Submitted:
03 May 2024
Posted:
07 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nano(bio)materials
2.2.1. LipImage™ 815 Synthesis and Characterisation
2.2.2. PACA Synthesis and Characterisation
2.3. Monocyte-Activation Test
2.3.1. Preparation of Standards, Controls, and Test Materials
2.3.2. PyroMAT® Cell Incubation/Stimulation of IL-6 Production
2.3.3. IL-6 ELISA Procedure
2.4. Monocyte Isolation and Differentiation to M1/M2 Macrophages
2.4.1. Blood Collection and Lymphocyte Isolation
2.4.2. CD14+ Monocyte Magnetic Labelling and Isolation
2.4.3. M1/M2 Macrophage Differentiation
2.5. Macrophage Exposure to NBMs, and Subsequent Cytokine Analysis
2.5.1. Cytokine Quantification
2.6. Statistics
3. Results
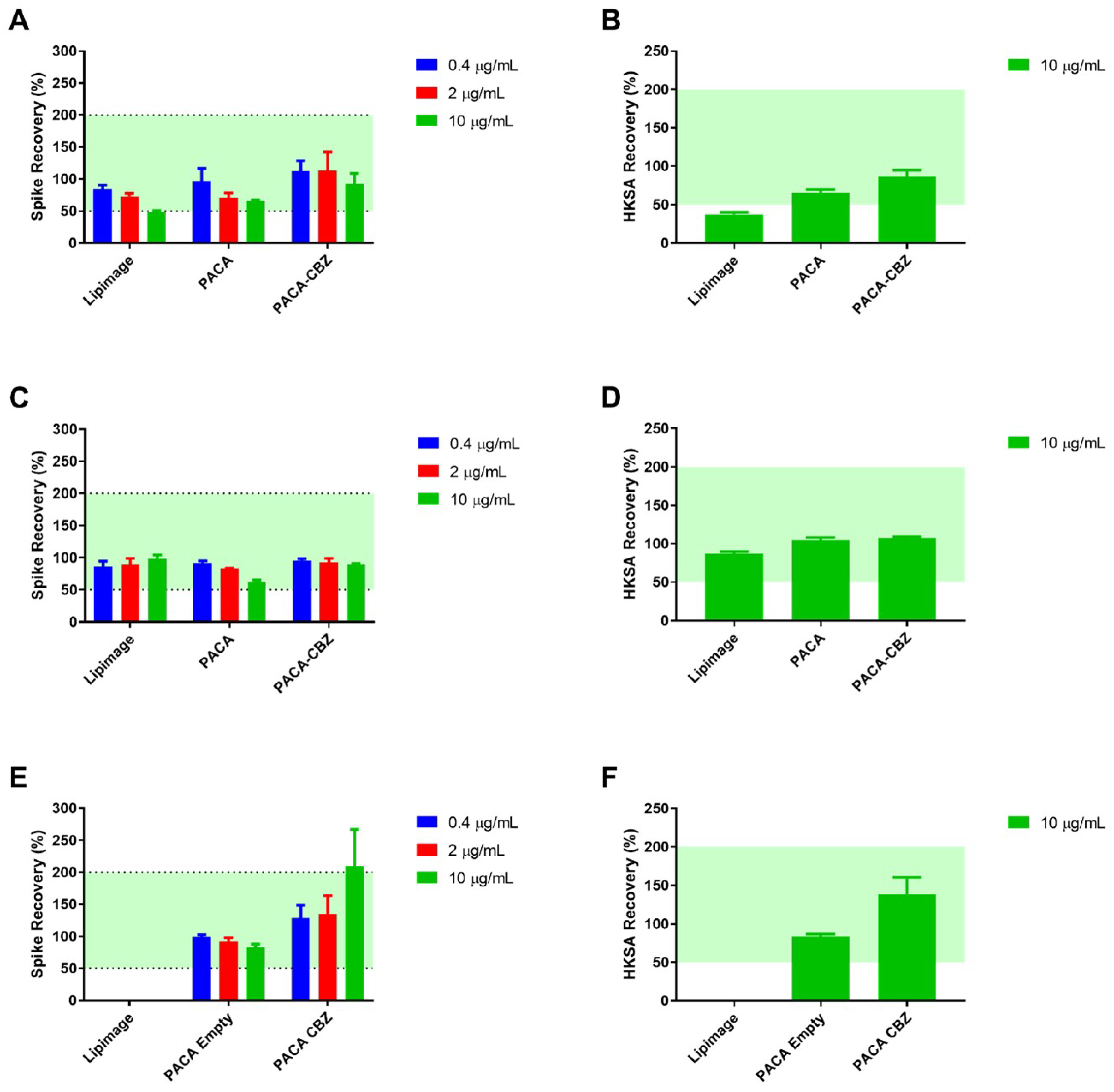
3.1. Monocyte-Activation Test
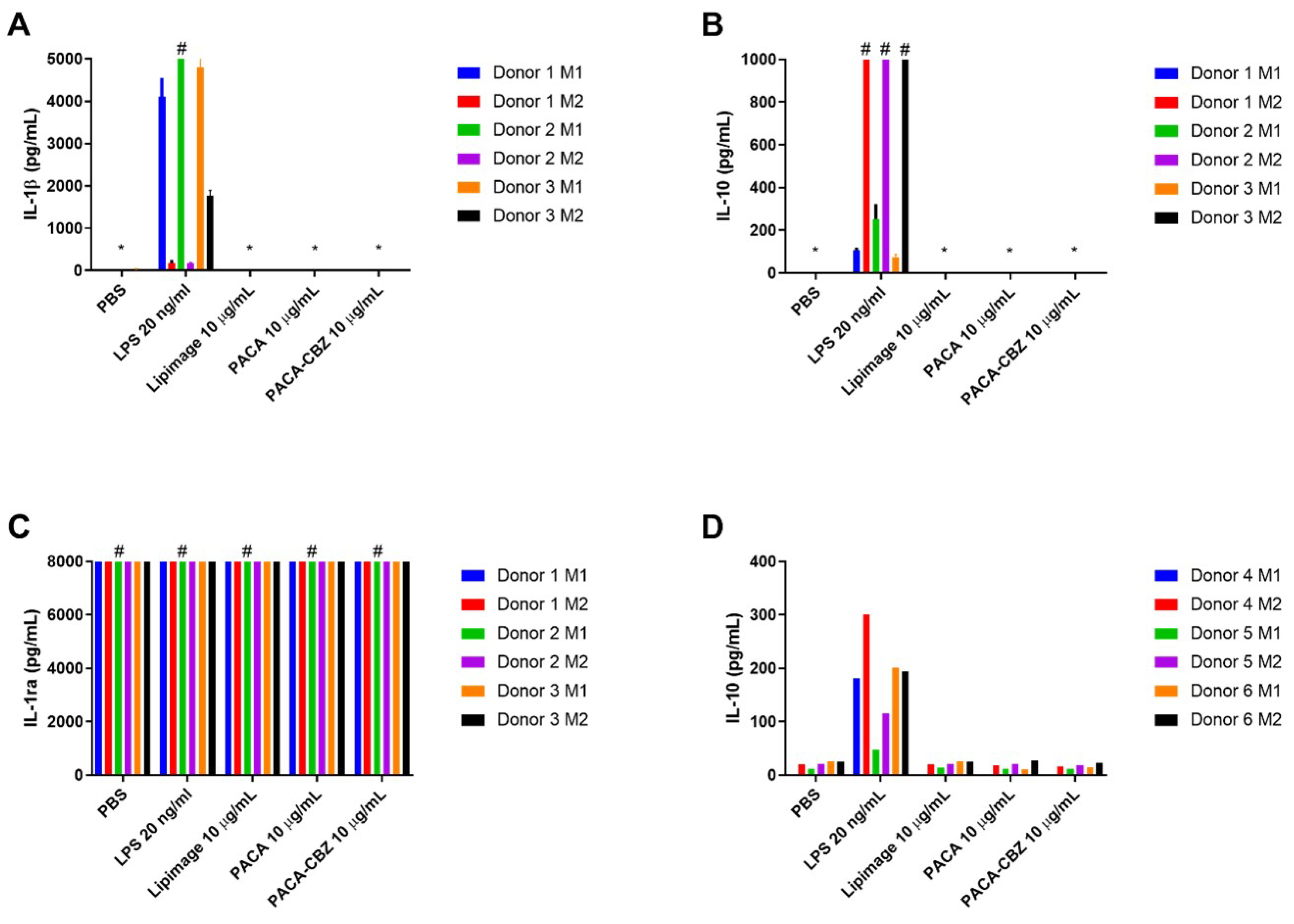
3.2. Macrophage Polarisation and Cytokines
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CARPA | Complement activation-related pseudoallergy |
| CBZ | Cabazitaxel |
| CD14 | Cluster of differentiation 14 |
| ELISA | Enzyme-linked immunosorbent assay |
| EU | Endotoxin units |
| FBS | Fetal bovine serum |
| FDA | Food and Drug Administration |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GMP | Good manufacturing practice |
| HBSS | Hank’s balanced salt solution |
| HIV | Human immunodeficiency virus |
| HSKA | Heat-killed Staphylococcus aureus |
| IL | Interleukin |
| LAL | Limulus amoebocyte lysate |
| LPS | Lipopolysaccharide |
| MAT | Monocyte-activation test |
| MDM | Monocyte-derived macrophage |
| M-CSF | Macrophage colony-stimulating factor |
| NBM | Nano(bio)materials |
| PACA | Poly(alkyl cyanoacrylate) |
| PBMC | Peripheral blood mononuclear cell |
| PEBCA | Poly(ethylbutyl cyanoacrylate) |
| PE | Phycoerythrin |
| PEG | Polyethylene glycol |
| PBS | Phosphate-buffered saline |
| REFINE | Regulatory Science Framework for Nano(bio)material-based |
