1. Introduction
CT image reconstruction has evolved from the original filtered back projection (FBP) to hybrid and model-based iterative reconstruction (IR) algorithms, with a significant decrease in the radiation dose [
1]. The main advantage of FBP is its computational efficiency, whereas its disadvantages include significant noise at low radiation doses and limited artifact reduction [
1]. Iterative reconstruction (IR) algorithms are widely employed in CT image reconstruction to preserve image quality, even in low-dose CT acquisitions, with reduced image noise and artifacts. [
1,
2]. Hybrid-IR algorithms employ both FBP and IR algorithms (ranging from 50% to 90% with complementary levels of FBP), and allow fast CT image reconstruction with a reduction in image noise [
1] and an improvement in image quality at lower radiation doses. Model-based IR algorithms are fully IR algorithms that use forward and backward reconstruction steps from the sinogram domain to the image domain [
1]. The main advantage of model-based IR is the maintenance of CT image quality with low noise, even at low doses; however, its disadvantage is the need for high computational power and low capability in the detection rate of low-contrast structures on low-dose CT images [
1,
2,
3,
4].
In recent years, there has been growing interest in the application of deep learning image reconstruction (DLIR) algorithms, which employ convolutional neural networks (CNNs) for CT image reconstruction to produce CT images with a very low noise level, even at low radiation doses [
4]. The performance of DLIR algorithms for CT image reconstruction relies mainly on the quality and quantity of the training data and high quality reference ground-truth CT images [
5,
6].
A marked reduction in radiation ED is particularly required in ICU patients who are exposed to high radiation exposure, frequently higher than 100 mSv during a single hospital admission, and particularly in those patients with prolonged hospitalization time [
7,
8], due to the extremely frequent use of X-ray imaging modalities. In particular, ICU patients undergo frequent CT scans, often with extended scanning lengths, hampered by low image quality and artifacts due to external or internal medical devices and patient arms placed along the body. No previous study has provided an intra-patient comparison between hybrid IR/FBP and DLIR CT reconstruction algorithms implemented in different CT scanners in terms of the radiation dose and image quality in ICU patients.
The aim of the present study was to assess whether the DLIR algorithm may reduce the CT effective dose (ED) and improve CT image quality in comparison with FBP and iterative reconstruction (IR) algorithms in ICU patients.
2. Materials and Methods
2.1. Patients
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of our hospital (Prot. n. 0000569 approved on january 4th 2023). Patient informed consent was waived due to retrospective nature of the study. We initially identified all consecutive patients referred to the ICU of our hospital because of their severe clinical status, major traumas, or even recent thoracic or abdominal major surgery (extended tumor resection or liver, cardiac, or lung transplant) between October 1, 2021, and February 28, 2023. Subsequently, we retrospectively selected only those patients who underwent at least two subsequent chest and/or abdominal contrast-enhanced CT scans with comparable scan lengths covering the same body region (chest, abdomen, or both chest and abdomen) during the same hospital admission. DLIR algorithm was used in the first CT scan for CT image reconstruction, whereas FBP or Adaptive Iterative Dose Reduction 3D (AIDR3D) hybrid or even Advanced Modeled Iterative Reconstruction (ADMIRE) model-based IR algorithms were used in the second CT scan. To ensure that significant physical changes in patient features, including body mass index, occurred between the two CT scans, only those obtained within a limited timeframe of 30 days were included.
2.2. CT Scanning Protocols
Because it was not possible to use different CT image reconstruction algorithms on the same raw data obtained from the same patients owing to the different CT acquisition technical settings related to the subsequent CT image reconstruction algorithm (DLIR) applied to CT scans acquired with a lower tube kV and current than IR or FBP, we compared different CT scanners equipped with different CT image reconstruction algorithms, according to the manufacturer’s technical solutions (
Table 1).
