Submitted:
06 May 2024
Posted:
07 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Procedure
2.1. Materials Methodology
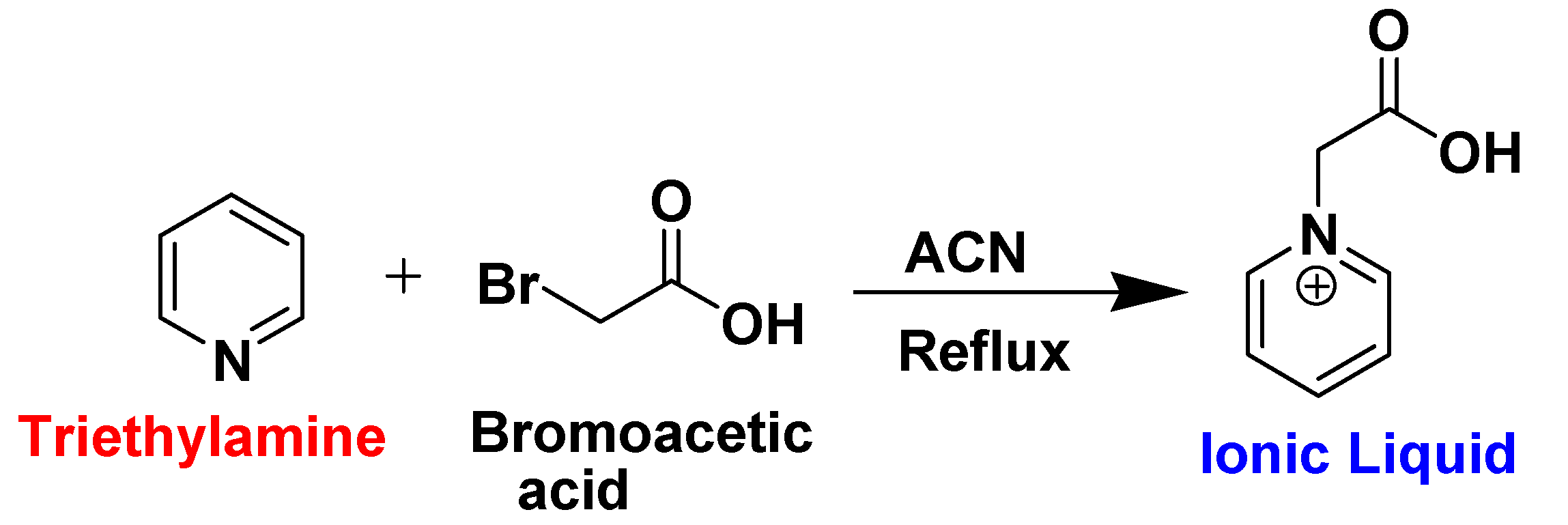
2.2. Synthesis of Ionic Liquid (IL) Electrolyte
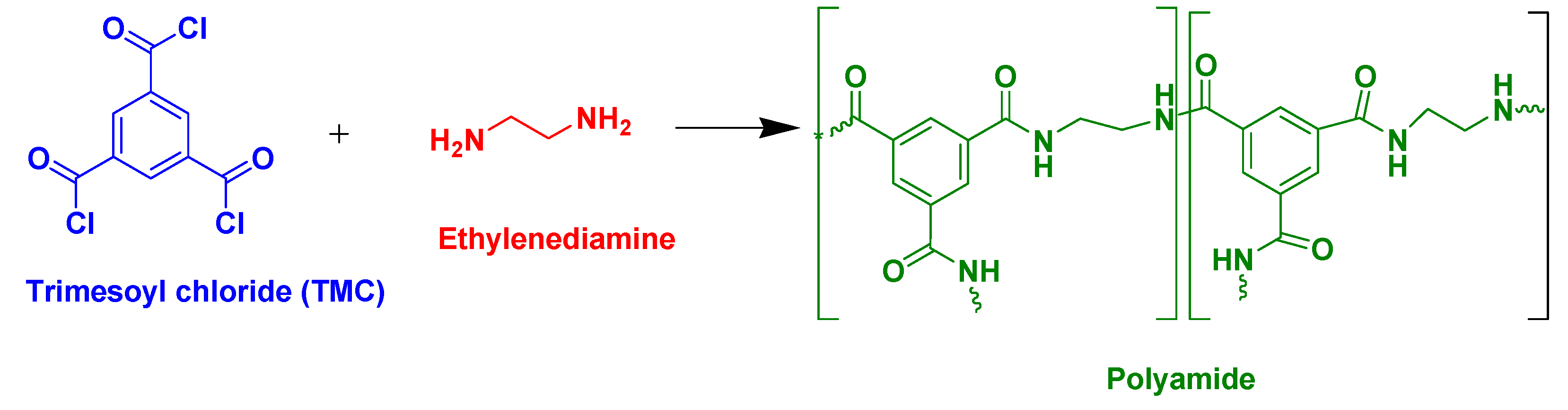
2.3. Synthesis of Dye
2.4. Preparation of ZnO Nanoparticles
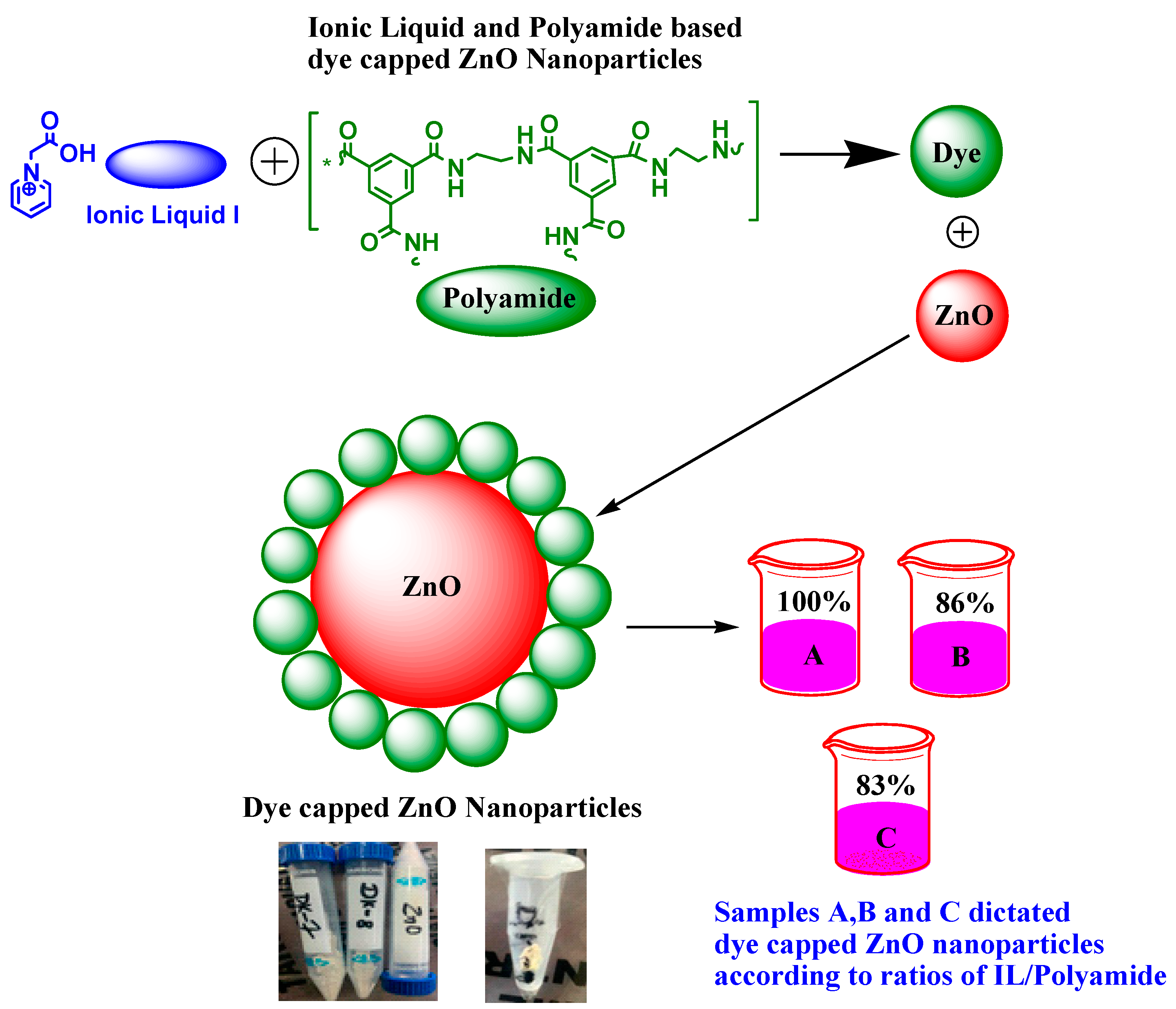
2.5. Capping of Dye on ZnO Nanoparticles (Formation of Samples A, B, and C)
| Name of Sample |
Ionic Liquid (in mg) |
Polyamide (in mg) |
Ratio: IL/Polyamide (in %) |
|---|---|---|---|
| Sample A | 500 mg | 500 mg | 100 |
| Sample B | 600 mg | 700 mg | 86 |
| Sample C | 500 mg | 600 mg | 83 |
2.6. Fabrication of Dye Capped ZnO DSSCs
3. Results and Discussions
3.1. Materials Development
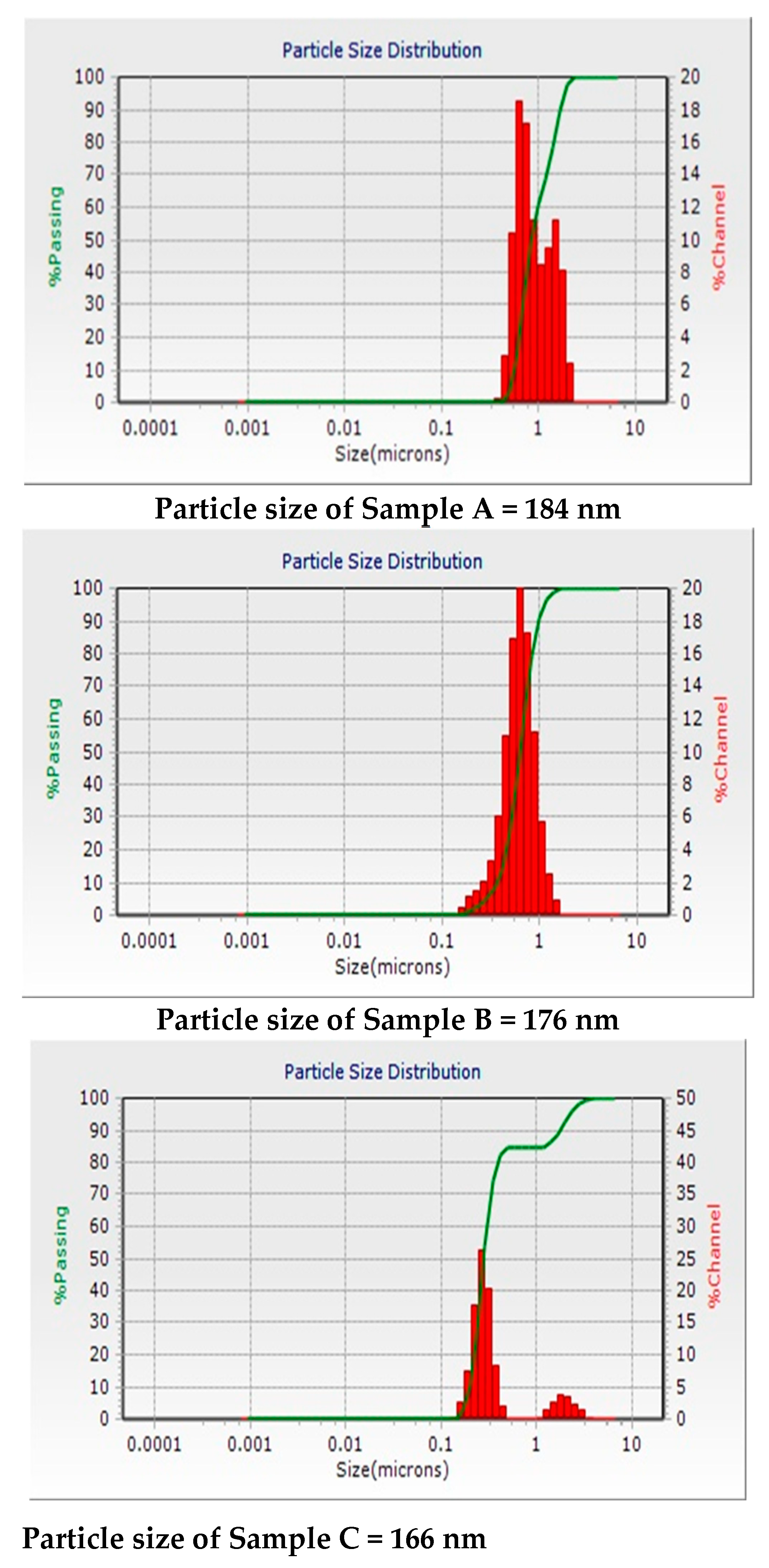
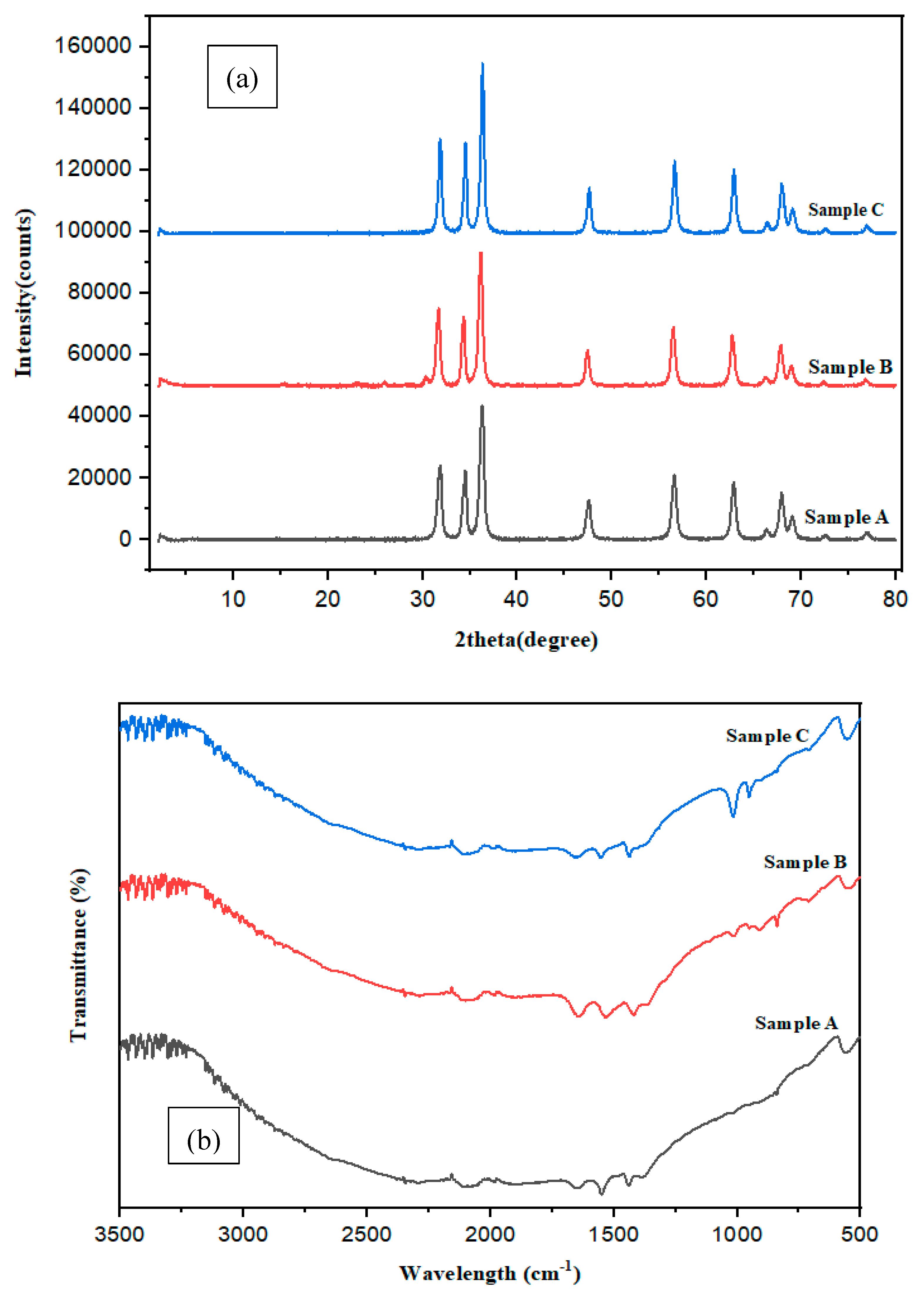
3.2. Characterizations of Samples A, B and C (DLS, XRD and FTIR)
| Polyamide dye capped ZnO Samples | Peak position (in degrees) |
