1. Introduction
The current world population is more than 8.1 billion as of 2024. The rapid increase seen in the growth of population fairly gives the idea of the skyrocketing news of food, fuel, and energy. Thus, there is an ever-increasing need to find efficient alternatives for complementing the existing food and energy sources to meet the scarcity that is evident to occur in the future. The statistics help us to understand the gravity of the problem to find an efficient alternative as early as possible. Microalgae and the products derived from them have proved to meet almost all of the requirements of an ideal alternative for meeting energy needs. Moreover, the economically feasible nature of the microalgal culturing processes.
1.1. Depletion of Fossil Fuels and the Need for Sustainable Alternatives
There is marked overexploitation of fossil fuels observed despite of being unsustainable sources of energy. This overexploitation is due to the rapid industrialization & skyrocketing growth of population. The present scenario is indicative of a global energy crisis in the near future. Statistics suggest that almost 85% of our energy demands have been met with fossil fuels which are harmful non-renewable energy sources [
1]. Thus, there is an increasing need to discover other energy sources to complement and eventually compensate for the existing fossil fuels which have adverse complications on the environment. The climate change instigated due to numerous anthropogenic factors has driven people’s attention to the bioproducts/biofuels obtained from microalgae. There have been evidence of exploitation of various foodstuffs for extracting energy but these sources are not enough to meet the increasing needs. Biofuel production from microalgae is coupled with carbon dioxide capture; this collective action helps in the elimination of carbon dioxide from the environment thereby overcoming the problem of global warming along with the energy crisis. The lipid yield from microalgae has been found to possess the potential to outgrow the efficiency of many other oil-producing crops. In addition, microalgae is also a rich source of carbon compounds such as biofuels, cattle feedstock, nutraceuticals et cetera. Thus, microalgae is proving to be an emerging alternative as an efficient alternative for fossil fuels.
1.2. Biofuels as a Promising Renewable Energy Source
The accelerated pace of increasing population is predicted to reach 9 billion by the year 2050. As a consequence, the emerging energy needs & greater global prices have put phenomenal pressure on the existing natural energy resources which eventually leads to their rapid depletion. Fossil fuel burning has led to innumerable environmental hazards, for instance, a surge in greenhouse gas (GHG) emissions more specifically carbon dioxide [
2]. As evident from the statistics, there is a marked increase observed in global primary energy utilization owing to higher standards of living & industrialization. Contemporarily, over 80% of the world’s energy needs are met by fossil fuel burning like coal, oil, and natural gas. Approximately 98% of this energy is extracted from the emissions of carbon compounds from fossil fuels. As per an estimate, the overall intensity & duration of drought-like conditions are predicted to be more severe due to plummeted water reserves by 5-fold in the 21st century. Due to the scarcity observed in the energy sources and to meet the rising needs for energy, alternative options for these energy sources are thought to be one of the most practical approaches for tackling this problem. An increased interest has been observed in culturing biofuels at a global & national level as a potential substitute for fossil fuels. These biofuels gained much popularity due to their capability of reversing impacts of climate change & reduction of emissions of GHG. These are considered as one of the profitable options due to their cheaper synthesis. This reduces the burden on the natural energy resources thereby conserving the environmental health. Biofuels or more specifically biodiesels have captured researcher’s attention owing to their tremendous advantages over fossil fuels & flexibility of feedstock conferred by them.
1.3. Advantages of Microalgae for Biofuel Production
The hunt for discovering potential energy alternatives for fossil fuels has led us to microalgae-derived biofuels & bioproducts [
3]. They have gained much attention due to ability to grow rapidly and thrive in diverse conditions, including extreme environments, makes cultivation cost-effective and versatile. Moreover, the natural oils it forms hold great potential for biodiesel production, while its lipid compounds offer promise as aviation fuel. Simultaneously, its high biomass cultivation aids in carbon dioxide sequestration, mitigating greenhouse gas emissions. Additionally, its application in wastewater treatment not only purifies water but also contributes to nutrient recycling. Ultimately, the production of biodegradable and eco-friendly biofuels underscores its significant role in fostering a greener, more sustainable future.
1.4. Chlorella Vulgaris: A Robust and Lipid-Rich Microalga
The experimental studies of microalgae have become a research topic of utmost importance in recent decades as they are primarily characterized as raw materials for chemical compounds; which have been influenced by their primary & secondary metabolism. In a nutshell, the innumerable advantages of the employment of a microalgae culturing system can be summarised as easy & rapid cultivation, and growth possible even from wastewater. The
Chlorella vulgaris are the species of microalgae of the order
Chlorococcales of the
Oocytaceae family. They belong to the genus
Chlorella. The shape of chloroplasts is spherical with a size of 1-10 microns & also contains chlorophyll which imparts a green color to the species [
4]. In conjunction with chlorophyll, they comprise of substantial amount of intracellular lipids, proteins, vitamins such as B1, B2, B6 & B12, β-carotenes, C, carbohydrates et cetera. This can be attributed to their large-scale usage of
Chlorella vulgaris for food supplement preparations, industrial cosmetic productions, clinical applications, wastewater treatment involving heavy metal detoxification & much more. These strains have been found to grow easily and relatively quickly based on their metabolism type whether it is mixotrophic, heterotrophic, or autotrophic. Experimental studies have shown that the highest biomass productivity has been observed in the case of mixotrophic medium or when photobioreactor systems have been employed. This data extracted from such studies significantly helps us to optimize the microalgal culturing system and to pool maximum benefits from these strains for improving the standard of living of the human race.
2. Sustainable Cultivation of Chlorella vulgaris for Biofuel Feedstock
The energy needs of the world are reaching skyrocketing limits with each passing day. There is an increasing need to find efficient alternatives for energy extraction to keep up with the pace of this robust development. In the present era, third-generation biofuels extracted from microalgae are considered as one of the most practical approaches for meeting energy needs [
5]. These third-generation biofuels possess the capability of overcoming the challenges faced by the first & second-generation biofuels. As per the extensive literature survey carried out, it was found that numerous
Chlorella vulgaris stresses have proven to be apt as a potential biofuel generator.
2.1. Strain Selection and Improvement Strategies
Microalgae is found to be an emerging sustainable energy alternative owing to their qualities like the capability to accommodate large-scale lipids, high productivity & rapid growth rate. Screening of suitable strains of
Chlorella vulgaris is pivotal for the success of exhaustive production under certain climate & environmental situations. In conjunction with high productivity & rapid growth rate, the ability of the strain to grow in wastewater should also be considered as it proves to be an important deciding factor for the economic feasibility of the specific production scheme [
6]. Microalgal bioprospecting is the foremost step while optimizing a novel biotechnological candidate strain. The crucial step in the bioprospecting process is sample collection from a pool of species inhabiting aquatic & terrestrial areas. The source of isolating rich microalgal strains can be enumerated as marine, freshwater & terrestrial environments, particularly including coastal areas, rivers, lakes & soil. It has also been found that microalgae can also be extracted from extreme habitats ranging from lithospheric, and cryospheric lands & desert sands due to their ability to withstand intense environmental conditions [
7].
Chlorella vulgaris is known to be isolated from the samples extracted from the environment using advanced microbiological techniques. Some of the isolation techniques employed for this task are single-cell isolation, direct plating & serial dilution. The technique of single-cell dilution can be carried out by generating clouds of aerosol in which each drop comprises a single cell of the target strains; microscopy & fluorescence-activated cell sorting (FACS) [
8]. In isolation based on microscopy, the cells are primarily observed under a microscope and then the individual cells are extracted via a micropipette. These sampled cells can be either inoculated into liquid media or plated on a solid media. The primary objective of these methods is to ensure the extraction of pure strains by separating possible confounding elements from the concerned projects [
9]. There is an increasing need to culture algal strains axenically for accurate characterization to produce high-yield products.
2.1.1. Natural Selection and Mutagenesis
Microalgal metabolites & biomass are considered excellent alternatives to renewable energy sources on the background of both economics & ecology. To achieve biotechnological optimization of
Chlorella vulgaris which is financially viable it is inevitable to improve the efficiency of the cell factories. The two primary approaches for improving the cell factories are- random mutagenesis & rational metabolic engineering [
10]. The central idea of random mutagenesis comprises an incessant exposure to chemical/physical mutagens; thus providing a phenotypic & genetic mutant diversity. This has to be further evaluated for improved metabolic functions & required cell attributes. The success of random mutagenesis is heavily dependent on a plethora of factors like the quality & quantity of the light supplied, nitrogen and carbon supply [
11]. Apart from the environmental factors, the nature of the mutagen involved, its exposure duration & concentration are some of the determining factors of the desired mutation.
This mutagen is responsible for inducing irreversible alterations in the genetic makeup of the algal strain [
12]. Its objective is to generate mutant strains possessing required phenotypic & genotypic characteristics. Mutagens impact the DNA in a specific sequence as intercalating agents can lead to the untwisting of DNA strands, disrupting the double helix structure. Meanwhile, physical mutagens like ionizing or UV radiations induce single- or double-stranded breaks in the DNA molecule, leaving it vulnerable to mutations. In some cases, physical mutagens can also cause covalent binding between two pyrimidine bases, further altering the DNA sequence. Moreover, various chemical mutagens are capable of causing alterations in DNA bases, introducing mismatches or substitutions. Alkylating agents, another type of chemical mutagen, can form cross-links between DNA strands, interfering with replication and repair processes, ultimately contributing to genetic instability.
2.1.2. Genetic Engineering Approaches
Genetic engineering has always been coupled with microalgal biotechnology to extrapolate maximal benefits from the process. The application of genetic engineering to microalgal strains has led to conquering the intrinsic challenges of the metabolic limits imposed on the accommodation capacity of biomolecules [
13]. This approach is aimed at boosting the economic feasibility of the overall production process. To elaborate, the data extrapolated from metabolic pathway maps, genomic sequencing & other sources are the fundamental factors pivotal for characterizing the target gene from the collected samples of microalgal strains. Considering the progress made in the genetic approach for microalgal production, there is still a need to explore gene functional experiments, and gene annotations even more to gain insights. In conjugation with the existing data, the multi-omics datasets for microalgal strains can also be investigated for ameliorating the quality of products derived from microalgae & biorefinery abilities [
14]. Another approach for devising strategies for strain improvement is the functional genetic investigation via high-throughput screening & genome-scale mutant libraries.
2.2. Optimization of Growth Conditions for Biomass and Lipid Production
To determine the optimal conditions for efficient growth of the
Chlorella vulgaris strain, numerous studies were carried out to determine the values. The microalgal strains were subjected to various pH, temperature, intensity of light, and salinity levels [
15]. The ones that showed the most efficient growth under certain sets of pH, temperature, light, and salinity conditions were considered ‘optimum’. The microalgal biomass was thus harvested & weighed and an attempt was made to establish a correlation between the biomass and lipid production.
2.2.1. Light Intensity and Quality
The optimum growth of the
Chlorella vulgaris strain & its associated lipid production was examined by exposing the samples to varied light qualities and intensities such as 100-500 flux [
16]. A direct relationship was observed between the growth of microalgal biomass and the growth & rate of photosynthesis carried by the strain depending on the light intensities and their durations. Experimentally, it was found that 400 flux is ascertained as the most optimal intensity supporting the growth of the microalgae
Chlorella vulgaris. The control variables for this experiment are
Scenedesmus obliquus,
Chlorophyta sp.,
Desmodesmus denticulatus, and,
Monoraphidium sp. as these recorded the highest values of absorbance [
17]. The data extracted also proved that the light and dark periods alternation is essential for photosynthesis as the formation of high-energy substrate nicotinamide adenine dinucleotide phosphate (NADPH) & adenosine-5'-triphosphate (ATP) requires light to stimulate the dark reaction to synthesize carbon skeletons.
2.2.2. Temperature and pH
Experimental conditions verified the fact that evaluation of temperature further leads to enhanced microalgal growth & lipid accommodation. It was found that the optimal temperature for the growth of the microalga
Chlorella vulgaris is 30-35ºC [
18]. These studies were under the previous experimental results obtained as evident from the extensive literature survey carried out.
Numerous pH levels were verified to check for the optimum value to significantly enhance the productivity of the process by comparing the absorbance value of the samples. As per the exhaustive experiments carried out, the microalgal strains are found to grow efficiently under acidic conditions. The microalgal strain Chlorella vulgaris has been observed to grow optimally under a neutral pH 7 level. Chlorella vulgaris is known to adapt to a wide range of pH levels from 4 to 10; the greatest productivity of biomass is observed at pH 9 to 10.
2.2.3. Nutrient Availability
The presence of essential nutrients during the growth of microalgal strains is a crucial determinant for optimum growth. Even deficiency of any one of these essential nutrients will significantly impact the growth of the strains thereby stunting the growth and compromising on the lipid accumulation [
19]. Nitrogen concentration is found to have a great impact on the growth & biochemical make-up of the strains like highly reduced biomass accumulation capability as a consequence of nitrogen deficiency. Thus, there is a close interrelationship between the nitrogen balance of the strain and the biomass accommodation. Phosphorous plays a key role in cell division activities, signal transduction, and most importantly induction of lipid accumulation.
2.3. Cultivation Systems for Chlorella Vulgaris
Microalgal strains possess the great ability to multiply at a faster pace. This ability can be exploited by coupling it with advanced culturing systems to make these bioproducts as a potential industrial starting material. Microalgal growth does not require pesticides, herbicides, freshwater & fertile land for growth. Microalgae can also be grown employing wastewater such as palm oil milling effluents & domestic sewage et cetera [
20]. This ensures both ensuring the optimum growth of microalgal strains as well as wastewater bioremediation. A model culturing system for microalgae particularly
The unicellular green algae Chlorella vulgaris is a prime option for sustainable biomass production because it can grow in a variety of conditions and is tolerant of several light sources, including artificial LED arrays and direct sunlight. This alga demonstrates effective gas exchange, facilitating the smooth transfer of CO2 and O2 over the liquid-gas interface, hence promoting its growth rate and overall productivity. We cultivate Chlorella vulgaris using simple methods like photobioreactors or open ponds, ensuring minimal contamination due to its resilient characteristics and the use of basic sterilizing processes. The organism's rapid growth in limited locations allows for low production costs, resulting in high yields on tiny land plots and maximum resource efficiency. Chlorella vulgaris possesses a unique set of characteristics that make it highly useful for a wide range of uses, including biofuels and nutritional supplements. Additionally, it promotes the use of cost-effective and sustainable production methods. On a broader perspective, the microalgal culturing systems are characterized into two classes: photo bioreactor & open pond.
2.3.1. Open Pond Systems
One of the most primitive and simplest ways for microalgae harvesting is the open pond system. The open pond is a primarily employed system for industrial use owing to its comparatively inexpensive operation, maintenance & construction costs. Allied advantages include decreased energy demand, easy of scale-up & operation. The open ponds which comprise natural water bodies like ponds & lakes; and artificial water bodies like raceways and circular ponds [
21,
22]. Occasionally, a tank is also used for microalgae culture. It is one of the most co-efficient microalgae culturing systems contemporarily. Though the need for cultivation area is high in the case of open pond systems, cultivation from natural water bodies possesses lower cell density; thereby highlighting the need for inventing a new efficient harvesting system. In conjunction, other challenges faced are salinity and pH of the rainwater runoff significantly affect microalgal growth. This results in increased turbidity of the water & leakage thus affecting the open pond system microalgae culturing productivity.
There are also greater chances of bacterial or protozoal contamination which leads to the generation of unstable & toxic bioproducts. The only solution to combat this problem is by cultivation of microalgae that possess the capability of withstanding extreme saline & alkaline conditions as a negligible amount of contaminants can thrive under such conditions [
23]. It is also extremely tedious to keep a check on the growth parameters such as intensity of the light & temperature due to the open nature of the culturing system. Microalgae cultivation from natural sources is one of the most common techniques known to man since time immemorial. The oldest evidence of harvesting microalgae was done by the Aztec community where
Spirulina was harvested from the lake Texcoco in Mexico [
24]. This culturing system is highly influenced by the growth condition provided by the natural water bodies which will in turn influence the productivity of the process.
