1. Introduction
Vermicomposting is scientifically decomposing the agricultural, municipality, and industrial wastes into nutrient riched agricultural amendments (Sharma and Grag 2017; Sharma and Grag 2018; Soobhany et al., 2017; Staggs, 2021). Vermicompost not only balance underground soil but also improves above ground environment for soil microbiota (Blouin et al., 2005; Sinha et al., 2008). Vermicompost is nutritive byproduct of biodegradation, stabilization and conversion of useful resources. The end product obtained is disinfected, detoxified and highly nutritive, enhancing the natural biodegradation and decomposition process of organic waste teeming the beneficial bacteria, fungi and actinomycestes (Visvanathan et al., 2005; DelaVega, 2016; Rakkini et al., 2017) contributing to soil health and nutrients availability. Vermiculture practice accomplish the waste management (60 to 80 %) soil health, plant vitality, and decrease chemical reliance (Hussaini, 2013). The earthworm contribution in vermicompost, decipher the concerns of economic stability, environmental sustainability and social acceptability together (Sinha et al., 2010; Saharia et al., 2015; Searchinger et al., 2019; Singh et al., 2020).
Synonymous to ‘environmental engineers’, earthworm (Crassiclitellata) as terrestrial oligochaetes, participate and promote the litter fragmentation, burrowing, casting, nutrient cycling, shaping soil structure, composition, water infiltration, soil carbon storage, plant growth and accelerating 40–90 % soil macro-faunal biomass of above and below ground ecosystems (Fragoso et al. 1999; Atiyeh et al., 2000; Edwards et al., 2004; Coleman et al., 2004; Sherman et al., 2018; Singh et al., 2019). Earthworm as ‘soil intestines’ or ‘soil conditioner’, improvise soil porosity, drainage and water holding capacity in eco-friendly mode, disposing the waste with no-energy or zero-waste technology (Edwards and Burrows 1988; Wang, 2000; Sinha et al., 2010; Arancon and Edwards 2011; Priyanka et al., 2022). As soil dweller, modifies the soil structure by ingestion, disintegration and ejection of soil as surface or subsurface cast (Nijhawan and Kanwar, 1952; Edwards and Lofty, 1977). Amid to basic behavior as waste engineers on grounds of vermicomposting, vermiremediation, wastewater and soil fertility, earthworm excels in bio-conversion, bio-degradation and bio-production technologies (Rajiv et al., 2010; Katiyar et al., 2017). Several earthworm species used in vermicomposting, include Eisenia fetida (Savigny), Eisenia andrei (Bouché), Dendrobaena veneta (Rosa), Dendrobaena hortensis (Michaelsen) Eudrilus eugeniae (Kinberg), and Perionyx excavatus (Perrier). Amongst the known species, Dendrobaena rubida (preferred for organic soil), Dendrobaena veneta (used in industrial vermicomposting), Lumbricus rubellus (preferred for moist surface), Perionyx excavates (suitable for high temperature range), Eisenia fetida (Savigny) (live and feed in litter layer) are preferred earthworm associated with vermicomposting (Gajalakshmi et al 2001; Domínguez et al., 2005; Chauhan et al., 2010; Deka et al., 2013; Rajendran and Thivyatharsan 2014). Although, Eisenia andrei and Eisenia fetida are the two most potential species used in vermicomposting and vermiculture facilities worldwide, E. fetida (Savigny) (Annelida: Lumbricidae) has recognized as most promising species used for vermicomposting in India (Domínguez, 2018). The species is epigeic, inhabit the soil of high organic content in patchy pattern (Elvira et al., 1996; Monroy et al., 2006). Amid to hardy nature, it is tolerant to wide range of temperature and humidity fluctuations, facilitating easy culture of the species (Domínguez, 2004; Domínguez and Edwards 2011). Nevertheless, the resilience to seasonal fluctuations exists in all the biological fauna of soil, thus, soil dwelling earthworms (Eisenhauer et al., 2009; Uvarov et al., 2011; Hughes et al., 2019). Being poikilothermic, the activity, growth, density, metabolism, respiration and reproduction of earthworms fluctuate with the surrounding seasonal environment (Baker et al., 1993; Edwards & Bohlen 1996; Tondoh, 2006; Millican and Lutterschmidt, 2007; Morón et al., 2010). The seasonal changes not only affect the spatial and temporal distribution of earthworms at local and regional scale but the seasonal activity also governs the dynamics of earthworm in a particular season (Jiménez et al., 1998; Walsh & Maynard, 2016; Walsh et al., 2019). The severe winters followed by hot, dry soils in summer produce dramatic seasonal fluctuations in soil from frozen surface to dry summer condition is expected to limit the activity and density of earthworm populations in this region. Therefore, the present study was conducted to assess the seasonal potential of E. fetida corresponding its activity in winter period and to quantify seasonal impact on vermicomposting in the region.
