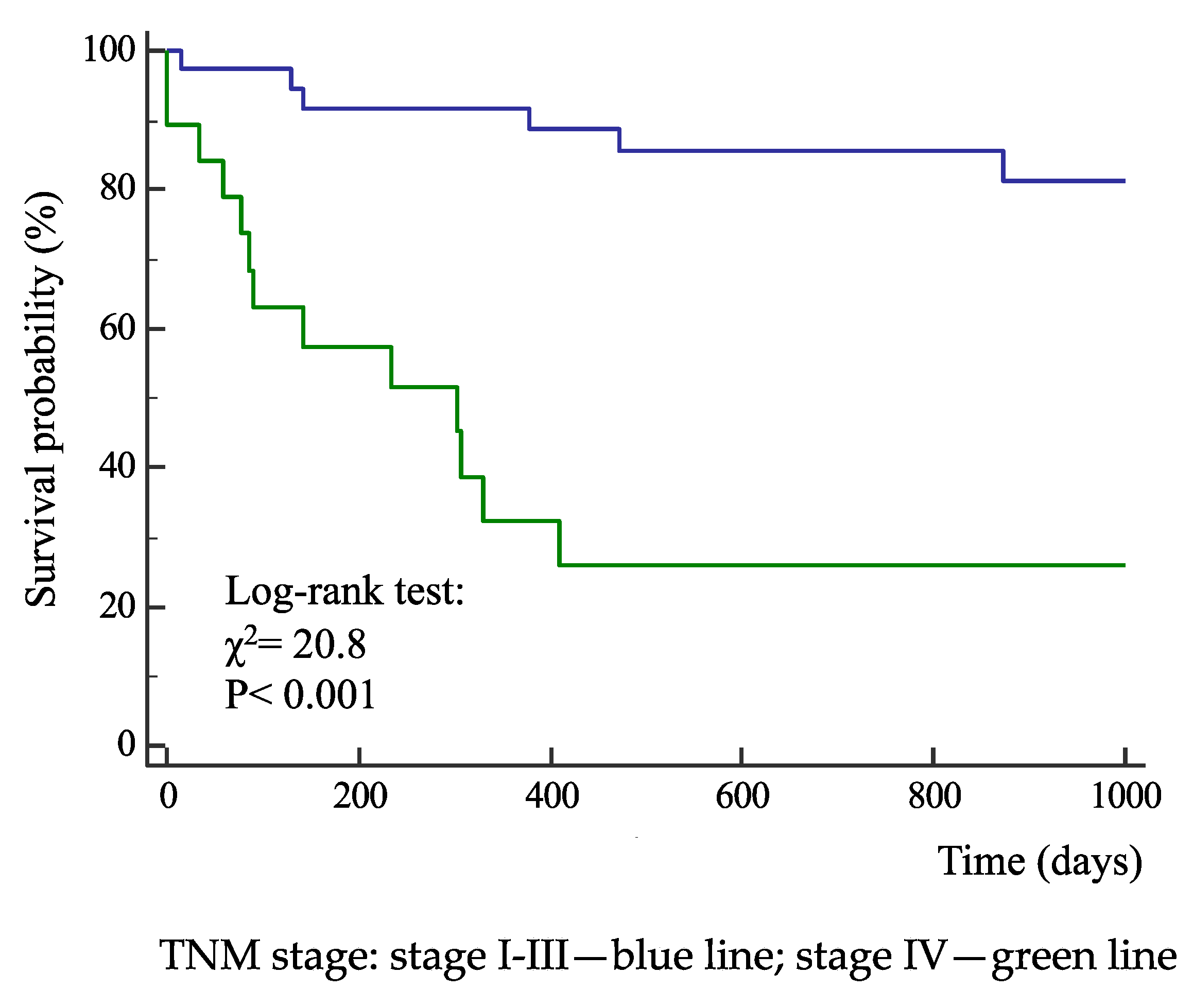
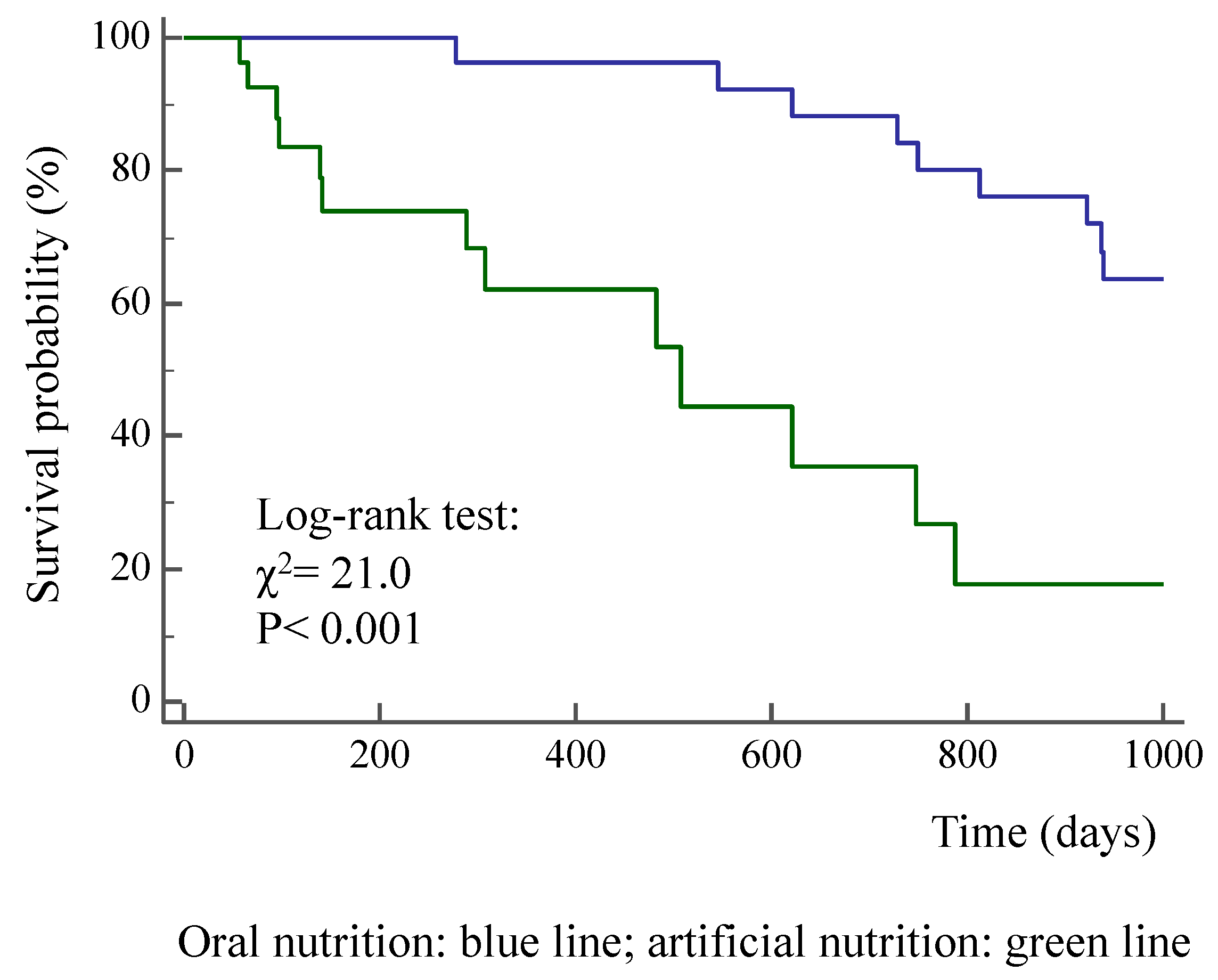
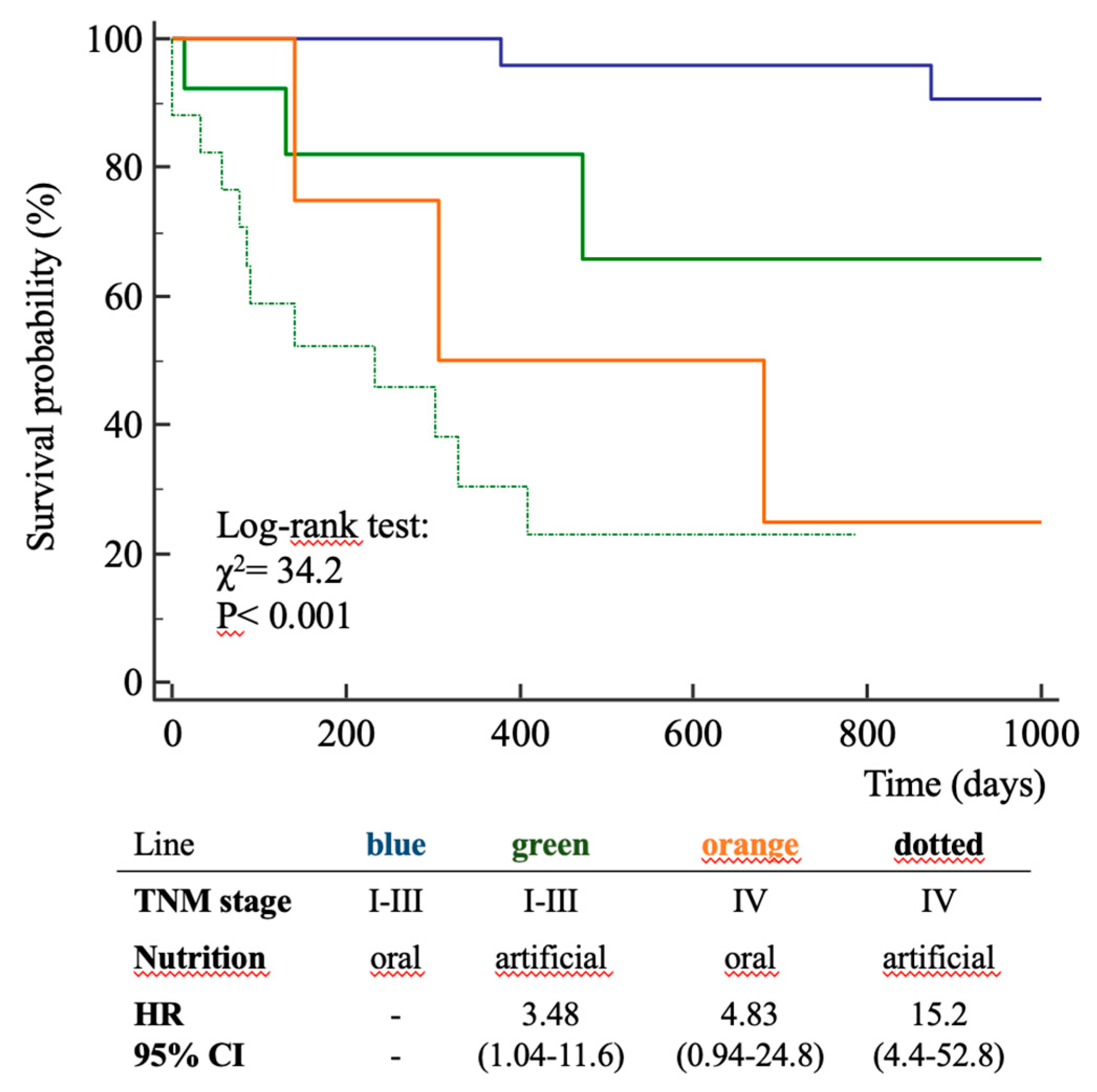
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer. World Health Organization. Global cancer observatory. URL:https://gco.iarc.fr/ (10/04/2024).
- Istituto Superiore di Sanità, Sistema Nazionale Linee Guida, aprile 2022. URL: https://www.iss.it/documents/20126/8403839/LG493-AIOM-Head-and-neck (10/04/2024).
- Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al., GLIM Core Leadership Committee; GLIM Working Group. GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. Clin Nutr. 2019; 38:1e9. [CrossRef]
- Ravasco P, Monteiro-Grillo I, Marques Vidal P, Camilo ME. Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 2005; 27(8):659-68. [CrossRef]
- Matsui R, Rifu K, Watanabe J, Inaki N, Fukunaga T. Impact of malnutrition as defined by the GLIM criteria on treatment outcomes in patients with cancer: A systematic review and meta-analysis. Clin Nutr. 2023; 42(5):615-624. [CrossRef]
- Pressoir M, Desné S, Berchery D, Rossignol G, Poiree B, Meslier M, Traversier S, Vittot M, Simon M, Gekiere JP, Meuric J, Serot F, Falewee MN, Rodrigues I, Senesse P, Vasson MP, Chelle F, Maget B, Antoun S, Bachmann P. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br J Cancer 2010; 102(6):966-71. [CrossRef]
- Martinovic D, Tokic D, Puizina Mladinic E, Usljebrka M, Kadic S, Lesin A, Vilovic M, Lupi-Ferandin S, Ercegovic S, Kumric M, Bukic J, Bozic J. Nutritional Management of Patients with Head and Neck Cancer-A Comprehensive Review. Nutrients 2023; 15(8):1864. [CrossRef]
- Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hütterer E, Isenring E, Kaasa S, Krznaric Z, Laird B, Larsson M, Laviano A, Mühlebach S, Muscaritoli M, Oldervoll L, Ravasco P, Solheim T, Strasser F, de van der Schueren M, Preiser JC. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017; 36(1):11-48. [CrossRef]
- Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, Erickson N, Laviano A, Lisanti MP, Lobo DN, McMillan DC, Muscaritoli M, Ockenga J, Pirlich M, Strasser F, de van der Schueren M, Van Gossum A, Vaupel P, Weimann A. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017; 36(5):1187-1196. [CrossRef]
- Caccialanza R, Cotogni P, Cereda E, Bossi P, Aprile G, Delrio P, Gnagnarella P, Mascheroni A, Monge T, Corradi E, Grieco M, Riso S, De Lorenzo F, Traclò F, Iannelli E, Beretta GD, Zanetti M, Cinieri S, Zagonel V, Pedrazzoli P. Nutritional Support in Cancer patients: update of the Italian Intersociety Working Group practical recommendations. J Cancer 2022;13(9):2705-2716. [CrossRef]
- Langius JA, Zandbergen MC, Eerenstein SE, van Tulder MW, Leemans CR, Kramer MH, Weijs PJ. Effect of nutritional interventions on nutritional status, quality of life and mortality in patients with head and neck cancer receiving (chemo)radiotherapy: a systematic review. Clin Nutr. 2013; 32:671-8. [CrossRef]
- Muscaritoli M, Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Hütterer E, Isenring E, Kaasa S, Krznaric Z, Laird B, Larsson M, Laviano A, Mühlebach S, Oldervoll L, Ravasco P, Solheim TS, Strasser F, de van der Schueren M, Preiser JC, Bischoff SC. ESPEN practical guideline: Clinical Nutrition in cancer. Clin Nutr. 2021; 40(5):2898-2913. [CrossRef]
- Hegazi R, Miller A, Sauer A. Evolution of the diagnosis of malnutrition in adults: a primer for clinicians. Front Nutr. 2024; 6;11:1169538. [CrossRef]
- Di Sebastiano, K.M., Mourtzakis M. A critical evaluation of body composition modalities used to assess adipose and skeletal muscle tissue in cancer. Appl Physiol Nutr Metab 2012; 37:811–821. [CrossRef]
- de Bree R, Meerkerk CDA, Halmos GB, Mäkitie AA, Homma A, Rodrigo JP, López F, Takes RP, Vermorken JB, Ferlito A. Measurement of sarcopenia in head and neck cancer patients and its association with frailty. Front Oncol. 2022; 12;12:884988. [CrossRef]
- Kuriyan, R. Body composition techniques. Indian J Med Res. 2018; 148(5):648-658. [CrossRef]
- Brierley JD, Gospodarowicz MK, Wittekind C, editors. The TNM classification of malignant tumors. 8. Wiley 2017.