Reflection (h k l) |
d-spacing (in nm) |
Size of particle D (in nm) |
|---|---|---|---|---|
| Sample A | 36.26° | 101 | 2.47 | 184.2 |
| Sample B | 36.12° | 101 | 2.48 | 176.3 |
| Sample C | 24.60° | 002 | 3.61 | 160.5 |
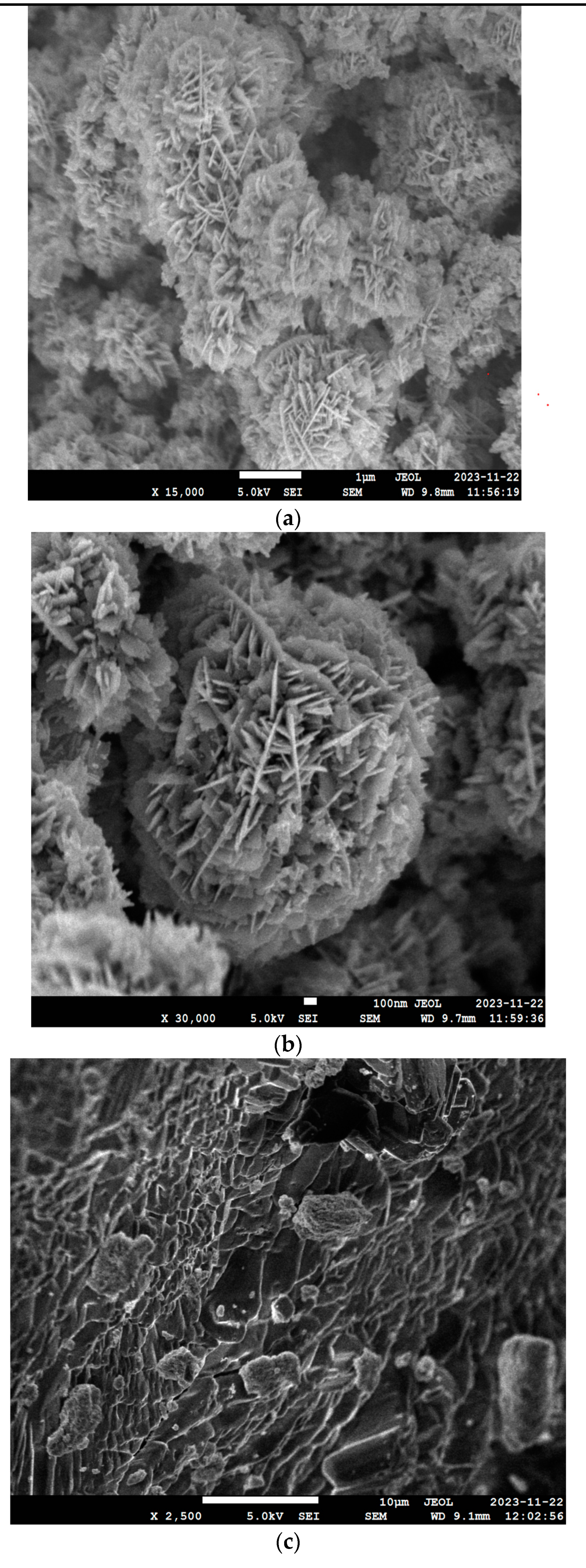
3.3. Morphological Studies (FE-SEM)
| Name of ZnO Sample | Width of ZnO Nanoparticle |
|---|---|
| Sample A | 9.8 nm |
| Sample B | 9.7 nm |
| Sample C | 9.1 nm |
| Sample Name |
XRD (size of ZnO nanoparticles) |
FE-SEM (size of ZnO nanoparticles) |
DLS (size of ZnO nanoparticles) |
|---|---|---|---|
| Sample A | 184.2 nm | 187 nm | 184 nm |
| Sample B | 176.3 nm | 180 nm | 176 nm |
| Sample C | 160.5 nm | 170 nm | 166 nm |
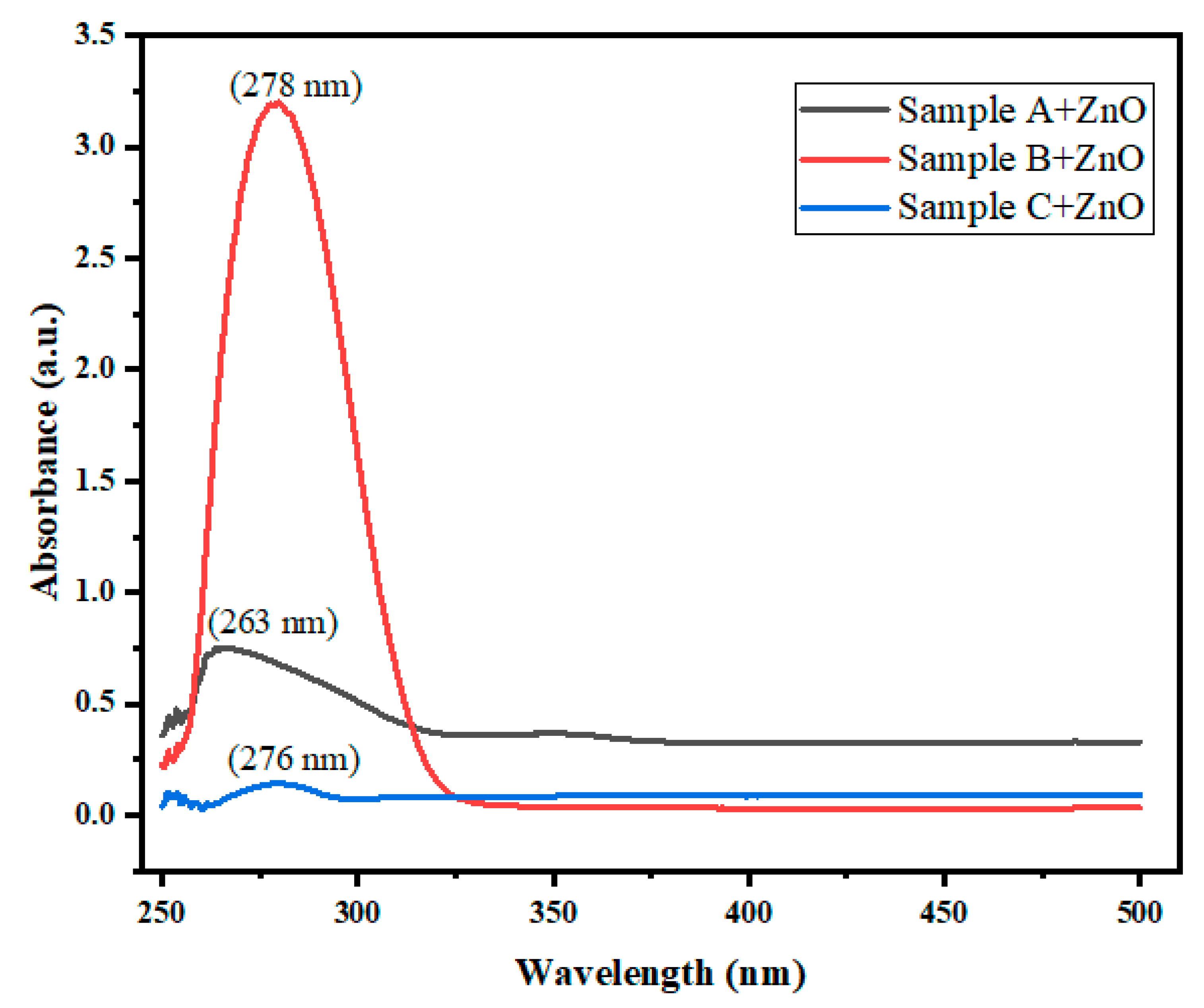
| Name of Sample | ZnO(in mg) | Ionic Liquid(in mg) | Polyamide(in mg) | UV-Visible spectraPeak 1 & Peak 2 |
| Sample A | 500 mg | 700 mg | 500 mg | 263 nm, 295 nm |
| Sample B | 500 mg | 600 mg | 700 mg | 278 nm, 344 nm |
