Submitted:
07 May 2024
Posted:
08 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Schiff Base-Based New Thiocarbohydrazones (1-10)
3. Results and Discussion
3.1. Physical Properties
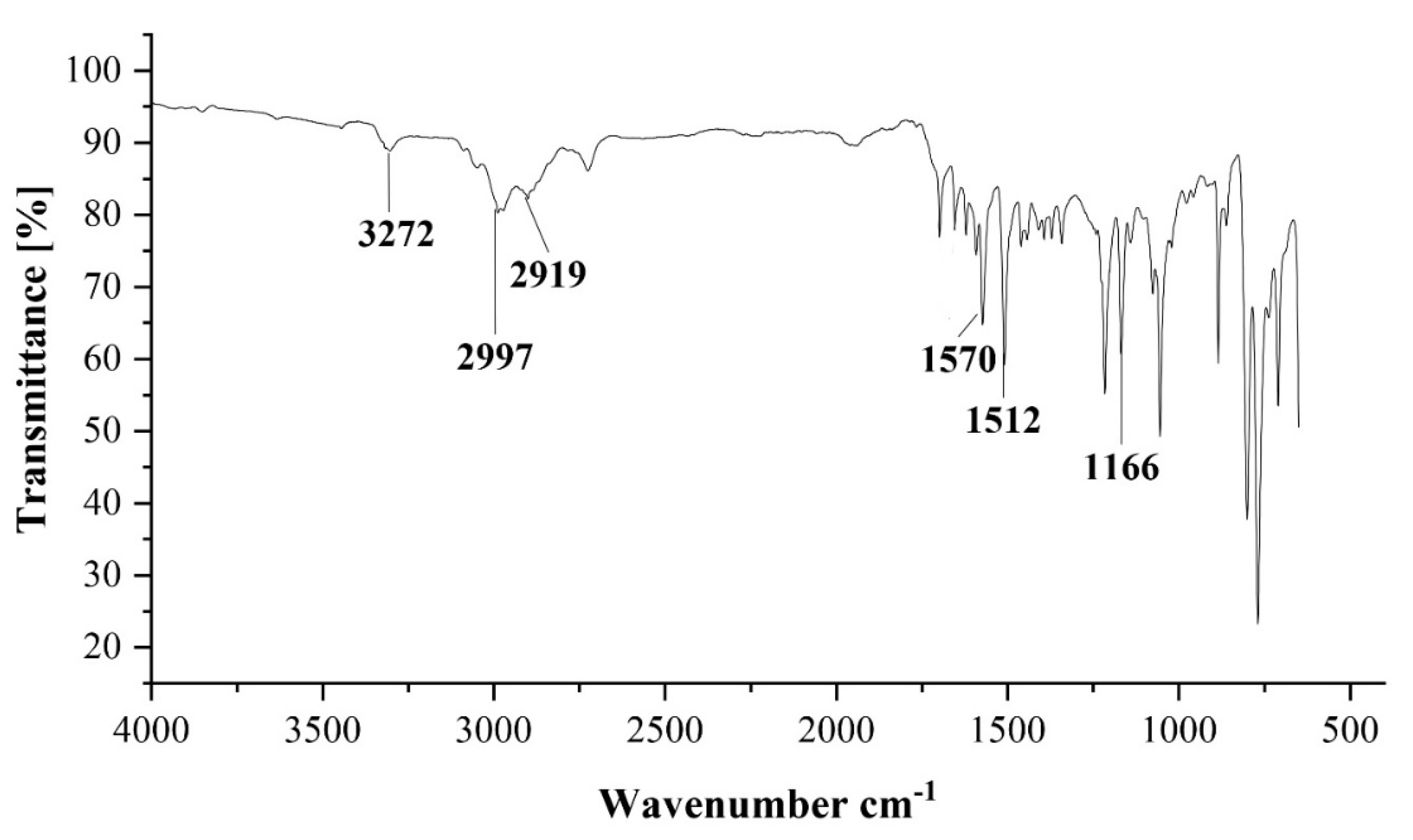
3.2. Vibration Frequency Ranges
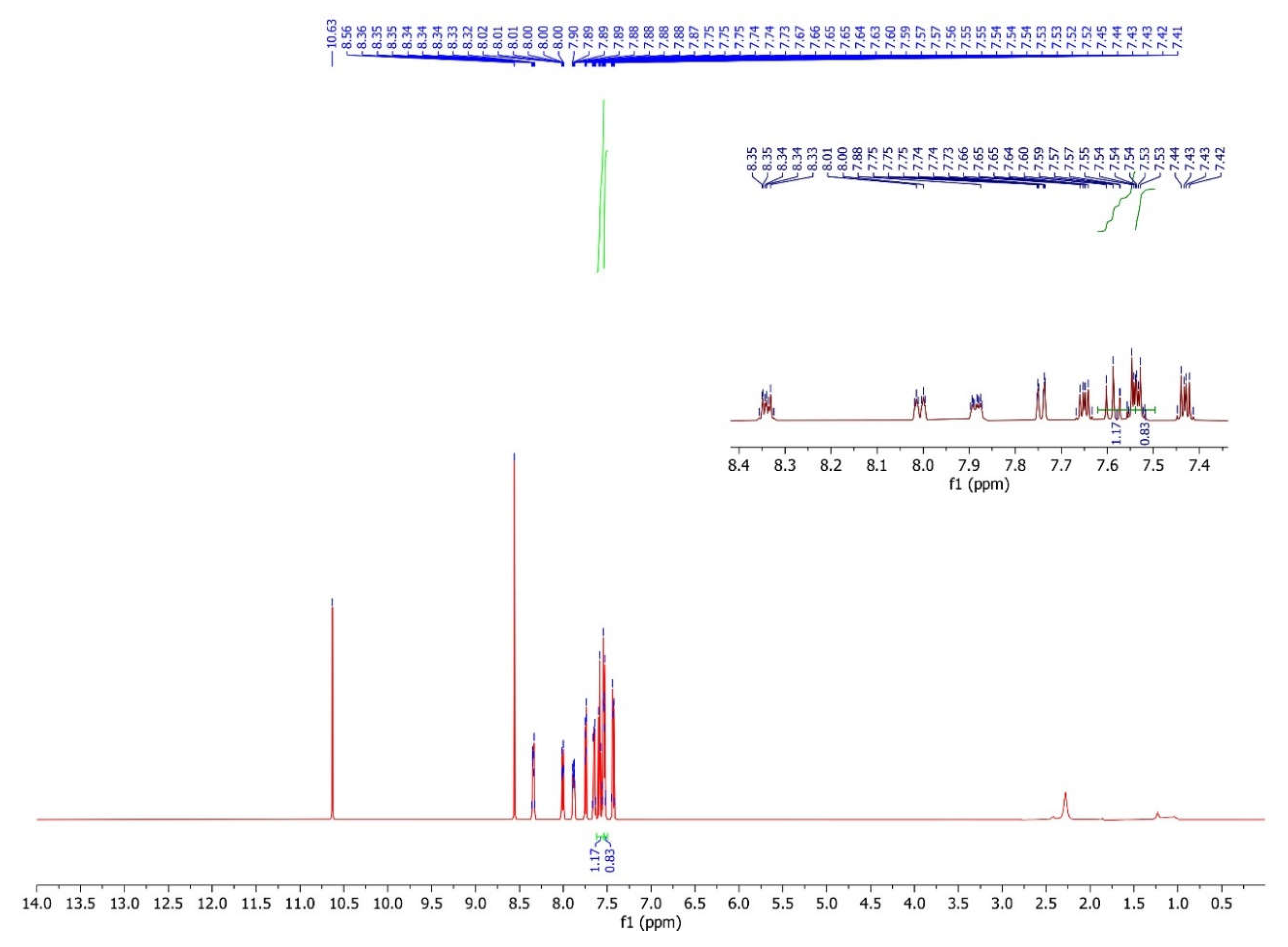
3.3. The Interpretation of the 1H Nuclear Magnetic Resonance Spectrum
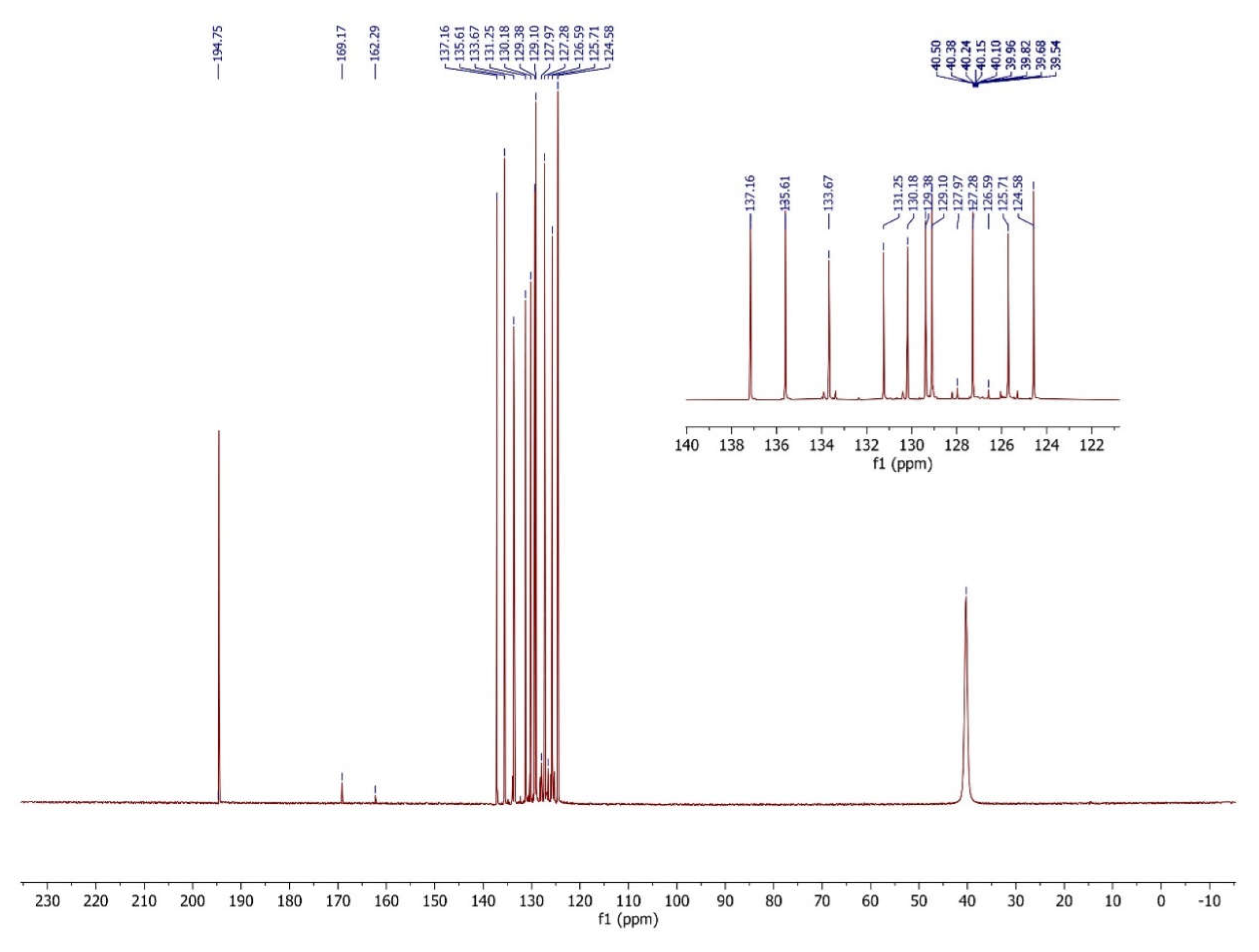
3.4. The interpretation of the 13C Nuclear Magnetic Resonance Spectrum
3.5. Analysis of the UV–Vis Spectra
3.6. Thermal Properties
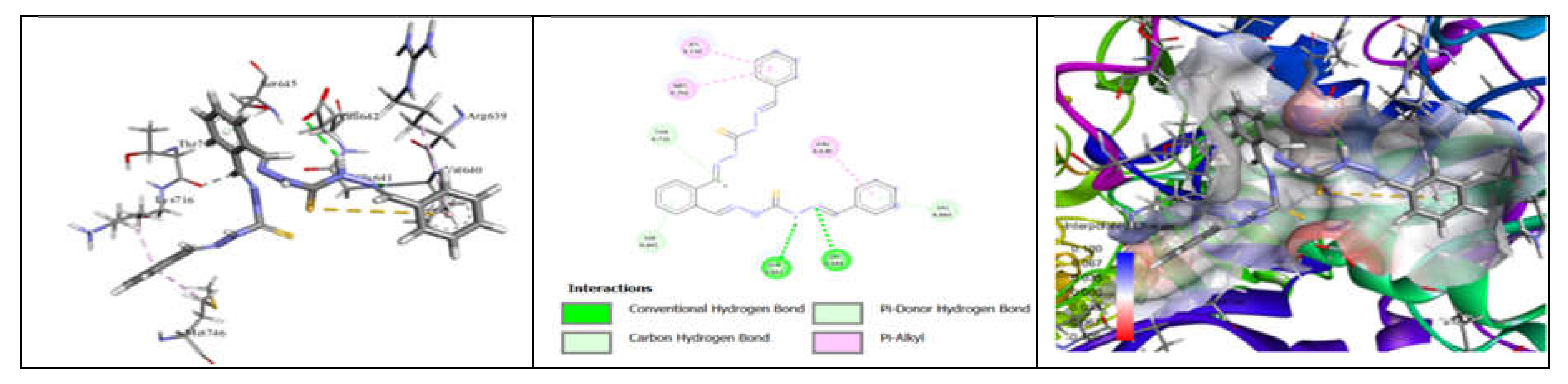
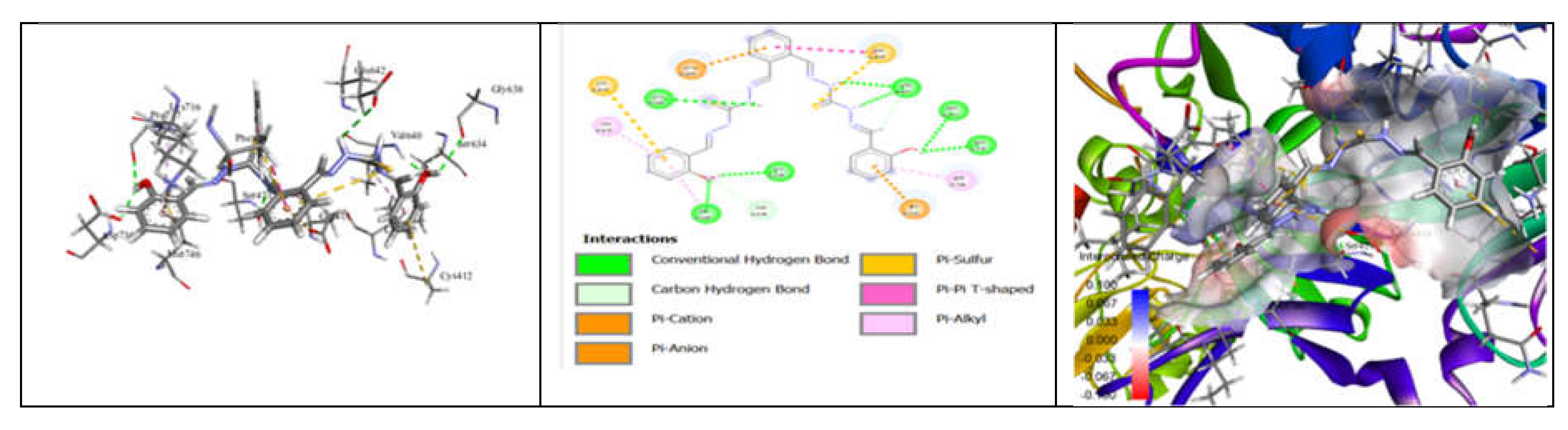
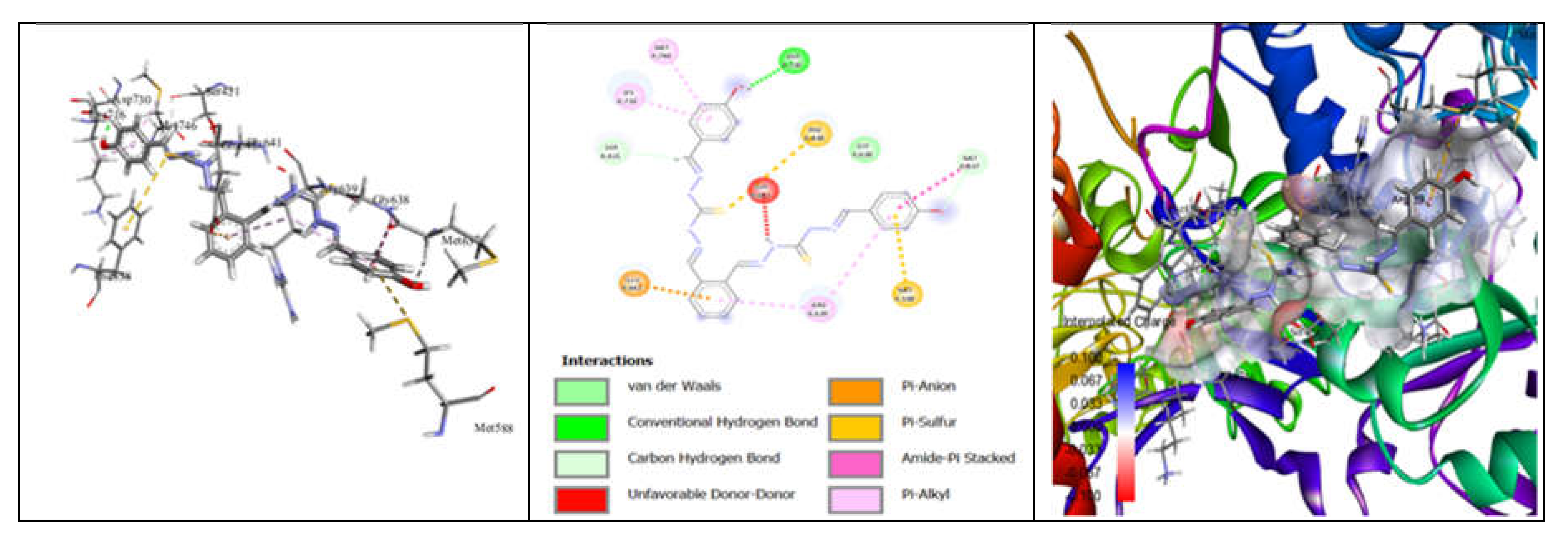
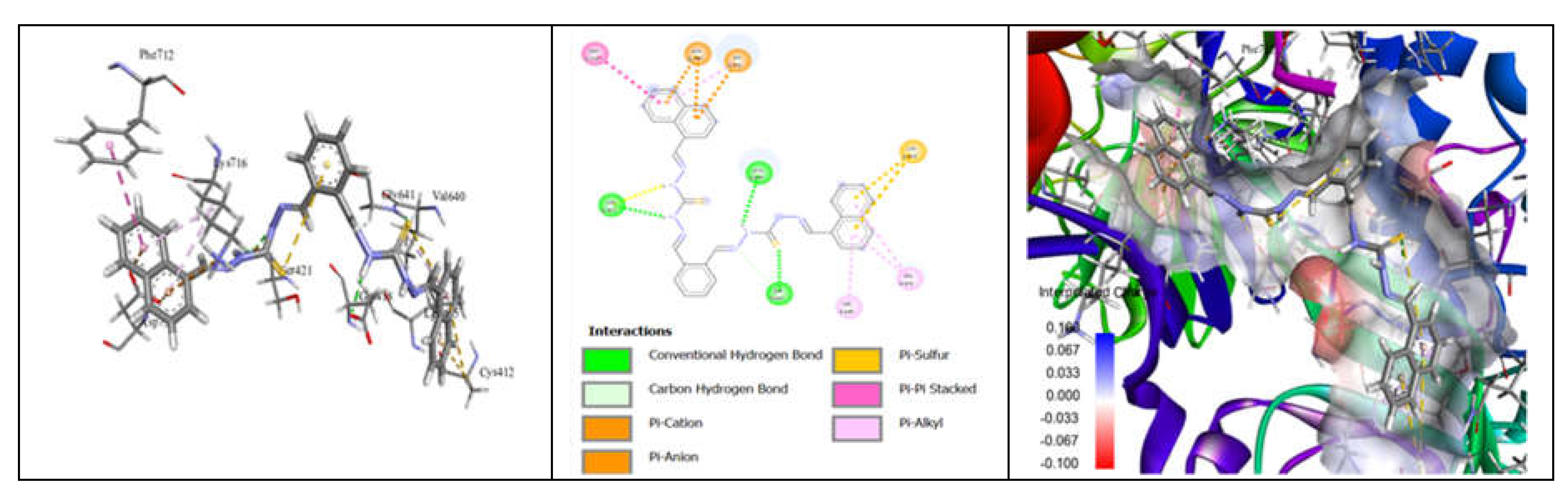
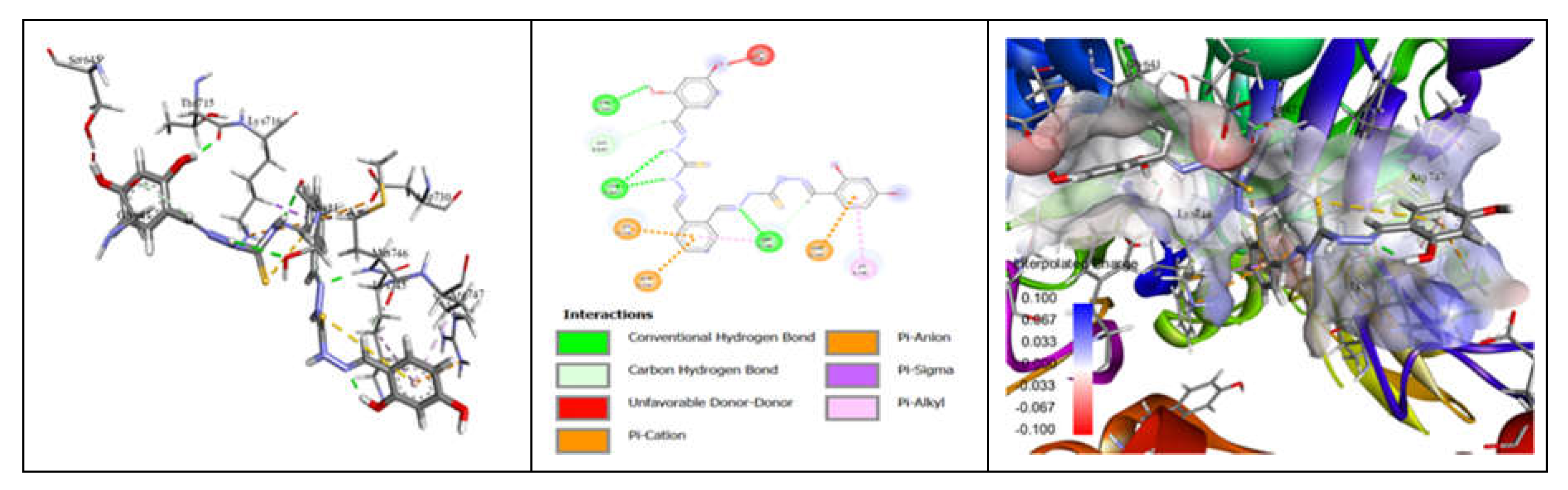
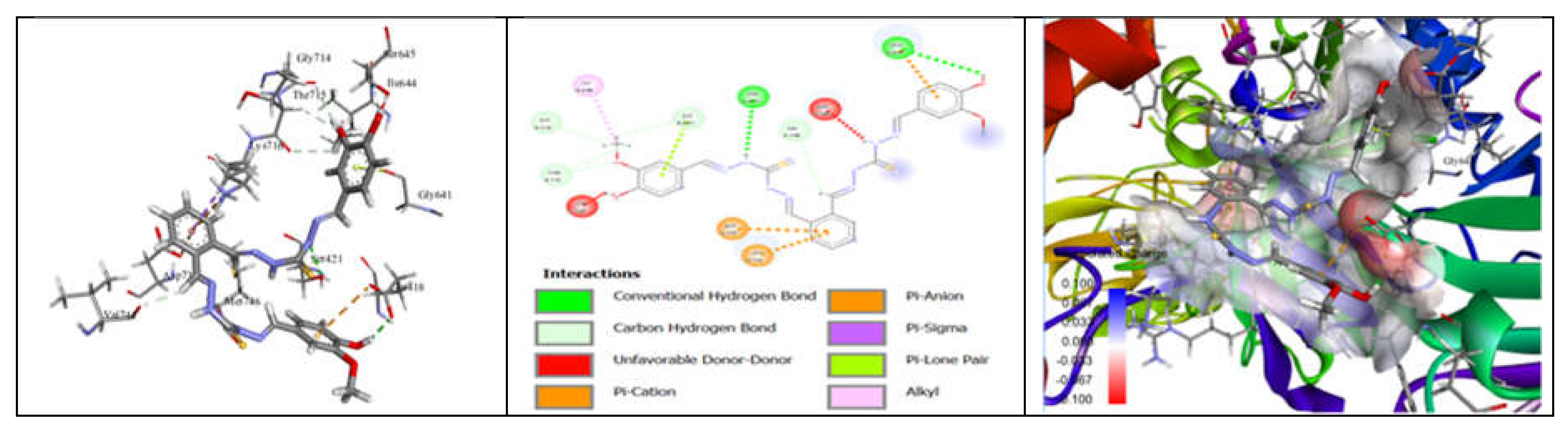
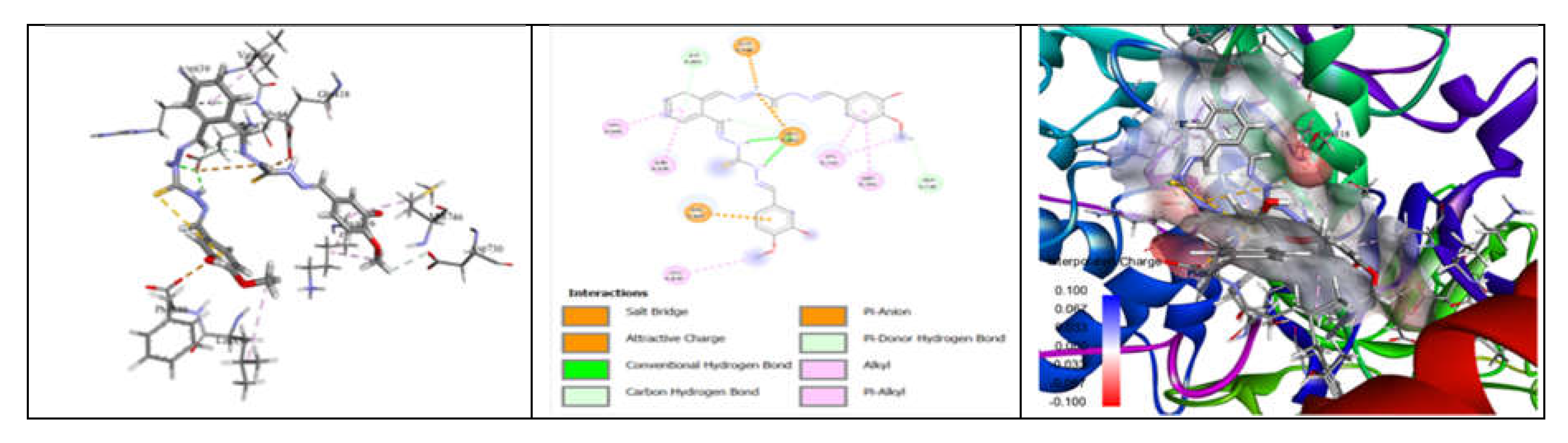
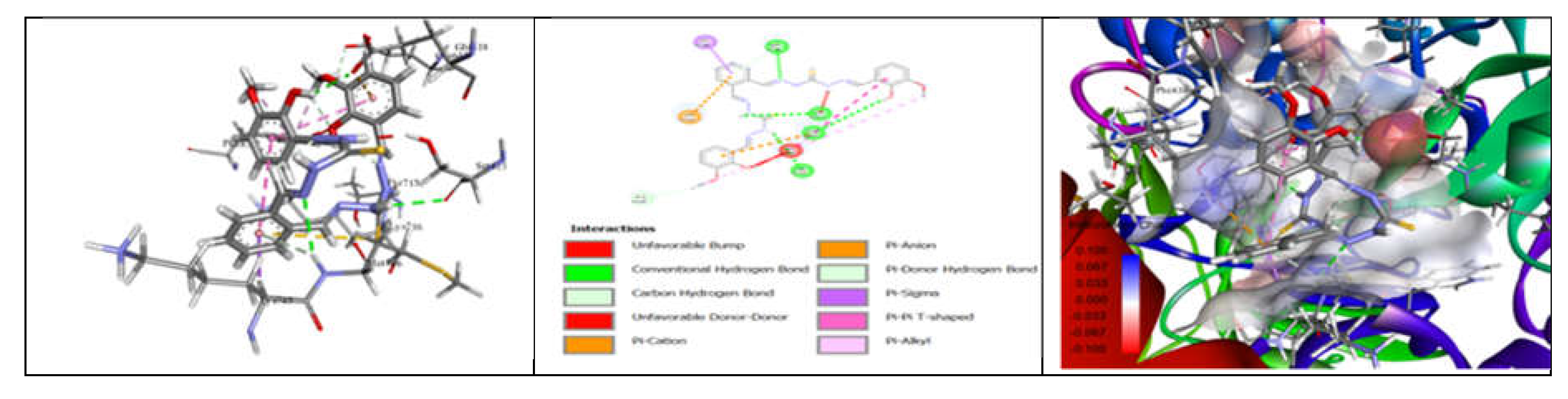
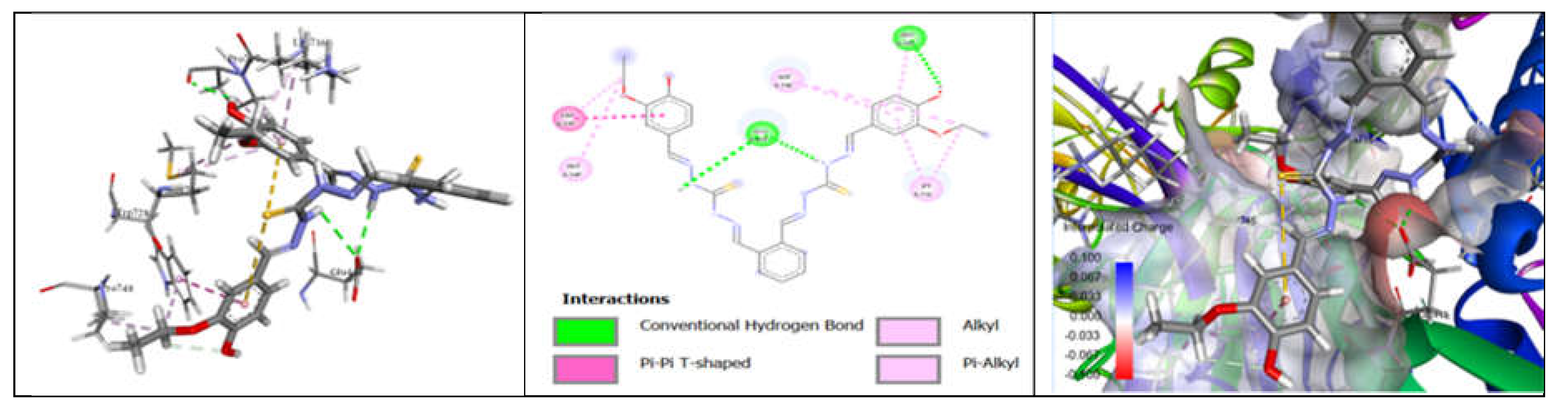
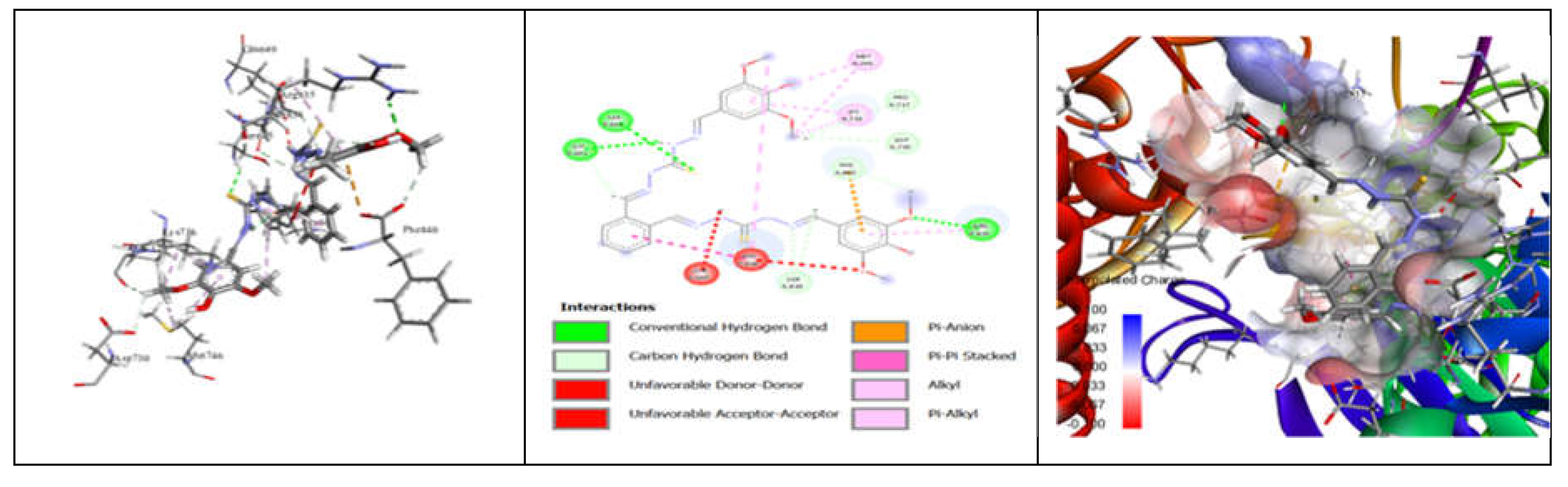
3.7. Molecular Docking and Urease Inhibition
| Comp. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | thiourea |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MolDock Score | -160 | -171 | -175 | -189 | -161 | -177 | -170 | -153 | -175 | -186 | - |
