1. Introduction
The Global Burden of Disease has considered migraines as one of the most prevalent and disabling burdens worldwide, especially among the working population. Because of their recurrent nature, long duration, and adverse effect on the quality of life, migraines were internationally ranked second with regards to the years-lived with disabilities, by the Global Burden of Disease study in 2016 [
1].
The neurophysiology of headaches indicates that the neuro-fibers, namely C-fibers and A-delta (Aδ) fibers play a significant role in the perception of headaches. Specifically, stimulation of C-fibers causes slow building aching, throbbing, or burning headaches, whereas stimulation of the Aδ fiber causes sharp and painful headaches [
2]. The sensory neurons, C-fibers and Aδ fibers, are found in the trigeminal ganglion and nerve terminals [
2]. Calcitonin gene-related peptides (CGRP) have recently been discovered to stimulate the trigeminal fibers [
2,
3]. Unmyelinated C-fiber sensory nerves are characterized by high CGRP expression, while myelinated Aδ-sensory nerves that are found in the peripheral nervous system have a high concentration of CGRP receptors. During the onset of a migraine, CGRP is released from the C-fiber of the trigeminal nerve ganglion and the trigeminal nerve terminal. This release subsequently stimulates the Aδ fiber, leading to the individual experiencing sharp, painful migraine headaches [
3,
4].
Triptans are 5-HT
1B and 5-HT
1D receptor agonists, and 5-HT
1D receptors are found at the Aδ-neurofiber terminal and commonly prescribed as migraine-specific abortive medications [
5]. Nonsteroidal anti-inflammatory drugs (NSAIDs) and nonspecific analgesia are only effective for treating mild headaches and migraine attacks. It is not uncommon for a migraine attack to remain unabated with a single tablet of triptan, which leads to the cycling of triptans or NSAIDs, which not only leads to a failure in controlling headaches associated with migraine attacks, but continuous intake of both triptans as well as NSAIDs can also result in medication overuse headaches (MOH). NSAIDs are easily available to patients as over-the-counter medications and do not require a prescription, whereas triptans require a prescription. Therefore, NSAIDs are more commonly associated with MOH [
5]. This conundrum has inspired medical researchers to search for a new specific medication that will be more selective in migraine treatment and will reduce the risk of MOHs for patients already suffering from an acute migraine attack.
Lasmiditan is a 5-HT
1F receptor agonist is a recently developed drug for treating acute migraines [
6]. 5-HT
1F receptor is present at C-fiber as well as Aδ-fiber terminals. Positive stimulation of the 5-HT
1F receptor on the C-fiber terminal by the drug inhibits CGRP release from the C-fiber terminal. In addition, the drug’s positive stimulation of 5-HT
1F receptor on Aδ-fiber terminal, inhibits pain signal transmission from the Aδ-fibers.
Both triptan and lasmiditan have exhibited high efficacies for the treatment of migraine attacks with varying intensity; however, triptans are contraindicated for some patients, such as those diagnosed with cardiovascular diseases, cerebrovascular diseases, hemiplegic migraines, and migraines with brainstem aura. Lasmiditan is a good medical alternative for these patients. In addition, time of administration of triptans plays a crucial role in their efficacy. Treatment with triptans needs to be initiated as early as possible after onset of migraine attack, as late intake of triptans has been observed to be ineffective [
7]. In contrast, the efficacy of lasmiditan is independent of the time of migraine onset [
8]. In addition, lasmiditan has shown to be effective in treating acute migraine attack in patients whose symptoms could not be abated by triptans [
9].
Currently, lasmiditan is commercially available at two doses (50 and 100 mg), and its efficacy and associated adverse events (AEs) have been observed to be dose-dependent [
10]. Although Japanese pharmaceuticals recommend the standard and starting dose of lasmiditan to be 100 mg [
11], this dosage has been associated with frequent AEs. The severity of AEs (dizziness and drowsiness) has resulted in discontinuation of treatment and hesitation among patients to continue with the treatment. In the United States, the recommended dose of lasmiditan is 50, 100, or 200 mg [
12].
Lasmiditan and triptan have different mechanisms of action, because of which the combination of these two different classes of drugs could be compensatory or synergistic. The combined use of lasmiditan and triptan has not yet been reported in the medical literature.
