1. Introduction
Magnesium ions (Mg2+), the fourth most abundant element in the human body and the second most prevalent cation in cellular environments after potassium ions, actively participate in vital physiological processes. They regulate enzymatic reactions involved in substance synthesis, influencing cell growth, differentiation, energy metabolism, excitability transmission in neurons, and cardiac excitability. Approximately 30%-50% of dietary magnesium is absorbed through the gut. It is facilitated by magnesium ion transport proteins, with normal plasma magnesium concentrations ranging between 0.75 and 0.85 mmol/L. Factors such as dietary intake, renal excretion, and skeletal muscle storage can impact magnesium homeostasis. As a calcium ion antagonist, magnesium ion deficiency in neurons leads to increased activity of N-methyl-D-aspartic acid receptors (NMDA), causing excessive influx of calcium ions, reactive oxygen species (ROS) production and mitochondrial damage. This heightened neuronal excitability is implicated in various diseases, including Parkinson’s disease, migraine, Alzheimer’s disease, anxiety, and depression. Epidemiological studies suggest that insufficient magnesium intake may increase the risk of cancer. In 1969, Bois et al. successfully induced thymic lymphoma in rats through a magnesium-deficient diet. In 1989, Kurata et al. confirmed in mice that magnesium supplementation in the diet effectively reduced the incidence of liver cancer, while a magnesium-deficient dietcould induce tumor formation. A higher magnesium intake in the diet (400 mg/day) has been shown to reduce the incidence of colorectal cancer in postmenopausal women, and elevated blood magnesium levels are negatively correlated with the risk of colorectal cancer in females. Moreover, recent research by Hess et al. , published in Cell, indicated that mice fed a magnesium-deficient diet exhibit significantly reduced efficacy of chimeric antigen receptor T-cell immunotherapy (CAR-T) against tumors, and B-cell lymphoma patients with lower serum magnesium levels have a shorter survival period.
As a cofactor for DNA repair enzymes, magnesium plays a crucial role in maintaining genomic stability and regulating cell proliferation, differentiation, and apoptosis. Magnesium deficiency may compromise DNA stability, contributing to tumor initiation and poor prognosis[
7,
8]. In vitro, Liu’s team reported that magnesium glycinate inhibits liver cancer cell proliferation by blocking the mitogen-activated protein kinase (MAPK) signaling pathway. Additionally, magnesium supplementation can inhibit the transcription of genes related to liver cancer cell proliferation by dephosphorylating p-Smad2/3 and blocking the transforming growth factor-β (TGF-β) signaling pathway.
Meanwhile, magnesium ions play a crucial regulatory role in mitochondrial function. According to the research conducted by Koning et al., magnesium sulfate can safeguard the mitochondrial respiratory chain, concurrently reducing the generation of ROS and inflammatory factors. Additionally, magnesium glycyrrhizinate is effective in preserving mitochondrial membrane potential and ultrastructure, while mitigating mitochondrial DNA (mtDNA) damage. Given the pivotal role of mitochondrial oxidative stress and mtDNA damage in tumor progression, magnesium ions hold promise as a novel target for cancer therapy. Further investigation into their protective effects on mitochondria and potential mechanisms in anticancer treatment is anticipated to offer new therapeutic avenues and hope for cancer patients.
This review aims to provide an overview of the mechanisms by which magnesium ions regulate tumors and discuss potential therapeutic applications, offering insights for clinical cancer treatment strategies.
2. Magnesium Transporters and Their Implications in Tumor Development
Magnesium (Mg) is predominantly distributed within cellular compartments, and its intracellular distribution and regulation rely heavily on magnesium ion transport proteins located on cellular and organelle membranes. Currently, major magnesium transporters include members of the transient receptor potential melastatin (TRPM) channel protein family, the human solute carrier (SLC) superfamily, magnesium transporter (MagT) proteins, cyclin M (CNNM) family proteins, and mitochondrial RNA splicing 2 (Mrs2) family genes.
2.1. TRPM Family
The TRPM family is widely expressed in numerous mammalian cells and is implicated in various biological functions, including redox reactions, inflammation, and insulin secretion. This channel protein family comprises numerous members with varying permeabilities to calcium ions (Ca2+) and magnesium ions (Mg2+). TRPM7 and TRPM9 play crucial roles in intestinal magnesium ion absorption. Moreover, the TRPM family participates in tumorigenesis by regulating the calcium ion balance, modulating cellular oxidative phosphorylation levels and influencing reactive oxygen species (ROS) generation within mitochondria.
In prostate cancer, the expression of TRPM8 and TRPM2 is significantly greater in tumor cells than in normal cells, whereas in lung cancer, TRPM5 upregulation is predominant. Additionally, the TRPM family plays a significant role in various cancers, such as breast cancer, pancreatic cancer, and melanoma, and is a potential target for antitumor therapies[
14,
19]. TRPM family may provide insights into its potential as a therapeutic target for combating tumorigenesis.
2.2. SLC Family
The SLC family comprises crucial membrane transport proteins on the cell membrane and consists of 52 subfamilies with more than 400 members. These proteins are widely distributed on cell membranes and various organelle membranes and function as typical transmembrane proteins. The SLC41 protein family facilitates the transmembrane transport of various metal ions, glucose, lipids, neurotransmitters and drugs, thus playing a crucial role in the development of various diseases, including metabolic disorders, neurological diseases, and tumors.