| IC50 (µg/mL) | 88.11±1.95 | 39.00±1.17 | 39.86±1.04 | 20.79±0.68 | 39.89±0.40 | 31.28±0.00 | 45.78±0.40 | 84.97±1.07 | 38.01±0.37 | 27.11±0.00 | 37.13±1.38 |
3.8. Pharmacokinetic Properties
| Comp. | Physicochemical Properties | Pharmacokinetic Properties | Drug-likeness | Lipophilicity | Solubility | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M.wt | TPSA | HBA | HBD | GI absorption | Log Kp | BBB | Druglike score | MolLogP | MolLogS (mg/L) | |
| 1 | 486.62 | 146.1 | 4 | 4 | Low | -6.04 | 2.05 | -1.27 | 6.55 | 0.17 |
| 2 | 518.61 | 202.2 | 6 | 6 | Low | -6.74 | 1.50 | -1.05 | 6.94 | 0.31 |
| 3 | 518.61 | 202.2 | 6 | 6 | Low | -6.74 | 1.17 | -0.74 | 5.42 | 1.41 |
| 4 | 568.73 | 161.74 | 4 | 4 | Low | -4.87 | 1.03 | -0.98 | 9.26 | 0.05 |
| 5 | 550.61 | 242.66 | 8 | 8 | Low | -7.44 | 1.08 | -0.64 | 5.91 | 0.74 |
| 6 | 587.67 | 220.66 | 8 | 6 | Low | -7.15 | 1.00 | -0.77 | 6.40 | 0.79 |
| 7 | 587.67 | 220.66 | 8 | 6 | Low | -7.15 | 1.00 | -0.75 | 7.10 | 0.69 |
| 8 | 587.67 | 220.66 | 8 | 6 | Low | -7.15 | 1.00 | -0.71 | 6.59 | 0.66 |
| 9 | 606.72 | 220.66 | 8 | 6 | Low | -6.8 | 0.93 | -0.40 | 7.59 | 0.72 |
| 10 | 638.72 | 239.12 | 10 | 6 | Low | -7.55 | 0.87 | -0.61 | 4.62 | 28.80 |
| Rule | ≤500 | <140 | <10 | <5 | - | -(3-5) | 0-6 | - | <5 | - |