In every patient, CT was performed craniocaudally with a scan range from the lower neck to the costophrenic angle level on chest CT and from the diaphragm level to the pelvis on abdominal CT before and after iodinated contrast agent injection (ioexol 350 mgI/mL; Omnipaque 350, GE HealthCare, Barrington, Illinois) or iodixanol 270 mg/mL (Visipaque 270, GE Healthcare, Barrington, Illinois, USA), iopromide 370 mgI/mL (Ultravist 370, Bayer, Leverkusen, Germany), or iomeprol 400 mgI/mL (Iomeron 400, Bracco, Milan, Italy). Patients were scanned with their arms placed along the body owing to their critical clinical status. The volume of contrast medium was calculated based on the patient’s lean body weight (LBW) which was estimated from the patient’s weight, height, and gender using Boer’s equation [
9]. The arterial phase was triggered by placing a region-of-interest (ROI) over the abdominal CT scan at the level of the second lumbar vertebral body and starting the scan when the density level achieves 100HU. The portal venous and late phases were obtained at 70 and 180 s after iodinated contrast injection. The contrast agent was injected into the antecubital vein (total contrast volume and injection speed adjusted by the patient’s body weight to 3-4 mL/sec) and saline push (10 s at the same rate). The following CT parameters were used: tube voltage, 100-120 kVp; automatic tube current modulation; gantry rotation period, 280 ms; detector collimation, 0.625 mm; and detector pitch, 1.53. The CT dataset was then reconstructed at 1.25 mm section thicknesses with 512×512 matrices, using standard kernels for soft tissues.
Although the same scanning protocol was generally used in both the first and second CT scans, a mismatch in scanning length, presence or absence of unenhanced CT scans, or even the number of contrast-enhanced dynamic phases (arterial, portal venous, or delayed phases) was possible between the two subsequent thoracic and/or abdominal CT scans. Therefore, these patients were excluded from analysis. In patients who underwent more than two repeated CT scans, only the two closest CT scans reconstructed using the DLIR and FBP or IR algorithms were considered for analysis.
Generally, the same iodinated contrast agent dosage and concentration were used in both the first and second CT scans unless the use of a different iodinated contrast agent type is required (e.g., suspicion of bleeding after major surgery, change in iodinated contrast type and/or injected contrast volume due to anaphylactoid reaction or incoming acute kidney injury, even suspicious pulmonary embolism). Patients in whom the iodinated contrast agent type, injected volume, and/or iodine dose was changed or modified were excluded from the analysis.
2.3. CT Reconstruction Algorithms
Different CT image reconstruction algorithms were employed according to the available CT equipment: Revolution Evo (GE Healthcare) CT with True Fidelity DLIR algorithm in the first CT scan; Somatom Sensation 64 (Siemens Healthineers) with Filtered Back Projection (FBP) or Aquilion ONE (Canon Medical Systems) with Adaptive Iterative Dose Reduction (AIDR 3D) IR hybrid reconstruction algorithm, or even Somatom Definition Edge (Siemens Healthineers) with Advanced Modeled Iterative Reconstruction (ADMIRE) IR hybrid reconstruction algorithm in the second CT scan (
Table 1).CT images were reconstructed at 3mm and 512x512 pixel matrix. DLIR strength was set to the highest level (DLIR-H), according to the manufacturer’s default settings.
2.4. Radiation Effective Dose Analysis
The CT dose index volume (CTDIvol) and dose-length product (DLP) were obtained from CT dose reporting produced automatically by the CT equipment at the end of the scan and archived on the PACS. The radiation ED was calculated by multiplying the DLP by. body region– specific conversion coefficient, k, according to the ICRP recommendations [
10,
11].
2.5. Visual Image Quality Analysis
Two radiologists with 3 and 10 years of experience performed subjective analyses of the three groups of images. The radiologists were blinded to the image reconstruction techniques and patient characteristics. The images are displayed in random order in a preset window, displaying a sequence at a time. The radiologists were able to scroll through the image and adjust the window width and position randomly. We used a 5-point scale to evaluate the subjective image quality of the soft and lung tissues. The scoring standard for soft tissue was as follows: 1 = poor definition of mediastinal / abdominal parenchyma borders and clearly visible noise, unacceptable image; 2 = moderate definition of mediastinal / abdominal parenchyma borders and moderately visible noise, suboptimal image; 3 = moderate definition of mediastinal / abdominal parenchyma borders and barely visible noise, acceptable image; 4 = good definition of mediastinal / abdominal parenchyma borders and barely visible noise, good image; and 5 = excellent definition of mediastinal / abdominal parenchyma borders and very low image noise, optimal image.
2.6. Quantitative Image Quality Analysis
CT image noise was calculated off-site on a dedicated PC using MATLAB (MATLAB version: 9.13. 0 (R2022b), Natick, Massachusetts: The MathWorks Inc.; 2022) with the Global Noise Level (GNL) algorithm for automatic noise measurement [
12,
13] by a medical student with specific competence in CT image quantitation software analysis over 4 years. The GN algorithm was used to analyze only the selected slice images. Observers selected similar slice locations, and therefore, approximately similar noise, by using anatomical landmarks for slice selection. This assumption was tested by measuring the variation in the selected slice locations across the observers. The slice locations selected by the observers were averaged, and the slice image closest to this location was selected for GN analysis.