The aim of this study was to investigate whether the acute treatment protocol involving sequential intake of lasmiditan after prior intake of triptan would result in better resolution of migraine pain. Patients whose acute migraine attacks were previously treated unsuccessfully with a single tablet of triptan were included in this study. Our secondary goal was to investigate the AEs associated with the administration of an additional 50 mg of lasmiditan for patients with persistent migraines even after taking triptans (Lasmiditan Addition for the Patients after POor Results of Triptan: LAPPORT). In this study, we selected 50 mg as the additional lasmiditan dose, because AEs of lasmiditan is dose dependent. Our hypothesis is additional 50 mg of lasmiditan is effective and safe for the patients whom one tablet of triptan is not effective enough.
2. Patients and Methods
2.1. Patients
The patients participating in this study had been previously diagnosed with migraines by board-certified headache specialists. The participating patients had no previous exposure to lasmiditan before their participation in this study. Only patients who had taken a single tablet of triptan (zolmitriptan, rizatriptan, or eletriptan) and self-reported unsatisfactory results were included in this study. All patients were Japanese and had no vascular disease.
2.2. Methods
The outcomes of this study were headache intensity evaluated using the numerical rating scale (NRS) and any AEs at 1, 2, and 4 hours after taking additional 50 mg of lasmiditan.
The following intake instructions were given to participants:
1. At the onset of a migraine attack, ingest a single tablet of triptan within 30 minutes after the initiation of the migraine attack. 2. At 1 hour following the intake of triptan, if headache relief is less than 50%, proceed to take lasmiditan 50 mg. 3. Patients were required to record headache intensity and any AEs on paper caused by lasmiditan at 1, 2, and 4 hours after taking an additional 50 mg of lasmiditan. Pain relief was defined as an improvement in NRS by more than 2 points, and pain-free condition was defined as an NRS value of less than 2. We instructed the participants to not consume more than one tablet of triptan and lasmiditan each on the same day.
