1. Introduction
Quinoa (
Chenopodium quinoa Willd.), an annual species of the Amaranthaceae family that originates from the Andean region of South America, has been cultivated for around 7000 years [
1]. It is a plant that stands out for its excellent nutritional value and has been known since ancient times in the Inca Empire. Despite having cereal characteristics, quinoa is designated as a “pseudo-cereal” as not belonging to the Gramineae family [
2]. Quinoa seeds have a higher nutritive value than most cereal grains and contain high-quality protein with the essential amino acid balance, high levels of fatty acids, vitamins, minerals, dietary fibers. In addition to presenting high nutritional quality, it is also characterized by being gluten-free, which is suitable as more nutritious food for patients with celiac disease [
2,
3]. Meanwhile, the genetic variability of quinoa is very plentiful, which can be adapted to a wide range of agroecological conditions, with cultivars of quinoa being grown from sea level to 4000 meters above sea level, from 40 ° S to 2 ° N latitude, from 40% to 80% humidity, and from cold, highland climate to subtropical conditions [
4]. It has a high tolerance to adverse environmental stress, such as drought, salinity and frost [
5]. The above-mentioned characteristics make quinoa a strategic crop for providing nutrition and playing a significant role in food security in the face of climate change, especially increasing salinization and aridity of the worldwide [
6,
7]. To focus world attention on the role that quinoa´s biodiversity and nutritional value play in providing food security and nutrition and in poverty eradication, the United Nations declared the year 2013 as “the International Year of Quinoa” (IYQ) [
8].
The number of countries growing quinoa has increased rapidly from eight in 1980, to 40 in 2010, and to 95 in 2015, which has been introduced in Europe, North America, Asia and Africa [
1]. In contrast, the history of quinoa cultivation in China is very short. Although experts from the Chinese Academy of Agricultural Sciences (CAAS) introduced quinoa germplasm in the 1960s, no relevant research was conducted due to a lack of knowledge about this pseudocereal. Until 1988, Tibet Agriculture and Animal Husbandry College introduced three quinoa lines from Bolivia and carried out preliminary planting experiments. However, these works were limited to simple studies of biological characteristics, cultivation and progeny selection [
9,
10,
11]. Since the turn of the 21st century, increased global research activities on quinoa combined with an explosion in the economic value of the crop have contributed to large-scale quinoa cultivation in Shanxi Province in 2008 and the breeding of some new cultivars adapted to the local environment. Henceforth, the number of provinces growing quinoa has risen rapidly to more than 20. The area under quinoa cultivation and the total output in China has increased from 1 300 ha and 2 100 t in 2014 to about 16 600 ha and 28 800 t in 2019, which indicates that China has become the third largest quinoa production country in the world, and is only surpassed by the traditional countries of origin: Peru and Bolivia.
There are large differences in quinoa farming systems and cultivation practices in China due to the vast territory, complex terrain and diverse climates. It is important to identify and develop of optimally adapted varieties. Meanwhile, study of the corresponding field farming practices is also needed. The objective of this research was to evaluate different introduced quinoa varieties for main agronomic traits performance under different altitude varied from 670 masl to 2200 masl in Ili state, Xinjiang province, which is located at northwest frontier of China. In addition, we also investigated the changes in the contents of total starch, protein, fat, ash, phenolic compounds and flavonoids of quinoa seeds harvested from different altitude.
2. Materials and Methods
2.1. Plant Material and Field Experiment
Six variety resources introduced from different provinces were evaluated in this study: two officially registered varieties named “Longli 1” and “Longli 4” were introduced from Gansu province; two resources named “Beijing 1” and “Beijing2” were introduced from Beijing; “Nongli 3” was introduced from Qinghai province; while “Jinli 1” was introduced from Shanxi province. Field experiment was conducted at four different stations of Ili institute of Agricultural Science in Yili State, Xinjiang province during the 2017 growing season. The climatic and environmental characterization of the four locations was shown in
Table 1. Before sowing, the soil required a fine preparation (flat, soft, moisture preservation), and adequate sole fertilizer application (300 kg / ha of granular organic fertilizer). The experiment was determined by the randomized blocks design of three replications. Each experiment plot was 20 m
2 (5 m long x 4 m wide), 50 cm between rows (9 rows per plot) and 15-20 cm between plants. The crop planting dates in the different locations were all early May in 2017 except location of Yining City which was 11 April 2017 (
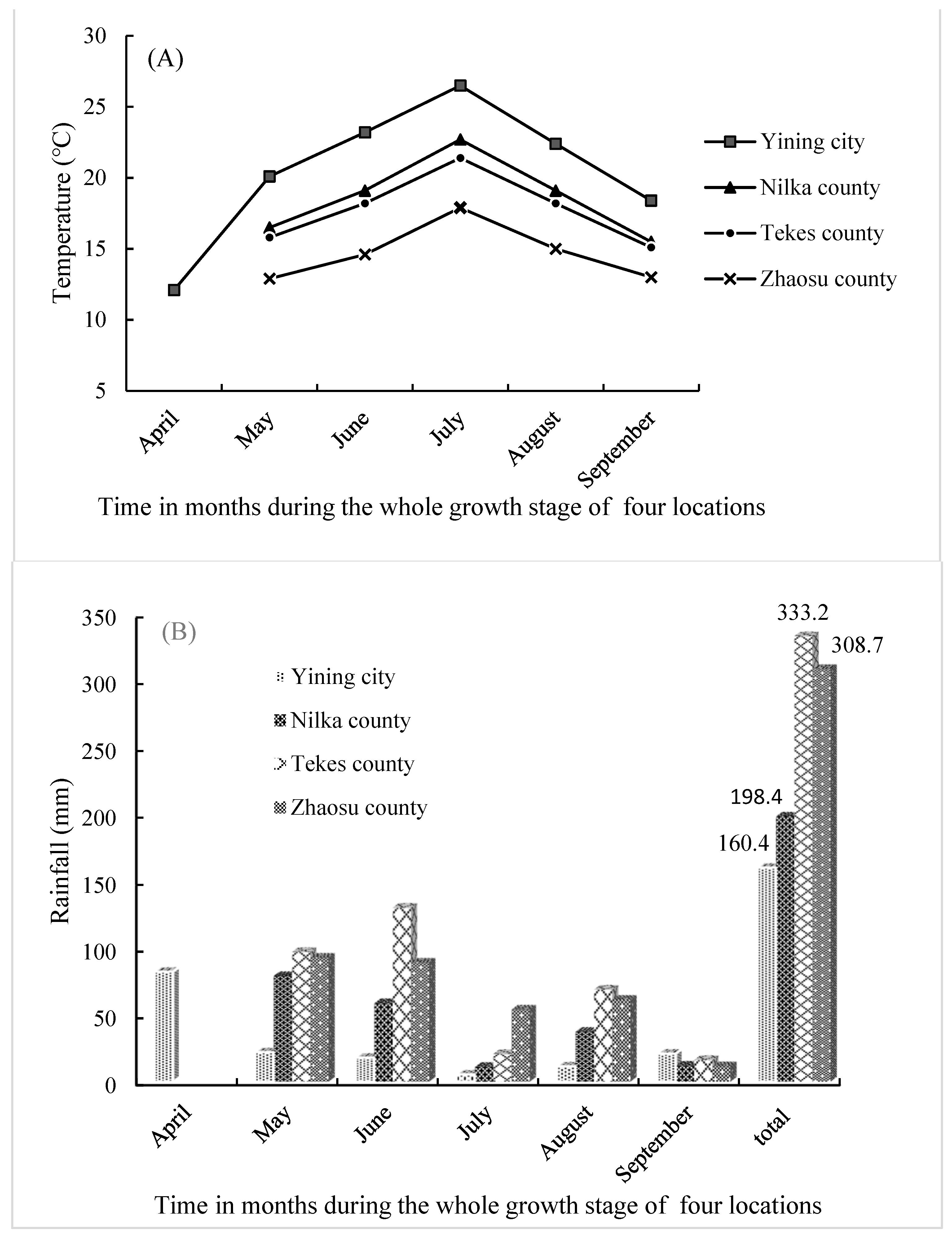
Table 1). The sowing depth was 2-3 cm with artificial ditching. To the seedling stage (4-6 leaves), the final singling of seedlings was made as 1 plant per pot. In squaring stage, granular organic fertilizer (75 kg / ha) was applied. The temperature and rainfall of each month of the four different locations during the whole growth stage were recorded in the present study (
Figure 1).
