Submitted:
06 May 2024
Posted:
08 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
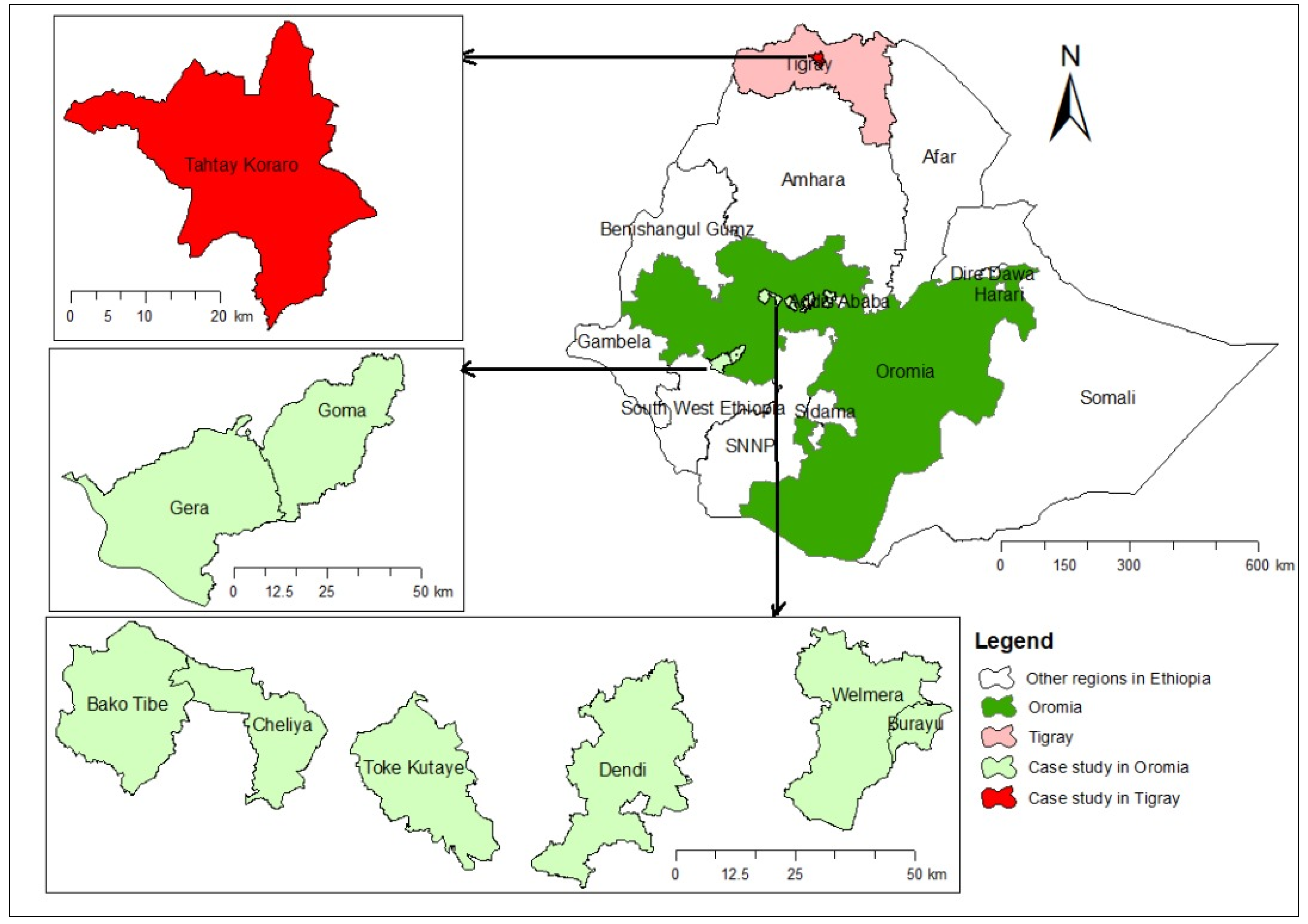
2.1. Study Regions and Description
2.2. Survey and Data Collection
2.3. Statistical Analysis
3. Results
3.1. Colony Management in Oromia and Tigray Regions
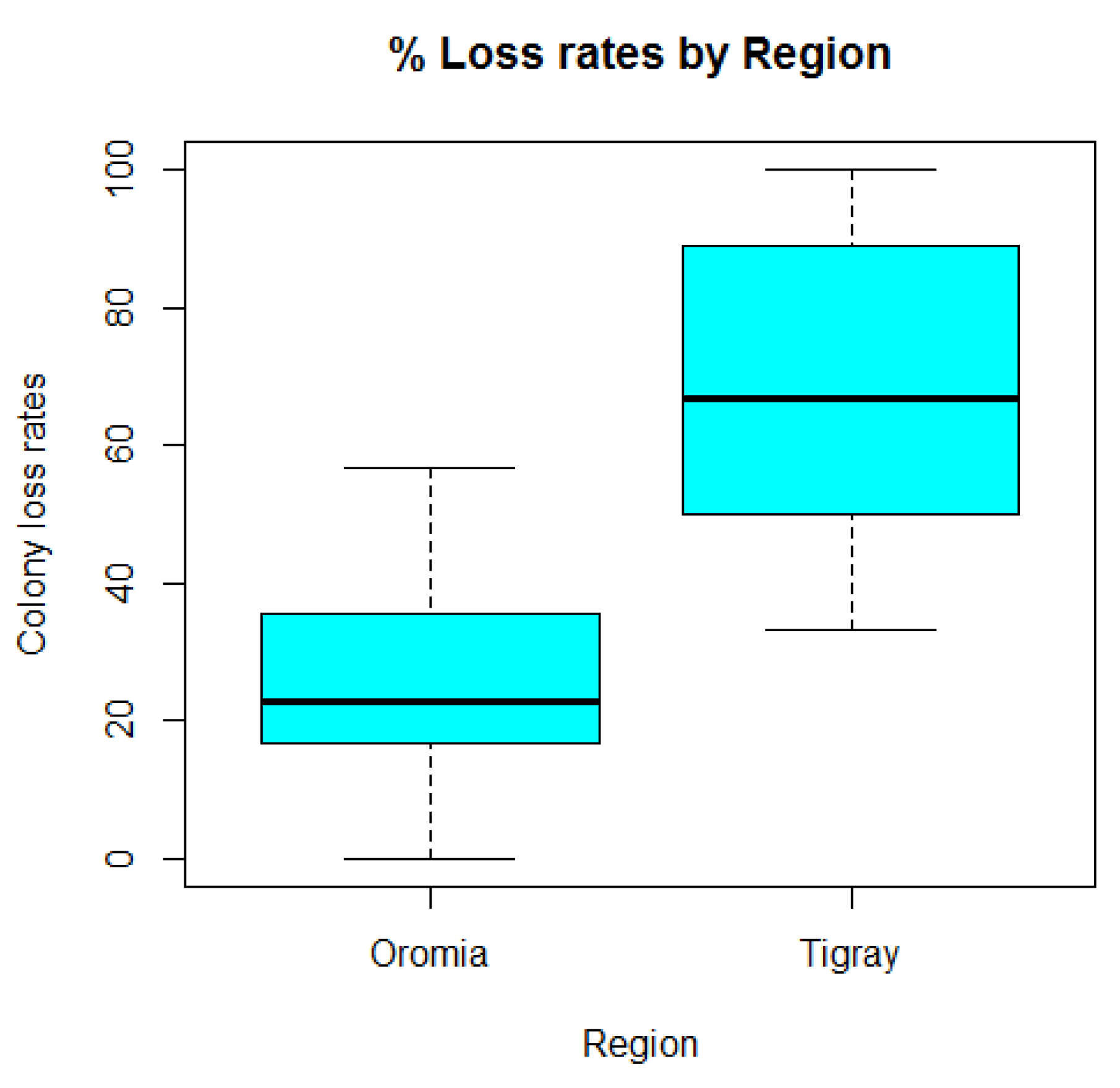
3.2. Colony Loss Rates, Components of Loss and Risk Factors
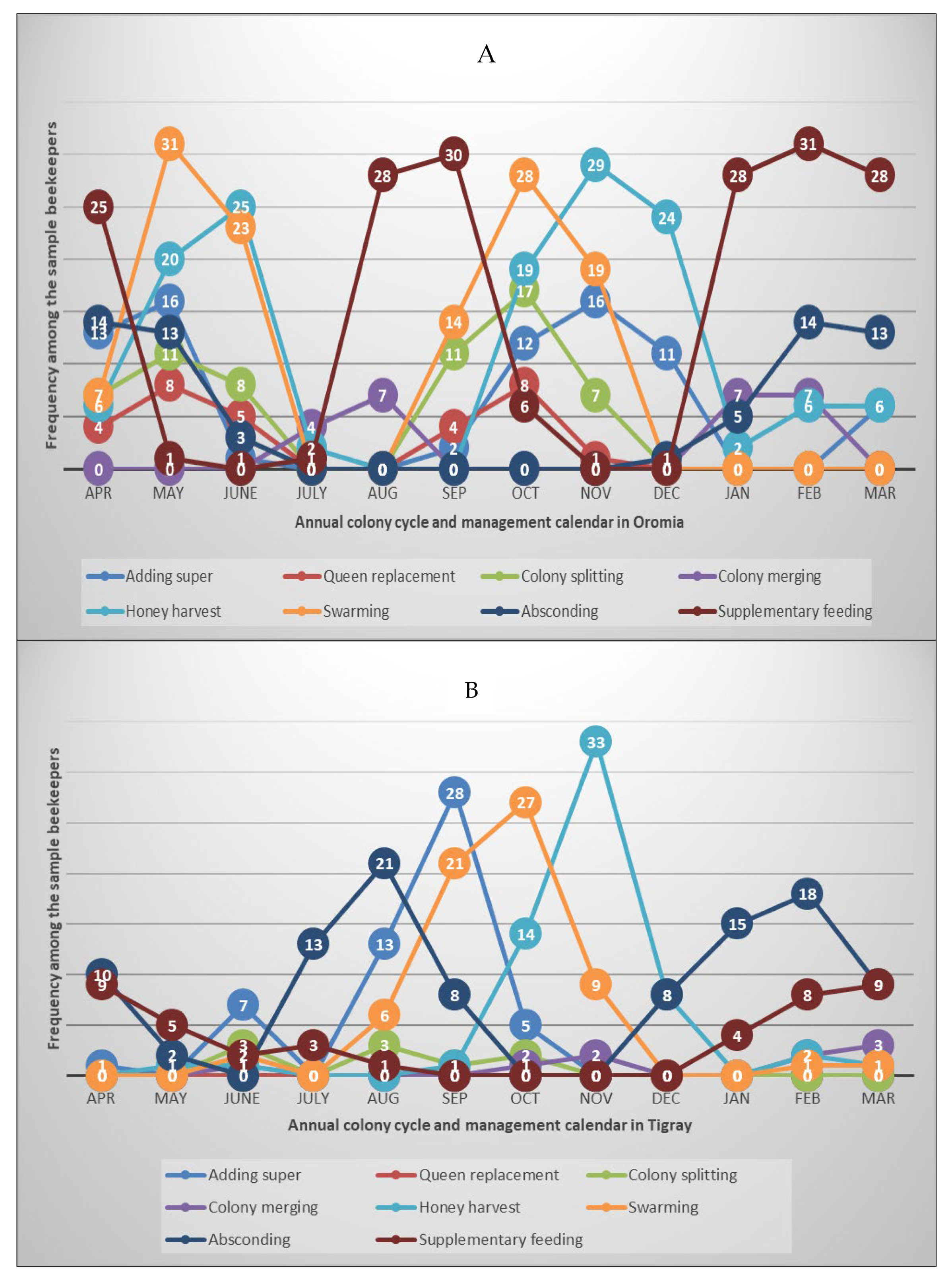
3.3. Annual colony development and management calendar
4. Discussion
Supplementary Materials
Author Contributions
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Van Engelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony Collapse Disorder: A Descriptive Study. PLoS One 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.D.; Evans, J.D.; Pettis, J. Colony Losses, Managed Colony Population Decline, and Colony Collapse Disorder in the United States. J Apic Res 2010, 49, 134–136. [Google Scholar] [CrossRef]
- Brodschneider, R.; Gray, A.; Adjlane, N.; Ballis, A.; Brusbardis, V.; Charrière, J.D.; Chlebo, R.; Coffey, M.F.; Dahle, B.; de Graaf, D.C.; et al. Multi-Country Loss Rates of Honey Bee Colonies during Winter 2016/2017 from the COLOSS Survey. J Apic Res 2018, 57, 452–457. [Google Scholar] [CrossRef]
- Gray, A.; Brodschneider, R.; Adjlane, N.; Ballis, A.; Brusbardis, V.; Charrière, J.D.; Chlebo, R.; F. Coffey, M.; Cornelissen, B.; Amaro da Costa, C.; et al. Loss Rates of Honey Bee Colonies during Winter 2017/18 in 36 Countries Participating in the COLOSS Survey, Including Effects of Forage Sources. J Apic Res 2019, 58, 479–485. [Google Scholar] [CrossRef]
- Gray, A.; Adjlane, N.; Arab, A.; Ballis, A.; Brusbardis, V.; Charrière, J.D.; Chlebo, R.; Coffey, M.F.; Cornelissen, B.; Amaro da Costa, C.; et al. Honey Bee Colony Winter Loss Rates for 35 Countries Participating in the COLOSS Survey for Winter 2018–2019, and the Effects of a New Queen on the Risk of Colony Winter Loss. J Apic Res 2020, 59, 744–751. [Google Scholar] [CrossRef]