The SLC41 family was discovered in 2003 and comprises SLC41A1, SLC41A2, and SLC41A3. The SLC41 family is capable of transmembrane transport of Mg
2+ and, along with other magnesium ion transporters, such as TRPM7, participates in the regulation of Mg
2+ homeostasis within the organism. Variations in the SLC41A1 gene within the PARK16 locus in Parkinson’s disease patients have a significant impact on the pathogenesis of Parkinson’s disease as well as on ion homeostasis and the function of dopamine neurons[
20,
22]. Additionally, the overexpression or downregulation of SLC41A1 is closely associated with the occurrence of preeclampsia and renal diseases. A study by Njiaju et al. reported the association between SLC41A2 and chemotherapeutic drug sensitivity, in which knocking down SLC41A2 significantly increased the sensitivity of tumor cells to paclitaxel. Conversely, high SLC41A3 expression is closely correlated with poor prognosis in hepatocellular carcinoma patients, leading to a significantly shorter survival period in patients with elevated SLC41A3 expression than in those with normal SLC41A3 expression. In summary, the SLC41 family participates in the onset of various diseases through the regulation of Mg
2+ homeostasis and has emerged as a potential target for drug therapy.
2.3. MagT Family
The MagT protein family primarily includes magnesium transporter 1 (MagT1), which is widely expressed in various human organs and tissues. It exhibits high similarity with human tumor suppressor candidate 3 (TUSC3). Both MagT1 and TUSC3 function in magnesium ion transport and protein N-terminal glycosylation. MagT1 was identified as a crucial target gene for X-linked immunodeficiency with magnesium defect (XMEN) disease. The absence of the MagT1 protein leads to impaired N-glycosylation of platelet glycoproteins, resulting in platelet dysfunction and accelerated arterial thrombosis formation. Simultaneously, the MagT1 protein regulates the intracellular magnesium ion concentration, influencing macrophage polarization and immune cell function.
MagT1 also plays a significant role in regulating the growth of tumor cells. Zheng et al. reported a close correlation between MagT1 expression and poor prognosis in patients with colorectal cancer, with patients expressing lower levels of MagT1 experiencing longer survival. In breast cancer research, Li et al. discovered that downregulation of MagT1 protein expression significantly inhibited the progression of breast cancer by suppressing Ki67. Additionally, MagT1 has been identified as an essential gene for cervical cancer cell proliferation, and low MagT1 expression inhibits the growth of cervical cancer cells and promotes cell apoptosis.
2.4. CNNM Family
The CNNM protein family is primarily distributed in brain tissues, kidneys, and intestines. As a conserved Mg2+ transport protein, the main function of this protein family is to transport magnesium ions out of cells. Additionally, CNNM family proteins promote the absorption of Mg2+ in the intestines and kidneys. The mechanism of magnesium transport by the CNNM family is complex. According to Bai et al , CNNM family proteins were regulated by the phosphatase of regenerating liver (PRLS), which is closely associated with tumor metastasis. CNNM selectively binds to the TRPM7 protein, thereby facilitating Mg2+ uptake. The intricate role of the CNNM family in Mg2+ transport is crucial for maintaining the intracellular Mg2+ balance. Dysfunction of CNNM family proteins often leads to hereditary hypomagnesemia, resulting in conditions such as hypertension, pulmonary arterial hypertension, epilepsy, Parkinson’s disease, and other disorders. In colon cancer tissues, the expression of the CNNM4 protein is significantly decreased compared to that in normal tissues. A lower level of CNNM4 implies a greater degree of malignancy of the tumor.
