Submitted:
06 May 2024
Posted:
06 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
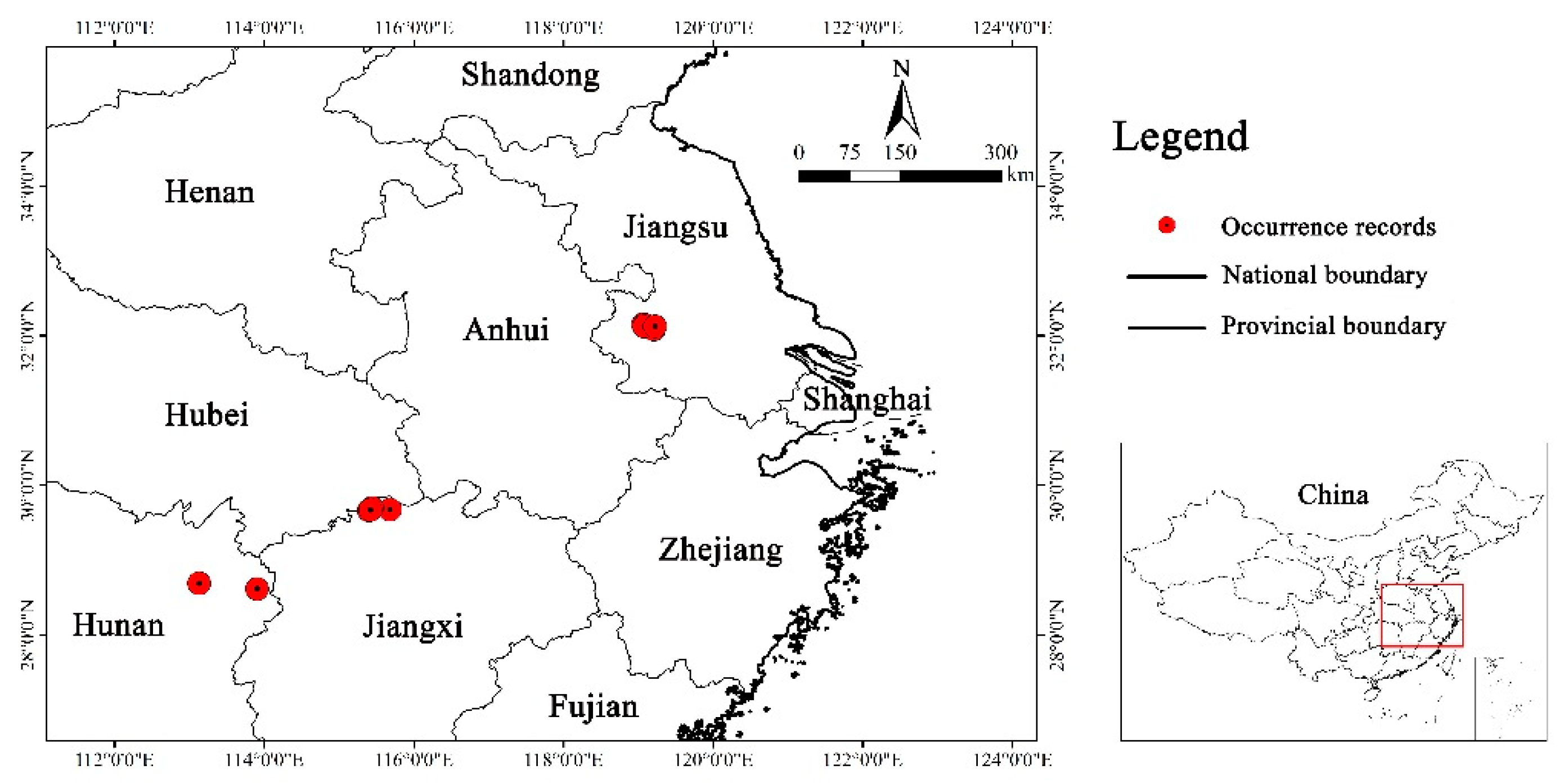
2.1. Species Occurrence Data
2.2. Environmental Variables
2.3. Modeling Process
2.4. Geospatial Data Analysis
3. Results
3.1. Model Performance
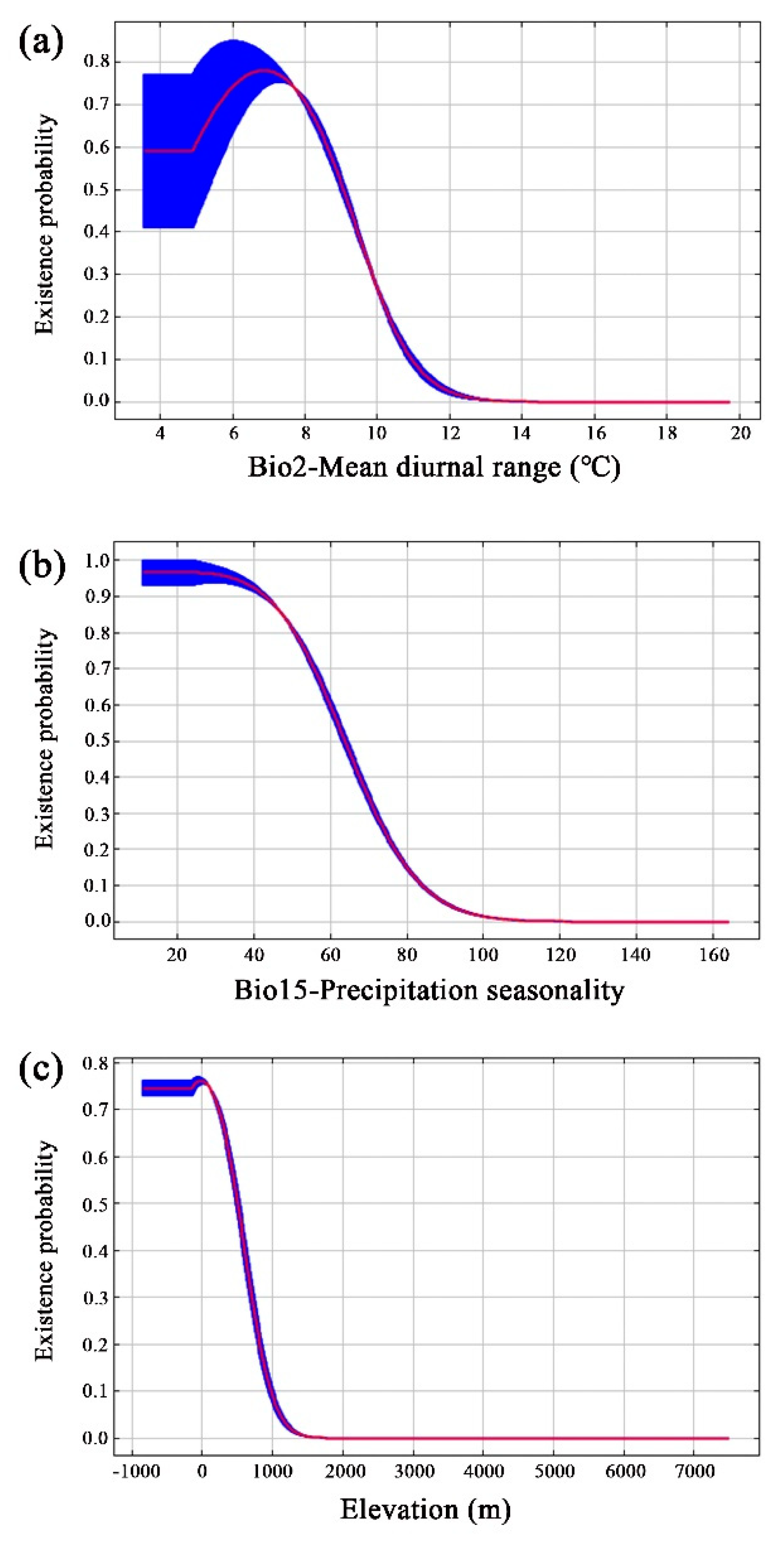
3.2. Main Environmental Factors
3.3. Current Potential Suitable Distribution
3.4. Potential Suitable Distribution in the Past
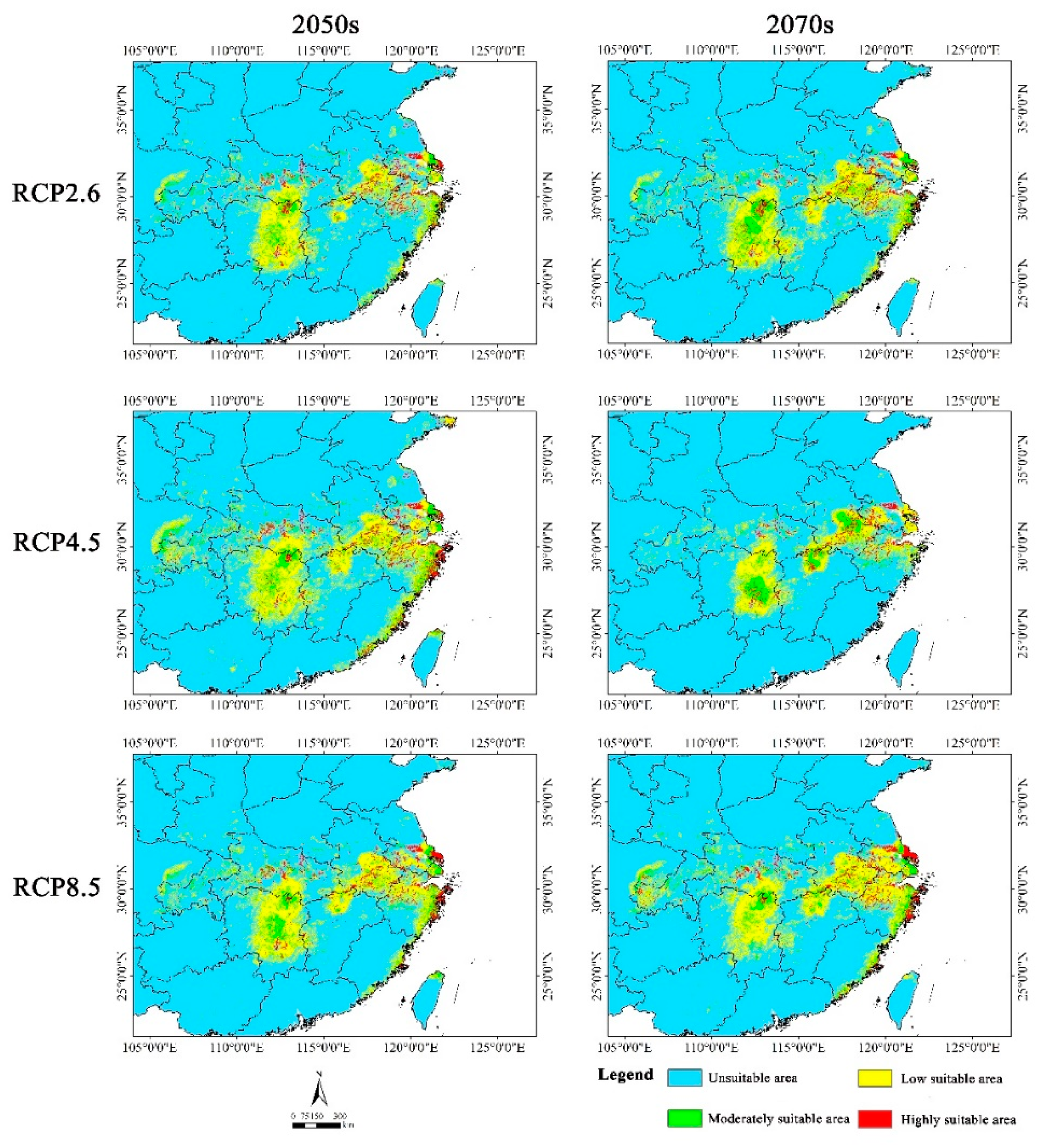
3.5. Potential Suitable Distribution in the Future
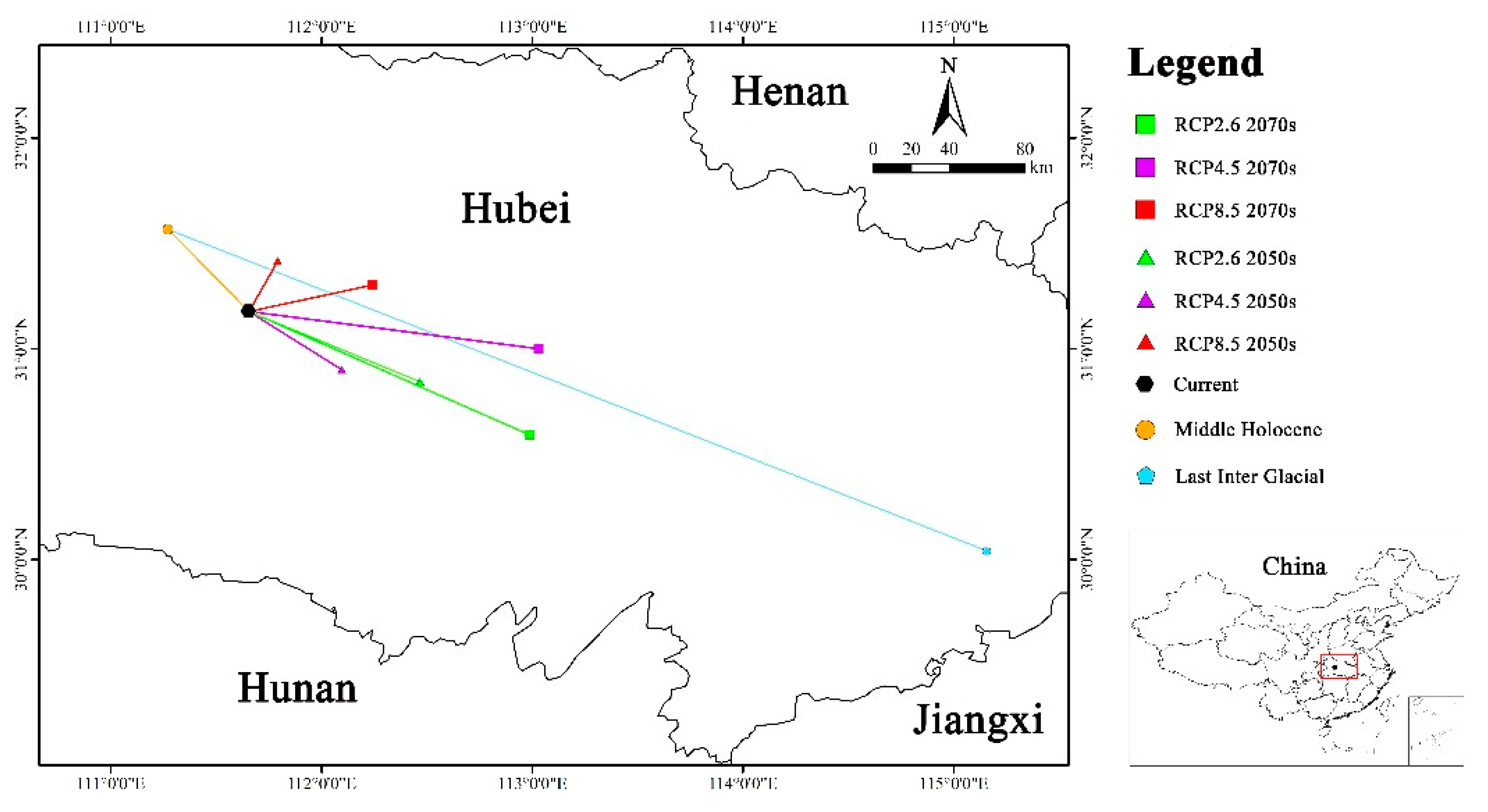
3.6. Centroid Migration under Different Scenarios
4. Discussion
4.1. Model Evaluation
4.2. Key Environmental Factors