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Da Silva, C.M.; Da Silva, D.L.; Modolo, L. V.; Alves, R.B.; De Resende, M.A.; Martins, C.V.B.; De Fátima, Â. Schiff Bases: A Short Review of Their Antimicrobial Activities. Journal of Advanced Research 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Abou-Melha, K.S.; Faruk, H. Bimetallic Complexes of Schiff Base Bis-[4-Hydroxycuomarin-3-Yl]- 1N,5N-Thiocarbohydrazone as a Potentially Dibasic Pentadentate Ligand. Synthesis, Spectral, and Antimicrobial Properties. Journal of the Iranian Chemical Society 2008, 5, 122–134. [Google Scholar] [CrossRef]
- Ahamad, I.; Prasad, R.; Quraishi, M.A. Experimental and Quantum Chemical Characterization of the Adsorption of Some Schiff Base Compounds of Phthaloyl Thiocarbohydrazide on the Mild Steel in Acid Solutions. Materials Chemistry and Physics 2010, 124, 1155–1165. [Google Scholar] [CrossRef]
- Aly, A.A.; Abdallah, E.M.; Ahmed, S.A.; Awad, M.K.; Rabee, M.M.; Mostafa, S.M.; Bräse, S. Metal Complexes of New Thiocarbohydrazones of Cu(I), Co(II), and Ni(II); Identification by NMR, IR, Mass, UV Spectra, and DFT Calculations. Journal of Sulfur Chemistry 2023, 44, 282–303. [Google Scholar] [CrossRef]