2.3.2. Photobioreactors
It is a photobioreactor system primarily employed for culturing microalgae in an enclosed system. The main objective of this system is restraining direct contact with the environment thereby curbing the degree of contamination of the cultured microalgae [
25]. It possesses the capability of overcoming most of the shortcomings of open pond systems. The size of the photobioreactor is more compact than the open pond system therefore increasing the efficiency of land utility. It provides a closed environment for avoiding contamination from the environment. It also provides highly controlled growth conditions which helps in the generation of single-strain, contaminant-free microalgae [
26]. In conjunction, high biomass production per substrate is a result of the translation of a controlled culture system into greater metabolic & nutrient efficiency. The major hurdle faced in this system is the highly limited scalability owing to the numerous design flaws; thereby making it financially unfeasible for industrial-scale production. The financial structure has also proved to be an obstacle in this system. The different types of photobioreactors are:
- (a)
Tubular photobioreactor
They consist of transparent glass or plastic tubes stacked in horizontal, vertical, or oblique alignment to maximize the process of capturing sunlight [
27].
- (b)
Vertical column photobioreactor
They consist of cylindrical transparent tubes in vertical orientation & an air bubble sparger [
28]. This system ensures the homogenization of the culture medium by allowing the effective transfer of oxygen and carbon dioxide with the microalgae culture.
- (c)
Flat-plate photobioreactor
They consist of transparent rectangular compartments of 1-5 cm depth. There is a recirculating air lift system that enables the mixing of the culture medium inside the reactor.
2.4. Integration with Wastewater Treatment and CO2 Capture
Microalgae have been studied since time immemorial; the various experiments carried out resulted in exploiting microalgae for biomass production, carbon dioxide capture, wastewater treatment management et cetera [
29]. The technology of carbon dioxide capture & wastewater treatment has been researched separately and was not integrated until this decade. The central idea behind carbon dioxide fixation using microalgae is that microalgae utilize carbon dioxide as their primary carbon source for all metabolic processes. This carbon dioxide bifurcation enhances algal biomass production & also decreases emissions of greenhouse gases correspondingly [
30,
31]. In wastewater treatment, to curb the expenses of tertiary treatment of wastewater, the rapidly growing microalgae culture approach-driven removal of nitrogen & phosphorus is employed. This two-way approach also removes unwanted nitrogen & phosphorous from the wastewater and also results in the formation of algal biomass.
Efficient microalgal production requires an ideal nutrient medium comprising all the essential macro- & micronutrients in it for maximizing biomass productivity. The wastewater coupled with carbon dioxide sparging proves to be an ideal medium for culturing microalgae. Various studies have cited that the microalgae grown from wastewater possess greater photosynthetic efficiencies when carbon dioxide is sparged into the culture medium [
32]. Any process leading to an increase in the pH leads to inhibition of the growth of the microalgae. Carbon dioxide sparging into the system helps to maintain the pH within the optimum range of 7.5-8.0.
3. Biomass Harvesting and Processing
Microalgae offer diverse environmental and energy applications, including wastewater bioremediation and biofuel production. Despite their potential, economic viability remains a challenge [
33]. Research focuses on utilizing wastewater for cultivation, adopting biorefinery approaches, and developing cost-effective harvesting methods. Harvesting costs, representing a significant portion of production expenses, are high due to microalgae's small size, low culture densities, and negatively charged surfaces [
34]. Efforts aim to identify economically viable and efficient harvesting techniques to advance sustainable microalgal biomass production [
35].
3.1. Harvesting Strategies
3.1.1. Flocculation
Substance-induced aggregation and settling are essential for economically optimizing microalgal harvesting processes, concentrating suspensions 20–100 times, and reducing energy demands for dewatering. This method involves adjusting pH levels or adding electrolytes to induce aggregation, while settling is encouraged through the addition of cationic polymers to promote particle settling. Factors influencing this process include cellular concentration, surface properties, coagulant/flocculant concentration, pH, and ionic strength. Magnesium ions, obtained from wastewater or lime addition, have been effective coagulants, initiating charge neutralization and sweep flocculation. Coagulant dose may vary with cell concentration and culture conditions, influenced by extracellular polymeric substances (EPS) and ionic strength. Polyelectrolyte flocculants, such as chitosan and cationic starch, bridge microalgal cells effectively in freshwater but may be inhibited by high salinity in marine environments. While chitosan is effective and non-contaminating, its cost limits large-scale applications, prompting exploration of alternatives like cationic starch.
Sedimentation
Gravity sedimentation is a simple and energy-efficient method used for harvesting microalgae, particularly for low-value end products like biofuels. However, its reliability is limited due to the slow settling rates of microalgae, ranging from 0.1 to 2.6 cm/h, which can result in biomass deterioration during the sedimentation process. To enhance microalgal settling, a coagulation/flocculation step is often applied beforehand. Lamella-type separators and sedimentation tanks are commonly used for microalgal harvesting through gravity sedimentation, but they typically yield low concentrations without prior coagulation/flocculation [
43]. Despite this, microalgal auto flocculation can lead to improved recovery rates, with sedimentation tanks considered a simple and cost-effective option [
44].
3.1.2. Centrifugation
Centrifugation is a rapid but costly method for microalgal harvesting, primarily suited for high-value products like unsaturated fatty acids and pharmaceuticals. While efficient in capturing microalgae, centrifuges can damage cell structures due to exposure to high gravitational and shear forces. Optimal harvesting efficiency requires longer retention times in the centrifuge bowl, especially for small microalgal cells [
45]. Although high capture efficiency demands more energy, lower energy conditions can decrease overall production costs. Combining coagulation/flocculation with centrifugation can reduce energy consumption and increase final algal concentration, making the process more cost-effective [
46].
3.1.3. Filtration
Filtration is crucial for dewatering microalgal suspensions after coagulation/flocculation to enhance harvesting efficiency. It relies on a pressure drop across a membrane to facilitate fluid flow, but membrane fouling by microalgal deposits and extracellular polymeric substances (EPS) hampers performance, necessitating regular cleaning. While suitable for certain microalgae, filtration is less common in large-scale processes due to high operational costs and membrane replacement needs [
47]. Tangential flow filtration (TFF) is preferred for smaller suspended algae, offering better anti-fouling performance. Microfiltration and ultrafiltration membranes are effective but energy-intensive and require frequent replacements. High gradient magnetic filtration shows promise for efficient microalgal harvesting, but major costs are associated with membrane replacement and pumping. Microfiltration could be a more cost-effective option for handling smaller volumes, while centrifugation emerges as a viable choice for larger volumes. Suggested pressure filtration designs include chamber filter presses, cylindrical sieves, and filter baskets models [
48].
3.2. Cell Disruption Methods
3.2.1. Mechanical Disruption (Bead Beating, Sonication)
Mechanical disruption methods such as pressing, bead-milling, ultrasound, autoclave, and homogenization are energy-intensive and are most effective at high cell density preparations (50–200 kg/m3 dry weight). These methods are often combined with solvent techniques to extract lipids from the cells. Additionally, pretreatments like acid/alkali and enzymatic actions are employed to improve recovery ratios. Mechanical disruption is preferred as it minimizes chemical contamination and preserves cellular functionality [
49].
Bead milling involves the use of a cylindrical compartment filled with high-velocity steel or glass beads, which spin rapidly to mechanically disrupt cells. This method has been applied to various microalgae species, including Scenedesmus obliquus, Spirulina platensis, and Monodus subterraneous [
50]. Bead milling, particularly with fine beads, has shown high efficiency in lipid extraction, comparable to other methods like microwave treatment. However, scaling up bead milling for industrial use poses challenges due to energy consumption [
51]. Despite this drawback, bead milling is considered one of the most efficient mechanical disruption methods for algae processing, especially when used alongside solvents and at high cell concentrations [
52].
Ultrasonication, or ultrasonic disruption, involves the application of high-frequency sound waves to disrupt cell structures and release intracellular contents. This method offers several advantages, including short extraction times, reduced solvent consumption, and enhanced penetration of solvents into cellular materials, resulting in improved extraction efficiency. Ultrasonication achieves this by inducing cavitation, which generates microbubbles that collapse violently, creating localized high temperatures and pressures that rupture cell walls [
53].
However, ultrasonication also has limitations, including high power consumption and difficulties in scaling up for industrial applications. The energy required for ultrasonication can be significant, especially for large-scale operations, which may increase operational costs. Additionally, challenges in maintaining consistent and uniform ultrasonic conditions across large volumes can hinder scalability. Despite these drawbacks, ultrasonication remains a valuable tool in laboratory settings and small-scale operations due to its effectiveness in extracting intracellular components from various biological materials [
54].
3.2.2. Chemical Disruption (Enzymes, Solvents)
Chemical solvents like alcohols, dimethyl sulfoxide, methyl ethyl ketone, or toluene can be used for cell lysis, extracting lipid components from the cell wall and releasing intracellular contents [
55]. However, it may denature some proteins, and it's not commonly used in large-scale processes. Another method involves hydrolysing the cell wall with alkali but it incurs high chemical costs for neutralization and may destabilize the product [
56].
Using digestive enzymes is another approach to achieve cell lysis, as they break down microbial cell walls. Different enzymes are used depending on the microbe; for example, lysozyme is effective for digesting the cell wall of gram-positive bacteria by hydrolysing β-1-4-glucosidic bonds in peptidoglycan. However, lysozyme is less efficient for gram-negative bacteria due to differences in their cell wall structure.
3.3. Biomass Dewatering and Drying
Flocculation is indeed used in both drinking water and wastewater treatment to aggregate particles, making subsequent sedimentation or flotation easier. It's particularly useful for separating microalgae from suspensions, making it a popular technology for microalgae dewatering.
Bio-flocculants, derived from bacteria, microalgae, and fungi, contain carbohydrates, proteins, humic substances, nucleic acids, lipids, and surfactants. They are used in various applications such as water treatment, wastewater flocculation, sludge dewatering, metal removal, and soil remediation [
57]. Bacterial bio-flocculants are effective for microalgae harvesting and wastewater treatment, surpassing chemical flocculants in effectiveness. However, their purification costs, involving centrifugation and solvent washing, can be high, prompting the need for low-cost purification methods [
58].
Electro-flocculation, widely used in wastewater treatment, offers advantages like easy operation, low chemical usage, and absence of residual anions in the solution. It has become a key alternative to conventional flocculation processes. Microalgal aggregates produced by electro-flocculation are typically collected using flotation. Operational factors include electrode material, electrolysis voltage, current density, electrolysis time, pH, and microalgae suspension composition. Using an aluminium electrode yields better dispersion destabilization compared to iron. Flocculation efficiency increases with energy consumption, voltage, current, and reaction time [
59]. Operational costs for electro-flocculation are relatively low, making it a promising option for large-scale applications.
Spray drying rapidly dries liquid droplets using hot gases in a vertical tower, suitable for algae production for human consumption. However, its high-pressure atomization process can rupture cells, affecting product quality. Despite its efficiency, spray drying has high operating costs and results in low digestibility compared to other methods like drum drying [
60].
Solar drying of algae is feasible in remote areas lacking energy grids, utilizing either direct solar radiation or solar water heating [
61]. Direct sunlight causes dehydration and alters the texture and colour of algae, while solar water heating systems help regulate biomass heating. Solar drying is weather-dependent and prone to overheating, but solar water heating systems offer better control [
62]. However, both methods are unreliable and may emit unpleasant odors, making them unsuitable for human consumption. They may be acceptable for animal feed production due to lower capital costs and simplicity [
63].
Cross-flow air drying was used to dry wet Spirulina algae slurry with 55–66% moisture content for 14 hours at 62°C, resulting in a good quality dried algae product 2–3 mm thick with 4–8% moisture content. The drying process maintained the integrity of the cell walls of Chlorella and Scenedesmus algae. Compared to drum drying, cross-flow air drying was found to be cheaper, and it was faster than solar heat drying [
64].
In a vacuum-shelf dryer, Spirulina algae slurry was dried to 4% moisture content at temperatures ranging from 50–65°C and a pressure of 0.06 atm. The resulting dried algae exhibited hygroscopic properties and a porous biomass structure. However, the study noted higher capital and operating costs associated with this drying method [
65].
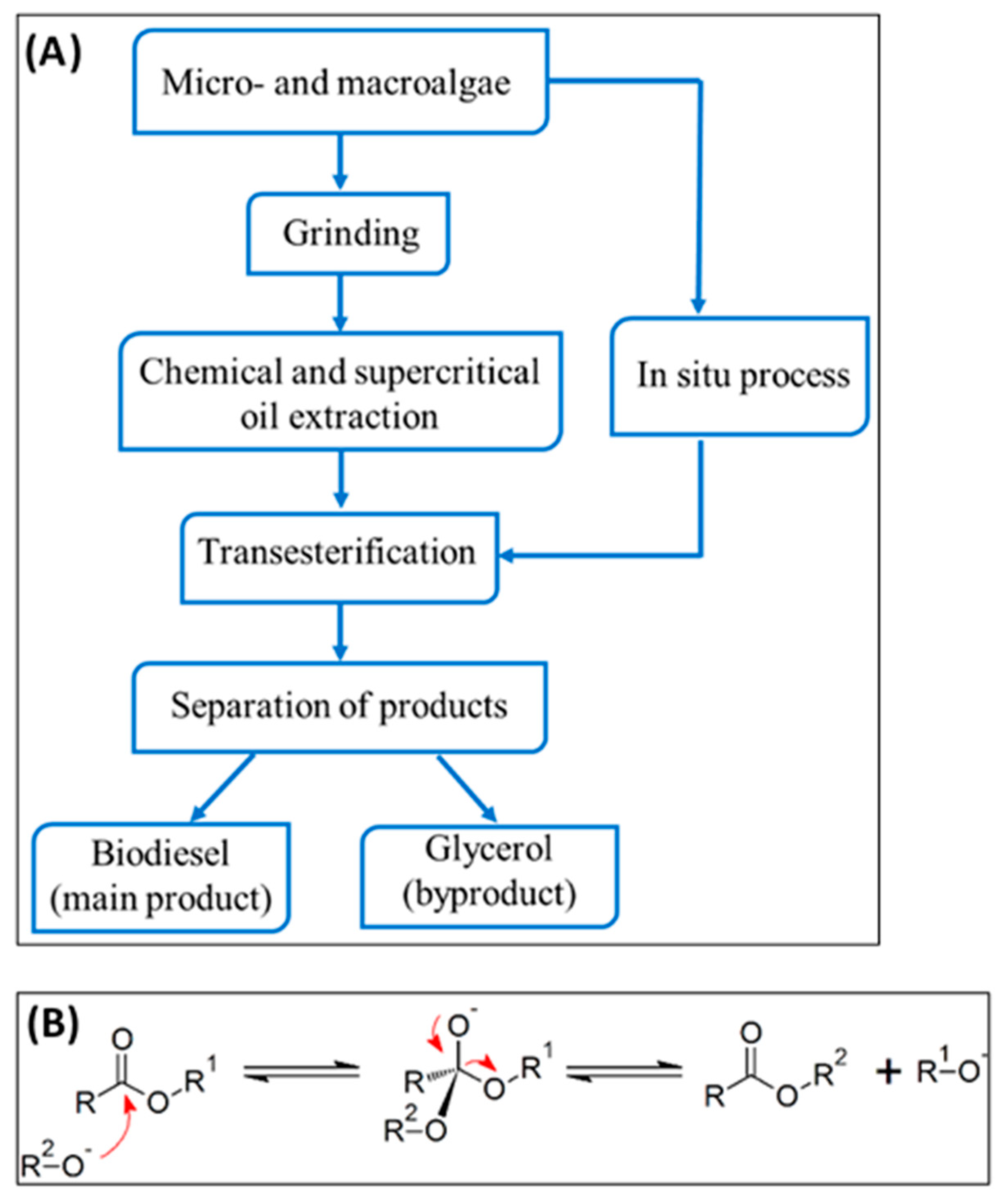
4. Biofuel Production from Chlorella Vulgaris Biomass and Lipids
4.1. Transesterification for Biodiesel Production and Catalysts
Extensive study has been conducted on the transesterification of lipids of Chlorella vulgaris for the purpose of biodiesel generation. In general, biodiesel refers to mono-alkyl esters of long chain fatty acids that are obtained from vegetable oils or animal fats [
66]. Biodiesel is synthesized by the transesterification process, also known as alcoholysis, wherein lipids (fats and oils) are reacted with alcohol (usually methanol or ethanol) to yield a mixture of fatty acid alkyl esters (FAAE) or fatty acid methyl esters (FAME) and some by product such as glycerol [
67].
he transesterification process involves a series of three reversible reactions, converting triglycerides to diglycerides, then to monoglycerides, and finally to glycerol. At each step, an ester is formed, resulting in the production of three ester molecules from one triglyceride molecule. This process efficiently converts triglycerides, such as those found in vegetable oils, into fatty acid methyl esters (FAME), commonly known as biodiesel [
68].