- Gray, A.; Adjlane, N.; Arab, A.; Ballis, A.; Brusbardis, V.; Bugeja Douglas, A.; Cadahía, L.; Charrière, J.D.; Chlebo, R.; Coffey, M.F.; et al. Honey Bee Colony Loss Rates in 37 Countries Using the COLOSS Survey for Winter 2019–2020: The Combined Effects of Operation Size, Migration and Queen Replacement. J Apic Res 2023, 62, 204–210. [Google Scholar] [CrossRef]
- Aurell, D.; Bruckner, S.; Wilson, M.; Steinhauer, N.; Williams, G.R. A National Survey of Managed Honey Bee Colony Losses in the USA: Results from the Bee Informed Partnership for 2020–21 and 2021–22. J Apic Res 2024, 63, 1–14. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and Control of Varroa destructor. J Invertebr Pathol 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Guzmán-Novoa, E.; Eccles, L.; Calvete, Y.; McGowan, J.; Kelly, P.G.; Correa-Benítez, A. Varroa destructor Is the Main Culprit for the Death and Reduced Populations of Overwintered Honey Bee (Apis mellifera) Colonies in Ontario, Canada. Apidologie 2010, 41, 443–450. [Google Scholar] [CrossRef]
- Noël, A.; Le Conte, Y.; Mondet, F. Varroa destructor: How Does It Harm Apis mellifera Honey Bees and What Can Be Done about It? Emerg Top Life Sci 2020, 4, 45–57. [Google Scholar] [CrossRef]
- Brodschneider, R.; Schlagbauer, J.; Arakelyan, I.; Ballis, A.; Brus, J.; Brusbardis, V.; Cadahía, L.; Charrière, J.D.; Chlebo, R.; Coffey, M.F.; et al. Spatial Clusters of Varroa destructor Control Strategies in Europe. J Pest Sci (2004) 2023, 96, 759–783. [Google Scholar] [CrossRef]
- Blacquière, T.; Boot, W.; Calis, J.; Moro, A.; Neumann, P.; Panziera, D. Darwinian Black Box Selection for Resistance to Settled Invasive Varroa destructor Parasites in Honey Bees. Biol Invasions 2019, 21, 2519–2528. [Google Scholar] [CrossRef]