- EL-Mahdy, K.M.; El-Kazak, A.M.; Abdel-Megid, M.; Seada, M.; Farouk, O. Synthesis, Characterization and Antimicrobial Activities of Some New Heterocyclic Schiff Bases Derived from Thiocarbohydrazide. Acta Chimica Slovenica 2016, 63, 18–25. [Google Scholar] [CrossRef]
- Gupta, A.K.; Prachand, S. Thiol Derivatives and Antifungal Activity. International Journal of Pharmacy & Life Sciences 2012, 3, 1848–1857. [Google Scholar]
- Hameed, A.; al-Rashida, M.; Uroos, M.; Abid Ali, S.; Khan, K.M. Schiff Bases in Medicinal Chemistry: A Patent Review (2010-2015). Expert Opinion on Therapeutic Patents 2017, 27, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Kajal, A.; Bala, S.; Kamboj, S.; Sharma, N.; Saini, V. Schiff Bases: A Versatile Pharmacophore. Journal of Catalysts 2013, 2013, 1–14. [Google Scholar] [CrossRef]
- Kalem, E.; Ağar, E. Schiff Bazlarinin Biyolojik Aktivitesi Biological Activity of Schiff Bases Öz : Abstract : 2021, 8, 57–76.
- Khan, S.A.; Kumar, P.; Joshi, R.; Iqbal, P.F.; Saleem, K. Synthesis and in Vitro Antibacterial Activity of New Steroidal Thiosemicarbazone Derivatives. European Journal of Medicinal Chemistry 2008, 43, 2029–2034. [Google Scholar] [CrossRef]
- Manjunatha, M.; Naik, V.H.; Kulkarni, A.D. Activities , and Spectroscopic Studies of Co ( II ), Ni ( II ), and Cu ( II ) Complexes of Biologically Potential Coumarin Schiff Bases. 2011, 64, 4264–4275.