To objectively compare image quality, the signal-to-noise ratio (SNR) was measured for different reconstruction algorithms. For thoracic evaluation, the SD of the values in Hounsfield units (HU) was measured in regions of interest (ROIs) measuring ≥ 1 cm2 drawn in the bilateral abdominal fat (SDax1 and SDax2) and the average HU values were measured in the bilateral paraspinal muscles (HUPSM1 and HUPSM2). The noise and SNR for each scan were calculated using the following equations.
2.7. Statistical Data Analyses
Statistical data analyses were performed using SciPy 1.11.2, an open-source software using Python 3.12 programming language, by a medical student with specific competence in statistical software analysis over 4 years. After the Shapiro-Wilk test failed to show a normal distribution, the Wilcoxon signed-rank test for paired data was used to assess the differences between the FBP, IR, and DLIR effective doses and image quality. Cohen’s kappa statistic was calculated for an agreement on the independent scoring of the image quality between the two radiologists. A kappa statistic of 0.81~1.00 implies an excellent agreement; 0.61~0.80, a substantial agreement; 0.41~0.60, a moderate agreement; 0.21~0.40, a fair agreement; and 0.00~0.20, a poor agreement. For all statistical tests, a P value <0.05 was set to indicate a statistically significant difference.
3. Results
3.1. Patients
Initially, we identified 14,431 patients who were admitted to the ICU. We excluded 13,417 patients due to a temporal distance of >30 days between the two subsequent CT scans; 860 patients due to a mismatch in the scanning length between the two CT scans (n=350), or differences in CT scanning protocols (n=251), including the absence of an unenhanced scan, different numbers of dynamic phases, or even changes in contrast agent type and/or contrast volume administration (n=259); and 71 patients due to CT scans that did not include the chest and/or abdomen (e.g., brain and limb). The total hospitalization period was 10 – 45 days (mean ± SD, 22 ± 10 days).
Finally, we included 83 patients (
Table 2) who underwent CT scans of the chest (n=14; 5 patients were scanned on unenhanced CT and during the arterial phase, while 9 patients underwent both unenhanced CT and contrast-enhanced CT on arterial and portal venous phases), abdomen (n=51; 32 patients scanned on unenhanced CT and arterial phase, and 19 patients scanned both on unenhanced CT and arterial and portal venous phases), and both chest and abdomen (n=18; 12 patients scanned on unenhanced CT and arterial phases, and 6 patients scanned both on unenhanced CT and arterial and portal venous phases). The timeframe between the two CT scans considered for quantitative analysis was 10.8 ± 8.6 days (range, 1 – 30 days).