Figure 1 illustrates the overall process of transesterification, which is a chemical reaction employed to transform triglycerides into biodiesel and glycerol. The process demonstrates the sequential reactivity of an alkoxide ion with the ester bonds of the triglyceride, resulting in the creation of methyl esters (biodiesel) and glycerol via a sequence of nucleophilic assaults and eliminations.
The conventional transesterification process starts by extracting lipids from algal biomass using a chemical solvent, and subsequently optimizing the reaction. Consequently, algal strains having a high concentration of lipids are chosen [
69]. The transesterification reaction employs two categories of homogeneous catalysts: (i) base catalysts, such as sodium hydroxide (NaOH) and potassium hydroxide (KOH) , and (ii) acid catalysts, including acids such as H
2SO
4, HF and HCl [
70] and an excess of dry methanol at atmospheric pressure and a specific temperature. Homogeneous catalysts (acid and alkali) are the preferred choice for biodiesel production due to their simplicity and shorter reaction time. Consequently, homogeneous catalysts are presently the catalysts that have been most extensively utilized in the industry. Base catalysts allow for effortless optimization of activity, contributing to their suitability for biodiesel production [
71]. However, the main disadvantages of base catalysts include the soap formation and requires extensive washing. Also, these types of catalyst are not suitable with the feedstock that are highly rich in FFA [
72]. In a study, Luna et al. [
73] explored the use of LiOH-pumice as a heterogeneous catalyst for in situ transesterification of Chlorella sp., achieving a fatty acid methyl ester (FAME) yield of 47% under optimized conditions. The catalyst was prepared via acid treatment and wet impregnation, showing effective lipid conversion with high surface area and porous morphology. Another similar study worker utilized calcium oxide (CaO) catalysts prepared from chicken egg shell waste for biodiesel production from Chlorella vulgaris. The study optimized the transesterification process using response surface methodology (RSM) and achieved a biodiesel yield of 92.03% under optimal conditions [
74]. Jazie et al. [
75] achieved higher yields of biodiesel through in-situ transesterification using dodecylbenzenesulfonic acid as a catalyst, highlighting its efficiency in reducing the cost of biodiesel production. The in-situ transesterification process yielded larger amounts of biodiesel compared to the two-step extraction-transesterification procedure. The ideal conditions for in situ transesterification involved using an alcohol to biomass ratio of 15, maintaining temperatures of 338.15 K for methanol and 348.15 K for ethanol, utilizing a catalyst ratio of 25%, conducting the reaction for a duration of 4 hours, and maintaining a mixing rate of 600 RPM. The results highlight the efficiency of in situ transesterification when appropriate operational parameters are used to improve biodiesel production. The key benefits of using homogeneous acid catalytic are high catalytic activity, productivity with high FFA feedstock, and no soap generation. However, their catalytic rate is slower than that of base catalysts [
76]. While homogeneous catalysts are widely used due to their efficiency and shorter reaction times, exploring heterogeneous catalysts offers significant environmental advantages and simplifies the separation process from the final products.
Heterogeneous catalysts, another significant group of catalysts, have been extensively investigated for biofuel production. They are gaining attention in biodiesel production due to their environmental benefits and ease of separation from the final product. For example, NaOH/zeolite, a heterogeneous catalyst for transesterification demonstrated significant efficiency, with a biodiesel yield of 98% from Chlorella vulgaris. Also, these catalyst are easy to separate from the obtained products [
77]. Supercritical methanol with oxide catalysts, is another heterogenous catalyst, having supercritical methanol in conjunction with oxide catalysts (α-Al2O3) has shown to enhance the yield of fatty acid methyl esters (FAMEs), suggesting that complete dewatering of Chlorella vulgaris biomass may not be necessary. This method promotes higher liquid product and FAME yields, highlighting its potential for one-pot biodiesel production [
78].
Table 2 provides a summary of the various catalysts used for biodiesel generation from Chlorella vulgaris, focusing on their reaction conditions and yields.
4.1.1. Catalysts and Reaction Mechanisms
Alkali-Catalysed Processes: Alkali catalysts like sodium hydroxide and potassium hydroxide are widely used due to their efficiency in lower reaction times and higher yield outcomes. Research by Salam et al. [
87] demonstrated that a high biodiesel yield of 96% could be achieved within 10 minutes using an alkali-catalysed reactive extraction method, despite the high free fatty acid content in Chlorella vulgaris.
Acid-Catalysed Processes: Acid catalysts, such as sulfuric acid, are beneficial for feedstocks with high free fatty acid content. A study by Kalsum et al. [
88] explored the effect of microwave irradiation on acid-catalyzed in situ transesterification, optimizing the yield to 31.56% under certain reaction conditions.
Enzymatic Processes: Enzymatic transesterification using lipases offers a more environmentally friendly alternative, with the potential for lower energy requirements and milder reaction conditions. Tran et al. [
89] cultivated Chlorella vulgaris ESP-31, in a photobioreactor with CO2 aeration, achieved high oil content (63.2%) and was used for biodiesel production via enzymatic transesterification with Burkholderia sp. C20 lipase. Direct transesterification of disrupted biomass (M-II method) yielded a higher biodiesel conversion (97.3%) compared to extracted oil (72.1%). The process effectively handled wet biomass with high water content and allowed multiple reuses of the lipase without significant loss of activity.
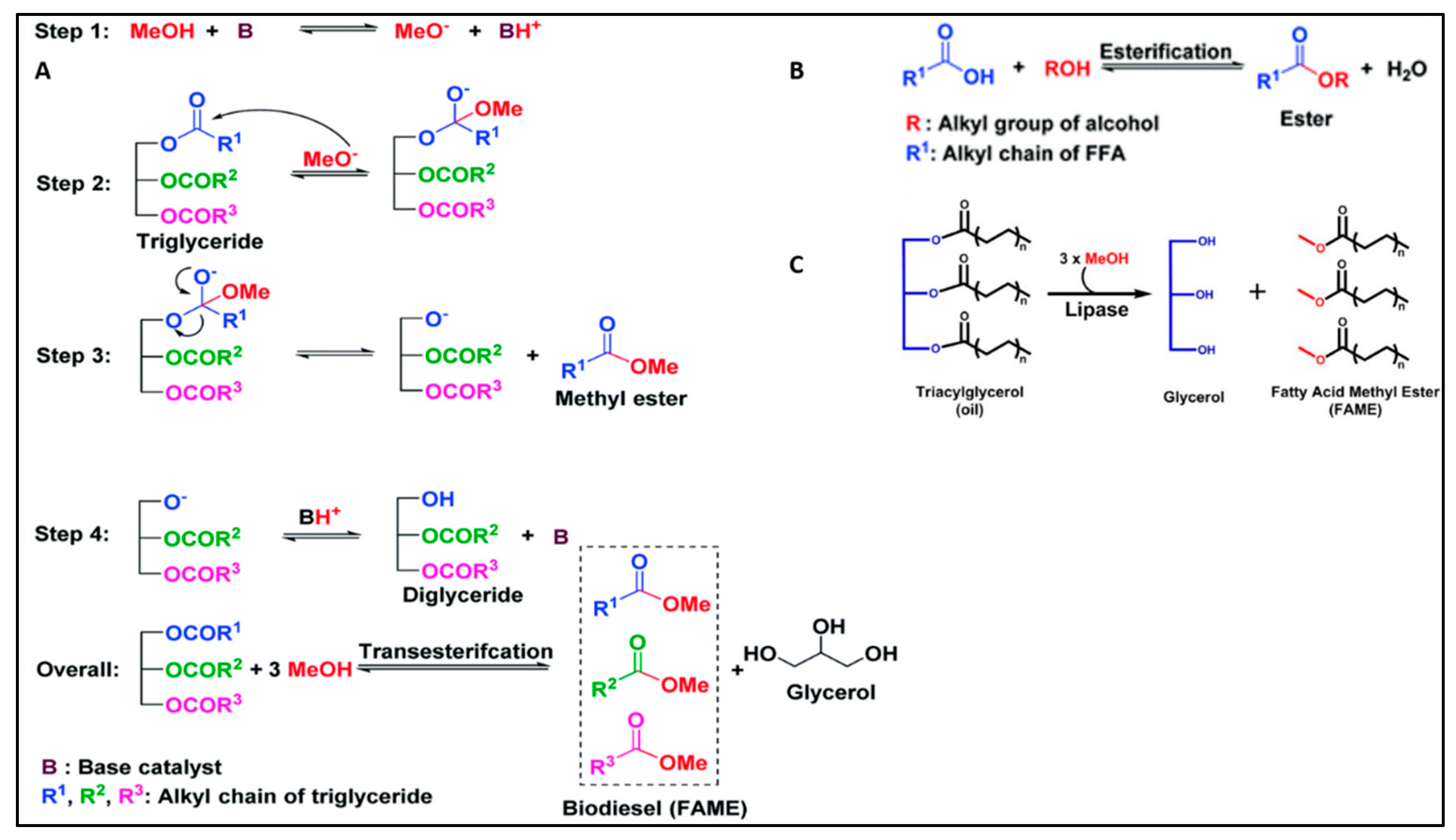
The alkali-catalyzed reaction mechanism, depicted in
Figure 2 (A), facilitates the transesterification of triglycerides (TGs) found in vegetable oil to produce biodiesel. In contrast,
Figure 2 (B) illustrates the acid-catalyzed esterification process, which targets the conversion of the free fatty acid (FFA) content of the vegetable oil into biodiesel. These two distinct mechanisms represent essential pathways for the conversion of vegetable oil into biodiesel, showcasing the versatility of transesterification and esterification processes in sustainable fuel production.
Figure 2 (C) depicts the mechanism of lipase-catalyzed transesterification reaction.
4.2. Alternative Biofuel Pathways
4.2.1. Hydrothermal Liquefaction
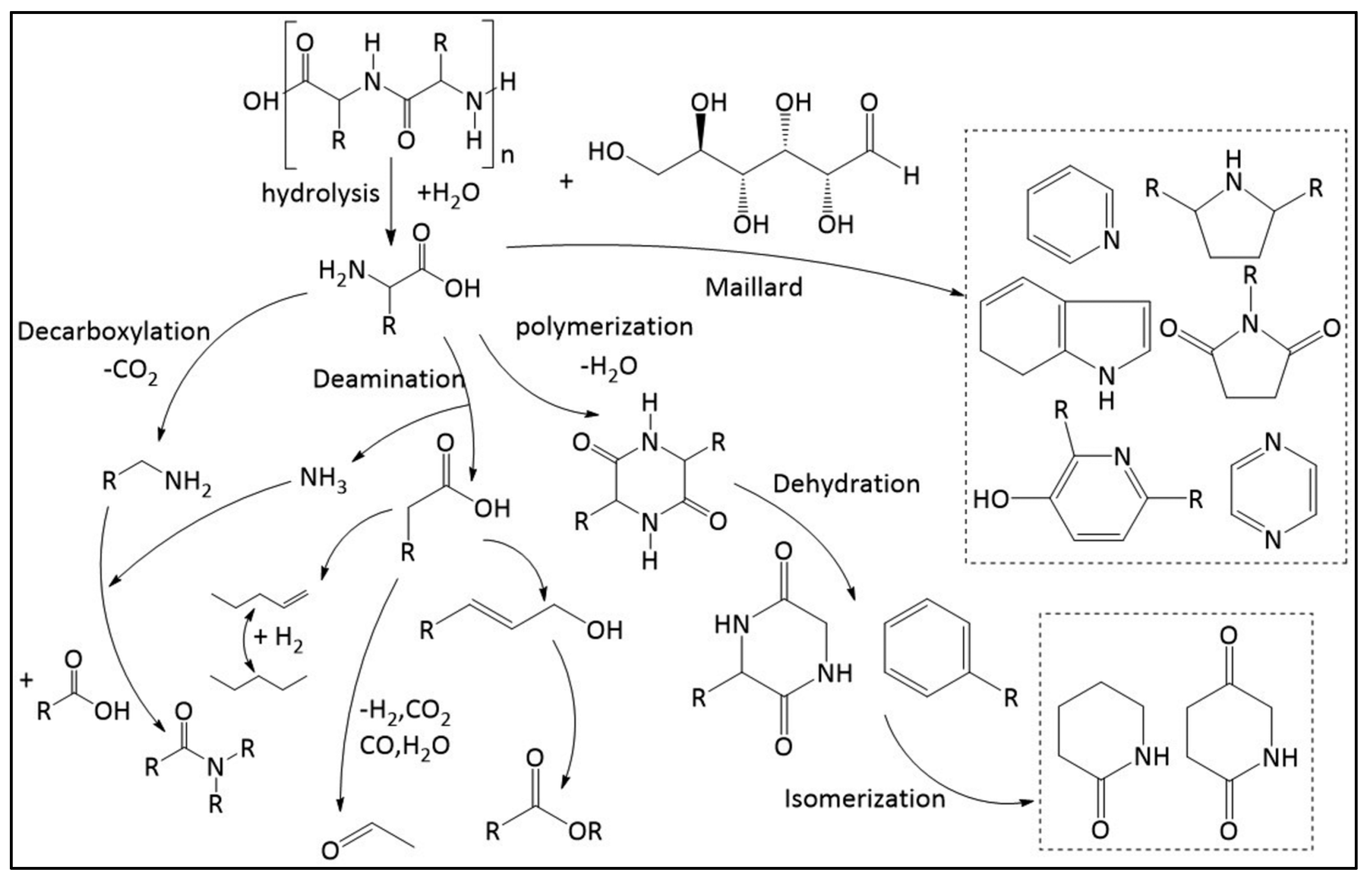
Hydrothermal processes, including hydrothermal liquefaction (HTL), effectively convert biomass into bio-crude oil by using water as a solvent under high temperatures (250 to 374°C) and pressures (up to 20 MPa). This method is particularly advantageous for processing high-moisture resources as it bypasses the need for drying the biomass. During HTL, the combination of heat and pressure hydrolyzes and breaks down lignocellulosic material, transforming it into a variety of biofuels and chemicals. This conversion not only utilize the inherent water content of the biomass but also enhances the efficiency and sustainability of biofuel production [
91]. Hydrothermal liquefaction (HTL) distinguishes itself from other thermochemical technologies like pyrolysis and gasification by its ability to process wet biomass directly, eliminating the costly and energy-intensive drying step required by other methods. This capability allows HTL to efficiently convert diverse wet feedstocks, including food waste, microalgae, lignocellulose, and sewage sludge, into bio-crude oil. This advantage not only rationalizes the conversion process but also enhances the economic feasibility of biofuel production from various biomass sources [
92,
93,
94,
95,
96].
Hydrothermal liquefaction (HTL) is a transformative technology that accelerates the natural geological process of converting organic matter into crude oil from millennia to mere minutes. This chemical process, similar to those occurring deep within the Earth over millions of years, efficiently converts 85%–90% of organic matter directly into bio-crude oil and natural gas, offering a viable alternative to traditional fuel production methods [
97]. HTL effectively processes a diverse array of wet biomass, such as food waste, microalgae, and sewage sludge, by breaking down complex molecules like cellulose and lignin through chemical reactions including hydrolysis, decarboxylation, and hydrogenation [
98,
99]. This conversion produces bio-crude oil with high energy density but also with impurities like oxygenates, nitrogen, and sulfur that require refining before the oil can be used as conventional fuel [
100]. HTL generates several valuable by-products, including a nutrient-rich aqueous phase, a gaseous phase primarily consisting of CO2, and a solid residue of minerals and char [
101]. This output supports the creation of a sustainable, closed-loop system where the aqueous phase can be recycled to grow more biomass or used in industrial applications, thereby enhancing environmental sustainability.