| Medical Products and Devices | |
| RPMI-1640 | Roswell Park Memorial Institute 1640 Medium |
| RPT | Rabbit pyrogen test |
| SOP | Standard operating procedure |
| TNF | Tumour necrosis factor alpha |
References
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.S. The Role of Macrophages in the Resolution of Inflammation. The Journal of Clinical Investigation 2019, 129, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. International Journal of Molecular Sciences 2018, 19, 1801. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liang, H.; Zen, K. Molecular Mechanisms That Influence the Macrophage M1–M2 Polarization Balance. Frontiers in Immunology 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Roszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators of Inflammation 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle Uptake: The Phagocyte Problem. Nano today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Germolec, D.R.; Weaver, J.L. Evaluation of Nanoparticle Immunotoxicity. Nature Nanotechnology 2009, 4, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Briley-Saebo, K.; Bjørnerud, A.; Grant, D.; Ahlstrom, H.; Berg, T.; Kindberg, G.M. Hepatic Cellular Distribution and Degradation of Iron Oxide Nanoparticles Following Single Intravenous Injection in Rats: Implications for Magnetic Resonance Imaging. Cell and Tissue Research 2004, 316, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Csukás, D.; Urbanics, R.; Wéber, G.; Rosivall, L.; Szebeni, J. Pulmonary Intravascular Macrophages: Prime Suspects as Cellular Mediators of Porcine CARPA. European Journal of Nanomedicine 2015, 7, 27–36. [Google Scholar] [CrossRef]
- Gordon, S. Phagocytosis: An Immunobiologic Process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M. Macrophage Recognition of Crystals and Nanoparticles. Frontiers in Immunology 2018, 9, 103. [Google Scholar] [CrossRef]