- Guigoz Y, Vellas B, Garry PJ. Mini Nutritional Assessment: a practical assessment tool for grading the nutritional state of elderly patients. Facts Res Gerontol 1994: 12–13.
- Buscemi S, Blunda G, Maneri R et al. Bioelectrical characteristics of type 1 and type 2 diabetic subjects with reference to body water compartments. Acta Diabetol. 1998; 35: 220–223. [CrossRef]
- Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin J-C, Pujol S, Bauer C, Jennings D, Fennessy F.M, Sonka M, Buatti J, Aylward S.R, Miller J.V, Pieper S, Kikinis R.3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn Reson Imaging 2012; 30:1323-41. [CrossRef]
- Reddon JR, Stefanyk WO, Gill DM, Renney C. Hand dynamometer: effects of trials and sessions. Percept Mot Skills. 1985; 61:1195-8.
- Talwar B, Donnelly R, Skelly R, Donaldson M. Nutritional management in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016; 130(S2):S32-S40. [CrossRef]
- Wendrich AW, Swartz JE, Bril SI, Wegner I, de Graeff A, Smid EJ, de Bree R, Pothen AJ. Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol. 2017; 71:26-33. [CrossRef]
- Buscemi S, Blunda G, Maneri R, Verga S. Bioelectrical characteristics of type 1 and type 2 diabetic subjects with reference to body water compartments. Acta Diabetol. 1998; 35:220. [CrossRef]
- Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008; 9:629-35.
- Fernández-Jiménez R, García-Rey S, Roque-Cuéllar MC, Fernández-Soto ML, García-Olivares M, Novo-Rodríguez M, González-Pacheco M, Prior-Sánchez I, Carmona-Llanos A, Muñoz-Jiménez C, Zarco-Rodríguez FP, Miguel-Luengo L, Boughanem H, García-Luna PP, García-Almeida JM. Ultrasound Muscle Evaluation for Predicting the Prognosis of Patients with Head and Neck Cancer: A Large-Scale and Multicenter Prospective Study. Nutrients. 2024; 16:387. [CrossRef]
- Swartz JE, Pothen AJ, Wegner I, Smid EJ, Swart KM, de Bree R, Leenen LP, Grolman W. Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol. 2016; 62:28-33. [CrossRef]
- Bril SI, van Beers MA, Chargi N, Carrillo Minulina N, Smid EJ, Dankbaar JW, de Bree R. Skeletal muscle mass at C3 is a strong predictor for skeletal muscle mass at L3 in sarcopenic and non-sarcopenic patients with head and neck cancer. Oral Oncol. 2021; 122:105558. [CrossRef]
- Zwart AT, Becker JN, Lamers MJ, Dierckx RAJO, de Bock GH, Halmos GB, van der Hoorn A. Skeletal muscle mass and sarcopenia can be determined with 1.5-T and 3-T neck MRI scans, in the event that no neck CT scan is performed. Eur Radiol. 2021; 31:4053-4062.
- Belavý DL, Miokovic T, Armbrecht G, Felsenberg D. Hypertrophy in the cervical muscles and thoracic discs in bed rest? J Appl Physiol 2013; 115:586-96.