In the hydrolysis stage of HTL, cellulose reacts with water under high temperatures, breaking hydrogen bonds and reducing crystallinity. This reaction transforms cellulose into simpler sugars like glucose and fructose, which further degrade into simpler hydrocarbons [
102]. The specific pathways of cellulose hydrolysis, including C–O–C bond cleavage, vary depending on whether the medium is acidic, basic, or neutral [
103]. For example, in an acidic medium, acid hydrolysis cleaves glycosidic bonds between glucose units, while in a basic medium, an OH- ion attacks the anomeric carbon. These variations illustrate the adaptable and complex chemistry of HTL, allowing for optimized biomass conversion into valuable products [
104,
105].
The hydrothermal liquefaction of cellulose primarily involves the impact of cellulose crystallinity on the degree of liquefaction, the yields of major reaction products such as glucose and 5-(hydroxymethyl)furfural (5-HMF), and the role of amorphous starting materials in the liquefaction process [
106]. On the other hand, the hydrothermal liquefaction of lipids, particularly in the context of algae, focuses on the efficient conversion of lipids, proteins, and carbohydrates into biofuels, highlighting the advantages of biofuels derived from renewable sources and the potential for generating new income and employment opportunities [
107,
108]
Figure 3.
This process shows potential for being both scalable and sustainable in the field of alternative fuel technologies. Exploring the ability of HTL technique to convert algal biomass into biofuel. Guo et al. [
110] investigated the continuous hydrothermal liquefaction (HTL) of
Chlorella vulgaris in a stirred tank reactor. They obtained an average biocrude yield of 36.2 wt% at the operating conditions of 350°C and 24 MPa. The biocrude obtained was subjected to additional refinement using nickel catalysts, resulting in a substantial enhancement of its appropriateness for the conversion into gasoline, kerosene, and diesel fuel. This demonstrates the effectiveness of continuous hydrothermal liquefaction (HTL) processes in the production of biofuels. In a similar study, Kumar et al. [
111] investigated the direct hydrothermal liquefaction (HTL) of microalgae to produce bio-oil using a high-pressure batch reactor under subcritical water conditions. Three different microalgae samples, namely
Chlorella vulgaris,
Botryococcus braunii, and
Scenedesmus quadricauda, were examined under hydrothermal liquefaction with different water concentrations (1:6, 1:7, 1:8, 1:9 & 1:10 ratio) at a temperature range of 200-320 °C, pressure of 60 bars, and a reaction time of 30 minutes. The highest bio-oil (BO) yield achieved with
S. quadricauda was 18 wt% at a 1:9 ratio. Analysis of the bio-oil via gas chromatography revealed the presence of furan, phenol, acid, and ester derivatives. It was observed that increasing temperatures led to higher BO yields due to polymerization reactions converting small biomass components into heavier molecules. Additionally, FTIR spectra showed a high percentage of aliphatic, phenolic, alcoholic, carboxylic, and hydroxyl groups in solid residues. These studies highlight the significant impact that HTL can have on the biofuel industry, specifically in terms of improving efficiency, promoting sustainability, and maximizing resource utilization.
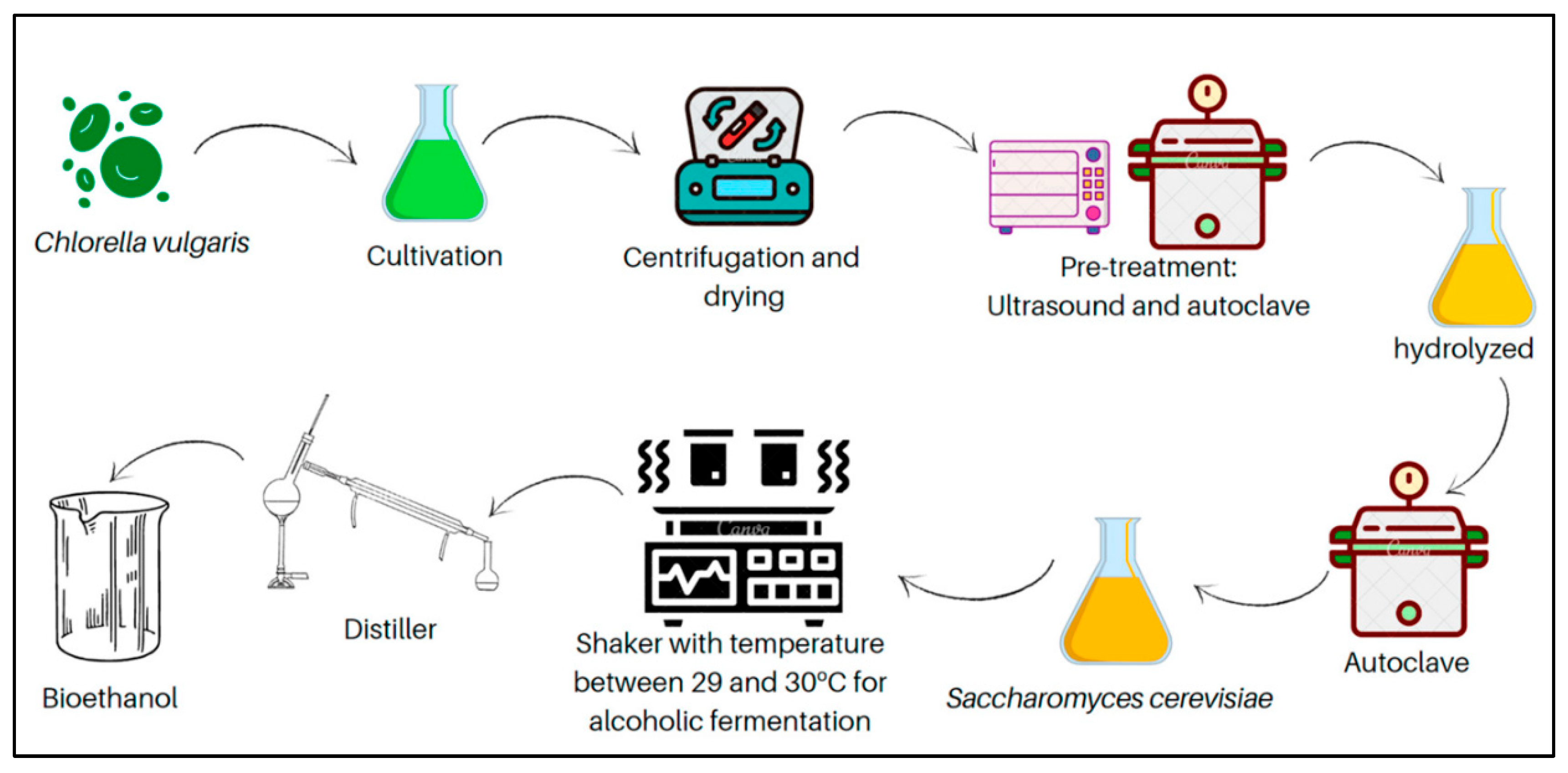
4.2.1. Microbial Fermentation
Microbial fermentation is a process that involves the use of microorganisms to transform sugars generated from lignocellulosic biomass into biofuels such as ethanol, butanol, acetone, iso-butanol, lipids, and other value-added biochemicals like organic acids.
Chlorella biomass, found to be a carbohydrate-rich microalga, has been considered as a potential feedstock for bioethanol production [
111]. Additionally, different ethanol-producing microorganisms have been screened for their potential in fermenting high-solids loadings of
Chlorella biomass [
112]. Building on the potential of
Chlorella biomass for bioethanol production, Rahman et al. [
113] carried out a novel study explored an integrated approach combining pretreatment, fermentation, and ethanol-assisted liquefaction to optimize biofuel yield from
Chlorella sp. In this study, the microalgal biomass was first pretreated with dilute sulfuric acid to enhance carbohydrate availability. The fermentation stage utilized
Saccharomyces cerevisiae and
Pichia stipitis, two yeast species known for their robust ethanol production capabilities. Notably, while both yeasts performed comparably at lower biomass concentrations,
P. stipitis demonstrated superior ethanol yields and more efficient glucose and xylose utilization at higher biomass loadings. This indicates a significant potential of
P. stipitis in processing
Chlorella-derived sugars into ethanol. Following fermentation, the introduction of ethanol-assisted liquefaction enhanced the conversion efficiency, yielding an increase of 40.7% in crude biodiesel production compared to traditional liquefaction methods. This integrated approach underscores the efficacy of using advanced fermentation techniques with specific microorganisms to maximize biofuel outputs from microalgal biomass. Furthermore, the high-density fed-batch cultivation of
Chlorella, particularly
Chlorella sorokiniana, has proven effective for achieving high lipid yields. This approach utilizes heterotrophic growth conditions to achieve dense algal cultures, which are essential for reducing costs and enhancing the feasibility of large-scale biofuel production [
114]. Additionally, the integration of waste substrates from other industrial processes, such as the by-products of brewer fermentation and crude glycerol, has been explored to cultivate
Chlorella protothecoides. This not only recycles waste but also maintains productivity, thereby supporting sustainable and economically viable biofuel production [
115]. Thus, these novel methods demonstrate the progressive developments in the industry of biofuel manufacturing through the utilization of microalgal biomass. These approaches provide avenues to increase output, decrease production expenses, and promote the sustainability of biofuel technologies.
5. Valorisation of Non-Lipid Biomass Components
The adoption of renewable energy is essential as it guarantees ecological sustainability, energy independence, and economic stability. The transition from using petroleum-based products to biomass-based materials enhances the availability of renewable carbon feedstock [
116]. The microalgae culture produces biomass that is composed of a variety of unique components, such as lipids, proteins, carbohydrates, and different inorganic species [
117]. The composition of microalgal biomass evolves depending on the unique species of microalgae and the environmental circumstances during their growth. It is crucial to acknowledge that the distinctive makeup of microalgal biomass can be utilized to determine its precise commercial value [
118].
5.1. Protein Extraction and Utilization
Microalgae rely on proteins for catalytic mechanisms that enable their metabolic processes to function optimally [
119]. These processes are vital for the survival and growth of microalgae. For instance, proteins are involved in the regulation of metabolic pathways, energy production, and the synthesis of essential molecules. The interaction and energy exchange between chloroplasts and mitochondria have been found to increase the growth of microalgae and the production of hydrogen, highlighting the significance of proteins in metabolic processes [
120]. Proteins also serve a structural role in microalgae by providing scaffolds for the assembly of chlorophyll molecules under visible light and for harvesting chloroplast complexes [
121]. This structural support is crucial for the efficient utilization of light energy in the process of photosynthesis, which is essential for the survival and growth of microalgae [
122]. The nutritional value of proteins in microalgae is determined by their composition and the quantity of amino acids they contain [
123]. Amino acids, as the building blocks of proteins, make up the majority of these essential macromolecules. The composition and quantity of amino acids in proteins are key factors in determining their nutritional value, which is significant for the overall health and growth of microalgae [
124]. The extraction and processing of basic protein from microalgae is a critical execution that commonly follows biomass generation in open or closed culture methods [
125]. Recently, attempts have been made to produce microalgae strains that are adept at secreting recombinant proteins in the growth medium, exhibiting encouraging results [
126,
127]. These findings have substantial ramifications for biotechnological applications and the food and feed industry. This specialized technique enables for the focused manufacturing of certain proteins, catering to the demands of niche markets with precision and efficiency. The generation of crude proteins utilizing microalgae farming based on the biorefinery idea is highlighted as a robust technology with easy scaling [
128,
129]. This technique strives to meet the demand of the food and feed markets efficiently. The integration of microalgae farming with the biorefinery idea offers a sustainable and economically effective approach for manufacturing crude proteins from microalgae [
130,
131]
Figure 4.
The varied content and structure of microalgae cell walls underscore the necessity to screen for commercial strains that can be disrupted by traditional means [
133,
134]. For example, T. suecica is more vulnerable to rupture than Chlorella sp. and Nannochloropsis sp., and the average energy for disruption of an individual cell of T. suecica is 17.4 pJ [
135], equating to a precise disruption energy of 673 J/kg of dry microalgal biomass. The establishment of highly effective methods for disruption of microalgae cells is vital as the energy required is substantially lower than the energy employed in a disruption process [
136,
137]. Mechanical procedures such as high and low-pressure homogenizers and bead mills offer considerable potential compared to other disruption approaches [
138,
139,
140]. Microalgae proteins are isolated from cellular detritus by their dispersibility in water and by the differentiation of the protein-rich aqueous phase from the solid phase. Centrifugation is primarily employed for this purpose, although its rapid acceleration and low temperatures may limit its scalability to microalgae farms. The dispersion of proteins in the aqueous phase is critical for successful separation. The connection between protein-protein and protein-water interactions drives protein dispersion. The most critical criteria for effective dispersion of proteins are ionic strength and pH [
141]. At low ionic strengths, charged ions diminish the dielectric constant of water and salt bridges connections between proteins, boosting interactions and dispersibility [
142]. However, in systems with high salt concentrations, proteins compete with salts for water molecules, limiting protein-water interactions and dispersibility. The degree of dispersibility diminishes as the pH is near their isoelectric points, resulting to more interactions between proteins. Conversely, pH values too divergent from the isoelectric points enhance dispersibility [
143,
144]. Most marketed microalgae proteins have specified isoelectric points, which can be modified to increase protein recovery yields. A contemporary technology for separation of microalgae proteins is the three-phase partitioning system. This technology fractionates microalgae components in nonpolar and polar phases, while proteins are retained in the intermediate phase using a mixture of ammonium sulfate and t-butanol [
145]. The utilization of optimal conditions resulted in a protein extract of Chlorella pyrenoidosa with a concentration of 78% (wt/wt), however significant concentrations of ammonium sulfate and solvents were necessary for high protein yields. Reducing chemical inputs is critical for the application of this technology in commercial synthesis of microalgae proteins [
146]. Protein purification and separation from microalgae, though complex and costly, yield high-purity proteins (90% to 98%) that find potential applications in health markets [
147]. Chromatographic methods, including molecular exclusion, ion-exchange, affinity, and hydrophobic interactions, enable the isolation of single proteins for diverse scientific studies, such as analyzing amino acid profiles, three-dimensional structures, and antigen activity [
148]. While affinity chromatography offers high purity and functionality, it necessitates specific stationary phases, potentially increasing operational costs [
149]. Additionally, hydrophobic interactions can be optimized by introducing salts to the medium to enhance the exposure of hydrophobic groups to the matrix, overcoming purification efficiency limitations.
The research and development of algal protein processing and utilization is a burgeoning subject in biotechnology and sustainable nutrition. Algal proteins have many potentials uses notably functional additives, nutrient-dense supplements, and animal feed. Innovations in biochemistry and technology enable large-scale manufacturing economically viable. The convergence of bioprocessing, genetic manipulation, and bioreactor engineering holds potential for commercializing algal protein commodities, encouraging a more sustainable approach to global food security and resource management.
