Submitted:
05 May 2024
Posted:
06 May 2024
You are already at the latest version
Abstract
Keywords:
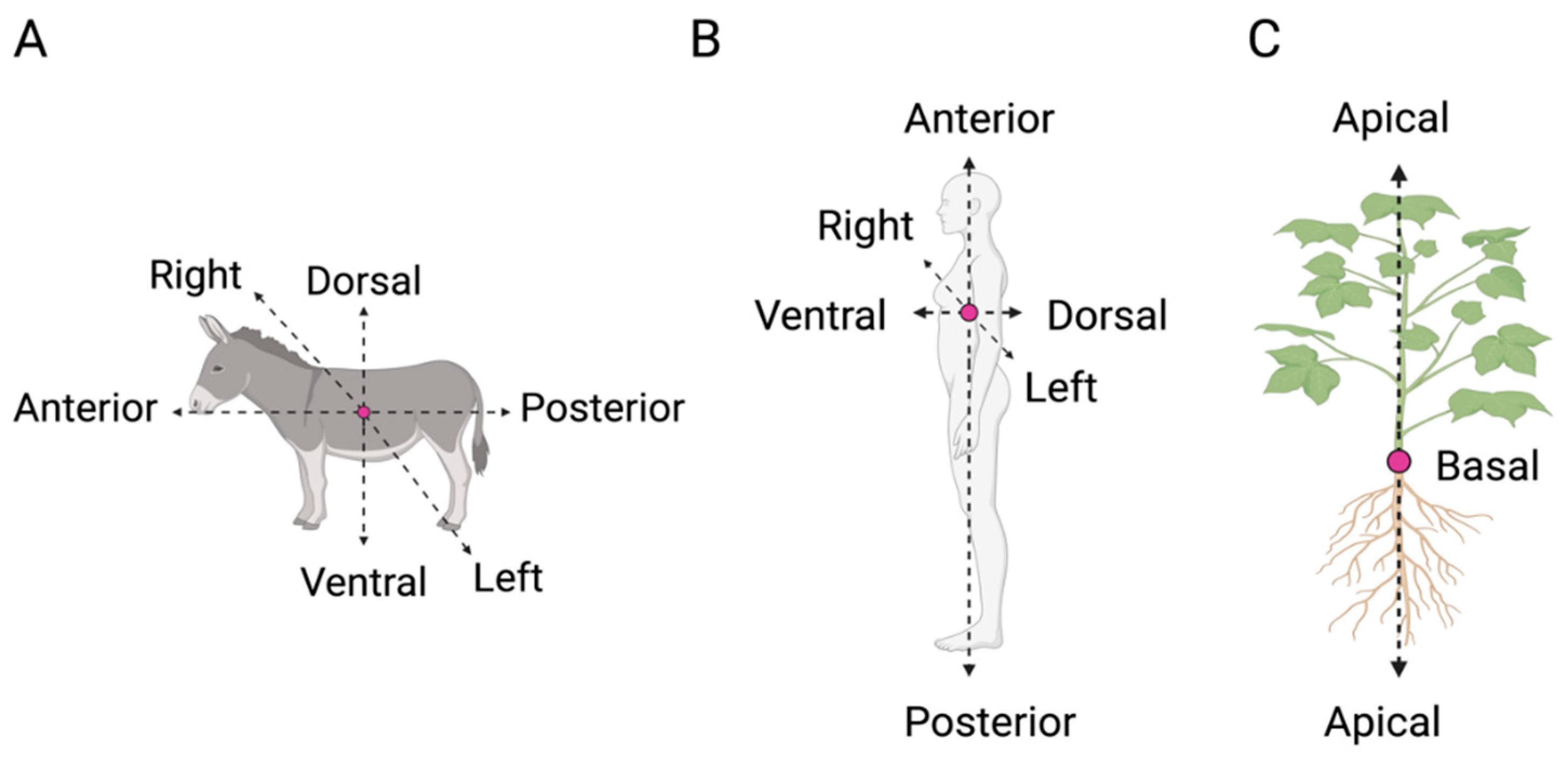
1. The Asymmetric Nature of the Universe
2. Handedness: One Term, Multiple Meanings
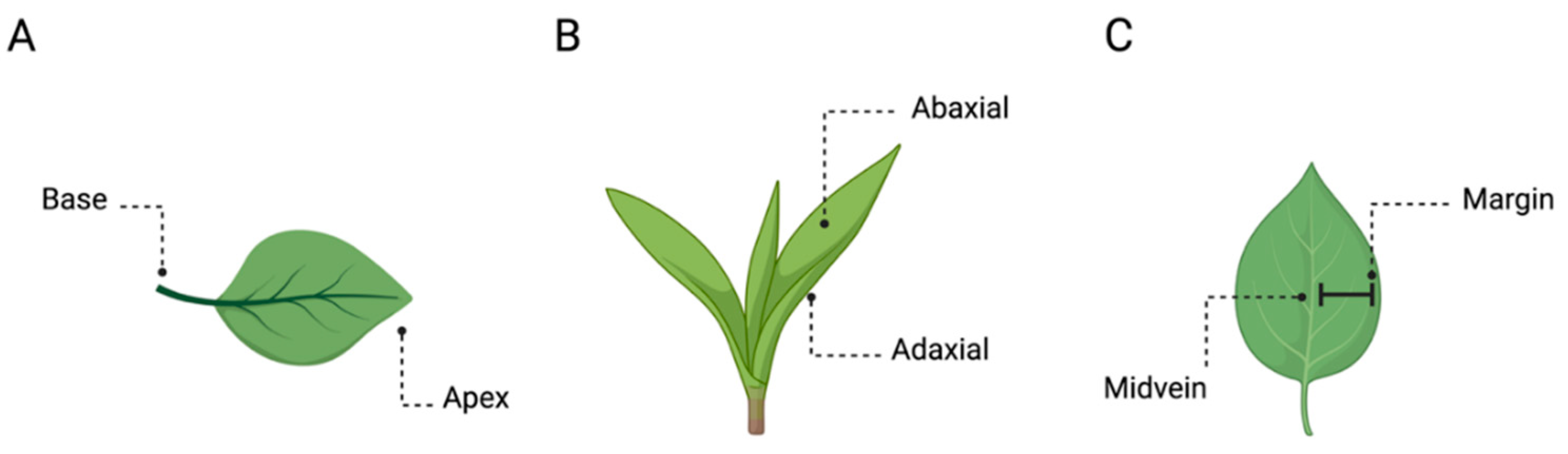
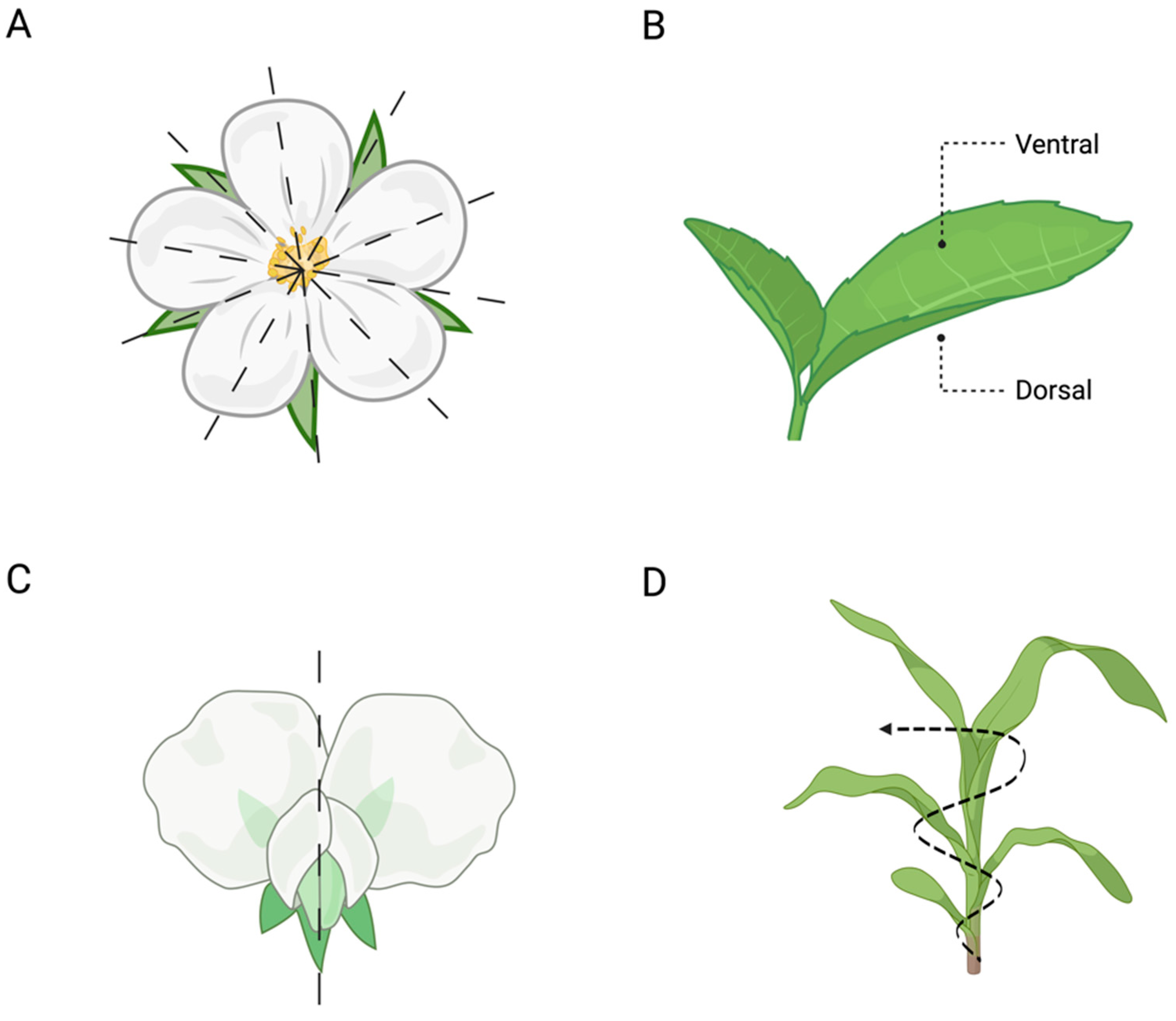
3. Handedness in Plants
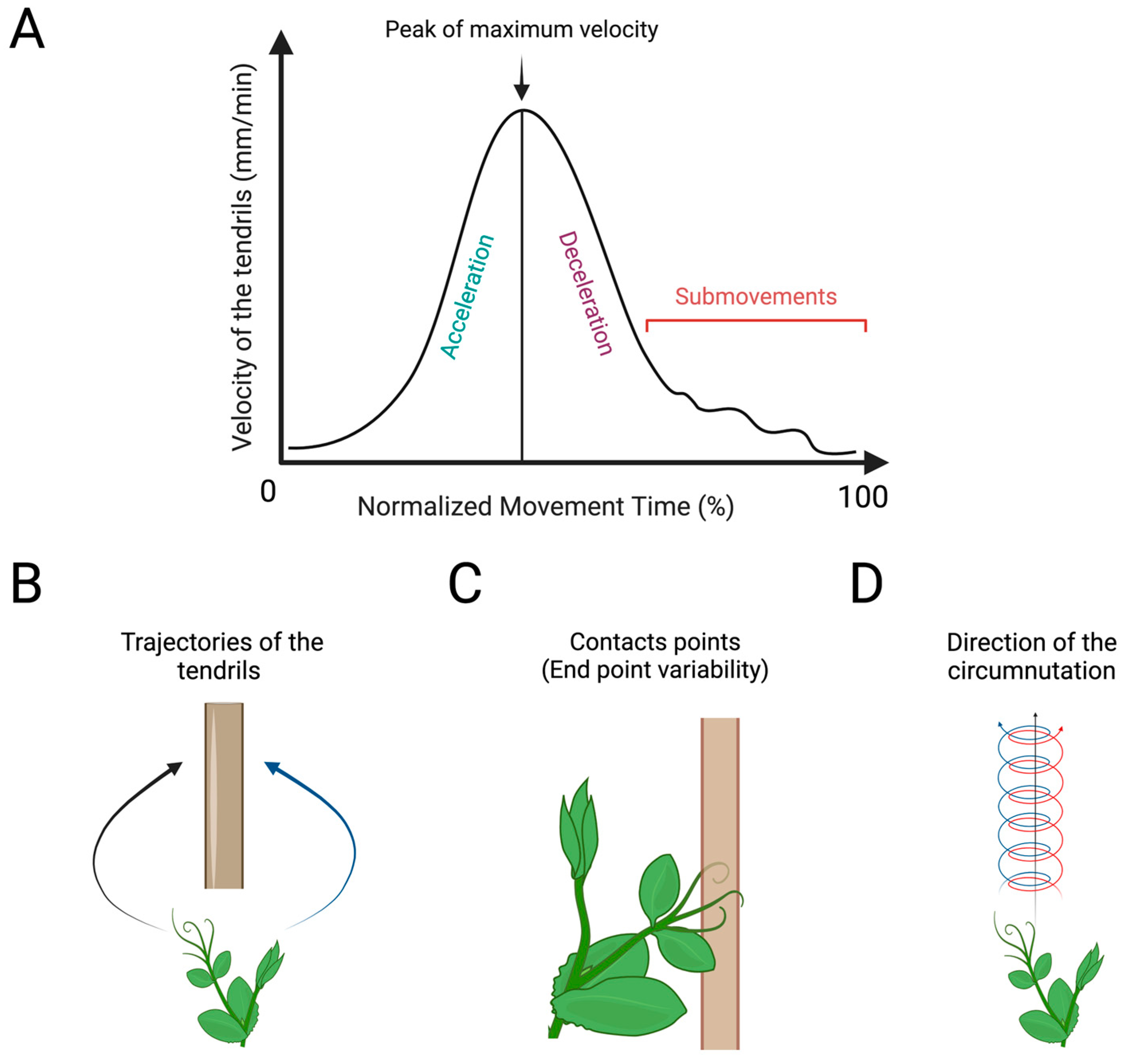
4. Toward a Comparative Study of Handedness in Animals and Plants
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hegstrom, R.A.; Kondepudi, D.K. The handedness of the universe. Sci. Am. 1990, 262, 108–115. [Google Scholar] [CrossRef]
- Hudson, A. Development of symmetry in plants. Annu. Rev. Plant Biol. 2000, 51, 349–370. [Google Scholar] [CrossRef]
- Jürgens, G. Axis formation in plant embryogenesis: cues and clues. Cell 1995, 81, 467–470. [Google Scholar] [CrossRef]
- Jeong, S.; Eilbert, E.; Bolbol, A.; Lukowitz, W. Going mainstream: How is the body axis of plants first initiated in the embryo? Dev. Biol. 2016, 419, 78–84. [Google Scholar] [CrossRef]
- Berger, F.; Brownlee, C. Establishment of the apical-basal axis in multicellular plant embryos. Biol. Cell. 1995, 84, 7–11. [Google Scholar] [CrossRef]