Three facilities, two community hospitals and one private clinic, participated in this study, and study approval was granted by the institutional review boards at each participating facility. This study was conducted at out-patient clinics in these three facilities.
This study had been registered in the public database of the University Hospital Medical Information Network (UMIN) as a clinical trial prior to the initiation of the study (UMIN ID UMIN000050092).
2.3. Statistical analysis
Statistical analysis for paired groups was conducted using a two-tailed t-test program on SPSS version 28.00 (IBM, Tokyo, Japan). A p value of <0.05 was considered statistically significant.
3. Results
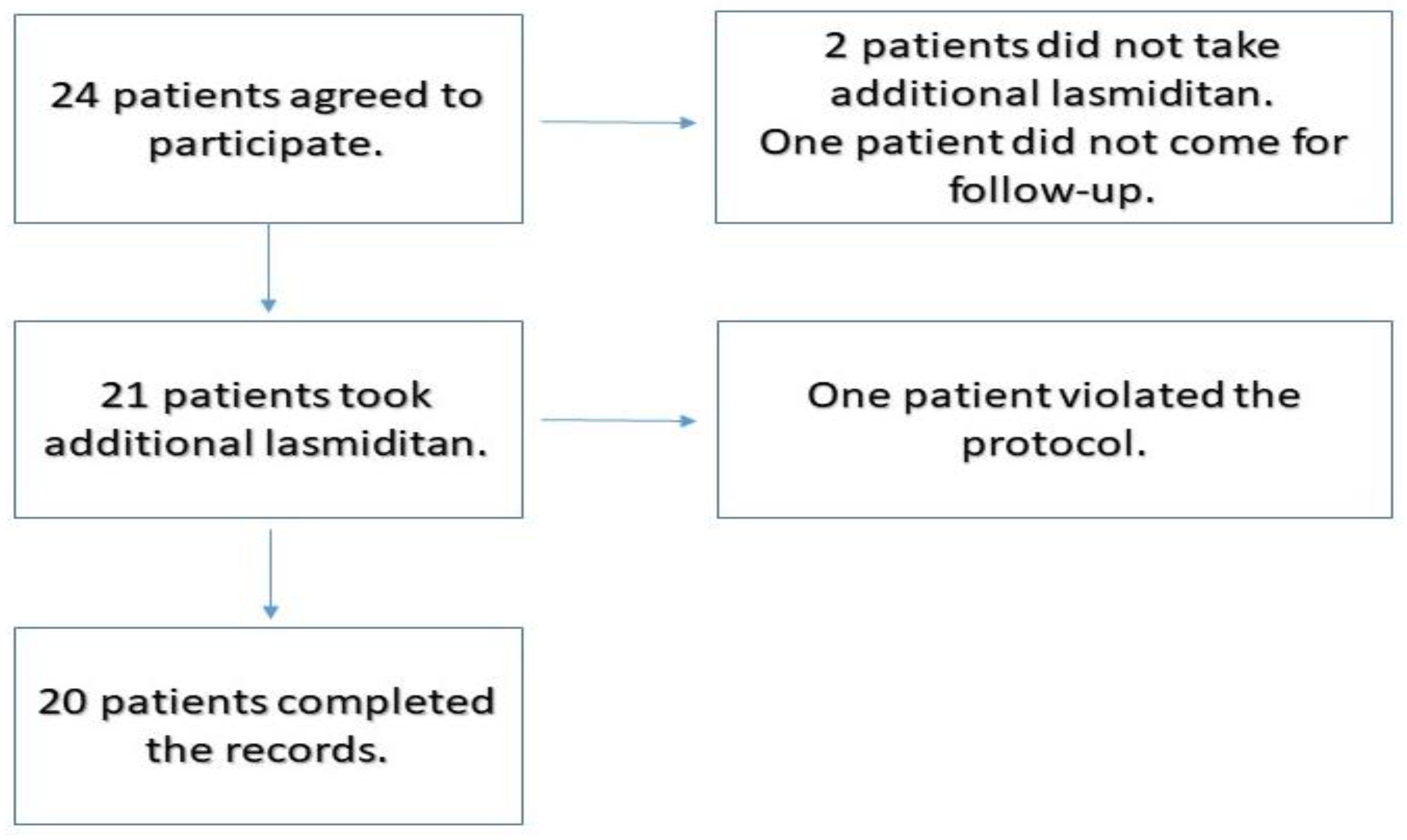
Twenty-four patients had participated in the study (
Figure 1). Two patients were excluded because they did not take the lasmiditan tablet as per the protocol. One patient did not return for follow up. Among the 21 patients who took additional lasmiditan and completed the reports, 1 patient violated the protocol and did not take additional lasmiditan at the designated time periods.
The final number of participants remaining in the study, whose results were recorded was 20. There were 4 males and 16 females in this group. The ages of the participants ranged from 14 years to 62 years, with the mean age being 43.1 years. A total of 40 migraine attacks were treated and recorded in the study. Eleven patients used this treatment protocol only once. Four patients used twice, 2 patients used three times and 3 patients used 5 times.
Among the 40 migraine attacks that were treated and recorded in the study, the records for the 2 hours post lasmiditan intake were partially missing for 12 migraine attacks in four patients, since they fell asleep. For these patients, records obtained until one hour after lasmiditan intake were included in the analysis.