5.2. Carbohydrate Conversion to Biofuels or Bio Products
Microalgae possess diverse kinds of carbohydrates, including sugars, glucose, starch, and polysaccharides [
150,
151]. These carbohydrates serve an integral part in the synthesis of other biochemical molecules in algae, both in terms of their structure and metabolism [
152,
153]. Microalgae species have the ability to create significant amounts of carbohydrates, which makes them a valuable resource that has the potential to be commercialized. Microalgae, with their lack of lignin and structural polysaccharides, are potential biofuel feedstocks [
154]. Starch, a more advantageous substrate, doesn't require costly pretreatment and can be processed using existing bioethanol infrastructure [
151,
155]. However, improving carbohydrate and starch productivity is needed for algal biofuel production to be economically efficient and sustainable. Microalgae that have an elevated level of polysaccharides can be utilized as a substrate for bioethanol production. Prior to therapy, the cell membrane might be disrupted, resulting in the conversion of intricate polysaccharides into uncomplicated sugars [
156]. The lack of lignin in microalgae can streamline the pretreatment and hydrolysis procedure [
157]. Hasin et al. (2020), performed a study on the utilization of Scenedesmus obliquus, a microalgae species rich in carbohydrates, as a raw material for the development of bio-ethanol. They did pre-treatment, fermentation, distillation, and characterisation of the raw sample. The cellulose percentage of the SO was determined to be 53.08%, indicating its viability for bio-ethanol production [
158]. Lee et al. (2017), performed an experimental investigation on carbohydrate-rich microalgae Scenedesmus dimorphus to examine the link among pretreatment and fermentation process. They performed distinct hydrolysis and fermentation and simultaneous saccharification and fermentation tests, examining the fermentation response of the microalgae biomass that received organosolv, enzymatic, and acidic pretreatment. Analysis demonstrated that an amalgamation of these treatments was most efficient in producing fermentable sugar for the ensuing fermentation process. The organosolv process, followed by SSF, provided a potential bioethanol yield above 90% [
159].
The study by Ghadain et al. (2021), concluded that 2% H2SO4 was the optimum treatment method, resulting in the highest amounts of reducing sugars, total carbohydrate, and bioethanol. They investigated the chemical and physical prior treatments of Chlorella vulgaris biomass. It discovered that acidic processing using 2% sulfuric acid generated the greatest reducing sugars and total carbohydrate concentrations, while pretreatment with alkaline solutions yielded the maximum reducing sugars, total carbohydrate, and bioethanol concentrations. Physical preliminary treatments were done utilizing microwaves and ultrasonication, with the greatest reducing sugars fermenting to bioethanol at the 4th fermentation day [
160]. Megawati et al. (2022), examined both the chemical and enzymatic hydrolysis of C. vulgaris, subsequently performing fermentation. They employed hydrochloric acid, sodium hydroxide, and potassium hydroxide catalysts and alpha-amylase + glucoamylase enzymes. The hydrolysate was fermented using “Separate Hydrolysis Fermentation (SHF) and Simultaneous Saccharification and Fermentation (SSF), both utilizing S. cerevisiae”. Following five hours of enzymatic hydrolysis, the maximal glucose content was 81.39%, whereas chemical hydrolysis achieved 73.39%. The study indicated that chemical hydrolysis was less successful than enzymatic hydrolysis. The ethanol generated from SHF and SSF fermentation techniques was 4.42 and 4.67 g/L, correspondingly [
161].
Silva et al. (2023) claimed cultivation of Chlorella vulgaris in scale-up with reclaimed water has considerable potential for third-generation biofuel production (
Figure 5). They established an approach to enhance the production scale of microalgae Chlorella vulgaris cultivated in distilled recycled water, complemented with a modified BG-11 medium, for bioethanol production. The biomass was pre-treated with sulfuric acid to promote carbohydrate extraction, and the hydrolyzed solution was supplemented with YPD medium and yeast. The pre-treated sample gave the best results, with an alcohol level of 68° Gay-Lussac) following distillation [
162].
The conversion of carbohydrates to biofuels presents a viable option for sustainable generation of energy and the synthesis of valuable chemical compounds. The complicated interplay of enzymatic and microbiological activities, coupled with modern bioreactor technologies, emphasizes the potential for scalable and efficient conversion methods. As research in this subject continues to untangle the intricacies of metabolic pathways and genetic manipulation, the prospects for improving yields and diversifying product portfolios become increasingly conceivable. The interdisciplinary nature of carbohydrate conversion to biofuels or bioproducts needs continual collaboration between biologists, chemists, engineers, and environmental scientists to propel this emerging subject toward economic feasibility and global influence.
6. Techno-Economic and Life Cycle Assessment of Chlorella vulgaris-Based Biofuels
6.1. Production Costs and Economic Feasibility
Microalgae, being lignocellulosic biomasses, are recognized as highly versatile raw materials for biorefinery systems. Consequently, the implementation of bio-refinery techniques becomes essential to isolate various cellular components for the extraction of non-biofuel lipids and other potential value-added products [
164,
165]. To achieve a successful biorefinery, comprehensive consideration of the entire process is imperative, encompassing the selection of appropriate feedstock, utilization of effective separation methods, and refinement of the end-product characteristics. Nonetheless, contemporary approaches leveraging nanotechnology, such as cell pre-treatments or the use of fuel additives, have the potential to mitigate costs. An alternative approach involves employing gentle cell disruption and extraction techniques that preserve the integrity of macromolecules within the cell, avoiding injury or denaturation. However, these methods must also be prioritized as being cost-effective and energy-efficient. Microalgae possess inherent properties such as the capacity to yield high amounts of lipids and adaptability to thrive in environments with low-quality soil and water, while also synergizing with carbon dioxide sources. This characteristic has sparked significant efforts to enhance the processing techniques for microalgae production, aiming to harness their potential for biofuel generation and the synthesis of other high-value products [
166]. Recent attention has been directed toward advancing and scaling up the commercial potential of converting microalgae-based feedstock into fuel, highlighting its significance in current research and industry endeavors. The composition of microalgae biomass and its conversion efficiency are pivotal factors for the success of biorefineries. However, environmental considerations also play a significant role in determining their viability and sustainability. During the transitional phase from laboratory-scale to commercial production, techno-economic analysis (TEA) focuses primarily on two major factors: the feedstock quality and availability, and the final productivity of the biofuel production process. These elements are crucial in assessing the feasibility and economic viability of scaling up the production process [
167]. Selecting the appropriate feedstocks is essential as the efficiency of biomass breakdown to produce fermentable sugars directly impacts the final cost. In biofuel facilities, annual operating costs reveal that raw feedstock expenses and facility-related costs, such as insurance, maintenance, and overhead, are the primary drivers influencing overall expenses [
168]. In a Techno-Economic Analysis (TEA) comparing the total production costs required for ethanol, butanol, and isobutanol, it was found that feedstock accounted for the largest proportion, approximately 32%, of the overall cost [
169]. Lignocellulosic biomass stands out as an abundant and cost-effective renewable resource capable of generating biofuel within diverse industrial settings [
170].
The production of biofuel from microalgae has undergone thorough examination via TEAs. Chen et al. [
171] conducted a review of numerous papers published between 2010 and 2013, which documented the costs associated with biodiesel production from microalgae, spanning from USD 2.52 to 85.36 per gallon. Algal biomass has proven to be an economically feasible feedstock source for the production of biodiesel or ethanol, with selling costs falling below
$5 per gallon for biodiesel and
$2.95 per gallon for ethanol [
172]. Based on the outcomes of TEAs, a financially sustainable biorefinery utilizing microalgae Chlorella vulgaris was established for treating paper industrial effluents and producing bioenergy. In this scenario, with assumed parameters of 3% photosynthetic efficiency, 75% lipid extraction efficiency, and 45% anaerobic digestion efficiency, the biorefinery yielded a net present value (NPV) of EUR 15.4 million and an internal rate of return (IRR) of 12% [
173]. Several companies, such as Sapphire Energy, Algenol, and Seambiotic, have successfully scaled up the production of bioethanol from algal biomass to a commercial level, achieving an annual output of 1 billion gallons. The production costs are reported to be approximately 85 cents per litre [
174].
7. Life Cycle Assessment-Based Biofuels
Life Cycle Assessment (LCA), also known as "cradle-to-grave" analysis, serves as an essential tool for evaluating the environmental impacts of biofuels throughout their entire life cycle, from resource extraction to final disposal. This comprehensive method assesses all phases of a product’s lifecycle, including raw material extraction, production, use, and disposal, ensuring a thorough understanding of its environmental footprint. Governed by the ISO 14,000 standards, LCAs provide a structured approach to compare the economic efficiency and environmental impacts of biofuels with fossil fuels, highlighting their potential benefits within changing energy matrices [
175]. Research in this field covers a variety of biofuels such as biodiesel, bioethanol, and biogas, offering detailed assessments of their life cycles and environmental impacts. These studies not only explore the effects of different biofuels across various production technologies but also contrast their impacts against traditional non-natural fuels, analyzing each phase of biofuel production from cultivation to energy conversion. The methodology of LCA itself significantly influences outcomes, affected by choices in goal definition, inventory analysis, and impact assessment. Current procedures require refinement to accommodate large-scale deployments, ensuring that LCAs can effectively guide decisions in biofuel use and policy making [
176,
177,
178].
Figure 6 illustrates the cradle-to-grave life cycle assessment (LCA) process for the production and use of algae biodiesel in a passenger vehicle with a diesel engine. The scope of the study encompasses various unit processes, starting with the cultivation of autotrophic algae in open pond reactors. The biodiesel production uses wet lipid extraction, a technique well-explored in LCA studies. Post-extraction, the algal residue undergoes anaerobic digestion to generate methane, which is then utilized on-site for generating heat and power. The diagram also details material and energy flows, highlighting waste and co-products that are released into the environment and inputs required from external sources [
179].
Efforts to understand and harmonize variability in life cycle assessment (LCA) studies on microalgae biofuels have been explored through comprehensive reviews of methodologies and results [
181,
182]. These reviews reveal that significant result variations stem from inconsistencies in scope definition, assumptions, technological choices, and data sources. Prior harmonization efforts focused on refining scope definitions [
183]. Meta-regression analyses demonstrated regional variations in greenhouse gas (GHG) emissions associated with producing third-generation biofuels [
184]. Resource assessments for microalgae biofuel scalability found wide-ranging GHG emissions for biofuel production [
14]. Recent LCAs have unveiled optimized microalgae-to-biofuels chains, with GHG emissions of 39 g CO2-eq MJ−1 for diesel and 112 g CO2-eq MJ−1 for ethanol [
185]. Integration of heat recovery, trainer recovery tower, and CO2 recycling effectively reduces life cycle GHG emissions. Evaluations of microalgae oil and carbohydrates as feedstock for biofuel production revealed the eco-friendliness of microalgae-to-butanol chains compared to microalgae-to-diesel chains [
186]. LCA of bioreactors for biodiesel production from microalgae indicated variations in fossil energy requirements and GHG emissions, with notable reductions observed in pilot substrate-based microalgal bioreactors [
187]. In scenarios with high photosynthetic efficiency and biomass productivity, microalgae biorefinery processes exhibit net CO2 consumption, highlighting their potential environmental benefits [
174].
7.1. Environmental Impact and Sustainability Considerations
Environmental impacts assess how various pathways react to stressors like resource depletion, acidification, and global warming. These analyses identify industries prone to stress and indicate the necessity for transitioning to more eco-friendly alternatives [
188].
7.1.1. Nutrient Source
Microalgae cultivation necessitates optimal light intensity, carbon, nitrogen, phosphorus, trace metals, and minerals. Typically, these nutrients are sourced from inorganic fertilizers, contributing to carbon emissions. Using wastewater as a nutrient source can mitigate emissions and production costs, offering an economically viable and environmentally friendly alternative [
189]. Energy analysis shows biodiesel production from Chlorella sp. in wastewater is energetically favourable, with significant potential for biomass production [
190].
7.1.2. Water Quality
Large-scale microalgae cultivation demands substantial water usage, potentially impacting water quality through eutrophication and groundwater contamination [
160]. Eutrophication, caused by nutrient-rich water, poses risks to aquatic ecosystems [
161]. Microalgae cultivation in open ponds may affect local weather and introduce pollutants to water bodies, necessitating proper pond construction to prevent environmental leakage [
162].
7.1.3. Land Use Change
While microalgae-based biofuel production offers land efficiency, pond construction can disrupt ecosystems, displacing fauna and introducing invasive species. Land clearance for cultivation may elevate greenhouse gas emissions, but innovative approaches such as biorefinery methods could mitigate these emissions. For example, the conversion of croplands to biofuel production facilities could potentially increase greenhouse gas emissions by up to 50% [
191]. However, the implementation of greenhouse gas sequestration methods could offset these emissions.
5. Conclusions
This Chlorella, a single-celled microorganism that undergoes photosynthesis, has attracted attention due to its ability to quickly store lipids under various environmental circumstances. This characteristic makes it ideal for biofuel production. This review paper examines the various parameters that influence chlorella's biomass and lipid productivity, such as growing techniques, carbon and nitrogen sources, and specific stress-inducing factors. In large-scale cultivation, the process commonly depends on heterotrophic conditions,additionally, the inclusion of glycerol can enhance lipid accumulation even more. Chlorella cultivated in the presence of light generally has higher levels of antioxidants. Additionally, chlorella is capable of absorbing nitrogen from both nitrate and ammonium, but the uptake of ammonium requires less energy. Customizing the nitrogen supply based on the genetic characteristics of the Chlorella strain can enhance the lipid content.
Despite its considerable potential, Chlorella production faces significant obstacles due to the exorbitant costs associated with cultivation, harvesting, and processing. In order to solve these issues, creative solutions are needed. For example, it is important to pick Chlorella strains that can produce a lot of biomass, do photosynthesis efficiently, and survive in harsh environments with high pH levels, temperature changes, or osmolarity. Implementing tactics that prioritize the enhancement of desirable by-product output can be advantageous for reducing production costs. In addition, the establishment of extensive Chlorella genome databases could facilitate genetic engineering endeavors aimed at producing strains with enhanced characteristics. Another issue of concern is the earthy and grassy flavor of chlorella, which has hindered its popularity in food applications. Developing cultivars with a less pronounced taste could increase its appeal.
Chlorella is a highly adaptable type of algae with great potential in renewable energy and nutrient-dense meal production. Further exploration and advancement in these fields have the potential to yield inventive techniques for cultivating chlorella, positioning it as a crucial contributor in the pursuit of sustainable solutions.