4.3. Current Suitable Area of Yulania zenii
4.4. Suitable Area Change in the Past and Future
4.5. Conservation Implications for Yulania zenii
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allouche, O; Tsoar, A; Kadmon, R. Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223-1232. [CrossRef]
- Brown, J.L.; Bennett J.R.; French C.M. SDMtoolbox 2.0: the next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [CrossRef]
- Chen, Q.H.; Yin, Y.J.; Zhao, R.; Yang, Y.; Teixeira da Silva, J.A.; Yu, X.N. Incorporating local adaptation into species distribution modeling of Paeonia mairei, an endemic plant to China. Front. Plant Sci. 2020, 10, 1717. [CrossRef]
- Chen, Y.X.; Nan, C.H. ISSR Analysis on genetic diversity of endangered plant Magnolia zenii. J. Sichuan Agr. Univ. 2016, 34, 445-449.
- Chen, Z.D.; Lu, A.M.; Liu, B.; Ye, J.F. Tree of Life for Chinese Vascular Plants; Science Press: Beijing, China, 2020; ISBN 9787030635600. [Google Scholar]
- Chu, X.L. Conservation and related research progress of rare and endangered plant Yulania zenii. Bot. Res. 2021, 10, 331.
- Dyderski, M.K.; Pawlik, Ł. Spatial distribution of tree species in mountain national parks depends on geomorphology and climate. Forest Ecol. Manag. 2020, 474, 118366. [CrossRef]
- Feng, L.; Sun, J.J.; El-Kassaby, Y.A.; Yang, X.Y.; Tian, X.N.; Wang, T.L. Predicting potential habitat of a plant species with small populations under climate change: Ostrya rehderiana. Forests 2022, 13, 129.
- He, X.; Ma, W.X.; Zhao, T.T.; Ma, Q.H.; Liang, L.S.; Wang, G.X.; Yang, Z. Prediction of potential distribution of endangered species Corylus chinensis Franch in climate change context. Forest Res. 2022, 35, 104-114.
- Hills, R.; Bachman, S.; Forest, F.; Moat, J.; Wilkin, P. Incorporating evolutionary history into conservation assessments of a highly threatened group of species, South African Dioscorea (Dioscoreaceae). S. Afr. J. Bot. 2019, 123, 296-307. [CrossRef]
- Jin, X.H.; Zhou, Z.H.; Yuan, L.C. National Key Protected Wild Plants of China; Hubei Science and Technology Press: Wuhan, China, 2023; ISBN 9787570625895.