2.5. MRS Family
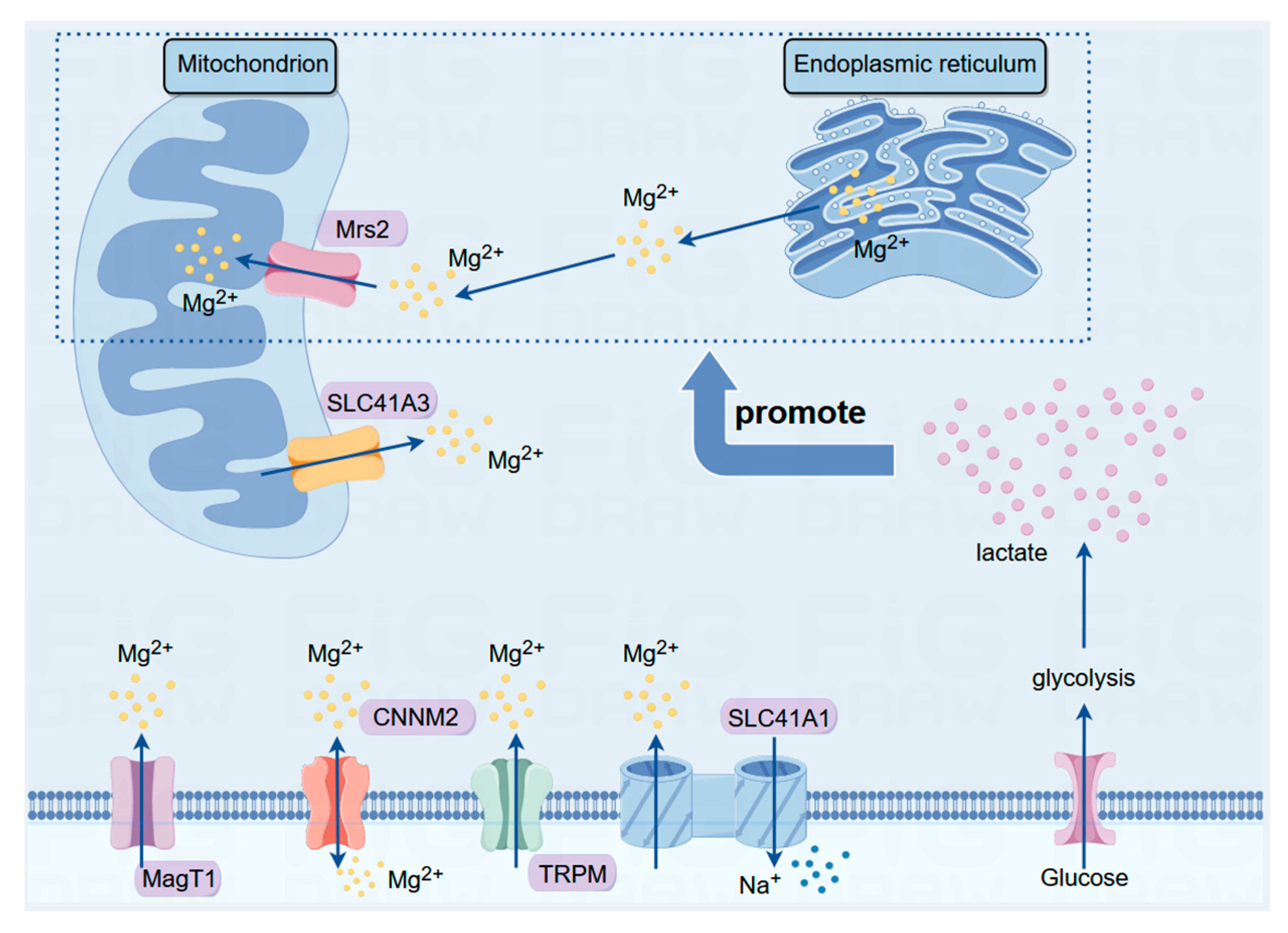
The Mrs2 protein is a magnesium ion transport protein located on the mitochondrial membrane. When stimulated by the glycolysis end product lactate, Mrs2 transports magnesium ions stored in the endoplasmic reticulum to the mitochondria. The spatiotemporal changes in magnesium ions within cells induced by Mrs2 result in reduced mitochondrial oxygen consumption and increased reactive oxygen species (ROS) production. Using electron microscopy, He et al. discovered that in addition to magnesium ions, Mrs2 also serves as a nonselective channel for calcium ions and can permeate sodium and potassium ions. The well-known Warburg effect demonstrates that most tumor cells exist in a microenvironment characterized by lactate accumulation. Therefore, subcellular magnesium ion transport mediated by Mrs2 is likely to become a crucial “switch” in regulating tumor cell growth.
Furthermore, Mrs2 plays a significant role in regulating mitochondrial function and cellular metabolism. According to the latest research by Madaris et al., in mice induced with a classic Western diet (a high-sugar, high-fat, high-cholesterol diet), knocking out the mitochondrial Mrs2 channel effectively inhibited western diet-induced obesity, metabolic syndrome, and spontaneous tumor incidence. This difference may be due to the loss of Mrs2 leading to the reprogramming of systemic energy metabolism regulated by hypoxia-inducible factor-1α (HIF-1α) in the liver and adipose tissue, enhancing mitochondrial activity, reducing hepatic fat accumulation, and inducing browning of white adipose tissue.
Due to the unique structural location of Mrs2 and its crucial role in subcellular magnesium ion transport and mitochondrial metabolic functions, Mrs2 has great potential for elucidating the mechanism of action of magnesium ions against cancer. Mrs2 may serve as a new target for energy metabolism-related tumor therapy (
Figure 1).
3. The Impact of Magnesium Ions on Mitochondrial Function
The intracellular magnesium ion distribution is regulated primarily by various magnesium ion transporter families. Currently, Mrs2 and solute carrier family 41 member A3 (SLC41A3) are the main magnesium transporters on the mitochondrial membrane. In 2016, Mastrototaro et al. first observed that the overexpression of SLC41A3 in human embryonic kidney 293 (HEK293) cells led to a 60% increase in mitochondrial magnesium ion efflux compared to that in normal cells. SLC41A3 functions as a Na+/Mg2+ exchanger, and the efflux of magnesium ions depends not only on the sodium ion concentration but also on temperature. At a temperature of 16°C, the ion flow nearly disappears. Moreover, overexpression of SLC41A3 may deplete mitochondrial magnesium ions, thereby impairing cellular respiration and reducing mitochondrial ATP production. The endoplasmic reticulum and mitochondria are important storage sites for magnesium ions within cells, and another mitochondrial magnesium ion transporter, Mrs2, acts as a messenger facilitating magnesium ion transfer between these two organelles. Under lactate stimulation, Mrs2 can transport magnesium ions from the endoplasmic reticulum to the mitochondria, further stimulating mitochondrial ROS production .
