1. Introduction
The increase in population in recent years has resulted in a search for more sustainable protein sources to cope with the increase in protein demand [
1]. Insects have been reported as a promising alternative due to the possibility of being raised with organic waste, low water consumption, high efficiency in their feed conversion, low emissions of greenhouse gases and ammonia, few animal welfare problems, and low risk of transmission of zoonotic infections [
2]. In addition, they have a high protein content and a good amino acid composition [
3].
One of the most studied and mass-produced species is
Hermetia illucens (Linneo, 1758) (Black Soldier Fly Larvae) [
4]. Both industry and research show interest in this specie due to its potential to contribute to the circular economy through waste management as they consume 25-500 mg of fresh matter/larva/day, in a wide range of decomposing organic materials (food or agricultural waste), as well as being a source of protein [
5,
6,
7]. It is a dipteran with a life cycle that includes the next stages: egg, larva, prepupa, pupa, and adult. However, the prepupal and larval stages are the most widely used in research as an alternative feed because it is a good source of protein (40-56.9 % in dry matter), lipids (35-40 % in DM) and minerals [
8,
9]. For that reason, its inclusion in the diet of terrestrial and aquatic animals has been studied [
8,
9,
10,
11]. This is the case for farm animals such as piglets, chickens, ducks and quail, and farmed fish such as seabass, turbot, jian carp, rainbow trout, and shrimp [
2,
9,
12,
13]. The optimal level of inclusion of BSFL seems to vary by species [
11], with full inclusion achieved for Atlantic salmon without compromising growth or sensory qualities [
14].
If new protein sources are used as food and feed, their digestibility should be assessed, as protein quality is determined by amino acid composition and digestibility. In a recent review, Rodríguez-Rodríguez et al. [
15] noted that BSFL shows very good results for crude protein digestibility (CPd), with a maximum of 89.7 % [
16] and lower data for prepupae, with 50 % [
12] using commercial enzymes simulating dog and duck digestion, respectively. These results have been obtained for raw insects, but if the aim is to market insect meal for human consumption, it is vitally important to investigate the most appropriate processing method. Kinyuru et al. [
17] state that processing methods can affect the nutritional composition of insects, especially the digestibility of their proteins.
The processing methods aim to improve the texture, palatability, nutrient bioavailability, or food safety of insect meals [
15]. Although in recent years, several researchers have studied the effect of treatments such as killing methods, drying methods, defatting or extrusion on the nutritional value of BSFL [
18,
19,
20,
21], results on the impact of different processing techniques, or combinations thereof, on their nutritional value or digestibility are still limited. As a result, a standard procedure for processing BSFL meal has not yet been established.
Currently, the method most companies use to slaughter different insect species is slow freezing, which can lead to enzymatic browning of insects [
20]. This browning is caused by the action of endogenous phenol oxidases (POs), which oxidize monophenols and diphenols to form
o-quinones [
19], which act during slow freezing. In living insects, this browning is a defense reaction or sclerotization [
22]. In addition to the color change, this browning could affect both protein quality and digestibility [
20]. This browning can be prevented by chemical inhibitors (such as ascorbic acid, sulfite, or Melacide
®) or physical treatments (boiling, blanching, or sonication) [
23].
Another critical process is heat treatment during drying prior to grinding, as protein-rich insects can show non-enzymatic browning (Maillard reaction), which can affect the nutritional value of BSFL meal [
24,
25]. Depending on temperature, heat treatment could improve protein digestibility by denaturing them, increasing enzyme susceptibility, or inactivating possible inhibitors of specific enzymes [
26]. However, if the temperatures are too high, reactions between amino acids can occur, which prevents hydrolysis [
27].
Therefore, it is necessary to investigate the optimal processing methods for each insect species. The aim of this work is to determine the effects of different types of slaughter methods and drying temperatures on the nutritional value and protein digestibility, in addition to the evaluation of the hygenic-sanitary quality of the BSFL meals as a feed source for farm animals.
2. Materials and Methods
BSFL were purchased from Entomo AgroIndustrial (Cehegín, Murcia, Spain). The insects were divided into different lots and subjected to different types of slaughter: Frozen: without any additional treatment, live larvae were placed in zipper bags in the freezer at -18 °C. Blanched: larvae were placed in boiling water at 100 °C for 1 minute. They were removed from the water with a strainer and placed in zipper bags in the freezer at -18 °C. Melacide
®: the larvae were mixed with Melacide
® (15 grams per kilo of BSFL). They were placed in zipper bags in the freezer at -18 °C. Liquid nitrogen: was poured into a container with the larvae. The larvae were placed in zipper bags in the freezer at -18 °C. Then each lot was subdivided into three different lots, each of which was dried at different temperatures (50, 70 and 90 °C) and ground (
Table 1).
In addition, soybean meal (SBM), albumin (A), and whey protein (W) were used as comparisons. Albumin and whey protein are pure proteins, they can serve as a control for the hydrolysis process. Soya, on the other hand, is a vegetable protein source traditionally used in animal feed.
2.1. Proximate Composition
The proximate composition was determined according to the Association of Official Analytical Chemists [
28]. Dry matter and ash were determined by gravimetry after drying at 105 °C (AOAC #934.01) in a conventional oven and combustion at 500 °C in a muffle furnace (AOAC #942.05). The crude protein (CP) was determined by the Kjeldahl method (AOAC #954.01). The CP data of the BSFL pretreatments were calculated using the conversion factor of 4.76. This coefficient has been used following the recommendation of Janssen et al. [
29] to avoid the overestimation of protein in insects. For the remainder of the materials analyzed (albumin, whey protein, and soybean meal), the usual conversion coefficient of 6.25 was used. The contents of acid detergent fiber (ADF) were determined according to the Van Soest et al. [
30] method using an Ankom
200 fiber analyzer. All analyzes were performed in triplicate.
2.2. In Vitro Protein Hydrolysis
The protocol described by Huang et al. [
18] was followed with some modifications, 100 g of BSFL meal was mixed with 4 mL NaCl (2 g/L) + HCl (7 mL/L), pH 2. The mixture was incubated at 38 ºC for 15 min in a shaking water bath. The gastric phase was carried out using porcine pepsin (P7000 Sigma‒Aldrich, St. Louis, MO) for 240 min under constant agitation at 38 ºC. After gastric digestion, the pH was adjusted to 7 with 0.2 M NaOH solution and the intestinal phase was carried out using porcine pancreatin (P1750 Sigma‒Aldrich) for 240 min under constant agitation at 38 ºC.