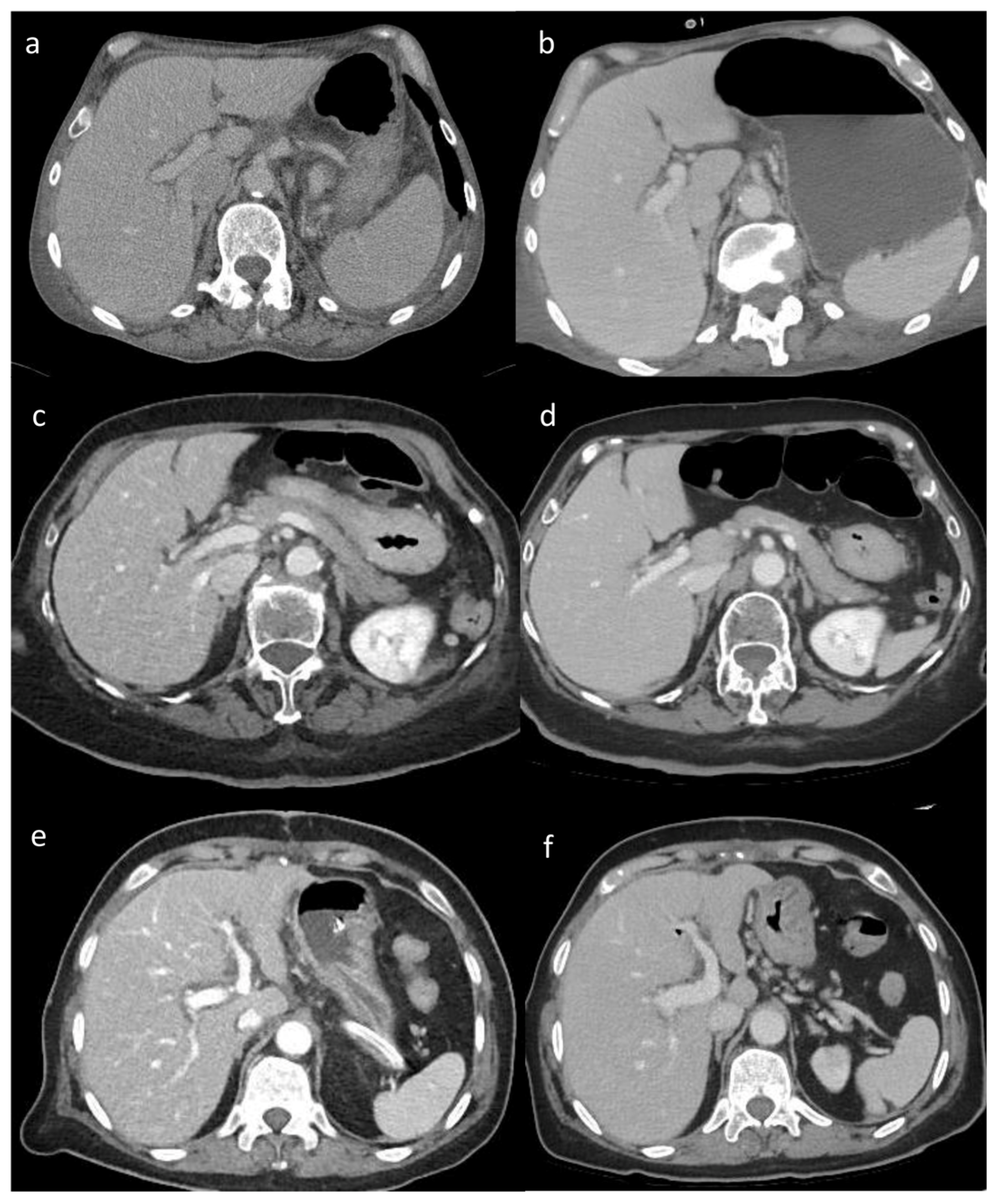
3.2. Visual Analysis
Visual analysis results showed significant differences in the image quality of soft tissue among the three reconstruction methods (all p < 0.05). The image score of DLIR (mean score = 5) was higher than that of ADMIRE (mean score = 4) and AIDR 3D (mean score = 3) and FBP (mean score = 3) by 50% of observers. Both radiologists believed that DLIR had outstanding noise reduction. The subjective scores of the two radiologists were consistent (kappa value range: 0.48–0.91) (
Figure 2).
3.3. Effective Dose and Quantitative Analysis
DLIR reduced the effective dose compared to FBP (
Table 3) and improved both the image noise and SNR compared to both the FBP and IR algorithms (
Table 3 and
Table 4). Among IR algorithms, compared to AIDR 3D, ADMIRE provided similar exposure data (
Table 3) with lower noise and higher SNR (
Table 4).
4. Discussion
Radiation ED in CT is determined by technical parameters (kV, mA, collimation, and pitch) employed in the in the acquisition phase. Reconstruction algorithms do not directly reduce the radiation dose but may compensate image quality loss due to reduction of radiation dose or may improve image quality by maintaining constant the radiation dose. In our study, we found that DLIR reduced radiation ED and improved SNR compared to FBP, whereas DLIR improved SNR compared to both the FBP and IR algorithms in ICU patients. DLIR is a recently introduced CT image-reconstruction algorithm based on deep learning. CNN handle millions of parameters trained with thousands of paired high-quality, high-dose radiation, and low-noise ground-truth CT images obtained from a large number of phantoms and patients. After training, a low-dose sinogram is provided to the CNN, and a final image with a very low noise level is obtained by comparing the output image to a ground truth image across multiple parameters such as image noise, low contrast resolution, low contrast detectability, and noise texture. The backpropagation operation reports the differences to the network, which then strengthens some equations and weakens others, and the process is repeated until there is a proximity between the output and ground-truth images. Commercially available DLIR algorithms include direct algorithms that use ground-truth images reconstructed by FBP and sinogram data directly fed into a CNN – True Fidelity (GE Healthcare) and Precise Image (Philips Healthcare) and indirect algorithms that use ground-truth images reconstructed by model-based IR algorithms (AiCE, Canon Medical System). The DLIR strengths of the FBP and IR algorithms can be selected by the operator as low (DLIR-L), medium (DLIR-M), or high (DLIR-H). In our study, we employed high DLIR strength according to the default settings of the CT equipment.