- Rana, K.; Pandurangan, A.; Singh, N.; Tiwari, A.K. A Systemic Review of Schiff Bases as an Analgesic, Anti-Inflammatory. Academic Sciences 2012, 4, 5–11. [Google Scholar]
- Sathisha, M.P.; Revankar, V.K.; Pai, K.S.R. Synthesis, Structure, Electrochemistry, and Spectral Characterization of Bis-Isatin Thiocarbohydrazone Metal Complexes and Their Antitumor Activity against Ehrlich Ascites Carcinoma in Swiss Albino Mice. Metal-Based Drugs 2008, 2008. [Google Scholar] [CrossRef] [PubMed]
- Tribak, Z.; Chda, A.; Skalli, M.K.; Haoudi, A.; Rodi, Y.K.; Senhaji, O.; Essassi, E.M.; Cheikh, R. Ben; EL Abida, K. Theoretical Approach Using DFT and Muscle Relaxant Effects of 5-Chloroisatin Derivatives. International Journal of Chemistry and Technology 2018, 2, 105–115. [Google Scholar] [CrossRef]
- Tuli, H.S.; Rani, A.; Kumar, M.; Khare, R. Schiff Bases as an Antimicrobial Agent: A Review Anti-Neoplastic Effects of Garcinol View Project Schiff Bases as an Antimicrobial Agent: A Review. Journal of Biological and Chemical Sciences 2015, 2, 62–91. [Google Scholar]
- Utreja, D.; Vibha, B.S.P.; Singh, S.; Kaur, M. Schiff Bases and Their Metal Complexes as Anti-Cancer Agents: A Review. Current Bioactive Compounds 2015, 11, 215–230. [Google Scholar] [CrossRef]
- Mathew, B.; Suresh, J.; Anbazhagan, S. Synthesis, in Silico Preclinical Evaluation, Antidepressant Potential of 5-Substituted Phenyl-3-(Thiophen-2-Yl)-4,5-Dihydro-1h-Pyrazole-1-Carboxamides. Biomedicine and Aging Pathology 2014, 4, 327–333. [Google Scholar] [CrossRef]
- Çavuş, M.S.; Yakan, H.; Özorak, C.; Muğlu, H.; Bakır, T.K. New N,N’-Bis(Thioamido)Thiocarbohydrazones and Carbohydrazones: Synthesis, Structure Characterization, Antioxidant Activity, Corrosion Inhibitors and DFT Studies. Research on Chemical Intermediates 2022, 48, 1593–1613. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Z.; Cheng, C.; Liu, C.; Ma, N.; Sun, D.; Li, D.; Wang, C. Bone Regeneration and Antibacterial Properties of Calcium-Phosphorus Coatings Induced by Gentamicin-Loaded Polydopamine on Magnesium Alloys. Biomedical Technology 2024, 5, 87–101. [Google Scholar] [CrossRef]
- Abu-Hussen, A.A.A.; Emara, A.A.A. Metal Complexes of Some Thiocarbohydrazone Ligands: Synthesis and Structure. Journal of Coordination Chemistry 2004, 57, 973–987. [Google Scholar] [CrossRef]
- Blumenkopf, T.A.; Harrington, J.A.; Koble, C.S.; Bankston, D.D.; Morrison, R.W.; Bigham, E.C.; Styles, V.L.; Spector, T. 2-Acetylpyridine Thiocarbonohydrazones. Potent Inactivators of Herpes Simplex Virus Ribonucleotide Reductase. Journal of Medicinal Chemistry 1992, 35, 2306–2314. [Google Scholar] [CrossRef]
- Bonaccorso, C.; Marzo, T.; La Mendola, D. Biological Applications of Thiocarbohydrazones and Their Metal Complexes: A Perspective Review. Pharmaceuticals 2020, 13. [Google Scholar] [CrossRef]
- Mosa, A.I.; Emara, A.A.A.; Yousef, J.M.; Saddiq, A.A. Novel Transition Metal Complexes of 4-Hydroxy-Coumarin-3- Thiocarbohydrazone: Pharmacodynamic of Co(III) on Rats and Antimicrobial Activity. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy 2011, 81, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim Tehrani, K.H.M.; Kobarfard, F.; Azerang, P.; Mehravar, M.; Soleimani, Z.; Ghavami, G.; Sardari, S. Synthesis and Antimycobacterial Activity of Symmetric Thiocarbohydrazone Derivatives against Mycobacterium Bovis BCG. Iranian Journal of Pharmaceutical Research 2013, 12, 331–346. [Google Scholar]
- Jarrahpour, A.; Sheikh, J.; Mounsi, I. El; Juneja, H.; Hadda, T. Ben Computational Evaluation and Experimental in Vitro Antibacterial, Antifungal and Antiviral Activity of Bis-Schiff Bases of Isatin and Its Derivatives. Medicinal Chemistry Research 2013, 22, 1203–1211. [Google Scholar] [CrossRef]
- Papadakis, M.; Barrozo, A.; Delmotte, L.; Straistari, T.; Shova, S.; Réglier, M.; Krewald, V.; Bertaina, S.; Hardré, R.; Orio, M. How Metal Nuclearity Impacts Electrocatalytic H2 Production in Thiocarbohydrazone-Based Complexes. Inorganics 2023, 11, 1–19. [Google Scholar] [CrossRef]
- Cvijetić, I.N.; Herlah, B.; Marinković, A.; Perdih, A.; Bjelogrlić, S.K. Phenotypic Discovery of Thiocarbohydrazone with Anticancer Properties and Catalytic Inhibition of Human DNA Topoisomerase IIα. Pharmaceuticals 2023, 16, 341. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.A.; Kareem, M.M.; Al-Noor, T.H.; Al-Muhimeed, T.; Alobaid, A.A.; Albukhaty, S.; Sulaiman, G.M.; Jabir, M.; Taqi, Z.J.; Sahib, U.I. Pt(Ii)-Thiocarbohydrazone Complex as Cytotoxic Agent and Apoptosis Inducer in Caov-3 and Ht-29 Cells through the P53 and Caspase-8 Pathways. Pharmaceuticals 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Shebl, M.; Khalil, S.M.E.; Al-Gohani, F.S. Preparation, Spectral Characterization and Antimicrobial Activity of Binary and Ternary Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Ce(III) and UO 2(VI) Complexes of a Thiocarbohydrazone Ligand. Journal of Molecular Structure 2010, 980, 78–87. [Google Scholar] [CrossRef]
- Cândido-Bacani, P. de M.; Reis, M.B. dos; Serpeloni, J.M.; Calvo, T.R.; Vilegas, W.; Varanda, E.A.; Cólus, I.M. de S. Mutagenicity and Genotoxicity of Isatin in Mammalian Cells in Vivo. Mutation Research - Genetic Toxicology and Environmental Mutagenesis 2011, 719, 47–51. [Google Scholar] [CrossRef]
- Bonaccorso, C.; Grasso, G.; Musso, N.; Barresi, V.; Condorelli, D.F.; La Mendola, D.; Rizzarelli, E. Water Soluble Glucose Derivative of Thiocarbohydrazone Acts as Ionophore with Cytotoxic Effects on Tumor Cells. Journal of Inorganic Biochemistry 2018, 182, 92–102. [Google Scholar] [CrossRef]
- Gabr, M.T.; El-Gohary, N.S.; El-Bendary, E.R.; El-Kerdawy, M.M.; Ni, N. Isatin-β-Thiocarbohydrazones: Microwave-Assisted Synthesis, Antitumor Activity and Structure-Activity Relationship. European Journal of Medicinal Chemistry 2017, 128, 36–44. [Google Scholar] [CrossRef]
- Mousavi, S.N.; Bahrami, A.; Sadri, M.; Alipour, A. Optimization of an Ecofriendly Coating Containing Chitosan and Gelatin as Corrosion Inhibitor of Carbon Steel Grade E by Response Surface Method. Journal of Particle Science & Technology 2019, 5, 23–31. [Google Scholar] [CrossRef]
- Yakan, H.; Koçyiğit, Ü.M.; Muğlu, H.; Ergul, M.; Erkan, S.; Güzel, E.; Taslimi, P.; Gülçin, İ. Potential Thiosemicarbazone-Based Enzyme Inhibitors: Assessment of Antiproliferative Activity, Metabolic Enzyme Inhibition Properties, and Molecular Docking Calculations. Journal of Biochemical and Molecular Toxicology 2022, 36. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Kaur, H.; Halliwell, B. Oxygen Free Radicals and Human Diseases. Journal of the Royal Society of Health 1991, 111, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Z.H.; Ibraheem, R.M. Anti-Oxidant Activity of Methanol Extracts of Arum Maculatum L. and Physalis Peruviana L. Plants. Ibn Al-Haitham J. for Pure & Appl. Sci. 2015, 28, 1–7. [Google Scholar]
- Knothe, G. Some Aspects of Biodiesel Oxidative Stability ☆. 2007, 88, 669–677. [CrossRef]
- Di Meo, F.; Lemaur, V.; Cornil, J.; Lazzaroni, R.; Duroux, J.L.; Olivier, Y.; Trouillas, P. Free Radical Scavenging by Natural Polyphenols: Atom versus Electron Transfer. Journal of Physical Chemistry A 2013, 117, 2082–2092. [Google Scholar] [CrossRef] [PubMed]
- Marković, Z.; Đorović, J.; Petrović, Z.D.; Petrović, V.P.; Simijonović, D. Investigation of the Antioxidant and Radical Scavenging Activities of Some Phenolic Schiff Bases with Different Free Radicals. Journal of Molecular Modeling 2015, 21. [Google Scholar] [CrossRef] [PubMed]
- Saur, I.M.L.; Panstruga, R.; Schulze-Lefert, P. NOD-like Receptor-Mediated Plant Immunity: From Structure to Cell Death. Nature Reviews Immunology 2021, 21, 305–318. [Google Scholar] [CrossRef] [PubMed]
- BIOVIA, D.S. No Title 2017, 779.
- Zhang, L.; Mulrooney, S.B.; Leung, A.F.K.; Zeng, Y.; Ko, B.B.C.; Hausinger, R.P.; Sun, H. Inhibition of Urease by Bismuth(III): Implications for the Mechanism of Action of Bismuth Drugs. BioMetals 2006, 19, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Yenigun, S.; Ipek, Y.; Marah, S.; Demirtas, I.; Ozen, T. DNA Protection, Molecular Docking, Antioxidant, Antibacterial, Enzyme Inhibition, and Enzyme Kinetic Studies for Parietin, Isolated from Xanthoria Parietina (L.) Th. Fr. Journal of Biomolecular Structure and Dynamics 2024, 42, 848–862. [Google Scholar] [CrossRef]
- Muğlu, H.; Yakan, H.; Misbah, A.G.A.; Çavuş, M.S.; Bakır, T.K. Synthesis, Structure Characterization and Quantum Chemical Study on Relationship between Structure and Antioxidant Properties of Novel Schiff Bases Bearing (Thio)/Carbohydrazones. Research on Chemical Intermediates 2021, 47, 4985–5005. [Google Scholar] [CrossRef]
- Yakan, H. Preparation, Structure Elucidation, and Antioxidant Activity of New Bis(Thiosemicarbazone) Derivatives. Turkish Journal of Chemistry 2020, 44, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Harald Küppers DuMonts Farben-Atlas. Über 5500 Farbnuancen Mit Kennzeichnung Und Mischanleitung; DuMont Reiseverlag, Ed.; Ostfildern, 1978; ISBN 9783770110582.