Before adding enzymes from the gastric and intestinal phases, samples were taken to be used as blanks. In this way, the data from both phases are not affected by the amino acids present at time 0. As the intestinal phase occurs after the gastric phase, we consider that intestinal digestibility includes the sum of the gastric + intestinal phase.
The reaction of the collected samples was stopped with an equal volume of 20 % trichloroacetic acid (TCA). Three replicates per sample were analyzed.
2.3. Organic Matter Digestibility (OMd)
After in vitro hydrolysis, the samples were centrifuged. They were washed with 80 ml of distilled water and again centrifuged. The residue was dried at 100 °C for 24 h to calculate the dry matter and then at 500 ºC to obtain the residue ash. The following formula was used to calculate the digestibility of organic matter:
where DM
i is the initial dry matter, DM
f is the final dry matter expressed in grams, Ash
i is the initial ash of the BSFL meal, and Ash
f is the final ash of the BSFL after hydrolysis.
2.4. O-Phthaldialdehyde (OPA) Method
The OPA methodology described by Church et al. [
31] is based on the reaction of OPA and 2-mercaptoethanol with amino groups. It was used for the calculation of α-amino groups, using L-leucine as a standard. α-Amino groups are found in most amino acids that make up proteins. This method measures the α-amino groups released after proteolysis.
The OPA results were expressed as % of the free amino groups in regard to total amino groups (DH/NH2) and % of the free amino groups in regard to dry matter (DH/DM) according to the following calculation.
The degree of hydrolysis (DH/100 NH
2) relates the number of peptide bonds broken during hydrolysis (h) to the total number of peptide bonds present in the sample (h
tot).
where h was calculated by measuring the free amino groups by OPA at the end time of hydrolysis (480 min), and h
tot is the total amino groups in the sample [
32]. These were determined by hydrolyzing 50 mg of the initial sample with 2.5 ml of 6 M HCl for 24 h at 100 °C.
DH/100 g of DM refers to the number of peptide bonds broken during protein hydrolysis (h) per 100 grams of dry matter.
where h was calculated by measuring the free amino groups with OPA at the beginning of hydrolysis (h
0) and after the end of the gastric (h
240) and intestinal (h
480) phases, and DM
i is the organic matter introduced at the start of protein hydrolysis.
Time 0 was used as a blank for the gastric and intestinal phases, while the intestinal phase is the sum of the gastric and intestinal phases.
The reason for expressing the results in dry matter is no other than to measure the yield of the sample, since the purpose of these meals is to be used in feed as a substitute for other proteins.
2.5. Total Hydrolysis (TH)
The degree of hydrolysis only measures the broken peptide bonds at the end of in vitro hydrolysis (480 min). However, the small peptides present that are susceptible to end hydrolysis are not considered. Therefore, to calculate the total hydrolysis, the final hydrolysis supernatant was completely hydrolyzed with the same amount of 12 M HCl for 24 h at 100 °C and neutralized with 6 M NaOH.
2.6. Hygienic-Sanitary Quality Study: Microbial Contamination Indicators
To establish the level of hygienic-sanitary safety provided by each of the slaughter and drying temperature combinations tested, the following microbial groups, usually considered indicators of contamination, were estimated. Sulfite-reducing clostridia (Clostridium perfringens) using SPS (Sulfite Polymyxin Sulfadiazine) agar as medium and incubated at 37 °C for 48 h. The potential presence of the Salmonella spp. pathogen was investigated according to the following protocol: preenrichment stage (incubation of the sample at 37 °C for 18 h, in buffered peptone water where the meal to nutrient medium ratio was 1:9); enrichment stage (incubation in Rappaport-Vassiliadis broth, a selective medium, at 41.5 °C for 24 h, extendable to 48 h in case of initial negative results); selective isolation stage (seeding from the presumptive positive selection medium grown on Hektoen Agar incubated at 37 °C for 24 h); and confirmation stage (study of suspected colonies by growth characteristics on Kligler Agar, incubated at 37 °C for 24 h). The presence of Enterobacteria was investigated in VRBG agar plates grown for 24 h at 37 °C. The presence of total aerobic mesophilic bacteria (TAMB) was investigated in PCA agar plates grown for 24 h at 37 °C.
2.7. Statistical Analyses
The experimental results were expressed as means ± SD. Statistical differences in insect digestibility between slaughtering and drying temperature were analyzed with the ANOVA multivariate test, followed by a comparison of means (Tukey test). Proximate composition, OMd, DH/NH
2, DH/100 g DM, TH and hygienic-sanitary results were analyzed using simple ANOVA followed by a multiple comparison of means (Tukey test). The correlations were analyzed using multivariate pairwise correlation analysis. Normality test was performed. The data did not follow a normal distribution, so a normalization of the data was performed. Data were normalized using a two-step transformation (first, the variable was transformed into a percentile rank and then the inverse-normal transformation) [
33]. The normal distribution of the residuals and of the transformed data was confirmed. The tests were carried out with IBM SPSS Statistics software (29.0.1.0). For graphic representation, all data in tables and figures were kept in raw form (prior to normalization) for clarity of presentation.
4. Discussion
To realize the full potential of insects as a novel and sustainable food in the near future, the insect mass-rearing industry needs to develop and optimize insect meal processing systems to maximize their nutritional potential and food safety. The high variability observed in the nutritional composition of the same insect species shows that, in addition to other factors, the slaughter and drying method can clearly affect it. All this makes it necessary to investigate the effects of common processing, slaughtering, and drying conditions on the nutritional characteristics and digestive proteolysis of BSLF meal.
It appears clear that the slaughter method and the drying temperature do not affect the protein content of BSLF (
Table 3). However, drying at 70 ºC showed a lower proportion of fibre and ash, and as pointed out by several studies [
34,
35], higher levels of fibre could be indicative of worse digestibility.