- Martin, S.J.; Hawkins, G.P.; Brettell, L.E.; Reece, N.; Correia-Oliveira, M.E.; Allsopp, M.H. Varroa destructor Reproduction and Cell Re-Capping in Mite-Resistant Apis mellifera Populations. Apidologie 2020, 51, 369–381. [Google Scholar] [CrossRef]
- Mondet, F.; Beaurepaire, A.; McAfee, A.; Locke, B.; Alaux, C.; Blanchard, S.; Danka, B.; Le Conte, Y. Honey Bee Survival Mechanisms against the Parasite Varroa destructor: A Systematic Review of Phenotypic and Genomic Research Efforts. Int J Parasitol 2020, 50, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.R.; Grindrod, I.; Webb, G.; Piñeiro, A.P.; Martin, S.J. Recapping and Mite Removal Behaviour in Cuba: Home to the World’s Largest Population of Varroa-Resistant European Honeybees. Sci Rep 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Begna, D. Occurrences and Distributions of Honeybee (Apis mellifera) Varroa Mite (Varroa destructor) in Tigray Region, Ethiopia. Journal of Fisheries & Livestock Production 2014, 03. [Google Scholar] [CrossRef]
- Shegaw, T.; Arke, A.; Belay, N.; Habte Giorgis, D. Diagnostic Survey on Varroa Mite (Varroa destructor) Prevalence in South-Western Ethiopia. Cogent Food Agric 2022, 8. [Google Scholar] [CrossRef]
- Gela, A.; Atickem, A.; Bezabeh, A.; Woldehawariat, Y.; Gebresilassie, A. Insights into Varroa Mite (Varroa destructor) Infestation Levels in Local Honeybee (Apis mellifera) Colonies of Ethiopia. Journal of Applied Entomology 2023, 147. [Google Scholar] [CrossRef]
- Robi, D.T.; Temteme, S.; Aleme, M.; Bogale, A.; Getachew, A.; Mendesil, E. Epidemiology, Factors Influencing Prevalence and Level of Varroosis Infestation (Varroa destructor) in Honeybee (Apis mellifera) Colonies in Different Agroecologies of Southwest Ethiopia. Parasite Epidemiol Control 2023, 23. [Google Scholar] [CrossRef] [PubMed]