2. Materials and Methods
2.1. Site specification
The study was carried out at the vermicompost unit (
Figure 1) of experimental field of Faculty of Agriculture, Mohammad Ali Jauhar University, Rampur, U.P, India. The region is located at coordinates of 28.79’ °N, 79.02’ °E, with humid, subtropical, dry winter climate
. The yearly temperature (average of 29.26 ºC) remains 3.29 % higher than India’s average temperature. The average relative humidity during summer and winter months ranges between 10.4 % to 98.6 %, with average of 61.4 % a year. The region is characterized by sandy laom coarse textured soil (ICAR-NBSS &LUP, 2018) of less water retention capacity. The selected site was shady (surrounded by long shrubs) and sufficiently humid. No prior activity of earthworm was noticed at the onset of experiment, also, the field was free from chemicals and fertilizers. The study was carried out in three following steps,
viz., a) Preparation of vermicompost unit b) Procurement of earthworm c) Production of vermiculture and Vermicompost. The study was conducted between 22 November 2022 to 10 March 2023 as date of inoculation and date of harvesting, respectively. Three months duration (November to March) was set as standard period for composting and experiment was conducted for 110 days without additional disturbance to the experimental site, besides water sprinkling at fifteen days interval.
2.2. Vermiculture and Feeding Material
Laboratory culture of E. fetida containing cocoon, juvenile and adults of earthworm was procured from Department of Agronomy, G.B Pant University of Agriculture and Technology, Pantnagar, Uttarakhand, India. The species E. fetida, was selected due to its native nature and easy culture in the region. One kg of E. fetida culture was brought and used for experiment. The material experimented as feed stock used for vermicomposting was cow dung, wheat straw and paddy straw obtained from Kam Dhenu, Dairy Farm Hamsafar Resort, Rampur, Uttar Pradesh.
2.3. Experimental Setup
Pit Method: Pit method of vermicomposting was used for inoculation of
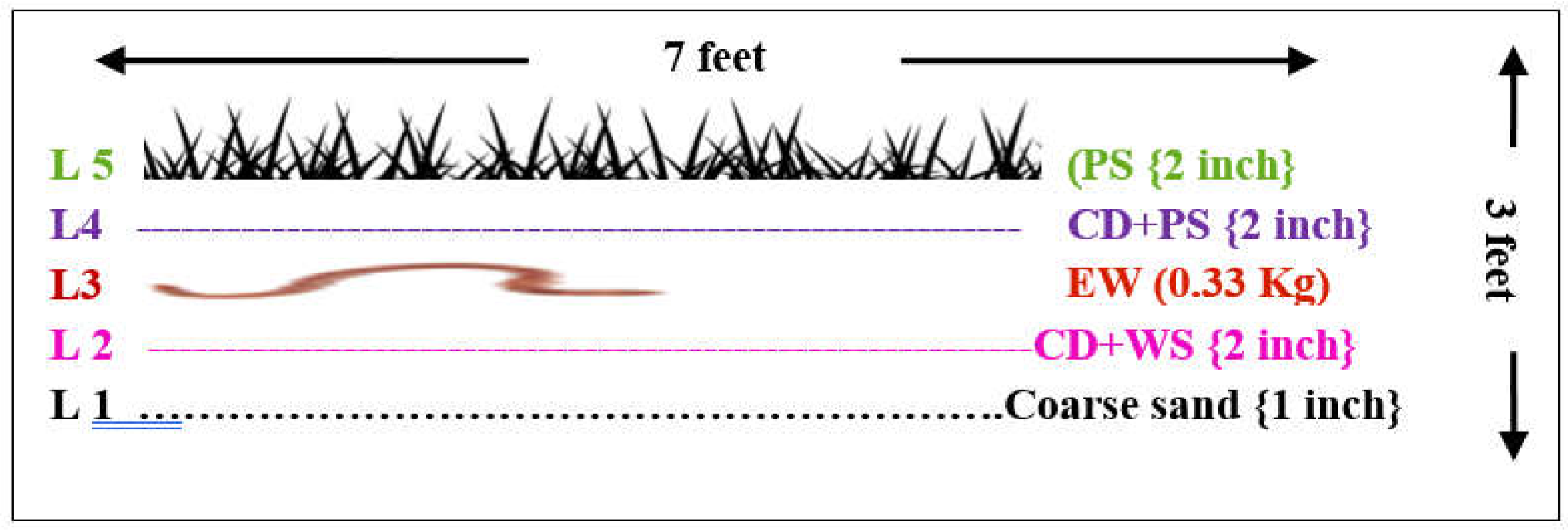
E. fetida in field conditions. Three Pits (P1, P2, P3) of size 7 X 3 X 1 (length X width X depth) feet were prepared at experimental field of Faculty of Agriculture. Each pit was filled with five layers (L 1, L 2, L 3, L 4, L 5) of bedding material containing Sand (S), Cow dung (CD) + Wheat Straw (CD+WS), Earthworm (EW), Cow dung+ Wheat Straw(CD+WS) and Paddy straw (PS). Sand (0.5 inch thick) was used as bedding material for covering the soil surface (L 1). The second layer (L 2) of 1” inch thickness was piled by mixing cow dung and wheat straw in 1:1 ratio. In third layer (L 3), the earthworm culture was inoculated by dividing the procured culture into three equal halves of 0.33 kg and introduced in three composting pits. Though, 1 kg/ m3 or 1:1 ratio of worms and wastes, by weight, has recommended for pit method of vermicomposting