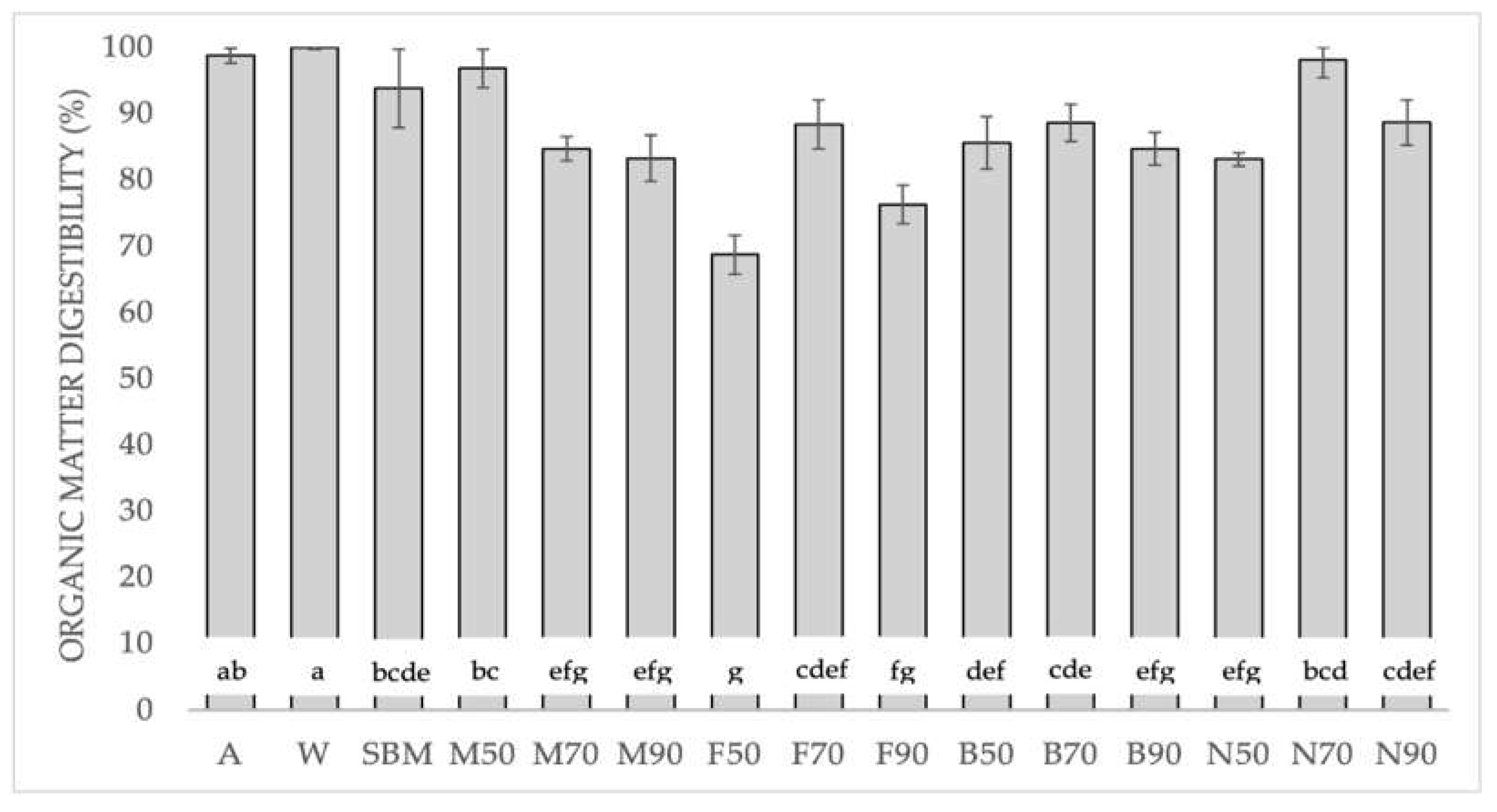
OMd has traditionally been used to assess the digestibility of food [
36]. Based on our OMd results (
Figure 1), Frozen is the worst slaughter method and 70 ºC is the best drying temperature. In general, our data when combining both processes were similar to those obtained by Bosch et al. [
37] who obtained 84.3 % for BSFL, and poultry meat meal (85.8 %). However, except for N70 (98.1%), they were less than SBM (93.8 %).
The DH data obtained (
Table 7) showed higher results for the Nitrogen and Blanched slaughter. Frozen and Nitrogen are both freezing slaughter treatments, and their difference is in the freezing temperature and therefore the speed of slaughter, as nitrogen slaughter is instantaneous. This could explain the differences in their results. As it is a fast slaughter, insects do not have time to develop the strategies they carry out when stressed, such as enzymatic browning, which can hinder protein hydrolysis. Browning can affect protein digestibility and quality, and the melanization process seems to be related to protein aggregation due to insect stress [
20]. According to Janssen et al. [
29], five enzymes may play an important role in enzymatic browning in insects: phenol oxidase, laccase, tyrosine hydroxylase, DOPA decarboxylase, and peroxidase. These are related to defense mechanisms and react quickly to stress. They are also involved in the sclerotization of the exoskeleton. Wessels et al. [
38] note that, of these, phenol oxidase is the key in browning and is likely to remain active after freezing. This is because there are anti-freeze proteins in insects that ensure that part of the water does not finish freezing, and that enzymatic reactions that favor the formation of aggregates and melanization continue to take place. This could explain the higher DH data in N with respect to F, but also the higher results for B (
Table 7). Some studies confirm the improvement in digestibility using blanching, such as that of Leni et al. [
20], who obtained higher DH results for BSFL in blanched (32 %) than frozen (16.5 %). In the industry, blanching has been used to inactivate enzymes and preserve food quality. This is because blanching blocks melanization by inactivating phenol oxidase [
20]. Different studies have shown less browning with blanching than with traditional freezing slaughter (-18 °C) [
20,
30,
39]. Other studies found no improvement in digestibility with blanching [
27,
40,
41]. Even in Janssen et al. [
19], DH data for
Alphitobius diaperinus were the same in raw and blanched but did not improve digestibility in BSFL or
Tenebrio molitor.