ICU patients are generally exposed to high radiation doses due to frequent and extended chest and/or abdominal CT scans, especially in patients who undergo major surgery or organ transplant, and in patients with prolonged hospitalization time, as in the ICU patients included in our study. Therefore, we focused on the ICU patient cohort because our aim was to analyze the major advantages of DLIR in terms of radiation dose exposure and CT image quality under extreme clinical conditions, which justified the use of repeated CT scans over a relatively restricted period.
Our study confirmed that DLIR reconstruction for CT images provides significant benefits in terms of dose reduction and image quality over FBP and improves image quality in comparison to both IR algorithms we used in ICU patients, both in terms of image noise and SNR, which emphasizes the advantage of the DLIR approach and its potential in daily clinical practice in keeping with previously published papers [
14,
15,
16].
In our study, DLIR did not show any reduction in radiation ED compared to both IR algorithms included in our study. This was due to the selected CT technical acquisition factors, including the tube voltage and, automatic tube current modulation grade according to the selected noise level, which were similar between the different CT scanners. Consequently, the radiation ED did not change significantly, with the advantage of reduced CT image noise, owing to the use of the DLIR algorithm. Most likely, DLIR may provide a reduction in the radiation dose, even when compared to IR algorithms, provided that a similar image quality in terms of both the noise level and SNR between the DLIR and IR algorithms would have been preliminarily planned. In this case, a comparable SNR between CT images produced by CT scanners employing DLIR vs those scanners employing IR algorithms would imply a higher patient radiation ED in CT scanners using IR, which is related to the higher tube current required to reduce noise. This reflects the generally higher attention paid to CT image quality than to patient radiation exposure in general clinical practice, even if repeated CT scans are required over a limited temporal range to strictly monitor clinical evolution, as in ICU patients.
DLIR provided a marked reduction in radiation ED in ICU patients who were frequently examined using different X-ray imaging modalities, including plain X-ray film and CT scans. CT scans provide the highest dose from medical exposure, although they are often penalized by low image quality in ICU critical patients. The main result is that all the advantages provided by DLIR algorithms translate into safer imaging practices, higher diagnostic confidence and more accurate diagnosis from radiologists, and ultimately, better patient care. Considering this increase, it is reasonable to expect a wider implementation of DLIR algorithms in the future given the increasing computational power of CT scanners. However, further evaluation is needed to investigate the potential differences between DLIR and IR algorithms, even in other anatomical locations such as the head or limbs, or in specific diseases, and to assess whether the improved image quality provided by DLIR may significantly affect subjective CT image quality, CT workflow, and efficiency in terms of the time needed to assess CT images or to achieve the correct diagnosis by a radiologist.
The first limitation of the present study includes the approximate approach we used for estimating radiation ED based on the DLP obtained by multiplying the CTDIvol by the scan length by the body region– specific conversion coefficient k [
10,
11]. Monte Carlo (MC) simulation is generally considered the most accurate method for estimating radiation ED, owing to its ability to provide an effective and realistic model of the physical interactions between radiation and tissues, considering the CT source, filtration, tube current, and scanner geometry [
10,
11]. Moreover, size specific dose estimate (SSDE) would have been useful in patient dose comparison, provided that patient lateral and anteroposterior diameter are known, although SSDE does not take the organs in the CT scan’s field of view into account and is not a measure of ED. SSDE is a better estimate of patient radiation dose from CT than CTDIvol in systems that use automated exposure control [
17].
The second limitation of this study corresponds to the wide variation in CT scanner characteristics and technical features related to the use of different CT image reconstruction algorithms, detector designs and configurations, dose modulation algorithms, and patient positioning/handling which may affect the outcomes of the study.
Other limitations are the retrospective nature of the study, and the wide patient population with very different clinical features.
5. Conclusions
In conclusion, CT scanners employing DLIR reduced radiation ED and improved SNR compared to CT scanners using FBP, whereas CT scanners using DLIR improved SNR compared to CT scanners using FBP or IR algorithms in ICU patients.
Author Contributions
Conceptualization, E.Q.; methodology, E.Q.; software, E.K.; validation, E.Q., E.K., E.A. and C.Z.; formal analysis, E.K.; investigation, E.Q., E.K., E.A. and C.Z.; resources, E.Q. and E.K.; data curation, E.K.; writing—original draft preparation, E.Q.; writing—review and editing, E.Q. and C.Z.; visualization, E.K.; supervision, E.Q.; project administration, E.Q.; funding acquisition, NA
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of our hospital (Prot. n. 0000569 approved on 4 January 2023).