E. fetida (Ndewa and Thomposon, 2000; Jicong, 2005), nevertheless, the reduced quantity of 0.33 kg/pit was evaluated to determine the potential of
E. fetida against the recommended rate of inoculation. The inoculated culture was covered with the combination of cow dung and paddy straw (CD+PS) mixed in same ratio (1:1) in fourth layer (L 4). The pits were finally covered (L 5) with crop remnants and long bushes (2 inches) grew in field (experimental field of MAJU) (
Table 1). All three pits were prepared by piling five layers following same procedure of filling and inoculation followed by sprinkling of water at regular intervals of fifteen days in order of moisture preservation for earthworm survival.
2.4. Vermiculture and Vermicompost harvesting
For harvesting of vermiculture and vermicompost, upper layer of long bushes was removed from all three Vermicompost pits. After removing bushes, the whole material was sieved through 3.0 mm mesh sieve in order of separating the vermiculture and vermicompost properly (
Figure 1). The harvested population of earthworm was initially sieved with 3 mm mesh size followed by 1 mm mesh sieve in order of separating immature and mature stages harvested from vermipits. The earthworm culture retained over the sieve was collected in plastic boxes, while the strained compost below the sieve, was used for further processing of packaging and commercializing (
Figure 3). The packed compost was utilized either in agri-experiments (crop cultivation for UG and PG standards) or for commercial purpose within University staff under the name of “
MAJU Vermicompost”. On the other hand, the vermiculture collected in boxes was further inoculated in previously prepared vermicompost pit to establish and maintain the earthworm culture and to quantify its seasonal variation during winter and summer months in subsequent seasons.
The productivity of vermicompost and Earthworm was calculate with the given formulae
3. Results and Discussion
The vermicompost produced in vermipits comprised of fine granules signifying the good manure quality. The fine granule certifies good aeration and respiration of soil, also ensures the mineral balance and availability of water soluble nutrients in vermicompost.
Table 2 & 3 illustrate the quantity of
E. fetida and vermicompost inoculated and produced in the field. The harvesting period of inoculated vermicompost and vermiculture was between 1
st to 10
th March 2023.
