1. Introduction
Nowadays, about one third of all reproductive-aged women are affected by obesity and the Increasing prevalence of obesity seems to have a negative impact on the gestational period, leading more and more pregnant patients to develop complications such as Gestational Diabetes (GDM) or Preeclampsia (PE), a serious hypertensive disorder of pregnancy [
1,
2].
Women with GDM bear elevated IL-6 levels [
3] implicated in various adverse conditions during pregnancy. Many studies have attempted to elucidate the role of these elevated circulating levels, combined with the presence of various gene polymorphisms, aiming to elucidate predisposing genetic factors for complications development in high-risk pregnancies. In obese pregnancies protein expression of IL-6 is also raised [
4] and must be clarified whether it is implicated in the inflammation generation for maternal obesity and subsequently leads to insulin resistance which is considered the causal and responsible mechanism for adverse outcomes during pregnancy. Certain markers with elevated levels are associated with maternal inflammation and have also been identified in preeclampsia [
5,
6]. Interleukin-6 involvement has been strongly suspected in various adverse conditions during pregnancy and many studies have attempted to elucidate the relation of certain IL-6 gene polymorphisms in preeclampsia. However, does not exist till today enough information in order various inflammatory markers, such as IL-6 to serve as predictive tools, because we are lacking studies investigating pregnancies with either preexisting type 2 diabetes or obesity.
The pathophysiology of preeclampsia involves both maternal and fetal/placental factors. Abnormalities in the development of placental vasculature early in pregnancy, will lead to placental hypoxia and ischemia, which subsequently generate the release of anti-angiogenic factors into the maternal circulation that alter maternal systemic endothelial function and results to hypertensive syndromes and other manifestations. A variety of pro-angiogenic (VEGF, PIGF) and anti-angiogenic factors (sFlt-1) act on the developing placenta with the balance between them to be considered critical for normal placental development. More than 30 different single nucleotide polymorphisms of VEGF gene have already been identified and many studies have attempted to evaluate their association with the increased risk and predisposition to preeclampsia. Studies are attempting to determine the expression of VEGF in women with preeclampsia, with a view to determine thoroughly its role in pregnancy complications.
It is reported that the levels of VEGF are higher in women with GDM compared to healthy pregnant women, while they were lower in cases with preexisting diabetes [
7]. Pregnancies complicated by GDM are characterized by increased placental expression of VEGF and it is also independently associated to maternal gestational weight gain, defining the concept of “placental diabesity” [
8]. It has also been demonstrated a related dysregulation of VEGF placental receptors expression in GDM and even more in pregnancies with minor degree of glucose intolerance (8). Changes in VEGF and its receptor act on the placenta, resulting in abnormal blood vessel proliferation and chronic hypoxia. It is strongly suggested that if VEGF and its corresponding receptors can be timely balanced at an early stage, the risk of pregnancy complication can be diminished [
9].
In the current study we investigated the genetic association of IL-6, which is a pro-inflammatory marker, in pregnant women with either pre-pregnancy obesity or type 2 diabetes and in pre-eclampsia cases. We also assessed the gene expression of the angiogenic markers VEGF and VEGF receptor gene expression during pregnancy mediated by insulin resistance and abnormal carbohydrate metabolism. Our aim is to support our hypothesis that insulin resistance state that is present to both preexisting diabetes and in obesity exerts the major pathophysiological role for adverse outcomes during gestation such as pre-eclampsia.
2. Materials and Methods
Subjects
We recruited to participate in the study 36 women who were divided into 4 study groups. The pre-eclampsia study group with 8 volunteers, 7 mothers with pre-existing type 2 diabetes to serve the DM2 group, 11 pregnant women who developed gestational diabetes to participate in the GDM group and 10 mothers who did not have any problems in pregnancy they served as the control group).
All women had given their signed consent for the participation in the study prior to their involvement.
Measurements
Muscle tissue biopsies were performed in the rectus abdominis muscles in the case of caesarean section and from the perineum muscles when given birth with normal vaginal delivery. All collected samples were then transported in sterile tubes and stored at −70 ℃ immediately after sampling.