The mitochondrial magnesium ion regulation system composed of SLC41A3 and Mrs2 allows cells to flexibly adjust magnesium ion levels within mitochondria under different physiological and environmental conditions to meet the metabolic needs of the cell. The functions and regulatory mechanisms of Mrs2 and SLC41A3 provide essential safeguards for maintaining cellular energy balance and normal metabolism, underscoring their irreplaceable roles in cell survival and functional maintenance.
3.1. The Impact of Magnesium Ions on Mitochondrial Energy Metabolism
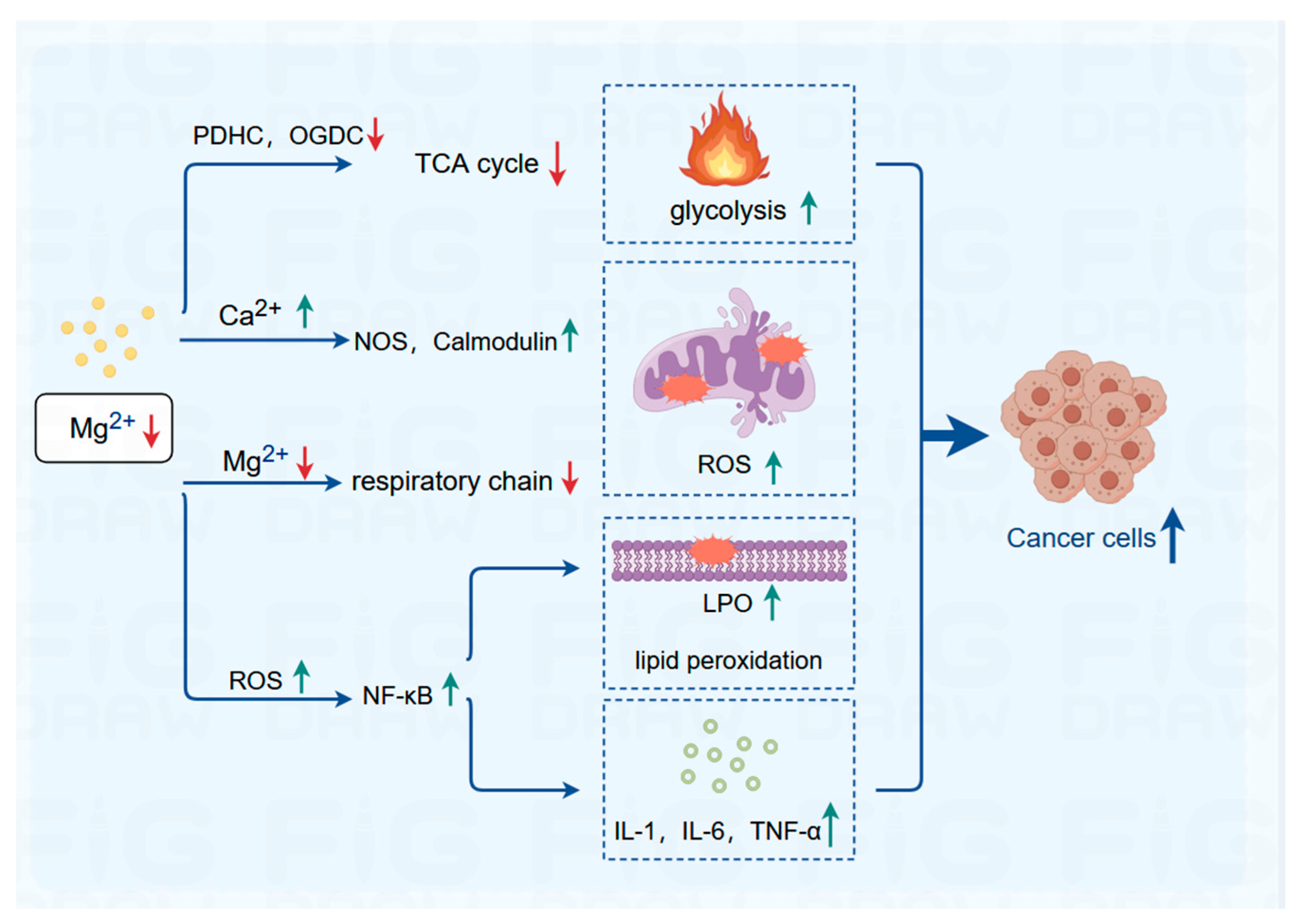
In 2016, Ha et al. discovered that magnesium-rich seawater effectively increases the activity of mitochondrial enzymes and the expression of mitochondrial DNA (mtDNA) in adipocytes, suggesting a potential regulatory and antiobesity role of magnesium ions. As a crucial co-factor for many key enzymes in glucose metabolism pathways, magnesium ions play a significant regulatory role in the energy metabolism of mitochondria. Free magnesium ions can indirectly stimulate the dephosphorylation of pyruvate dehydrogenase (PDH) by pyruvate dehydrogenase phosphatase, thereby upregulating the activity of the pyruvate decarboxylase component within the pyruvate dehydrogenase complex (PDHC). This, in turn, affects ATP synthesis by enhancing the activity of the rate-limiting enzyme in the tricarboxylic acid cycle, the α-ketoglutarate dehydrogenase complex (OGDC), ultimately regulating cellular energy metabolism.