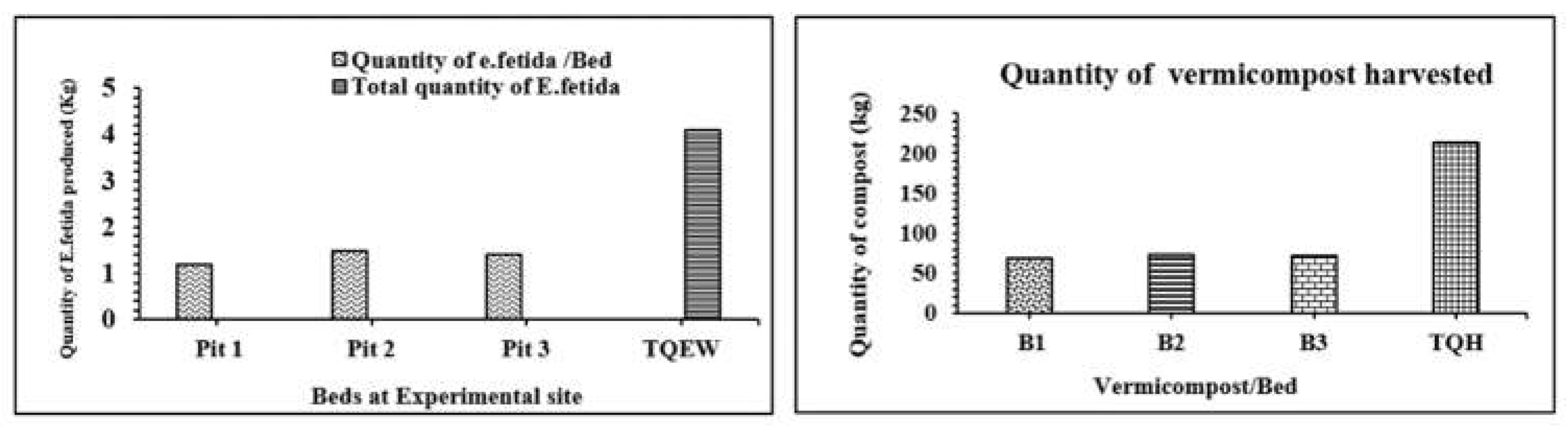
Quantity of E. fetida produced in field: The population of earthworm was comprising eggs, cocoon, juveniles and adults. The harvested population of mixed juvenile and matured earthworms was weighed on digital electric weighing balance (HG precision scale, India Pvt limited). The population was weighed individually and separately for B1, B2 and B3. The results indicate the quantity of earthworm inoculation (QOI) to each pit in November and the corresponding quantity of harvesting (QOH) in March for each pit applied as feed stock of earthworm. On inoculation of 0.33 kg E. fetida, in each pit, the harvesting yield from pit I quantified to 1.2 kg, indicating the significant increase of the applied inoculation. Similar to P1, P2 and P3 produced 1.5 and 1.4 kg earthworms, respectively. The three pits produced total quantity (TQH) of 4.1 kg E. fetida with an average of 1.36 kg/pits. The productivity however was weighed 4 times (410) to the inoculation quantity which provide the potential insight of E. fetida in the three pitss (Figure 6).
Quantity of Vermicompost produced in field: The vermicompost produced during the process was sieved through 3 mm mesh sieve in order of separating the coarse weeds mixed with compost near the field per se. The sieved compost was filled in gunny bags and brought to laboratory. The compost was spread over concrete floor to dry up the moisture present if any. The dried compost was filled in 1, 2, 5 and 10 kg polythene bags and weighed on digital weighing balance, followed by labeling with ‘MAJU Vermicompost’ and sealed with heat sealer (Cozen bag sealer, Boutique Series, MJP-198). The quantity of vermicompost (QOV) produced in three respective pits (B1, B2, B3) was 69, 73 and 71 kg. However, the total quantity (TQH) of Vermicompost (B1+ B2+ B3) produced by earthworm inoculation was quantified 2.13 quintal (213 kg) from all three pits. The productivity of compost on inoculation of 56.5 kg waste input was calculated as 3 times (376) to the quantity of introduced material (Figure 7).
Figure 4 &5.
Quantity of E. fetida Produced in field; Quantity of Vermicompost Produced in field.
Figure 4 &5.
Quantity of E. fetida Produced in field; Quantity of Vermicompost Produced in field.
Figure 6 &7.
Input quantity of waste and E. fetida in pits; Productivity of compost and E. fetida in pits.
Figure 6 &7.
Input quantity of waste and E. fetida in pits; Productivity of compost and E. fetida in pits.
