Submitted:
03 May 2024
Posted:
07 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Chemicals
Cell Culture
Pharmacological Patterning of Dopaminergic Neurons
Cellular ET Uptake and Liquid Chromatography Mass Spectrometry
MTT Assay
Fluorescence Microscopy
Quantitative RT-PCR
Flow Cytometry
ATP Assay
Western Blot
Protein Carbonylation Assay
Tyrosine Hydroxylase Protein Assay
Dopamine Detection ELISA Kit
Statistical Analysis
3. Results
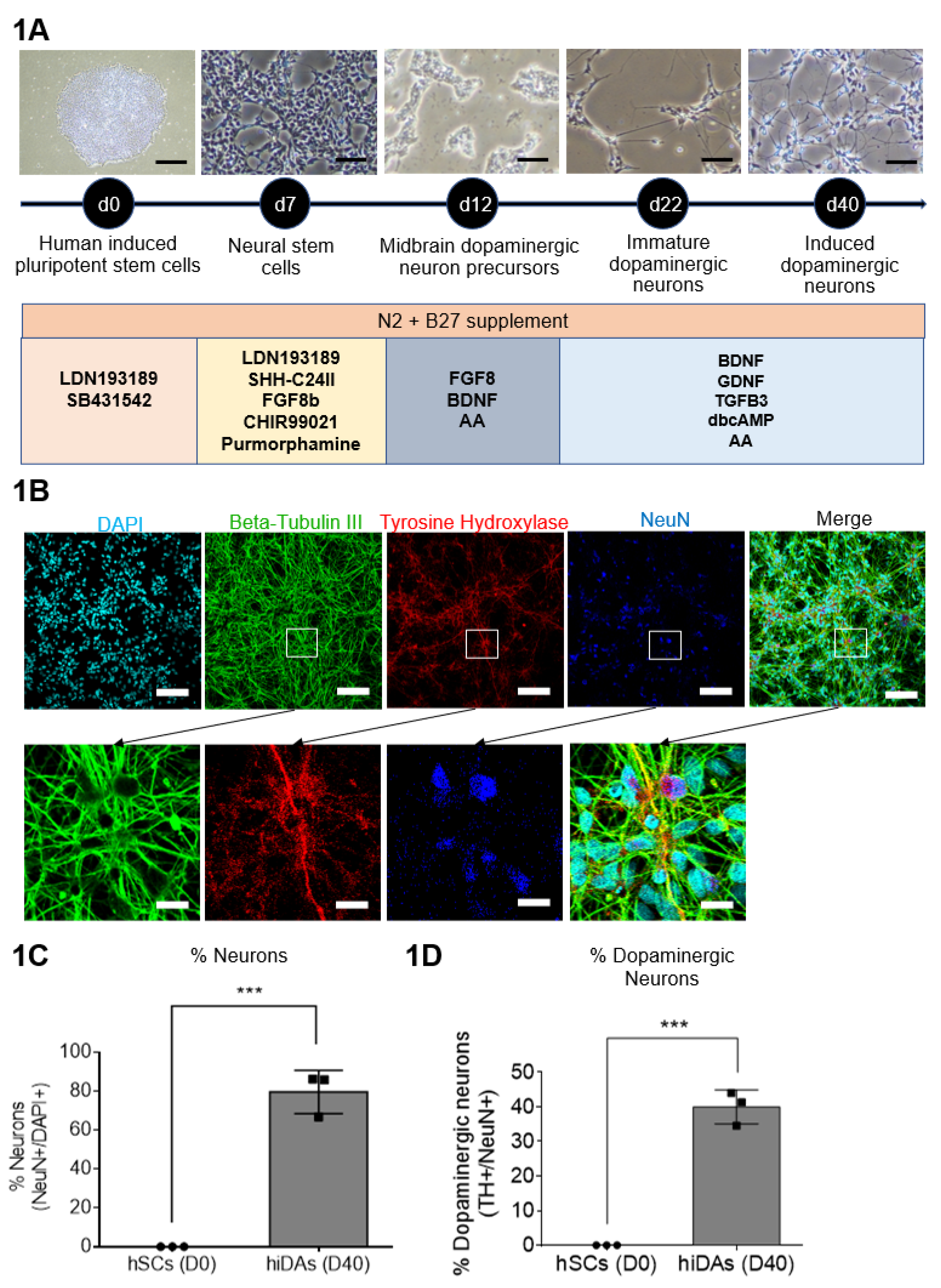
3.1. Generation of hiPSC-Derived Day 40 Dopaminergic Neurons
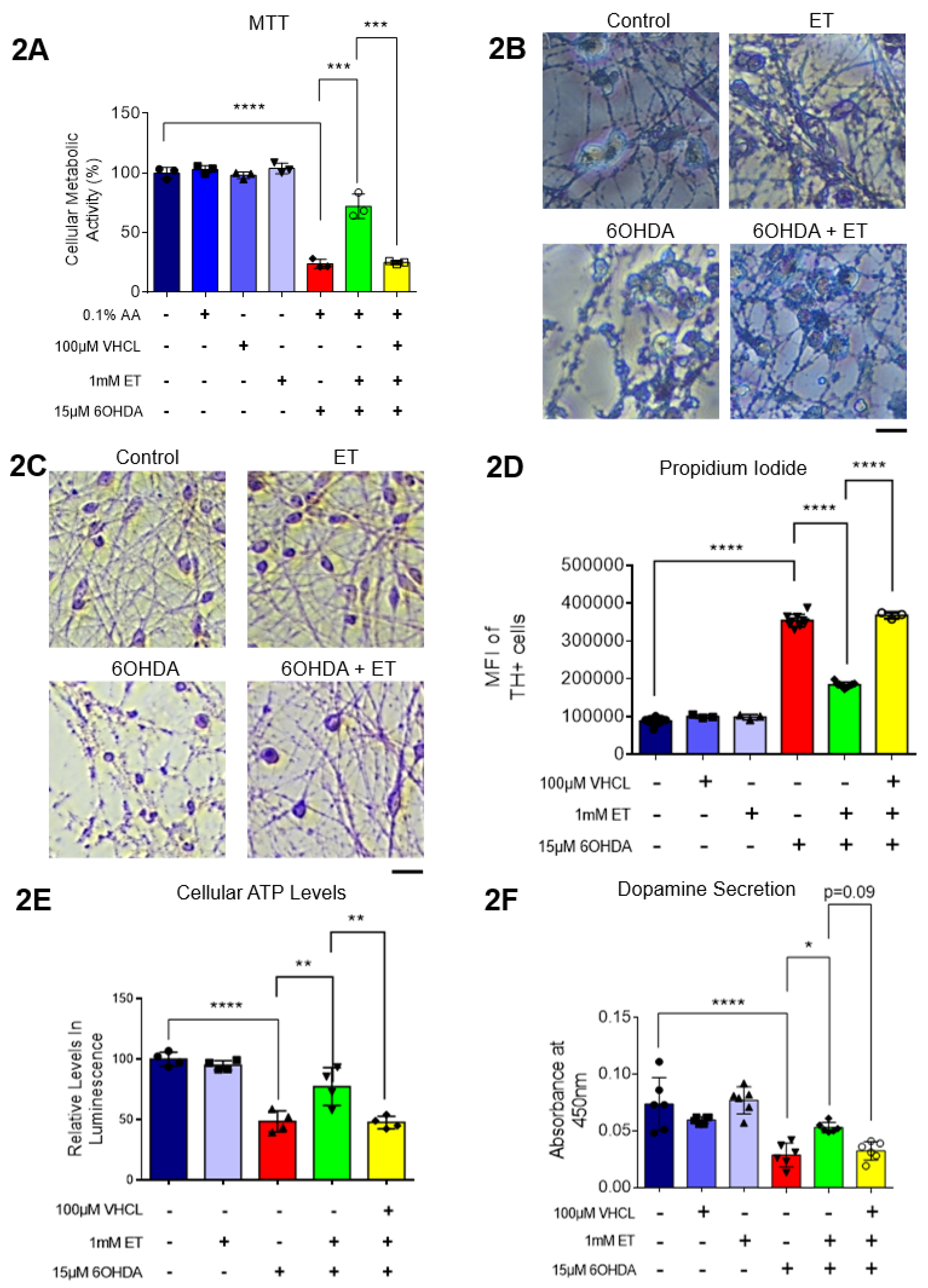
3.2. ET Protects iDAs Against 6-OHDA Induced Cell Death and Loss of Dopamine Secretion
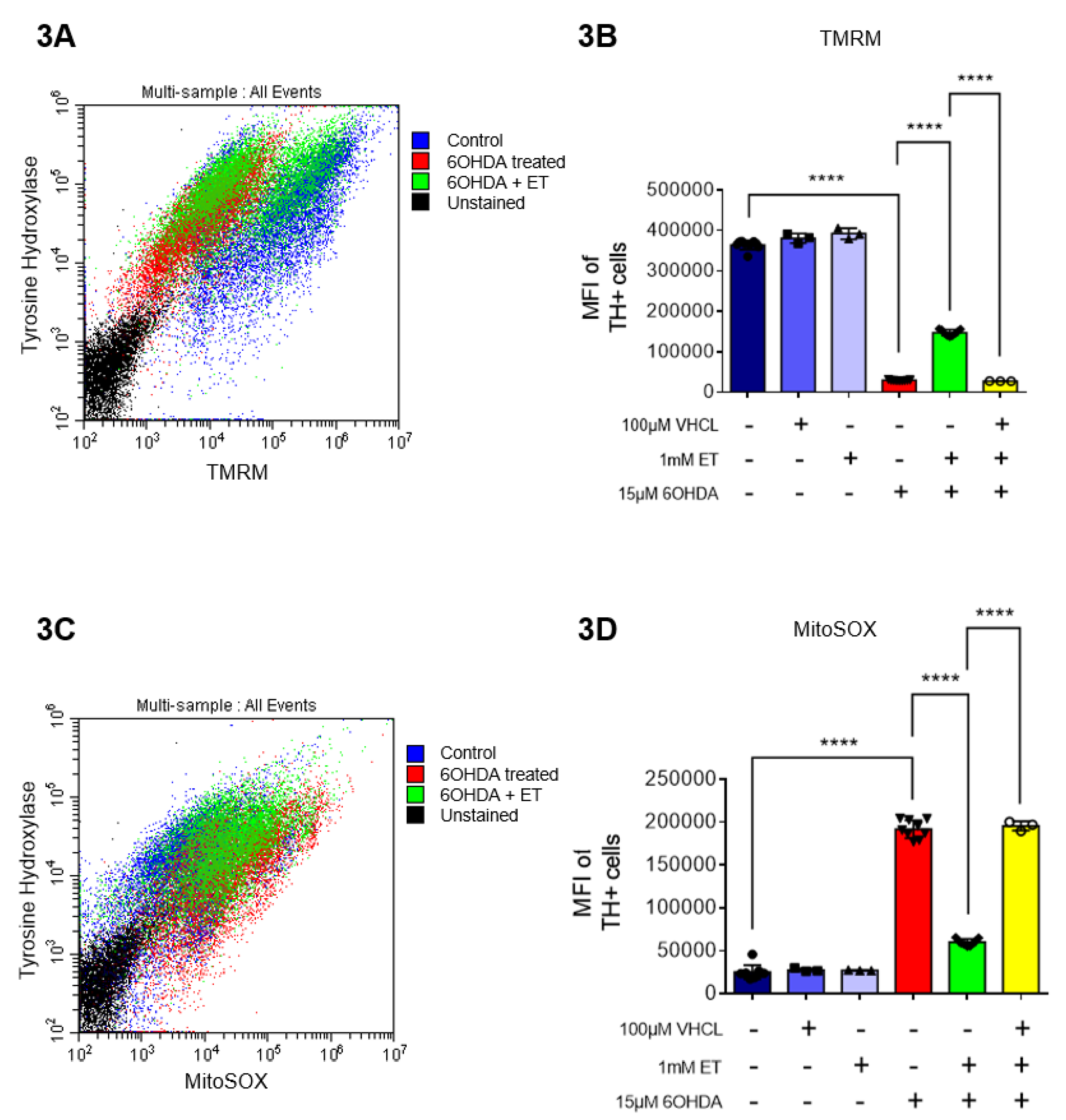
3.3. ET Also Protects Day 40 iDAs Against 6-OHDA-Induced Increase in mROS and Loss of MMP.
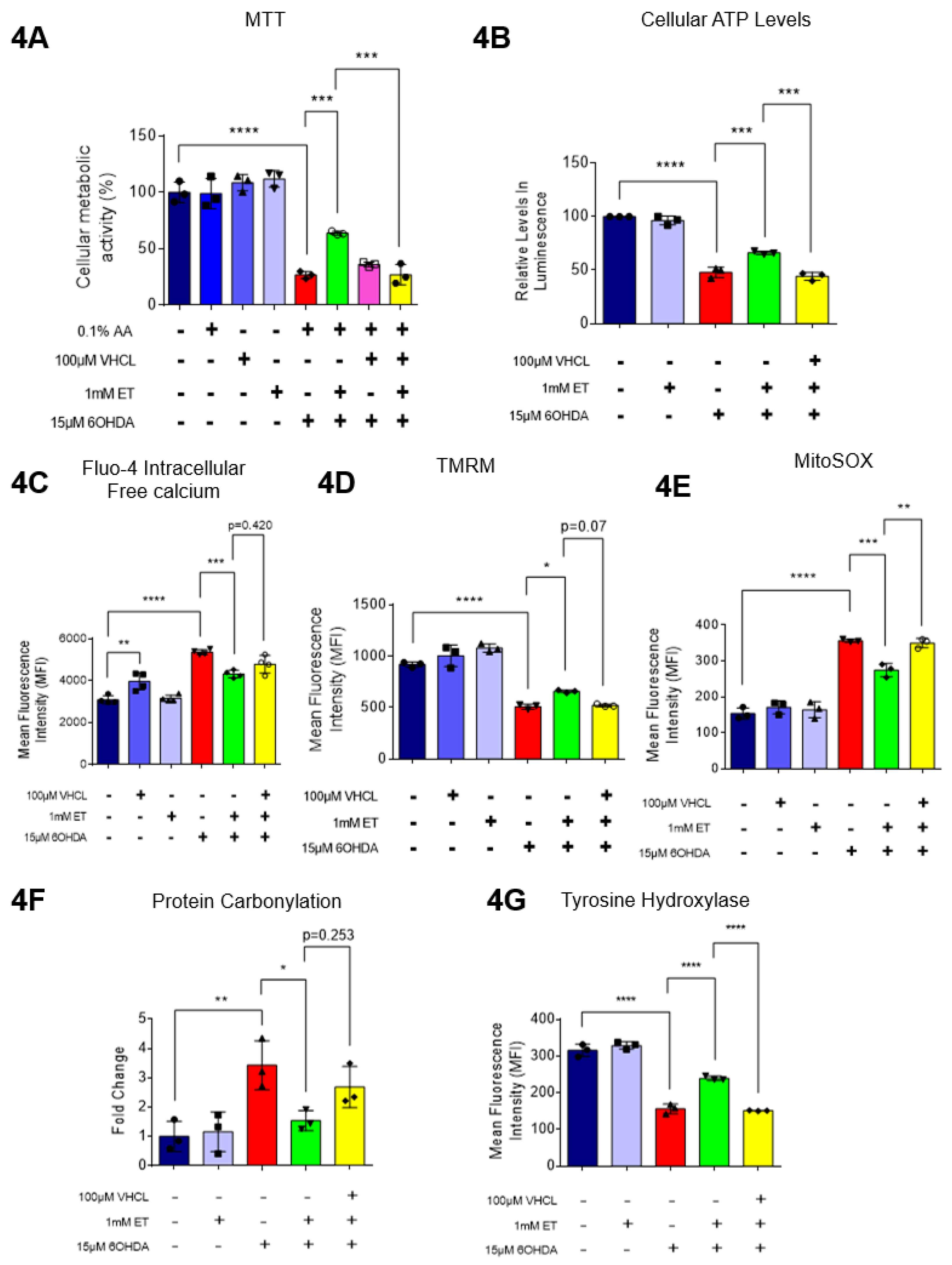
3.4. ET Uptake Protects TH+ SH-SY5Y Cells Against 6-OHDA Induced Cell Death, Metabolic Dysfunction, and Oxidative Stress