2.2. Measurement of Agronomic Traits
Growth and development stages were recorded as: period of seedling, branching, squaring, anthesis, and maturity (50% of the plants show and reach these traits from each plot). Before and after harvest, data on the following traits were measured, plant height (cm), stem diameter (cm), branches, effective branches, panicle length (cm), and 1000 seed weight (g). Harvested seed per plot was weighed and grain yield per hectare was estimated at 10-12% moisture content.
2.3. Quality Traits Analysis
Before analysis, whole quinoa seeds of each variety resources were grounded and passed through 60 mesh screens. The compositions of moisture and ash were determined according to the methods of [
12,
13]. The protein and fat contents were determined by the kjeldahl method with FOSS 2300 type automatic azotometer [
14], and the Soxhlet extraction method [
15], respectively. The total starch content was determined by commercially-available assays (Megazyme International Ireland Ltd, Wicklow, Ireland).
Total polyphenol and flavonoid were extracted according our previously study [
16]. The total polyphenol content was determined using the Folin-Ciocalteus method which was expressed as mg Gallic Acid Equivalents (GAE) per gram of dry weight (r
2 = 0.9931) [
17].
The total flavonoid content was analyzed according to a colorimetric assay described by [
18] with some modifications. Briefly, the appropriate dilution of extractions (0.2 mL) was mixed with 2mL of 0.125% (2-aminoethyl) diphenylborate solution. The mixture was incubated at room temperature for 10 min and the absorbance was measured at 405 nm using the UV/VIS spectrometer. The total flavonoid content was expressed as mg quercetin Equivalents (QE) per gram of dry weight (r
2 = 0.9955).
Extraction of total saponins was performed according to a previous method with some modifications [
19]. Briefly, 1 g sample was extracted with 40 mL of methanol at 60 °C under reflux for 3 h. Then, the solution was centrifuged at 2500×g for 10 min to separate the supernatant. The total saponins content was determined as described previously, with some modifications [
20]. The supernatant (1 mL) or oleanolic acid standard solution (0.1–0.5 mg/mL) was mixed with 0.8 mL of 5% (w/v) vanillin in acetic acid and 3.2 mL of 72% perchloric acid. After incubated in a water bath at 50 °C for 10 min, the mixture was then cooled by circulating water. The absorbance was measured at 560 nm using the UV/VIS spectrometer. The total saponins content was expressed as mg oleanolic acid equivalents (OAE) per gram of dry weight (r
2 = 0.9972).
2.4. Statistical Analysis
Results are expressed as means ± standard deviations of triplicate determinations. ANOVA and Tukey’s test were performed using IBM SPSS Statistics version 22.0 software. Statistical significance was established at p < 0.05.
3. Results
The influence of different elevations on both agronomic and quality characteristics of quinoa in the four northwestern parts including Yining city, Nilka County, Tekes County and Zhaosu county of Xinjiang province of China were evaluated. By conducting a thorough analysis, the effect of elevation on the performance of six varieties of quinoa (Nongli 3, Beijing 1, Longli 4, Beijing 2, Longli 1, Jinli 1) with a particular emphasis on agronomic attributes and quality metrics were determined.
3.1. Agronomic Traits of Quinoa Genotypes in Various Altitudes
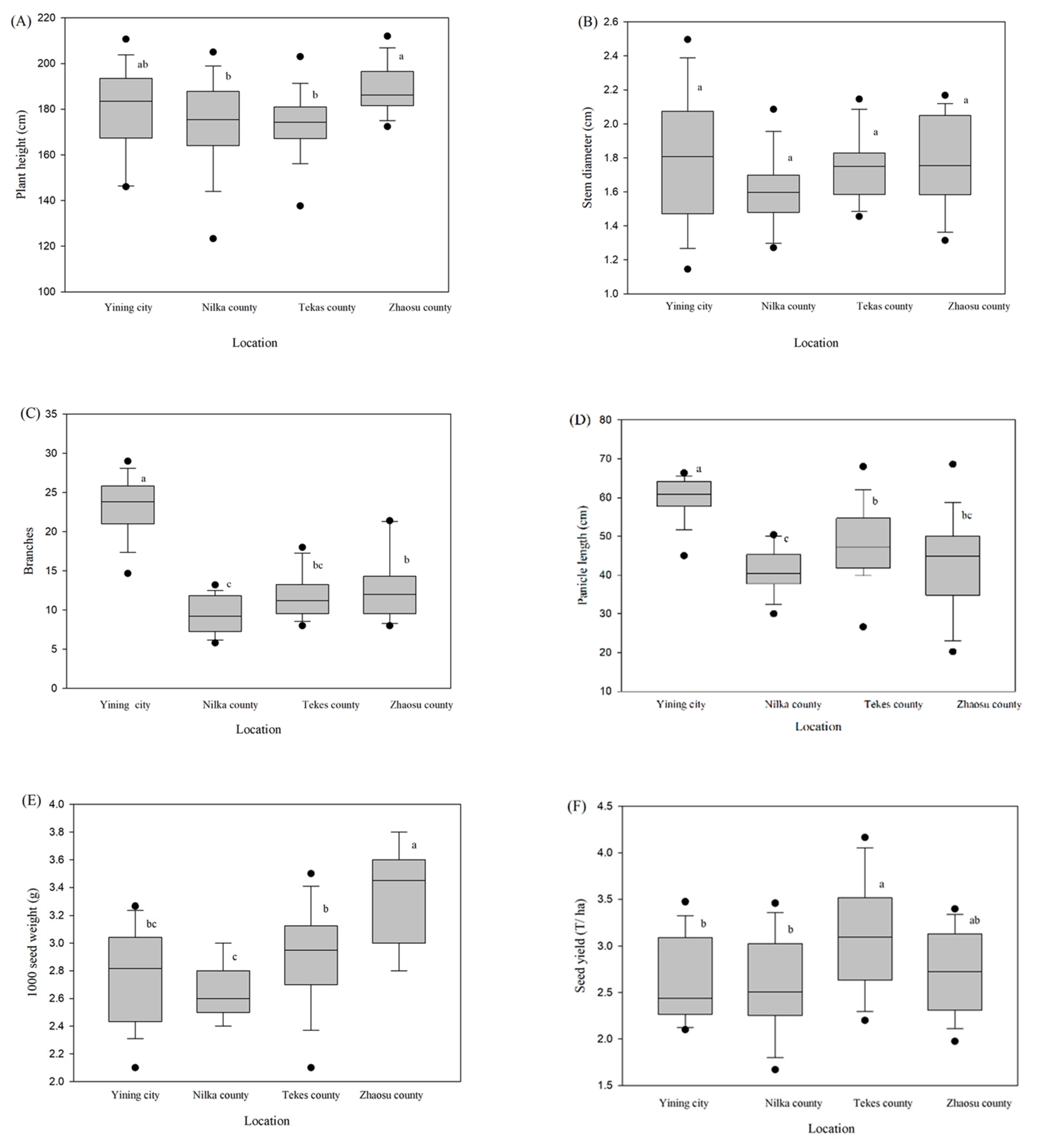
The agronomic traits of different quinoa genotypes in various altitudes of Xiangjiang province were significantly different (
Table 2). The average height of all six genotypes was highest in Zhaosu County, measuring 189.38, followed by Yining city with a height of 179.35 (
Figure 2A). Jinli 1 and Beijing 2 achieved unprecedented height in Zhaosu County and Yining city, surpassing the 200 mark (
Table 2). The stem diameter did not significantly vary between altitudes, with the exception of Zhaosu County, where Jinli 1 recorded a diameter of 2.12 cm (
Figure 2B;
Table 2). With a mean of 23.43 and no discernible genotypic variations, Yining city had the highest Branch number; nevertheless, the Beijing 2 genotype had the highest Branch number (26.00), followed by Beijing 1, respectively. Nilka County exhibited the lowest branch number among the four zones, with a mean of 9.43 across the genotypes (
Figure 2C;
Table 2). The length of the panicle for each genotype in the Zhaosu and Nilka counties were not statistically different from one another, however, the longest panicle was found in Yining city, with a mean length of 62.44 cm, and genotype Longli 4 recorded 64.78 cm, the longest, followed by Beijing 1 (