- Arjunan, V.; Mohan, S.; Subramanian, S.; Thimme Gowda, B. Synthesis, Fourier Transform Infrared and Raman Spectra, Assignments and Analysis of N-(Phenyl)- and N-(Chloro Substituted Phenyl)-2,2-Dichloroacetamides. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy 2004, 60, 1141–1159. [Google Scholar] [CrossRef] [PubMed]
- Berthomieu, C.; Hienerwadel, R. Fourier Transform Infrared (FTIR) Spectroscopy. Photosynthesis Research 2009, 101, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Bee, S.; Gupta, A.; Tandon, P. Comparative Vibrational Spectroscopic Studies, HOMO-LUMO and NBO Analysis of N-(Phenyl)-2,2-Dichloroacetamide, N-(2-Chloro Phenyl)-2,2-Dichloroacetamide and N-(4-Chloro Phenyl)-2,2-Dichloroacetamide Based on Density Functional Theory. Computational and Theoretical Chemistry 2013, 1016, 8–21. [Google Scholar] [CrossRef]
- Fábián, B.; Kudar, V.; Csámpai, A.; Nagy, T.Z.; Sohár, P. Synthesis, IR-, NMR-, DFT and X-Ray Study of Ferrocenyl Heterocycles from Thiosemicarbazones. Part 21: Study on Ferrocenes. Journal of Organometallic Chemistry 2007, 692, 5621–5632. [Google Scholar] [CrossRef]
- Lobana, T.S.; Sharma, R.; Bawa, G.; Khanna, S. Bonding and Structure Trends of Thiosemicarbazone Derivatives of Metals-An Overview. Coordination Chemistry Reviews 2009, 253, 977–1055. [Google Scholar] [CrossRef]
- Nuwan De Silva, N.W.S.V.; Albu, T. V. A Theoretical Investigation on the Isomerism and the NMR Properties of Thiosemicarbazones. Central European Journal of Chemistry 2007, 5, 396–419. [Google Scholar] [CrossRef]
- Srinivasan, B.R.; Raghavaiah, P.; Nadkarni, V.S. Reinvestigation of Growth of Urea Thiosemicarbazone Monohydrate Crystal. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy 2013, 112, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Wolinski, K.; Hinton, J.F.; Pulay, P. Efficient Implementation of the Gauge-Independent Atomic Orbital Method for NMR Chemical Shift Calculations. Journal of the American Chemical Society 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Barreto Bastos, A.M.; De Carvalho Alcântara, A.F.; Beraldo, H. Structural Analyses of 4-Benzoylpyridine Thiosemicarbazone Using NMR Techniques and Theoretical Calculations. Tetrahedron 2005, 61, 7045–7053. [Google Scholar] [CrossRef]
- Ebrahimi, H.P.; Hadi, J.S.; Alsalim, T.A.; Ghali, T.S.; Bolandnazar, Z. A Novel Series of Thiosemicarbazone Drugs: From Synthesis to Structure. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy 2015, 137, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, T.K.; Pierens, G.K.; Reutens, D.C. Synthesis, NMR Structural Characterization and Molecular Modeling of Substituted Thiosemicarbazones and Semicarbazones Using DFT Calculations to Prove the Syn/Anti Isomer Formation. Magnetic Resonance in Chemistry 2014, 52, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Chee, D.N.A.; Affan, M.A.; Ahmad, F.B.; Asaruddin, M.R.; Sam, N.; Salam, M.A.; Ismail, A.; Tan, S.H. Synthesis, Characterization, and Antibacterial Activity of Organotin(IV) Complexes with 2-Hydroxyacetophenone Thiocarbohydrazone. Journal of Coordination Chemistry 2011, 64, 4191–4200. [Google Scholar] [CrossRef]
- Kaya, Y.; Erçağ, A.; Kaya, S.; Katin, K.P.; Atilla, D. New Mixed-Ligand Iron(III) Complexes Containing Thiocarbohydrazones: Preparation, Characterization, and Chemical Reactivity Analysis through Theoretical Calculations. Applied Organometallic Chemistry 2022, 36, 1–12. [Google Scholar] [CrossRef]
- Li, M.; Xu, J.; Li, R.; Wang, D.; Li, T.; Yuan, M.; Wang, J. Simple Preparation of Aminothiourea-Modified Chitosan as Corrosion Inhibitor and Heavy Metal Ion Adsorbent. Journal of Colloid and Interface Science 2014, 417, 131–136. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Khan, S.; Nami, S.A.A.; El-ajaily, M.M. Polynuclear Transition Metal Complexes with Thiocarbohydrazide and Dithiocarbamates. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy 2007, 67, 995–1002. [Google Scholar] [CrossRef]
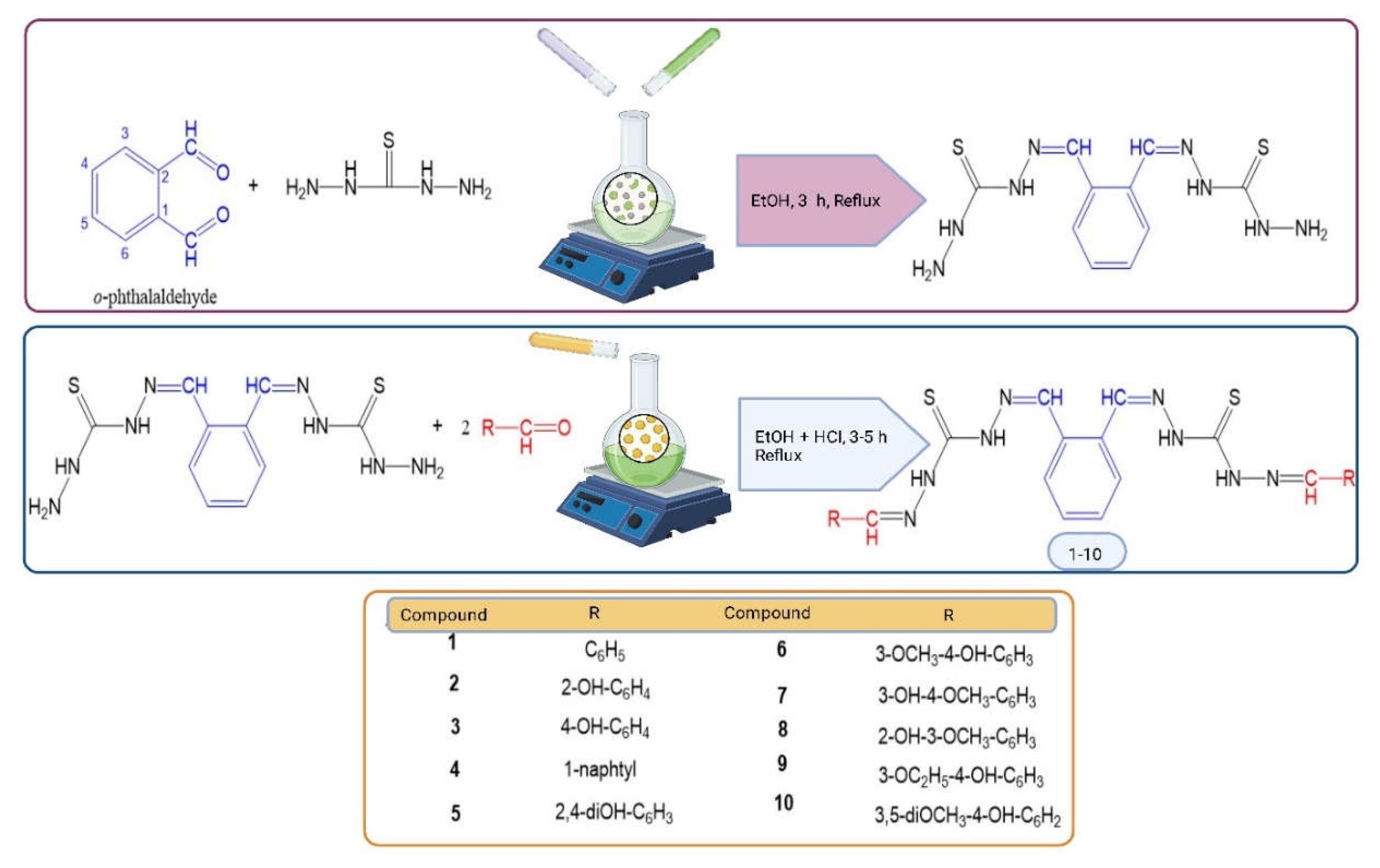