- Kanitkar, T.; Mythri, A.; Jayaraman, T. Equity assessment of global mitigation pathways in the IPCC Sixth Assessment Report. Clim. Policy 2024, 1-20. [CrossRef]
- Kiser, A.H.; Cummings, K.S.; Tiemann, J.S.; Smith, C.H.; Johnson, N.A.; Lopez, R.R.; Randklev, C.R. Using a multi-model ensemble approach to determine biodiversity hotspots with limited occurrence data in understudied areas: An example using freshwater mussels in Mexico. Ecol. Evol. 2022, 12, e8909. [CrossRef]
- Kong, F. Spatial and temporal variation characteristics and regional differences of days of diurnal temperature range in China from 1961 to 2018. J. Zhejiang Univ. (Sci. Edition), 2020, 47, 422-434.
- Kong, W.Y.; Li, X.H.; Zou, H.F. Optimizing MaxEnt model in the prediction of species distribution. Chin. J. Appl. Ecol. 2019, 30, 2116-2128.
- Lang, X.P.; Fan, R.Y.; Li, Q.F. Analysis of potential suitable areas of Allium mongolicum in Northern China. Acta Agrestia Sin. 2023, 31, 3525-3534.
- Liu, C.R.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778-789. [CrossRef]
- Liu, Q.X. Flora of Jiangsu; Jiangsu Phoenix Science and Technology Press: Nanjing, China, 2015; Volume 2, ISBN 9787553701073. [Google Scholar]
- Lu, X.; Jiang, R.Y.; Zhang, G.F. Predicting the potential distribution of four endangered holoparasites and their primary hosts in China under climate change. Front. Plant Sci. 2022, 13, 942448. [CrossRef]
- Luo, M.; Wang, H.; Lyu, Z. Evaluating the performance of species distribution models Biomod2 and MaxEnt using the giant panda distribution data. Chin. J. Appl. Ecol. 2017, 28, 4001-4006.
- Moss, R.H.; Edmonds, J.A.; Hibbard, K.A.; Manning, M.R.; Rose, S.K.; Van Vuuren, D.P.; Carter, T.R.; Seita, E.; Mikiko, K.; Kram, T; et al. The next generation of scenarios for climate change research and assessment. Nature, 2010, 463, 747-756.
- Peng, Y. A critically endangered species of Yulania zenii was discovered in Hunan Province for the first time. Forestry Ecol. 2022, 4, 48.
- Poirazidis, K.; Bontzorlos, V.; Xofis, P.; Zakkak, S.; Xirouchakis, S.; Grigoriadou, E.; Kechagioglou, S.; Gasteratos, I.; Alivizatos, H.; Panagiotopoulou, M. Bioclimatic and environmental suitability models for Capercaillie (Tetrao urogallus) conservation: Identification of optimal and marginal areas in Rodopi Mountain-Range National Park (Northern Greece). Glob. Ecol. Conserv. 2019, 17, e00526. [CrossRef]
- Qin, A.L.; Liu, B.; Guo, Q.S.; Bussmann, R. W.; Ma, F.Q.; Jian, Z.J.; Xu, G.X.; Pei, S.X. Maxent modeling for predicting impacts of climate change on the potential distribution of Thuja sutchuenensis Franch., an extremely endangered conifer from southwestern China. Glob. Ecol. Conserv. 2017, 10, 139-146. [CrossRef]
- Qin, H.N. Seed Plants of China: Checklist, Uses and Conservation Status; Hebei Science and Technology Press: Shijiazhuang, China, 2020; ISBN 9787571706494.
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629-643. [CrossRef]
- Rathore, M.K., Sharma, L.K. Efficacy of species distribution models (SDMs) for ecological realms to ascertain biological conservation and practices. Biodivers. Conserv. 2023, 32, 3053-3087. [CrossRef]
- Ren, Z.C.; Zagortchev, L.; Ma, J.X.; Yan, M.; Li, J.M. Predicting the potential distribution of the parasitic Cuscuta chinensis under global warming. BMC Ecol. 2020, 20, 1-14. [CrossRef]
- Shabani, F.; Kumar, L.; Ahmadi, M. A comparison of absolute performance of different correlative and mechanistic species distribution models in an independent area. Ecol. Evol. 2016, 6, 5973-5986. [CrossRef]
- Shi, X.D.; Yin, Q.; Sang, Z.Y.; Zhu, Z.G.; Jia, Z.G.; Ma, L.Y. Prediction of potentially suitable areas for the introduction of Magnolia wufengensis under climate change. Ecol. Indic. 2021, 127, 107762. [CrossRef]
- Sillero, N.; Arenas-Castro, S.; Enriquez-Urzelai, U.; Vale, C.G.; Sousa-Guedes, D.; Martínez-Freiría, F.; Real, R.; Barbosa, A.M. Want to model a species niche? A step-by-step guideline on correlative ecological niche modelling. Ecol. Model. 2021, 456, 109671. [CrossRef]
- Sillero, N.; Barbosa, A.M. Common mistakes in ecological niche models. Int. J. Geogr. Inf. Sci. 2021, 35, 213-226. [CrossRef]
- Singh, M.; Arunachalam, R.; Kumar, L. Modeling potential hotspots of invasive Prosopis juliflora (Swartz) DC in India. Ecol. Inform. 2021, 64, 101386. [CrossRef]
- Stanton, J.C.; Pearson, R.G.; Horning, N.; Ersts, P.; Reşit Akçakaya, H. Combining static and dynamic variables in species distribution models under climate change. Methods Ecol. Evol. 2012, 3, 349-357. [CrossRef]
- Turney, C.S.M.; Jones, R.T. Does the Agulhas current amplify global temperatures during Super-Interglacials? J. Quaternary Sci. 2010, 25, 839-843.