RNA Isolation and cDNA Synthesis by Reversetranscription PCR (RT-PCR)
RNA extraction was performed using the commercial kit Nucleospin®RNA Plus (Macherey-Nagel). cDNA was synthesized using Luna Script® RT SuperMix Kit (New England Biolabs). A 5 μL aliquot of purified RNA was added to 4 μL of the Luna Script® RT SuperMix Kit. The reaction was performed in a total volume of 20 μl. The RT-PCR was performed by combining cDNA, Platinum Blue PCR Super Mix (Invitrogen, Paisley, UK) and the forward and reverse primers for VEGF, and VEGFR. The forward and reverse primer sequences used are shown in the following sequence table. Beta actin (β-actin), a housekeeping gene, was used as internal control. Relative quantification of gene expression was calculated using the ΔΔCT method. Analysis of relative gene expression data was performed using quantitative real-time PCR and method 2 (−Delta Delta C(T)) The reaction was continued for 40 cycles under the following thermal conditions: 95˚C for 5 minutes (initial denaturation), followed by 40 cycles of 45 seconds at 95˚C (denaturation), 45 seconds at 60˚C (annealing) and 45 seconds at 72˚C (extention). Negative controls (Nontemplate water instead of cDNA) were also included to ensure lack of reagent DNA contamination.
Sequence Table
The sequences, annealing temperatures, and product sizes of the primers used to amplify genes of interest.
Biomedical Ethics Issues
The collection of clinical data was correlated with the laboratory research results and was conducted in such a way as to fully guarantee the patients’ anonymity and personal data confidentiality.
Statistical Analysis
Quantitative variables were tested for normality of distribution with the Kolmogorov-Smirnov test. Variables with a normal distribution are expressed as mean values and standard deviations (Standard Deviation = SD), while those following a normal distribution are expressed as median values and interquartile ranges (interquartile range). Absolute (N) and relative (%) frequencies were used to describe qualitative variables. Comparison of proportions was performed with Fisher’s exact test where appropriate. For the comparison of quantitative variables between more than two groups, the parametric test of analysis of variance (ANOVA) or the non-parametric Kruskal-Wallis test was used. Testing of the relationship between pairs of quantitative variables was assessed with Spearman’s correlation coefficient (rho). Significance levels were two-sided and statistical significance was set at 0.05. The statistical program SPSS 22.0 was used for the analysis. Polymerase chain reaction (Real time PCR), agarose gel electrophoresis and Restriction Fragment Length Polymorphism (RFLP) were used to process the samples.
3. Results
-
A)
Analysis of the -174 G/C polymorphism of the IL-6 gene
We investigated the occurrence rates of the GG, GC and CC genotypes of the -174 G/C polymorphism of the IL-6 gene in the muscle tissue of the volunteers.
The majority (80.6%) of the women were carrying the GG genotype (
Table 1). Women with preeclampsia, and with type 2 diabetes or gestational diabetes predominantly expressed the GG genotype. In contrast, the occurrence rate of the CC genotype was significantly higher in the control group with a normal pregnancy (50%).
Further analysis was performed on women with gestational diabetes mellitus, dividing to those treated with diet only or with insulin. Even more the control group with normal pregnancies, were compared according to whether they had a normal or elevated BMI (
Table 2). According to the results, women with gestational diabetes mellitus predominantly expressed the GG genotype regardless of diet or insulin intake. In contrast, of particular interest is the finding that among women with normal pregnancies, those with a normal BMI expressed the CC genotype at a rate of 100%, while the obese carried the GG genotype at a rate of 100%.