| Sample C | 500 mg | 500 mg | 600 mg | 276 nm, 305 nm |
| Ionic liquid based ZnO samples | Wavelength(λ)( in nm) | Absorbance(α) | (αhν)2 | Energy band gap(Eg)(in eV) |
| Sample A | 263.8 | 0.746 | 3.50 | 4.70 |
| Sample B | 278.8 | 3.185 | 14.16 | 4.44 |
| Sample C | 276.6 | 0.136 | 0.61 | 4.48 |
3.4. Optoelectronic Properties of Samples A, B and C Based ZnO Nanoparticles
UV-Visible Spectra Studies
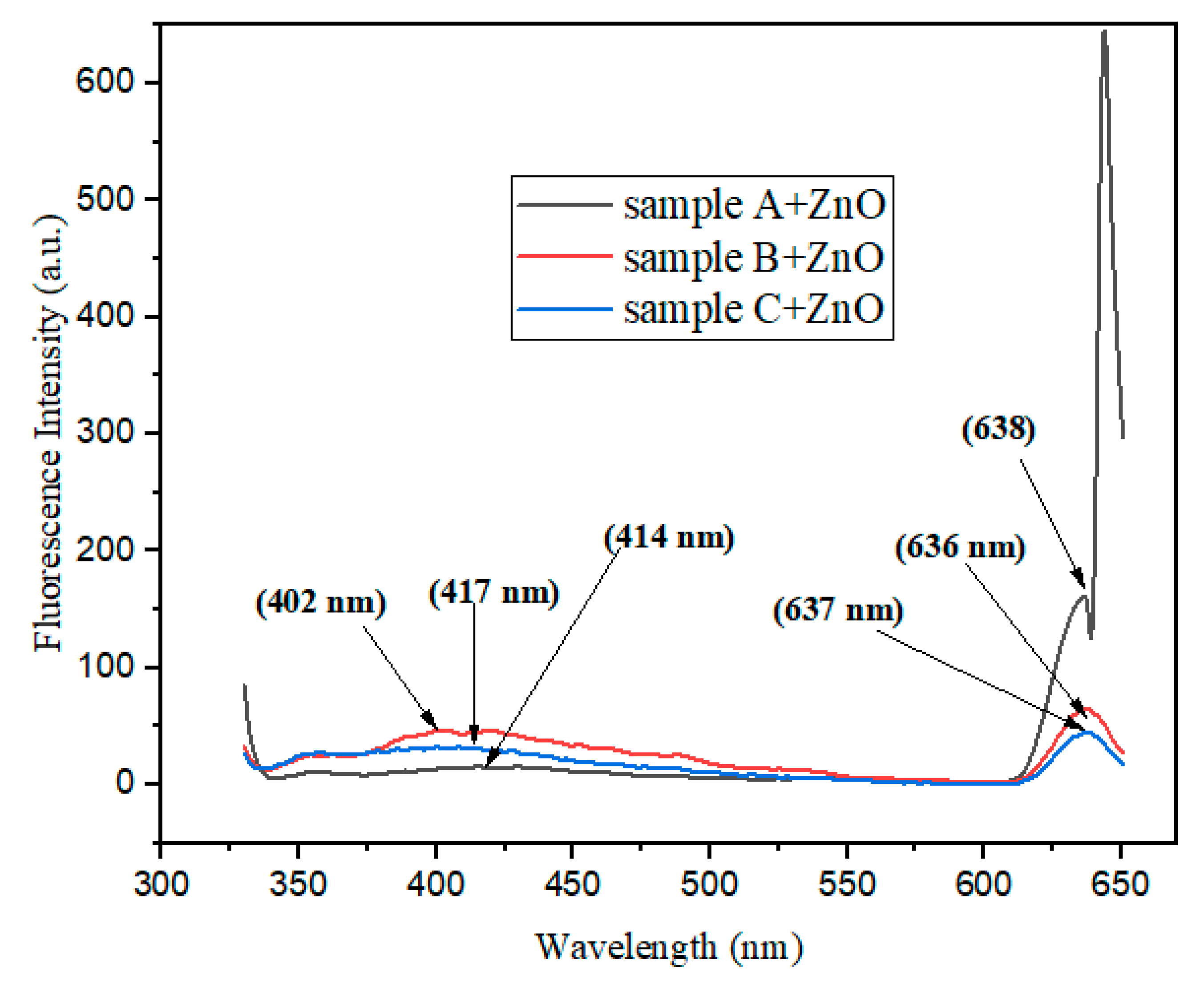
Fluorescence Spectra Studies
3.5. Photovoltaic Performances of ZnO DSSCs
3.6. Conclusions
Funding
Acknowledgements
Conflict of Interest
Notes
References
- Cornelia Sima, Constantin Grigoriu, Stefan Antohe, Comparison of the dye-sensitized solar cells performances based on transparent conductive ITO and FTO, Thin Solid Films, 519 (2010), p. 595-597.
- Zhicai He, Chengmei Zhong, Xun Huang, Wai-Yeung Wong, Hongbin Wu., Liwei Chen, Shijian Su, Yong Cao, Simultaneous Enhancement of open circuit voltage, short-circuit current density, and Fill Factor in Polymer Solar Cells, Advanced Materials, 23 (2011), p. 4636-4643.