- Veloz, S.D.; Williams, J.W.; Blois, J.L.; He, F.; Otto-Bliesner, B.; Liu, Z.Y. No-analog climates and shifting realized niches during the late quaternary: implications for 21st-century predictions by species distribution models. Global Change Biol. 2012, 18, 1698-1713. [CrossRef]
- Vincent, H.; Bornand, C.N.; Kempel, A.; Fischer, M. Rare species perform worse than widespread species under changed climate. Biol. Conserv. 2020, 246, 108586. [CrossRef]
- Wang, S. Study on the Reproductive Biology of the Critically Endangered Plant Magnolia zenii 'Cheng'; Nanjing Forestry University: Nanjing, China, 2020.
- Wang, X.F.; Duan, Y.X.; Jin, L.L.; Wang, C.Y.; Peng, M.C.; Li, Y.; Wang, X.H.; Ma, Y.F. Prediction of historical, present and future distribution of Quercus sect. Heterobalanus based on the optimized MaxEnt model in China. Acta Ecol. Sin. 2023, 43, 6590-6604.
- Wang, Y.M. A study on Subgenus Yulania; Northwest Sci-Tech University of Agriculture and Forestry: Yangling, China, 2003.
- Wang, Z.W.; Yin, J.; Wang, X.; Chen, Y.; Mao, Z.K.; Lin, F.; Gong, Z.Q.; Wang, X.G. Habitat suitability evaluation of invasive plant species Datura stramonium in Liaoning Province: Based on Biomod2 combination model. Chin. J. Appl. Ecol. 2023, 34, 1272-1280. [CrossRef]
- Wu, Y.M.; Shen, X.L., Tong, L.; Lei, F.W.; Mu, X.Y.; Zhang, Z.X. Impact of past and future climate change on the potential distribution of an endangered montane shrub Lonicera oblata and its conservation implications. Forests 2021, 12, 125. [CrossRef]
- Wu, Z.Y.; Raven, P.H.; Hong, D.Y. Flora of China; Science Press: Beijing, China, 2008; Volume 7, ISBN 9781930723818.
- Xu, Y.D.; Huang, Y.; Zhao, H.R.; Yang, M.L.; Zhuang, Y.Q.; Ye, X.P. Modelling the effects of climate change on the distribution of endangered Cypripedium japonicum in China. Forests 2021, 12, 429. [CrossRef]
- Yackulic, C.B.; Chandler, R.; Zipkin, E.F.; Royle, J.A.; Nichols, J.D.; Campbell Grant, E.H.; Veran S. Presence-only modelling using MAXENT: When can we trust the inferences? Methods Ecol. Evol. 2013, 4, 236-243.
- Yan, G.; Zhang, G.F. Predicting the potential distribution of endangered Parrotia subaequalis in China. Forests 2022, 13, 1595. [CrossRef]
- Yan, H.; Ma, S.M.; Wei, B.; Zhang, H.X.; Zhang, D. Historical distribution patterns and environmental drivers of relict shrub Amygdalus pedunculata. Chin. J. Plant Ecol. 2022, 46, 766. [CrossRef]
- Yang, J.; Cai, L.; Liu, D.T.; Chen, G.; Gratzfeld, J.; Sun, W.B. China's conservation program on plant species with extremely small populations (PSESP): progress and perspectives. Biol. Conserv. 2020, 244, 108535.
- Yang, Y.; Wu, Y.X.; Chen, Q.H.; Liu, C.; Liu, G.L.; Cheng, S.P.; Wang, L.S. Potential spatiotemporal distribution changes and conservation recommendations of two connected endangered tree peony species (Paeonia decomposita & P. rotundiloba). Flora 2022, 294, 152131. [CrossRef]
- Ye, X.Z.; Zhao, G.H.; Zhang, M.Z.; Cui, X.Y.; Fan, H.H.; Liu, B. Distribution pattern of endangered plant Semiliquidambar cathayensis (Hamamelidaceae) in response to climate change after the last interglacial period. Forests 2020, 11, 434. [CrossRef]
- Yin, H. Rare and Endangered Plants in China; China Forestry Publishing House: Beijing, China, 2013; ISBN 9787503870262.