-
B)
Analysis of VEGF and VEGF-R gene expression
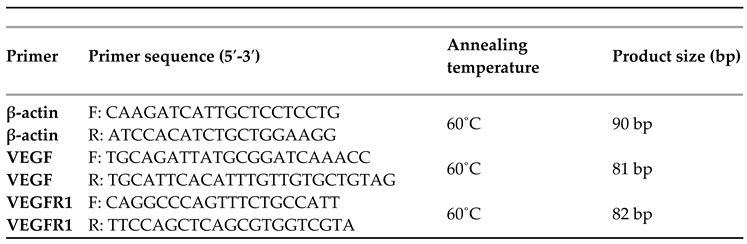
VEGF and VEGF-R receptor gene expression were quantified and compared between groups of women participating in the study. This analysis was performed on the women’s muscle tissue, obtained during childbirth. Expression of both the VEGF gene and the VEGF-R receptor differed significantly between the 4 study groups (
Table 3,
Figure 1). According to the pairwise comparison of VEGF expression in the study groups of women, its levels were significantly lower in women with preeclampsia compared to women with type 2 diabetes mellitus (p=0.001), with gestational diabetes mellitus (p <0.001), as well as compared to women with a normal pregnancy (p<0.001). In addition, women with type 2 diabetes had significantly lower VEGF expression compared to those with gestational diabetes (p=0.012) and normal pregnancies (p=0.015). There was no significant difference in VEGF expression between women with gestational diabetes mellitus and those with normal pregnancy.