- Xuanhua Li, Wallace C. H. Choy, Lijun Huo, Fengxian Xie, Wei E. I. Sha, Baofu Ding, Xia Guo, Yongfang Li, Jianhui Hou, Jingbi You, Yang Yang, Dual Plasmonic Nanostructures for High Performances Invested Organic Solar Cells, Advanced Materials, 24 (2012), p. 3046-3052.
- Zhicai He, Changmei Zhong, Shijian Su, Miao Xu, Hongbin Wu and Yong Cao, Enhanced power conversion efficiency in polymer solar cells using an inverted device structure, Nature Photonics, 6 (2012), p. 591-595.
- Jingbi You, Letian Dou, Ken Yoshimura, Takehito Kato, Kenichiro Ohya, Jing Gao, Gang Li, Yang Yang, A polymer tandem solar cell with 10.6% power conversion efficiency, Nature Communications, 4:1446 (2013), p. 1-10.
- H. Park, Y. Park, W. Kim, W. Choi, Surface modification of TiO2 photocatalyst for environment applications, Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 15 (2013), p. 1-20.
- J. Fan, Y. Hao, A. Cabot, E. M. J. Johansson, G. Boschloo, A. Hagfeldt, Cobalt(II/III) redox electrolyte in ZnO nanowire-based dye sensitized solar cells, ACS Applied Materials & Interfaces, 5 (6) (2013), p. 1902-1906.
- Guillaume Bousrez, Olivier Renier, Brando Adranno, Volodymyr Smetana, and Anja-Verena Mudring, Ionic Liquid-Based Dye-Sensitized Solar Cells-Insights into Electrolyte and Redox Mediator Design, ACS Sustainable Chemistry Engineering, 9 (2021), p. 8107-8114.
- Denizalti, Serpil; Ali, Abdulrahman Khalaf; Ela, Cagatay; Ekmekci, Mesut; Erten-Ela, Sule, Dye-Sensitized Solar Cells Using Ionic Liquids as Redox Mediator, Chemical Physics Letters, 691 (2018), p. 373-378.
- Annkatrin Lennert, Michelle Sternberg, Karsten Meyer, Ruben D. Costa, Dirk M. Guldi, Iodine-Pseudohalogen Ionic Liquid-Based Electrolytes for Quasi-Solid State Dye-Sensitized Solar Cells, ACS Applied Material Interfaces, 9 (39) (2017), p. 33437-33445.