- Rosales, C.; Uribe-Querol, E. Phagocytosis: A Fundamental Process in Immunity. BioMed Research International 2017, 2017, 9042851. [Google Scholar] [CrossRef] [PubMed]
- Godec, J.; Tan, Y.; Liberzon, A.; Tamayo, P.; Bhattacharya, S.; Butte, A.J.; Mesirov, J.P.; Haining, W.N. Compendium of Immune Signatures Identifies Conserved and Species-Specific Biology in Response to Inflammation. Immunity 2016, 44, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; Finnerty, C.C.; López, C.M.; Honari, S.; Moore, E.E.; Minei, J.P.; Cuschieri, J.; Bankey, P.E.; Johnson, J.L.; Sperry, J.; Nathens, A.B.; Billiar, T.R.; West, M.A.; Jeschke, M.G.; Klein, M.B.; Gamelli, R.L.; Gibran, N.S.; Brownstein, B.H.; Miller-Graziano, C.; Calvano, S.E.; Mason, P.H.; Cobb, J.P.; Rahme, L.G.; Lowry, S.F.; Maier, R.V.; Moldawer, L.L.; Herndon, D.N.; Davis, R.W.; Xiao, W.; Tompkins, R.G.; Inflammation and Host Response to Injury, Large Scale Collaborative Research Program. Genomic Responses in Mouse Models Poorly Mimic Human Inflammatory Diseases. Proceedings of the National Academy of Sciences of the United States of America 2013, 110, 3507–3512. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, S.; De Majo, F.; Kim, J.; Trenti, A.; Trevisi, L.; Fadini, G.P.; Bolego, C.; Zandstra, P.W.; Cignarella, A.; Vitiello, L. Convenience versus Biological Significance: Are PMA-Differentiated THP-1 Cells a Reliable Substitute for Blood-Derived Macrophages When Studying in Vitro Polarization? Frontiers in Pharmacology 2018, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.C.; Andersen, M.N.; Møller, H.J. Monocyte Isolation Techniques Significantly Impact the Phenotype of Both Isolated Monocytes and Derived Macrophages in Vitro. Immunology 2020, 159, 63–74. [Google Scholar] [CrossRef] [PubMed]
- FDA.; CDER.; CBER. Drug Products, Including Biological Products, That Contain Nanomaterials - Guidance for Industry, 2022.