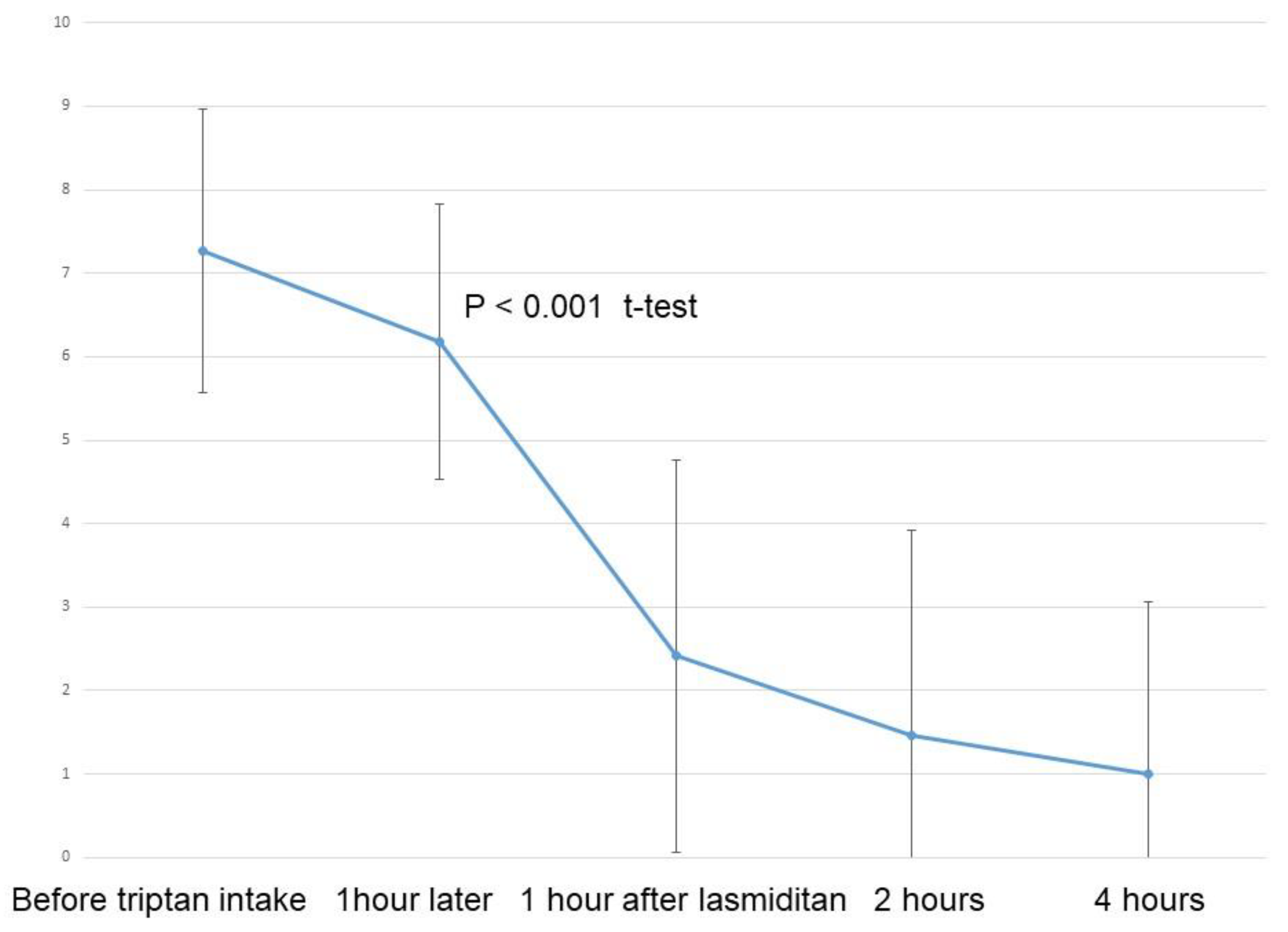
Figure 2 depicts the results for NRS for all 40 migraine attacks. A significant reduction in NRS was observed following intake of lasmiditan 50 mg (p < 0.001, t-test). Pain relief, defined as an improvement in NRS by more than 2 points, was reported for 32 migraine attacks (80%) at one hour, and for 24 migraine attacks (86%) at two hours after additional lasmiditan intake (
Figure 3).
Relief from pain status (pain-free), defined as NRS value of less than 2, was reported in 17 migraine attacks (43%) at one hour and for 16 migraine attacks (57%) at two hours following additional lasmiditan intakes (
Figure 3).
The AEs, which were mostly dizziness and drowsiness, were observed in 25 migraine attacks (63%) at one hour after additional lasmiditan intake (
Figure 3). However, a reduction in these AEs was observed in six migraine attacks (21%), two hours following additional lasmiditan intake. No same-day migraine headache recurrence was observed in our study population.
The results of 17 migraine attacks that were initially treated with this protocol were also analyzed and a significant reduction in NRS was observed (p < 0.001, t-test), after the intake of 50 mg lasmiditan (
Figure 4). Pain relief, defined as reduction in NRS by 2 points, was achieved in 16 migraine attacks (94%) at one hour and in 13 migraine attacks (93%) at two hours following additional lasmiditan intakes (
Figure 3). Pain-free status, defined as NRS less than 2, was observed in nine migraine attacks (53%) at one hour and 11 migraine attacks (79%) at two hours after additional lasmiditan intakes. The AEs, mostly dizziness and drowsiness, were observed in 14 migraine attacks (82%) at one hour after additional lasmiditan intake. However, a reduction in these AEs was observed for five among the 14 migraine attacks (36%) at two hours after the additional lasmiditan intake.