- Yu, S.X.; Wang, Z.H.; Peng, Y.D.; Zhao, H. Color Illustrations of Endangered and Protected Plants of China; China Customs Press: Beijing, China, 2023; ISBN 9787517507147.
- Zhang, G.F.; Xiong, T.S.; Sun, T.; Li, K.D.; Shao, L.Y. Diversity, distribution, and conservation of rare and endangered plant species in Jiangsu Province. Biodivers. Sci. 2022, 30, 21335. [CrossRef]
- Zhang, G.F.; Yi, X.G. Vegegraphy of Jiangsu; Fujian Science and Technology Publishing House: Fuzhou, China, 2023; ISBN 9787533571337.
- Zhang, G.F. The rare plant Magnolia zenii endemic to China. China Nature 2007, 6, 45-47.
- Zhang, J.H.; Li, K.J.; Liu, X.F.; Yang, L.; Shen, S.K. Interspecific variance of suitable habitat changes for four alpine Rhododendron species under climate change: Implications for their reintroductions. Forests 2021, 12, 1520. [CrossRef]
- Zhang, W.X.; Kou, Y.X.; Zhang, L.; Zeng, W.D.; Zhang, Z.Y. Suitable distribution of endangered species Pseudotaxus chienii (Taxaceae) in five periods using niche modeling. Chin. J. Ecol. 2020, 39, 600-613.
- Zhao, G.H.; Cui, X.Y.; Sun, J.J.; Li, T.T.; Wang, Q.; Ye, X.Z.; Fan, B.G. Analysis of the distribution pattern of Chinese Ziziphus jujuba under climate change based on optimized biomod2 and MaxEnt models. Ecol. Indic. 2021, 132, 108256. [CrossRef]
- Zhao, R.N.; Chu, X.J.; He, Q.Q.; Tang, Y.; Song, M.; Zhu, Z.L. Modeling current and future potential geographical distribution of Carpinus tientaiensis, a critically endangered species from China. Forests 2020, 11, 774.
- Zhou, Y.Z.; Lu, X.; Zhang, G.F. Potentially differential impacts on niche overlap between Chinese endangered Zelkova schneideriana and its associated tree species under climate change. Front. Ecol. Evol. 2023, 11, 1218149. [CrossRef]



| Category | Variable | Description | Unit | Percent Contribution (%) | ||
|---|---|---|---|---|---|---|
| LIG | MH | Current | ||||
| Bioclimate | Bio1 | Annual mean temperature | °C | 0.7 | ||
| Bio2 | Mean diurnal range (mean of monthly (max temp–min temp)) | °C | 25.2 | 32.1 | 32.9 | |
| Bio3 | Isothermality ( (Bio2/Bio7)×100) | % | 27.1 | 19.4 | 9.8 | |
| Bio4 | Temperature seasonality (standard deviation × 100) |
- | ||||
| Bio5 | Max temperature of warmest month | °C | 0 | 3.1 | 0 | |
| Bio6 | Min temperature of coldest month | °C | ||||
| Bio7 | Temperature annual range (Bio5–Bio6) | °C | ||||
| Bio8 | Mean temperature of wettest quarter | °C | 2.3 | |||
| Bio9 | Mean temperature of driest quarter | °C | ||||
| Bio10 | Mean temperature of warmest quarter | °C | 2.2 | |||
| Bio11 | Mean temperature of coldest quarter | °C | ||||
| Bio12 | Annual precipitation | mm | ||||
| Bio13 | Precipitation of wettest month | mm | ||||
| Bio14 | Precipitation of driest month | mm | ||||
| Bio15 | Precipitation seasonality (coefficient of variation) | - | 43.1 | 39.9 | 21.1 | |
| Bio16 | Precipitation of wettest quarter | mm | ||||
| Bio17 | Precipitation of driest quarter | mm | 4.9 | |||
| Bio18 | Precipitation of warmest quarter | mm | ||||
| Bio19 | Precipitation of coldest quarter | mm | ||||
| Terrain | Elevation | - | m | 14.8 | ||
| Slope | - | ° | 0.4 | |||
| Soil | T-BS | Topsoil Base Saturation | % | |||