- Overturf, K.A.; Steinhauer, N.; Molinari, R.; Wilson, M.E.; Watt, A.C.; Cross, R.M.; Williams, G.R.; Rogers, R. Winter Weather Predicts Honey Bee Colony Loss at the National Scale. Ecol Indic 2022, 145, 109709. [Google Scholar] [CrossRef]
- Insolia, L.; Molinari, R.; Rogers, S.R.; Williams, G.R.; Chiaromonte, F.; Calovi, M. Honey Bee Colony Loss Linked to Parasites, Pesticides and Extreme Weather across the United States. Sci Rep 2022, 12, 20787. [Google Scholar] [CrossRef]
- Landaverde, R.; Rodriguez, M.T.; Parrella, J.A. Honey Production and Climate Change: Beekeepers’ Perceptions, Farm Adaptation Strategies, and Information Needs. Insects 2023, 14. [Google Scholar] [CrossRef]
- Neumann, P.; Straub, L. Beekeeping under Climate Change. J Apic Res 2023, 62, 963–968. [Google Scholar] [CrossRef]
- Rafferty, N.E.; Diez, J.M.; Bertelsen, C.D. Changing Climate Drives Divergent and Nonlinear Shifts in Flowering Phenology across Elevations. Current Biology 2020, 30, 432–441.e3. [Google Scholar] [CrossRef]
- McNally, L.C.; Schneider, S.S. Seasonal Cycles of Growth, Development and Movement of the African Honey Bee, Apis mellifera scutellata, in Africa. Ins. Soc. 1992, 39, 167–179. [Google Scholar] [CrossRef]
- Hailu, T.G.; Wakjira, K.; Gray, A. Honey Bee Colony Population Annual Dynamics in Northern Ethiopia’s Semi-Arid Region, Tigray. J Apic Res 2024. [Google Scholar] [CrossRef]
- Gebretinsae, T.; Tesfay, Y. Honeybee Colony Marketing and Its Implications for Queen Rearing and Beekeeping Development in Tigray, Ethiopia. Int J Livestock Production 2014, 5, 117–128. [Google Scholar] [CrossRef]
- Jara, L.; Ruiz, C.; Martín-Hernández, R.; Muñoz, I.; Higes, M.; Serrano, J.; De la Rúa, P. The Effect of Migratory Beekeeping on the Infestation Rate of Parasites in Honey Bee (Apis mellifera) Colonies and on Their Genetic Variability. Microorganisms 2021, 9, 1–18. [Google Scholar] [CrossRef]