- Comelli A, Bignardi S, Stefano A, Russo G, Sabini MG, Ippolito M, Yezzi A. Development of a new fully three-dimensional methodology for tumours delineation in functional images. Comput. Biol. Med. 2020; 120:103701. [CrossRef]
- Buscemi S, Batsis JA, Parrinello G, Massenti FM, Rosafio G, Sciascia V, Costa F, Pollina Addario S, Mendola S, Barile AM, Maniaci V, Rini N, Caimi G. Nutritional predictors of mortality after discharge in elderly patients on a medical ward. Eur. J. Clin Invest. 2016; 46:609-18. [CrossRef]
- Van Dijk BA, Gatta G, Capocaccia R, Pierannunzio D, Strojan P, Licitra L; RARECARE Working Group. Rare cancers of the head and neck area in Europe. Eur. J. Cancer. 2012; 48:783-96. [CrossRef]
| Oral Cancer Stage | |||
|---|---|---|---|
| I-III n= 41 |
IV n= 22 |
Pa | |
| Males (%) | 53.7 | 45.5 | 0.54 |
| Age (years) | 65 ± 17 | 64 ± 11 | 0.85 |
| Actual survival from surgery (days) | 764 ± 465 | 317 ± 360 | < 0.001 |
| All-cause death (%) | 19.5 | 72.7 | < 0.001 |
| Nutrition (%): | |||
| oral | 63.4 | 13.6 | |
| artificial | 36.6 | 86.4 | < 0.001 |
| Body weight (kg) | 68.0 ± 17.5 | 60.3 ± 13.2 | 0.07 |
| Body mass index (kg/m2) | 25.3 ± 5.4 | 22.2 ± 5.8 | 0.06 |
| Bioimpedance analysis: | n=11 | n= 12 | |
| resistance (Ohm) | 641 ± 114 | 544 ± 142 | 0.08 |
| reactance (Ohm) | 65 ± 21 | 44 ± 16 | < 0.01 |
| phase angle (°) | 5.7 ± 1.1 | 4.5 ± 0.9 | < 0.01 |
| fat mass (%) | 26.6 ± 10.3 | 17.5 ± 10.2 | 0.06 |
| fat-free mass (kg) | 44.2 ± 8.7 | 45.6 ± 8.4 | 0.70 |
| fat-free mass index (kg/m2) | 16.8 ± 2.3 | 16.8 ± 3.2 | 0.98 |
| Hand-grip test (kg): | n=12 | n= 14 | |