Figure 2D;
Table 2). The correlation analysis unveiled significant positive correlation between plant heigh and stem diameter (r = 0.39, p <0.000). Additionally, a strong positive correlation (r = 0.59, p < 0.000) was observed between number of branches and panicle length, underscoring the relationship between branch development and the length of the panicle (
Table 3).
3.2. Yield Performances and Phenology for All Tested Varieties by Site
The yield performances, including 1000 seed weight (g) and seed yield (T/ha), as well as phenology, including flowering days and crop cycle of all the examined cultivars exhibited considerable variations across different sites. The genotype and location displayed significant statistical differences, as did the genotype by location interaction (P < 0.001;
Table 2 and
Table 4).
3.1.1. Yining City
Among the genotypes examined in Yining city, it was noted that the average time for flowering among the tested genotypes was remarkably fast, with an average of 80 days. This length was the shortest among the four unique altitudes being studied. Beijing 2 had the earliest blossoming, followed closely by Longli 4. Conversely, Longli 1 had a longer flowering period, needing roughly 100 days to reach flowering stage. The crops with the shortest growth cycles were Longli 4, Beijing 2, and Jinli 1, which took a total of 125.33, 127.67, and 128.00 days to mature, respectively (
Table 5). As shown in
Table 2, Jinli 1 and Beijing 2 showcased remarkable performance in seed yield, surpassing all other quinoa varieties with recorded yields of 3.34 and 3.06 t/ha, respectively. However, it's noteworthy that the 1000-grain weight for Beijing 1 and Longli 1 was significantly lower compared to the other genotypes.
3.1.2. Nilka County
All investigated genotypes showed statistically significant variations in phenological and seed yield parameters in Nilka County, according to the research. Among the genotypes, Jinli 1 and Beijing 2 illustrated the lowest blossoming flowering time and crop cycle (
Table 5). Similarly, Jinli 1 and Beijing 2 had significantly higher 1000-seed weights of 2.87 and 2.83 g, respectively, indicating their superior performance, while Longli 4 and Jinli 1 were particularly notable among all the genotypes, as they exceeded a yield of 3 t/ha (
Table 2).
3.1.3. Tekes County
The county of Tekes displayed significant variability in both phenological and seed yield indices. The Beijing 1 genotype had a significantly shortened blooming period, finishing the process in a mere 71.00 days (
Table 2). In addition, Longli 4 and Jinli 1 had the shortest crop cycles overall, with durations of 108.00 and 109.00 days, respectively. Considering Jinli 1’s noteworthy accomplishment of having shortest crop cycle and 1000 seed weight (g), it substantiated its excellence by yielding tremendously impressive 4.05 t/ha, surpassing all other evaluated genotype at various altitudes. Furthermore, Longli 1 and Beijing 2 ranked 3rd in 4th in Tekes County and showed notable seed production, with yields of 3.46 and 3.38 tons per hectare, respectively, closely tracking Jinli 1 (
Table 2).
3.1.4. Zhaosu County
Blooming period in the Zhaosu County was the longest compared to the various latitudes. Comparatively, Nongli 3 and Longli 1 showed the longest flowering and crop cycle. Among them, shortest crop cycle was observed by Longli 4 with 116 days (
Table 5). Despite having the lengthiest crop cycle in Zhaosu County, all the quinoa genotypes displayed a relatively high 1000-seed weight (g) at the tested elevation sites. Jinli 1 emerged as the highest achiever, surpassing all other varieties and yielding an impressive 3.28 t/ha of seeds. Closely behind, Longli 1 also demonstrated commendable performance, yielding 3.14 t/ha (
Table 2).
The correlations between the seed yield and agronomic traits revealed that seed yield was not associated with stem diameter and branches, but it was negatively associated with crop cycle (r = 0.40, p < 0.001) and positively correlated with plant height (r = 0.40, p < 0.001). 1000-seed weight showed no correlation with either agronomic and phenological traits (
Table 3). An affirmative correlation was detected between the duration of the crop cycle and the number of days in which flowering occurs (r = 0.29, p < 0.05), suggesting that a greater length of time for blooming generally corresponds to a longer overall duration of the crop cycle. On the other hand, there was a strong positive association (r = 0.48, p < 0.001) between the number of branches and crop cycle.