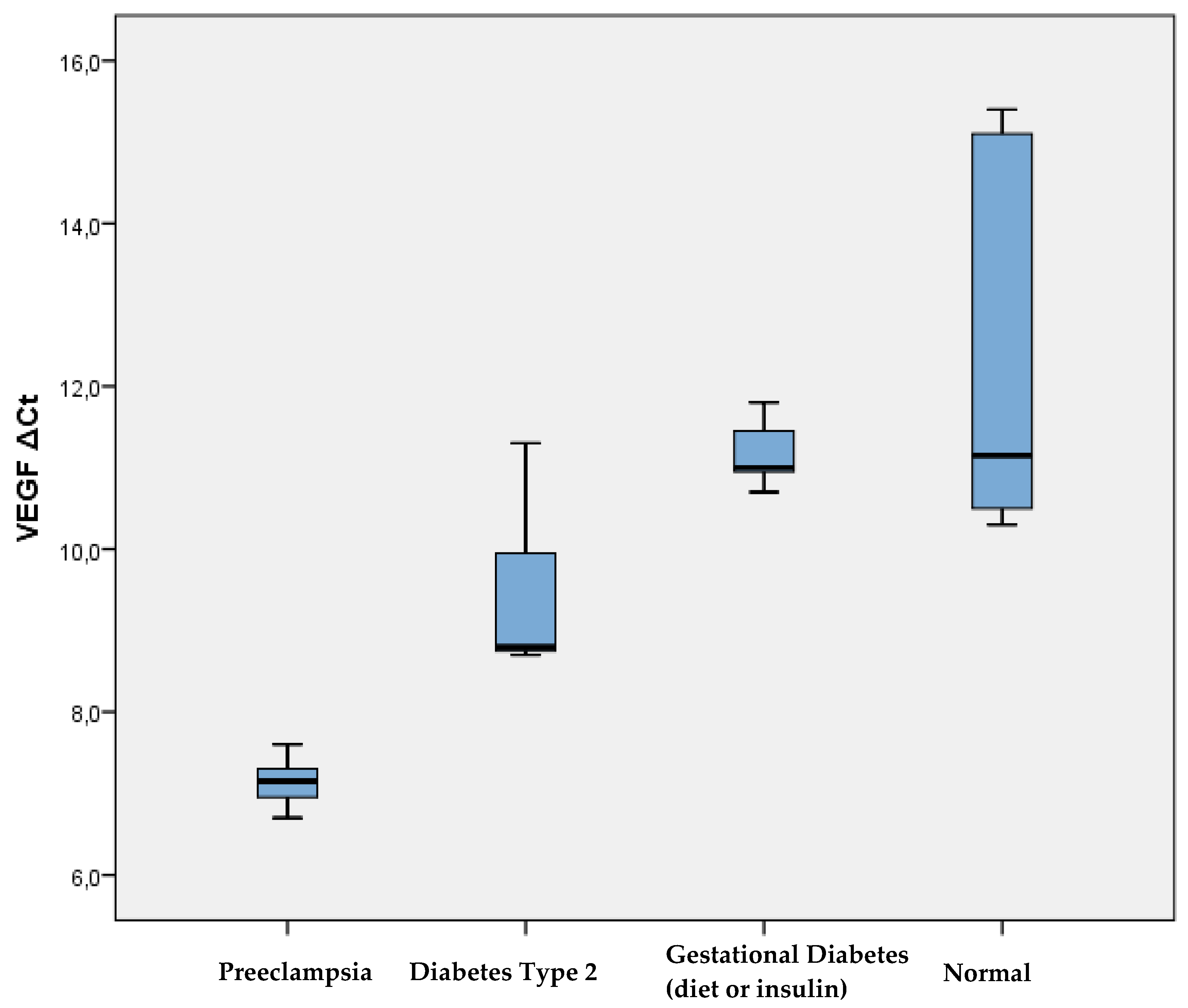
Further comparison between the 6 study patient groups showed significantly lower expression of VEGF in women with preeclampsia compared to those with type 2 diabetes mellitus (p=0.001), with gestational diabetes mellitus on diet (p=0.003), with gestational diabetes mellitus women on insulin (p=0.002), as well as to women with a normal pregnancy (p=0.003) and to women with a normal pregnancy and increased BMI (p=0.003) (
Table 4,
Figure 2). In addition, women with type 2 diabetes mellitus had significantly lower levels of VEGF expression compared to women with gestational diabetes mellitus who were on diet (p=0.027), with gestational diabetes mellitus on insulin (p=0.043), as well as to those with a normal pregnancy (p=0.012). VEGF