As indicated in the Materials and Methods section, in this experiment, we used Melacide
®. It is an antioxidant, antimelanosis, color, and texture stabilizer additive used for frozen crustaceans and mollusks. No studies to date using Melacide
® in insects have been found in the literature. This treatment was carried out precisely to try to reduce the possible melanization of the insects during slaughter. DH data showed higher results with Melacide
® (M) than without Melacide
® (F) (
Table 7); therefore, it is a desirable treatment if slaughter is to be carried out by freezing.
Regarding drying temperatures, the highest DH were obtained at 70 ºC and the lowest when increasing the temperature to 90 ºC (
Table 7). These data follow the same line as those obtained by Mancini et al. [
40], in which they obtained a better digestibility for TM in a traditional oven at 70 °C than at 150 °C. High heat treatments appear to make proteins more compact by polymerizing them [
18]. Janssen et al. [
19] observed that this denaturation and aggregation reduced the accessibility of digestive enzymes, decreasing protein digestibility.
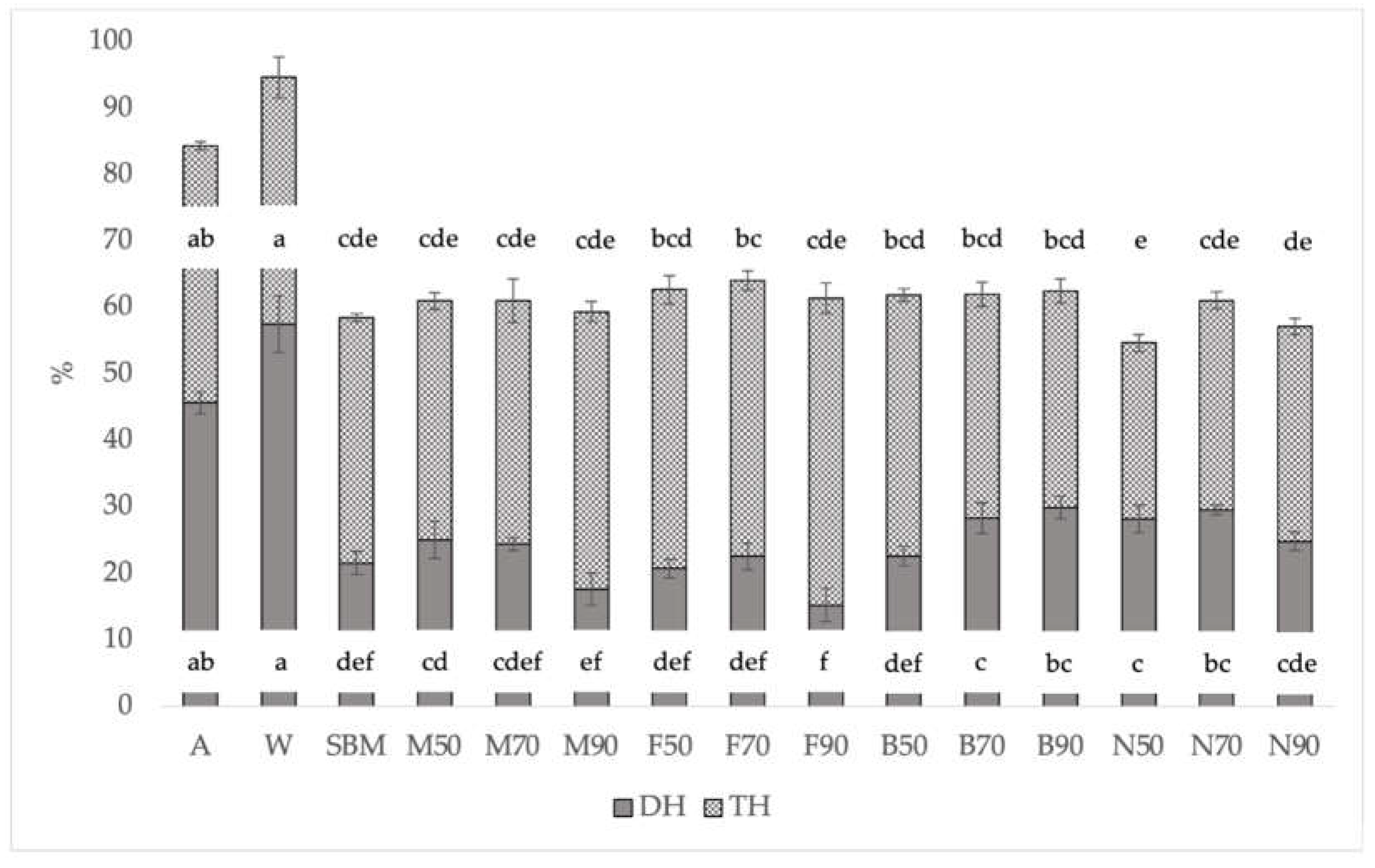
When the treatments were compared with SBM, all showed similar or even higher DH (B70, B90, N50, and N70) (
Figure 2).
However, when these data are referred to as TH, which considers DH plus other small peptides that have been solubilized after hydrolysis and are expected to be digestible, the statistical differences disappear, showing similar digestibility among almost all treatments. TH was higher in the treatments with lower DH (
Figure 2). This could indicate a slower velocity of digestion for these samples, maybe because they have a higher content and/or a less digestible structure of the scleroprotein in their exoskeleton. However, the TH data indicate that these differences are due to small peptides, not accounted for by DH, which are likely to be absorbed. This may be relevant if we want to value these insect meals as feed. Protein digestibility may be underestimated if only DH is taken into account. Differences were found between slaughter with N and the rest of the treatments. Regarding the drying temperature, again the best results were obtained at 70 °C (
Table 7).
When evaluating free amino groups per g of sample (in DM), high initial values were observed (
Table 9). This may indicate that the endogenous enzymes of the insect are active from the beginning. Therefore, as these enzymes are inactivated by blanching (B), this method of slaughter releases the fewest amino groups. According to Leni et al. [
42] it was observed partial solubilization of insect protein under hydrolysis conditions without enzymatic action below 50 %. As stated above, these data were higher than those obtained by Hall et al. [
43] (20 % for cricket) and similar to those of a previous study by Caligiani et al. [
8] (43 % for BSFL). They suggested that these differences may depend on temperature, time, pH, and enzyme conditions. After the gastric and intestinal phases, it seems clear that the slaughter method with the lowest release of amino groups was F, and the methods with the highest release were B and N (
Table 9).