Author Contributions
“Conceptualization, P.K, H.S. and M.J.; software, H.S, S.P,A.Y and M.J.; validation, K.D. and M.J.; formal analysis, M.S.; investigation, P.K.; resources, writing—H.S., S.P., and P.K. draft preparation, K.D., and M.J.; writing—review and editing,.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Randrianarison, G.; Ashraf, M.A. Microalgae: a potential plant for energy production. Geol Ecol Landscapes. 2017, 1(2), 104–120. [Google Scholar] [CrossRef]
- Zhu, L.D.; Li, Z.H.; Hiltunen, E. Strategies for Lipid Production Improvement in Microalgae as a Biodiesel Feedstock. Biomed. Res. Int. 2016, 2016, 8792548. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal Triacylglycerols as Feedstocks for Biofuel Production: Perspectives and Advances. Plant. J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.-L. Strategies to Enhance Production of Microalgal Biomass and Lipids for Biofuel Feedstock. Eur. J. Phycol. 2017, 52, 419–437. [Google Scholar] [CrossRef]
- Kumar, G.; Shekh, A.; Jakhu, S.; Sharma, Y.; Kapoor, R.; Sharma, T.R. Bioengineering of Microalgae: Recent Advances, Perspectives, and Regulatory Challenges for Industrial Application. Front. Bioeng. Biotechnol. 2020, 8, 914. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-M.; Ren, L.-J.; Zhao, Q.-Y.; Ji, X.-J.; Huang, H. Enhancement of Lipid Accumulation in Microalgae by Metabolic Engineering. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, C.; Dubey, K.K.; Shukla, P. Metabolic Engineering of Microalgal Based Biofuel Production: Prospects and Challenges. Front. Microbiol. 2016, 7, 432. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, W.; Mao, X.; Li, Y.; Wu, T.; Chen, F. High-Value Biomass from Microalgae Production Platforms: Strategies and Progress Based on Carbon Metabolism and Energy Conversion. Biotechnol. Biofuels 2018, 11, 227. [Google Scholar]
- Xue, J.; Balamurugan, S.; Li, T.; Cai, J.-X.; Chen, T.-T.; Wang, X.; Yang, W.-D.; Li, H.-Y. Biotechnological Approaches to Enhance Biofuel Producing Potential of Microalgae. Fuel 2021, 302, 121169. [Google Scholar] [CrossRef]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.-Y.; Raman, A.A.A.; Ibrahim, S. Microalgae Lipid and Biomass for Biofuel Production: A Comprehensive Review on Lipid Enhancement Strategies and Their Effects on Fatty Acid Composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232. [Google Scholar] [CrossRef]
- Atabani, A.E.; Tyagi, V.K.; Fongaro, G.; Treichel, H.; Pugazhendhi, A.; Hoang, A.T. Integrated Biorefineries, Circular Bio-Economy, and Valorization of Organic Waste Streams with Respect to Bio-Products. Biomass Convers. Biorefin. 2022, 12, 565. [Google Scholar] [CrossRef]
- Beal, C.M.; Hebner, R.E.; Webber, M.E.; Ruoff, R.S.; Seibert, A.F.; King, C.W. Comprehensive Evaluation of Algal Biofuel Production: Experimental and Target Results. Energies 2012, 5, 1943–1981. [Google Scholar] [CrossRef]
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from Microalgae: The Potential of Domestication towards Sustainable Biofactories. Microb. Cell Fact. 2018, 17, 173. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Agrawal, K.; Verma, P. Algal Biofuels: An Economic and Effective Alternative of Fossil Fuels. In Clean Energy Production Technologies; Springer: Singapore, 2020; pp. 207–227. ISBN 9789811571893. [Google Scholar]
- Bogen, C.; Klassen, V.; Wichmann, J.; La Russa, M.; Doebbe, A.; Grundmann, M.; Uronen, P.; Kruse, O.; Mussgnug, J.H. Identification of Monoraphidium contortum as a Promising Species for Liquid Biofuel Production. Bioresour. Technol. 2013, 133, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Brar, A.; Kumar, M.; Soni, T.; Vivekanand, V.; Pareek, N. Insights into the Genetic and Metabolic Engineering Approaches to Enhance the Competence of Microalgae as Biofuel Resource: A Review. Bioresour. Technol. 2021, 339, 125597. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.R.; Ong, H.C.; Chew, K.W.; Show, P.L.; Phang, S.-M.; Ling, T.C.; Nagarajan, D.; Lee, D.-J.; Chang, J.-S. Sustainable Approaches for Algae Utilisation in Bioenergy Production. Renew. Energy 2018, 129, 838–852. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, H.; Loganathan, B. Third-Generation Biofuels from Microalgae: A Review. Curr. Opin. Green Sustain. Chem. 2019, 20, 39–44. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Freitas, B.C.B.; Rosa, G.M.; Moraes, L.; Morais, M.G.; Mitchell, B.G. Operational and Economic Aspects of Spirulina-Based Biorefinery. Bioresour. Technol. 2019, 292, 121946. [Google Scholar] [CrossRef]
- Daroch, M.; Geng, S.; Wang, G. Recent Advances in Liquid Biofuel Production from Algal Feedstocks. Appl. Energy 2013, 102, 1371–1381. [Google Scholar] [CrossRef]
- Gan, S.Y.; Maggs, C.A. Random Mutagenesis and Precise Gene Editing Technologies: Applications in Algal Crop Improvement and Functional Genomics. Eur. J. Phycol. 2017, 52, 466–481. [Google Scholar] [CrossRef]
- Gao, C.; Zhai, Y.; Ding, Y.; Wu, Q. Application of Sweet Sorghum for Biodiesel Production by Heterotrophic Microalgae Chlorella protothecoides. Appl. Energy 2010, 87, 756–761. [Google Scholar] [CrossRef]
- Gimpel, J.A.; Specht, E.A.; Georgianna, D.R.; Mayfield, S.P. Advances in Microalgae Engineering and Synthetic Biology Applications for Biofuel Production. Curr. Opin. Chem. Biol. 2013, 17, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Jiang, M. Biodiesel Production with Microalgae as Feedstock: From Strains to Biodiesel. Biotechnol. Lett. 2011, 33, 1269–1284. [Google Scholar] [CrossRef] [PubMed]
- Jagadevan, S.; Banerjee, A.; Banerjee, C.; Guria, C.; Tiwari, R.; Baweja, M.; Shukla, P. Recent Developments in Synthetic Biology and Metabolic Engineering in Microalgae towards Biofuel Production. Biotechnol. Biofuels 2018, 11, 1–21. [Google Scholar] [CrossRef]
- Ji, M.-K.; Kim, H.-C.; Sapireddy, V.R.; Yun, H.-S.; Abou-Shanab, R.A.I.; Choi, J.; Lee, W.; Timmes, T.C.; Inamuddin; Jeon, B. -H. Simultaneous Nutrient Removal and Lipid Production from Pretreated Piggery Wastewater by Chlorella vulgaris YSW-04. Appl. Microbiol. Biotechnol. 2013, 97, 2701–2710. [Google Scholar] [CrossRef] [PubMed]
- Přibyl, P.; Cepák, V.; Zachleder, V. Production of Lipids in 10 Strains of Chlorella and Parachlorella, and Enhanced Lipid Productivity in Chlorella vulgaris. Appl. Microbiol. Biotechnol. 2012, 94, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, S.M.; Laamanen, C.A.; Basiliko, N.; Scott, J.A. Utilization of Lipid-Extracted Biomass (LEB) to Improve the Economic Feasibility of Biodiesel Production from Green Microalgae. Environ. Rev. 2020, 28, 325–338. [Google Scholar] [CrossRef]
- Mehrabadi, A.; Craggs, R.; Farid, M.M. Biodiesel Production Potential of Wastewater Treatment High Rate Algal Pond Biomass. Bioresour. Technol. 2016, 221, 222–233. [Google Scholar] [CrossRef]
- Naghshbandi, M.P.; Tabatabaei, M.; Aghbashlo, M.; Aftab, M.N.; Iqbal, I. Metabolic Engineering of Microalgae for Biofuel Production. Methods Mol. Biol. 2020, 1980, 153–172. [Google Scholar] [PubMed]
- Singh, H. M., M. Sharma, V. V. Tyagi, K. Goria, D. Buddhi, A. Sharma, F. Bruno, S. Sheoran, & R. Kothari. "Potential of biogenic and non-biogenic waste materials as flocculant for algal biomass harvesting: Mechanism, parameters, challenges and future prospects." J Environ Manage (2023) 337: 117591. [CrossRef]
- Xu, Z., H. Wang, P. Cheng, T. Chang, P. Chen, C. Zhou, & R. Ruan. "Development of integrated culture systems and harvesting methods for improved algal biomass productivity and wastewater resource recovery - a review." Sci Total Environ (2020) 746: 141039. [CrossRef]
- Banerjee, C., S. Ghosh, G. Sen, S. Mishra, P. Shukla, & R. Bandopadhyay. "Study of algal biomass harvesting using cationic guar gum from the natural plant source as flocculant." Carbohydr Polym (2013) 92: 675-81. [CrossRef]
- Lai, Z. , & K. Yin. "Physical-biological coupling induced aggregation mechanism for the formation of high biomass red tides in low nutrient waters." Harmful Algae (2014) 31: 66-75. [CrossRef]
- Morris, J. D., S. S. Daood, S. Chilton, & W. Nimmo. "Mechanisms and mitigation of agglomeration during fluidized bed combustion of biomass: A review." Fuel (2018) 230: 452-73. [CrossRef]
- Gutiérrez, R., I. Ferrer, E. Uggetti, C. Arnabat, H. Salvadó, & J. García. "Settling velocity distribution of microalgal biomass from urban wastewater treatment high rate algal ponds." Algal Research (2016) 16: 409-17. [CrossRef]
- Zhao, F., Z. Li, X. Han, Z. Shao, & Z. Li. "Optimization of air flotation and the combination of air flotation and membrane filtration in microalgae harvesting." Processes (2022) 10: 1594. [CrossRef]
- Golberg, A., M. Sack, J. Teissie, G. Pataro, U. Pliquett, G. Saulis, T. Stefan, D. Miklavcic, E. Vorobiev, & W. Frey. "Energy-efficient biomass processing with pulsed electric fields for bioeconomy and sustainable development." Biotechnology for Biofuels (2016) 9: 94. [CrossRef]
- Wahlen, B. D., M. S. Roni, K. G. Cafferty, L. M. Wendt, T. L. Westover, D. M. Stevens, & D. T. Newby. "Managing variability in algal biomass production through drying and stabilization of feedstock blends." Algal Research (2017) 24: 9-18. [CrossRef]
- Silva, M. P., I. J. Badruddin, T. Tonon, S. Rahatekar, & L. D. Gomez. "Environmentally benign alginate extraction and fibres spinning from different European brown algae species." International Journal of Biological Macromolecules (2023) 226: 434-42. [CrossRef]
- Banerjee, C., P. Gupta, S. Mishra, G. Sen, P. Shukla, & R. Bandopadhyay. "Study of polyacrylamide grafted starch based algal flocculation towards applications in algal biomass harvesting." Int J Biol Macromol 51 (2012): 456-61. [CrossRef]
- de Godos, I., H. O. Guzman, R. Soto, P. A. Garcia-Encina, E. Becares, R. Munoz, & V. A. Vargas. "Coagulation/flocculation-based removal of algal-bacterial biomass from piggery wastewater treatment." Bioresour Technol 102 (2011): 923-7. [CrossRef]
- Najjar, Y. S. H. , & A. Abu-Shamleh. "Harvesting of microalgae by centrifugation for biodiesel production: A review." Algal Research 51 (2020): 102046. [CrossRef]
- Cromar, N. J. , & H. J. Fallowfield. "Separation of components of the biomass from high rate algal ponds using percollr density gradient centrifugation." J Appl Phycol 4 (1992): 157-63. [CrossRef]
- Deepa, P., K. Sowndhararajan, & S. Kim. "A review of the harvesting techniques of microalgae." Water 15 (2023): 3074. [CrossRef]
- Riaza, J., P. E. Mason, J. M. Jones, A. Williams, J. Gibbins, & H. Chalmers. "Shape and size transformations of biomass particles during combustion." Fuel 261 (2020): 116334. [CrossRef]
- Elnajjar, E., S. T. P. Purayil, F. Alnuaimi, H. Al Khawaja, L. Shaikhoun, N. Arnaoud, & S. Almutawa. "Mechanistic investigation of efficient cell disruption methods for lipid extraction from various macro and micro species of algae." Biore-source Technology Reports 22 (2023): 101482. [CrossRef]
- Alavijeh, R. S., K. Karimi, R. H. Wijffels, C. van den Berg, & M. Eppink. "Combined bead milling and enzymatic hydrolysis for efficient fractionation of lipids, proteins, and carbohydrates of Chlorella vulgaris microalgae." Bioresource Technology 309 (2020): 123321. [CrossRef]
- Ranjith Kumar, R., P. Hanumantha Rao, & M. Arumugam. "Lipid extraction methods from microalgae: A comprehensive review." Frontiers in Energy Research 2 (2015): Article 61. [CrossRef]
- Jeon, B.-H., J. -A. Choi, H.-C. Kim, J.-H. Hwang, R. A. I. Abou-Shanab, B. A. Dempsey, J. M. Regan, & J. R. Kim. "Ultrasonic disintegration of microalgal biomass and consequent improvement of bioaccessibility/bioavailability in microbial fermentation." Biotechnology for Biofuels 6 (2013): 37. [CrossRef]
- Rahman, M. M., N. Hosano, & H. Hosano. "Recovering microalgal bioresources: A review of cell disruption methods and extraction technologies." Molecules 27 (2022): 2786. [CrossRef]
- Shehadul Islam, M., A. Aryasomayajula, & P. R. Selvaganapathy. "A review on macroscale and microscale cell lysis methods." Micromachines (Basel) 8 (2017): Article 83. [CrossRef]
- Sierra, L. S., C. K. Dixon, & L. R. Wilken. "Enzymatic cell disruption of the microalgae Chlamydomonas reinhardtii for lipid and protein extraction." Algal Research 25 (2017): 149-59. [CrossRef]
- Zhu, L., Z. Li, & E. Hiltunen. "Microalgae Chlorella vulgaris biomass harvesting by natural flocculant: Effects on biomass sedimentation, spent medium recycling and lipid extraction." Biotechnology for Biofuels 11 (2018): 183. [CrossRef]
- Prajapati, S. K., A. Bhattacharya, P. Kumar, A. Malik, & V. K. Vijay. "A method for simultaneous bioflocculation and pretreatment of algal biomass targeting improved methane production." Green Chemistry 18 (2016): 5230-38. [CrossRef]
- Inostroza, C., N. El Bahraoui, R. Rivera-Tinoco, & F. G. Acién. "Uses of electro-coagulation-flocculation (ECF) for the pre-concentration of microalgae biomass." Process Biochemistry 122 (2022): 1-7. [CrossRef]
- Qin, S., K. Wang, F. Gao, B. Ge, H. Cui, & W. Li. "Biotechnologies for bulk production of microalgal biomass: From mass cultivation to dried biomass acquisition." Biotechnol Biofuels Bioprod 16 (2023): 131. [CrossRef]
- Kalaiselvan, N. , & Mathimani, T. (2022). Design and fabrication of box-type passive solar dryer (btpsd) with thermal insulation material for valorizing biomass and neutral lipids of marine chlorella vulgaris for biodiesel application. Sci Rep, 12, 6046. [CrossRef]
- López Pastor, R. , Pinna-Hernández, M. G., & Acién Fernández, F. G. (2023). Technical and economic viability of using solar thermal energy for microalgae drying. Energy Reports, 10, 989-1003. [CrossRef]
- Schmid, B. , Navalho, S., Schulze, P. S. C., Van De Walle, S., Van Royen, G., Schüler, L. M.,... & Maia, I. B. (2022). Drying microalgae using an industrial solar dryer: A biomass quality assessment. Foods, 11, 1873. [CrossRef]
- Machado, L. , Carvalho, G., & Pereira, R. N. (2022). Effects of innovative processing methods on microalgae cell wall: Prospects towards digestibility of protein-rich biomass. Biomass,2, 80-102.
- Schmid, B. , Navalho, S., Schulze, P. S. C., Van De Walle, S., Van Royen, G., Schüler, L. M.,... & Maia, I. B. (2022). Drying microalgae using an industrial solar dryer: A biomass quality assessment. Foods, 11, 1873.