- Kitty Y. Chen, Phil A. Schauer, Brian O. Patrick and Curtis P. Berlinguette, Correlating cobalt redox couple to photovoltage in the dye-sensitized solar cell, Dalton Transactions, 47 (2018), p. 11942-11952.
- Dianlu Jiang, Narek Darabedian, Sevak Ghazarian, Yuanqiang Hao, Maxim Zhgamadze, Natalie Majaryan, Rujuan Shen, and Feimeng Zhou, Dyes and Redox Couples with Matched Energy Levels: Elimination of the Dye-Regeneration Energy Loss in Dye-Sensitized Solar Cells, ChemPhysChem, 16 (2015), p. 3385-3388.
- Jun-Ho Yum, Etienne Baranoff, Florian Kesslar, Thomas Moehl, Shahzada Ahmad, Takeru Bessho, Arianna Marchioro, Elham Ghadiri, Jacques-E. Moser, Chenyi Yi, Md. K. Nazeeruddin, and Michael Grätzel, A cobalt complex redox shuttle for dye-sensitized solar cells with high open circuit potentials, Nature Communication, 3 (631) (2012), p. 1-8.
- Rodrigo García-Rodríguez, Roger Jiang, Esdras J. Canto-Aguilar, Gerko Oskam, and Gerrit Boschloo, Improving the mass transport of copper-complex redox mediators in dye-sensitized solar cells by reducing the inter-electrode distance, Physical Chemistry Chemical Physics, 19 (2017), p. 32132-32142.
- Marina Freitag, Fabrizio Giordano, Wenxing Yang, Meysam Pazoki, Burkhard Ziete, Michael Grӓtzel, Anders Hagfeldt, and Gerrit Boschloo, Copper Phenanthroline as a Fast and High-Performance Redox Mediator for Dye-Sensitized Solar Cells, Journal of Physical Chemistry C, 18 (120) (2016), p. 9595-9603.
- Tomohiro Higashino, Hitomi Liyama, Shimpei Nimura, Yuma Kurumisawa, and Hiroshi Imahori, Effect of Ligand Structures of Copper Redox Shuttles on Photovoltaic Performance of Dye-Sensitized Solar Cells, Inorganic Chemistry, 59 (1) (2020), p.452-459.
- Tae-Yeon Cho, Soon-Gil Yoon, S.S. Sekhon, and Chi-Hwan Han, Effect of Ionic Liquid with Different Cations in I-/I3- Redox Electrolyte on the Performance of Dye-Sensitized Solar Cells, Bulletin of the Korean Chemical Society, 32 (6) (2011), p. 2058-2062.
- Genevieve P.S. Lau, Jean-David Décoppet, Thomas Moehl, Shaik M. Zakeeruddin, Michael Grätzel, and Paul J. Dyson, Robust High-Performance Dye-Sensitized Solar Cells Based on Ionic Liquid-Sulfolane Composite Electrolytes, Scientific Reports, 5 (18156) (2016), p. 1-8.
- Mini Thomas and Sheeja Rajiv, Dye-Sensitized Solar Cells Based on an Electrospun Polymer Nanocomposite Membrane as Electrolyte, New Journal of Chemistry, 43 (2019), p. 4444-4454.
- Ruwaida Asyikin Abu Talip, Wan Zaireen Nisa Yahya, and Mohamad Azmi Bustam, Ionic Liquids Roles and Perspectives in Electrolyte for Dye-Sensitized Solar Cells, Sustainability, 20 (18) (2020), p. 1-23.
- Mikhail Gorlov and Lars Kloo, Ionic Liquid electrolytes for dye-sensitized solar cells, Dalton Transactions, 20 (2008), p. 2655-2666.
- Zhigang Lei, Biaohua Chen, Yoon-Mo Koo, Douglas R. MacFarlane, Introduction: Ionic Liquids, Chemical Reviews, 117 (2017), p. 6633-6635.
- Amal-Al-Kalout, Thermal treatment optimization of ZnO nanoparticles-photoelectrodes for high photovoltaic performance of dye-sensitized solar cells, Journal of the Association of Arab Universities for Basic and Applied Sciences, 17 (2014), p. 66-72.
- A.K. Chandiran, M. Abdi-Jalebi, M. K. Nazeeruddin, M. Grätzel, Analysis of electron transfer properties of ZnO and TiO2 photoanodes for dye sensitized solar cells, ACS Nano 8 (3) (2014), p. 2261-2268.
- Sarita Bose, Virendra Soni and K.R. Genwa, Recent Advances and Future Prospects for Dye Sensitized Solar Cells: Review, 5 (2015), p. 1-9.
- Satbir Singh, Tilak Raj, Amarpal Singh, Navneet Kaur, Optoelectronic and Photovoltaic Performance of Pyridine Based Monomer and Polymer capped ZnO Dye-Sensitized Solar Cells, 16(6) (2016), p. 5975-5983.
- R. Vittal, Kuo-chuan Ho, Zinc Oxide based dye-sensitized solar cells: Review, Renewable and Sustainable Energy Reviews, 70 (2016), p. 920-935.
- Ardiansyah Taufik, Alfred Albert, Roseri Saleh, Sol-gel synthesis of ternary CuO/TiO2/ZnO nanocomposites for enhanced photocatalytic performance under UV and visible light irradiation, Journal of Photochemistry and Photobiology A: Chemistry, 344 (2017), p. 149-162.
- Khushboo Sharma, Vinay Sharma and S.S Sharma, Dye-Sensitized Solar Cells: Fundamental and Current Status, Nanoscale Research Letters, 13 (2018), p. 1-46.
- Seong II Cho, Hye Kyeong Sung, Sang-Ju Lee, Wook Hyun Kim, Dae-Hwan Kim and Yoon Soo Han, Photovoltaic Performance of Dye-Sensitized Solar Cells Containing ZnO Microrods, Nanomaterials, 9 (2019), p. 1-12.
- Sabastine C. Ezike, Clement N. Hyelnasinyi, Mufutau A. Salawu, John F. Wansah, Amarachukwu N. Ossai, Nnabuike N. Agu, Synergistic effect of chlorophyll and anthocyanin co-sensitizers in TiO2-based dye-sensitized solar cells, Surfaces and Interfaces, 22 (2020), p. 1-39.
- Amarachukwu N. Ossai, Sabastine C. Ezike, Pascal Timtere, Abubakar D. Ahmed, Enhanced photovoltaic performance of dye-sensitized solar cells-based carica papaya leaf and black cherry fruit co-sensitizers, Chemical Physics Impact, 2 (2021), p. 1-8.