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ou, Z.; et al. Global Trends in the Incidence, Prevalence, and Years Lived With Disability of Parkinson's Disease in 204 Countries/Territories From 1990 to 2019. Frontiers in Public Health 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; et al. Parkinson disease. Nat Rev Dis Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Chinta, S.J.; Andersen, J.K. Redox imbalance in Parkinson's disease. Biochim Biophys Acta 2008, 1780, 1362–1367. [Google Scholar] [CrossRef]
- Jenner, P.; Olanow, C.W. Oxidative stress and the pathogenesis of Parkinson's disease. Neurology 1996, 47 (6_suppl_3), 161S–170S. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.T.; Jenner, P. Parkinson disease: From pathology to molecular disease mechanisms. Free Radic Biol Med 2013, 62, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Seet, R.C.; et al. Oxidative damage in Parkinson disease: Measurement using accurate biomarkers. Free Radic Biol Med 2010, 48, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Alam, Z.I.; et al. Oxidative DNA damage in the parkinsonian brain: An apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem 1997, 69, 1196–1203. [Google Scholar] [CrossRef]
- Alam, Z.I.; et al. A generalised increase in protein carbonyls in the brain in Parkinson's but not incidental Lewy body disease. J Neurochem 1997, 69, 1326–1329. [Google Scholar]
- Henchcliffe, C.; Beal, M.F. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 2008, 4, 600–609. [Google Scholar] [CrossRef]
- Abou-Sleiman, P.M.; Muqit, M.M.K.; Wood, N.W. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nature Reviews Neuroscience 2006, 7, 207–219. [Google Scholar] [CrossRef]
- Bindoff, L.A.; et al. Mitochondrial function in Parkinson's disease. Lancet 1989, 2, 49. [Google Scholar] [CrossRef]