- Åslund, A.K.O.; Vandebriel, R.J.; Caputo, F.; de Jong, W.H.; Delmaar, C.; Hyldbakk, A.; Rustique, E.; Schmid, R.; Snipstad, S.; Texier, I.; Vernstad, K.; Borgos, S.E.F. A Comparative Biodistribution Study of Polymeric and Lipid-Based Nanoparticles. Drug Delivery and Translational Research 2022, 12, 2114–2131. [Google Scholar] [CrossRef] [PubMed]
- Liptrott, N.J.; Giardiello, M.; McDonald, T.O.; Rannard, S.P.; Owen, A. Lack of Interaction of Lopinavir Solid Drug Nanoparticles with Cells of the Immune System. Nanomedicine 2017, 12, 2043–2054. [Google Scholar] [CrossRef] [PubMed]
- Liptrott, N.J.; Giardiello, M.; McDonald, T.O.; Rannard, S.P.; Owen, A. Assessment of Interactions of Efavirenz Solid Drug Nanoparticles with Human Immunological and Haematological Systems. Journal of Nanobiotechnology 2018, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hobson, J.J.; Rannard, S.P.; Owen, A.; Liptrott, N.J. Safety Assessment of a New Nanoemulsion-Based Drug-Delivery System Reveals Unexpected, Drug-Free Anticoagulant Activity. Nanomedicine 2020, 15, 1361–1373. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Frontiers in Immunology 2014, 5. [Google Scholar] [CrossRef]
- EDQM. Monocyte-Activation Test. European Pharmacopoeia, 6th Edition (6.7), 2010.
- Jacquart, A.; Keramidas, M.; Vollaire, J.; Boisgard, R.; Pottier, G.; Rustique, E.; Mittler, F.; Navarro, F.; Boutet, J.; Coll, J.L.; Texier, I. LipImage™ 815: Novel Dye-Loaded Lipid Nanoparticles for Long-Term and Sensitive in Vivo near-Infrared Fluorescence Imaging. Journal of Biomedical Optics 2013, 18, 101311. [Google Scholar] [CrossRef] [PubMed]
- Varache, M.; Escudé, M.; Laffont, C.; Rustique, E.; Couffin, A.C. Development and Validation of an HPLC-fluorescence Method for the Quantification of IR780-oleyl Dye in Lipid Nanoparticles. International Journal of Pharmaceutics 2017, 532, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Klymchenko, A.S.; Roger, E.; Anton, N.; Anton, H.; Shulov, I.; Vermot, J.; Mely, Y.; Vandamme, T.F. Highly Lipophilic Fluorescent Dyes in Nano-Emulsions: Towards Bright Non-Leaking Nano-Droplets. RSC advances 2012, 2, 11876–11886. [Google Scholar] [CrossRef] [PubMed]
- Halamoda-Kenzaoui, B.; Holzwarth, U.; Roebben, G.; Bogni, A.; Bremer-Hoffmann, S. Mapping of the Available Standards against the Regulatory Needs for Nanomedicines. Wiley Interdisciplinary Reviews. Nanomedicine and Nanobiotechnology 2019, 11, e1531. [Google Scholar] [CrossRef] [PubMed]
- Halamoda-Kenzaoui, B.; Vandebriel, R.J.; Howarth, A.; Siccardi, M.; David, C.A.W.; Liptrott, N.J.; Santin, M.; Borgos, S.E.; Bremer-Hoffmann, S.; Caputo, F. Methodological Needs in the Quality and Safety Characterisation of Nanotechnology-Based Health Products: Priorities for Method Development and Standardisation. Journal of Controlled Release 2021, 336, 192–206. [Google Scholar] [CrossRef] [PubMed]
- David, C.A.; Owen, A.; Liptrott, N.J. Determining the Relationship between Nanoparticle Characteristics and Immunotoxicity: Key Challenges and Approaches. Nanomedicine 2016, 11, 1447–1464. [Google Scholar] [CrossRef] [PubMed]
- EMA.; CHMP. ICH Guideline Q4B Annex 14 to Note for Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions on Bacterial Endotoxins Tests – General Chapter, 2013.