3.3. Nutritional Quality Analysis
The nutritional metrics, encompassing indicators such as total starch, Protein, fat, ash, total polyphenol, total flavonoids, total saponins showcased significant variations across the various examination sites (
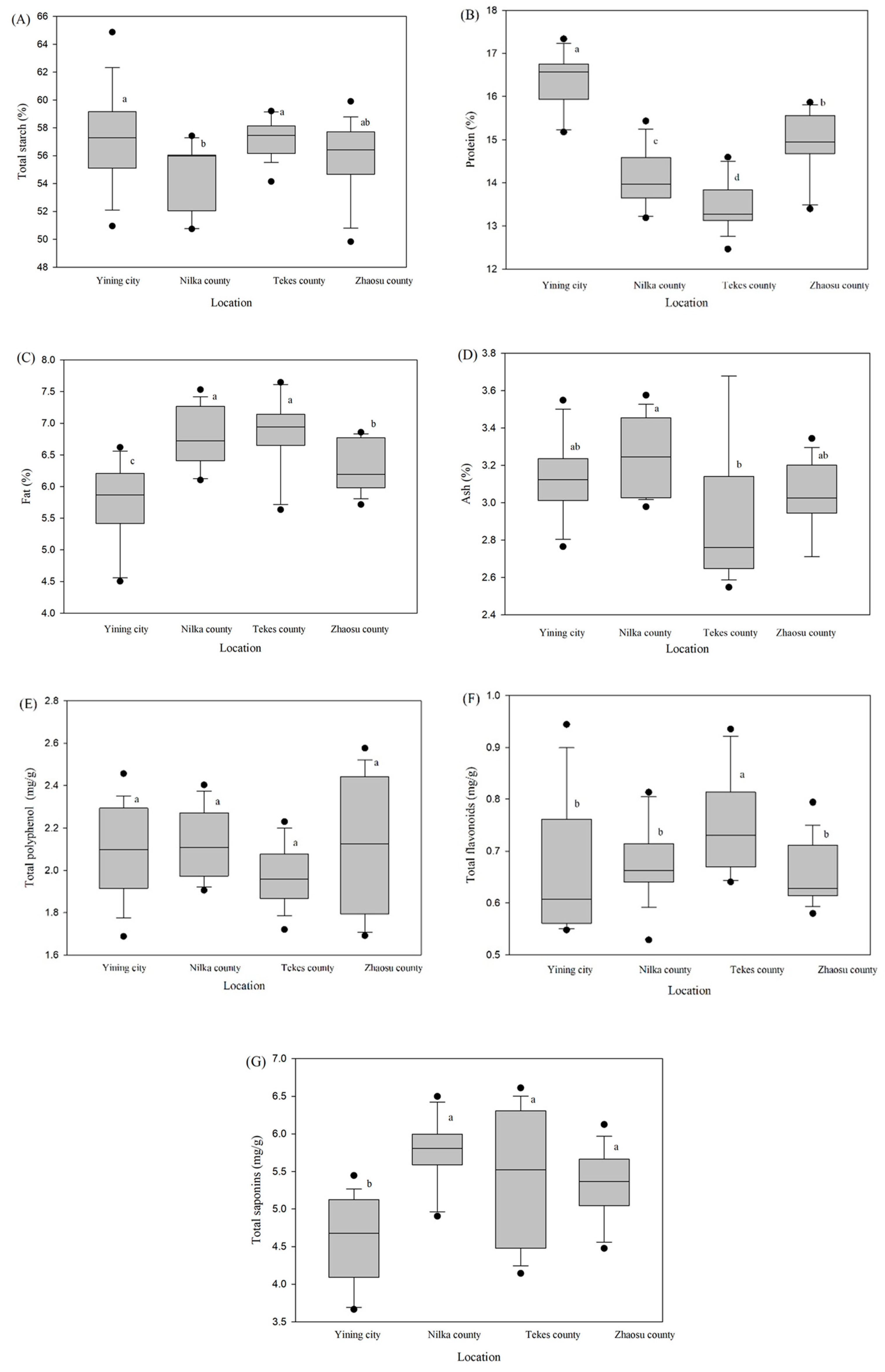
Table 6). In Zhaosu County, no substantial differences were detected in total starch content among the various genotypes. However, Longli 4 stood out with the highest total starch content of 62.04% and 57.27% in both Yining city and Nilka County compared to the other quinoa genotypes, but among the seeds in Tekes County, the Beijing 2 genotype substantially indicated the greatest concentration of total starch (59.13%). The Longli 1 genotype consistently displayed the lowest overall starch content at three distinct altitudes, with recorded values of 52.23%, 51.65%, and 55.66% in Yining city, Nilka County, and Tekes County, respectively. The Jinli 1 genotype demonstrated the highest protein content in two distinct altitudes and climates, with recorded values of 17.21% in Yining city and 15.22% in Nilka County. Nevertheless, under the most frigid environments and at the greatest elevation in Zhaosu County, Nongli 3, Beijing 2, and Jinli 1 exhibited notably increased protein concentrations in their seeds, measuring 15.72%, 15.52%, and 15.46%, respectively. Significant variations in fat content were detected across the different genotypes and altitudes, notably, Beijing 1 in Nilka County and Zhaosu County, while Jinli 1 across all altitudes emerged as an exceptional performer with the highest fat reserves of (6.55%, 7.41%, 7.61%, and 6.79%) in its seeds. Statistically significant variations in ash percentage were observed across different genotypes and altitudes. Statistically significant variations in ash percentage were observed across different genotypes and altitudes. Precisely, Longli 1 exhibited an ash percentage of 3.50% in Yining city, whereas both Longli 1 and Nongli 3 recorded values of 3.52% and 3.46% in Nilka County. In Tekes County, Nongli 3 presented the highest ash percentage, reaching 3.68%. Furthermore, in Zhaosu County, Beijing 1 and Longli 1 demonstrated ash percentages of 3.29% and 3.19%, respectively.
Total polyphenol was highest in Nongli 3 in moderate climatic regions Nilka County and Tekes County, with recorded values 2.36 and 2.21 mg/g, respectively. In Yining city, Beijing 2 and Jinli 1 had the lowest total polyphenol content, recording values of 1.89 mg/g and 1.82 mg/g, respectively. Conversely, in Zhaosu County, the same genotypes along with Nongli 3 outclassed all other genotypes, with Beijing 2, Jinli 1, and Nongli 3 presenting higher total polyphenol values of 2.43 mg/g, 2.37 mg/g, and 2.50 mg/g, respectively. Beijing 2 had exceptional performance in both Yining city and Zhaosu County, with reported values of 0.90 mg/g and 0.72 mg/g, respectively, surpassing all other genotypes in terms of total polyphenol content. On the other hand, Beijing 1 had the greatest total flavonoid levels at all elevations, with the exception of Yining city, at 0.78 mg/g, 0.91 mg/g, and 0.74 mg/g, respectively. Significantly low saponin content was observed in various genotypes in various altitudes. In Yining city, genotype Jinli 1, Beijing 2, and Longli 3 revealed the lowest saponin content, measuring 3.83 mg/g, 4.26 mg/g and 4.02 mg/g. Similarly, in Nilka County and Tekes County, Beijing 2 had significantly low amounts of saponin, with recorded values of 6.34 mg/g and 4.29 mg/g, respectively. Among the genotypes studied, Longli 4 had the lowest saponin content in Zhaosu County.