3.1. Multiplication of E. fetida and compost production in field
The cowdung added in vermipits was the basic food source for the survival, growth and reproduction of E. fetida in field. The rate of addition of E. fetida to vermicompost pits lower to recommended rate was the basis for the study to be conducted in the region. The inoculation of 0.33 kg/ pits of 2.1 meter (approx 7 feet) against the recommended rate of 1 kg/m3 was carried out to determine the real time potential of E. fetida during winter months. The results showed the equivalent number of earthworm production in three Vermipits. The population reproduced was three times of inoculated number. The number effect on its production may be explained by two edaphic mechanisms. First, the regional effect, the area surrounded by kosi river, being rich in sandy laom soil of coarse textured, granular, high humus and good water passage that would have accelerated the survival and reproduction which in turn had reduced the generation time of earthworm cycle, thus increased earthworm count in vermipits. The loose and perforated soil might have facilitated easy penetration of earthworm in deeper layer thus altering soil structure into fertile compost. Secondly, the increased population of E. fetida would have accelerated the decomposition of agri-waste, thus increased production of compost in vermipits. This suggests that the low population of E. fetida might be able to reproduce at faster pace in the region, and its persistence would produce sufficient population in one inoculation of relatively small count without chronic addition of earthworm population in vermipits in forthcoming seasons. In semiarid ecosystems, the potential of earthworms is highly dependent on soil moisture, also the seasonal variation governs its growth and activity in response to changes in soil moisture (Perreault and Whalen 2006, Kale and Karmegam 2010; Singh et al., 2019; Singh et al., 2020). According to Singh et al., (2019), survival of earthworms is highly dependent on soil moisture in arid ecosystems, while in temperate zones the survival is at stake, and resumes as soil dries out Moreover, the earthworm activity is strongly constrained and coupled to seasonal dynamics largely based on hydrological status of soil (Jiménez et al., 1998; Walsh and Maynard 2016; Ruiz et al., 2021; Mangiarotti et al., 2021), which ultimately link the mechanical ability of moving and burrowing through soil subsurface, to decomposing residue layers (Lavelle, et al 2007; Ruiz et al., 2015; Ruiz et al., 2017). The penetration of earthworm to deeper burrows also vary with soil type and hydration thus affecting its sensitivity to soil compaction, moisture retention likely linked to exceptionally high potential of E. fetida in soil classified as sandyloam in this region (Abbott, 1994; Hendrix, P. F. & Bohlen 2002; Phillips, et al., 2019).
The productivity record of compost vs E. fetida illustrate the 3 and 4 times effectiveness, respectively, indicating the two way advantage, by aiding in decomposition process along augmented count of initial population indicating the success rate vermicomposting to several folds in this region. The inoculation in winter window insight its winter activity on seasonal grounds of Rampur region of Uttar Pradesh, India. The reference potential of cold months determines the earthworm activity which also inflicts the better output in upcoming hot periods. In addition, the compost quantity produced in particular season establish the seasonal relevance for improved process and production of vermicomposting. Furthermore, the improved practice along alteration in feeding stocks in forthcoming summer months would uncover insights for its optimization, both in terms of compost and earthworm production and augmentation.
4. Conclusions
It is important to note that earthworm population and the period of activity likely to change, on account of spatial variability and soil properties linked to the moisture availability and retention capacity. The regional difference also governs quality of compost produced in both of physical features and nutritional characters as well. The current studies depicts the winter potential of E. fetida, however, the followup studies from same experimental site would explain its summer potential and compost quantity production at high soil temperature and moisture. Moreover, the hospitable site and season for the activity of E. fetida in the region would suggest the seasonal relevance of compost quantity with improved and altered feeding stocks turning trash to treasure for enhanced agricultural efficacy.
Acknowledgements
Authors gratefully acknowledge the funding from Mohammad Ali Jauhar University for establishing the vermicompost unit in the University, Rampur, Uttar Pradesh, India. The authors would like to acknowledge the help from G.B Pant University of Agriculture and Technology, Pantnagar, Uttarakhand, India, for providing earthworm culture and other staff involved in accomplishing the work.
References
- Abbott, I. (1994). Distribution of the native earthworm fauna of Australia-a continent-wide perspective. Soil Research, 32(1), 117-126.
- Atiyeh, R. M., Subler, S., Edwards, C. A., Bachman, G., Metzger, J. D., & Shuster, W. (2000). Effects of vermicomposts and composts on plant growth in horticultural container media and soil. Pedobiologia, 44(5), 579-590.
- Baker, G. H., Barrett, V. J., Carter, P. J., Williams, P. M. L., & Buckerfield, J. C. (1993). Seasonal changes in abundance of earthworms (Annelida: Lumbricidae and Acanthodrilidae) in soils used for cereal and lucerne production in South Australia. Australian Journal of Agricultural Research, 44(6), 1291-1301.
- Blouin, M., Zuily-Fodil, Y., Pham-Thi, A. T., Laffray, D., Reversat, G., Pando, A., ... & Lavelle, P. (2005). Belowground organism activities affect plant aboveground phenotype, inducing plant tolerance to parasites. Ecology letters, 8(2), 202-208.
