Submitted:
06 May 2024
Posted:
06 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
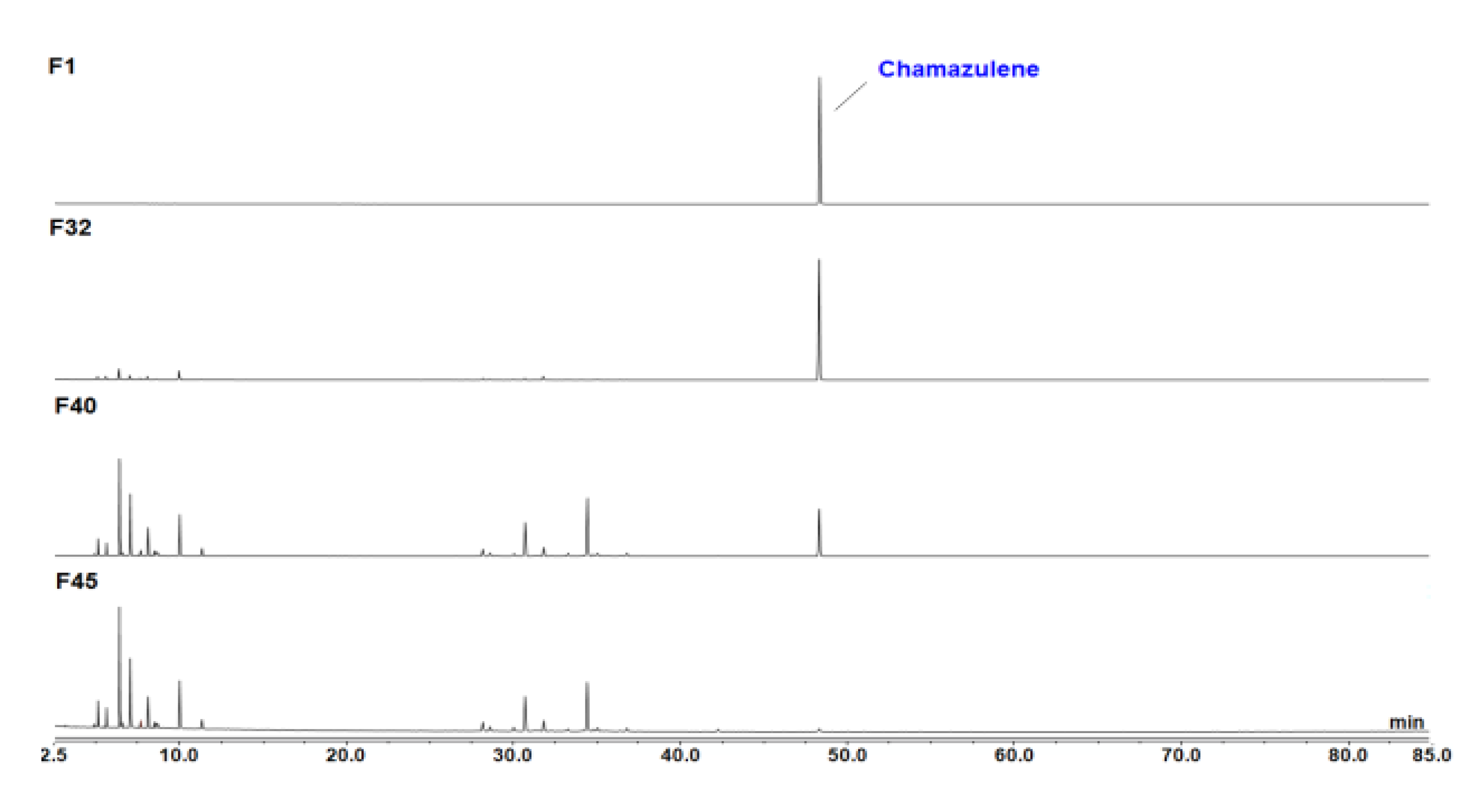
2.1. CA Isolation and Purification
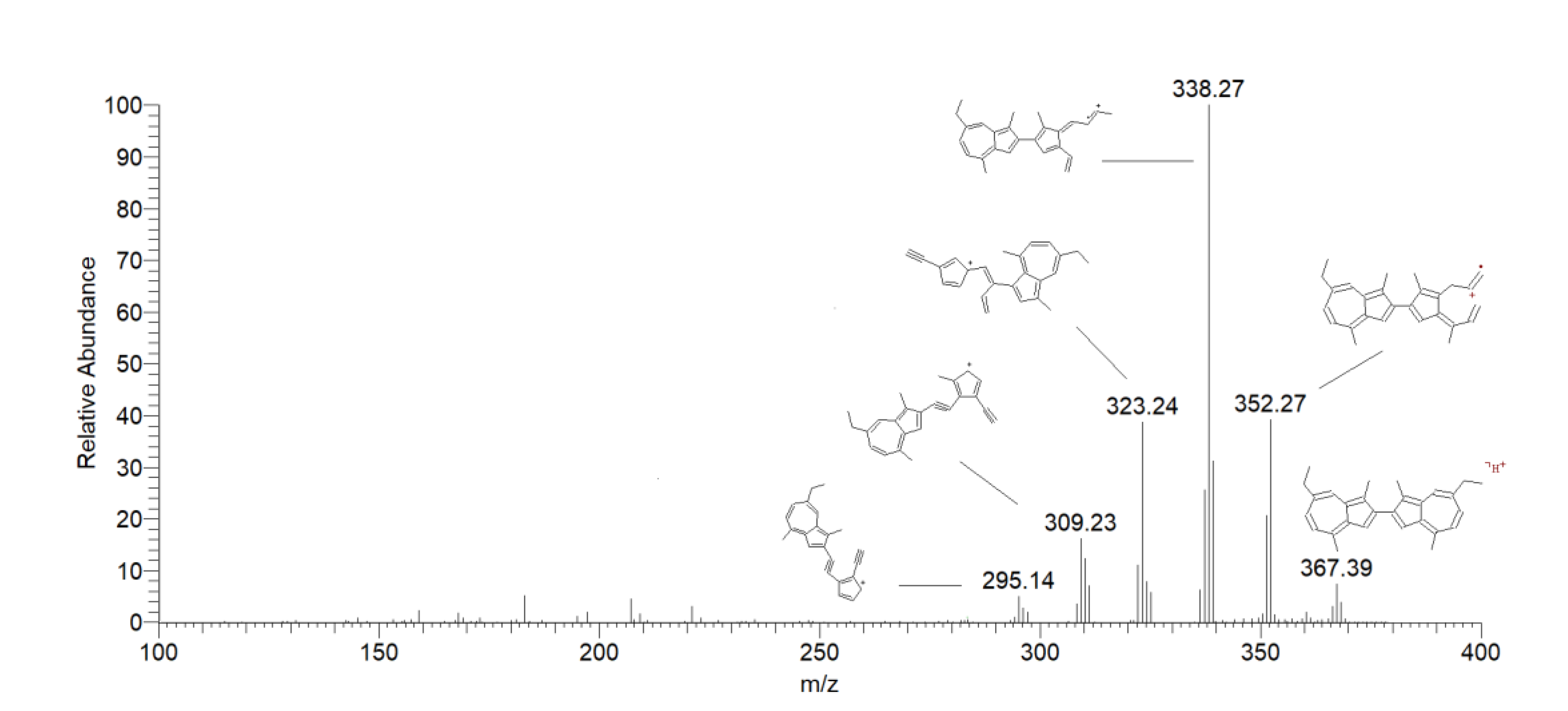
2.2. Generation of Photo-Degradation Products of CA
2.3. Photostability Test
2.3.1. Effect of Solvent and Oxygen
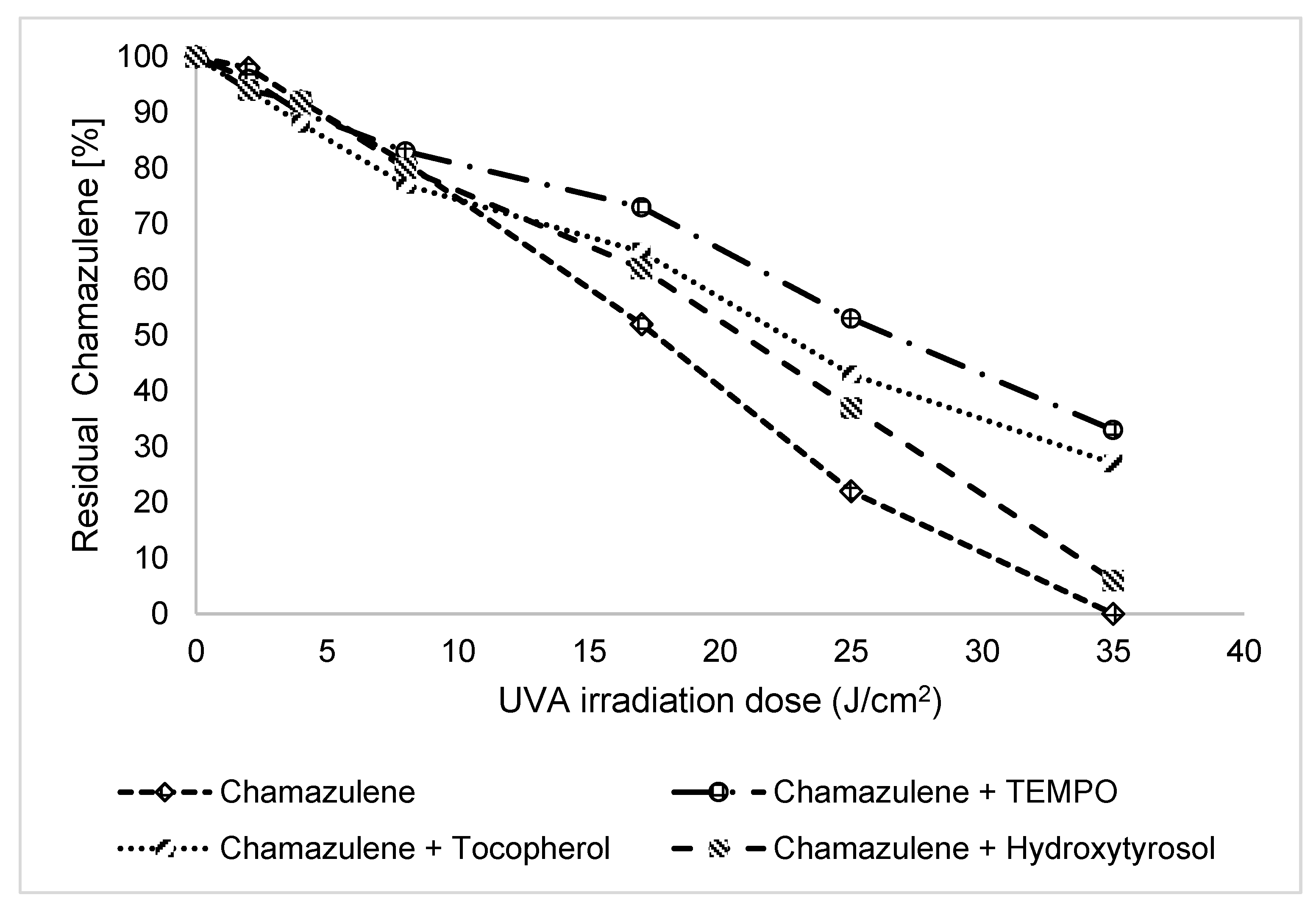
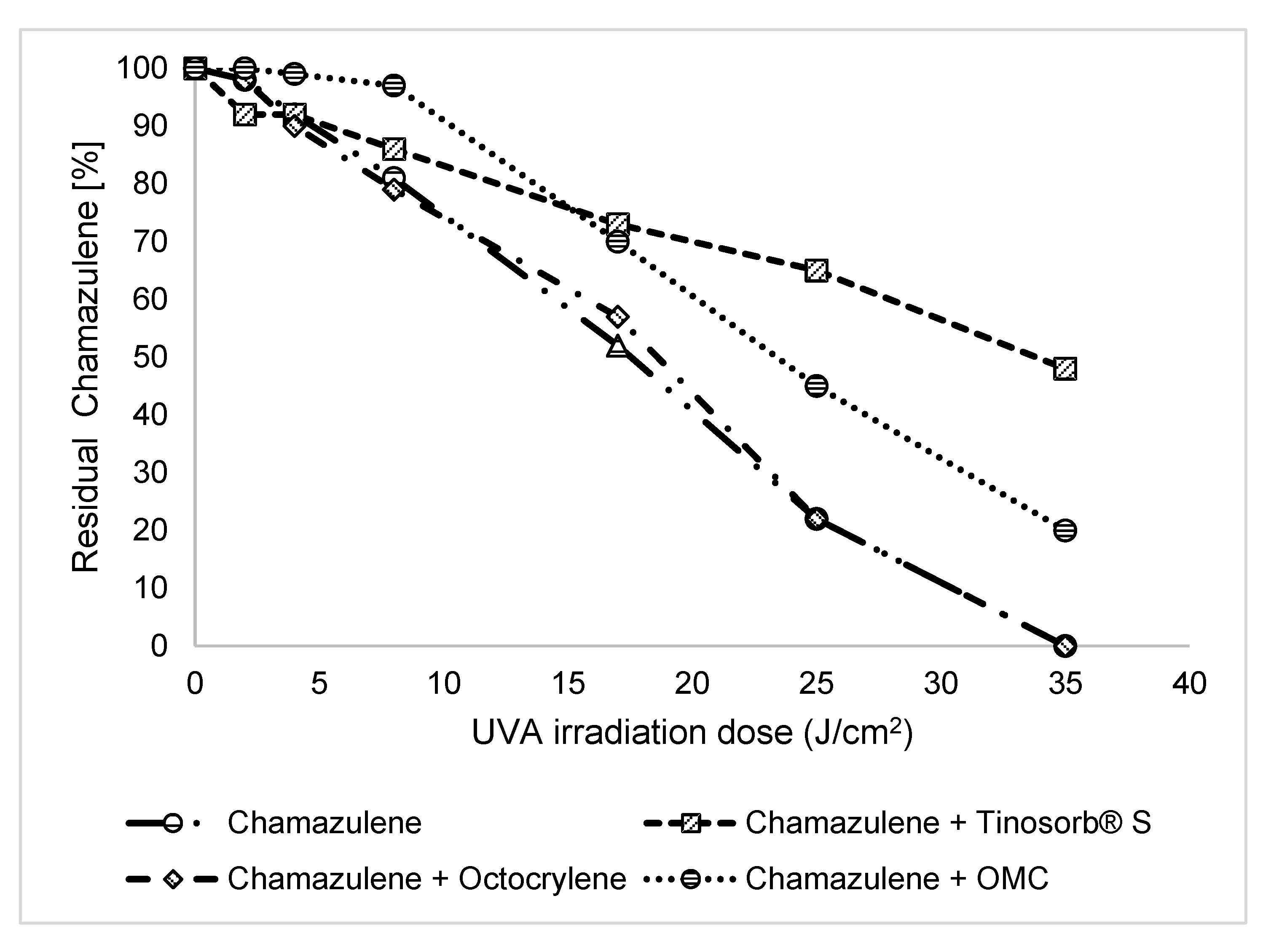
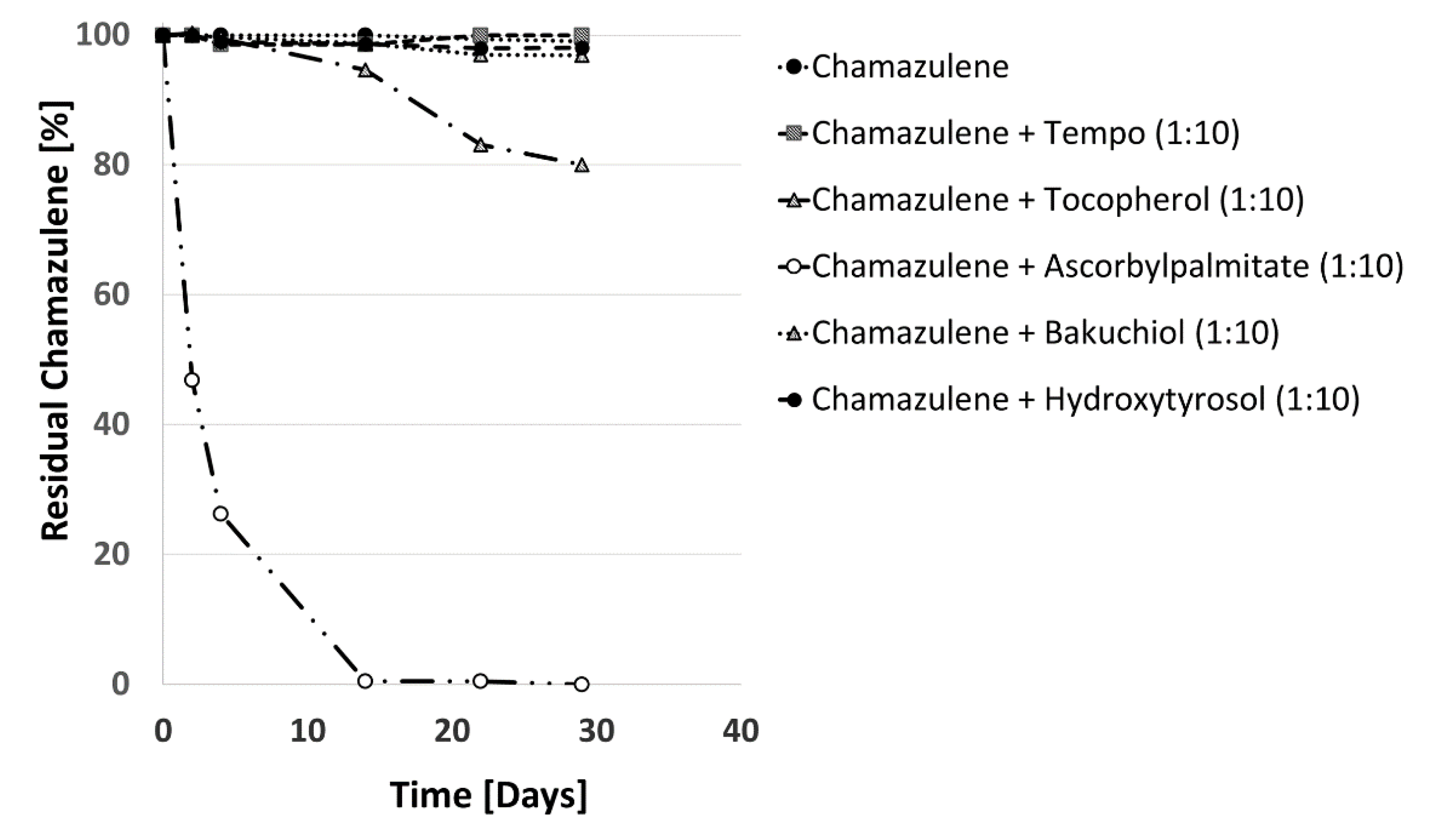
2.3.2. Effect of Antioxidants and Sunscreen
2.4. Thermal Stability Of Chamazulene
3. Materials and Methods
3.1. Materials
3.2. Isolation of Chamazulene (CA)
3.3. Photolysis Experiments: Equipment and General Procedures
3.4. GC-MS Analysis
3.5. UPLC-ESI-MSn, ESI-MSn Analysis
3.6. LC-PDA Analysis
3.7. UV-Vis Spectrophotometry
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Slon, E.; Slon, B.; Kowalczuk, D. Azulene and Its Derivatives as Potential Compounds in the Therapy of Dermatological and Anticancer Diseases: New Perspectives against the Backdrop of Current Research. Molecules 2024, 29, 2020. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Q.; Bao, W.; Pa, B. Antihyperlipidemic Effect, Identification and Isolation of the Lipophilic Components from Artemisia integrifolia. Molecules 2019, 24, 725. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Zheng, Y.; Feng, Y.; Chen, H.; Dai, S.-X.; Wang, Y.; Xu, M. Comparative Analysis of Active Ingredients and Potential Bioactivities of Essential Oils from Artemisia argyi and A. verlotorum. Molecules 2023, 28, 3927. [Google Scholar] [CrossRef] [PubMed]
- De, J.; Lu, Y.; Ling, L.; Peng, N.; Zhong, Y. Essential Oil Composition and Bioactivities of Waldheimia glabra (Asteraceae) from Qinghai-Tibet Plateau. Molecules 2017, 22, 460. [Google Scholar] [CrossRef] [PubMed]
- Qasem, A.; Assaggaf, H.; Montesano, D.; Khalil, Z.; Al-Mijalli, S.H.; Baaboua, A.E.; El Omari, N.; El Menyiy, N.; Bakrim, S.; Sheikh, R.A.; et al. Determination of Chemical Compounds and Investigation of Biological Properties of Matricaria chamomilla Essential Oils, Honey, and Their Mixture. Molecules 2022, 27, 5850. [Google Scholar] [CrossRef]
- Mohammed, H.A. Phytochemical Analysis, Antioxidant Potential, and Cytotoxicity Evaluation of Traditionally Used Artemisia absinthium L. (Wormwood) Growing in the Central Region of Saudi Arabia. Plants 2022, 11, 1028. [Google Scholar] [CrossRef] [PubMed]
- Konarska, A.; Weryszko-Chmielewska, E.; Sulborska-Różycka, A.; Kiełtyka-Dadasiewicz, A.; Dmitruk, M.; Gorzel, M. Herb and Flowers of Achillea millefolium subsp. millefolium L.: Structure and Histochemistry of Secretory Tissues and Phytochemistry of Essential Oils. Molecules 2023, 28, 7791. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.; Goeters, S.; Watzer, B.; Krause, E.; Lohmann, K.; Bauer, R.; Hempel, B.; Imming, P. Chamazulene carboxylic acid and matricin: a natural profen and its natural prodrug, identified through similarity to synthetic drug substances. Journal of Natural Products 2006, 69, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Sizova, N.V. Composition and antioxidant activity of essential oils containing azulene derivatives. Pharm. Chem. J. 2012, 46, 42–44. [Google Scholar] [CrossRef]