3.2. The Impact of Magnesium Ions on Oxidative Stress
In both hypertonic and hypotonic environments, magnesium ions and potassium ions play a protective role in the permeability of the mitochondrial outer membrane, effectively safeguarding mitochondrial activity. Conversely, magnesium deficiency inhibits the magnesium transporter protein on the mitochondrial membrane (Mrs2) . As a result, the magnesium ion content within the mitochondria decreases, leading to reduced electron transport chain activity, increased mitochondrial ROS, inhibition of key antioxidant enzymes, and subsequent initiation of oxidative stress . Simultaneously, magnesium ions deficiency, which antagonize calcium ions, increases intracellular calcium overload, activating numerous calcium-dependent kinases and proteins, such as nitric oxide synthase and calcium-dependent calcium-binding proteins, further augmenting ROS production. Additionally, magnesium deficiency-induced ROS overload activates transcription factors such as nuclear factor kappa-B (NF-κB), which induces lipid peroxidation and stimulates the secretion of proinflammatory cytokines, including interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α, triggering inflammatory responses and exacerbating ROS production. Supplementation with magnesium sulfate has been shown to effectively protect newborn neurons by preserving mitochondrial respiration and reducing ROS production and inflammation.
4. Relationships between Mitochondrial Function and Cancer
As the primary energy-producing organelle within cells, mitochondria play a crucial role in regulating energy metabolism, biosynthesis, and cellular life activities. The well-known Warburg effect suggests that tumor cells preferentially utilize glycolysis for glucose metabolism, indicating a potential association between mitochondrial dysfunction and the pathogenesis of cancer. Due to the limited energy provided by glycolysis, tumor cells require increased mitochondrial activity to meet their energy demands. Hasumi and Lang confirmed a substantial increase in mitochondrial quantity in malignant pheochromocytoma, accompanied by a significant increase in respiratory frequency compared to that in normal cells. Cannino et al. observed an increased number and enlarged volume of mitochondria in tumor cells, with notable structural differences from those of normal mitochondria, including swelling, shrinkage, and alterations in the outer membrane. These abnormalities further impact mitochondrial respiratory function and stimulate the accumulation of lactate.
Therefore, mitochondria have emerged as novel targets for cancer therapy. Antitumor drugs can inhibit cancer cell energy supply, promote apoptosis, and impede tumor proliferation and invasion by suppressing mitochondrial respiratory chain activity, inducing excessive ROS production, damaging mitochondrial DNA, and interfering with tumor cell metabolic pathways. Given the crucial role of magnesium ions in regulating mitochondrial function, magnesium ion therapy is expected to become a new “switch” in tumor treatment.
5. Prospects of Magnesium Ions and Their Transport Proteins in Cancer Therapy
Currently, magnesium ions have been employed as anti-tumor implant materials for interventional therapy. During degradation, magnesium generates magnesium hydroxide, hydrogen gas, and magnesium ions. Magnesium hydroxide alkalinizes the acidic tumor microenvironment, while hydrogen gas and magnesium ions exhibit significant anti-inflammatory and antioxidant effects.
An increase in the blood magnesium concentration can inhibit mitochondrial oxidative stress, restraining tumor cell proliferation and invasion. Additionally, magnesium ions can modulate the function of T cells, promoting the activation of immune functions and inhibiting tumor growth. However, the application of magnesium ions in clinical tumor treatment is currently quite limited, with most related research findings still occurring in the early stages of basic experiments and a considerable distance from clinical application. In recent years, researches on the regulation of mitochondrial structure and function by magnesium ions, which impact mitochondrial metabolism and oxidative stress, has gradually increased. Given the close association between tumor cell proliferation and invasion and mitochondrial dysfunction, magnesium ions and their crucial transporters are poised to become key “switches” in regulating tumor growth. Magnesium ions and their transporters may emerge as novel targets for tumor therapy.
6. Conclusions
In general, the dysfunction of mitochondria is closely associated with the occurrence and progression of tumors. Given the pivotal role of mitochondria as the “engine” in cellular energy metabolism, targeted regulation of mitochondrial function is poised to emerge as a novel direction in cancer therapy. The imbalance and inter-organelle trafficking of intracellular magnesium ions can affect mitochondrial function by influencing the generation of ROS, oxidative stress levels, stability of mtDNA, and the activity of key mitochondrial metabolic enzymes. Magnesium ion therapy holds promise as a novel therapeutic modality for metabolic tumors, offering advantages such as low toxicity and anti-inflammatory properties, thus exhibiting significant clinical application potential (
Figure 2).
Abbreviations
| ROS |
Reactive oxygen species |
| TRPM |
Transient receptor potential melastatin channel protein family |
| SLC |
Human solute carrier superfamily |
| MagT |
Magnesium transporter proteins |
| CNNM |
Cyclin M family proteins |
| Mrs2 |
Mitochondrial RNA splicing 2 family genes |
| PDHC |
Pyruvate dehydrogenase complex |
| OGDC |
Oxoglutarate dehydrogenase complex |
| NOS |
Nitricoxide synthase |
| LPO |
Lipid hydroperoxide |
| NF-κB |
Nuclear factor kappa-B |
| IL |
Interleukin |
| TNF |
Tumor necrosis factor |
Funding
This study was funded by grants from the Natural Science Foundation of Fujian, China (Grant No. 2018J0838, Ling Lin); the Natural Science Foundation of Fujian, China (Grant No. 2022J011036, Leyi Huang); and Startup Fund for scientific research, Fujian Medical University (Grant No. 2021QH1172, Leyi Huang).