4. Discussion
The results of this study indicate that participants who continued to experience migraine even after taking a triptan benefited from the intake of lasmiditan 50 mg, leading to a significant improvement in headache severity. Although AEs were observed in 63% of the patients who took an additional 50 mg of lasmiditan, most were mild and resolved 1 hour after lasmiditan intake.
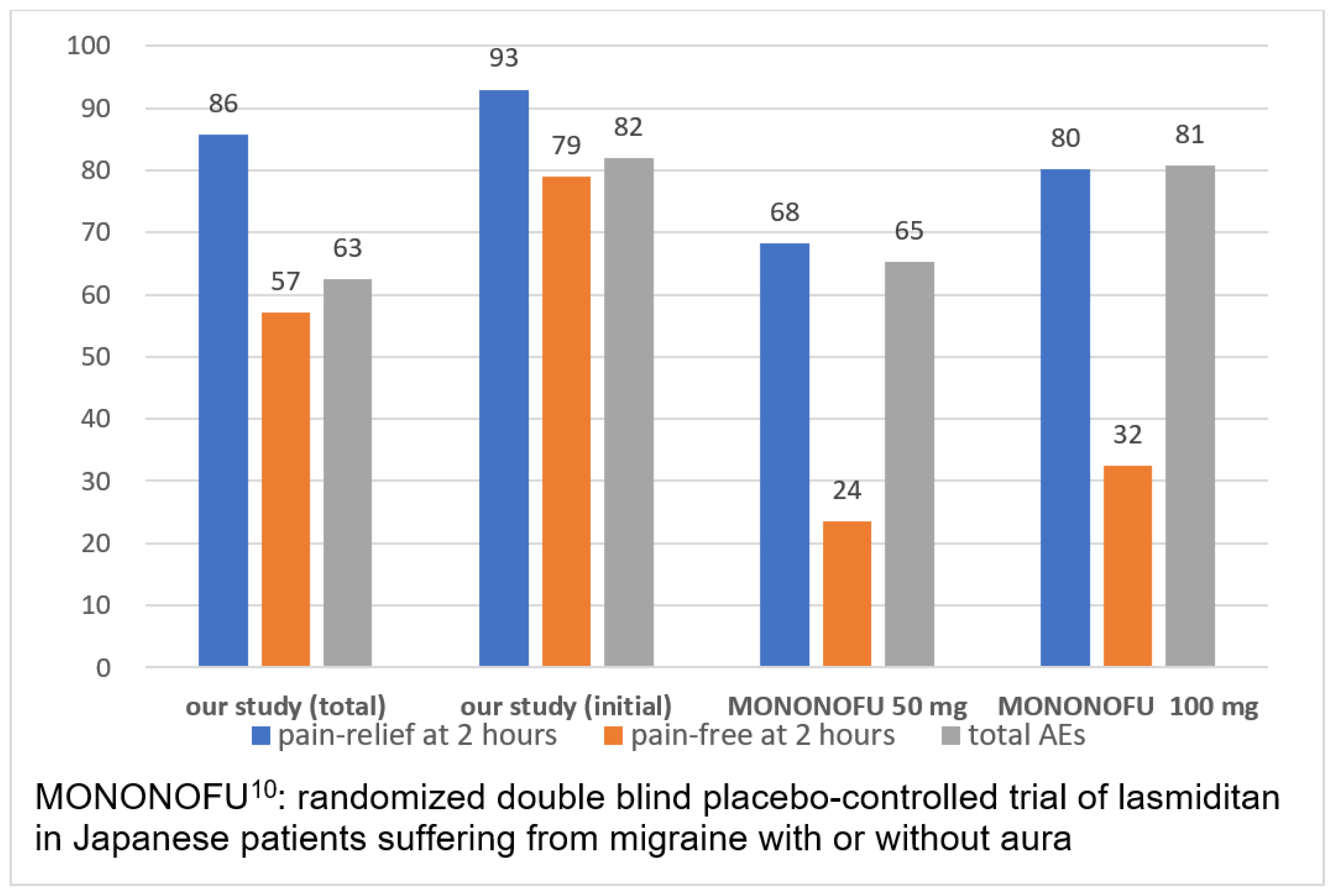
In a phase 2 randomized placebo-controlled study, MONONOFU, the acute treatment of migraine in Japanese patients with lasmiditan was investigated [
8]. Pain-relief rates observed 2 hours post-dose were 55.0% for the placebo, 68.2% for lasmiditan 50 mg, 80.2% for lasmiditan 100 mg, and 78.2% for lasmiditan 200 mg. Pain-free rates observed 2 hours post-dose were 16.6% for placebo, 23.5% for lasmiditan 50 mg, 32.4% for lasmiditan 100 mg, and 40.8% for lasmiditan 200 mg (
Figure 3). Pain-free rates observed after administration of lasmiditan 50 mg were not significantly different from those of placebo. However, as the sample size for this study was small, calculations to ensure a certain statistical power in comparison with placebo could not be achieved. Pain-free and pain-relief rates showed significant improvement compared with those obtained with a placebo. In our study, 80% of patients achieved pain relief, defined as an improvement in the NRS of more than 2 points, at 1 hour and 86% at 2 hours after additional lasmiditan intake. Pain-free status, defined as a NRS of less than 2, was achieved by 43% of patients at 1 hour and 57% at 2 hours after additional lasmiditan intake. In the MONONOFU study, migraine severity was recorded using a 4-point headache severity rating scale, whereas the NRS was used to rate headache pain in our study. This complicates the comparison of results obtained in this study with those obtained in the MONONOFU study [
8]. Conversely, our study suggests better headache elimination with an additional 50 mg of lasmiditan.
In global phase 3 studies (SAMURAI and SPARTAN), 14%–15% of participants experienced headache recurrence after lasmiditan intake [
10,
13]. In the MONONOFU study, sustained pain-free rates at 24 and 48 hours were 14.9% for lasmiditan 50 mg and around 20% for lasmiditan 100 mg [