- Querio, G.; Antoniotti, S.; Foglietta, F.; Levi, R.; Bertea, C.M.; Canaparo, R. , Gallo, M.P. Chamazulene prevents ROS production in human dermal fibroblast and bovine aortic endothelial cells exposed to oxidative stress. Vascul. Pharmacol. 2018, 56, 103–105. [Google Scholar] [CrossRef]
- Capuzzo, A.; Occhipinti, A.; Maffei, M.E. Antioxidant and radical scavenging activities of chamazulene. Nat. Prod. Res. 2014, 28, 2321–2323. [Google Scholar] [CrossRef] [PubMed]
- Matera, R.; Lucchi, E.; Valgimigli, L. Plant Essential Oils as Healthy Functional Ingredients of Nutraceuticals and Diet Supplements: A Review. Molecules 2023, 28, 901. [Google Scholar] [CrossRef] [PubMed]
- Gabbanini, S.; Lucchi, E.; Carli, M.; Berlini, E.; Minghetti, A.; Valgimigli, L. In vitro evaluation of the permeation through reconstructed human epidermis of essentials oils from cosmetic formulations. Journal of Pharmaceutical and Biomedical Analysis 2009, 50, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, L.; Wang, W.; Wei, G.; Ma, L.; Liu, H.; Yao, L. Artemisia sieversiana Ehrhart ex Willd. Essential Oil and Its Main Component, Chamazulene: Their Photoprotective Effect against UVB-Induced Cellular Damage and Potential as Novel Natural Sunscreen Additives. ACS Sustainable Chem. Eng. 2023, 11, 17675–17686. [Google Scholar] [CrossRef]
- Fiori, J.; Gotti, R.; Valgimigli, L.; Cavrini, V. Guaiazulene in health care products: determination by GC-MS and HPLC-DAD and photostability test. Journal of Pharmaceutical and Biomedical Analysis 2008, 47, 710–715. [Google Scholar] [CrossRef]
- Valgimigli, L. Lipid Peroxidation and Antioxidant Protection. Biomolecules 2023, 13, 1291. [Google Scholar] [CrossRef] [PubMed]
- Fiori, J.; Gotti, R.; Albini, A.; Cavrini, V. Study on the photostability of guaiazulene by high-performance liquid chromatography/mass spectrometry and gas chromatography/mass spectrometry. Rapid Communications in Mass Spectrometry 2008, 22, 2698–2706. [Google Scholar] [CrossRef]
- Korenberg, C. The photo-ageing behaviour of selected watercolour paints under anoxic conditions. The British Museum Technical Research Bulletin 2008, 2, 49–57. [Google Scholar]
- Arney, J.S.; Jacobs, A.J.; Newman, R. The Influence of Oxygen on the Fading of Organic Colorants. Journal of the American Institute for Conservation 1979, 18, 108–117. [Google Scholar] [CrossRef]