Ethical Approval and Consent to Participate
This manuscript is a review article and does not involve a research protocol requiring approval from the relevant institutional review board or ethics committee.
Consent for Publication
Not applicable.
Availability of Data
Not applicable.
Authors’ Contributions and Materials
Conceptualization, HLY, QYL, and LL.; writing—review and editing, HLY, LRX, and CJX; supervision, QYL, and LL. All the authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors declare no conflicts of interest.
Acknowledgements
We would like to thank Weibin Lin, Yihan Shen, Shaoqing Zheng, Zeyu Wu, and Yiyang Wang from Fujian Medical University for their assistance.
References
- J.H. de Baaij, J.G. Hoenderop, and R.J. Bindels. Magnesium in man: implications for health and disease. Physiol Rev 2015; 95: 1-46. [CrossRef]
- D. Fiorentini, C. Cappadone, G. Farruggia, and C. Prata. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021; 13. [CrossRef]
- L. Huang, Study on the relationship between the changes of retinaldopamine neurons and magnesium ionsin 6-OHDA-induced Parkinsonian rats (Ed.)^(Eds.), Fujian Medical University, 2021, Fuzhou.
- P. Bois, E. Sandborn, and P. Messier. A study of thymic lymphosarcoma developing in magnesium-deficient rats. Cancer research 1969; 29: 763-75.
- J. Auwercx, P. Rybarczyk, P. Kischel, I. Dhennin-Duthille, D. Chatelain, H. Sevestre, et al. Mg Transporters in Digestive Cancers. Nutrients 2021; 13.
- J. Lötscher, A. Martí I Líndez, N. Kirchhammer, E. Cribioli, G. Giordano Attianese, M. Trefny, et al. Magnesium sensing via LFA-1 regulates CD8 T cell effector function. Cell 2022; 185: 585-602.e29. [CrossRef]
- S. Castiglioni, and J. Maier. Magnesium and cancer: a dangerous liason. Magnesium research 2011; 24: S92-100.
- U. Blaszczyk, and A. Duda-Chodak. Magnesium: its role in nutrition and carcinogenesis. Roczniki Panstwowego Zakladu Higieny 2013; 64: 165-71.
- Y. Liu, X. Li, Q. Zou, L. Liu, X. Zhu, Q. Jia, et al. [Inhibitory effect of magnesium cantharidate on human hepatoma SMMC-7721 cell proliferation by blocking MAPK signaling pathway]. Xi bao yu fen zi mian yi xue za zhi = Chinese journal of cellular and molecular immunology 2017; 33: 347-351.
- Y. Liu, Y. Xu, H. Ma, B. Wang, L. Xu, H. Zhang, et al. Hepatitis B virus X protein amplifies TGF-β promotion on HCC motility through down-regulating PPM1a. Oncotarget 2016; 7: 33125-35. [CrossRef]
- G. Koning, A. Leverin, S. Nair, L. Schwendimann, J. Ek, Y. Carlsson, et al. Magnesium induces preconditioning of the neonatal brain via profound mitochondrial protection. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2019; 39: 1038-1055.
- X. Wang, H. Zhu, J. Hu, H. Li, S. Guo, B. Chen, et al. Magnesium Isoglycyrrhizinate Reduces the Target-Binding Amount of Cisplatin to Mitochondrial DNA and Renal Injury through SIRT3. International journal of molecular sciences 2022; 23. [CrossRef]
- S. Reuter, S. Gupta, M. Chaturvedi, and B. Aggarwal. Oxidative stress, inflammation, and cancer: how are they linked? Free radical biology & medicine 2010; 49: 1603-16.
- T. Ciaglia, V. Vestuto, A. Bertamino, R. González-Muñiz, and I. Gómez-Monterrey. On the modulation of TRPM channels: Current perspectives and anticancer therapeutic implications. Frontiers in Oncology 2023; 12. [CrossRef]
- Y. Arancibia-Hernández, E. Hernández-Cruz, and J. Pedraza-Chaverri. Magnesium (Mg) Deficiency, Not Well-Recognized Non-Infectious Pandemic: Origin and Consequence of Chronic Inflammatory and Oxidative Stress-Associated Diseases. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 2023; 57: 1-23. [CrossRef]
- Y. Gao, and P. Liao. TRPM4 channel and cancer. Cancer letters 2019; 454: 66-69.
- Y. Chen, L. Liu, L. Xia, N. Wu, Y. Wang, H. Li, et al. TRPM7 silencing modulates glucose metabolic reprogramming to inhibit the growth of ovarian cancer by enhancing AMPK activation to promote HIF-1α degradation. Journal of experimental & clinical cancer research : CR 2022; 41: 44. [CrossRef]
- B. Miller. TRPM2 in Cancer. Cell calcium 2019; 80: 8-17.
- A. Hantute-Ghesquier, A. Haustrate, N. Prevarskaya, and V.y. Lehen’kyi. TRPM Family Channels in Cancer. Pharmaceuticals 2018; 11. [CrossRef]
- M. Schweigel-Röntgen, and M. Kolisek. SLC41 transporters--molecular identification and functional role. Current topics in membranes 2014; 73: 383-410.