- FDA. ; CDER.; CBER.; CVM.; CDRH.; ORA. Guidance for Industry - Pyrogen and Endotoxins Testing: Questions and Answers, 2012. [Google Scholar]
- Dobrovolskaia, M.A.; Neun, B.W.; Clogston, J.D.; Ding, H.; Ljubimova, J.; McNeil, S.E. Ambiguities in Applying Traditional Limulus Amoebocyte Lysate Tests to Quantify Endotoxin in Nanoparticle Formulations. Nanomedicine (London, England) 2010, 5, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Smulders, S.; Kaiser, J.P.; Zuin, S.; Van Landuyt, K.L.; Golanski, L.; Vanoirbeek, J.; Wick, P.; Hoet, P.H. Contamination of Nanoparticles by Endotoxin: Evaluation of Different Test Methods. Particle and Fibre Toxicology 2012, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- van der Bruggen, T.; Nijenhuis, S.; van Raaij, E.; Verhoef, J.; van Asbeck, B.S. Lipopolysaccharide-Induced Tumor Necrosis Factor Alpha Production by Human Monocytes Involves the Raf-1/MEK1-MEK2/ERK1-ERK2 Pathway. Infection and Immunity 1999, 67, 3824–3829. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the Mechanism of IL-1β Secretion. Cytokine & Growth Factor Reviews 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A. Pre-Clinical Immunotoxicity Studies of Nanotechnology-Formulated Drugs: Challenges, Considerations and Strategy. Journal of Controlled Release: Official Journal of the Controlled Release Society 2015, 220, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Tarique, A.A.; Logan, J.; Thomas, E.; Holt, P.G.; Sly, P.D.; Fantino, E. Phenotypic, Functional, and Plasticity Features of Classical and Alternatively Activated Human Macrophages. American Journal of Respiratory Cell and Molecular Biology 2015, 53, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Plüddemann, A. Tissue Macrophages: Heterogeneity and Functions. BMC Biology 2017, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Anchordoquy, T.J.; Barenholz, Y.; Boraschi, D.; Chorny, M.; Decuzzi, P.; Dobrovolskaia, M.A.; Farhangrazi, Z.S.; Farrell, D.; Gabizon, A.; Ghandehari, H.; Godin, B.; La-Beck, N.M.; Ljubimova, J.; Moghimi, S.M.; Pagliaro, L.; Park, J.H.; Peer, D.; Ruoslahti, E.; Serkova, N.J.; Simberg, D. Mechanisms and Barriers in Cancer Nanomedicine: Addressing Challenges, Looking for Solutions. ACS nano 2017, 11, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; McNeil, S.E. Understanding the Correlation between in Vitro and in Vivo Immunotoxicity Tests for Nanomedicines. Journal of Controlled Release: Official Journal of the Controlled Release Society 2013, 172, 456–466. [Google Scholar] [CrossRef]
- Buscher, K.; Ehinger, E.; Gupta, P.; Pramod, A.B.; Wolf, D.; Tweet, G.; Pan, C.; Mills, C.D.; Lusis, A.J.; Ley, K. Natural Variation of Macrophage Activation as Disease-Relevant Phenotype Predictive of Inflammation and Cancer Survival. Nature Communications 2017, 8, 16041. [Google Scholar] [CrossRef] [PubMed]
- Gharib, S.A.; McMahan, R.S.; Eddy, W.E.; Long, M.E.; Parks, W.C.; Aitken, M.L.; Manicone, A.M. Transcriptional and Functional Diversity of Human Macrophage Repolarization. The Journal of Allergy and Clinical Immunology 2019, 143, 1536–1548. [Google Scholar] [CrossRef]
- Maes, E.; Landuyt, B.; Mertens, I.; Schoofs, L. Interindividual Variation in the Proteome of Human Peripheral Blood Mononuclear Cells. PLOS ONE 2013, 8, e61933. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