- Abd Rabu, R. , Janajreh, I., & Honnery, D. (2013). Transesterification of waste cooking oil: Process optimization and conversion rate evaluation. Energy Convers. Manag., 65, 764-769. [CrossRef]
- Shu, Q. , Gao, J., Liao, Y., & Wang, J. (2011). Reaction kinetics of biodiesel synthesis from waste oil using a carbon-based solid acid catalyst. Chinese J. Chem. Eng., 19, 163-168. [CrossRef]
- Patel, N. K. , & Shah, S. N. (2015). Biodiesel from Plant Oils. In Food, Energy, Water Chem. Connect. (pp. 277–307). Elsevier. [CrossRef]
- Ghedini, E. , Taghavi, S., Menegazzo, F., & Signoretto, M. (2021). A review on the efficient catalysts for algae transesterification to biodiesel. Sustain., 13, 10479. [CrossRef]
- Changmai, B. , Vanlalveni, C., Ingle, A. P., Bhagat, R., & Rokhum, L. (2020). Widely used catalysts in biodiesel production: a review. RSC Adv., 10, 41625–41679. [CrossRef]
- Thangaraj, B. , Solomon, P. R., Muniyandi, B., Ranganathan, S., & Lin, L. (2019). Catalysis in biodiesel production - A review. Clean Energy, 3, 2–23. [CrossRef]
- Rizwanul Fattah, I. M. , Ong, H. C., Mahlia, T. M. I., Mofijur, M., Silitonga, A. S., Rahman, S. M. A., & Ahmad, A. (2020). State of the Art of Catalysts for Biodiesel Production. Front. Energy Res., 8, 546060. [CrossRef]
- De Luna, M. D. G. , Doliente, L. M. T., Ido, A. L., & Chung, T. W. (2017). In situ transesterification of Chlorella sp. microalgae using LiOH-pumice catalyst. J. Environ. Chem. Eng., 5, 2830–2835. [CrossRef]
- Pandit, P. R. , & Fulekar, M. H. (2019). Biodiesel production from microalgal biomass using CaO catalyst synthesized from natural waste material. Renew. Energy., 136, 837–845. [CrossRef]
- Jazie, A. A. , Alshebaney, E. J., & Abed, S. A. (2019). In-situ dodecylbenzenesulfonic acid-Catalyzed transesterification of micro algae chlorella Sp. for biodiesel production. In 5th Int. Conf. Power Gener. Syst. Renew. Energy Technol. PGSRET 2019. [CrossRef]
- Dianursanti, Sistiafi, A. G., & Putri, D. N. (2018). Biodiesel synthesis from nannochloropsis oculata and chlorella vulgaris through transesterification process using NaOH/zeolite heterogeneous catalyst. In IOP Conf. Ser. Earth Environ. Sci. [CrossRef]
- Han, F. Y. , Komiyama, M., Uemura, Y., & Rabat, N. E. (2018). One pot biodiesel production from wet Chlorella vulgaris using supercritical methanol with oxide catalysts. In AIP Conf. Proc. [CrossRef]
- Kalsum, U. , Mahmuddin, Mahfud, M., & Roesyadi, A. (2018). Biodiesel Production from Chlorella vulgaris via Homogenous Acid Catalyzed in situ Transesterification with Microwave Irradiation. In IOP Conf. Ser. Earth Environ. Sci. [CrossRef]
- Al-Humairi, S. T. , Lee, J. G. M., Salihu, M., & Harvey, A. P. (2022). Biodiesel Production through Acid Catalyst In Situ Reactive Extraction of Chlorella vulgaris Foamate. Energies, 15. [CrossRef]
- Ma, G. , Hu, W., Pei, H., Jiang, L., & Ji, Y. (2015). Study of KOH/Al2O3 as heterogeneous catalyst for biodiesel production via in situ transesterification from microalgae. Environ. Technol. (United Kingdom), 36, 622–627. Environ. Technol. (United Kingdom). [CrossRef]
- Tsigie, Y. A. , Huynh, L. H., Ismadji, S., Engida, A. M., & Ju, Y. H. (2012). In situ biodiesel production from wet Chlorella vulgaris under subcritical condition. Chem. Eng. J., 213, 104–108. H. ( 213, 104–108. [CrossRef]
- Malekghasemi, S. , Kariminia, H. R., Plechkova, N. K., & Ward, V. C. A. (2021). Direct transesterification of wet microalgae to biodiesel using phosphonium carboxylate ionic liquid catalysts. Biomass and Bioenergy., 150. [CrossRef]
- Davoodbasha, M. A. , Pugazhendhi, A., Kim, J. W., Lee, S. Y., & Nooruddin, T. (2021). Biodiesel production through transesterification of Chlorella vulgaris: Synthesis and characterization of CaO nanocatalyst. Fuel, 300. [CrossRef]
- Aghilinategh, M. , Barati, M., & Hamadanian, M. (2020). The modified supercritical media for one-pot biodiesel production from Chlorella vulgaris using photochemically-synthetized SrTiO3 nanocatalyst. Renew. Energy., 160, 176–184. [CrossRef]
- Chang, C. H. , Wei, H. Y., Chen, B. Y., & Tan, C. S. (2020). In situ catalyst-free biodiesel production from partially wet microalgae treated with mixed methanol and castor oil containing pressurized CO2. J. Supercrit. Fluids., 157. [CrossRef]
- Salam, K. A., Velasquez-Orta, S. B., & Harvey, A. P. (2016). Kinetics of fast alkali reactive extraction/in situ transesterification of Chlorella vulgaris that identifies process conditions for a significant enhanced rate and water tolerance. Fuel Process. Technol., 144, 212–219. [CrossRef]
- Tran, D. T., Yeh, K. L., Chen, C. L., & Chang, J. S. (2012). Enzymatic transesterification of microalgal oil from Chlorella vulgaris ESP-31 for biodiesel synthesis using immobilized Burkholderia lipase. Bioresour. Technol., 108, 119–127. Bioresour. Technol. [CrossRef]
- Korman, T. P., Sahachartsiri, B., Charbonneau, D. M., Huang, G. L., Beauregard, M., & Bowie, J. U. (2013). Dieselzymes: Development of a stable and methanol tolerant lipase for biodiesel production by directed evolution. Biotechnol. Biofuels., 6, 1–13. [CrossRef]
- Kumar, M., Oyedun, A. O., & Kumar, A. (2018). A review on the current status of various hydrothermal technologies on biomass feedstock. Renew. Sustain. Energy Rev., 81, 1742–1770. [CrossRef]
- Watson, J., Wang, T., Si, B., Chen, W. T., Aierzhati, A., & Zhang, Y. (2020). Valorization of hydrothermal liquefaction aqueous phase: pathways towards commercial viability. Prog. Energy Combust. Sci., 77, 100819. [CrossRef]
- Huang, H. J., & Yuan, X. Z. (2015). Recent progress in the direct liquefaction of typical biomass. Prog. Energy Combust. Sci., 49, 59–80. [CrossRef]
- Chen, W. H., Lin, Y. Y., Liu, H. C., Chen, T. C., Hung, C. H., Chen, C. H., & Ong, H. C. (2019). A comprehensive analysis of food waste derived liquefaction bio-oil properties for industrial application. Appl. Energy., 237, 283–291. [CrossRef]
- Huang, H. J., & Yuan, X. Z. (2016). The migration and transformation behaviors of heavy metals during the hydrothermal treatment of sewage sludge. Bioresour. Technol., 200, 991–998. 200. [CrossRef]
- Chen, W. T., Zhang, Y., Zhang, J., Schideman, L., Yu, G., Zhang, P., & Minarick, M. (2014). Co-liquefaction of swine manure and mixed-culture algal biomass from a wastewater treatment system to produce bio-crude oil. Appl. Energy., 128, 209–216. [CrossRef]
- Liiv, J., Mäeorg, U., Vaino, N., & Rikmann, E. (2024). Low-Temperature and Low-pressure HydroThermal Liquefaction (L-HTL) of biomass using ultrasonic cavitation to achieve a local supercritical state in water. Sci. Technol. Energy Transit., 79. [CrossRef]
- Ellersdorfer, M. (2020). Hydrothermal co-liquefaction of chlorella vulgaris with food processing residues, green waste and sewage sludge. Biomass and Bioenergy., 142. [CrossRef]
- da Costa Magalhães, B., Matricon, L., Ramirez Romero, L. A., Checa, R., Lorentz, C., Chambonniere, P., ... & Geantet, C. (2023). Catalytic hydrotreatment of bio-oil from continuous HTL of Chlorella sorokiniana and Chlorella vulgaris microalgae for biofuel production. Biomass and Bioenergy., 173, 106798. [CrossRef]
- Hegdahl, S. H., Ghoreishi, S., Løhre, C., & Barth, T. (2023). Exploring hydrothermal liquefaction (HTL) of digested sewage sludge (DSS) at 5.3 L and 0.025 L bench scale using experimental design. Sci. Rep., 13, 1–14. [CrossRef]
- Mishra, R. K., Kumar, V., Kumar, P., & Mohanty, K. (2022). Hydrothermal liquefaction of biomass for bio-crude production: A review on feedstocks, chemical compositions, operating parameters, reaction kinetics, techno-economic study, and life cycle assessment. Fuel, 316, 123377. [CrossRef]
- Ghadge, R., Nagwani, N., Saxena, N., Dasgupta, S., & Sapre, A. (2022). Design and scale-up challenges in hydrothermal liquefaction process for biocrude production and its upgradation. Energy Convers. Manag. X., 14, 100223. [CrossRef]
- de Caprariis, B., Scarsella, M., Bavasso, I., Bracciale, M. P., Tai, L., & De Filippis, P. (2021). Effect of Ni, Zn and Fe on hydrothermal liquefaction of cellulose: Impact on bio-crude yield and composition. J. Anal. Appl. Pyrolysis., 157. [CrossRef]
- Yin, S., & Tan, Z. (2012). Hydrothermal liquefaction of cellulose to bio-oil under acidic, neutral and alkaline conditions. Appl. Energy., 92, 234–239. [CrossRef]
- Jin, F., Wang, Y., Zeng, X., Shen, Z., & Yao, G. (2014). Water Under High Temperature and Pressure Conditions and Its Applications to Develop Green Technologies for Biomass Conversion. In: pp. 3–28. [CrossRef]
- Marković, Z., & Joksimović, G. (2007). Investigation of the Mechanism of Acidic Hydrolysis of Cellulose. Acta Agric. Serbica., 24, 51–57. https://scindeks.ceon.rs/article.aspx?artid=0354-95420724051J (accessed April 28, 2024).
- Möller, M., Harnisch, F., & Schröder, U. (2013). Hydrothermal liquefaction of cellulose in subcritical water-the role of crystallinity on the cellulose reactivity. RSC Adv., 3, 11035–11044. [CrossRef]
- Yang, W., Wang, Z., Han, J., Song, S., Zhang, Y., & Gong, W. (2019). The role of polysaccharides and proteins in bio-oil production during the hydrothermal liquefaction of algae species. RSC Adv., 9, 41962–41969. [CrossRef]
- Grande, L., Pedroarena, I., Korili, S. A., & Gil, A. (2021). Hydrothermal liquefaction of biomass as one of the most promising alternatives for the synthesis of advanced liquid biofuels: A review. Materials (Basel), 14. [CrossRef]
- Hao, B., Xu, D., Jiang, G., Sabri, T. A., Jing, Z., & Guo, Y. (2021). Chemical reactions in the hydrothermal liquefaction of biomass and in the catalytic hydrogenation upgrading of biocrude. Green Chem., 23, 1562–1583. [CrossRef]
- Guo, B. (2019). Hydrothermal liquefaction within a microalgae biorefinery.
- Kiran Kumar, P., Vijaya Krishna, S., Verma, K., Pooja, K., Bhagawan, D., Srilatha, K., & Himabindu, V. (2018). Bio oil production from microalgae via hydrothermal liquefaction technology under subcritical water conditions. J. Microbiol. Methods., 153, 108–117. [CrossRef]
- Ho, S. H., Huang, S. W., Chen, C. Y., Hasunuma, T., Kondo, A., & Chang, J. S. (2013). Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour. Technol., 135, 191–198. [CrossRef]
- Condor, B. E., de Luna, M. D. G., Chang, Y.-H., Chen, J.-H., Leong, Y. K., Chen, P.-T., Chen, C.-Y., Lee, D.-J., & Chang, J.-S. (2022). Bioethanol Production from Microalgae Biomass at High Solid Loadings. SSRN Electron. J. [CrossRef]
- Rahman, Q. M., Zhang, B., Wang, L., & Shahbazi, A. (2019). A combined pretreatment, fermentation and ethanol-assisted liquefaction process for production of biofuel from Chlorella sp. Fuel., 257. [CrossRef]
- Zheng, Y., Li, T., Yu, X., Bates, P. D., Dong, T., & Chen, S. (2013). High-density fed-batch culture of a thermotolerant microalga chlorella sorokiniana for biofuel production. Appl. Energy., 108, 281–287. [CrossRef]
- Feng, X., Walker, T. H., Bridges, W. C., Thornton, C., & Gopalakrishnan, K. (2014). Biomass and lipid production of Chlorella protothecoides under heterotrophic cultivation on a mixed waste substrate of brewer fermentation and crude glycerol. Bioresour. Technol., 166, 17–23. 166. [CrossRef]
- Raj, S., Sajith, A., Sreenikethanam, A., Vadlamani, S., Satheesh, A., Ganguly, A., Banu, J. R., Varjani, S., Gugulothu, P., & Bajhaiya, A. K. (2023). Renewable biofuels from microalgae: technical advances, limitations and economics. Environ. Technol. Rev., 12, 18–36. [CrossRef]
- Ghosh, D., Ghorai, P., Debnath, S., Indrama, T., Kondi, V., & Tiwari, O. N. (2022). Algal biofertilizer towards green sustainable agriculture. In: New Futur. Dev. Microb. Biotechnol. Bioeng. Sustain. Agric. Revital. through Org. Prod. [CrossRef]
- Eldiehy, K. S. H., Bardhan, P., Borah, D., Gohain, M., Rather, M. A., Deka, D., & Mandal, M. (2022). A comprehensive review on microalgal biomass production and processing for biodiesel production. Fuel., 324, 124773. [CrossRef]
- Gimpel, J. A., Henríquez, V., & Mayfield, S. P. (2015). In metabolic engineering of eukaryotic microalgae: Potential and challenges come with great diversity. Front. Microbiol., 6, 150322. [CrossRef]
- Sun, H., Zhao, W., Mao, X., Li, Y., Wu, T., & Chen, F. (2018). High-value biomass from microalgae production platforms: Strategies and progress based on carbon metabolism and energy conversion. Biotechnol. Biofuels., 11, 1–23. [CrossRef]
- S.F. Han, W.B. Jin, R.J. Tu, W.M. Wu, Biofuel production from microalgae as feedstock: Current status and potential, Crit. Rev. Biotechnol. 35 (2015) 255–268. [CrossRef]
- P.J.L.B. Williams, L.M.L. Laurens, Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics, Energy Environ. Sci. 3 (2010) 554–590. [CrossRef]
- M.L. Amorim, J. Soares, J.S. dos Reis Coimbra, M. de Oliveira Leite, L.F.T. Albino, M.A. Martins, Microalgae proteins: production, separation, isolation, quantification, and application in food and feed, Crit. Rev. Food Sci. Nutr. 61 (2021) 1976–2002. [CrossRef]
- A.K. Koyande, K.W. Chew, K. Rambabu, Y. Tao, D.T. Chu, P.L. Show, Microalgae: A potential alternative to health supplementation for humans, Food Sci. Hum. Wellness. 8 (2019) 16–24. [CrossRef]
- M.E.M. Soudagar, T.S. Kiong, L. Jathar, N.N.N. Ghazali, S. Ramesh, U. Awasarmol, H.C. Ong, Perspectives on cultivation and harvesting technologies of microalgae, towards environmental sustainability and life cycle analysis, Chemosphere. 353 (2024) 141540. [CrossRef]
- S.B. Grama, Z. Liu, J. Li, Emerging Trends in Genetic Engineering of Microalgae for Commercial Applications, Mar. Drugs. 20 (2022) 285. [CrossRef]
- S.V.V. Bharadwaj, S. Ram, I. Pancha, S. Mishra, Recent trends in strain improvement for production of biofuels from microalgae, in: Microalgae Cultiv. Biofuels Prod., Academic Press, 2019: pp. 211–225. [CrossRef]
- M. Bhattacharya, S. Goswami, Microalgae – A green multi-product biorefinery for future industrial prospects, Biocatal. Agric. Biotechnol. 25 (2020) 101580. [CrossRef]
- S.Y.A. Siddiki, M. Mofijur, P.S. Kumar, S.F. Ahmed, A. Inayat, F. Kusumo, I.A. Badruddin, T.M.Y. Khan, L.D. Nghiem, H.C. Ong, T.M.I. Mahlia, Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: An integrated biorefinery concept, Fuel. 307 (2022) 121782. [CrossRef]