expression had no significant difference between women with gestational diabetes mellitus either on diet or on insulin. In contrast, women with normal pregnancy and increased BMI had significantly lower VEGF expression compared to women with normal pregnancy who were not obese (p=0.047).
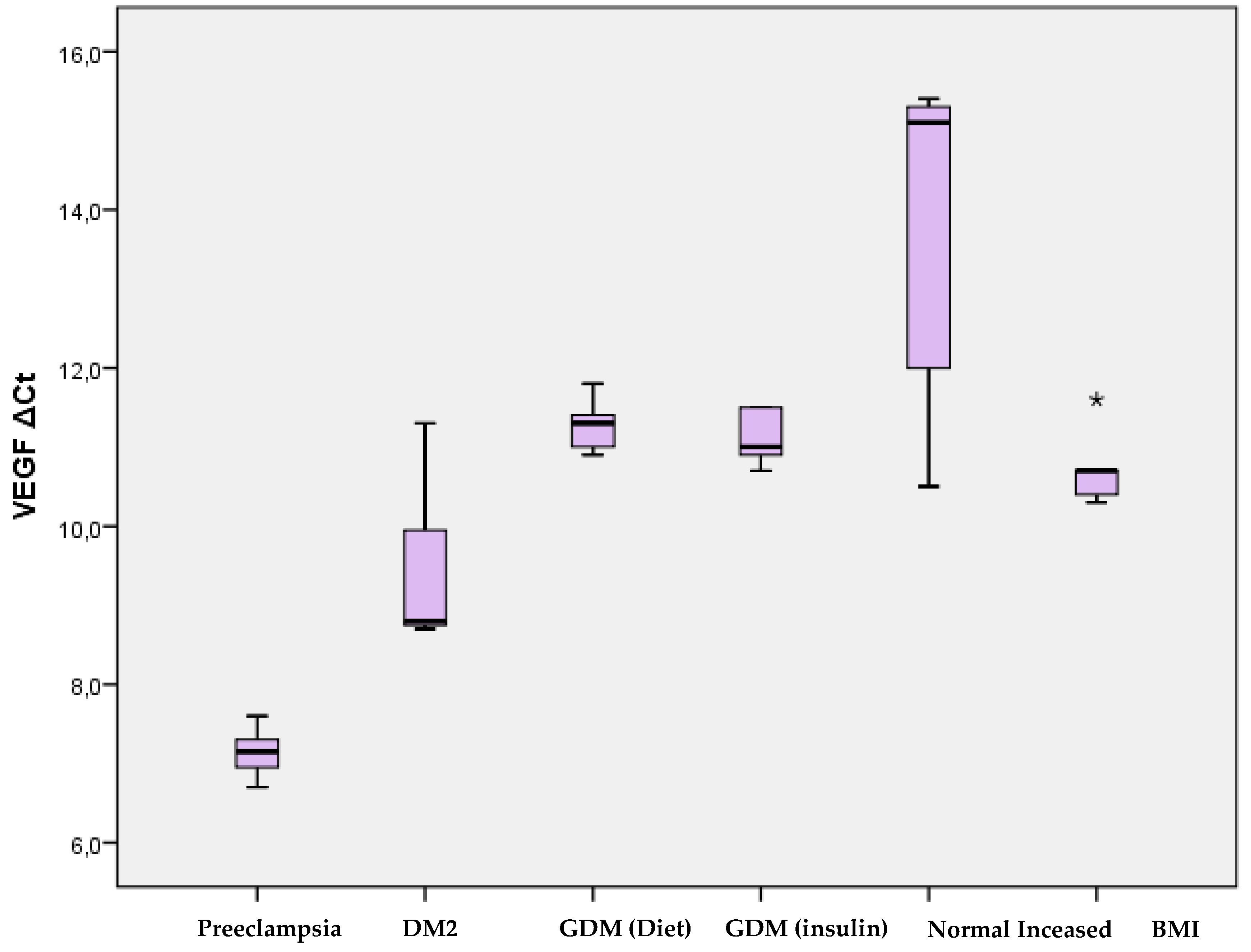
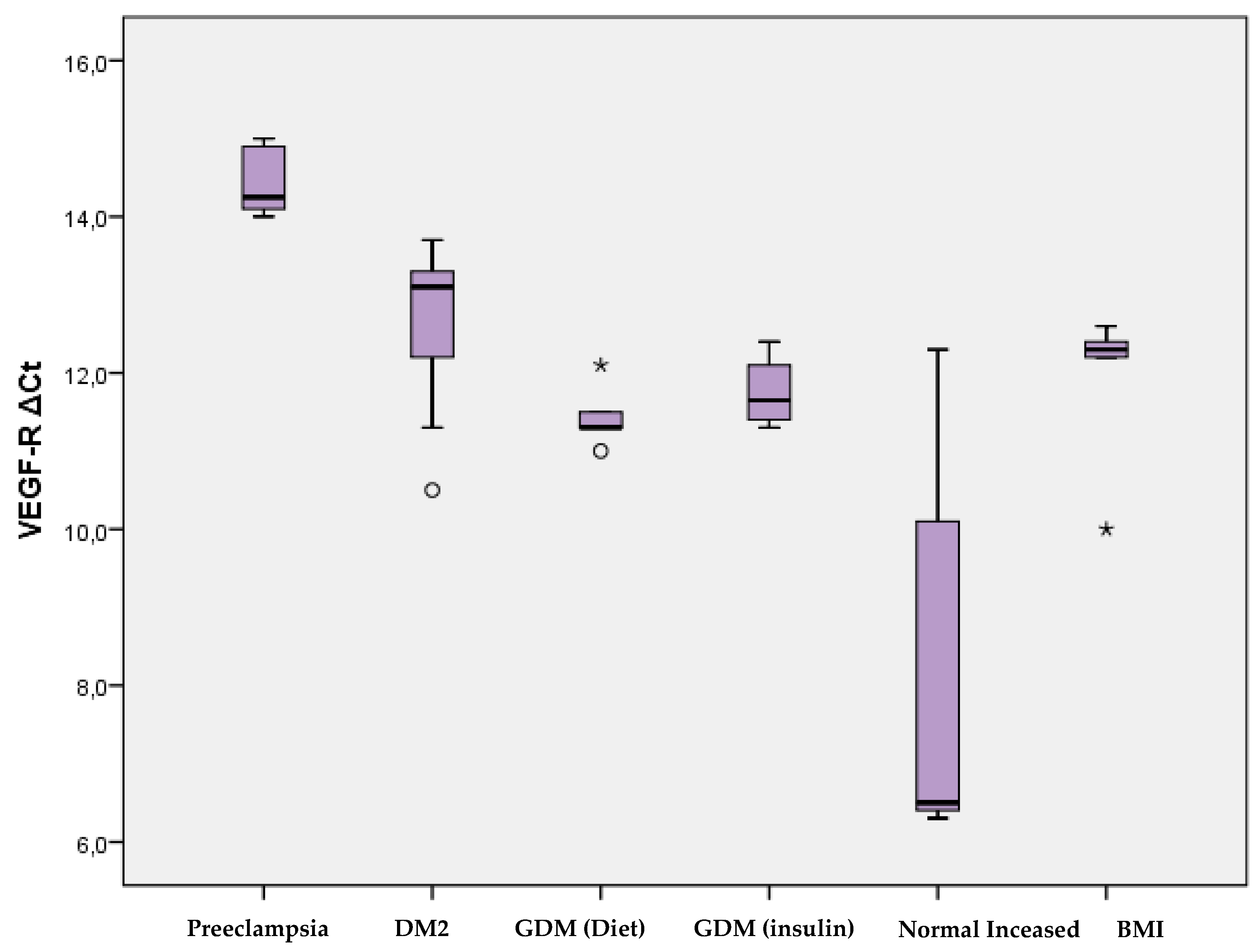
Regarding the expression of the VEGF-R receptor, significantly higher levels were observed in women with preeclampsia compared to women with type 2 diabetes mellitus (p=0.001), with gestational diabetes mellitus (p<0.001), and with normal pregnancy (p <0.001). In addition, women with type 2 diabetes mellitus had significantly higher levels of VEGF-R expression compared to women with normal pregnancy (p=0.015). There was no significant difference in VEGF-R expression between women with gestational diabetes mellitus and those with normal pregnancy (
Table 3,
Figure 3).
According to the analysis in the 6 groups of women in the study, significantly higher levels of VEGF-R expression were recorded in women with preeclampsia compared to women with type 2 diabetes mellitus (p=0.001), with gestational diabetes mellitus on diet (p= 0.003) and on insulin (p=0.002), as well as to women with normal pregnancy and normal BMI (p=0.003), but also with normal pregnancy and increased BMI (p=0.003). Women with type 2 diabetes had significantly higher expression of VEGF-R than women with normal pregnancy (p=0.012). Of particular interest is the observation that the expression of VEGF-R is similar among women with gestational diabetes mellitus treated with diet and those treated with insulin. In contrast, expression levels differ dramatically in women with normal versus obese pregnancies, with significantly higher levels in those with high BMI (
Table 3,
Figure 4).