- Sachs, T. Cell polarity and tissue patterning in plants. Dev. 1991, 113, 83–93. [Google Scholar] [CrossRef]
- Dahdul, W.M.; Cui. H.; Mabee. P.M.; Mungall. C.J.; Osumi–Sutherland. D.; Walls. R.L.; Haendel, M.A. Nose to tail, roots to shoots: spatial descriptors for phenotypic diversity in the Biological Spatial Ontology. J. Biomed. Semant. 2014, 5, 1–13. [Google Scholar] [CrossRef]
- Hofmeister, W. AllgemeineMorphologie der Gewächse. Handbuch der physiologischen Botanik 1868, 1, 405–664. [Google Scholar]
- Braybrook, S.A.; Kuhlemeier, C. How a plant builds leaves. The Plant Cell. 2010, 22, 1006–1018. [Google Scholar] [CrossRef]
- Endress, P.K.; Doyle, J.A. Floral phyllotaxis in basal angiosperms: development and evolution. Curr. Opin. Plant Biol. 2007, 10, 52–57. [Google Scholar] [CrossRef]
- Warren, J.M. Handedness and laterality in humans and other animals. Physiol. Psychol. 1980, 8, 351–359. [Google Scholar] [CrossRef]
- Michel, G.F.; Babik, I.; Nelson, E.L.; Campbell, J.M.; Marcinowski, E.C. How the development of handedness could contribute to the development of language. Dev. Psychobiol. 2013, 55, 608–620. [Google Scholar] [CrossRef]