Informed Consent Statement
Patient informed consent was waived due to retrospective nature of the study.
Data Availability Statement
Data are not publicly available due due to privacy or ethical restrictions although can be provided after anonymisation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Den Harder AM, Willemink MJ, Budde RPJ, et al. Hybrid and Model-Based Iterative Reconstruction Techniques for Pediatric CT. AJR Am J Roentgenol 2015; 204 (3): 645 – 653. [CrossRef]
- Patino M, Fuentes JM, Singh S, et al. Iterative Reconstruction Techniques in Abdominopelvic CT: Technical Concepts and Clinical Implementation. AJR Am J Roentgenol 2015; 205: W19 – W31. [CrossRef]
- Koetzier LR, Mastrodicasa D, Szczykutowicz TP, et al. Deep learning image reconstruction for CT: Technical Principles and Clinical Prospects. Radiology 2023; 306 (3): e221257. [CrossRef]
- Cao J, Mroueh N, Pisuchpen P, et al. Can 1.25 mm thin-section images generated with a Deep Learning Image Reconstruction technique replace standard-of-care 5 mm images in abdominal CT? Abdominal Radiology 2023; 48:3253–3264. [CrossRef]
- Parakh A, Cao J, Pierce TT, et al. Sinogram-based deep learning image reconstruction technique in abdominal CT: image quality considerations. Eur Radiol 2021; 31(11):8342-8353. [CrossRef]
- Jensen CT, Liu X, Tamm EP, et al. Image Quality Assessment of Abdominal CT by Use of New Deep Learning Image Reconstruction: Initial Experience AJR Am J Roentgenol 2020;215 (1):50- 57. [CrossRef]
- Krishnan S, Moghekar A, Duggal A, et al. Radiation Exposure in the Medical ICU Predictors and Characteristics. Chest 2018; 153(5): 1160-1168. Epub 2018 Jan 31. [CrossRef]
- Moloney F, Fama D, Twomey M, et al. Cumulative radiation exposure from diagnostic imaging in intensive care unit patients. World J Radiol 2016; 28; 8(4): 419–427. [CrossRef]
- Boer, P. Boer P. Estimated lean body mass as an index for normalization of body fluid volumes in humans. American Journal of Physiology-Endocrinology and Metabolism 1984; 247(4): F632–636. [CrossRef]
- Christner JA, Kofler JM, McCollough CH. Estimating Effective Dose for CT Using Dose–Length Product Compared With Using Organ Doses: Consequences of Adopting International Commission on Radiological Protection Publication 103 or Dual-Energy Scanning. AJR Am J Roentgenol 2010; 194: 881 – 889. [CrossRef]
- Deak PD, Smal Y, Kalender WA. Multisection CT Protocols: Sex- and Age-specific Conversion Factors Used to Determine Effective Dose from Dose-Length Product. Radiology 2010; 257:158–166. [CrossRef]
- Christianson O, Winslow J, Frush DP, Samei E. Automated technique to measure noise in clinical CT examinations. AJR Am J Roentgenol 2015; 205 (1): W93–W99. [CrossRef]
- Malkus A, Szczykutowicz TP. A Method to extract image noise level from patient images in CT. Med Phys 2017; 44 (6): 2173–2184. [CrossRef]
- Shehata MA, Saad AM, Kamel S, et al. Deep learning CT reconstruction in clinical scans of the abdomen: a systematic review and meta analysis. Abdominal Radiology 2023; 48: 2724 – 2756. [CrossRef]
- Greffier J, Hamard A, Pereira F, et al. Image quality and dose reduction opportunity of deep learning image reconstruction algorithm for CT: A Phantom Study. Eur Radiology 2020; 30 (7): 3951–3959. [CrossRef]
- Benz DC, Ersözlü S, Mojon FLA, et al.Radiation dose reduction with deep-learning image reconstruction for coronary computed tomography angiography. Eur Radiol 2022; (32) 4: 2620–2628. [CrossRef]
- Rajaraman V, Ponnusamy M, Halanaik D. Size specific dose estimate (SSDE) for estimating patient dose from CT used in myocardial perfusion SPECT/CT. Asia Ocean J Nucl Med Biol. 2020 Winter; 8(1): 58–63. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).