The relationships between nutritional, agronomic, and phenological traits yielded interesting insights. As shown in
Table 3, the total starch demonstrated no association with most of the traits, except for negative association with flowering days (r = 0.39, p < 0.001) and a positive association with 1000-seed weight (r = 0.33, p < 0.001). The protein content of quinoa seeds showed a positive correlation with both the crop cycle (r = 0.49, p < 0.001) and the number of branches (r = 0.63, p < 0.001), whereas fat content displayed the opposite pattern and showed a negative correlation with both variables (r = 0.77, p < 0.001; r = 0.48, p < 0.001). Significant strong positive relationship was displayed between Ash and total polyphenol with flowering days and crop cycle, while both were negatively associated with seed yield. Moreover, total flavonoids detected no relationship with agronomic and phenological traits, while strong negative association was observed with protein (r = 0.33, p < 0.001). Total saponin exhibited strong negative correlated with several traits, including branches (r = 0.53, p < 0.001), panicle length (r = 0.59, p < 0.001), total starch (r = 0.48, p < 0.001) and protein (r = 0.42, p < 0.001). Conversely, it presented positive correlation with ash (r = 0.37, p < 0.001) and total polyphenol (r = 0.31, p < 0.001).
3.3. Traits across the Study Area
The results demonstrate a complex connection between altitude and different genotypes of quinoa, providing insight into the crop's ability to adapt and respond to a wide range of environmental conditions. The 4 distinct elevations (Yining city at 650 masl, Nilka County at 1050 masl, Tekes County at 1400 masl, and Zhaosu County at 2200 masl) had a substantial impact on both agronomic traits and nutritional quality traits, as detailed in
Table 1 and
Table 4. The analysis revealed that genotypic variation was not significant for branches and panicle length, however, with the exception of stem diameter, the interaction between locations and genotypes was significant for all agronomic, phonologic, and nutritional traits (
Table 4). From the warmest to the coldest and lowest altitude to the highest (
Figure 1A), there were substantial differences among Yining City, Nilka County, Tekes County, and Zhaosu County, except for stem diameter and total polyphenol (
Figure 2 and
Figure 3). The warmest city, Yining city, revealed the highest branch number and panicle length. On the other hand, the coldest location, Zhaosu County, followed by Tekes County, recorded the highest 1000-seed weight. However, Tekes County had the maximum seed output per hectare across all genotypes (
Figure 2C–F). Variations in nutritional traits content were remarkably dissimilar between the various climates (
Figure 3). Warmer climate of Yining city received more protein and less fat and Low saponin in their seeds followed by the colder, higher altitude region of Zhaosu County (
Figure 3B,C,G).
4. Discussion
4.1. Ecological Adaptability of Quinoa in Different Regions
Testing quinoa's ability to thrive in various environmental and geographical conditions is of significant interest due to the rising international demand for the grain, which is boosted by both agricultural development and the Andean export commodity [
21,
22,
23]. Improvements in crop agronomy for various climates are largely responsible for the rise in quinoa cultivation areas around the world. There has been a recent uptick in the practice of selectively breeding plant types for increased demand in nations with certain environmental requirements [
22,
24]. Quinoa is an exceptional crop for many parts of the world because of its remarkable adaptability to a wide variety of climates and soil types [
25]. Quinoa exhibited remarkable ecological adaptability in various geographical areas, as indicated by the significant variations in phenological, agronomic, and nutritional quality characteristics identified in Xinjiang province, China, focusing on its response to variations in elevation, temperature, soil, precipitation (rainfall), and other environmental factors. The flexibility of quinoa genotypes to respond to varied environmental conditions was reflected in the large variability in agronomic parameters that were noticed between and across different altitudes. Our findings demonstrate quinoa's various environmental adaptations. Quinoa genotypes in colder, higher altitude places like Zhaosu County were taller, possibly to maximise light interception and photosynthesis in low-light situations. It has been observed that due to shorter daylight hours and lower light intensity, plants in colder temperatures impede plant development but may stimulate taller plants to optimise light interception for photosynthesis [
26,
27,
28]. In contrast, quinoa plants in lower altitude areas such as Yining city showed increased branch growth and longer panicle lengths, possibly due to less rainfall and the necessity to enhance reproductive success. Nevertheless, Nilka and Zhaosu County received a significant quantity of rainfall and are much colder climates in comparison to the other two regions, leading to greater 1000 seed weight and seed production per hectare. Similar results were also reported by [
29].
Although Yining city had the earliest flowering time (80 days), it also had the longest crop cycle (132 days) among the genotypes (
Table 5). Yining city's Jinli 1 and Beijing 2 genotypes produced the earliest flowers and the maximum seed yields in comparison to other varieties. Jinli 1, Beijing 2, and Longli 4 presented a uniformly shorter crop cycle across all geographical regions, whereas Longli 4 produced the maximum seed yield in Nilka, followed by Jinli 1. It is noteworthy that Jinli 1 exhibited the maximum seed yield in both Zhaosu County and Tekes County when compared to the other genotypes. The varieties cultivated in Yining City have the highest number of branches, longest panicle length, respectable stem thickness, and plant height, as well as the highest protein content in their seeds, making it a prime area for fodder production. In order to identify cultivars and develop breeding programmes that aim to enhance protein quantity and quality, protein-based methodologies have been implemented to characterise storage proteins in quinoa seeds [
30,
31]. However, it has been proposed that grain yield and grain size, which are predictors of crop commercial quality, are widely utilised as selection criteria for quinoa breeding [
32]. Nevertheless, our findings demonstrated diverse responses of the cultivars to changes in the environment. Similar observations were noted by [
31], who noticed cultivar-specific responses to environmental variation and the cultivar performance was primarily impacted by the crop cycle. In this study, quinoa illustrates exceptional resilience and adaptability in spite of diverse climatic conditions, spanning from the comparatively milder and lower altitude regions of Yining city to the colder and higher altitude regions of Zhaosu County.
4.2. Selection of Agronomic Traits for High-Yield Quinoa
In order to optimise quinoa yield at varying altitudes, our research emphasises the significance of selecting suitable agronomic characteristics. Significant variation in yield performance and phenology was observed among quinoa genotypes at various locations. The influence of plant height on the crop performance of quinoa genotypes is elucidated by the existence of a strong positive correlation between seed yield and plant height. Similar conclusions were also reported by [
33,
34]. Quinoa varieties are short-day plants with varying temperature and photoperiod sensitivity [
35,
36,
37]. Crop longevity is dependent on the length of the basic vegetative phase (BVP) for each cultivar during photoperiods less than a threshold (<11 h). A photoperiod-sensitive phase (PSP) concludes with the initiation of floral buds if conditions are not inductive [
37,
38]. The inverse relationship between seed yield and phenological parameters, specifically flowering time and crop cycle, can be explained by the fact that both exhibit a negative correlation with seed yield (
Table 3). In general, plants that exhibit reduced reproductive periods and crop cycles demonstrate enhanced productivity. Previous studies emphasized that the days to flowering was identified as the most critical determinant influencing the productivity of quinoa grains in various environmental conditions [
32,
39,
40]. These results suggest that genotypes that demonstrate earlier flowering in each particular environment are associated with the improvement of grain weight. Grain weight determination mostly took place during the post-flowering stage. As a result, genotypes exhibiting extended periods of time until flowering in each natural setting were exposed to detrimental circumstances, including reduced mean temperature and radiation, which might have compromised the processes involved in grain filling [
41]. Similarly, Quinoa varieties with short to medium life cycles were capable of evading high temperatures during these crucial phases of development, and produced better grain yield than long crop cycle genotypes (
Table 2;
Table 5). Similar observations were reported by [
42], suggesting short or medium life cycle varieties produces higher yield and completed life cycle before detrimental environmental circumstance mainly heat stress.