4. Discussion
The sample of our study consisted of 36 pregnant women who were divided into 4 groups (8 women who developed preeclampsia, 7 who suffered from pre-existing type 2 diabetes mellitus, 11 who developed gestational diabetes and 10 who did not have any problems during pregnancy and served as the control group). The analysis of the gene expression of the VEGF factor and the VEGFR receptor in the groups of women leads to the following findings: In consistency with available literature data, VEGF and VEGF-R expression is inversely proportional. Most studies agree that in pathological conditions, such as pregnancies complicated by preeclampsia or Type 2 Diabetes Mellitus, low expression of VEGF and high expression of the VEGF-R receptor are observed [
10]. Likewise, our study supports that the expression of VEGF is higher, while that of VEGF-R is lower mainly in pregnant women with preeclampsia but also in those with preexisting Diabetes Mellitus, compared to women with a normal pregnancy. It should be noted that no statistically significant difference is found between women with gestational diabetes treated with insulin or diet alone. The above data support the theory that the VEGF factor plays an important role for normal angiogenesis in the placenta, while in conditions of hypoxia and hypoperfusion, such as in preeclampsia, its production decreases and VEGF-R levels increase reactively. There is also a huge effect of obesity, with women who are not obese having very high expression of the VEGF factor and very low expression of the VEGF-R receptor. A correspondingly significant difference in the expression of the factors also results from the comparison between pregnant women with a normal BMI and those with a high BMI, with higher levels of VEGF-R expression in obese women, that approach the levels of VEGF-R in women with Gestational Diabetes Mellitus. These results lead to the conclusion that obesity in pregnancy reflects a state of disturbed angiogenesis and that the genetic profile of the obese pregnant woman refers to that of the diabetic pregnant woman, creating a background for complications in pregnancy.
Interesting findings also arise from the analysis for the presence of the -174 G/C polymorphism of the IL-6 gene in the study sample: Regarding the presence of the polymorphism in muscle tissue, the most frequent genotype is GG in all groups of women, except for women with normal pregnancy and normal BMI, who express the CC genotype at a rate of 100%. This reinforces the theory that the C allele probably functions protectively, while on the contrary the G allele (and especially in its homozygous form) is associated with the occurrence of problems in pregnancy. It is worth noting that obesity, even in women who complete a normal pregnancy, seems to be associated with an unfavorable gene profile of IL-6 with an increased frequency of the G allele, which is found in women who experience problems in pregnancy, as we found in our study. The above findings are in accord with Pacheco-Romero et al., 2021 [
11], who showed that the GG genotype and the G allele appeared to be associated with the risk for preeclampsia. Also, the study by Sowmya et al., 2015 [
12] showed a strong association of the polymorphism with preeclampsia and suggested that it may be an important genetic regulator in the etiology of premature preeclampsia. The meta-analysis by Veisian et al., 2020 [
13] showed that the interleukin-6 -174 G/C polymorphism was associated with an increased risk for preeclampsia in Asians, but not in Caucasians and mixed populations. This observation may reflect differences in the genetic profile of the populations. However, data for Caucasians are too limited to draw reliable conclusions. Also, there are no previous data for the Greek population, as this is the first study.
The results of the study are consistent with the theory that complicated pregnancies are characterized by a disturbance in the balance between pro-angiogenic and anti-angiogenic factors, and by an inflammatory environment resulting in the induction of systemic endothelial dysfunction with possible adverse effects. Genetic modifications, either in the presence of inherited polymorphisms or at the level of gene expression, may be potential risk factors or predisposing factors for the occurrence of Preeclampsia and pregnancy disorders.
Given the relatively high incidence of Gestational Diabetes Mellitus and Preeclampsia in the general population and the potential adverse consequences on pregnancy outcome, elucidating the underlying mechanisms and identifying potential biomarkers for increased risk of pregnancy disorders is of paramount importance and warrants further research.
As with most similar studies conducted in women with pregnancy complications, the present study has as its main limitation the relatively small sample size. However, it is important to note that the study was conducted in a homogeneous population of European descent, a characteristic that is a key advantage in genetic studies.
5. Conclusions
The present study supports the hypothesis that the vascular endothelial factor VEGF plays an important role for normal placental development, as its expression is reduced, with a simultaneous increase in the expression of the VEGF-R receptor, in complicated pregnancies such as Preeclampsia and in pregnancies with pre-existing Diabetes Mellitus Type 2. This observation is consistent with the fact that both conditions are characterized by increased insulin resistance and share a common hemodynamic profile. Furthermore, similar findings are observed in pregnant women with an increased body mass index, leading to the conclusion that the genetic profile of obese pregnant women has a common background with that of the diabetic ones.