- H. Karan, J. Roles, I.L. Ross, M. Ebrahimi, D. Rackemann, T. Rainey, B. Hankamer, Solar biorefinery concept for sustainable co-production of microalgae-based protein and renewable fuel, J. Clean. Prod. 368 (2022) 132981. [CrossRef]
- Saravanan, P.S. Kumar, M. Badawi, G. Mohanakrishna, T.M. Aminabhavi, Valorization of micro-algae biomass for the development of green biorefinery: Perspectives on techno-economic analysis and the way towards sustainability, Chem. Eng. J. 453 (2023) 139754. [CrossRef]
- E.S. Okeke, O. Ejeromedoghene, C.O. Okoye, T.P.C. Ezeorba, R. Nyaruaba, C.K. Ikechukwu, A. Oladipo, J.I. Orege, Micro-algae biorefinery: An integrated route for the sustainable production of high-value-added products, Energy Convers. Manag. X 16 (2022) 100323. [CrossRef]
- S. Mehariya, R.K. Goswami, P. Verma, R. Lavecchia, A. Zuorro, Integrated Approach for Wastewater Treatment and Biofuel Production in Microalgae Biorefineries, Energies 14 (2021) 2282. [CrossRef]
- G. Ezhumalai, M. Arun, A. Manavalan, R. Rajkumar, K. Heese, A Holistic Approach to Circular Bioeconomy Through the Sustainable Utilization of Microalgal Biomass for Biofuel and Other Value-Added Products, Microb. Ecol. 87 (2024) 1–16. [CrossRef]
- K. Gaurav, K. Neeti, R. Singh, Microalgae-based biodiesel production and its challenges and future opportunities: A review, Green Technol. Sustain. 2 (2024) 100060. [CrossRef]
- A.K. Lee, D.M. Lewis, P.J. Ashman, Force and energy requirement for microalgal cell disruption: An atomic force microscope evaluation, Bioresour. Technol. 128 (2013) 199–206. [CrossRef]
- P.S. Saravana, V. Ummat, P. Bourke, B.K. Tiwari, Emerging green cell disruption techniques to obtain valuable compounds from macro and microalgae: a review, Crit. Rev. Biotechnol. 43 (2023) 904–919. [CrossRef]
- M.M. Rahman, N. Hosano, H. Hosano, Recovering Microalgal Bioresources: A Review of Cell Disruption Methods and Extraction Technologies, Molecules 27 (2022) 2786. [CrossRef]
- R.I.M. Almoselhy, High-Speed and High-Pressure Homogenization Techniques for Optimization of Food Processing, Quality, and Safety, Open Access J. Microbiol. Biotechnol. 7 (2022) 1–4. [CrossRef]
- T.A. Gomes, C.M. Zanette, M.R. Spier, An overview of cell disruption methods for intracellular biomolecules recovery, Prep. Biochem. Biotechnol. 50 (2020) 635–654. [CrossRef]
- G. Nemer, N. Louka, E. Vorobiev, D. Salameh, J.M. Nicaud, R.G. Maroun, M. Koubaa, Mechanical cell disruption technologies for the extraction of dyes and pigments from microorganisms: A review, Fermentation 7 (2021) 36. [CrossRef]
- J. Stack, A.V. Le Gouic, P.R. Tobin, F. Guihéneuf, D.B. Stengel, R.J. FitzGerald, "Protein extraction and bioactive hydrolysate generation from two microalgae, Porphyridium purpureum and Phaeodactylum tricornutum," J. Food Bioact. 1 (2018) 153–165. [CrossRef]
- S. Pylaeva, M. Brehm, D. Sebastiani, "Salt Bridge in Aqueous Solution: Strong Structural Motifs but Weak Enthalpic Effect," Sci. Rep. 8 (2018) 1–7. [CrossRef]
- A.V. Ursu, A. Marcati, T. Sayd, V. Sante-Lhoutellier, G. Djelveh, P. Michaud, "Extraction, fractionation and functional properties of proteins from the microalgae Chlorella vulgaris," Bioresour. Technol. 157 (2014) 134–139. [CrossRef]
- S. Kulkarni, Z. Nikolov, "Process for selective extraction of pigments and functional proteins from Chlorella vulgaris," Algal Res. 35 (2018) 185–193. [CrossRef]
- A.G. Waghmare, M.K. Salve, J.G. LeBlanc, S.S. Arya, "Concentration and characterization of microalgae proteins from Chlorella pyrenoidosa," Bioresour. Bioprocess. 3 (2016) 1–11. [CrossRef]
- N. Ferrer-Miralles, P. Saccardo, J.L. Corchero, E. Garcia-Fruitós, "Recombinant Protein Production and Purification of Insoluble Proteins," in: Methods Mol. Biol., Humana, New York, NY, 2022: pp. 1–31. [CrossRef]
- L. Hovgaard, L. Skriver, S. Frokjaer, "Protein purification," in: Pharm. Formul. Dev. Pept. Proteins, Second Ed., InTech, 2012: pp. 35–48. [CrossRef]
- D. Agyei, R. Potumarthi, M.K. Danquah, "Production of Lactobacilli Proteinases for the Manufacture of Bioactive Peptides: Part II-Downstream Processes," in: Mar. Proteins Pept. Biol. Act. Appl., John Wiley & Sons, Ltd, 2013: pp. 231–251. [CrossRef]
- C.Y. Chen, X.Q. Zhao, H.W. Yen, S.H. Ho, C.L. Cheng, D.J. Lee, F.W. Bai, J.S. Chang, "Microalgae-based carbohydrates for biofuel production," Biochem. Eng. J. 78 (2013) 1–10. [CrossRef]
- G.E. Lakatos, K. Ranglová, J.C. Manoel, T. Grivalský, J. Kopecký, J. Masojídek, "Bioethanol production from micro-algae polysaccharides," Folia Microbiol. (Praha). 64 (2019) 627–644. [CrossRef]
- C. Debnath, T.K. Bandyopadhyay, B. Bhunia, U. Mishra, S. Narayanasamy, M. Muthuraj, "Microalgae: Sustainable resource of carbohydrates in third-generation biofuel production," Renew. Sustain. Energy Rev. 150 (2021) 111464. [CrossRef]
- G. Markou, I. Angelidaki, D. Georgakakis, "Microalgal carbohydrates: An overview of the factors influencing carbo-hydrates production, and of main bioconversion technologies for production of biofuels," Appl. Microbiol. Biotechnol. 96 (2012) 631–645. [CrossRef]
- Y. Tang, J.N. Rosenberg, P. Bohutskyi, G. Yu, M.J. Betenbaugh, F. Wang, "Microalgae as a feedstock for biofuel pre-cursors and value-added products: Green fuels and golden opportunities," BioResources. 11 (2016) 2850–2885. [CrossRef]
- D. Soto, G. Revilla, "Viability Assesment of Bioethanol Production From Algae," (2020) 216. [Online]. Available: https://upcommons.upc.edu/handle/2117/332231.
- S. Maity, N. Mallick, "Trends and advances in sustainable bioethanol production by marine microalgae: A critical review," J. Clean. Prod. 345 (2022) 131153. [CrossRef]
- K. Kusmiyati, H. Hadiyanto, A. Fudholi, "Treatment updates of microalgae biomass for bioethanol production: A comparative study," J. Clean. Prod. 383 (2023) 135236. [CrossRef]
- M. Hasin, M. Gohain, D. Deka, "Bio-Ethanol Production from Carbohydrate-Rich Microalgal Biomass: Scenedesmus Obliquus," in: Springer, Singapore, 2021: pp. 1215–1224. [CrossRef]
- L.M. Chng, K.T. Lee, D.J.C. Chan, "Synergistic effect of pretreatment and fermentation process on carbohydrate-rich Scenedesmus dimorphus for bioethanol production," Energy Convers. Manag. 141 (2017) 410–419. [CrossRef]
- G.W.A. El-Souod, E.M. Morsy, L.H.S. Hassan, M.M. El-Sheekh, "Efficient Saccharification of the Microalga Chlorella vulgaris and its Conversion into Ethanol by Fermentation," Iran. J. Sci. Technol. Trans. A Sci. 45 (2021) 767–774. [CrossRef]
- M. Megawati, D.A.P.R.D.A.T.B.& P. H Bahlawan Z. A. S., "Comparative study on the various hydrolysis and fermentation methods of Chlorella vulgaris biomass for the production of bioethanol," Int. J. Renew. Energy Dev. 11 (2022) 515.
- G. Silva, K. Cerqueira, J. Rodrigues, K. Silva, D. Coelho, R. Souza, "Cultivation of Microalgae Chlorella vulgaris in Open Reactor for Bioethanol Production," Phycology. 3 (2023) 325–336. [CrossRef]
- H.W. Yen, I.C. Hu, C.Y. Chen, S.H. Ho, "Microalgae-based biorefinery–from biofuels to natural products," Elsevier. (n.d.). [Online]. Available: https://www.sciencedirect.com/science/article/pii/S096085241201601X.
- G. Zanchett, E.C.O.-F.- Toxins, "Cyanobacteria and cyanotoxins: from impacts on aquatic ecosystems and human health to anticarcinogenic effects," Mdpi.ComG Zanchett, EC Oliveira-FilhoToxins. 5 (2013) 1896–1917. [CrossRef]
- R. Zhang, J. Wang, X. Zhai, J. Che, Z. Xiu, "Carbonate assisted lipid extraction and biodiesel production from wet microalgal biomass and recycling waste carbonate for CO2 supply in microalgae cultiva-tion," ElsevierR Zhang, J Wang, X Zhai, J Che, Z Xiu, Z ChiScience Total Environ. 2021•Elsevier. (n.d.). [Online]. Available: https://www.sciencedirect.com/science/article/pii/S0048969721015138.
- V.O. Adesanya, E. Cadena, S.A. Scott, A.G.S.-B. technology, "Life cycle assessment on microalgal biodiesel production using a hybrid cultivation system," ElsevierVO Adesanya, E Cadena, SA Scott, AG SmithBioresource Technol. 2014•Elsevier. (n.d.). [Online]. Available: https://www.sciencedirect.com/science/article/pii/S0960852414005562.
- N.R. Boyle, M.D. Page, B. Liu, I.K. Blaby, D. Casero, J. Kropat, S.J. Cokus, A. Hong-Hermesdorf, J. Shaw, S.J. Kar-powicz, S.D. Gallaher, S. Johnson, C. Benning, M. Pellegrini, A. Grossman, S.S. Merchant, "Three acyltransferases and ni-trogen-responsive regulator are implicated in nitrogen starvation-induced triacylglycerol accumulation in Chlamydomonas," ASBMB. 287 (2012) 15811–15825. [CrossRef]
- J.L. Blatti, J. Michaud, M.D.B.-C. opinion in chemical biology, "Engineering fatty acid biosynthesis in microalgae for sustainable biodiesel," ElsevierJL Blatti, J Michaud, MD BurkartCurrent Opin. Chem. Biol. 2013•Elsevier. (n.d.). [Online]. Available: https://www.sciencedirect.com/science/article/pii/S1367593113000641.
- Y. Gong, M. Jiang, Biodiesel production with microalgae as feedstock: From strains to biodiesel, Biotechnol. Lett. 33 (2011) 1269–1284. [CrossRef]
- J. Chen, J. Li, W. Dong, X. Zhang, … R.D.T.-, … S.E., undefined 2018, The potential of microalgae in biodiesel production, Elsevier. (n.d.). https://www.sciencedirect.com/science/article/pii/S1364032118301576.
- S.S. Rahpeyma, J. Raheb, Microalgae Biodiesel as a Valuable Alternative to Fossil Fuels, Bioenergy Res. 12 (2019) 958–965. [CrossRef]
- M.I. Silva, A.L. Gonçalves, V.J.P. Vilar, J.C.M.P.- Sustainability, undefined 2021, Experimental and techno-economic study on the use of microalgae for paper industry effluents remediation, Mdpi.ComMI Silva, AL Gonçalves, VJP Vilar, JCM PiresSustainability (2021). [CrossRef]
- Z. Zhang, H. Ji, G. Gong, X. Zhang, T.T.-B. technology, undefined 2014, Synergistic effects of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for enhancement of biomass and lipid yields, Elsevier. (n.d.). https://www.sciencedirect.com/science/article/pii/S0960852414005446.
- D. Mu, C. Xin, W.Z.-M.C. for Biofuels Production, undefined 2020, Life cycle assessment and techno-economic analysis of algal biofuel production, Elsevier. (n.d.). https://www.sciencedirect.com/science/article/pii/B9780128175361000187.
- L. Reijnders, Life Cycle Assessment of Biofuels, in: Methods Mol. Biol., Humana Press Inc., 2021: pp. 53–67. [CrossRef]
- V.K. Garlapati, S. Tewari, R. Ganguly, Life cycle assessment of first-, second-generation, and microalgae biofuels, in: Adv. Feed. Convers. Technol. Altern. Fuels Bioprod. New Technol. Challenges Oppor., Elsevier, 2019: pp. 355–371. [CrossRef]
- L.A.J. Letti, J.C. De Carvalho, S.J. Da Costa, T.S. Martins, N. da Silva Ramos, M.C. Dotto, A.L. Woiciechowski, C.R. Soccol, Life-cycle assessment of biofuels, in: Green Energy Technol., Springer Verlag, 2016: pp. 485–500. [CrossRef]
- D. Mu, R. Ruan, M. Addy, S. Mack, P. Chen, Y.Z.-B. technology, undefined 2017, Life cycle assessment and nutrient analysis of various processing pathways in algal biofuel production, Elsevier. (n.d.). https://www.sciencedirect.com/science/article/pii/S0960852417300020.
- D. Mu, C. Xin, W. Zhou, Life cycle assessment and techno-economic analysis of algal biofuel production, in: Micro-algae Cultiv. Biofuels Prod., Academic Press, 2019: pp. 281–292. [CrossRef]
- J.C. Quinn, R.D.-B. technology, undefined 2015, The potentials and challenges of algae based biofuels: a review of the techno-economic, life cycle, and resource assessment modeling, ElsevierJC Quinn, R DavisBioresource Technol. 2015•Elsevier. (n.d.).
- F. Menten, B. Chèze, L. Patouillard, F.B.- Renewable, sustainable, undefined 2013, A review of LCA greenhouse gas emissions results for advanced biofuels: the use of meta-regression analysis, Renewable Sustain. Energy Rev. 2013•Elsevier. (n.d.). https://www.sciencedirect.com/science/article/pii/S1364032113002736.
- Q. Tu, M. Eckelman, J. Zimmerman, Meta-analysis and Harmonization of Life Cycle Assessment Studies for Algae Biofuels, Environ. Sci. Technol. 51 (2017) 9419–9432. [CrossRef]
- W. Wu, K.H. Lin, J.S.C.-B. technology, undefined 2018, Economic and life-cycle greenhouse gas optimization of microalgae-to-biofuels chains, ElsevierW Wu, KH Lin, JS Chang. Technol. 2018•Elsevier. (n.d.).
- W. Wu, Y.C. Lei, J.S.C.-B. technology, undefined 2019, Life cycle assessment of upgraded microalgae-to-biofuel chains, Elsevier. (n.d.). https://www.sciencedirect.com/science/article/pii/S0960852419307229.
- G. Saranya, T.V.R.-E. Conversion, M. X, undefined 2020, Life cycle assessment of biodiesel from estuarine microalgae, ElsevierG Saranya, TV RamachandraEnergy Convers. Manag. X, 2020•Elsevier. (n.d.).
- L. Barsanti, P.G.-A. research, undefined 2018, Is exploitation of microalgae economically and energetically sustainable?, ElsevierL Barsanti, P GualtieriAlgal Res. 2018•Elsevier. (n.d.). https://www.sciencedirect.com/science/article/pii/S2211926417309980.
- E.G. Arenas, M.C.R. Palacio, A.U. Juantorena, S.E.L. Fernando, P.J. Sebastian, Microalgae as a potential source for biodiesel production: techniques, methods, and other challenges, Int. J. Energy Res. 41 (2017) 761–789. [CrossRef]
- B.S.M. Sturm, S.L.L.-A. energy, undefined 2011, An energy evaluation of coupling nutrient removal from wastewater with algal biomass production, ElsevierBSM Sturm, SL LamerApplied Energy, 2011•Elsevier. (n.d.). https://www.sciencedirect.com/science/article/pii/S0306261910005763.
- P.K. Usher, A.B. Ross, A. Camargo-Valero, A.S. Tomlin, W.F. Gale, "An overview of the potential environmental impacts of large-scale microalgae cultivation," Biofuels, 5 (2014) 331–349. [CrossRef]
- K.W. Chew, J.Y. Yap, P.L. Show, N.H. Suan, J.C.J.-B., "Microalgae biorefinery: high value products perspectives," Technol. (n.d.). [Online]. Available: link.
- T.J. Lundquist, I.C. Woertz, N.W.T. Quinn, J.R. Benemann, "A realistic technology and engineering assessment of algae biofuel production," Energy Biosci. Institute (2010). [Online]. Available: link.
- T. Searchinger, R. Heimlich, R.A. Houghton, F.D., "Use of US croplands for biofuels increases greenhouse gases through emissions from land-use change," Sci. 319 (2008) 1238–1240. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).