| Comp. | Compound Name | -R | Yield % | Melting Point (◦C) | Color | Color Code [46] |
|---|---|---|---|---|---|---|
| 1 | N',N'''-(1,2-phenylenebis(methaneylylidene))bis(N'-benzylidenemethanebis(thiohydrazide)) | C6H5 | 80 | 205.26 | Brown | S70O26G02 |
| 2 | N',N'''-(1,2-phenylenebis(methaneylylidene))bis(N'-(2-hydroxybenzylidene)methanebis(thiohydrazide)) | 2-OH-C6H4 | 84 | 214.01 | Dark Brown | S80O07G15 |
| 3 | N',N'''-(1,2-phenylenebis(methaneylylidene))bis(N'-(4-ydroxybenzylidene)methanebis(thiohydrazide)) | 4-OH-C6H4 | 55 | 241.44 | Black | S99O11G33 |
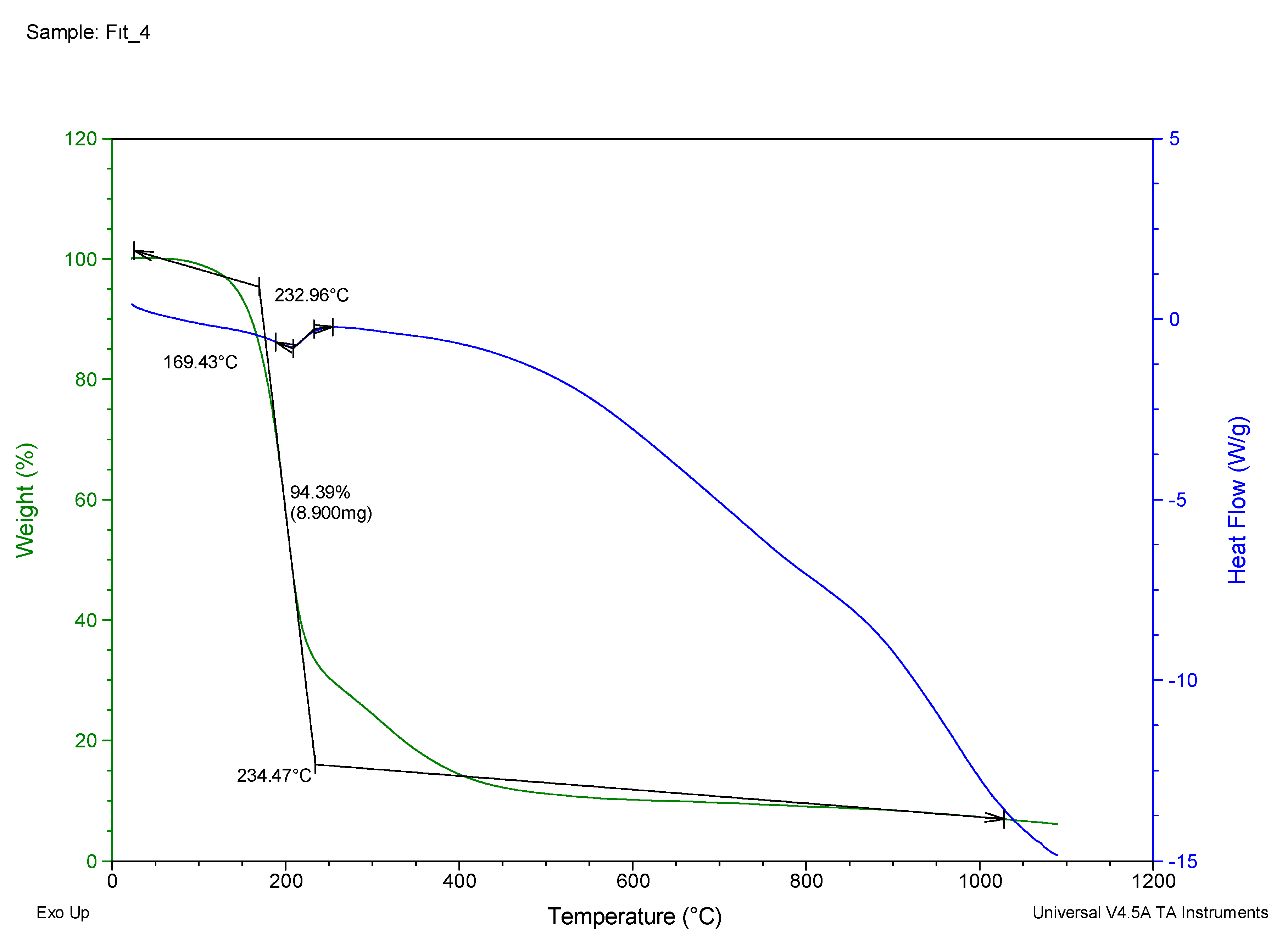
| 4 | N',N'''-(1,2-phenylenebis(methaneylylidene))bis(N'-(naphthalen-1-ylmethylene)methanebis(thiohydrazide)) | C11H10 | 61 | 232.96 | Dark Black | S99O00G02 |
| 5 | N',N'''-(1,2-phenylenebis(methaneylylidene))bis(N'-(2,4-dihydroxybenzylidene)methanebis(thiohydrazide)) | 2,4-diOH-C6H3 | 77 | 261.42 | Brown | S90O60G11 |
| 6 | N',N'''-(1,2-phenylenebis(methaneylylidene))bis(N'-(4-hydroxy-3-methoxybenzylidene)methanebis(thiohydrazide)) | 3-OCH3-4-OH-C6H3 | 81 | 239.74 | Brown | S90O02G50 |
| 7 | N',N'''-(1,2-phenylenebis(methaneylylidene))bis(N'-(3-hydroxy-4-methoxybenzylidene)methanebis(thiohydrazide)) | 3-OH-4OCH3-C6H3 | 73 | 231.96 | Brown | S90O11G20 |
| 8 | N',N'''-(1,2-phenylenebis(methaneylylidene))bis(N'-(2-hydroxy-3-methoxybenzylidene)methanebis(thiohydrazide)) | 2-OH-3OCH3-C6H3 | 87 | 298.97 | Light Brown | S70O26G33 |
| 9 | N',N'''-(1,2-phenylenebis(methaneylylidene))bis(N'-(3-ethoxy-4-hydroxybenzylidene)methanebis(thiohydrazide)) | 3-OC2H5-4-OH-C6H3 | 78 | 212.59 | Brown | S80O33G33 |
| 10 | N',N'''-(1,2-phenylenebis(methaneylylidene))bis(N'-(4-hydroxy-3,5-dimethoxybenzylidene)methanebis(thiohydrazide)) | 3,5-diOCH3-4-OH-C6H2 | 62 | 295.93 | Pale Black | S90O07G41 |
| Comp. | Molecular mass (g/mol) | Molecular formula | Calculated | Experimental | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (C)% | (H)% | (N)% | (S)% | (O)% | (C)% | (H)% | (N)% | (S)% | (O)% | |||
| 1 | 486.62 | C24H22N8S2 | 59.24 | 4.56 | 23.03 | 13.18 | - | 59.30 | 4.52 | 23.06 | 13.12 | - |
| 2 | 518.61 | C24H22N8O2S2 | 55.58 | 4.28 | 21.61 | 12.36 | 6.17 | 55.57 | 4.29 | 21.63 | 12.37 | 6.14 |
| 3 | 518.61 | C24H22N8O2S2 | 55.58 | 4.28 | 21.61 | 12.36 | 6.17 | 55.60 | 4.28 | 21.58 | 12.37 | 6.17 |
| 4 | 586.74 | C32H26N8S2 | 65.51 | 4.47 | 19.10 | 10.93 | - | 65.53 | 4.45 | 19.11 | 10.91 | - |
| 5 | 550.61 | C24H22N8O4S2 | 52.35 | 4.03 | 20.35 | 11.65 | 11.62 | 52.33 | 4.04 | 20.37 | 11.64 | 11.62 |
| 6 | 578.67 | C26H26N8O4S2 | 53.97 | 4.53 | 19.36 | 11.08 | 11.06 | 53.97 | 4.54 | 19.35 | 11.08 | 11.06 |
| 7 | 578.67 | C26H26N8O4S2 | 53.97 | 4.53 | 19.36 | 11.08 | 11.06 | 53.96 | 4.53 | 19.34 | 11.09 | 11.08 |
| 8 | 578.67 | C26H26N8O4S2 | 53.97 | 4.53 | 19.36 | 11.08 | 11.06 | 53.97 | 4.54 | 19.33 | 11.09 | 11.07 |
| 9 | 606.72 | C28H30N8O4S2 | 55.43 | 4.98 | 18.47 | 10.57 | 10.55 | 55.40 | 4.99 | 18.50 | 10.56 | 10.55 |
| 10 | 638.72 | C28H30N8O6S2 | 52.65 | 4.73 | 17.54 | 10.04 | 15.03 | 52.65 | 4.72 | 17.57 | 10.05 | 15.01 |
| Comp. | ν(OH) | ν(NH) | Ar. CH | Aliph. CH | ν(C=N) | ν(NH-C=S) | ν(C-N) | Spec. vib. |
|---|---|---|---|---|---|---|---|---|
| 1 | - | 3216 | 3080 | 2991 | 1492 | 1451 | 1120 | - |
| 2 | 3679 | 3201 | 2991 | 2929 | 1491 | 1464 | 1191 | C-O:1069 |
| 3 | 3680 | 3207 | 2990 | 2921 | 1514 | 1456 | 1166 | C-O:1068 |
| 4 | - | 3272 | 2997 | 2919 | 1570 | 1512 | 1166 | - |
| 5 | 3685 | 3254 | 2990 | 2911 | 1509 | 1411 | 1100 | - |
| 6 | 3679 | 3221 | 2984 | 2904 | 1513 | 1461 | 1190 | C-O:1065 |
| 7 | 3679 | 3219 | 2990 | 2905 | 1512 | 1409 | 1195 | C-O:1068 |
| 8 | 3679 | 3232 | 2984 | 2911 | 1481 | 1405 | 1185 | C-O:1076 |
| 9 | 3678 | 3218 | 2990 | 2905 | 1512 | 1444 | 1195 | C-O:1068 |
| 10 | 3679 | 3234 | 2990 | 2904 | 1512 | 1465 | 1199 | C-O:1077 |
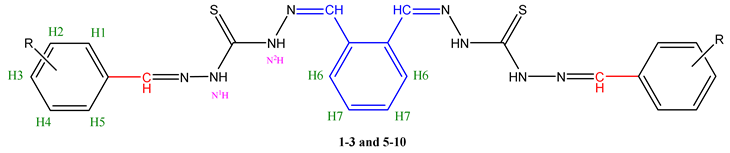 | ||||||||||||
| Comp. | H1 | H2 | H3 | H4 | H5 | H6 | H7 | N1H-N | N2H-N | CH=N | O-H | Spec. peaks |