- Chauhan, A., Kumar, S., Singh, A. P., & Gupta, M. (2010). Vermicomposting of vegetable wastes with cowdung using three earthworm species Eisenia foetida, Eudrilus eugeniae and Perionyx excavatus. Nature and science, 8(1), 34-42.
- Coleman, D. C., Callaham, M. A., & Crossley Jr, D. A. (2017). Fundamentals of soil ecology. Academic press.
- De la Vega, A. (2016). Vermicomposting: The Future of Sustainable Agriculture and Organic Waste Management. Lessons from the USA and Cuba, 1-48.
- Deka, H., Deka, S., & Baruah, C. K. (2013, December). Vermicomposting of water hyacinth eichhornia crassipes (mart. solms) employing indigenous earthworm species. In International Conference on Chemical, Agricultural and Medical Sciences (CAMS-2013), Kuala Lumpur (Malaysia) (pp. 29-30).
- Domínguez, J. (2023). State-of-the-Art and New Perspectives on Vermicomposting Research: 18 Years of Progress. In Vermicomposting for Sustainable Food Systems in Africa (pp. 27-44). Singapore: Springer Nature Singapore.
- Dominguez, J. E. C. A., & Edwards, C. A. (2011). Biology and ecology of earthworm species used for vermicomposting. Vermiculture technology: earthworms, organic waste and environmental management. CRC Press, Boca Raton, 27-40.
- Domínguez, J., Velando, A., & Ferreiro, A. (2005). Are Eisenia fetida (Savigny, 1826) and Eisenia andrei (Oligochaeta, Lumbricidae) different biological species?. Pedobiologia, 49(1), 81-87. Domínguez, J. (2018). Earthworms and vermicomposting (pp. 63-77). London (UK): IntechOpen.
- Edwards, C. A., & Bohlen, P. J. (1996). Biology and Ecology of Earthworms, Chapman and Hall, London, UK.
- Edwards, C. A., & Bohlen, P. J. (1996). Biology and ecology of earthworms (Vol. 3). Springer Science & Business Media. Chapman and Hall; London: p. 426.
- Edwards, C. A., & Burrows, I. (1988). Potential of earthworm composts as plant growth media. Earthworms in waste and environmental management/edited by Clive A. Edwards and Edward F. Neuhauser.
- Edwards, C. A., & Burrows, I. (1988). Potential of earthworm composts as plant growth media. Earthworms in waste and environmental management/edited by Clive A. Edwards and Edward F. Neuhauser.
- Edwards, C. A., Dominguez, J., & Aranconl, N. Q. (2004). 18. The influence ofvermicomposts on plant growth and pest incidence. Soil Zoology for Sustainable Development in the 21st Century; Hanna, HSH, Mikhail, WZA, Eds.
- Eisenhauer, N., Milcu, A., Sabais, A. C., Bessler, H., Weigelt, A., Engels, C., & Scheu, S. (2009). Plant community impacts on the structure of earthworm communities depend on season and change with time. Soil Biology and Biochemistry, 41(12), 2430-2443.
- Elvira, C., Dominguez, J., & Briones, M. J. I. (1995). Earthworm community composition in an uncontrolled rubbish dump, a pig farm dunghill, and a deposit of primary sludge. Nova Acta Cientifica Compostelana(NACC Bioloxia), 6, 123-129.
- Elvira, C., Dominguez, J., & Briones, M. J. I. (1995). Earthworm community composition in an uncontrolled rubbish dump, a pig farm dunghill, and a deposit of primary sludge. Nova Acta Cientifica Compostelana(NACC Bioloxia), 6, 123-129.
- Gajalakshmi, S., Ramasamy, E. V., & Abbasi, S. A. (2001). Potential of two epigeic and two anecic earthworm species in vermicomposting of water hyacinth. Bioresource technology, 76(3), 177-181.
- Hau, J., Qiao, Y., Liu, G., & Dong, R. (2005). The influence of temperature, pH and C/N ratio on the growth and survival of earthworms in municipal solid waste. Agricultural Engineering International: CIGR Journal.
- Hendrix, P. F., & Bohlen, P. J. (2002). Exotic earthworm invasions in North America: ecological and policy implications: expanding global commerce may be increasing the likelihood of exotic earthworm invasions, which could have negative implications for soil processes, other animal and plant species, and importation of certain pathogens. Bioscience, 52(9), 801-811.