- Knecht, S.; Dräger, B.; Deppe, M.; Bobe, L.; Lohmann, H.; Flöel, A. Handedness and hemispheric language dominance in healthy humans. Brain 2000, 123, 2512–2518. [Google Scholar] [CrossRef]
- Annett, J. Motor Learning: A Review. In: Heuer, H. Kleinbeck, U. Schmidt, KH. (eds) Motor Behavior. Springer, Berlin, Heidelberg, 1985. [CrossRef]
- McManus, I.C. Handedness, language dominance and aphasia: a genetic model. Psychol. Med. Monogr. Suppl. 1985, 8, 3–40. [Google Scholar] [CrossRef]
- Rat-Fischer, L.; O'Regan, J.K.; Fagard, J. Handedness in infants' tool use. Dev. Psychobiol. 2013, 55, 860–868. [Google Scholar] [CrossRef]
- Domellöf, E.; Johansson, A.M.; Rönnqvist, L. Handedness in preterm born children: a systematic review and a meta–analysis. Neuropsychologia 2011, 49, 2299–2310. [Google Scholar] [CrossRef]
- Raymond, M.; Pontier, D. Is there geographical variation in human handedness? Laterality 2004, 9, 35–51. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Russell, J.L.; Lambeth, S.; Schapiro, S.J. Handedness and neuroanatomical asymmetries in captive chimpanzees: a summary of 15 years of research. Int. J. Primatol. 2007, 5, 146–181. [Google Scholar] [CrossRef]