- Y.T.K. Nguyen, H.T.T. Ha, T.H. Nguyen, and L.N. Nguyen. The role of SLC transporters for brain health and disease. Cell Mol Life Sci 2021; 79: 20. [CrossRef]
- T. Nemoto, H. Tagashira, T. Kita, S. Kita, and T. Iwamoto. Functional characteristics and therapeutic potential of SLC41 transporters. J Pharmacol Sci 2023; 151: 88-92. [CrossRef]
- J. Sahni, and A. Scharenberg. The SLC41 family of MgtE-like magnesium transporters. Molecular aspects of medicine 2013; 34: 620-8.
- U. Njiaju, E. Gamazon, L. Gorsic, S. Delaney, H. Wheeler, H. Im, et al. Whole-genome studies identify solute carrier transporters in cellular susceptibility to paclitaxel. Pharmacogenetics and genomics 2012; 22: 498-507. [CrossRef]
- Q. Li, D. Xiong, H. Wang, W. Jin, Y. Ma, and X. Fan. High Expression of SLC41A3 Correlates with Poor Prognosis in Hepatocellular Carcinoma. OncoTargets and therapy 2021; 14: 2975-2988. [CrossRef]
- H. Ding, Y. Li, M. Fang, J. Chen, L. Liu, Z. Lu, et al. Epigenetic activation of the TUSC3 gene as a potential therapy for XMEN disease. The Journal of allergy and clinical immunology 2023; 151: 1622-1633.e10. [CrossRef]
- S. Gotru, E. Mammadova-Bach, G. Sogkas, M. Schuhmann, K. Schmitt, P. Kraft, et al. MAGT1 Deficiency Dysregulates Platelet Cation Homeostasis and Accelerates Arterial Thrombosis and Ischemic Stroke in Mice. Arteriosclerosis, thrombosis, and vascular biology 2023; 43: 1494-1509. [CrossRef]
- M. Oh, J. Jang, and J. Lee. Polarization of THP-1-Derived Macrophage by Magnesium and MAGT1 Inhibition in Wound Healing. Archives of plastic surgery 2023; 50: 432-442. [CrossRef]
- K. Zheng, Q. Yang, L. Xie, Z. Qiu, Y. Huang, Y. Lin, et al. Overexpression of MAGT1 is associated with aggressiveness and poor prognosis of colorectal cancer. Oncology letters 2019; 18: 3857-3862. [CrossRef]
- L. Li, X. Zhang, Y. Li, B. Xiao, S. Pei, H. Jiang, et al. Transcription factor KLF16 activates MAGT1 to regulate the tumorigenesis and progression of breast cancer. International journal of molecular medicine 2022; 50. [CrossRef]
- J. Zhang, X. Yang, and H. Ni. Long non-coding RNA FLVCR1-AS1 functions as a ceRNA to aggravate cervical cancer cell growth by the miR-381-3p/MAGT1 axis. Archives of gynecology and obstetrics 2022; 306: 2093-2103. [CrossRef]
- C. Bi, X. Zhang, Y. Chen, Y. Dong, Y. Shi, Y. Lei, et al. MAGT1 is required for HeLa cell proliferation through regulating p21 expression, S-phase progress, and ERK/p38 MAPK MYC axis. Cell cycle (Georgetown, Tex.) 2021; 20: 2233-2247. [CrossRef]
- Y. Funato, and H. Miki. Molecular function and biological importance of CNNM family Mg2+ transporters. J Biochem 2019; 165: 219-225. [CrossRef]
- Z. Bai, J. Feng, G.A.C. Franken, N. Al’Saadi, N. Cai, A.S. Yu, et al. CNNM proteins selectively bind to the TRPM7 channel to stimulate divalent cation entry into cells. PLoS Biol 2021; 19: e3001496. [CrossRef]
- Y. Hirata, Y. Funato, Y. Takano, and H. Miki. Mg2+-dependent interactions of ATP with the cystathionine-β-synthase (CBS) domains of a magnesium transporter. J Biol Chem 2014; 289: 14731-9. [CrossRef]
- Y. Funato, D. Yamazaki, S. Mizukami, L. Du, K. Kikuchi, and H. Miki. Membrane protein CNNM4-dependent Mg2+ efflux suppresses tumor progression. The Journal of clinical investigation 2014; 124: 5398-410. [CrossRef]
- M. Li, Y. Li, Y. Lu, J. Li, X. Lu, Y. Ren, et al. Molecular basis of Mg permeation through the human mitochondrial Mrs2 channel. Nature communications 2023; 14: 4713. [CrossRef]
- Z. He, Q. Chen, W. He, J. Cao, S. Yao, Q. Huang, et al. Hepatocellular carcinoma subtypes based on metabolic pathways reveals potential therapeutic targets. Frontiers in oncology 2023; 13: 1086604. [CrossRef]
- M. Liberti, and J. Locasale. The Warburg Effect: How Does it Benefit Cancer Cells? Trends in biochemical sciences 2016; 41: 211-218.