- Adgaba, N. Selling Honeybee Colonies as a Source of Income for Subsistence Beekeepers. Bees for Development 2002, 64. [Google Scholar]
- Central Statistical Agency (CSA) Agricultural Sample Survey 2017/18 [2010 E.C.]: Report on Livestock and Livestock Characteristics; Addis Ababa, 2018; Vol. II.
- Hailu, T.G.; Chagunda, M.; Rosenkranz, P. Sustainable Development Outlooks to Subsistent Apiculture in a Transition: The Case of Ethiopia. J Apic Res 2023, 62, 730–740. [Google Scholar] [CrossRef]
- Bahadur Poudel, P.; Ram Poudel, M.; Gautam, A.; Phuyal, S.; Krishna Tiwari, C.; Bashyal, N.; Bashyal, S. COVID-19 and Its Global Impact on Food and Agriculture. J Biol Today’s World 2020, 9, 221. [Google Scholar]
- Kassegn, A.; Endris, E. Review on Socio-Economic Impacts of ‘Triple Threats’ of COVID-19, Desert Locusts, and Floods in East Africa: Evidence from Ethiopia. Cogent Soc Sci 2021, 7. [Google Scholar] [CrossRef]
- World Peace Foundation Report 2021, Starving Tigray. World Peace Foundation, Somerville, MA.
- Demissie, B.; Nyssen, J.; Annys, S.; Negash, E.; Gebrehiwet, T.; Abay, F.; Wolff, E. Geospatial Solutions for Evaluating the Impact of the Tigray Conflict on Farming. Acta Geophysica 2022, 70, 1285–1299. [Google Scholar] [CrossRef]
- Gesesew, H.; Berhane, K.; Siraj, E.S.; Siraj, D.; Gebregziabher, M.; Gebre, Y.G.; Gebreslassie, S.A.; Amdes, F.; Tesema, A.G.; Siraj, A.; et al. The Impact of War on the Health System of the Tigray Region in Ethiopia: An Assessment. BMJ Glob Health 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Gebrekirstos, A.; Birhane, E. The War on Tigray Wiped out Decades of Environmental Progress: How to Start Again 2023. The Conversation, April 2.