- Crow, T. Directional asymmetry is the key to the origin of modern Homo sapiens (the Broca-Annett axiom): A reply to Rogers' review of The Speciation of Modern Homo Sapiens. Laterality: asymmetries of body, brain and cognition, 2004, 9, 233–242. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Cantalupo, C. Handedness in chimpanzees (Pan troglodytes) is associated with asymmetries of the primary motor cortex but not with homologous language areas. Behav. Neurosci. 2004, 118, 1176. [Google Scholar] [CrossRef]
- Caspar, K.R.; Pallasdies, F.; Mader, L.; Sartorelli, H.; Begall, S. The evolution and biological correlates of hand preferences in anthropoid primates. Elife 2022, 11, e77875. [Google Scholar] [CrossRef]
- Vallortigara, G.; Rogers, L. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. BBS, 2005. [CrossRef]
- Watson, S.L.; Hanbury, D.B. Prosimian primates as models of laterality. Primatol. 2007, 5, 228–250. [Google Scholar] [CrossRef]
- Bisazza, A.; Sovrano, V.A.; Vallortigara, G. Consistency among different tasks of left–right asymmetries in lines of fish originally selected for opposite direction of lateralization in a detour task. Neuropsychologia 2001, 39, 1077–1085. [Google Scholar] [CrossRef]