- Mythri, R.B.; et al. Mitochondrial complex I inhibition in Parkinson's disease: How can curcumin protect mitochondria? Antioxid Redox Signal 2007, 9, 399–408. [Google Scholar] [CrossRef]
- Schapira, A.H.; et al. Mitochondrial complex I deficiency in Parkinson's disease. Lancet 1989, 1, 1269. [Google Scholar] [CrossRef]
- Grünewald, A.; et al. Mitochondrial DNA Depletion in Respiratory Chain–Deficient Parkinson Disease Neurons. Annals of Neurology 2016, 79, 366–378. [Google Scholar] [CrossRef]
- González-Rodríguez, P.; et al. Disruption of mitochondrial complex I induces progressive parkinsonism. Nature 2021, 599, 650–656. [Google Scholar] [CrossRef]
- Thomas, B.; Beal, M.F. Mitochondrial therapies for Parkinson's disease. Movement Disorders, 2010, 25(S1), S155-S160.
- Matthews, R.T.; et al. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp Neurol 1999, 157, 142–149. [Google Scholar] [CrossRef]
- Klivenyi, P.; et al. Additive neuroprotective effects of creatine and a cyclooxygenase 2 inhibitor against dopamine depletion in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. J Mol Neurosci 2003, 21, 191–198. [Google Scholar] [CrossRef]
- Cheah, I.K.; Halliwell, B. Ergothioneine, recent developments. Redox Biol 2021, 42, 101868. [Google Scholar] [CrossRef]
- Halliwell, B.; Cheah, I. Ergothioneine, where are we now? FEBS Letters 2022, 596, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Cheah, I. Are age-related neurodegenerative diseases caused by a lack of the diet-derived compound ergothioneine? Free Radic Biol Med 2024, 217, 60–67. [Google Scholar] [CrossRef]
- Halliwell, B.; Tang, R.M.Y.; Cheah, I.K. Diet-Derived Antioxidants: The Special Case of Ergothioneine. Annual Review of Food Science and Technology 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Hartman, P.E., [32] Ergothioneine as antioxidant, in Methods in Enzymology. 1990, Academic Press. p. 310-318.