- Zielinski, Z.; Presseau, N.; Amorati, R.; Valgimigli, L.; Pratt, D.A. Redox Chemistry of Selenenic Acids and the Insight It Brings on Transition State Geometry in the Reactions of Peroxyl Radicals. J. Am. Chem. Soc. 2014, 136, 1570–1578. [Google Scholar] [CrossRef]
- Matsubara, Y.; Morita, M.; Takekuma, S.; Nakano, T.; Yamamoto, H.M.; Nozoe, T. Oxidation of 4,6,8-Trimethylazulene and Guaiazulene with Hydrogen Peroxide in Pyridine. Bulletin of the Chemical Society of Japan 1991, 64, 3497–3499. [Google Scholar] [CrossRef]
- Kaufmann, A.; Butcher, P.; Maden, K.; Walker, S.; Widmer, M. Using in silico fragmentation to improve routine residue screening in complex matrices. J. Am. Mass Spectrom. 2017, 28, 2705–2715. [Google Scholar] [CrossRef]
- [23] Matsubara, Y.; Yamamoto, H.; Nozoe, T. Oxidation Products of Guaiazulene and Other Azulenic Hydrocarbons. Studies in Natural Products Chemistry 1994, 14, 313–354. [Google Scholar] [CrossRef]
- Chiang, H.-. M-; Yin, J.-J.; Xia, Q.; Zhao, Y.; Fu, P.P.; Wen, K.-C.; Yu, H. Photoirradiation of azulene and guaiazulene—Formation of reactive oxygen species and induction of lipid peroxidation. Journal of Photochemistry and Photobiology A: Chemistry 2010, 211, 123–128. [Google Scholar] [CrossRef]
- Warren, J.J.; Tronic, T.A.; Mayer, J.M. Thermochemistry of Proton-Coupled Electron Transfer Reagents and its Implications. Chem. Rev. 2010, 110, 6961–7001. [Google Scholar] [CrossRef]
- Amorati, R.; Baschieri, A.; Morroni, G.; Gambino, R.; Valgimigli, L. Peroxyl Radical Reactions in Water Solution: A Gym for Proton-Coupled Electron-Transfer Theories. Chem. Eur. J. 2016, 22, 7924–7934. [Google Scholar] [CrossRef]
- Singha, A.; Kaishyop, J.; Khan, T.S.; Chowdhury, B. Visible-Light-Driven Toluene Oxidation to Benzaldehyde over WO3 Nanostructures. ACS Appl. Nano Mater. 2023, 6, 21818–21828. [Google Scholar] [CrossRef]
- Battin-Leclerc, F.; Warth, V.; Bounaceur, R.; Husson, B.; Herbinet, O.; Glaude, P.-A. The oxidation of large alkylbenzenes: An experimental and modeling study. Proceedings of the Combustion Institute 2015, 35, 349–356. [Google Scholar] [CrossRef]
- Zhao, J. Plant Troponoids: Chemistry, Biological Activity, and Biosynthesis. Current Medicinal Chemistry 2007, 14, 2597–2621. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Zhao, Y.; Wang, S.; Yu, H.; Chiang, H.-M. Phototoxicity of Herbal Plants and Herbal Pr oducts. Journal of Environmental Science and Health C 2013, 31, 213–255. [Google Scholar] [CrossRef]
- Bolton, J.L.; Dunlap, T. Formation and Biological Targets of Quinones: Cytotoxic versus Cytoprotective Effects. Chem. Res. Toxicol. 2017, 30, 13–37. [Google Scholar] [CrossRef]
- ICH Q1B Guideline. Photostability Testing of New Drug Substances and Products. http://www.columbiapharma.com/reg_updates/international/ich/q1a.pdf (Accessed on st 2024). 1 May.