- Mikko Kokkonen, Parisa Talebi, Jin Zhou, Somayyeh Asgari, Sohail Ahmed Soomro, Farid Elsehrawy, Janne Halme, Shahzada Ahmad, Anders Hagfeldt and Syed Ghufran Hashmi, Advanced research trends in dye-sensitized solar cells, Journal of Material Chemistry A, 9 (2021), p. 10527-10545.
- Dr. Satbir Singh, Dr. Tilak Raj, Indra Bahadur, Dr. Harpreet Singh, Prof. Rajender S. Varma, Improved Power Conversion Efficiencies of Dye-Capped and Sensitized ZnO Solar Cells, ChemistrySelect, 7 (2022), p.1-11.
- Salisu. I. Kunya, Yunusa Abdu, Mohd Kamarulzaki Mustafa, and Mohd Khairul Ahmad, Survey of the Achievement of a ZnO Dye-Synthesized Solar Cell, Journal of Chemistry Studies, 2 (1) (2023), p. 1-12.
- Satbir Singh, Talik Raj, Indra Bahadur, Harpreet Singh, and Rajender S. Varma, Improved Power Conversion Efficiencies of Dye-Capped and Sensitized Solar Cells, Chemistry Select, 7 (38) (2022), p. 1-11.
- T. Kato, A. Okazaki, S. Hayase, Latent gel electrolyte precursors for quasi-solid dye sensitized solar cells: The comparison of nano-particle cross-linkers with polymers cross-linkers, Journal of Photochemistry and Photobiology A: Chemistry, 179 (1-2) (2006), p. 42-48.
- M. Wang, X. Xiao, X. Zhou, X. Li and Y. Lin, The Effect of Ionic Liquid to Dye-Sensitized Solar Cell with Fluorinated Oligomer Electrolyte, Solar Energy Material, 91 (2007), p. 785.
- Peng Wang, Shaik M. Zakeeruddin, Ivan Exnar and Michael Grätzel, High efficiency dye-sensitized nanocrystalline solar cells based on ionic liquid polymer gel electrolyte, Chemical Communications, 22 (2002), p. 2972-2973.
- E. Stathatos, P. Lianos, S. M. Zakeeruddin, P. Liska, and M. Grätzel, A Quasi-Solid-State Dye-Sensitized Solar Cell Based on a Sol-Gel Nanocomposite Electrolyte Containing Ionic Liquid, Chemistry of Materials, 15 (9) (2003), p. 1825-1829.
- Serpil Denizalti, Abdurrahman K. Ali, Ҫağatay Ela, Mesut Ekmekei, Sule Erten Ela, Dye-Sensitized Solar Cells using Ionic Liquids as Redox Mediator, Chemical Physics Letters, 691 (2018), p. 373-378.