The decrease in amino group content with increasing drying temperature (
Table 9) could be due to an aggregation of proteins that hinders their breakage and therefore the release of their amino groups. It is known that increasing or decreasing temperature destabilizes proteins and can promote their aggregation [
44]. Temperatures above 60-70 °C can destroy hydrogen bonds and electrostatic and hydrophobic interactions of proteins, and disorganize their structure, thus favoring protein denaturation and aggregation [
45]. Santiago et al. [
46], studying protein aggregation in
Gryllus assimilis, observed that aggregation progressively increased from practically non-existent at 65 °C to reaching its maximum at 90-95 °C. Janssen et al. [
19] linked protein denaturation and aggregation with worsening digestibility, as aggregates hinder the action of enzymes for protein degradation.
After the gastric and intestinal phases, there was a higher percentage of amino groups released in A and W as expected. In general, the BSFL meals had similar protein yields for the same amount of dry matter as SBM, and only a few BSFL treatments (M90, F50, F70, F90 y B50) showed significantly lower data (
Table 9).
The diversity in the way authors approach protein digestibility makes it difficult to compare results. Therefore, we consider it of interest to study the potential correlation between the different parameters. A positive correlation was found between CP and the different protein digestibility measurements (
Table 10). This could indicate that the higher the protein content, the higher the protein digestibility. According to Finke [
34], the ADF of insects consists of chitin and associated cuticular proteins, and since the determination of chitin is complex, the ADF can be used to estimate digestibility. Protein digestibility is affected because chitin is neither degraded nor absorbed in the digestive tract [
47]. Similar to us, Marono et al. [
35] found a negative correlation between ADF and digestibility for BSFL. Therefore, although it is not a parameter that measures digestibility, when deep studies are not possible, determining ADF can be a rough predictive tool for the degree of protein digestibility of the insect. In general, ash and ADF showed a negative correlation with the different protein digestibility parameters analysed; nevertheless, this would require further research. The correlation between CP and TH or DH showed that both parameters can be used interchangeably.
The presence of certain microbial species in final products made from insects and destined for the food market is of great importance from a hygienic-sanitary perspective. In this sense, guaranteeing the suitability of the processing protocols applied to ensure their microbial quality gives these protocols additional value in the food sector. The efficiency in the progressive disappearance of the Enterobacteriaceae group seems to be mainly a consequence of the application of drying temperatures, as noted in other studies [
48], which describe the high efficiency of thermal protocols, even with low potential, to control the presence of this type of bacteria. In the case of TAMB, the observed impact was not as considerable, although the reductions in the counts obtained were more significant as the drying temperature increased (
Table 11). Thus, the important role of temperature in relation to the microbiological quality of the insect seems to be confirmed. However, the method of slaughter also contributed to the observed differences, with nitrogen treatment being the most favorable, compared to freezing. Treatments performed at 50 °C were not sufficient to ensure the avoidance of
Salmonella (
Table 11). Therefore, it is important to ensure the correct application of the treatment; otherwise, the resistance of this bacterium tends to increase [
49]. Although there are few studies that combine the study of microbial quality with slaughter techniques based on the use of liquid nitrogen, the presence of SRC in insects processed by this type of methodology has been described [
50]. Taking into account what was mentioned, and from a microbial perspective, it seems logical to conclude that the temperature applied during the drying stage is more decisive than the method of slaughtering. Temperatures of approximately 70 °C should ensure the hygienic-sanitary quality of insects in terms of their use in the food sector.
Figure 1.
Organic matter digestibility (%) of BSFL pretreatments, soybean meal, albumin and whey protein and relative standard deviation (±SD). Significant differences in the Tukey test are represented by different letters (p<0.05). A: Albumin; W: Whey protein; SBM: Soybean meal; M: Melacide®; F: Frozen; B: Blanched; N: Nitrogen; 50, 70, 90: dried at 50 °C, 70 °C, 90 °C.
Figure 1.
Organic matter digestibility (%) of BSFL pretreatments, soybean meal, albumin and whey protein and relative standard deviation (±SD). Significant differences in the Tukey test are represented by different letters (p<0.05). A: Albumin; W: Whey protein; SBM: Soybean meal; M: Melacide®; F: Frozen; B: Blanched; N: Nitrogen; 50, 70, 90: dried at 50 °C, 70 °C, 90 °C.
Figure 2.
DH (% of total amino groups) and Total hydrolysis (% of total amino groups) of BSFL pretreatments, fishmeal, soybean meal, albumin, and whey protein and relative standard deviation (±SD). Significant differences in the Tukey test are represented by different letters (p<0.05). A: Albumin; W: Whey protein; SBM: Soybean meal; M: Melacide®; F: Frozen; B: Blanched; N: Nitrogen; 50, 70, 90: dried at 50 °C, 70 °C, 90 °C; DH: Hydrolysis Degree; TH: Total Hydrolysis.
Figure 2.
DH (% of total amino groups) and Total hydrolysis (% of total amino groups) of BSFL pretreatments, fishmeal, soybean meal, albumin, and whey protein and relative standard deviation (±SD). Significant differences in the Tukey test are represented by different letters (p<0.05). A: Albumin; W: Whey protein; SBM: Soybean meal; M: Melacide®; F: Frozen; B: Blanched; N: Nitrogen; 50, 70, 90: dried at 50 °C, 70 °C, 90 °C; DH: Hydrolysis Degree; TH: Total Hydrolysis.
Table 1.
Treatments according to the slaughter method and drying temperature.
Table 1.
Treatments according to the slaughter method and drying temperature.
| Slaughtering |
Frozen |
Blanched+Frozen |
Melacide®+Frozen |
Liquid Nitrogen |
| Tª dried (°C) |
50 |
70 |
90 |
50 |
70 |
90 |
50 |
70 |
90 |
50 |
70 |
90 |
| Treatment |
F50 |
F70 |
F90 |
B50 |
B70 |
B90 |
M50 |
M70 |
M90 |
N50 |
N70 |
N90 |
Table 2.
MANOVA and ANOVA fit statistics CP, ADF, Ash in BSFL treatments, soybean meal, albumin, and whey protein. CP: Crude protein; ADF: Acid detergent fiber.