- Caroprese, A.; Gabbanini, S.; Beltramini, C.; Lucchi, E.; Valgimigli, L. HS-SPME-GC-MS analysis of body odor to test the efficacy of foot deodorant formulations. Skin Research and Technology 2009, 15, 503–510. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L.; Panzella, L.; Napolitano, A.; d’Ischia, M. 5-S-Lipoylhydroxytyrosol, a Multidefense Antioxidant Featuring a Solvent-Tunable Peroxyl Radical-Scavenging 3-Thio-1,2-dihydroxybenzene Motif J. Org. Chem. 2013, 78, 9857–9864. [Google Scholar] [CrossRef]
- Cariola, A.; El Chami, M.; Granatieri, J.; Valgimigli, L. Anti-tyrosinase and antioxidant activity of meroterpene bakuchiol from Psoralea corylifolia (L.). Food Chemistry 2023, 405, 134953. [Google Scholar] [CrossRef]
- Foti, M.C.; Ingold, K.U. Mechanism of Inhibition of lipid peroxidation by γ-terpinene, an unusual and potentially useful hydrocarbon antioxidant. Journal of Agriculture and Food Chemistry 2003, 51, 2758–2765. [Google Scholar] [CrossRef]
- Guo, Y.; Baschieri, A.; Amorati, R.; Valgimigli, L. Synergic antioxidant activity of γ-terpinene with phenols and polyphenols enabled by hydroperoxyl radicals. Food Chemistry 2021, 345, 128468. [Google Scholar] [CrossRef]
- Baschieri, A.; Valgimigli, L.; Gabbanini, S.; DiLabio, G.A.; Romero-Montalvo, E.; Amorati, R. Extremely Fast Hydrogen Atom Transfer between Nitroxides and HOO· Radicals and Implication for Catalytic Coantioxidant Systems J. Am. Chem. Soc. 2018, 140, 10354–10362. [Google Scholar] [CrossRef]
- Valgimigli, L.; Lucarini, M.; Pedulli, G.F.; Ingold, K.U. Does β-Carotene Really Protect Vitamin E from Oxidation? J. Am. Chem. Soc. 1997, 119, 8095–8096. [Google Scholar] [CrossRef]
- Johansson, H.; Shanks, D.; Engman, L.; Amorati, R.; Pedulli, G.F.; Valgimigli, L. Long-lasting antioxidant protection: A regenerable BHA analogue. Journal of Organic Chemistry 2010, 75, 7535–7541. [Google Scholar] [CrossRef] [PubMed]
- Pniewska, A.; Kalinowska-Lis, U. A Survey of UV Filters Used in Sunscreen Cosmetics. Appl. Sci. 2024, 14, 3302. [Google Scholar] [CrossRef]
- Nitulescu, G.; Lupuliasa, D.; Adam-Dima, I.; Nitulescu, G.M. Ultraviolet Filters for Cosmetic Applications. Cosmetics 2023, 10, 101. [Google Scholar] [CrossRef]
- Durand, L. , Habran, N., Henschel, V., & Amighi, K. (2010). Encapsulation of ethylhexyl methoxycinnamate, a light-sensitive UV filter, in lipid nanoparticles. Journal of Mi-croencapsulation, 27(8), 714–725. [CrossRef]
- Dencausse, L.; Galland, A.; Clamou J., L.; J. Basso, J. Validation of HPLC method for quantitative determination of Tinosorb S and three other sunscreens in a high protec-tion cosmetic product. International Journal of Cosmetic Science 2008, 30, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Hüglin, D. Advanced UV Absorbers for the Protection of Human Skin. Chimia 2016, 70, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Arimoto-Kobayashi, S.; Anma, N.; Yoshinaga, Y.; Douki, T.; Cadet, J.; Hayatsu, H. Oxidative damage and induced mutations in m13mp2 phage DNA exposed to N-nitrosopyrrolidine with UVA radiation. Mutagenesis 2000, 15, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.B.; Jin, W.; Ho, H.L.; Chan, K.C.; Chan, C.C.; Demokan, M.S.; Stewart, G.; Culshaw, B.; Liao, Y.B. Multiplexing of optical fiber gas sensors with a frequency-modulated continuous-wave technique. Appl. Opt. 2001, 40, 1011. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.G.K.; Sereli, M.; Anstöter, C.S.; Dessent, C.E.H. Photochemical Degradation of the UV Filter Octyl Methoxy Cinnamate Probed via Laser-Interfaced Mass Spectrometry. Molecules 2022, 27, 8796. [Google Scholar] [CrossRef] [PubMed]
- Jentzsch, F.; Reich, M.; Kümmerer, K.; Olsson, O. Photolysis of mixtures of UV filters octocrylene and ethylhexyl methoxycinnamate leads to formation of mixed transformation products and different kinetics. Science of The Total Environment 2019, 697, 134048. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.M.; Narayanan, S.; Nichols, V.M.; Bardeen C., J. Photochemical degradation of the UV filter octyl methoxycinnamate in solution and in aggregates. Photochem. Photobiol. Sci. 2015, 14, 1607–1616. [Google Scholar] [CrossRef]
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. https://eur-lex.europa.eu/eli/reg/2009/1223/oj (accessed on May 2nd 2024).