| 1 | 7.74-7.73 d | 7.50-7.48 dd | 7.45-7.43 dd | 7.48-7.46 dd | 7.73–7.72 d | 7.66-7.64 d | 7.43–7.42 dd | 9.83 s |
11.62 s |
8.58 s |
- |
- |
| 2 | - | 6.95-6.93 d |
7.31-7.28 dd |
6.93-6.91 dd |
7.52-7.50 d |
7.66-7.64 d |
7.44-7.42 dd |
10.64 s | 10.64 s |
8.56 s |
11.04 s |
- |
| 3 | 7.53-7.52 d |
6.89-6.88 dd |
- | 6.87-6.87 dd |
7.54-7.54 d |
7.66-7.63 d |
7.45-7.41 dd |
9.83 s | 11.50 s |
8.56 s |
9.69 s |
- |
| 5 | - | 6.56-6.55 s |
- | 6.55-6.53 d |
7.49-7.48 d |
7.67-7.63 d |
7.45-7.41 dd |
10.63 s | 10.63 s |
8.56 s |
11.69 s 10.11 s |
- |
| 6 | 7.25-7.24 s | - | - | 6.89-6.87 d |
7.27-7.25 d |
7.67-7.63 d |
7.45-7.41 dd |
9.83 s | 11.62 s |
8.56 s |
9.50 s |
-OCH3: 3.79, s |
| 7 | 7.28 s | - | - | 7.05-7.03 d |
7.40-7.38 7.20-7.17 m |
7.66-7.64 d |
7.44-7.42 dd |
9.86 s | 11.62 s |
8.54 s |
9.24 s |
-OCH3: 3.80, s |
| 8 | - | - | 6.99-6.97 d |
6.96-6.94 dd |
7.16-7.14 dd |
7.67-7.63 d |
7.45-7.41 dd |
10.66 s | 10.66 s |
8.54 s |
13.46 s |
-OCH3: 3.80, s |
| 9 | 7.25-7.24 s |
- | - | 6.88-6.86 d |
7.27-7.25 d |
7.67-7.63 d |
7.45-7.41 dd |
9.74 s | 10.98 s |
8.53 s |
9.62s | -CH2: 4.07-4.03 dd -CH3: 1.33-1.31t |
| 10 | 7.00-6.99 s |
- | - | - | 7.00-6.99 s |
7.67-7.63 | 7.45-7.41 | 9.95 s | 11.50 s | 8.52 s | 8.75 s | -OCH3: 3.83 s |
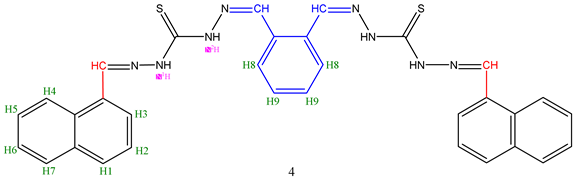 | |||||||||||||
| Comp. | H1 | H2 | H3 | H4 | H5 | H6 | H7 | N1H-N | N2H-N | CH=N | O-H | Spec. peaks | |
| 4 | 8.01-7.88 d |
7.55-7.54 dd |
7.66-7.64 d |
8.35-8.33 d |
7.54-7.53 dd | 7.75-7.73 d |
10.63 s |
10.63 s |
8.55 s |
- |
H8 7.60-7.57 d |
H9 7.44-7.42 dd |
|
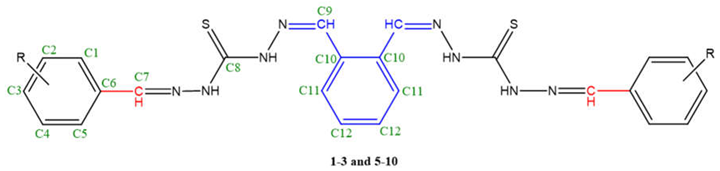 | |||||||||||||
| Comp. | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | C11 | C12 | R |
| 1 | 129.02 | 128.62 | 132.01 | 128.62 | 129.02 | 133.51 | 147.53 | 184.25 | 145.64 | 134.30 | 129.02 | 132.01 | - |
| 2 | 158.88 | 117.99 | 132.80 | 121.06 | 127.84 | 118.78 | 147.14 | 185.75 | 142.96 | 136.19 | 129.02 | 130.51 | - |
| 3 | 132.43 | 116.0 | 160.77 | 116.0 | 132.43 | 125.95 | 148.32 | 184.96 | 142.08 | 134.69 | 129.02 | 133.90 | |
| 5 | 163.12 | 103.82 | 162.62 | 110.03 | 133.67 | 111.76 | 146.24 | 185.11 | 144.54 | 134.14 | 129.25 | 131.46 | - |
| 6 | 114.44 | 149.66 | 151.55 | 117.91 | 122.64 | 131.93 | 146.75 | 185.67 | 144.30 | 134.69 | 129.73 | 132.49 | -OCH3: 57.64 |
| 7 | 125.08 | 153.60 | 155.03 | 120.35 | 127.33 | 131.46 | 150.50 | 184.19 | 145.34 | 136.11 | 129.49 | 131.46 | -OCH3: 56.21 |
| 8 | 155.81 | 151.39 | 115.94 | 118.38 | 126.26 | 120.67 | 148.48 | 187.64 | 145.49 | 137.84 | 130.75 | 133.43 | -OCH3: 56.31 |
| 9 | 113.26 | 148.48 | 153.60 | 116.41 | 123.55 | 130.99 | 146.27 | 186.14 | 143.30 | 135.64 | 129.49 | 132.96 | O-CH2: 65.75CH2-CH3: 15.15 |
| 10 | 107.35 | 149.90 | 140.84 | 149.90 | 107.35 | 128.23 | 146.98 | 185.90 | 143.87 | 135.87 | 129.25 | 131.46 | -OCH3: 56.61 |
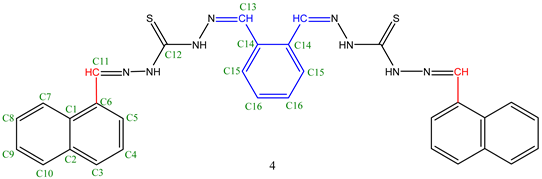 | ||||||||||||||||
| Comp. | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | C11 | C12 | C13 | C14 | C15 | C16 |
| 4 | 135.61 | 137.16 | 131.25 | 126.59 | 124.58 | 133.67 | 125.71 | 120.10 | 127.28 | 130.18 | 162.29 | 194.75 | 169.17 | 135.61 | 127.97 | 129.38 |
| Compound | π → π*, B band | n → π*, C=N | n → π*, C=S |
|---|---|---|---|
| 1 | 240 | 280 | 330 |
| 2 | 240 | 280 | 340 |
| 3 | 240 | 280 | 330 |
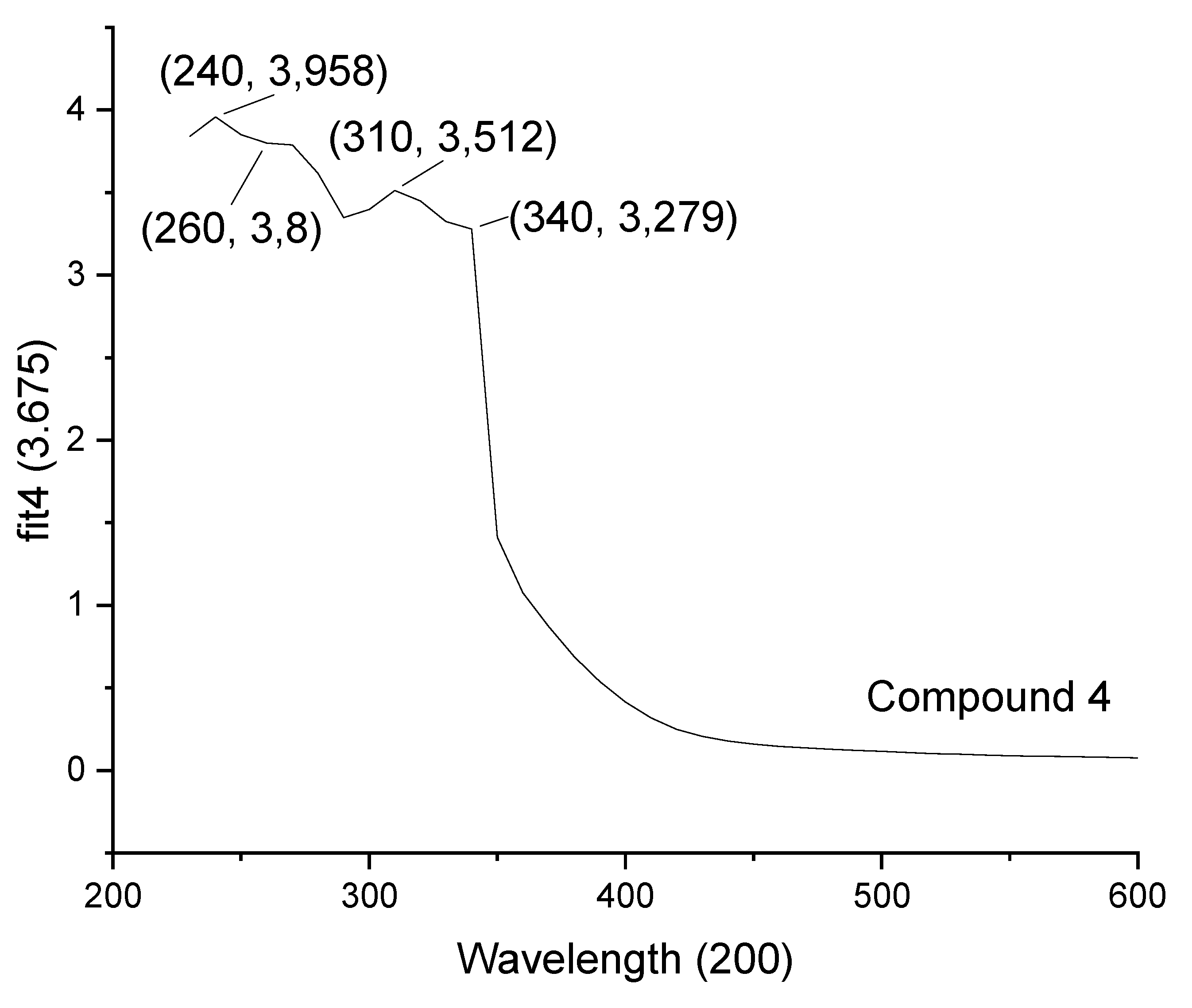
| 4 | 240 | 260 | 310, 340 |
| 5 | 250 | 280 | 350 |
| 6 | 260 | 280 | 340 |
| 7 | 240 | 280 | 340 |
| 8 | 260 | 280 | 330 |
| 9 | 250 | 280 | 340 |
| 10 | 240 | 280 | 320 |
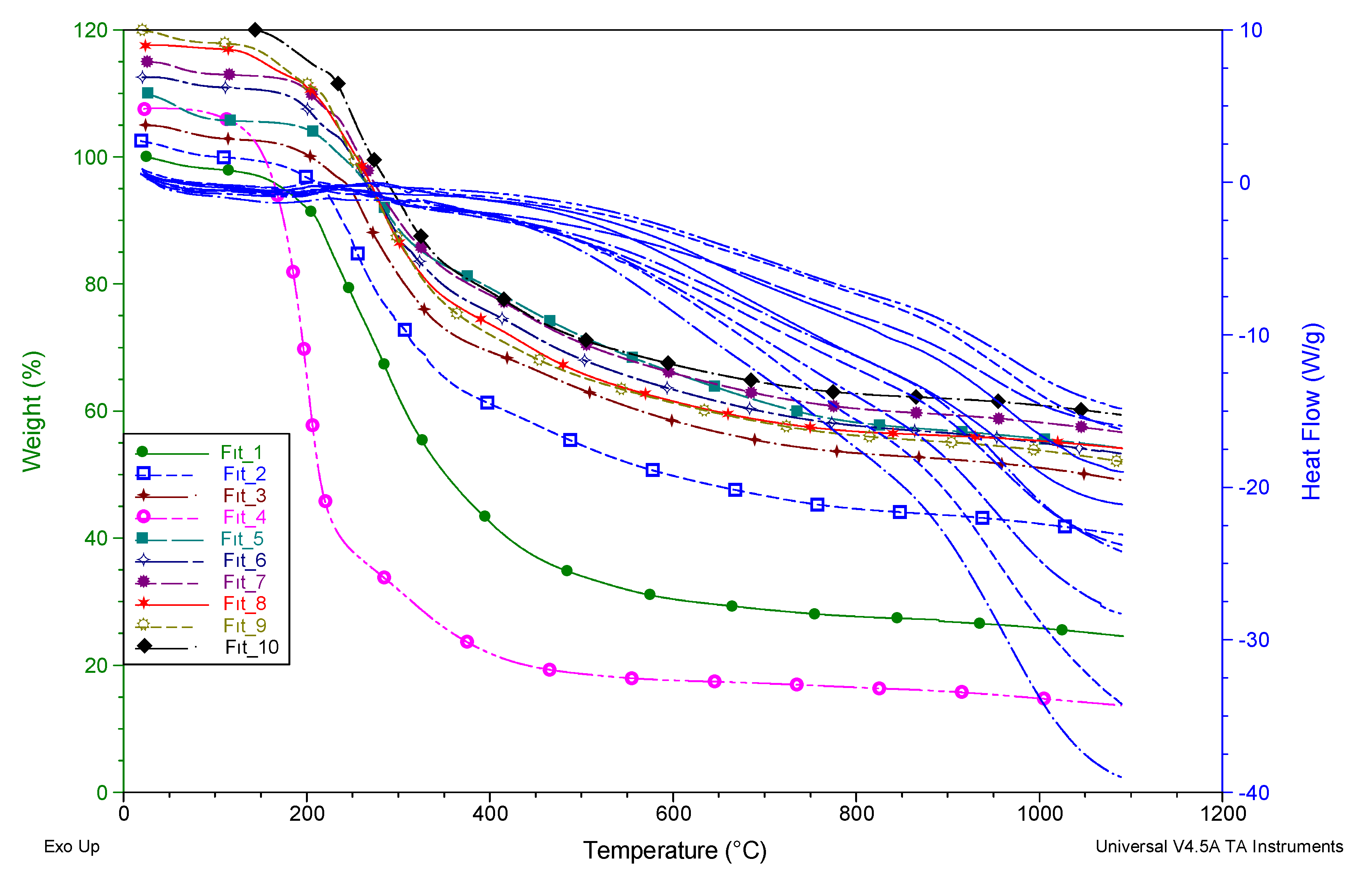
| Compounds | TGA | TGA | DSC |
|---|---|---|---|
| aTon | bWeight loss % at 1100 °C | cTm (°C) | |
| 1 | 195.69 | 74.20 | 205.26 |
| 2 | 200.98 | 58.42 | 214.01 |
| 3 | 228.08 | 54.63 | 241.44 |
| 4 | 169.43 | 94.39 | 232.96 |
| 5 | 236.94 | 54.93 | 261.42 |
| 6 | 211.38 | 58.83 | 239.74 |
| 7 | 217.53 | 58.02 | 231.96 |
| 8 | 207.42 | 63.82 | 298.97 |
| 9 | 200.22 | 65.13 | 212.59 |
| 10 | 211.37 | 61.56 | 295.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