Table 2.
MANOVA and ANOVA fit statistics CP, ADF, Ash in BSFL treatments, soybean meal, albumin, and whey protein. CP: Crude protein; ADF: Acid detergent fiber.
| |
Factor |
F |
df |
p-value |
Test |
| CP |
Temperature |
0.029 |
2 |
0.971 |
ANOVA multivariate |
| Sacrifice |
0.234 |
3 |
0.872 |
| T x S |
0.767 |
6 |
0.604 |
| ADF |
Temperature |
32.662 |
2 |
<0.001 |
| Sacrifice |
0.717 |
3 |
0.552 |
| T x S |
6.952 |
6 |
<0.001 |
| Ash |
Temperature |
48.620 |
2 |
<0.001 |
| Sacrifice |
28.390 |
3 |
<0.001 |
| T x S |
3.905 |
6 |
<0.001 |
| CP |
- |
4.772 |
14 |
<0.001 |
ANOVA simple |
| ADF |
- |
25.780 |
14 |
<0.001 |
| Ash |
- |
31.305 |
14 |
<0.001 |
Table 3.
Proximate composition (g/100 g dry matter) of BSFL treatments, soybean meal, albumin, whey protein and relative standard deviation (±SD). Significant differences in the Tukey test (p<0.05) are represented by different letters. A: Albumin; W: Whey protein; SBM: Soybean meal; M: Melacide®; F: Frozen; B: Blanched; N: Nitrogen; 50, 70, 90: dried at 50 °C, 70 °C, 90 °C; CP: Crude protein; ADF: Acid detergent fiber; nsd: no significative differences.
Table 3.
Proximate composition (g/100 g dry matter) of BSFL treatments, soybean meal, albumin, whey protein and relative standard deviation (±SD). Significant differences in the Tukey test (p<0.05) are represented by different letters. A: Albumin; W: Whey protein; SBM: Soybean meal; M: Melacide®; F: Frozen; B: Blanched; N: Nitrogen; 50, 70, 90: dried at 50 °C, 70 °C, 90 °C; CP: Crude protein; ADF: Acid detergent fiber; nsd: no significative differences.
| |
|
CP |
ADF |
Ash |
Test |
| Slaughtering |
Melacide®
|
nsd |
nsd |
16.2 ± 1.8 a
|
ANOVA multivariate |
| Frozen |
nsd |
nsd |
13.8 ± 1.7 b |
| Blanched |
nsd |
nsd |
14.0 ± 1.6 b
|
| Nitrogen |
nsd |
nsd |
13.6 ± 0.3 b |
| Temperature |
50 ºC |
nsd |
5.8 ± 0.8 a
|
16.0 ± 1.9 a
|
| 70 ºC |
nsd |
4.6 ± 0.3 c
|
13.6 ± 1.3 b
|
| 90 ºC |
nsd |
5.1 ± 0.5 b
|
13.6 ± 0.7 b
|
| Sample |
A |
98.9 ± 0.9 a
|
0.0 ± 0.0 g
|
1.1 ± 0.0 h
|
ANOVA simple |
| W |
90.5 ± 1.3 b
|
0.0 ± 0.0 g
|
3.2 ± 0.1 gh
|
| SBM |
50.0 ± 0.5 c
|
8.4 ± 0.8 a
|
6.9 ± 0.0 fgh
|
| M50 |
37.2 ± 3.5 d
|
6.1 ± 0.3 bc
|
18.6 ± 0.3 a
|
| M70 |
34.7 ± 3.3 d
|
4.3 ± 0.2 fg
|
15.5 ± 0.8 bc
|
| M90 |
34.0 ± 0.5 d
|
5.2 ± 0.5 def
|
14.7 ± 0.0 bcd
|
| F50 |
34.9 ± 1.7 d
|
6.6 ± 0.3 b
|
15.7 ± 1.5 b
|
| F70 |
34.6 ± 1.3 d
|
4.5 ± 0.3 fg
|
12.6 ± 0.8 efgh
|
| F90 |
33.9 ± 0.4 d
|
4.9 ± 0.5 def
|
13.2 ± 0.3 defg
|
| B50 |
34.2 ± 1.5 d
|
5.9 ± 0.3 bcd
|
16.0 ± 0.2 b
|
| B70 |
34.6 ± 0.4 d
|
4.9 ± 0.5 def
|
12.7 ± 0.6 efgh
|
| B90 |
35.8 ± 1.8 d
|
4.9 ± 0.4 def
|
13.3 ± 0.2 defg
|
| N50 |
34.8 ± 1.3 d
|
4.6 ± 0.1 efg
|
13.8 ± 0.1 bcde
|
| N70 |
34.5 ± 0.2 d
|
4.7 ± 0.1 ef
|
13.5 ± 0.5 cdef
|
| N90 |
34.8 ± 0.6 d
|
5.5 ± 0.4 cde
|
13.3 ± 0.0 defg
|
Table 4.
MANOVA fit statistics Organic Matter Digestibility in BSFL treatments. ANOVA fit statistics Organic Matter Digestibility in BSFL treatments, albumin, whey protein and soybean meal. OMd: Organic Matter digestibility.
Table 4.
MANOVA fit statistics Organic Matter Digestibility in BSFL treatments. ANOVA fit statistics Organic Matter Digestibility in BSFL treatments, albumin, whey protein and soybean meal. OMd: Organic Matter digestibility.
| |
Factor |
F |
|
df |
p-value |
Test |
| OMd |
Temperature |
10.635 |
|
2 |
<0.001 |
ANOVA multivariate |
| Sacrifice |
10.705 |
|
3 |
<0.001 |
| T x S |
10.423 |
|
6 |
<0.001 |
| - |
19.395 |
|
14 |
<0.001 |
ANOVA simple |
Table 5.
Organic matter digestibility of BSFL treatments, and relative standard deviation (±SD). Significant differences in the Tukey test (p<0.05) are represented by different letters. OMd: Organic Matter digestibility.
Table 5.