- Bowry, V.W.; Ingold, K.U. The Unexpected Role of Vitamin E (α-Tocopherol) in the Peroxidation of Human Low-Density Lipoprotein. Acc. Chem. Res. 1999, 32, 1–27. [Google Scholar] [CrossRef]

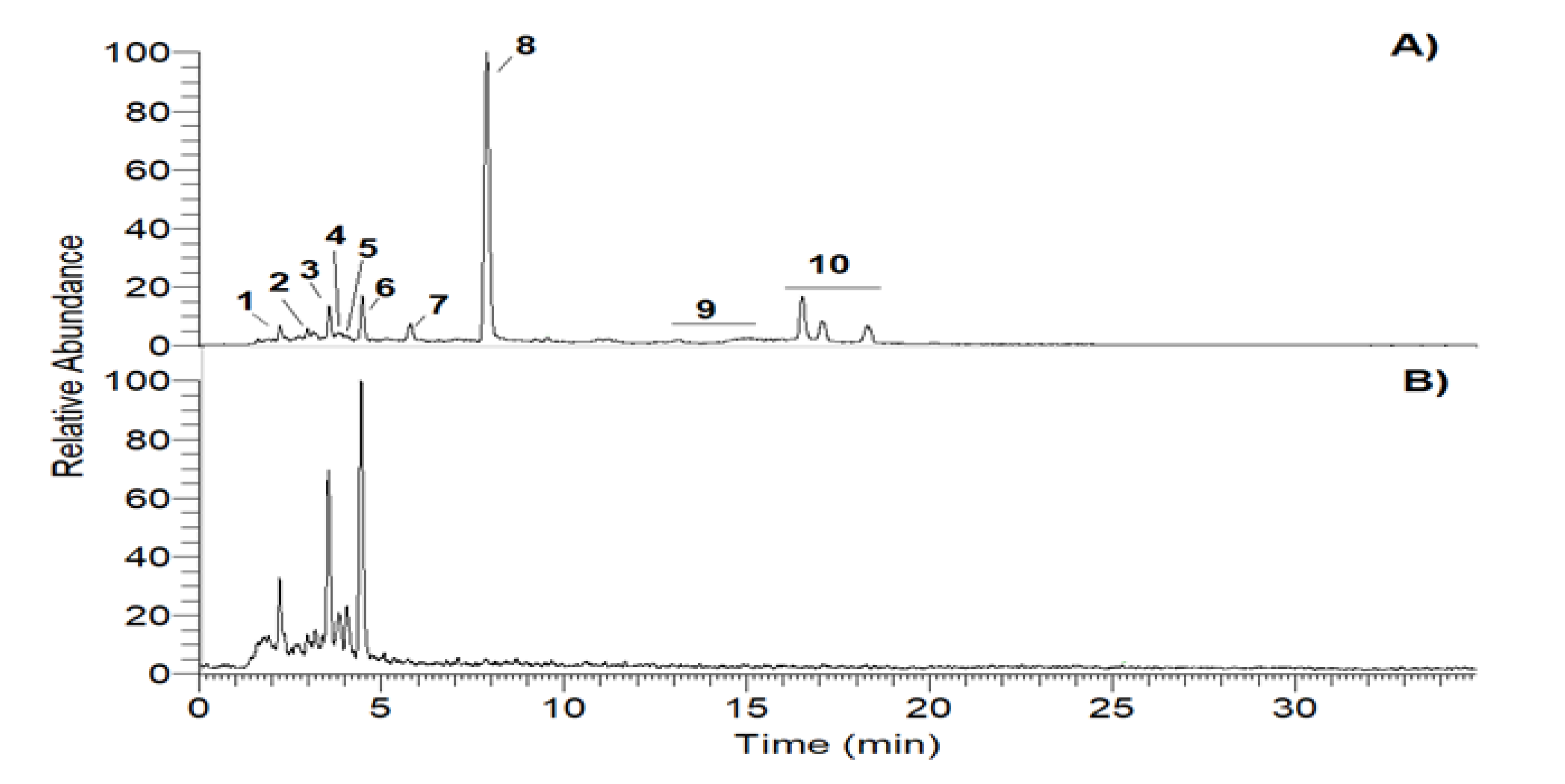
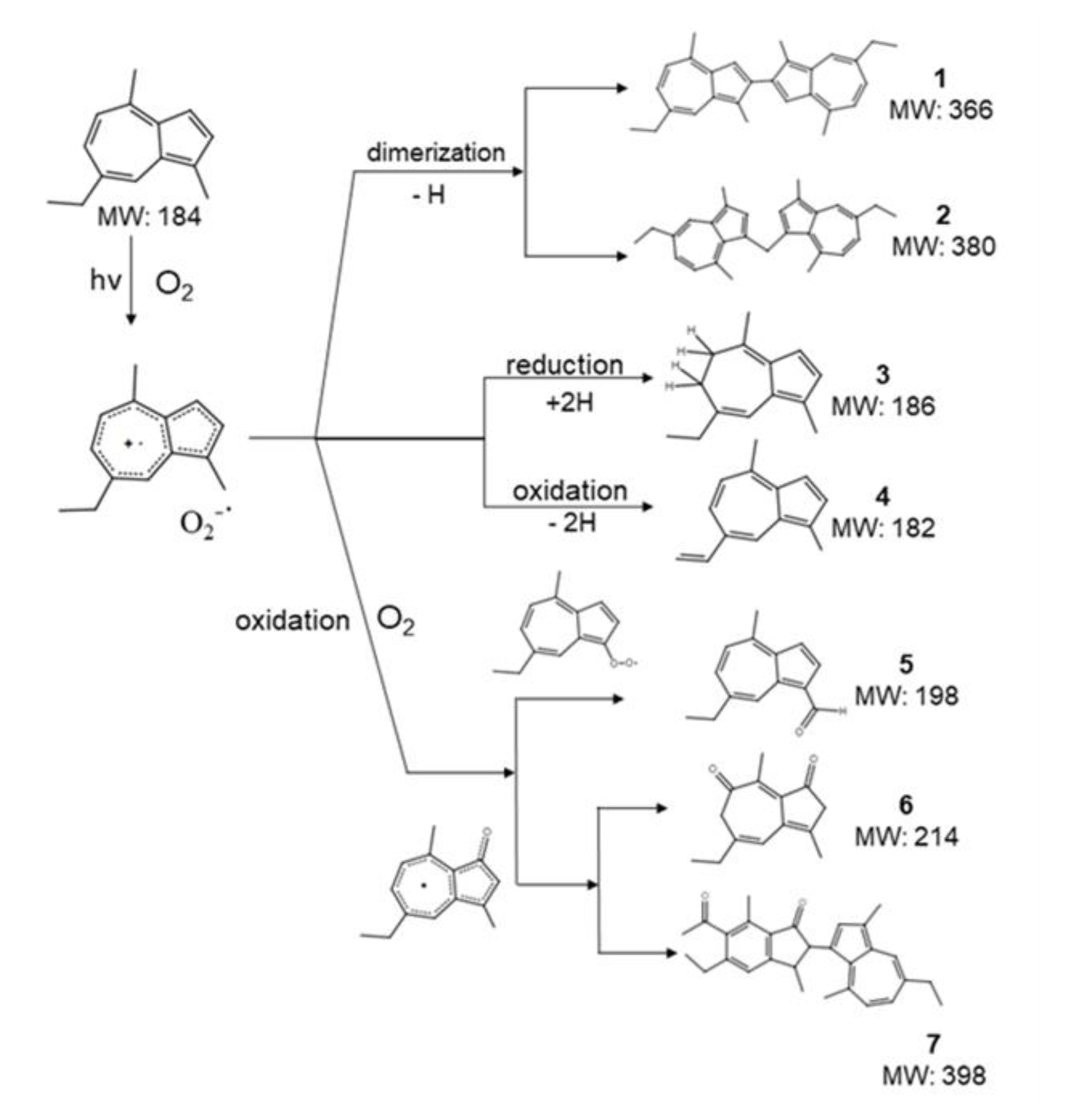
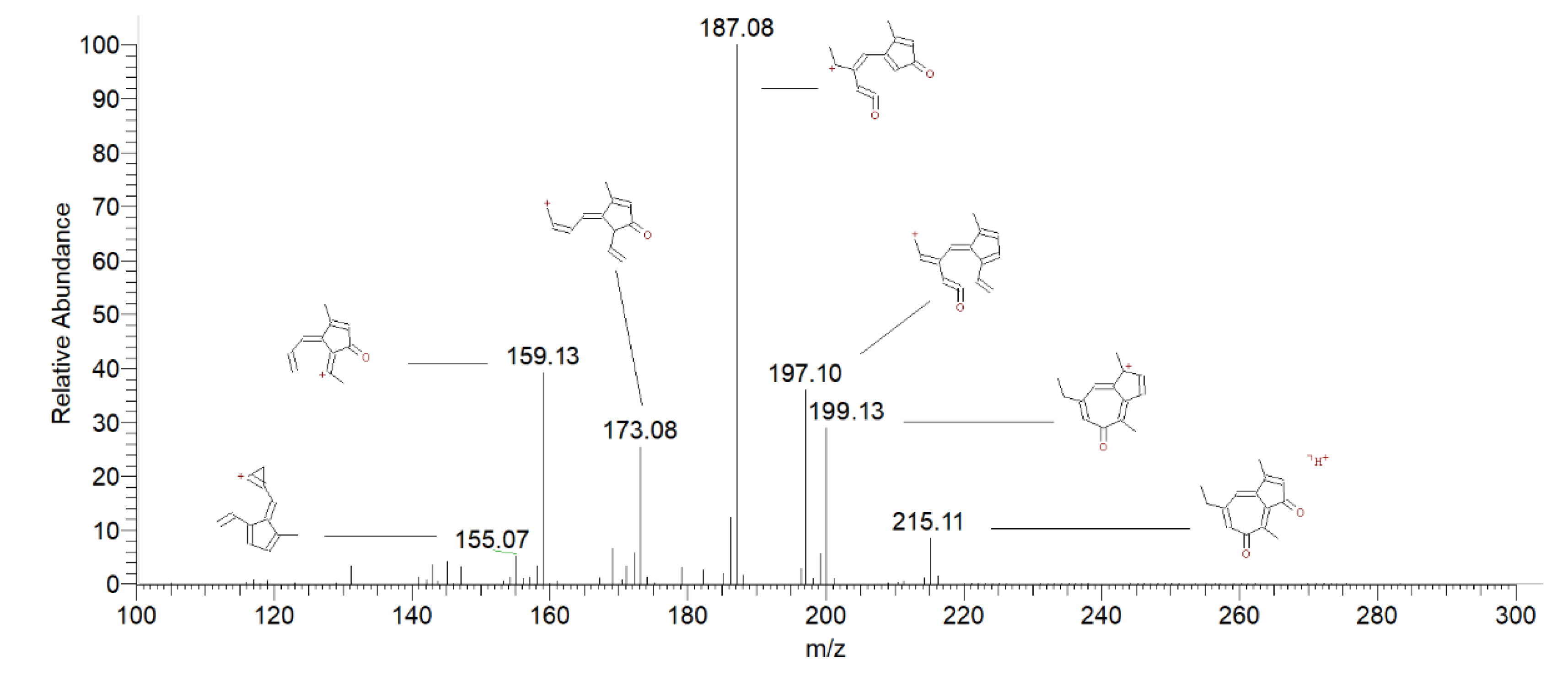
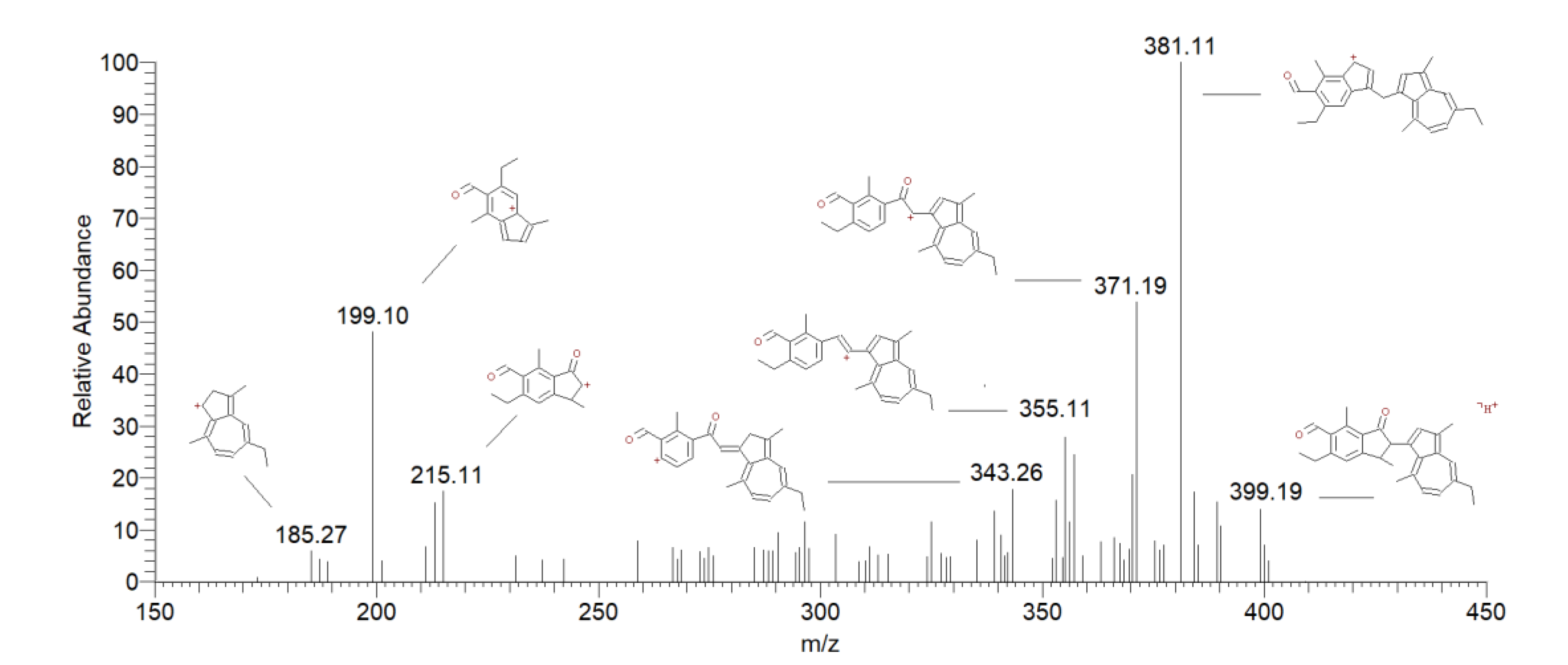
| Peak # | Compound | Retention Time (min) |
Parent peak m/z [M + H] + | Main Product ion | RA (%) |
|---|---|---|---|---|---|
| 2 | Oxidized chamazulene | 2.88 | 183 | 169 [M+H-CH2] + | 48 |
| 155 [M+H-CH2=CH2] + | |||||
| 141 [M+H-C3H6] + | 38 35 |
||||
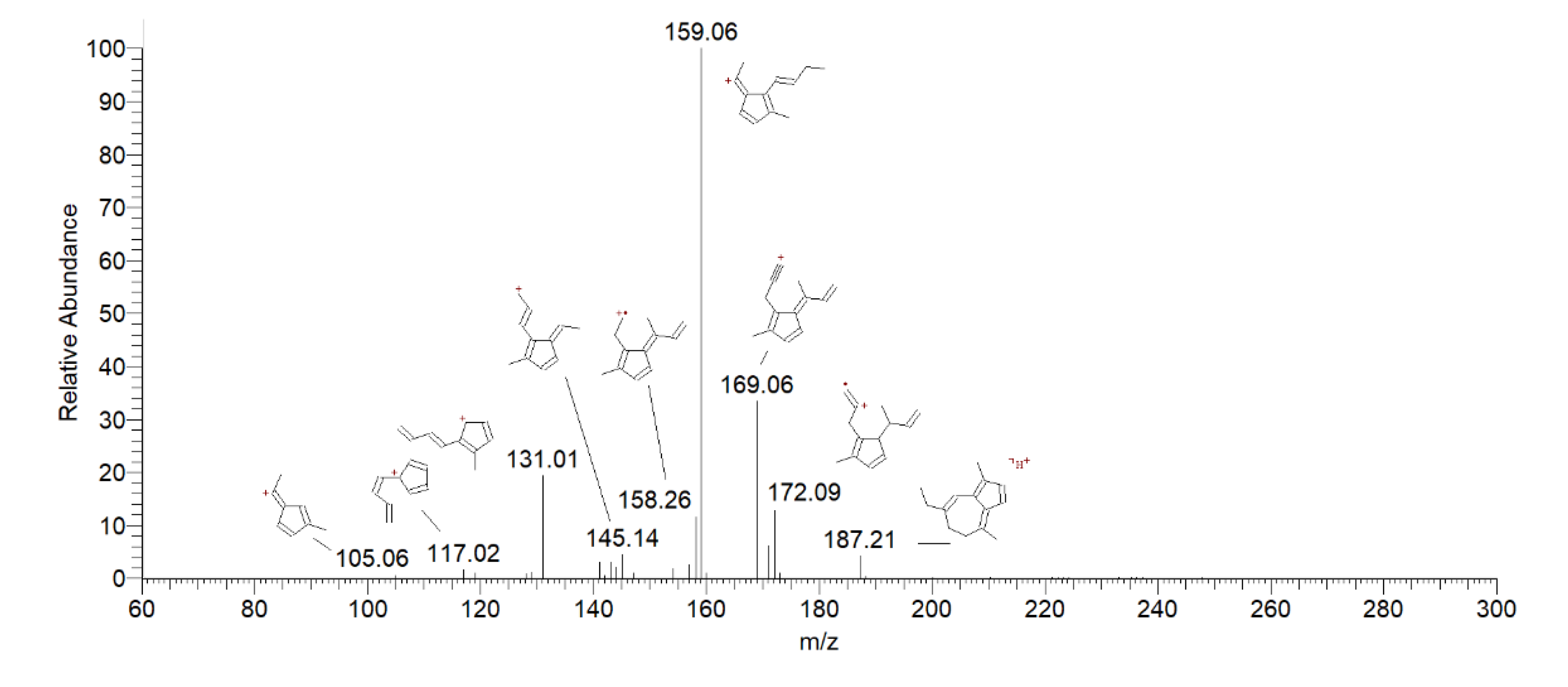
| 3 | Chamazulene quinone | 3.61 | 215 | 197 [M+H-H2O] + | 40 |
| 187 [M+H-CO] + | 100 | ||||
| 173 [M+H-CH2CO]+ | 33 38 |
||||
| 159 [M+H-CH2=CH2-CO] + | |||||
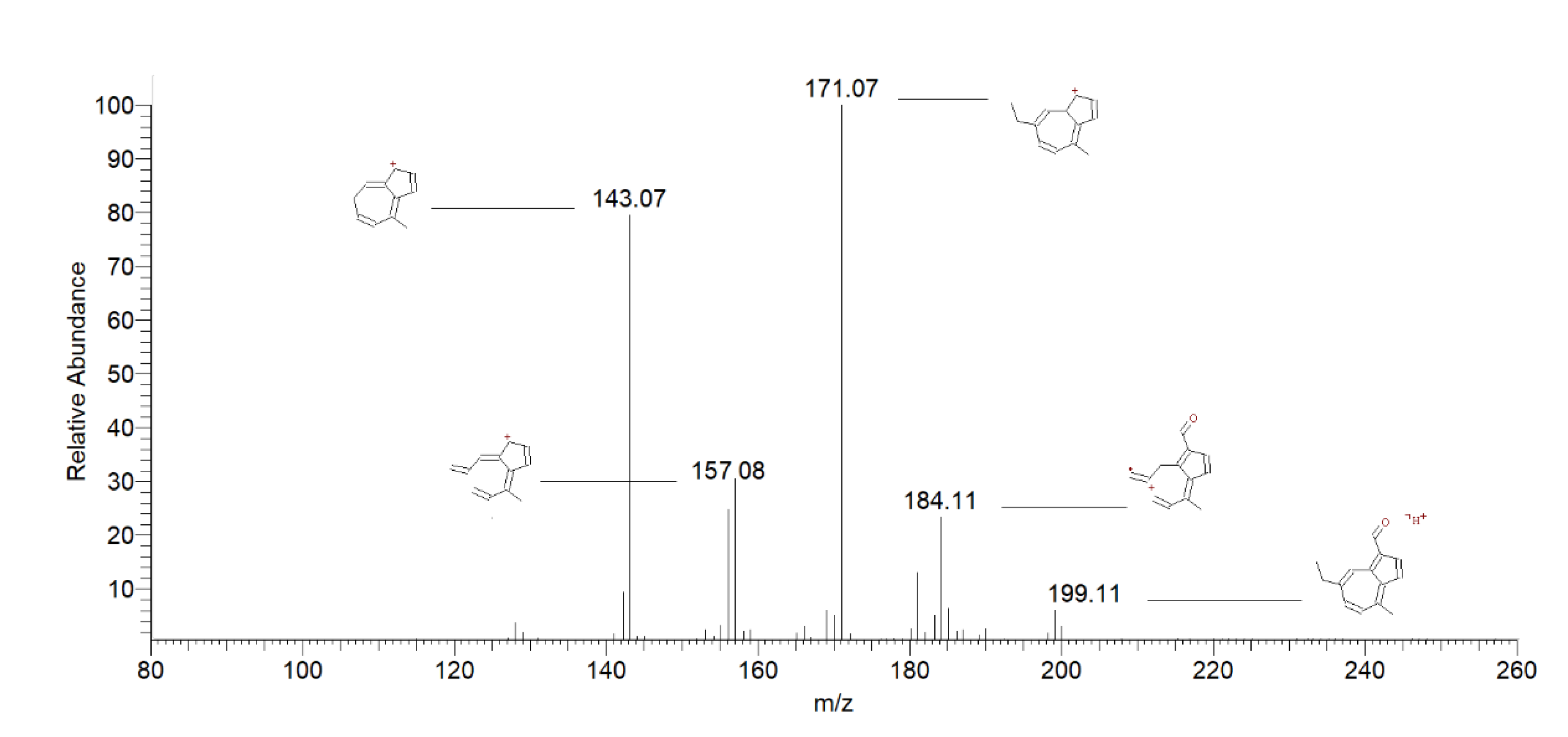
| 4,5 | Chamazulene Carbaldehyde | 3.75 | 199 | 184 [M+H-CH3] + | 25 |
| 3.85 | 171 [M+H-CO] + | 100 | |||
| 143 [M+H-CH2=CH2-CO] + | 78 | ||||
| 6 | Dihydrochamazulene | 4.44 | 187 | 172 [M+H-CH3] + | 15 |
| 159 [M+H-C2H4] + | 100 | ||||
