1. Introduction
Groundwater is the world’s main resource for drinking, irrigation, and industrial uses (Mcdonald et al., 2019). It is one of the most valuable natural resources both in developed and developing countries including Ethiopia. In fact, in arid and semiarid areas, groundwater is a vital resource for domestic, industrial, and irrigation uses (Delgado et al., 2010; Yousefi et al., 2018). In Ethiopia, in most cities and towns including Addis Ababa, groundwater serves as a major and/or alternative to surface water supplies (Berehanu et al., 2018). For instance, in the Middle and Lower Awash and in some parts of the Oromia and Afar regions, where there is no enough potable surface water for the population’s daily needs, notably drinking, groundwater is the main water supply source. According to several studies, the previously projected groundwater potential was understated, coming in at nearly 2.6 BMC. However, groundwater researchers estimate/show that it may be greater than the previous numbers and predict roughly 12 to 30 BMC, or even more if we evaluate all of the lowland aquifers (Birhanu et al., 2006).
The groundwater chemistry of the study area is essential for providing insight into water types for the groundwater data scarce basins, geochemical process might be taking place, and characteristics based on hydro-geochemical benchmarks. Accordingly, by integrating the groundwater chemistry with geochemical characteristics, in the study the possibility of identifying trends, environmental challenges, might enhance understanding of the groundwater resource water quality and suitability to use and contamination as well. The Awash Basin, partly located in the Main Ethiopian Rift (MER), is a densely populated and industrialized area where numerous enterprises rely on groundwater for their operations (MoWE-WLRC, 2023). Therefore, the majority of human development initiatives in the basin will continue to depend heavily on the quantity and quality of groundwater.
In the Middle Awash Basin and the country at large, the water quality of most groundwater sources is inadequately monitored and insufficiently regulated. Consequently, areas within the upstream Awash Basin, particularly around Modjo, Bishoftu, Gelan, and Addis Ababa are highly susceptible to unregulated abstraction and pollution of groundwater (Kidist et al., 2023). Researches indicate that these groundwater sources are at risk of contamination from industrial waste, sewage, and solid waste leachates due to the prevalent discharge of poorly treated wastewater by industries (Yimer and Geberekidan, 2020). Moreover, factors such as excessive groundwater abstraction, the overuse of agricultural chemicals, mismanagement of human waste, and rapid population growth further exacerbate groundwater contamination (Subba Rao et al., 2017; Adimalla and Venkatayogi, 2018).
The Ministry of Water Resources (MoWR, 2011) report identifies key groundwater quality issues in Ethiopia as predominantly natural, notably high fluoride concentrations in certain areas and iodine deficiencies in others, alongside general groundwater salinity. Studies suggest that the sources of some pollutant chemical concentrations in ground water may vary, but research conducted by the MoWR suggests that the analysis of groundwater contamination should concentrate on soil characteristics, hydrology, and geological conditions (confined or unconfined). For example, certain substances, like Fe, K, Cl, and SO4, are well-known in the concentrations found in the Awassa-Yirgachfie, Butajera-Assela, Afderra & Dallol, and Ogaden basin, respectively (MoWR, 2009). These naturally occurring elements, particularly fluoride and arsenic, when concentrated in drinking water, can detrimentally impact human health, causing widespread suffering globally (Adimalla, N., Vasa, S.K., and Li, P., 2018). However, attributing these groundwater quality issues solely to natural causes may oversimplify the complexity of the underlying geology and topography, especially in regions like the Rift Valley. Pollution sources such as heavy metals, pesticides, and pharmaceutical wastes can permanently degrade groundwater, making its purification significantly more difficult. Therefore, preventing groundwater pollution is essential and should be prioritized to safeguard this vital resource for future generations. Groundwater management requires at most attention and proactive measures due to the global challenges posed by rapid population growth, urbanization, climate change, and various human activities (Sophocleous, 2004; Giordano, 2009; Taylor et al., 2013).