| right | 21.3 ± 9.7 | 23.3 ± 11.4 | 0.64 |
| left | 20.2 ± 10.2 | 21.1 ± 10.1 | 0.82 |
| Mini Nutritional Assessment: | |||
| risk of malnutrition | 47.1 | 38.9 | |
| malnutrition | 52.9 | 61.1 | 0.54 |
| Serum concentration of: | |||
| proteins (g/dl) | 6.1 ± 1.0 | 5.4 ± 0.8 | < 0.005 |
| albumin (g/dl) | 3.8 ± 0.6 | 3.3 ± 0.7 | < 0.01 |
| hemoglobin (g/dl) | 11.0 ± 2.3 | 10.2 ± 2.2 | 0.25 |
| creatinine (mg/dl) | 0.74 ± 0.24 | 0.69 ± 0.42 | 0.61 |
| Mean ± SD; aunpaired Student’s t-test or χ2 when appropriate. | |||
| Nutrition | |||
|---|---|---|---|
| Oral n= 29 |
Artificial n= 34 |
Pa | |
| Males (%) | 65.5 | 38.2 | < 0.05 |
| Age (years) | ± | ± | |
| Actual survival from surgery (days) | 994 ± 353 | 289 ± 299 | < 0.001 |
| All-cause death (%) | 20.7 | 52.9 | < 0.01 |
| TNM stage I-III (%) | 89.7 | 44.4 | < 0.001 |
| Body mass index (kg/m2) | 26.4 ± 5.7 | 22.5 ± 5.1 | < 0.01 |
| Bioimpedance analysis: | n= 6 | n= 17 | |
| phase angle (°) | 5.9 ± 0.9 | 4.8 ± 1.1 | < 0.05 |
| fat-free mass index (kg/m2) | 17.4 ± 2.5 | 16.6 ± 2.8 | 0.53 |
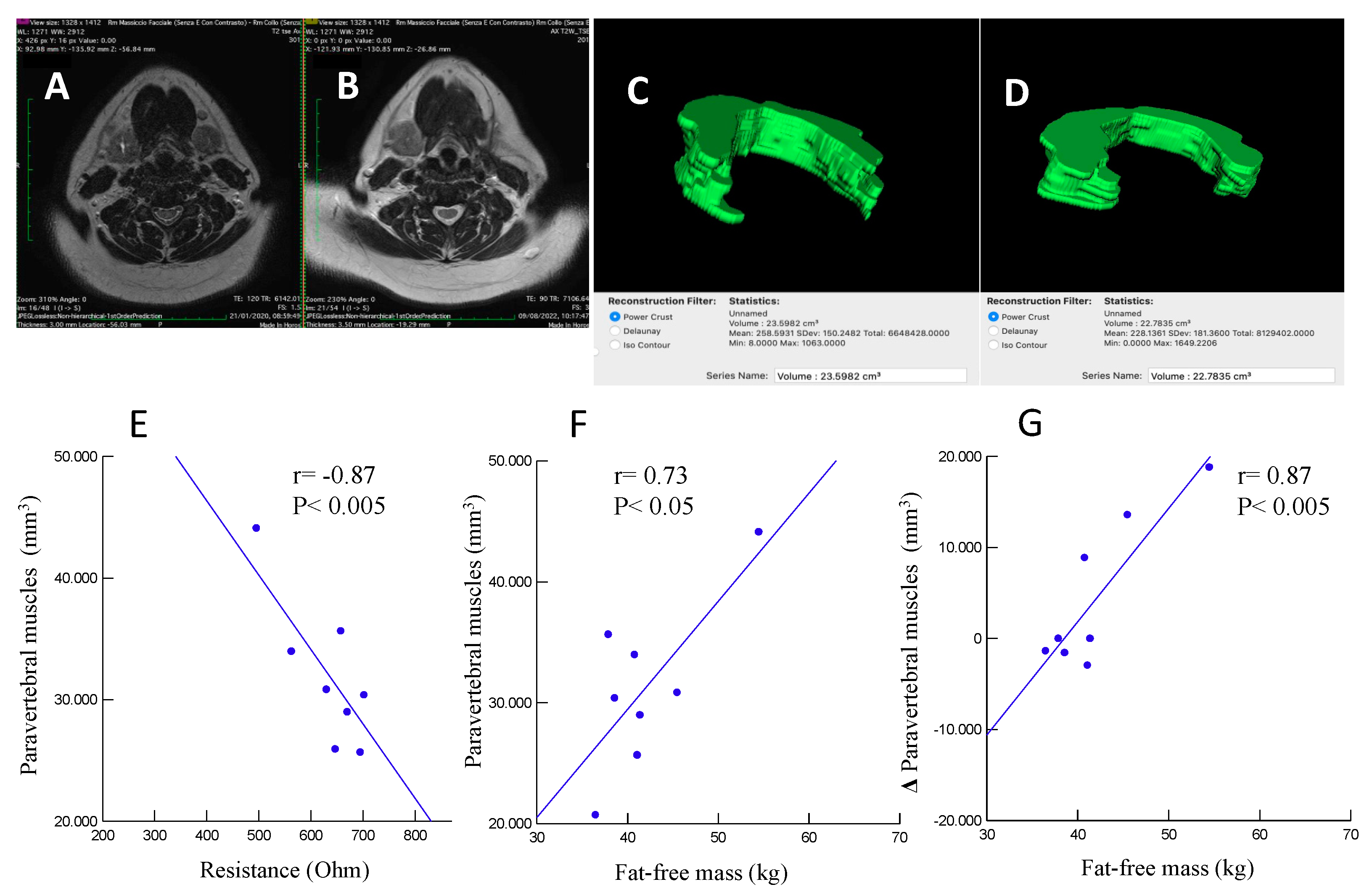
| MRI_C3 (mm3): | n= | n= | |
| before surgery | 35586 ± 5063 | 28728 ± 6151 | < 0.05 |
| after (3-12 months) surgery | 44822 ± 10685 | 26088 ± 5123 | < 0.001 |
| Δ MRI_C3 | 9236 ± 7313 | -2640 ± 4184 | < 0.001 |
| Serum creatinine (mg/dl) | 0.79 ± 0.23 | 0.67 ± 0.35 | 0.14 |
| Mean ± SD; aunpaired Student’s t-test or χ2 when appropriate. MRI_C3, volumetric magnetic resonance imaging of posterior paravertebral muscles at the C3 level. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