| 131 [M+H-C4H8] + | 22 | ||||
| 7 | Chamazulene benzenoid | 5.79 | 399 | 381 [M + H-H2O] + | 100 |
| 371 [M + H-CO] + | 57 | ||||
| 215 [M+H-Chamazulene] + | 10 | ||||
| 199 [M+H-CH3Chamazulene] + | 63 | ||||
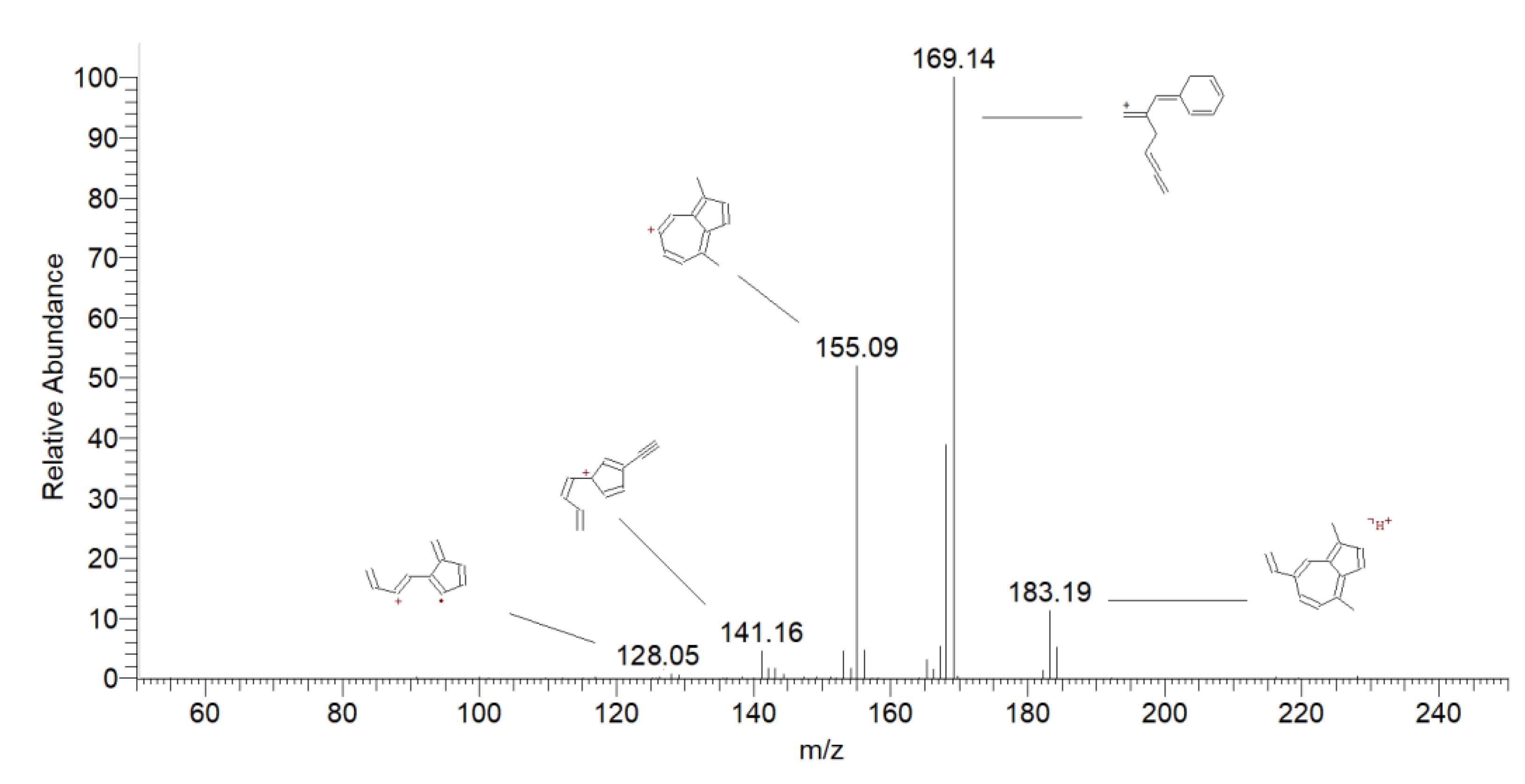
| 8 | Chamazulene | 7.89 | 185 | 169 [M + H-CH4] + | 100 |
| 129 [M + H-CH2=CHCH2CH3] + | 10 | ||||
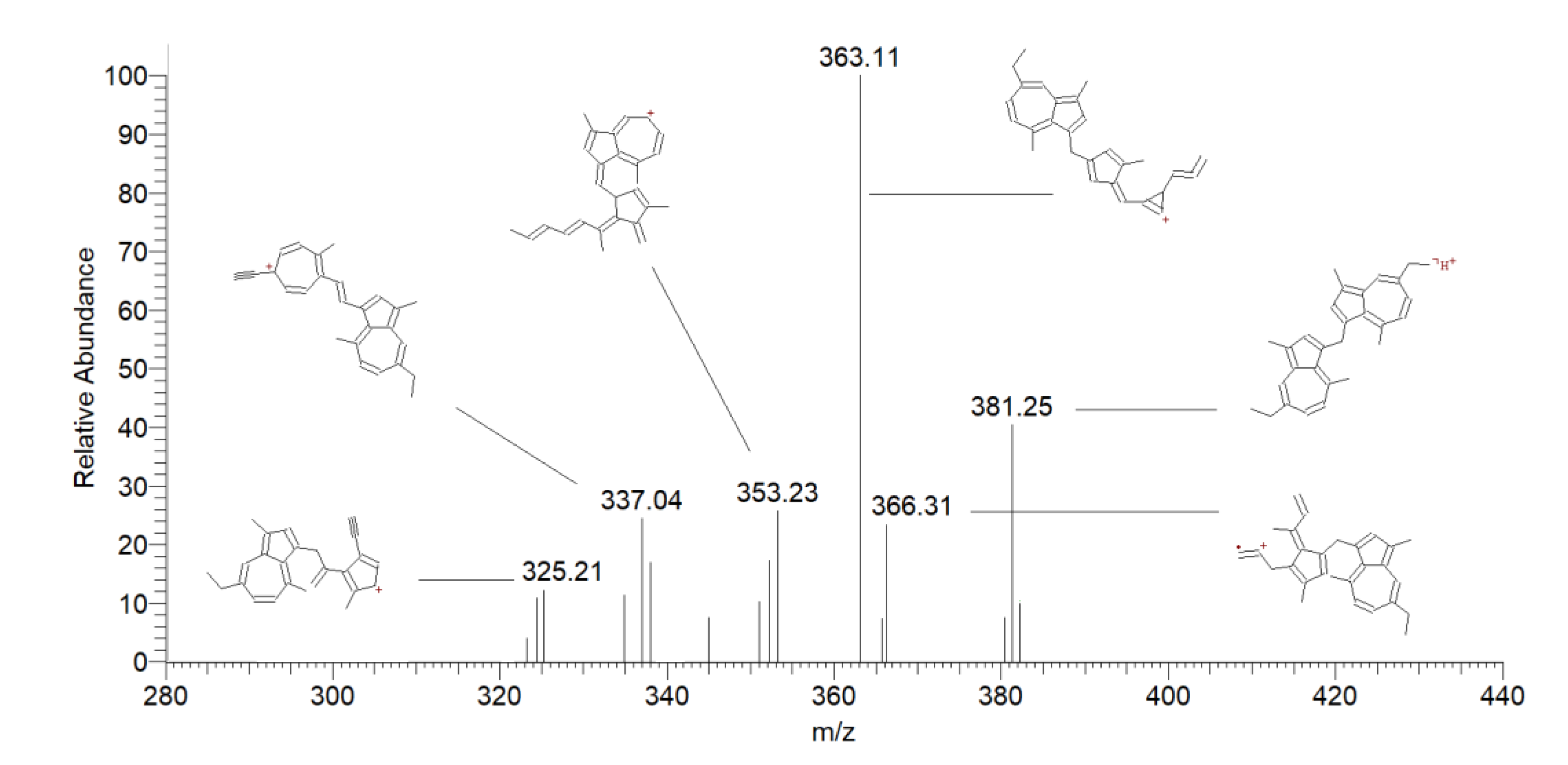
| 9 | Methylene dimers of chamazulene | 13.06 | 381 | 366 [M + H-CH3] + | 38 |
| 363 [M + H-H2O] + | 100 | ||||
| 353 [M + H-CH2=CH2] + | 22 | ||||
| 14.90 | 337 [M + H-CH2=CHCH3] + | 30 | |||
| 321 [M + H-C6H6] + | 33 | ||||
| 10 | Chamazulene dimers | 16.51 | 367 | 352 [M + H-CH3] + | 60 |
| 17.05 | 338 [M + H-CH3CH2] + | 100 | |||
| 18.28 | 309 [M + H-C4H10] + | 25 |
| Type of UV | Wavelength [nm] |
Radiance [mWcm-2 sr-1] |
Irradiance [mWcm-2] |
Energy absorbed [Jcm-2] (t = 15 min) |
Energy absorbed [Jcm-2] (t = 4 hrs.) |
|---|---|---|---|---|---|
| UVA | 320 - 400 | 35.2 | 2.4 | 2.2 | 34.6 |
| UVB | 290 – 320 | 7.5 | 0.5 | 0.5 | 8 |
| Air saturated | |||||
| HS 0 cm3 | HS 0.17 cm3 | HS 0.34 cm3 | |||