The expanding urban-industrial centers in Awash Basin including Addis Ababa, Modjo, Bishoftu, and Addama taking water from the Awash River and groundwater. Accordingly the groundwater withdrawals are extremely accelerating and not well governed. Not only this but also the groundwater contamination has become a significant issue and cause for concern in recent years (Mukherjee et al., 2020). There are many different types of sources that might contaminate groundwater. For example, the main causes of groundwater contamination in developed nations include the use of pesticides and fertilizers, the discharge of hazardous microbes and chemical pollutants in urban areas, and domestic and industrial wastes (Bhubaneswar et al., 2023). The study area is mainly known by sugar farm estate, because the Wonji, Methara, and Kessum sugar factories are situated in this basin. Unregulated sugar mill effluent contains considerable amount of potentially harmful substances including soluble salts and heavy metals such as Fe, Cu, Zn, Mn, Pb (Bhatnagar, A., et al., 2016). Perhaps, the long-term use of this sugar mill effluent for irrigation must be discouraged, as this improper wastewater usage results in the contamination of soils and water if released into water bodies (Fakayode 2005).
Similar to this, unchecked human activities, industrial activities, and geological activities cause groundwater contamination in developing nations including, Ethiopia. It has been increasing and has got less emphasis. For instance, the Lake Beseka expansion affects the surface and groundwater dynamics and soil properties of the region and the condition is specifically dangerous for the sustainability of Methara Sugar Estate and Matahara town in particular and the Awash basin irrigation in general (Demlie, M., and Wohnlich, S. (2006). Protecting groundwater from diverse contamination sources necessitates an integrated approach, emphasizing the need for better data collection and a strategic framework for contamination prevention and management. In fact, once contaminated, groundwater presents a complex challenge for remediation, more so than surface water due to its relatively stable quality that does not fluctuate. Perhaps understanding and maintaining groundwater quality is crucial, particularly in regions experiencing significant pollution like Awash Basin. However, comprehensive data on groundwater quality in Ethiopia are scarce, which complicates efforts to assess and manage this resource effectively (BGS, 2001).
Due to pollution in surface water and its shrinkage, unregulated groundwater abstraction, and contamination from both anthropogenic and geogenic sources, ensuring equitable access to safe and affordable drinking water in the Middle Awash Basin presents significant challenges; directly impacting the achievement of Sustainable Development Goal 6 (SDG6). Additionally, the goals of halving the proportion of untreated wastewater, eliminating the dumping and minimizing the release of hazardous chemicals, including heavy metals, into water bodies, and significantly increasing the recycling and safe reuse of water in Africa, as outlined in SDG 6.3, face substantial obstacles in regions like Ethiopia. Therefore, a comprehensive assessment of both groundwater and surface water quality in water-scarce areas such as the Middle Awash is essential to support sustainable development and maintain reliable water sources. The primary objective of this research is to evaluate the groundwater quality in the Middle Awash Basin, focusing on its suitability for both irrigation and drinking purposes. This study is critical due to the prevailing high significant reliance on groundwater in regions where access to potable water is frequently unreliable. By analyzing a range of physicochemical and heavy metal parameters in boreholes and hand-dug wells, this research aims to provide a comprehensive assessment of groundwater quality. Both natural and anthropogenic causes of groundwater pollution were thoroughly analyzed. The findings are expected to inform the development of regulatory guidelines and risk reduction strategies that will ensure a reliable supply of clean drinking water.