As for the analysis of interleukin-6 polymorphisms, it appears that the G allele is associated with the occurrence of pregnancy complications, either in the form of diabetes mellitus or in the form of preeclampsia. It is interesting to observe that obese pregnant women also carry the same polymorphism, supporting its possible contribution to the occurrence of problems during pregnancy.
Abbreviations
IL-6: interleukin 6, VEGF: vascular endothelial growth factor, VEGF-R: endothelial growth factor receptor, BMI: body mass index, PCR: polymerase chain reaction, RFLP: restriction fragment length polymorphism, sFlt-1: soluble fms -like tyrosine kinase-1
Author Contributions
Conceptualization, P.H., T.TS. D.H; methodology, P.H., T.TS. D.H; validation, P.H., T.TS. D.H, G,TS.; formal analysis, P.H., T.TS. D.H, I.TS.; investigation, T.TS, V.TS,L.P, O.P.; resources, G.TS, D,H, data curation, T.TS, D.H.; writing—original draft preparation, T,TS, P.H; writing—review and editing, D.H.G.TS; visualization, I.TS, O.P, L.P.; supervision, P.H.; project administration, P.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki, and it was approved by the ethics committee of University General Hospital Ethical Committee with the protocol number 1235 (15 April 2020).
Informed Consent Statement
Written informed consent has been obtained from the patients to participate in this study.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships, that could be construed as a potential conflict of interest.
References
- Chu SY, Kim SY, Bish CL. Prepregnancy obesity prevalence in the United States, 2004-2005. Matern Child Health J 2009; 13: 614-20; PMID:18618231. [CrossRef]
- Gibson KS, Waters TP, Catalano PM. Maternal weight gain in women who develop gestational diabetes mellitus. Obstet Gynecol 2012; 119:560- 5; PMID:22353954. [CrossRef]
- Tutar D, Cintesun F N I, et al.: The association of interleukin-6, interleukin-27, and body roundness index with gestational diabetes mellitus J Obst Gynaecology 2022, 42:6. [CrossRef]
- Lob S, Knabl J, et al.: Obesity in pregnancy is associated with macrophage influx and an upregulated GRO-alpha and IL-6 expression in the decidua J Reprod Immunol 2023 Mar:156:103800. [CrossRef]
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 2005; 308:1592. [CrossRef]
- Germain SJ, Sacks GP, Sooranna SR, et al. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol 2007; 178: 5949. [CrossRef]
- Dong PP. Association of vascular endothelial growth factor expression and polymorphisms with the risk of gestational diabetes mellitus. J Clin Lab Anal 2019; 33: e22686. [CrossRef]
- Sirico A, Rossi ED, Degennaro VA, et al. Placental diabesity: placental VEGF and CD31 expression according to pregestational BMI and gestational weight gain in women with gestational diabetes. Arch Gynecol Obstet. 2023 Jun;307(6):1823-1831. [CrossRef]
- Marini M, Vichi D, Toscano A, et al. Effect of impaired glucose tolerance during pregnancy on the expression of VEGF receptors in human placenta Reproduction, Fertility and Development 20(7). [CrossRef]
- Bolatai, A., He, Y. & Wu, N. Vascular endothelial growth factor and its receptors regulation in gestational diabetes mellitus and eclampsia. J Transl Med 20, 400 (2022). [CrossRef]
- Pacheco-Romero J, Acosta O, Huerta D, et al. Genetic markers for preeclampsia in Peruvian women. Colomb Med (Cali). 2021; 52(1): e2014437 https://doi.org/10.25100%2Fcm.v52i1.4437.
- Sowmya S, Ramaiah A, Nallari P et al. Role of IL-6-174 (G/C) promoter polymorphism in the etiology of early-onset preeclampsia. Inflamm Res. 2015 Jun; 64(6): 433-9. [CrossRef]
- Veisian M, Javaheri A, Amjadi N, et al. Association of IL-6 -176G > C polymorphism with susceptibility to preeclampsia: a systematic review and meta-analysis. Fetal Pediatr Pathol. 2020 Dec; 39(6): 491-502. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).