- Fine, M.L.; Friel, J.P.; McElroy, D.; King, C.B.; Loesser, K.E.; Newton, S. Pectoral spine locking and sound production in the channel catfish Ictalurus punctatus. Copeia 1994, 777–790. [Google Scholar] [CrossRef]
- Shine, R.; Olsson, M.M.; LeMaster, M.P.; Moore, I.T.; Mason, R.T. Are snakes right–handed? Asymmetry in hemipenis size and usage in gartersnakes (Thamnophis sirtalis). Behav. Ecol. 2000, 11, 411–415. [Google Scholar] [CrossRef]
- Roth, E.D. ‘Handedness’ in snakes? Lateralization of coiling behaviour in a cottonmouth, Agkistrodon piscivorus leucostoma, population. Animal Behav. 2003, 66, 337–341. [Google Scholar] [CrossRef]
- Darwin, C.; Darwin, F. The power of movement in plants. John Murray, London. 1880. [CrossRef]
- Darwin, C. The movements and habits of climbing plants. John Murray, London. 1875.
- Gianoli, E. The behavioural ecology of climbing plants. AoB PLANTS 2015, 7, plv013. [Google Scholar] [CrossRef]
- Edwards, W.; Moles, A.T.; Franks, P. The global trend in plant twining direction. Glob. Ecol. Biogeogr. 2007, 16, 95–800. [Google Scholar] [CrossRef]
- Gardner, M. The ambidextrous universe. Penguin, Victoria, Australia. 1970.