2. Study Area
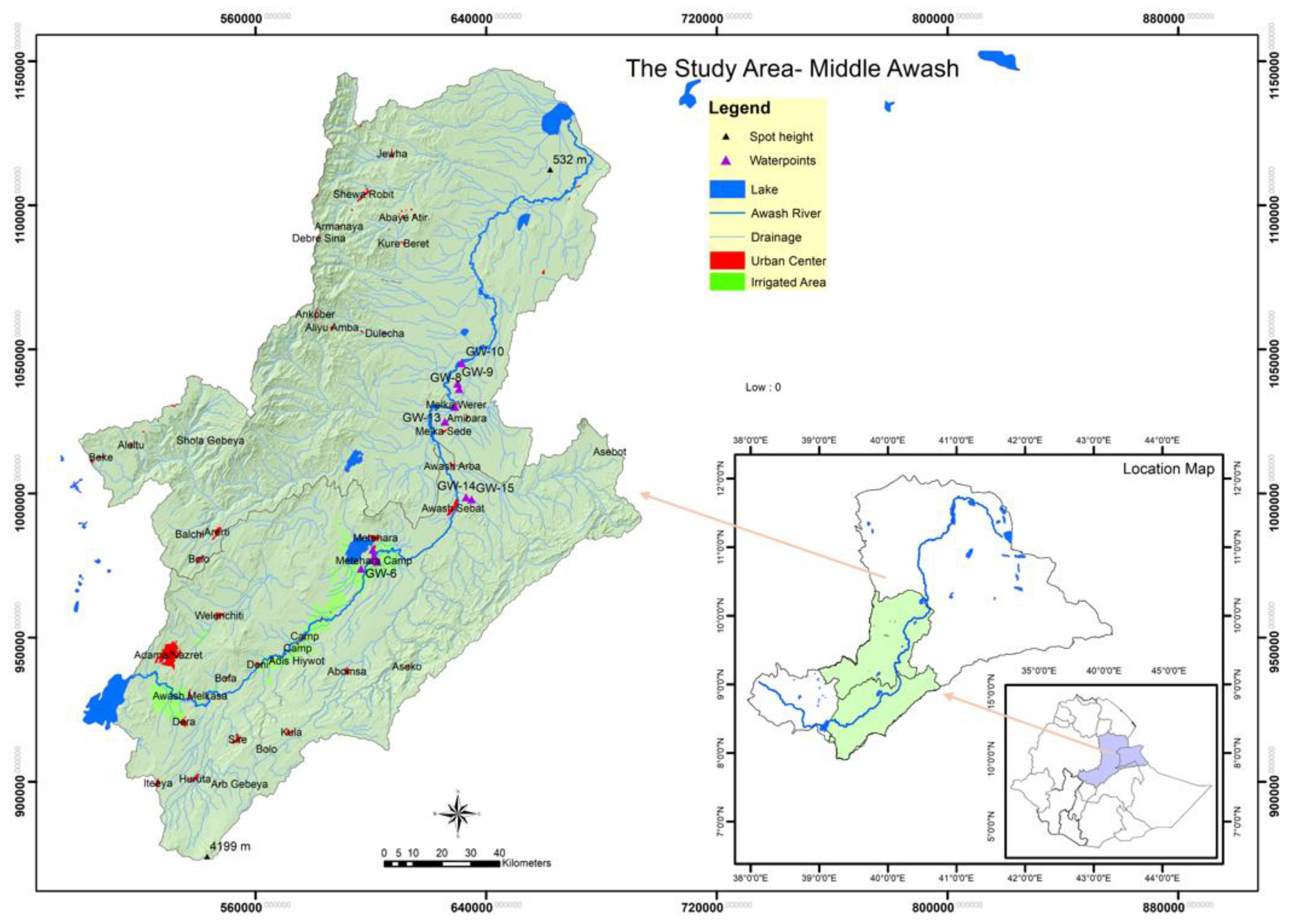
The Awash River basin lies between 37° 57′ E and 43° 25′ E longitude, and between latitudes 7° 53′ N and 12° 00′N (
Figure 1). The Awash River is 1200 kilometers long overall. It starts in Ethiopia’s central highlands at an elevation of 3000 meters and passes through a number of locations before joining Lake Abe at a height of 250 meters. The Awash River Basin, the basin where the study was undertaken is divided into three sections: upper, middle, and lower. It is one of the most heavily utilized basins in the country, situated at a lower course of the most urbanized and industrialized Upper Awash. Middle Awash is known for having both large- and small-scale irrigation, as well as agroindustry and sugar factories (Wenji, Methara, and Kesem Sugar factories) are located. The study area’s elevation gradient covered a range of elevations, from high mountains approximately 4199 m to low points 532 m above sea level (
Figure 1). The Middle Awash Basin is primarily known for being water-scarce and occasionally flood-prone. There are tributary rivers, lakes, hot springs, and swamps in the Middle Awash basin. One of the basin’s natural lakes, for example, is Lake Beseka, Ethiopia’s Lake, was a saline lake located in the East African Rift Valley (Middle Awash). It has no natural exits and has grown significantly since the 1960s as a result of subsurface flows bringing excess irrigation water into the lake from subterranean flows, hot spring inflow, and groundwater discharge. Since 2004, a constructed canal (10.8 km) has connected the lake to the Awash River.