According to studies, panicles that are longer than shorter ones may produce more grain [
34,
43]. However, no significant differences were observed for panicle lengths between genotypes in different altitudes except Tekes County. Compared to Longli 1 and Jinli 1, Beijing 2 has the longest panicle length but lower seed production. Furthermore, in terms of panicle length and seed yields, Longli 4 in Nilka County and Jinli 1 in Zhaosu County exhibited the shortest and most productive, respectively. A large, open inflorescence may be more susceptible to seed loss and evaporate more rapidly after morning dew and precipitation than a small, compact one [
1]. Similar findings were also reported by [
44]. Despite the lack of statistical significance in the relationship between panicle length and seed yield, a positive correlation was observed, with the maximum seed yield being produced by panicles of medium length. [
44] reported comparable findings and noted that the best seed output was achieved with a medium-sized panicle. Genotypes characterized by shorter flowering and crop cycles, as well as taller plant stature, are recommended for cultivation across diverse altitudes.
4.3. Factors Affecting the Quality of Quinoa in Different Regions
The investigation we conducted on the quality indicators of quinoa in different geographical areas illuminates the complex ways in which environmental factors impact nutritional characteristics. The observed discrepancies in nutritional quality underscore the intricate interplay among genetic elements, environmental circumstances including temperature, altitude, soil and precipitation (rainfall) in determining the nutritional characteristics of quinoa. Nevertheless, photoperiod and temperature are the two key factors influencing quinoa production [
37], output is typically negligible in areas where temperatures exceed 32 °C [
1,
45]. However, performance of a genotype is primarily determined by the genetic makeup of the cultivar, the surrounding environment, and their interaction [
32,
37,
46]. The nutritional characteristics varied between genotypes and altitudes, as indicated by the significant genotypic effect, locations, and genotype-location interactions (G × L) values for all nutritional parameters (
Table 4). Genotype, environmental factors, agroecological circumstances, and nitrogen availability in the soil have been suggested to play a role in determining amino acid and protein content [
30,
47]. The protein content decreased significantly while the fat content increased along a gradient that ran from the lowest to the highest altitude and from the hottest to the coldest places, all of which had the same type of soil (sierozem). Intriguingly, it is worth noting that Zhaosu County, characterised by its chernozem type soil and the highest altitude and lowest temperatures, demonstrated a more favorable response in protein content and a lower fat content in comparison to Nilka County and Tekes County (
Figure 3B,C). This discrepancy may potentially be ascribed to the fertile soil composition and nitrogen availability in Zhaosu County. However, the influence of environmental factors on protein and amino acid levels is mostly determined by the genotype [
48]. Our study conducted in Yining City unveiled that quinoa varieties displayed the highest protein content and lowest fat content, suggesting a potential influence of elevated temperatures and the interaction between genotype and environment. These results are consistent with the findings of [
47], who examined the effect of temperature on various quinoa cultivars and observed an uptick in protein content when the plant was subjected to thermal stress.
Strong genetic variability and environmental differences in Xinjiang province contribute to the variation in the nutritional composition of quinoa ecotypes. Previous studies have documented comparable impacts of agricultural practices, agroclimatic factors, and soil types on the ecotype composition of quinoa [
49]. Although all nutritional characteristics (with the exception of total polyphenol) at different altitudes illustrated statistically significant findings, no discernible pattern (except for proteins and fats) in relation to altitude and temperature was noticed in terms of nutritional properties. Saponins, which impart a characteristic bitter flavor to seeds, are secondary metabolites whose concentrations may vary in response to environmental conditions. In Yining City, saponins were found lowest compared to other geographical regions (
Figure 3G). this could be attributed to the lowest rainfall received by Yining City. [
30] observed similar trend in saponin content during low rainfall in 2019 in their experiment, however, [
19] observed 45% reduction in saponin concentration during a water deficit experiment carried out in southern Europe. The variability of saponin content is primarily determined by genotype, although it can also be influenced by stressors such as soil salinity or drought [
50,
51]. Similar trend was observed in our findings, Jinli 1, Beijing 2 and Longli 4 were considered sweet varieties and showed lower values across the sites except for Zhaosu County, where no significant differences were observed between genotypes except for Longli 4 (
Table 6).
Any recommended varieties in each site? In Yining City, the varieties Jinli 1 and Beijing 2 are recommended due to their short crop cycles, high seed yields, elevated protein content, and lower saponin levels. In Nilka County, Jinli 1, Longli 4, and Beijing 2 are suggested as they exhibit the shortest crop cycles. Longli 4 and Jinli 1 notably surpass the 3 t/ha seed yield mark. Moreover, Jinli 1 and Beijing 2 seeds boast high protein content and low saponin levels. In Tekes County, Jinli 1, Longli 1, and Beijing 2 produced the greatest seed yield, whereas Nongli 3 had the highest protein content and total saponin. In Zhaosu County, Longli 4, Beijing 2, and Jinli 1 offer the shortest crop cycles. Among these, Beijing 2 and Jinli 1 demonstrate the highest protein content. Longli 4, on the other hand, exhibits the lowest total saponin content compared to other varieties.
In the province of Xinjiang, quinoa has proven to be truly adaptable and possesses remarkable nutritional value, rendering it a prime contender for prospective cultivation in any location. Breeding initiatives should be undertaken to augment the quality of forage and yield, with an emphasis on lowering saponin levels and increasing protein content, in order to further exploit its potential.
5. Conclusions
In conclusion, this study presents comprehensive insights into the ecological adaptability, agronomic performance and nutritional characteristics of quinoa across various altitudes in the northwestern part of China. Yining City, which is distinguished by its lower altitudes and higher temperatures, appears to offer optimal circumstances for the forage production of quinoa. Due to the superior characteristics exhibited by quinoa genotypes in this region, including longer panicles, increased branch count, sturdy stems, and high seed protein content, making it an ideal location for enhancing livestock feed availability. Additionally, it is worth noting that Nilka and Tekes counties illustrate considerable potential as locations for quinoa seed production due to their comparatively brief crop cycles and substantial yields. The genotype Jinli 1, in particular, demonstrates remarkable yield performance, surpassing 4 tons per hectare (T/ha) in Tekes County, coupled with high protein and fat content and low saponin levels. Interestingly, Zhaosu County, characterized by cooler climates and fertile chernozem soil, illustrates a robust protein response and high 1000-seed weight (g), highlighting the impact of soil fertility on nutritional composition in quinoa. Correlation analyses highlight the significance of agronomic traits in determining seed yield and quality. Plants with shorter flowering times, crop cycles, compact inflorescence, and taller heights tend to exhibit superior seed yields, emphasizing the need for selecting genotypes with optimal agronomic characteristics in order to enhance productivity. Overall, our study contributes significant insights into the ecological adaptability and nutritional dynamics of quinoa, with implications for sustainable crop production and food security in diverse agroecosystems.