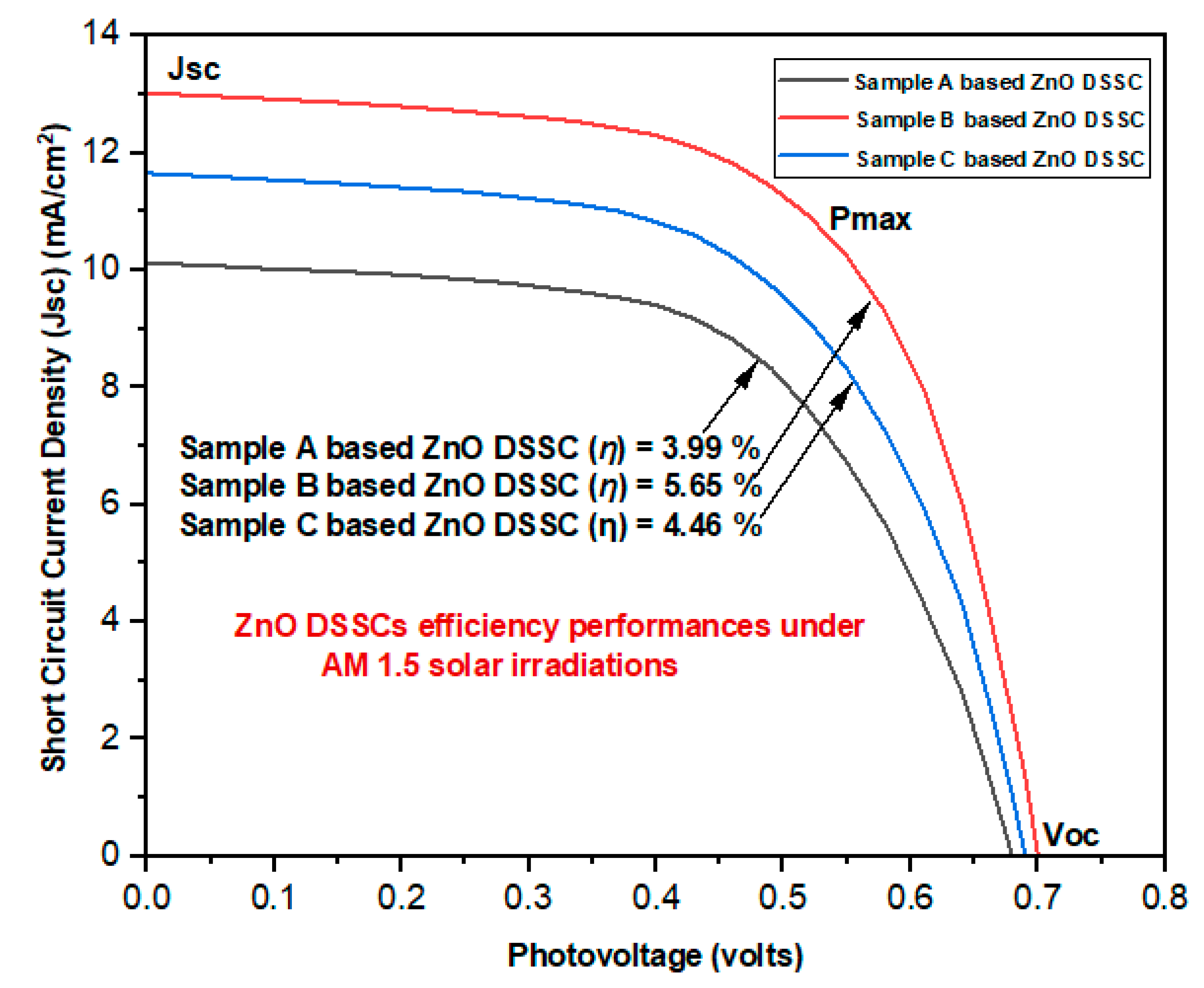
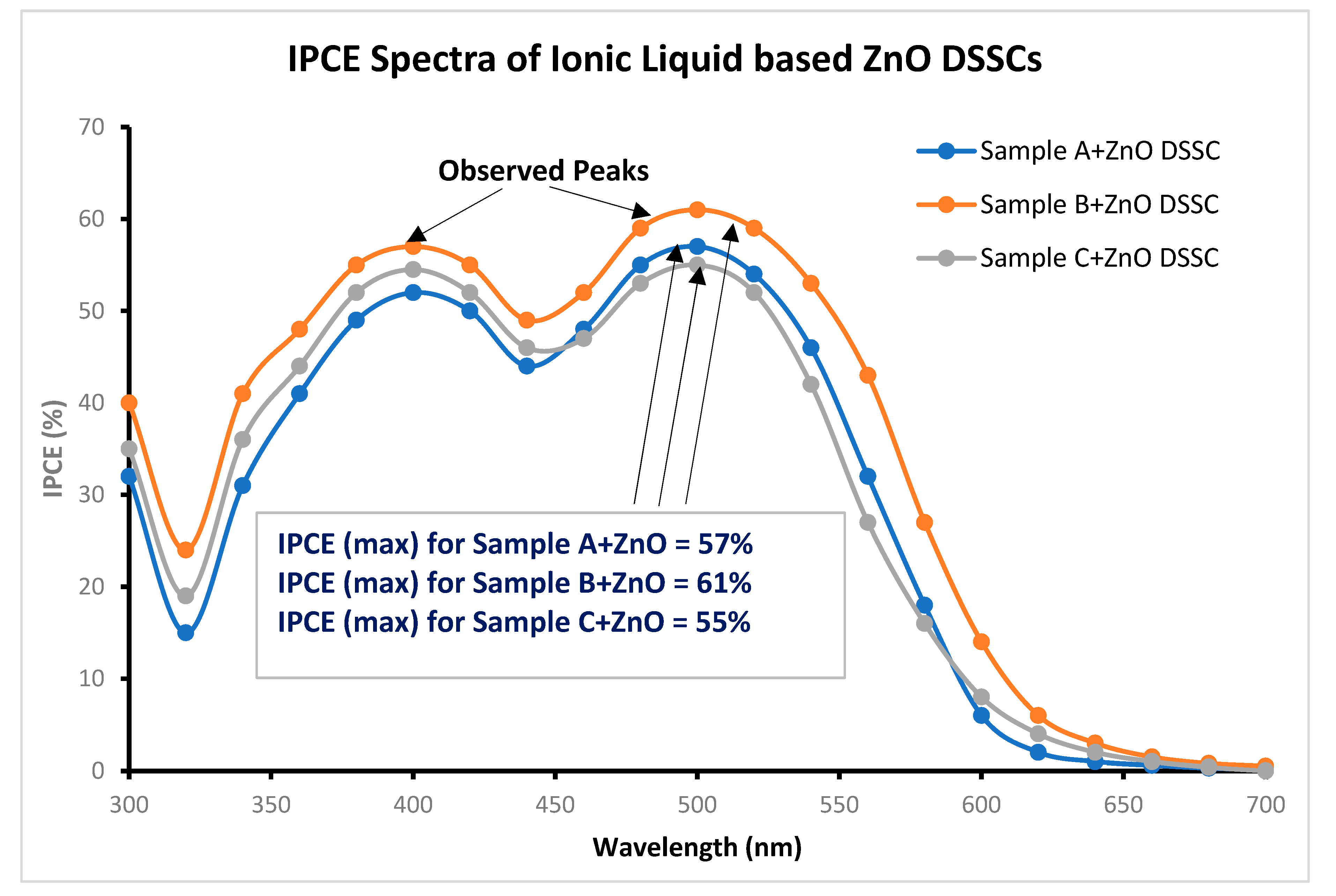
| Name of ZnO DSSC | Open Circuit voltage Voc (volts) | Short Circuit Current Density (Jsc) (mA/cm2) | Maximum short circuit current density (Jmax) (mA/cm2) |
Maximum open circuit voltage (Vmax) (volts) |
Fill Factor (FF) | Efficiency (η %) |
|---|---|---|---|---|---|---|
| SCA | 0.68 | 10.12 | 8.82 | 0.46 | 0.58 | 3.99 |
| SCB | 0.70 | 13.02 | 10.92 | 0.52 | 0.62 | 5.65 |
| SCC | 0.69 | 11.65 | 9.74 | 0.49 | 0.59 | 4.46 |
| ZnO DSSC Description | Electrolyte/Dye/Sensitizer | Value of IPCE (%) |
|---|---|---|
| SCA | Ionic liquid/polyamide | 57% |
| SCB | Ionic liquid/polyamide | 61% |
| SCC | Ionic liquid/polyamide | 55% |
| ZnO DSSC | Electrolytes Mixture (IL) used | Dye used |
Voc (volts) |
Jsc (mA/cm2) | FF | η% |
|---|---|---|---|---|---|---|
| SCA | Triethylamine (1g)+Bromoacetic acid (1g) | Trimesoyl chloride (10 ml) +Ethylenediamine (5 ml) |
0.68 | 10.12 | 0.58 |
3.99 (present result) |
| SCB | Triethylamine (0.5g)+Bromoacetic acid (0.5g) | Trimesoyl chloride (10 ml) + Ethylenediamine (5 ml) |
0.7 | 13.02 | 0.62 |
5.65 (present result) |
| SCC | Triethylamine (1g)+Bromoacetic acid (0.5g) | Trimesoyl chloride (5 ml) + Ethylenediamine (5 ml) |
0.69 | 11.65 | 0.59 |
4.46 (present result) |
| Other DSSCs | Electrolyte Mixture (IL) | Used dyes | η% | Ref. | ||
| SC1 | 0.3 M I2, PVP (2 wt%), HOOC(CH2)16COOH(4 wt%), H2O (5 wt%) in PMImI | N-3 | 4.4 | [37] | ||
| SC2 | 1 M Lil, 0.5 M I2 in PEO(750)MimCl | N-3 | 3.1 | [38] | ||
| SC3 | PVDF-HFP (10 wt%), I2 in PMImI | Z-907 | 5.3 | [39] | ||
| SC4 | CH3CO2H, TMOS, 0.5 M PMImI, 0.04 Mm NMBI, 20 MMI2 in PC/Triton X-100 (molar ratio 4:1) | N-3 | 5.4 | [40] | ||
| SC5 | 0.6 M ionic liquid + 0.1 M LiI + 0.05 M I2 in MPN | ----- | 5.17 | [41] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