Organic matter digestibility of BSFL treatments, and relative standard deviation (±SD). Significant differences in the Tukey test (p<0.05) are represented by different letters. OMd: Organic Matter digestibility.
| |
|
OMd |
Test |
| Slaughtering |
Melacide®
|
88.3 ± 6.9 a
|
ANOVA multivariate |
| Frozen |
77.8 ± 9.0 b
|
| Blanched |
86.3 ± 3.2 a
|
| Nitrogen |
90.0 ± 6.9 a
|
| Temperature |
50 ºC |
83.6 ± 10.7 b
|
| 70 ºC |
89.9 ± 5.7 a
|
| 90 ºC |
83.3 ± 5.4 b
|
Table 6.
MANOVA fit statistics Hydrolysis degree and Total hydrolysis in BSFL treatments. ANOVA fit statistics Hydrolysis degree and Total hydrolysis in BSFL treatments, albumin, whey protein and soybean meal.
Table 6.
MANOVA fit statistics Hydrolysis degree and Total hydrolysis in BSFL treatments. ANOVA fit statistics Hydrolysis degree and Total hydrolysis in BSFL treatments, albumin, whey protein and soybean meal.
| |
Factor |
F |
|
df |
p-value |
Test |
| DH |
Temperature |
11.781 |
|
2 |
<0.001 |
ANOVA multivariate |
| Sacrifice |
32.258 |
|
3 |
<0.001 |
| T x S |
8.391 |
|
6 |
<0.001 |
| TH |
Temperature |
3.273 |
|
2 |
0.057 |
| Sacrifice |
6.224 |
|
3 |
0.003 |
| T x S |
1.341 |
|
6 |
0.281 |
| DH |
- |
26.423 |
|
14 |
<0.001 |
ANOVA simple |
| TH |
- |
8.875 |
|
14 |
<0.001 |
Table 7.
Hydrolysis degree and Total hydrolysis between different slaughtering methods and drying temperatures. Significant differences in the Tukey test (p<0.05) are represented by different letters. DH: Hydrolysis degree; TH: Total hydrolysis.
Table 7.
Hydrolysis degree and Total hydrolysis between different slaughtering methods and drying temperatures. Significant differences in the Tukey test (p<0.05) are represented by different letters. DH: Hydrolysis degree; TH: Total hydrolysis.
| |
|
DH |
TH |
Test |
| Slaughtering |
Melacide®
|
22.4 ± 3.9 b
|
60.4 ± 2.3 ab
|
ANOVA multivariate |
| Frozen |
19.5 ± 3.7 c
|
62.6 ± 1.9 a
|
| Blanched |
26.9 ± 3.8 a
|
62.0 ± 1.5 a
|
| Nitrogen |
27.5 ± 2.8 a
|
57.6 ± 3.2 b
|
| Temperature |
50 ºC |
24.1 ± 3.3 b
|
nsd |
| 70 ºC |
26.2 ± 3.6 a
|
nsd |
| 90 ºC |
21.9 ± 6.2 b
|
nsd |
Table 8.
MANOVA fit statistics Amino groups (g / 100 g DM) in BSFL treatments. ANOVA fit statistics Amino groups (g / 100 g DM) in BSFL treatments, albumin, whey protein and soybean meal.
Table 8.
MANOVA fit statistics Amino groups (g / 100 g DM) in BSFL treatments. ANOVA fit statistics Amino groups (g / 100 g DM) in BSFL treatments, albumin, whey protein and soybean meal.
| |
Factor |
F |
|
df |
p-value |
Test |
| Initial (0’) |
Temperature |
47.222 |
|
2 |
<0.001 |
ANOVA multivariate |
| Sacrifice |
15.712 |
|
3 |
<0.001 |
| T x S |
4.721 |
|
6 |
0.003 |
| Gastric (240’) |
Temperature |
0.300 |
|
2 |
0.744 |
| Sacrifice |
23.388 |
|
3 |
<0.001 |
| T x S |
7.631 |
|
6 |
<0.001 |
| Intestinal (480’) |
Temperature |
12.982 |
|
2 |
<0.001 |
| Sacrifice |
42.667 |
|
3 |
<0.001 |
| T x S |
8.487 |
|
6 |
<0.001 |
| Initial (0’) |
- |
19.021 |
|
14 |
<0.001 |
ANOVA simple |
| Gastric (240’) |
- |
24.055 |
|
14 |
<0.001 |
| Intestinal (480’) |
- |
26.855 |
|
14 |
<0.001 |
|
Table 9.
Amino groups (g / 100 g DM) of BSFL treatments, soybean meal, albumin, and whey protein at the start and end of the gastric and intestinal phases of protein hydrolysis and relative standard deviation (±SD). Significant differences in the Tukey test (p<0.05) obtained in the different ANOVAS are represented by different letters. A: Albumin; W: Whey protein; SBM: Soybean meal; M: Melacide®; F: Frozen; B: Blanched; N: Nitrogen; 50, 70, 90: dried at 50 °C, 70 °C, 90 °C; nsd: no significative differences.
Table 9.
Amino groups (g / 100 g DM) of BSFL treatments, soybean meal, albumin, and whey protein at the start and end of the gastric and intestinal phases of protein hydrolysis and relative standard deviation (±SD). Significant differences in the Tukey test (p<0.05) obtained in the different ANOVAS are represented by different letters. A: Albumin; W: Whey protein; SBM: Soybean meal; M: Melacide®; F: Frozen; B: Blanched; N: Nitrogen; 50, 70, 90: dried at 50 °C, 70 °C, 90 °C; nsd: no significative differences.