Author Contributions
Conceptualization, C.H. and P.Q.; methodology, Q.Y. and B.X.; validation, X.S., S.E.A. and D.T.; formal analysis, B.Z. and S.S.S; investigation, C.H. Q.Y. and B.X.; writing—original draft preparation, P.Q.; writing—review and editing, B.Z. and S.S.S; supervision, G.X. P.Q.; project administration, M.G.; funding acquisition, C.H. P.Q. and B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Jiangsu Agricultural Science and Technology Innovation Fund (Grant No. CX (22) 2013), Secondary introduction project in Xinjiang Uygur Autonomous Region (2023004), Jilin Provincial Development and Reform Commission Project (2022C037-8) and Oat Industry Technology System Project of Jilin Provincial Department of Agriculture and Rural Affairs (JARS-2024-0501).
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bazile, D.; Jacobsen, S.-E.; Verniau, A. The global expansion of quinoa: trends and limits. Frontiers in plant science 2016, 7, 622. [Google Scholar] [CrossRef]
- Bhargava, A.; Srivastava, S. Quinoa: Botany, production and uses; CABI: 2013. eds S. Jain, A. Lainsbury, and S. Hill (London), 3-6.
- Filho, A.M.M.; Pirozi, M.R.; Borges, J.T.D.S.; Pinheiro Sant'Ana, H.M.; Chaves, J.B.P.; Coimbra, J.S.D.R. Quinoa: Nutritional, functional, and antinutritional aspects. Critical reviews in food science and nutrition 2017, 57, 1618–1630. [Google Scholar] [CrossRef]
- Jacobsen, S.-E. The worldwide potential for quinoa (Chenopodium quinoa Willd.). Food reviews international 2003, 19, 167–177. [Google Scholar] [CrossRef]
- Jacobsen, S.-E.; Mujica, A.; Jensen, C. The resistance of quinoa (Chenopodium quinoa Willd.) to adverse abiotic factors. Food reviews international 2003, 19, 99–109. [Google Scholar] [CrossRef]
- Li, G.; Zhu, F. Quinoa starch: Structure, properties, and applications. Carbohydrate polymers 2018, 181, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuña-Rodríguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Coulibaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A. Quinoa biodiversity and sustainability for food security under climate change. A review. Agronomy for sustainable development 2014, 34, 349–359. [Google Scholar] [CrossRef]
- FAO. 2013 international year of quinoa. Available online: http://www.fao.org/quinoa-2013/en/ (accessed on 12 June 2019).
- Gongbuzhaxi, W. , Zhang, C.X., Yang Q. S.. Biological characteristics of south american pelican in Tibet. Southwest China Journal of Agricultural Sciences 1994, 3, 54–62. [Google Scholar]
- Gongbuzhaxi, W.m. Biological characteristics and cultivation techniques of South American quinoa. Tibet’s Science and Technology 1995, 4, 19–22. [Google Scholar]
- Gongbuzhaxi, W.m. , Wang, L. Research on the original materials of South American alfalfa breeding. Tibet Science and Technology.
- 12. GB5009.4-2016. National food safety standard - Determination of ash in food. National Standard of the People’s Republic of China, Beijing.
- 13. GB20264-2006. Grain and oilseed - Determination of moisture content - Twice drying method. National Standard of the People’s Republic of China, Beijing.
- GB5009.5-2016. National food safety standard - Determination of protein in food. National Standard of the People’s Republic of China, Beijing. (: Available online.
- NY4-1982. Method for the determination of crude fats in cereals and oil crop seeds. the Agricultural Trade Standard of the People’s Republic of China, Beijing.
- Qin, P.; Wang, Q.; Shan, F.; Hou, Z.; Ren, G. Nutritional composition and flavonoids content of flour from different buckwheat cultivars. International Journal of Food Science & Technology 2010, 45, 951–958. [Google Scholar] [CrossRef]
- Emmons, C.L.; Peterson, D.M. Antioxidant activity and phenolic contents of oat groats and hulls. Cereal Chemistry 1999, 76, 902–906. [Google Scholar] [CrossRef]
- Oomah, B.D.; Mazza, G. Flavonoids and antioxidative activities in buckwheat. Journal of Agricultural and Food Chemistry 1996, 44, 1746–1750. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Iafelice, G.; Lavini, A.; Pulvento, C.; Caboni, M.F.; Marconi, E. Phenolic compounds and saponins in quinoa samples (Chenopodium quinoa Willd.) grown under different saline and nonsaline irrigation regimens. Journal of Agricultural and Food Chemistry 2012, 60, 4620–4627. [Google Scholar] [CrossRef]
- Hiai, S.; Oura, H.; Nakajima, T. Color reaction of some sapogenins and saponins with vanillin and sulfur1c acid. Planta medica 1976, 29, 116–122. [Google Scholar] [CrossRef]
- Angeli, V.; Miguel Silva, P.; Crispim Massuela, D.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium quinoa Willd.): An overview of the potentials of the “golden grain” and socio-economic and environmental aspects of its cultivation and marketization. Foods 2020, 9, 216. [Google Scholar] [CrossRef]
- Bazile, D.; Biaggi, M.C.; Jara, B. Quinoa’s spreading at global level: State of the art, trends, and challenges. Biology and Biotechnology of Quinoa: Super Grain for Food Security. [CrossRef]
- Jacobsen, S.E. The scope for adaptation of quinoa in Northern Latitudes of Europe. Journal of Agronomy and Crop Science 2017, 203, 603–613. [Google Scholar] [CrossRef]
- Afzal, I.; Basra, S.M.A.; Rehman, H.U.; Iqbal, S.; Bazile, D. Trends and limits for quinoa production and promotion in Pakistan. Plants 2022, 11, 1603. [Google Scholar] [CrossRef] [PubMed]
- Fita, A.; Rodríguez-Burruezo, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Breeding and domesticating crops adapted to drought and salinity: a new paradigm for increasing food production. Frontiers in Plant Science 2015, 6, 978. [Google Scholar] [CrossRef]
- Davis, P.A.; Burns, C. Photobiology in protected horticulture. Food and Energy Security 2016, 5, 223–238. [Google Scholar] [CrossRef]
- Ferrante, A.; Mariani, L. Agronomic management for enhancing plant tolerance to abiotic stresses: High and low values of temperature, light intensity, and relative humidity. Horticulturae 2018, 4, 21. [Google Scholar] [CrossRef]