| n-Hexane | y = -0.0551x + 99.638 R2 = 0.9368 |
y = -0.0654x + 100.94 R2 = 0.9958 |
y = -0.0736x + 101.04 R2 = 0.9692 |
||
| Methanol | y = -0.1107x + 101.17 R2 = 0.9659 | ||||
| Acetonitrile | y = -0.3173x + 102.56 R2 = 0.9811 | ||||
| N2 purged | |||||
| HS 0 cm3 | |||||
| n-Hexane | y = -0.0009x + 100.72 R2 = 0.0033 | ||||
| Methanol | y = -0.0417x + 101.75 R2 = 0.7921 | ||||
| Acetonitrile | y = -0.1146x + 103.17 R2 = 0.8943 | ||||
| UV irradiated solutions of CA | Percentage of CA after UVA irradiation | ||||||
|---|---|---|---|---|---|---|---|
| 0 J/cm2 | 2 J/cm2 | 4 J/cm2 | 8 J/cm2 | 17 J/cm2 | 25 J/cm2 | 35 J/cm2 | |
| CA | 100 | 98±1 | 92±2 | 81±2 | 52±3 | 22±1 | 0 |
| MIXED WITH ANTIOXIDANTS | |||||||
| CA + TEMPO (1:10) | 100 | 96±3 | 90±2 | 83±4 | 73±3 | 53±1 | 33±1 |
| CA + Tocopherol (1:10) | 100 | 94±2 | 88±2 | 77±1 | 65±2 | 43±1 | 27±1 |
| CA + Ascorbyl palmitate (1:10) | 100 | 95±2 | 94±4 | 71±3 | 49±3 | 2±1 | 0 |
| CA + Bakuchiol (1:10) | 100 | 94±3 | 91±3 | 62±3 | 11±3 | 0 | 0 |
| CA + Hydroxytyrosol (1:10) | 100 | 94±3 | 92±3 | 80±2 | 62±2 | 37±1 | 6±1 |