| T-CaCO3 | Topsoil Calcium Carbonate | % | ||||
| T-CaSO4 | Topsoil Calcium Sulfate | % | ||||
| T-CEC-CLAY | Topsoil CEC (clay) | - | ||||
| T-CEC-SOIL | Topsoil CEC (soil) | - | ||||
| T-CLAY | Topsoil Clay Fraction | % | ||||
| T-ECE | Topsoil Electrical Conductivity | S/m | ||||
| T-ESP | Topsoil Sodicity | - | ||||
| T-GRAVEL | Topsoil Gravel Content | % | ||||
| T-OC | Topsoil Organic Carbon | % | 0.5 | |||
| T-PH-H2O | Topsoil PH (H2O) | - | 9.8 | |||
| T-REF-BULK | Topsoil Reference Bulk Density | kg/m3 | ||||
| T-SAND | Topsoil Sand Fraction | % | ||||
| T-SILT | Topsoil Silt Fraction | % | 9.5 | |||
| T-TEB | Topsoil Exchangeable Base | - | ||||
| T-TEXTURE | Topsoil TEXTURE | - | 1.2 | |||
| T-USDA-TEX | Topsoil USDA Texture Classification | - | ||||
| Model name | Model code | AUC | TSS |
|---|---|---|---|
| Artificial neural networks model | ANN | 0.8231±0.3113 | 0.4679±0.3114 |
| Classification tree analysis model | CTA | 0.7913±0.3111 | 0.5828±0.3112 |
| Flexible discriminant analysis model | FDA | 0.9549±0.3109 | 0.6893±0.3106 |
| Generalized additive model | GAM | Modeling failure | Modeling failure |
| Generalized boosting model | GBM | 0.9836±0.3118 | 0.4847±0.3115 |
| Generalized linear model | GLM | 0.8387±0.3121 | 0.6780±0.3118 |
| Maximum entropy model | MaxEnt | 0.9783±0.0244 | 0.8913±0.0889 |
| Multivariate adaptive regression splines model | MARS | 0.8571±0.3112 | 0.6377±0.3109 |
| Random forest model | RF | 0.9827±0.3114 | 0.5291±0.3112 |
| Surface range envelope model | SRE | 0.6056±0.3105 | 0.2110±0.3121 |
| Scenarios | AUC | TSS | |
|---|---|---|---|
| Last Inter Glacial | 0.9848±0.0077 | 0.9517±0.0020 | |
| Middle Holocene | 0.9770±0.0126 | 0.9250±0.0037 | |
| Current | 0.9783±0.0244 | 0.8913±0.0889 | |
| 2050s | RCP2.6 | 0.9837±0.0213 | 0.8783±0.1149 |
| RCP4.5 | 0.9775±0.0251 | 0.8994±0.0618 | |
| RCP8.5 | 0.9830±0.0194 | 0.9008±0.0845 | |
| 2070s | RCP2.6 | 0.9800±0.0221 | 0.8981±0.0840 |
| RCP4.5 | 0.9894±0.0146 | 0.9238±0.0669 | |
| RCP8.5 | 0.9846±0.0185 | 0.9080±0.0759 | |
| Scenarios | Low Suitable Area |
Moderately Suitable Area |
Highly Suitable Area |
Suitable Area (Moderately and Highly) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Area (×104 km2) |
Trend (%) | Area (×104 km2) |
Trend (%) | Area (×104 km2) |
Trend (%) | Area (×104 km2) |
Trend (%) | ||
| Last Inter Glacial | 19.09 | ↓18.80 | 12.16 | ↑28.95 | 11.42 | ↑117.52 | 23.58 | ↑60.63 | |
| Middle Holocene | 31.19 | ↑32.67 | 20.95 | ↑122.16 | 14.73 | ↑180.57 | 35.68 | ↑143.05 | |
| Current | 23.51 | - | 9.43 | - | 5.25 | - | 14.68 | - | |
| 2050s | RCP2.6 | 22.66 | ↓3.62 | 6.54 | ↓30.65 | 4.08 | ↓22.29 | 10.62 | ↓27.66 |
| RCP4.5 | 31.46 | ↑33.82 | 9.69 | ↑2.76 | 4.69 | ↓10.67 | 14.38 | ↓2.04 | |
| RCP8.5 | 26.80 | ↑13.99 | 7.78 | ↓17.50 | 3.85 | ↓26.67 | 11.63 | ↓20.78 | |
| 2070s | RCP2.6 | 24.93 | ↑6.04 | 8.16 | ↓13.47 | 3.81 | ↓27.43 | 11.97 | ↓18.46 |
| RCP4.5 | 17.63 | ↓25.01 | 6.10 | ↓35.31 | 2.74 | ↓47.81 | 8.84 | ↓39.78 | |
| RCP8.5 | 27.63 | ↑17.52 | 8.68 | ↓7.95 | 4.12 | ↓21.52 | 12.80 | ↓12.81 | |
| The mean value of six future climate scenarios | 25.19 | ↑7.12 | 7.83 | ↓17.02 | 3.88 | ↓26.07 | 11.71 | ↓20.26 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