- Pedersen, P.; Lauer, J.G. Soybean growth and development in various management systems and planting dates. Crop Science 2004, 44, 508–515. [Google Scholar] [CrossRef]
- Lesjak, J.; Calderini, D.F. Increased night temperature negatively affects grain yield, biomass and grain number in Chilean quinoa. Frontiers in Plant Science 2017, 8, 352. [Google Scholar] [CrossRef]
- Granado-Rodríguez, S.; Aparicio, N.; Matías, J.; Pérez-Romero, L.F.; Maestro, I.; Gracés, I.; Pedroche, J.J.; Haros, C.M.; Fernandez-Garcia, N.; Navarro del Hierro, J. Studying the impact of different field environmental conditions on seed quality of quinoa: The case of three different years changing seed nutritional traits in southern Europe. Frontiers in Plant Science 2021, 12, 649132. [Google Scholar] [CrossRef] [PubMed]
- Zurita-Silva, A.; Fuentes, F.; Zamora, P.; Jacobsen, S.-E.; Schwember, A.R. Breeding quinoa (Chenopodium quinoa Willd.): potential and perspectives. Molecular Breeding 2014, 34, 13–30. [Google Scholar] [CrossRef]
- Bertero, H.D.; De la Vega, A.; Correa, G.; Jacobsen, S.; Mujica, A. Genotype and genotype-by-environment interaction effects for grain yield and grain size of quinoa (Chenopodium quinoa Willd.) as revealed by pattern analysis of international multi-environment trials. Field crops research 2004, 89, 299–318. [Google Scholar] [CrossRef]
- Afiah, S.A.; Hassan, W.A.; Al Kady, A. Assessment of six quinoa (Chenopodium quinoa Willd.) genotypes for seed yield and its attributes under Toshka conditions. Zagazig Journal of Agricultural Research 2018, 45, 2281–2294. [Google Scholar] [CrossRef]
- Maliro, M.F.; Guwela, V.F.; Nyaika, J.; Murphy, K.M. Preliminary studies of the performance of quinoa (Chenopodium quinoa Willd.) genotypes under irrigated and rainfed conditions of central Malawi. Frontiers in Plant Science 2017, 8, 227. [Google Scholar] [CrossRef]
- Bois, J.-F.; Winkel, T.; Lhomme, J.-P.; Raffaillac, J.-P.; Rocheteau, A. Response of some Andean cultivars of quinoa (Chenopodium quinoa Willd.) to temperature: Effects on germination, phenology, growth and freezing. European Journal of agronomy 2006, 25, 299–308. [Google Scholar] [CrossRef]
- Christiansen, J.; Jacobsen, S.-E.; Jørgensen, S. Photoperiodic effect on flowering and seed development in quinoa (Chenopodium quinoa Willd.). Acta Agriculturae Scandinavica, Section B-Soil & Plant Science 2010, 60, 539–544. [Google Scholar] [CrossRef]
- Thiam, E.; Allaoui, A.; Benlhabib, O. Quinoa productivity and stability evaluation through varietal and environmental interaction. Plants 2021, 10, 714. [Google Scholar] [CrossRef]
- Bendevis, M.A.; Sun, Y.; Shabala, S.; Rosenqvist, E.; Liu, F.; Jacobsen, S.-E. Differentiation of photoperiod-induced ABA and soluble sugar responses of two quinoa (Chenopodium quinoa Willd.) cultivars. Journal of Plant Growth Regulation 2014, 33, 562–570. [Google Scholar] [CrossRef]
- Curti, R.N.; De la Vega, A.; Andrade, A.J.; Bramardi, S.J.; Bertero, H.D. Multi-environmental evaluation for grain yield and its physiological determinants of quinoa genotypes across Northwest Argentina. Field Crops Research 2014, 166, 46–57. [Google Scholar] [CrossRef]
- Curti, R.N.; De la Vega, A.; Andrade, A.J.; Bramardi, S.J.; Bertero, H.D. Adaptive responses of quinoa to diverse agro-ecological environments along an altitudinal gradient in North West Argentina. Field Crops Research 2016, 189, 10–18. [Google Scholar] [CrossRef]
- Capristo, P.R.; Rizzalli, R.H.; Andrade, F.H. Ecophysiological yield components of maize hybrids with contrasting maturity. Agronomy Journal 2007, 99, 1111–1118. [Google Scholar] [CrossRef]
- Nanduri, K.R.; Hirich, A.; Salehi, M.; Saadat, S.; Jacobsen, S.E. Quinoa: a new crop for harsh environments. Sabkha Ecosystems: Volume VI: Asia/Pacific. [CrossRef]
- De Santis, G.; Ronga, D.; Caradonia, F.; Ambrosio, T.D.; Troisi, J.; Rascio, A.; Fragasso, M.; Pecchioni, N.; Rinaldi, M. Evaluation of two groups of quinoa (Chenopodium quinoa Willd.) accessions with different seed colours for adaptation to the Mediterranean environment. Crop and Pasture Science 2018, 69, 1264–1275. [Google Scholar] [CrossRef]
- Manjarres-Hernández, E.H.; Arias-Moreno, D.M.; Morillo-Coronado, A.C.; Ojeda-Pérez, Z.Z.; Cárdenas-Chaparro, A. Phenotypic characterization of quinoa (Chenopodium quinoa Willd.) for the selection of promising materials for breeding programs. Plants 2021, 10, 1339. [Google Scholar] [CrossRef]
- Hinojosa, L.; Matanguihan, J.B.; Murphy, K.M. Effect of high temperature on pollen morphology, plant growth and seed yield in quinoa (Chenopodium quinoa Willd.). Journal of agronomy and crop science 2019, 205, 33–45. [Google Scholar] [CrossRef]
- Hakeem, K.R. Crop production and global environmental issues; Springer: 2015.
- Matías, J.; Rodríguez, M.J.; Cruz, V.; Calvo, P.; Reguera, M. Heat stress lowers yields, alters nutrient uptake and changes seed quality in quinoa grown under Mediterranean field conditions. Journal of Agronomy and Crop Science 2021, 207, 481–491. [Google Scholar] [CrossRef]
- Präger, A.; Munz, S.; Nkebiwe, P.M.; Mast, B.; Graeff-Hönninger, S. Yield and quality characteristics of different quinoa (Chenopodium quinoa Willd.) cultivars grown under field conditions in Southwestern Germany. Agronomy 2018, 8, 197. [Google Scholar] [CrossRef]
- Miranda, M.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Rodríguez, M.J.; Maureira, H.; Martínez, E.A. Nutritional aspects of six quinoa (Chenopodium quinoa Willd.) ecotypes from three geographical areas of Chile. Chilean journal of agricultural research 2012, 72, 175. [Google Scholar] [CrossRef]
- Abidi, I.; Daoui, K.; Abouabdillah, A.; Belqadi, L.; Mahyou, H.; Bazile, D.; Douaik, A.; Gaboun, F.; Hassane Sidikou, A.A.; Alaoui, S.B. Quinoa–Olive Agroforestry System Assessment in Semi-Arid Environments: Performance of an Innovative System. Agronomy 2024, 14, 495. [Google Scholar] [CrossRef]
- Kaur, H.; Grewal, S.K.; Gill, R.K.; Gill, P.S. Characterization of quinoa (Chenopodium quinoa Willd.) genotypes for nutritional quality and antioxidant potential. Agricultural Research Journal 2022, 59. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).