- Tang, R.M.Y.; et al. Distribution and accumulation of dietary ergothioneine and its metabolites in mouse tissues. Sci Rep 2018, 8, 1601. [Google Scholar] [CrossRef] [PubMed]
- Novotny, B.C.; et al. Metabolomic and lipidomic signatures in autosomal dominant and late-onset Alzheimer's disease brains. Alzheimers Dement 2023, 19, 1785–1799. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.S.; et al. Effect of Ergothioneine on 7-Ketocholesterol-Induced Endothelial Injury. NeuroMolecular Medicine 2021, 23, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Leow, D.M.; et al. Protective Effect of Ergothioneine against 7-Ketocholesterol-Induced Mitochondrial Damage in hCMEC/D3 Human Brain Endothelial Cells. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.Y.; et al. Protective Effect of Ergothioneine Against Stroke in Rodent Models. Neuromolecular Med 2023, 25, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, P.K.; et al. Eugenol attenuates ischemia-mediated oxidative stress in cardiomyocytes via acetylation of histone at H3K27. Free Radic Biol Med 2023, 194, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; et al. Ergothioneine is associated with reduced mortality and decreased risk of cardiovascular disease. Heart 2020, 106, 691–697. [Google Scholar] [CrossRef]
- Brancaccio, M.; et al. First evidence of dermo-protective activity of marine sulfur-containing histidine compounds. Free Radic Biol Med 2022, 192, 224–234. [Google Scholar] [CrossRef]
- Gao, Y.; et al. l-Ergothioneine Exhibits Protective Effects against Dextran Sulfate Sodium-Induced Colitis in Mice. ACS Omega 2022, 7, 21554–21565. [Google Scholar] [CrossRef]
- Roda, E.; et al. Cognitive Healthy Aging in Mice: Boosting Memory by an Ergothioneine-Rich Hericium erinaceus Primordium Extract. Biology 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.-C.; et al. Ergothioneine protects against neuronal injury induced by β-amyloid in mice. Food and Chemical Toxicology 2012, 50, 3902–3911. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; et al. TrkB phosphorylation in serum extracellular vesicles correlates with cognitive function enhanced by ergothioneine in humans. npj Science of Food 2024, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Apparoo, Y.; et al. Ergothioneine and its prospects as an anti-ageing compound. Exp Gerontol 2022, 170, 111982. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; et al. Identification of novel biomarkers for Parkinson's disease by metabolomic technologies. J Neurol Neurosurg Psychiatry 2016, 87, 295–301. [Google Scholar] [CrossRef]
- Gründemann, D.; et al. Discovery of the ergothioneine transporter. Proc Natl Acad Sci U S A 2005, 102, 5256–5261. [Google Scholar] [CrossRef] [PubMed]
- Gründemann, D.; Hartmann, L.; Flögel, S. The ergothioneine transporter (ETT): Substrates and locations, an inventory. FEBS Letters 2022, 596, 1252–1269. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; et al. Gene knockout and metabolome analysis of carnitine/organic cation transporter OCTN1. Pharm Res 2010, 27, 832–840. [Google Scholar] [CrossRef]
- Park, J.S.; Davis, R.L.; Sue, C.M. Mitochondrial Dysfunction in Parkinson's Disease: New Mechanistic Insights and Therapeutic Perspectives. Curr Neurol Neurosci Rep 2018, 18, 21. [Google Scholar] [CrossRef]
- Hiller, B.M.; et al. Optimizing maturity and dose of iPSC-derived dopamine progenitor cell therapy for Parkinson’s disease. npj Regenerative Medicine 2022, 7, 24. [Google Scholar] [CrossRef]
- Salari, S.; Bagheri, M. In vivo, in vitro and pharmacologic models of Parkinson's disease. Physiol Res 2019, 68, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; et al. The Parkinsonian mimetic, 6-OHDA, impairs axonal transport in dopaminergic axons. Molecular Neurodegeneration 2014, 9, 17. [Google Scholar] [CrossRef]
- Mendes-Pinheiro, B.; et al. Unilateral Intrastriatal 6-Hydroxydopamine Lesion in Mice: A Closer Look into Non-Motor Phenotype and Glial Response. International Journal of Molecular Sciences 2021, 22, 11530. [Google Scholar] [CrossRef] [PubMed]
- Blum, D.; et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson's disease. Prog Neurobiol 2001, 65, 135–172. [Google Scholar] [CrossRef] [PubMed]
- Latchoumycandane, C.; et al. Dopaminergic neurotoxicant 6-OHDA induces oxidative damage through proteolytic activation of PKCδ in cell culture and animal models of Parkinson's disease. Toxicol Appl Pharmacol 2011, 256, 314–323. [Google Scholar] [CrossRef]
- Weihe, E.; et al. Three types of tyrosine hydroxylase-positive CNS neurons distinguished by dopa decarboxylase and VMAT2 co-expression. Cell Mol Neurobiol, 2006; 26, 659–678. [Google Scholar]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Molecular Neurodegeneration 2017, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.R., L.S. Hu, and G.Y. Li, SH-SY5Y human neuroblastoma cell line: In vitro cell model of dopaminergic neurons in Parkinson's disease. Chin Med J (Engl) 2010, 123, 1086–1092.