- Tekulu, F.B.; Asgedom, D.B.; Gebre, H.T.; Desta, G.M.; Shifare, T.H.; Hadigu, T.B. The Effect of War on Educational Institutions of Eastern Tigray Zone, Tigray State, Ethiopia. Research Square, preprint, 2023. [Google Scholar] [CrossRef]
- Ngussie, H.G.; Hailu, T.G. Tigray War Put Honey Bees in Peril. Bee World 2023, 100, 51–55. [Google Scholar] [CrossRef]
- Lewoyehu, M.; Amare, M. Comparative Assessment on Selected Physicochemical Parameters and Antioxidant and Antimicrobial Activities of Honey Samples from Selected Districts of the Amhara and Tigray Regions, Ethiopia. Int J Food Sci 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- R Core Team 2020. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/.
- JMP®, Version Pro 16. SAS Institute Inc., Cary, NC. 1989–2023.
- Gratzer, K.; Wakjira, K.; Fiedler, S.; Brodschneider, R. Challenges and Perspectives for Beekeeping in Ethiopia. A Review. Agron. Sustain. Dev 2021, 41. [Google Scholar] [CrossRef]
- Kükrer, M.; Kence, M.; Kence, A. Honey Bee Diversity Is Swayed by Migratory Beekeeping and Trade Despite Conservation Practices: Genetic Evidence for the Impact of Anthropogenic Factors on Population Structure. Front Ecol Evol 2021, 9. [Google Scholar] [CrossRef]
- Cánovas, F.; De La Rúa, P.; Serrano, J.; Galián, J. Microsatellite Variability Reveals Beekeeping Influences on Iberian Honeybee Populations. Apidologie 2011, 42, 235–251. [Google Scholar] [CrossRef]
- Melicher, D.; Wilson, E.S.; Bowsher, J.H.; Peterson, S.S.; Yocum, G.D.; Rinehart, J.P. Long-Distance Transportation Causes Temperature Stress in the Honey Bee, Apis mellifera (Hymenoptera: Apidae). Environ Entomol 2019, 48, 691–701. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.; Strand, M.K.; Tarpy, D.R.; Rueppell, O. Impact of Honey Bee Migratory Management on Pathogen Loads and Immune Gene Expression Is Affected by Complex Interactions with Environment, Worker Life History, and Season. Journal of Insect Science 2022, 22. [Google Scholar] [CrossRef] [PubMed]
- Medina-Flores, C.A.; López-Carlos, M.; Carrillo-Muro, O.; Gray, A. Honey Bee Colony Losses in Mexico’s Semi-Arid High Plateau for the Winters 2016–2017 to 2021–2022. Insects 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Akyol, E.; Yeninar, H.; Korkmaz, A.; Çakmak, I. An Observation Study on the Effects of Queen Age on Some Characteristics of Honey Bee Colonies. Ital J Anim Sci 2008, 7, 19–25. [Google Scholar] [CrossRef]
- Lodesani, M.; Costa, C.; Besana, A.; Dall’Olio, R.; Franceschetti, S.; Tesoriero, D.; Vaccari, G. Impact of Control Strategies for Varroa destructor on Colony Survival and Health in Northern and Central Regions of Italy. J Apic Res 2014, 53, 155–164. [Google Scholar] [CrossRef]