Geological Setting and Structures
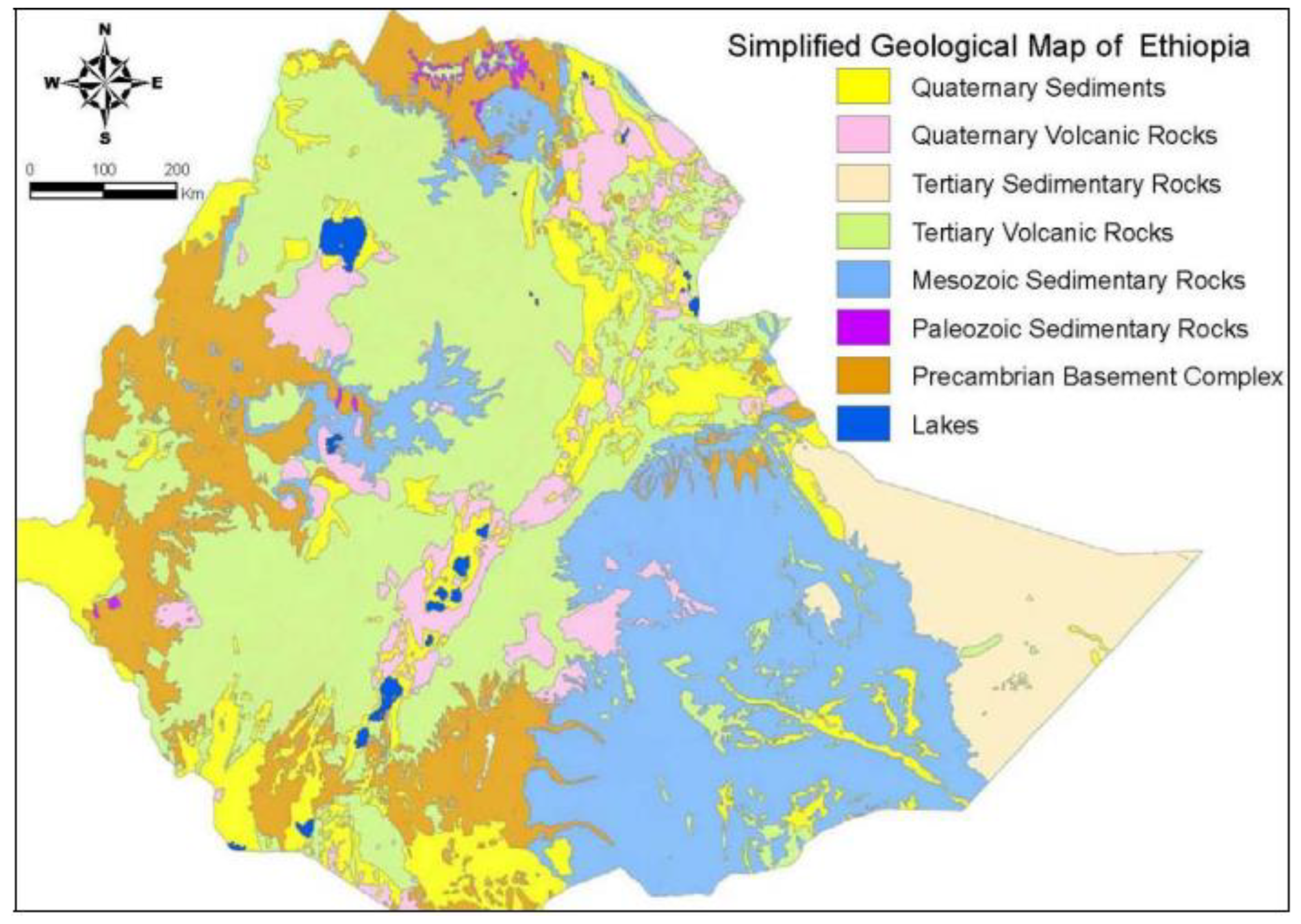
Ethiopia is home to a wide variety of volcanic, sedimentary, and metamorphic rocks, as seen in
Figure 2 above. According to studies, there are four distinct rock groups that can be distinguished based on their coverage and extent. Precambrian basement complex, Paleozoic and Mesozoic sedimentary rock, territory volcanic rocks, and quaternary sediments and volcanic rocks can all be shown with percentage compositions of 18%, 25%, 40%, and 17%, respectively. But their presence might also differ from basin to basin or from one place to another. For example, the largest groups of volcanic rocks in the study area are the Trap Series, which formed in the early and middle Tertiary when cracks opened up. In the Rift valley, the Plio-Quaternary volcanics are mostly contained. The geology of the study area surrounding the lake is comprised of volcanic rocks, mainly recent to sub-recent basalts, ignimbrite, rhyolite, and tuff. The floor and edges of Lake Beseka are constituted by Pleistocene lacustrine sediments (Gebremichale et al., 2022). Most of the volcano-tectonic process-controlled lakes found in the Ethiopian Rift Valley are found in the part of the Ethiopian rift system located to the south of the Afar T
riangle, called the Main Ethiopian Rift (MER) (Bonini et al., 2005, Gasse and Street, 1978). The study area’s topography is a reflection of the Middle Awash Valley’s recent geomorphic history, which saw the formation of a vast alluvial plain from deposits from the Awash River (Mamo et al., 2019). Slope gradients are often very low, with most of them falling between 1% and 2% (Mamo et al., 2019). Volcanic rocks make up the parent materials of the alluvial deposits in the study area’s rift valley. These comprise rhyolite parent materials, alkaline olivine-and dolerite-andesite basaltic magmas, carbonatite, volcanic ash, tuff, pumice, and granites, feldspars, and aluminosilicates of sodium and potassium (Italconsult, 1969; Heluf, 1985).