- Hughes, F. M., Cortes-Figueira, J. E., & Drumond, M. A. (2018). Anticipating the response of the Brazilian giant earthworm (Rhinodrilus alatus) to climate change: implications for its traditional use. Anais da Academia Brasileira de Ciências, 91.
- Hussaini, A. (2013). Vermiculture bio-technology: An effective tool for economic and environmental sustainability. African Journal of Environmental Science and Technology, 7(2), 56-60.
- Jiménez, J. J., Moreno, A. G., Decaëns, T., Lavelle, P., Fisher, M. J., & Thomas, R. J. (1998). Earthworm communities in native savannas and man-made pastures of the Eastern Plains of Colombia. Biology and Fertility of soils, 28, 101-110.
- Jiménez, J. J., Moreno, A. G., Decaëns, T., Lavelle, P., Fisher, M. J., & Thomas, R. J. (1998). Earthworm communities in native savannas and man-made pastures of the Eastern Plains of Colombia. Biology and Fertility of soils, 28, 101-110.
- Kale, R. D., & Karmegam, N. (2010). The role of earthworms in tropics with emphasis on Indian ecosystems. Applied and Environmental Soil Science, 2010.
- Katiyar, R. B., Suresh, S., & Sharma, A. K. (2017). A review on vermicomposting of different leaf litters. In Biofuels and Bioenergy (BICE2016) International Conference, Bhopal, India, 23-25 February 2016 (pp. 305-312). Springer International Publishing.
- Lavelle, P., Barot, S., Blouin, M., Decaëns, T., Jimenez, J. J., & Jouquet, P. (2007). Earthworms as key actors in self-organized soil systems. Ecosystem Engineers: Plants to Protists, 405.
- Mangiarotti, S., Fu, E., Jouquet, P., Tran, M. T., Huc, M., & Bottinelli, N. (2021). Earthworm activity and its coupling to soil hydrology: A deterministic analysis. Chaos: An Interdisciplinary Journal of Nonlinear Science, 31(1).
- Millican, D. S., & Lutterschmidt, W. I. (2007). Comparative seasonal observations of soil temperature and moisture and the occurrence of two earthworms inhabiting prairie and deciduous woodland sites. The Southwestern Naturalist, 52(4), 468-474.
- Monroy, F., Aira, M., Domínguez, J., & Velando, A. (2006). Seasonal population dynamics of Eisenia fetida (Savigny, 1826)(Oligochaeta, Lumbricidae) in the field. Comptes Rendus Biologies, 329(11), 912-915.
- Morón-Ríos, A., Rodríguez, M. Á., Pérez-Camacho, L., & Rebollo, S. (2010). Effects of seasonal grazing and precipitation regime on the soil macroinvertebrates of a Mediterranean old-field. European Journal of Soil Biology, 46(2), 91-96.
- Ndewa, P.M. and S.A. Thomposon. 2000. Effect of C-to-N ratio on vermicomposting of biosolids. Bioresource Technology. 75(1): 7-12.
- Phillips, H. R., Guerra, C. A., Bartz, M. L., Briones, M. J., Brown, G., Crowther, T. W., ... & Eisenhauer, N. (2019). Global distribution of earthworm diversity. Science, 366(6464), 480-485.
- Rajendran, M., & Thivyatharsan, R. (2014). Performance of different species of earthworm on vermicomposting. International Journal of Research, 2, 2311-2476.
- Rakkini, V. M., Vincent, S., Kumar, A. S., & Baskar, K. (2017). An overview: organic waste management by earthworm. J Civil Eng Environ Sci, 3(1), 013-017.
- Ruiz, S. A., Bickel, S., & Or, D. (2021). Global earthworm distribution and activity windows based on soil hydromechanical constraints. Communications biology, 4(1), 612.
- Ruiz, S., Or, D., & Schymanski, S. J. (2015). Soil penetration by earthworms and plant roots—mechanical energetics of bioturbation of compacted soils. PloS one, 10(6), e0128914.
- Ruiz, S., Schymanski, S. J., & Or, D. (2017). Mechanics and energetics of soil penetration by earthworms and plant roots: Higher rates cost more. Vadose Zone Journal, 16(8), 1-16.
- S.K.Singh, S. Chatterji, S. Chattaraj, and P.S. Butte (2018). ICAR-NBSS&LUP Technologies. NBSS Publ. No.176, ICAR–NBSS&LUP, Nagpur p.90.
- Saha, P., Barman, A., & Bera, A. (2022). Vermicomposting: a step towards sustainability. Sustainable Crop Production: Recent Advances, 53.