- T. Madaris, M. Venkatesan, S. Maity, M. Stein, N. Vishnu, M. Venkateswaran, et al. Limiting Mrs2-dependent mitochondrial Mg uptake induces metabolic programming in prolonged dietary stress. Cell reports 2023; 42: 112155. [CrossRef]
- L. Mastrototaro, A. Smorodchenko, J. Aschenbach, M. Kolisek, and G. Sponder. Solute carrier 41A3 encodes for a mitochondrial Mg(2+) efflux system. Scientific reports 2016; 6: 27999. [CrossRef]
- I. Pilchova, K. Klacanova, Z. Tatarkova, P. Kaplan, and P. Racay. The Involvement of Mg in Regulation of Cellular and Mitochondrial Functions. Oxidative medicine and cellular longevity 2017; 2017: 6797460. [CrossRef]
- C. Daw, K. Ramachandran, B. Enslow, S. Maity, B. Bursic, M. Novello, et al. Lactate Elicits ER-Mitochondrial Mg Dynamics to Integrate Cellular Metabolism. Cell 2020; 183: 474-489.e17. [CrossRef]
- B. Ha, D. Moon, H. Kim, and Y. Shon. Magnesium and calcium-enriched deep-sea water promotes mitochondrial biogenesis by AMPK-activated signals pathway in 3T3-L1 preadipocytes. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2016; 83: 477-484. [CrossRef]
- A. Panov, and A. Scarpa. Independent modulation of the activity of alpha-ketoglutarate dehydrogenase complex by Ca2+ and Mg2+. Biochemistry 1996; 35: 427-32.
- V. Gorgoglione, D. Laraspata, G. La Piana, D. Marzulli, and N. Lofrumento. Protective effect of magnesium and potassium ions on the permeability of the external mitochondrial membrane. Archives of biochemistry and biophysics 2007; 461: 13-23. [CrossRef]
- M. Liu, and S. Dudley. Magnesium, Oxidative Stress, Inflammation, and Cardiovascular Disease. Antioxidants (Basel, Switzerland) 2020; 9.
- A. Mazur, J. Maier, E. Rock, E. Gueux, W. Nowacki, and Y. Rayssiguier. Magnesium and the inflammatory response: potential physiopathological implications. Archives of biochemistry and biophysics 2007; 458: 48-56. [CrossRef]
- C. Xie, X. Li, J. Zhu, J. Wu, S. Geng, and C. Zhong. Magnesium isoglycyrrhizinate suppresses LPS-induced inflammation and oxidative stress through inhibiting NF-κB and MAPK pathways in RAW264.7 cells. Bioorganic & medicinal chemistry 2019; 27: 516-524.
- Y. Wang, and G. Patti. The Warburg effect: a signature of mitochondrial overload. Trends in cell biology 2023; 33: 1014-1020. [CrossRef]
- H. Hasumi, M. Baba, Y. Hasumi, Y. Huang, H. Oh, R. Hughes, et al. Regulation of mitochondrial oxidative metabolism by tumor suppressor FLCN. Journal of the National Cancer Institute 2012; 104: 1750-64. [CrossRef]
- M. Lang, C. Vocke, M. Merino, L. Schmidt, and W. Linehan. Mitochondrial DNA mutations distinguish bilateral multifocal renal oncocytomas from familial Birt-Hogg-Dubé tumors. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2015; 28: 1458-69.
- G. Cannino, F. Ciscato, I. Masgras, C. Sánchez-Martín, and A. Rasola. Metabolic Plasticity of Tumor Cell Mitochondria. Frontiers in oncology 2018; 8: 333. [CrossRef]
- J. Winter, T. Yadav, and J. Rutter. Stressed to death: Mitochondrial stress responses connect respiration and apoptosis in cancer. Molecular cell 2022; 82: 3321-3332. [CrossRef]
- I. Jain, L. Zazzeron, R. Goli, K. Alexa, S. Schatzman-Bone, H. Dhillon, et al. Hypoxia as a therapy for mitochondrial disease. Science (New York, N.Y.) 2016; 352: 54-61. [CrossRef]
- B. Perillo, M. Di Donato, A. Pezone, E. Di Zazzo, P. Giovannelli, G. Galasso, et al. ROS in cancer therapy: the bright side of the moon. Experimental & molecular medicine 2020; 52: 192-203.
- C. Li, Y. Zhang, J. Liu, R. Kang, D. Klionsky, and D. Tang. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy 2021; 17: 948-960. [CrossRef]
- J. Lee, A. Yesilkanal, J. Wynne, C. Frankenberger, J. Liu, J. Yan, et al. Effective breast cancer combination therapy targeting BACH1 and mitochondrial metabolism. Nature 2019; 568: 254-258. [CrossRef]
- M. Chen, Y. Sun, H. Liu. Cell membrane biomimetic nanomedicines for cancer phototherapy. Interdiscip Med. 2023, 1, e20220012.
- R. Zan, H. Wang, W. Cai, J. Ni, B. Luthringer-Feyerabend, W. Wang, et al. Controlled release of hydrogen by implantation of magnesium induces P53-mediated tumor cells apoptosis. Bioactive materials 2022; 9: 385-396. [CrossRef]
- B. Xu, Y. Song, K. Yang, Y. Li, B. Chen, X. Liao, et al. Magnesium metal and its corrosion products: Promising materials for tumor interventional therapy. Journal of Magnesium and Alloys 2023; 11: 763-775. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).