- Jackson, D.B. A glossary of botanical terms. London. 4th ed. 1953. [Google Scholar]
- Jaffe, M.J. Physiological Studies on Pea Tendrils VIII. The Relationship of Circumnutation to Contact Coiling – With a Description of a Laboratory Intervalometer Using Integrated Digital Circuits. Physiol. Plant. 1972, 26, 73–80. [Google Scholar] [CrossRef]
- Kihara, H. Right–and left–handedness in plants–A review. ISO 690. 1973. [Google Scholar]
- Smyth, D.R. Helical growth in plant organs: mechanisms and significance. Dev. 2016, 143, 3272–3282. [Google Scholar] [CrossRef]
- Davis, T.A.; Davis, B. Association of coconut foliar spirality with latitude. Math. Model. 1987, 8, 730–733. [Google Scholar] [CrossRef]
- Hashimoto, T. Molecular genetic analysis of left–right handedness in plants. Philosophical Philos. Trans. R. Soc. 2002, 357, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Minorsky, P.V. Latitudinal differences in coconut foliar spiral direction: a re–evaluation and hypothesis. Ann. Bot. 1998, 82, 133–140. [Google Scholar] [CrossRef]
- Minorsky, P.V.; Bronstein, N.B. Natural experiments indicate that geomagnetic variations cause spatial and temporal variations in coconut palm asymmetry. Plant Physiol. 2006, 142, 40–44. [Google Scholar] [CrossRef]
- Schuster, J.; Engelmann, W. Circumnutations of Arabidopsis thaliana seedlings. Biol. Rhythm Res. 1997, 28, 422–440. [Google Scholar] [CrossRef]
- Millet, B.; Melin, D.; Badot, P.M. Circumnutation in Phaseolus vulgaris. I. Growth, osmotic potential and cell ultrastructure in the free-moving part of the shoot. Physiol. Plant. 1988, 72, 133–138. [Google Scholar] [CrossRef]
- Bonato, B.; Wang, Q. ; Guerra, S.; Simonetti, V.; Bulgheroni, M.; Quaggiotti, S.; Ruperti, B.; Castiello, U. ‘United we stand, divided we fall’: intertwining as evidence of joint actions in pea plants. AoB Plants 2024, 16, plad088. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S. Bruno, G. Spoto, A. Panzeri, A. Wang, Q. Bonato, B. Simonetti, V. Castiello, U. Ascent and attachment in pea plants: a matter of iteration. Research square preprint. [CrossRef]
- Wang, Q.; Guerra, S.; Bonato, B.; Simonetti, V.; Bulgheroni, M.; Castiello, U. Decision–making underlying support searching in pea plants. Plants 2023, 12, 1597. [Google Scholar] [CrossRef] [PubMed]
- Silk, W.K.; Holbrook, N.M. The importance of frictional interactions in maintaining the stability of the twining habit. AJB. 2005, 92, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Bonato, B.; Simonetti, V.; Bulgheroni, M.; Wang, Q.; Guerra, S.; Quaggiotti, S.; Ruperti, B.; Castiello, U. Evidence of motor intentions in plants: a kinematical study. J Comp Psycho. 2023. [Google Scholar] [CrossRef] [PubMed]
- Bonato, B.; Castiello, U.; Guerra, S.; Wang, Q. Motor cognition in plants: from thought to real experiments. Theor. Exp. Plant Physiol. 2024. [Google Scholar] [CrossRef]
- Castiello, U. (Re) claiming plants in comparative psychology. J. Comp. Psychol. 2021, 135, 127. [Google Scholar] [CrossRef]
- Ceccarini, F.; Guerra, S.; Peressotti, A.; Peressotti, F.; Bulgheroni, M.; Baccinelli, W.; Bonato, B.; Castiello, U. Speed–accuracy trade-off in plants. Psychon. Bull. Rev. 2020, 27, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Ceccarini, F.; Guerra, S.; Peressotti, A.; Peressotti, F.; Bulgheroni, M.; Baccinelli, W.; Bonato, B.; Castiello, U. On-line control of movement in plants. Biochem. Biophys. Res. Commun. 2020, 564, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; Bonato, B.; Wang, Q.; Ceccarini, F.; Peressotti, A.; Peressotti, F.; Bacinelli, W.; Bulgheroni, M.; Castiello, U. The coding of object thickness in plants: when roots matter. J Comp Psycho. 2021, 135, 495–504. [Google Scholar] [CrossRef]
- Guerra, S.; Bonato, B.; Wang, Q.; Ceccarini, F.; Peressotti, A.; Peressotti, F.; Bacinelli, W.; Bulgheroni, M.; Castiello, U. Kinematic evidence of root–to–shoot signaling for the coding of support thickness in pea plants. Biology 2022, 11, 405. [Google Scholar] [CrossRef]