- Sahariah, B., Goswami, L., Kim, K. H., Bhattacharyya, P., & Bhattacharya, S. S. (2015). Metal remediation and biodegradation potential of earthworm species on municipal solid waste: A parallel analysis between Metaphire posthuma and Eisenia fetida. Bioresource Technology, 180, 230-236.
- Searchinger, T., Hanson, C., Ranganathan, J., Lipinski, B., Waite, R., Winterbottom, R., ... & Heimlich, R. (2013). Creating a sustainable food future: World resources report 2013-2014: Interim findings. World Resources Institute, Washington, DC.
- Sharma, K., & Garg, V. K. (2017). Vermi-modification of ruminant excreta using Eisenia fetida. Environmental Science and Pollution Research, 24, 19938-19945.
- Sharma, K., & Garg, V. K. (2018). Comparative analysis of vermicompost quality produced from rice straw and paper waste employing earthworm Eisenia fetida (Sav.). Bioresource technology, 250, 708-715.
- Singh, A., Karmegam, N., Singh, G. S., Bhadauria, T., Chang, S. W., Awasthi, M. K., & Ravindran, B. (2020). Earthworms and vermicompost: an eco-friendly approach for repaying nature’s debt. Environmental Geochemistry and Health, 42, 1617-1642.
- Singh, J., Schädler, M., Demetrio, W., Brown, G. G., & Eisenhauer, N. (2019). Climate change effects on earthworms-a review. Soil organisms, 91(3), 114.
- Singh, J., Schädler, M., Demetrio, W., Brown, G. G., & Eisenhauer, N. (2019). Climate change effects on earthworms-a review. Soil organisms, 91(3), 114.
- Singh, S., Sharma, A., Khajuria, K., Singh, J., & Vig, A. P. (2020). Soil properties changes earthworm diversity indices in different agro-ecosystem. BMC ecology, 20, 1-14.
- Sinha, R. K., Bharambe, G., & Ryan, D. (2008). Converting wasteland into wonderland by earthworms—a low-cost nature’s technology for soil remediation: a case study of vermiremediation of PAHs contaminated soil. The Environmentalist, 28, 466-475.
- Sinha, R. K., Herat, S., Valani, D., & Chauhan, K. (2010). Earthworms–the environmental engineers: review of vermiculture technologies for environmental management and resource development. International Journal of Global Environmental Issues, 10(3-4), 265-292.
- Smith, K. M. (2011). How to Build, Maintain, and Use a Compost System: Secrets and Techniques You Need to Know to Grow the Best Vegetables. Atlantic Publishing Company. Pp 163–170. ISBN 978-1-60138-354-9).
- Soobhany, N., Gunasee, S., Rago, Y. P., Joyram, H., Raghoo, P., Mohee, R., & Garg, V. K. (2017). Spectroscopic, thermogravimetric and structural characterization analyses for comparing Municipal Solid Waste composts and vermicomposts stability and maturity. Bioresource technology, 236, 11-19.
- Staggs, H. (2021). Vermiculture: A Viable Solution for Sustainable Agriculture.
- The Worm Farmers Handbook. White River Junction, VT. Chelsea Green Publishing.
- Tondoh, J. E. (2006). Seasonal changes in earthworm diversity and community structure in central Côte d’Ivoire. European Journal of Soil Biology, 42, S334-S340.
- Uvarov, A. V., Tiunov, A. V., & Scheu, S. (2011). Effects of seasonal and diurnal temperature fluctuations on population dynamics of two epigeic earthworm species in forest soil. Soil Biology and Biochemistry, 43(3), 559-570.
- Visvanathan, C., Trankler, J., Jospeh, K., & Nagendran, R. (2005). Vermicomposting as an eco-tool in sustainable solid waste management. Asian Institute of Technology, Anna University, India.
- Walsh, C. L., & Johnson-Maynard, J. L. (2016). Earthworm distribution and density across a climatic gradient within the Inland Pacific Northwest cereal production region. Applied Soil Ecology, 104, 104-110.
- Walsh, C. L., & Johnson-Maynard, J. L. (2016). Earthworm distribution and density across a climatic gradient within the Inland Pacific Northwest cereal production region. Applied Soil Ecology, 104, 104-110.
- Walsh, C., Johnson-Maynard, J. L., & Leslie, I. N. (2019). Seasonal variations in exotic earthworm populations in wheat fields of the Inland Pacific Northwest, USA. Pedobiologia, 76, 150569.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).