| CA + Tocopherol + Bakuchiol (1:1:1) | 100 | 94±1 | 92±1 | 77±3 | 41±1 | 10±1 | 0 |
| CA + Ascorbyl palmitate + Tocopherol (1:1:1) | 100 | 91±2 | 80±2 | 68±3 | 17±1 | 0 | 0 |
| CA + Tocopherol + Hydroxytyrosol (1:1:1) | 100 | 93±2 | 81±4 | 72±3 | 37±2 | 8±3 | 0 |
| CA + Tocopherol +TEMPO (1:1:1) | 100 | 96±3 | 90±2 | 76±1 | 19±1 | 0 | 0 |
| CA + Ascorbyl palmitate + TEMPO (1:1:1) | 100 | 96±3 | 90±2 | 75±2 | 17±1 | 0 | 0 |
| CA + γ-terpinene + Ascorbyl palmitate (1:5:1) | 100 | 94±2 | 92±3 | 76±2 | 28±4 | 0 | 0 |
| CA + γ-terpinene + Hydroxytyrosol+ (1:5:1) | 100 | 96±2 | 90±3 | 78±1 | 38±2 | 0 | 0 |
| CA + γ-terpinene + TEMPO+ (1:5:1) | 100 | 93±1 | 93±2 | 80±2 | 57±2 | 24±1 | 6±1 |
| MIXED WITH UV FILTERS | |||||||
| CA + Tinosorb® S (5%) | 100 | 92±2 | 92±3 | 86±2 | 73±4 | 65±2 | 48±1 |
| CA + Octocrylene (5%) | 100 | 98±3 | 90±2 | 79±3 | 57±1 | 22±1 | 0 |
| CA + Octyl methoxycinnamate (5%) | 100 | 100±1 | 99±3 | 97±3 | 70±3 | 45±2 | 20±2 |
| CA in the dark (negative control) | 100 | 100±2 | 99±2 | 99±2 | 100±1 | 99±3 | 100±2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