- Avazzadeh, S.; et al. Modelling Parkinson's Disease: iPSCs towards Better Understanding of Human Pathology. Brain Sci 2021, 11. [Google Scholar] [CrossRef]
- Mahajani, S.; et al. Homogenous generation of dopaminergic neurons from multiple hiPSC lines by transient expression of transcription factors. Cell Death & Disease 2019, 10, 898. [Google Scholar]
- Yang, N.-C., A Convenient One-Step Extraction of Cellular ATP Using Boiling Water for the Luciferin–Luciferase Assay of ATP. Analytical Biochemistry 2002, 306, 323–327. [CrossRef]
- Engel, M.; et al. Common pitfalls of stem cell differentiation: A guide to improving protocols for neurodegenerative disease models and research. Cellular and Molecular Life Sciences 2016, 73, 3693–3709. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; et al. Combined small-molecule inhibition accelerates the derivation of functional cortical neurons from human pluripotent stem cells. Nat Biotechnol 2017, 35, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Gaia, S.; et al. Mutant LRRK2 Toxicity in Neurons Depends on LRRK2 Levels and Synuclein But Not Kinase Activity or Inclusion Bodies. The Journal of Neuroscience 2014, 34, 418. [Google Scholar]
- Fu, T.-T.; L. Shen, Ergothioneine as a Natural Antioxidant Against Oxidative Stress-Related Diseases. Frontiers in Pharmacology 2022, 13.
- Nguyen, T.H., R. Nagasaka, and T. Ohshima, CHAPTER 12 - The Natural Antioxidant Ergothioneine: Resources, Chemical Characterization, and Applications, in Lipid Oxidation, A. Logan, U. Nienaber, and X. Pan, Editors. 2013, AOCS Press. p. 381-415.
- Murphy, M.P.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nature Metabolism 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Suzuki, Y.J., M. Carini, and D.A. Butterfield, Protein carbonylation. Antioxid Redox Signal 2010, 12, 323–325.