- Mortensen, A.N.; Jack, C.J.; Bustamante, T.A.; Schmehl, D.R.; Ellis, J.D. Effects of Supplemental Pollen Feeding on Honey Bee (Hymenoptera: Apidae) Colony Strength and Nosema Spp. Infection. J Econ Entomol 2019, 112, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Gregorc, A. Monitoring of Honey Bee Colony Losses: A Special Issue. Diversity (Basel) 2020, 12, 403. [Google Scholar] [CrossRef]
- Negash, E.; Birhane, E.; Gebrekirstos, A.; Gebremedhin, M.A.; Annys, S.; Rannestad, M.M.; Berhe, D.H.; Sisay, A.; Alemayehu, T.; Berhane, T.; et al. Remote Sensing Reveals How Armed Conflict Regressed Woody Vegetation Cover and Ecosystem Restoration Efforts in Tigray, Ethiopia. Science of Remote Sensing 2023, 100108. [Google Scholar] [CrossRef]
- Manaye, A.; Afewerk, A.; Manjur, B.; Solomon, N. The Effect of the War on Smallholder Agriculture in Tigray, Northern Ethiopia. Cogent Food Agric 2023, 9. [Google Scholar] [CrossRef]
- Nyssen, J.; Negash, E.; Van Schaeybroeck, B.; Haegeman, K.; Annys, S. Crop Cultivation at Wartime–Plight and Resilience of Tigray’s Agrarian Society (North Ethiopia). Defence and Peace Economics 2023, 34, 618–645. [Google Scholar] [CrossRef]
- Schneider, S.S.; McNally, L.C. Factors Influencing Seasonal Absconding in Colonies of the African Honey Bee, Apis mellifera scutellata. Insectes. Soc 1992, 39, 403–423. [Google Scholar] [CrossRef]
- Spiewok, S.; Neumann, P.; Hepburn, H.R. Preparation for Disturbance-Induced Absconding of Cape Honeybee Colonies (Apis mellifera capensis Esch.). Insectes Soc 2006, 53, 27–31. [Google Scholar] [CrossRef]
- McMenamin, A.; Mumoki, F.; Frazier, M.; Kilonzo, J.; Mweu, B.; Baumgarten, T.; Patch, H.; Torto, B.; Masiga, D.; Tumlinson, J.; et al. The Impact of Hive Type on the Behavior and Health of Honey Bee Colonies (Apis mellifera) in Kenya. Apidologie 2017, 48, 703–715. [Google Scholar] [CrossRef]
- Loftus, J.C.; Smith, M.L.; Seeley, T.D. How Honey Bee Colonies Survive in the Wild: Testing the Importance of Small Nests and Frequent Swarming. PLoS One 2016, 11, e0150362. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, O.; Mekonnen, E. Floral Calendar of Honeybee Plants in Kellem and West Wollega Zone, Western Ethiopia. International Journal of Forestry Research 2023, 2797159. [Google Scholar] [CrossRef]
- Bareke, T.; Roba, K.; Addi, A. Identification and Establishing Floral Calendar in Jimma Zone of Oromia Ethiopia. Journal of Global Ecology and Environment 2023, 19, 48–61. [Google Scholar] [CrossRef]
- Gebremedhn, H.; Tesfay, Z.; Murutse, G.; Estifanos, A. Seasonal Honeybee Forage Availability, Swarming, Absconding and Honey Harvesting in Debrekidan and Begasheka Watersheds of Tigray, Northern Ethiopia. Livestock Research for Rural Development 2013, 25, 61. [Google Scholar]