| |
|
Initial (0’) |
Gastric (240’) |
Intestinal (480’) |
Test |
| Slaughtering |
Melacide |
4.5 ± 0.9 a
|
6.1 ± 2.0 b
|
8.4 ± 1.5 c
|
ANOVA multivariate |
| Frozen |
4.2 ± 0.4 b
|
4.6 ± 0.3 c
|
7.2 ± 1.4 d
|
| Blanched |
3.3 ± 0.9 c
|
8.3 ± 1.1 a
|
9.8 ± 1.3 b
|
| Nitrogen |
4.2 ± 0.9 b
|
6.5 ± 1.0 b
|
11.2 ± 1.1 a
|
| Temperature |
50 ºC |
4.9 ± 0.4 a
|
nsd |
9.2 ± 1.6 ab
|
| 70 ºC |
4.1 ± 0.8 b
|
nsd |
9.9 ± 1.6 a
|
| 90 ºC |
3.2 ± 0.5 c
|
nsd |
8.3 ± 2.4 b
|
| Sample |
A |
4.0 ± 0.1 cdef
|
18.8 ± 0.5 ab
|
39.3 ± 0.9 ab
|
ANOVA simple |
| W |
4.7 ± 0.1 abc
|
18.5 ± 0.8 a
|
46.0 ± 1.5 a
|
| SBM |
1.8 ± 0.0 g
|
9.4 ± 0.2 abc
|
11.2 ± 1.6 cd
|
| M50 |
5.1 ± 0.1 ab
|
8.6 ± 0.7 cde
|
9.4 ± 0.5 def
|
| M70 |
5.1 ± 0.4 a
|
4.6 ± 0.3 g
|
9.3 ± 1.0 def
|
| M90 |
3.4 ± 0.1 defg
|
5.0 ± 0.6 fg
|
6.6 ± 0.6 fg
|
| F50 |
4.7 ± 0.1 abc
|
4.5 ± 0.0 g
|
7.6 ± 0.7 efg
|
| F70 |
4.2 ± 0.2 cde
|
4.6 ± 0.4 g
|
8.3 ± 1.0 efg
|
| F90 |
3.7 ± 0.1 cdefg
|
4.7 ± 0.4 fg
|
5.6 ± 0.5 g
|
| B50 |
4.4 ± 0.4 bcd
|
6.8 ± 0.2 defg
|
8.2 ± 0.5 efg
|
| B70 |
3.1 ± 0.2 efg
|
8.9 ± 0.2 cde
|
10.2 ± 0.8 cde
|
| B90 |
2.4 ± 0.1 fg
|
9.1 ± 0.1 bcd
|
10.8 ± 0.8 cd
|
| N50 |
5.3 ± 0.3 a
|
6.4 ± 0.4 defg
|
11.5 ± 0.6 bcd
|
| N70 |
3.9 ± 0.2 cdef
|
7.3 ± 0.3 cdef
|
12.0 ± 0.9 bc
|
| N90 |
3.3 ± 0.3 defg
|
5.6 ± 1.2 efg
|
10.0 ± 0.9 cde
|
Table 10.
Correlations between proximal composition, organic matter digestibility, and protein digestibility (DH and TH). The correlation is significant at the 0.05 level. CP: Crude Protein; ADF: Acid Detergent Fibre; OMd: Organic Matter digestibility; DH: Hydrolysis Degree; TH: Total Hydrolysis; p: p-value.
Table 10.
Correlations between proximal composition, organic matter digestibility, and protein digestibility (DH and TH). The correlation is significant at the 0.05 level. CP: Crude Protein; ADF: Acid Detergent Fibre; OMd: Organic Matter digestibility; DH: Hydrolysis Degree; TH: Total Hydrolysis; p: p-value.
| |
TH |
p |
DH |
p |
OMd |
p |
Ash |
p |
ADF |
CP |
| CP |
0.329 |
0.031 |
0.479 |
0.001 |
0.607 |
<0.001 |
-0.408 |
0.006 |
nsd |
- |
| ADF |
-0.385 |
0.011 |
-0.489 |
<0.001 |
-0.383 |
0.011 |
0.450 |
0.002 |
- |
- |
| Ash |
-0.302 |
0.047 |
-0.374 |
0.012 |
nsd |
- |
- |
- |
- |
- |
| OMd |
0.454 |
0.002 |
0.636 |
<0.001 |
- |
- |
- |
- |
- |
- |
| DH |
0.456 |
0.002 |
- |
- |
- |
- |
- |
- |
- |
- |
Table 11.
Hygienic-sanitary quality, according to the levels of microbial contamination indicators, of insects after processing according to the different treatments. Values are expressed as Log10 CFU mL-1. Mean values ± SD are shown. Significant differences in the Tukey test are represented by different letters (p<0.05). A: Albumin; W: Whey protein; SBM: Soybean meal; M: Melacide®; F: Frozen; B: Blanched; N: Nitrogen; 50, 70, 90: dried at 50 °C, 70 °C, 90 °C. TAMB: Thermophilic Acidophilic Bacteria; SRC: sulfite-reducing Clostridia. *Detection limit: 1 log CFU mL-1.
Table 11.
Hygienic-sanitary quality, according to the levels of microbial contamination indicators, of insects after processing according to the different treatments. Values are expressed as Log10 CFU mL-1. Mean values ± SD are shown. Significant differences in the Tukey test are represented by different letters (p<0.05). A: Albumin; W: Whey protein; SBM: Soybean meal; M: Melacide®; F: Frozen; B: Blanched; N: Nitrogen; 50, 70, 90: dried at 50 °C, 70 °C, 90 °C. TAMB: Thermophilic Acidophilic Bacteria; SRC: sulfite-reducing Clostridia. *Detection limit: 1 log CFU mL-1.
| |
TAMB |
Enterobacteria |
SRC* |
Salmonella |
| Control |
5.92 ± 0,35 ab
|
5.32 ± 0.13 a
|
Negative |
Positive |
| M50 |
7.25 ± 0.33 a
|
< 1 b
|
Negative |
Positive |
| M70 |
2.92 ± 2.53 cde
|
< 1 b
|
Negative |
Negative |
| M90 |
1.65 ± 1.43 ef
|
< 1 b
|
Negative |
Negative |
| F50 |
6.14 ± 0.25 ab
|
< 1 b
|
Negative |
Positive |
| F70 |
4.84 ± 0.50 bc
|
< 1 b
|
Negative |
Negative |
| F90 |
2.11 ± 1.92 de
|
< 1 b
|
Negative |
Negative |
| B50 |
6.97 ± 0.13 a
|
< 1 b
|
Negative |
Positive |
| B70 |
3.54 ± 0.92 cd
|
< 1 b
|
Negative |
Negative |
| B90 |
< 1 f
|
< 1 b
|
Negative |
Negative |
| N50 |
1.71 ± 0.06 ef
|
< 1 b
|
Positive |
Negative |
| N70 |
< 1 f
|
< 1 b
|
Positive |
Negative |
| N90 |
< 1 f
|
< 1 b
|
Positive |
Negative |