- Guerra, S.; Peressotti, A.; Peressotti, F.; Bulgheroni, M.; Bacinelli, W.; D’Amico, E.; Gómez, A.; Massaccesi, S.; Ceccarini, F.; Castiello, U. Flexible control of movement in plants. Sci Rep. 2019, 9, 16570. [Google Scholar] [CrossRef]
- Falik, O.; Reides, P.; Gersani, M.; Novoplansky, A. Root navigation by self inhibition. Plant, Cell Environ. 2005, 28, 562–569. [Google Scholar] [CrossRef]
- Gilroy, S.; Trewavas, T. Agency, teleonomy and signal transduction in plant systems. Biological J. Linn. Soc. 2023, 139, 514–529. [Google Scholar] [CrossRef]
- Novoplansky, A. Picking battles wisely: plant behaviour under competition. Plant, Cell Environ. 2009, 32, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Parise, A.G.; De Toledo, G.R.A.; de Carvalho Oliveira, T.F.; Souza, G.M.; Castiello, U, Gagliano, M. ; Marder, M. Do plants pay attention? A possible phenomenological–empirical approach. Prog. Biophys. Mol. Biol. 2022, 173, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Parise, A.G.; Marder, M. Extended plant cognition: a critical consideration of the concept. Theor. Exp. Plant Physiol. 2023. [Google Scholar] [CrossRef]
- Parise, A.G.; Reissig, G.N.; Basso, L.F.; Senko, L.G.S.; Oliveira, T.F.D.C.; De Toledo, G.R.A. Detection of different hosts from a distance alters the behaviour and bioelectrical activity of Cuscuta racemosa. Front. Plant Sci. 2021, 12, 594195. [Google Scholar] [CrossRef] [PubMed]
- Saito, K. A study on diameter-dependent support selection of the tendrils of Cayratia japonica. Sci. Rep. 2022, 12, 1–8. [Google Scholar] [CrossRef]
- Wang, Q.; Barbariol, T.; Susto, G.A.; Bonato, B.; Guerra, S.; Castiello, U. Classifying circumnutation in pea plants via supervised machine learning. Plants 2023, 12, 965. [Google Scholar] [CrossRef]
- Parma, V.; Brasselet, R.; Zoia, S.; Bulgheroni, M.; Castiello, U. The origin of human handedness and its role in pre-birth motor control. Sci. Rep. 2017, 7, 16804. [Google Scholar] [CrossRef]
- Cox, R.F.A.; Smitsman, W. Action planning in young children’s tool use. Developmental Science 2006, 9, 628–641. [Google Scholar] [CrossRef]
- Flindall, J. W.; Gonzalez, C. L. On the evolution of handedness: evidence for feeding biases. PLoS One 2013, 8, e78967. [Google Scholar] [CrossRef]
- Leconte, P.; Fagard, J. Which factors affect hand selection in children’s grasping in hemispace? Combined effects of task demand and motor dominance. Brain Cogn. 2006, 60, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Morange–Majoux, F.; Peze, A.; Bloch, H. Organisation of left and right hand movement in a prehension task: A longitudinal study from 20 to 32 weeks. Laterality: Asymmetries of Body, Brain Cogn. 2000, 5, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Rönnqvist, L.; Domellöf, E. Quantitative Assessment of Right and Left Reaching Movements in Infants: A Longitudinal Study from 6 to 36 Months. Dev. Psychobiol. 2006, 48, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Sacrey, L.A.R.; Arnold, B.; Whishaw, I.Q.; Gonzalez, C. Precocious hand use preference in reach–to–eat behavior versus manual construction in 1– to 5–yearold children. Dev. Psychobiol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Begliomini, C.; Nelini, C.; Caria, A.; Grodd, W.; Castiello, U. Cortical activations in humans grasp–related areas depend on hand used and handedness. PLoS ONE 2008, 3, e3388. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Cantalupo, C.; Wesley, M.J.; Hostetter, A.B.; Pilcher, D.L. Grip morphology and hand use in chimpanzees (Pan troglodytes): Evidence of a left hemisphere specialization in motor skill. J. Exp. Psychol. Gen. 2002, 131, 412. [Google Scholar] [CrossRef]
- Simonetti, V.; Bulgheroni, M.; Guerra, S.; Peressotti, A.; Peressotti, F.; Baccinelli, W.; Ceccarini, F.; Bonato, B.; Wang, Q.; Castiello, U. Can plants move like animals? A three–dimensional stereovision analysis of movement in plants. Animals 2021, 11, 1854. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