- Zhang, X.; et al. Tau Pathology in Parkinson's Disease. Front Neurol 2018, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Callizot, N.; et al. Necrosis, apoptosis, necroptosis, three modes of action of dopaminergic neuron neurotoxins. PLoS ONE 2019, 14, e0215277. [Google Scholar] [CrossRef]
- Wang, S.-F.; et al. Baicalein prevents 6-OHDA/ascorbic acid-induced calcium-dependent dopaminergic neuronal cell death. Scientific Reports 2017, 7, 8398. [Google Scholar] [CrossRef]
- Halliwell, B., I.K. Cheah, and C.L. Drum, Ergothioneine, an adaptive antioxidant for the protection of injured tissues? A hypothesis. Biochem Biophys Res Commun 2016, 470, 245–250. [CrossRef]
- Li; et al., Uptake and protective effects of ergothioneine in human endothelial cells. J Pharmacol Exp Ther 2014, 350, 691–700. [CrossRef] [PubMed]
- Cheah; et al., Liver ergothioneine accumulation in a guinea pig model of non-alcoholic fatty liver disease. A possible mechanism of defence? Free Radic Res 2016, 50, 14–25. [CrossRef] [PubMed]
- Shinozaki, Y.; et al. Impairment of the carnitine/organic cation transporter 1–ergothioneine axis is mediated by intestinal transporter dysfunction in chronic kidney disease. Kidney International 2017, 92, 1356–1369. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; et al. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res 2013, 8, 2003–2014. [Google Scholar] [PubMed]
- Blum, D.; et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson's disease. Progress in Neurobiology 2001, 65, 135–172. [Google Scholar] [CrossRef] [PubMed]
- Park, B.C.; et al. Protective effects of fustin, a flavonoid from Rhus verniciflua Stokes, on 6-hydroxydopamine-induced neuronal cell death. Experimental & Molecular Medicine 2007, 39, 316–326. [Google Scholar]
- Kwon, S.H.; et al. Suppression of 6-Hydroxydopamine-Induced Oxidative Stress by Hyperoside Via Activation of Nrf2/HO-1 Signaling in Dopaminergic Neurons. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.Y.; et al. Protective Effect of Ergothioneine Against Stroke in Rodent Models. Neuromolecular Med, 2022.
- Yuzawa, S.; et al. Ergothioneine Prevents Neuronal Cell Death Caused by the Neurotoxin 6-Hydroxydopamine. Cells 2024, 13. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.S.; et al. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. American Journal of Physiology-Cell Physiology 2004, 287, C817–C833. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; et al. Nimodipine, an L-type calcium channel blocker attenuates mitochondrial dysfunctions to protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in mice. Neurochem Int 2016, 99, 221–232. [Google Scholar] [CrossRef]
- Epand, R.M. The role of dietary ergothioneine in the development of diabetes mellitus. Med Hypotheses 1982, 9, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, M., R.C. Bollineni, and R. Hoffmann, Protein carbonylation as a major hallmark of oxidative damage: Update of analytical strategies. Mass Spectrom Rev 2014, 33, 79–97. [CrossRef]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nature Reviews Molecular Cell Biology 2012, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Estébanez, B.; et al. Endoplasmic Reticulum Unfolded Protein Response, Aging and Exercise: An Update. Front Physiol 2018, 9, 1744. [Google Scholar] [CrossRef]
- Holtz, W.A.; et al. Oxidative stress-triggered unfolded protein response is upstream of intrinsic cell death evoked by parkinsonian mimetics. Journal of Neurochemistry 2006, 99, 54–69. [Google Scholar] [CrossRef]
- Bizzozero, O.A., Protein Carbonylation in Neurodegenerative and Demyelinating CNS Diseases, in Handbook of Neurochemistry and Molecular Neurobiology: Brain and Spinal Cord Trauma, A. Lajtha, N. Banik, and S.K. Ray, Editors. 2009, Springer US: Boston, MA. p. 543-562.
- Di Rita, A.; et al. AMBRA1-Mediated Mitophagy Counteracts Oxidative Stress and Apoptosis Induced by Neurotoxicity in Human Neuroblastoma SH-SY5Y Cells. Frontiers in Cellular Neuroscience 2018, 12. [Google Scholar] [CrossRef]
- Elkon, H., E. Melamed, and D. Offen, Oxidative stress, induced by 6-hydroxydopamine, reduces proteasome activities in PC12 cells: Implications for the pathogenesis of Parkinson's disease. J Mol Neurosci 2004, 24, 387–400. [CrossRef]
- Smith, M.P.; W.A. Cass, Oxidative stress and dopamine depletion in an intrastriatal 6-hydroxydopamine model of Parkinson's disease. Neuroscience 2007, 144, 1057–1066. [CrossRef]
- Lamhonwah, A.M.; I. Tein, Novel localization of OCTN1, an organic cation/carnitine transporter, to mammalian mitochondria. Biochem Biophys Res Commun 2006, 345, 1315–1325. [CrossRef]
- Shitara, Y.; et al. Role of Organic Cation/Carnitine Transporter 1 in Uptake of Phenformin and Inhibitory Effect on Complex I Respiration in Mitochondria. Toxicological Sciences 2012, 132, 32–42. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