8]. So significant proportion of the patients reported migraine headache recurrence on the same day. No migraine headache recurrence on the same day was observed in our study population. So our results suggested better control of migraine headache may be obtained using our treatment protocol.
AEs were also found to be dose-dependent in the MONONOFU study. Our study found similar rates of AEs with the MONOFOFU study at lasmiditan doses of 50 mg. A higher treatment-emergent rate of AEs was reported in the MONONOFU study compared to other global phase 3 studies [
8,
13,
14]. The cause of this difference can most likely be attributed to the differences in data collection methods, informed consent methods, and the body mass indices of Japanese participants in comparison with non-Asian population participants [
8,
15]. Mean body mass indices of the participants in the MONONOFU study was 22.6 kg/m
2 and mean body mass indices of global phase 3 studies were from 30 to 31 kg/m
2.
Our study analyzed the results for headache relief observed in migraine attacks treated with secondary treatment of 50 mg lasmiditan after unsatisfactory results observed with triptan. The numbers of migraine attacks reported in this study varied for each patient. The patients with favorable effect of additional lasmiditan might have recorded more migraine attacks in this study. In addition, repeat intake of lasmiditan is effective in reducing the AEs caused by lasmiditan [
9]. To prevent these factors from introducing a bias in our study results, we conducted a separate analysis that included patients who took additional lasmiditan for the first time using this treatment protocol as this treatment study for migraine headaches. Our analyses revealed higher rates of headache relief, pain-free patients, and higher incidence of AEs for the patients who took additional lasmiditan for the first time in this study. These results indicate reduced incidence of AEs might with repeat intake of lasmiditan as anticipated. An important finding from this study is that the clinical effects of lasmiditan were observed from the first intake of lasmiditan, confirming its efficacy as an anti-migraine medication.