| Description | Regional State | Total | |
|---|---|---|---|
| Oromia | Tigray | ||
| No. of Kebelles | 10 | 4 | 14 |
| No. of Beekeepers | 31 | 33 | 64 |
| No. of Apiaries | 35 | 33 | 68 |
| No of Honey bee colonies | 1,305 | 408 | 1,713 |
| Average no. of Colonies per apiary | 42.10 | 12.36 | 26.77 |
|
Variables |
Oromia |
Tigray |
Test of difference | |
|---|---|---|---|---|
| Method | P-value | |||
Colonies per beekeeper in spring 2022
|
27.00 17.00 7-125 |
9.03 6.00 2-47 |
Mann-Whitney test |
p<0.001 |
Colonies per beekeeper before winter 2023
|
42.10 27.00 10-200 |
12.36 8.00 2-60 |
Mann-Whitney test |
p<0.001 |
Colonies per beekeeper in spring 2023
|
30.58 16.00 5-160 |
5.24 3.00 0-37 |
Mann-Whitney test |
p<0.001 |
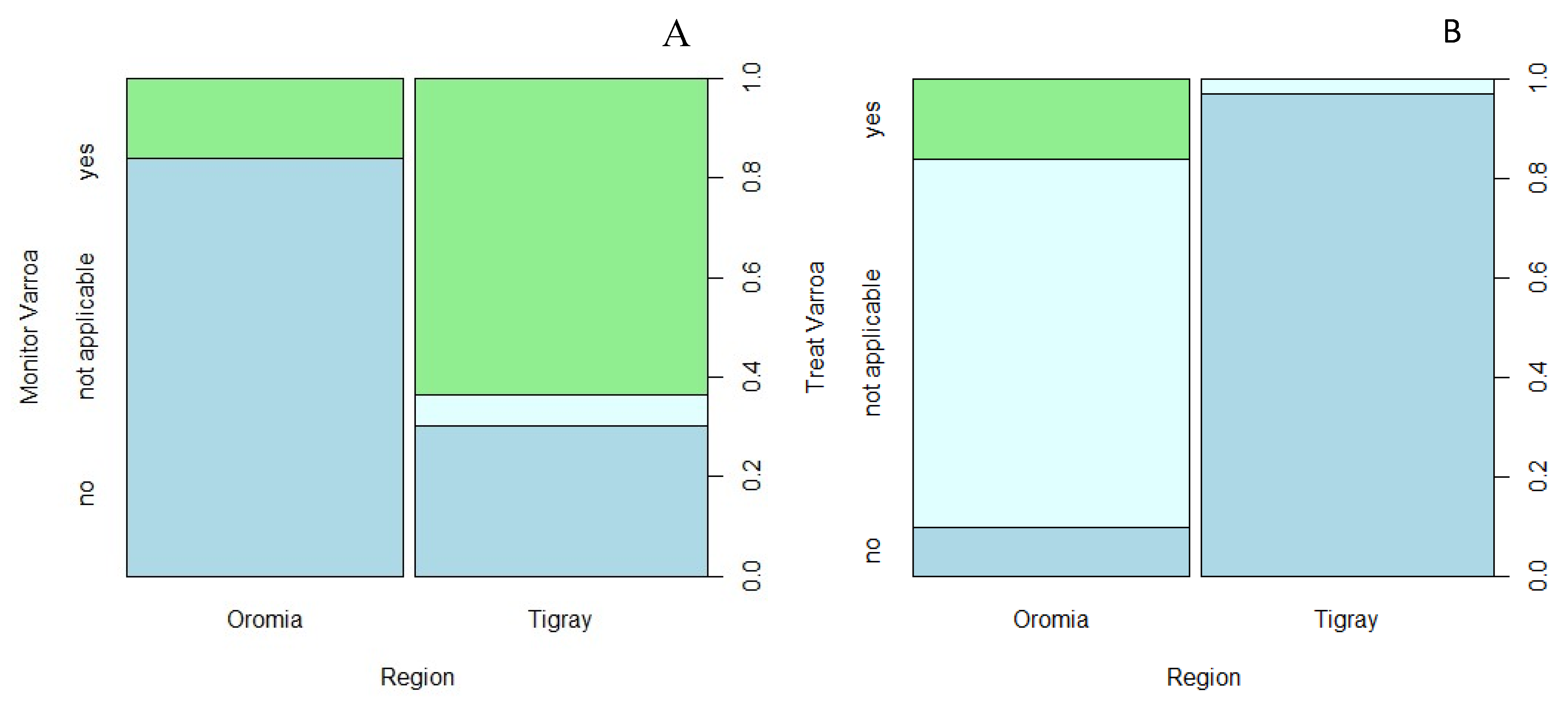
Monitoring Varroa destructor (frequency; % of total sample beekeepers)
|
5; 7.81 26; 40.63 0; 0.00 |
21; 32.81 10; 15.63 2; 3.13 |
Fisher’s test |
p<0.0001 |
Treating against V. destructor (frequency; % of total sample beekeepers)
|
5; 7.81 3; 4.69 23; 35.94 |
0; 0.00 32; 50.00 1; 1.56 |
Fisher’s test |
p<0.0001 |
Bees with deformed wings (frequency; % of total sample beekeepers)
|
0; 0.00 31; 48.44 0; 0.00 0; 0.00 |
7; 10.94 15; 23.44 10; 15.63 1; 1.56 |
Fisher’s test |
p<0.0001 |
Use of natural comb (frequency; % of total sample beekeepers)
|
31; 48.44 0; 0.00 |
14; 21.88 19; 29.69 |
Fisher’s test |
p<0.0001 |
Purchase of external wax (frequency; % of total sample beekeepers)
|
18; 28.13 13; 20.31 |
30; 46.88 3; 4.69 |
Fisher’s test |
p<0.01 |
Presence of Vespa velutina (frequency; % of total sample beekeepers)
|
8; 12.50 13; 20.31 10; 15.63 |
29; 45.31 4; 6.25 0; 0.00 |
Fisher’s test |
p<0.0001 |
Colony merging (frequency; % of total sample beekeepers)
|
21; 32.81 10; 15.63 |
6; 9.38 27; 42.19 |
Fisher’s test |
p<0.001 |
Colony splitting (frequency; % of total sample beekeepers)
|
26; 40.63 5; 7.81 |
5; 7.81 28; 43.75 |
Fisher’s test |
p<0.0001 |
Feed supplementation (frequency; % of total sample beekeepers)
|
31; 48.44 0; 0.00 |
17; 26.56 16; 25.00 |
Fisher’s test |
p<0.0001 |
Queen replacement (frequency; % of total sample beekeepers)
|
19; 29.69 12; 18.75 |
0; 0.00 33; 51.56 |
Fisher’s test |
p<0.0001 |
| #Colonies before winter | #Colony loss due to queen problems | #Colony loss due to natural disaster | #Empty hive or dead colonies | #Colonies in spring 2022 | |
|---|---|---|---|---|---|
| #Colony loss due to queen problems | 0.1353 | ||||
| #Colony loss due to natural disaster | 0.2777 | 0.4102 | |||
| #Empty hive or dead colonies | 0.6773 | 0.1340 | 0.0558 | ||
| #Colonies in spring 2022 | 0.9228 | 0.0421 | 0.2798 | 0.5245 | |
| #Colonies in spring 2023 | 0.9336 | 0.0105 | 0.1087 | 0.5783 | 0.9249 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