Based on the results obtained, we would recommend a step care treatment, consisting of initial triptan followed by lasmiditan if needed, for treating the symptoms of migraines. The Disability in Strategies of Care study investigated stratified care (strategy of rigid predetermined medications to give an ailing patient with no rescue measures) versus step care strategies for treating acute migraine attacks [
16]. In this randomized, controlled, parallel-group clinical trial, three strategies were compared. In accordance with this stratified care program, grade II patients based on Migraine Disability Assessment Scale (MIDAS), would be treated with aspirin plus metoclopramide, and patients with MIDAS grade III and IV would be treated with zolmitriptan. MIDAS is a self-assessment questionnaire aimed at measuring the impact of headaches. MIDAS grade II, III, and IV define mild, moderate, and severe disabilities. In step care plan, across attacks, initial treatment was aspirin plus metoclopramide. Patients who did not achieve satisfactory results in at least two of the first three attacks are switched to zolmitriptan. In step care within attacks, initial treatment was aspirin and metoclopramide. Patients not responding to this treatment after two hours at the beginning of each attack were shifted to zolmitriptan. As the results indicated, stratified care provided significantly better clinical outcomes compared with step care strategies within or across attacks as indicated by headache response and disability time. In these step care strategies, aspirin plus metoclopramide were used as initial treatment agents. In step care strategy within attacks, triptan was not administered within 2 hours in each attack. However, our study used triptan as an initial medication. As step care within attacks, lasmiditan was taken within 1.5 hours after the initiation of migraine attack. We believe that migraine-specific medication should be administered as early as possible. Our study results indicated to recommend a step care treatment consisting of initial triptan followed by lasmiditan if needed for acute treatment of migraine. Treatment strategy of migraine should be tailor made for each migraine attacks, not each migraine patients, because every migraine patient has various migraine attacks.
Although easy access and cost of aspirin and NSAIDs are the major factors based on which it is recommended as the first medication treatment for migraines, it is associated with several AEs, making it inferior as an abortive treatment. We believe that migraine-specific drugs should be used to treat migraine attacks instead for the abortive treatments. Rothrock recommended different therapies for acute migraine treatment, since symptoms of a migraine attack might vary across attacks [
17]. He proposed additional rescue therapy despite initial treatment as “stratified” care. We believe that this additional therapeutic strategy is referred to as step care. From the view-points of shared decision making and patient education of self-medication, this treatment strategy is beneficial and rapport will be obtained.
As an acute medication for migraine attack, triptans are commercially available in most developed countries. The benefits of triptan are their long history and experiences in clinical applications, availability of several brands, and the availability of drugs in various forms including hard tablets, orally disintegrating tablets, subcutaneous injections and nasal sprays. Gepants, such as rimegapant, are new oral CGRP antagonists used for acute treatment as well as the prevention of migraine attacks. While gepants are commercially available in some countries, all gepants are currently under clinical trials in Japan. A prior meta-analysis has indicated favorable outcomes in terms of pain freedom and pain relief 2 hours after the intake of gepants [
18].
Our study had some limitations. This was a single-armed study and did not involve comparison with a placebo or other medications. Furthermore, triptans may not be effective within 1 hour after consumption. The effect of additional lasmiditan in our study may partially reflect the late effect of triptans. However, in our study, only patients who had experienced frequent unsatisfactory effects of triptans were included. Whether the results of this study can be generalized for all patients remains unclear. In the future, we plan to conduct a study that compares the efficacy of patients receiving lasmiditan as a second-line drug in comparison to patients receiving a placebo. Since our study was a single-armed study, the cost benefits of our treatment strategy were not evaluated. This aspect will be also analyzed in future studies. Since the study population was small, various sub-analyses, including migraine types, disease durations, concomitant prophylactic medications, triptan brand, and MIDAS class were not possible. However, most patients in our study needed frequent intake of triptans, and standard dosing recommendations of triptans were not enough. Having a large-scale data can provide the answers for these questions. Our study population was limited to migraine patients who have some experiences of triptans. Most these patients had not experienced any severe AEs of triptans. Our study result may not be applicable for the patients who did not have a history of triptan intake. Some patients could not record their headache information and AEs after two hours of additional lasmiditan intake. So long term effects and AEs beyond two hours following additional lasmiditan intake need to be clarified in future studies. Our study used NRS for self-evaluating headache. Some clinical trials used a 4-point pain scale [
8,
9,
10,
19]. Therefore, comparison of our study with these studies may be difficult. However, we believe that an 11-point NRS is more sensitive than a 4-point scale. Gepants are not commercially available in Japan, hence the clinical effects of gepants could not be evaluated. In future studies, combination or sequential studies of these acute medications of migraine-specific drugs should be studied.