This area’s geological makeup includes volcanic ash and a variety of rock types, such as sedimentary and igneous rocks, which significantly contribute to groundwater contamination. For example, in the Afar section of the Rift and much of the adjacent plateau areas, the dominant basaltic rocks rich in Fe and Mn are often interbedded with sediments (Tefera et al., 1996). This interplay of volcanic activity and rifting has led to the formation of the low-lying Afar Depression. Additionally, young (Quaternary) unconsolidated alluvial and lake sediments within the Rift zone can further influence water quality (BGS, 2001). Thus, both natural conditions and complex geological processes must be considered to fully understand and address the challenges of groundwater quality in Ethiopia.
Climate and Hydrology
The basin covers a wide climatic zone from humid subtropical to arid and has a relatively long-running and dense meteorological and hydrological observation network. Climate variability is one of the severe environmental changes of the twenty first century (Mall RK, et al., 2006; Ekwueme BN, et al., 2021). Thus, parts of the water cycle are probably accelerating due to climate change because rising global temperatures accelerate global evaporation. On average, there may be greater precipitation due to increased evaporation. In actuality, there are differences in the global distribution of higher rates of precipitation and evaporation. Some countries like Ethiopia, such as the upper Awash (Upper Awash > Middle Awash > Lower Awash), may get precipitation that is heavier than usual. As a result of the complex topography, the intertropical convergent zone (ITCZ) and associated atmospheric circulation are important contributors to the Awash River basin climate (Taye et al., 2018). Studies also revealed that the annual temperatures in the basin showed a significant increase (Daba et al., 2020). The elevated temperatures in the research region linked to climate change may result in modifications to precipitation and evaporation patterns. For this reason, the research area is extremely vulnerable to significant flooding as well as limited water accesses.
In fact, Ethiopia is one of the nation’s most susceptible to the effects of climate change. Regarding climate change, the Ethiopian governments have to carry out SDG Goal 13 (urgent action to address climate change and its consequences). However, groundwater quality is predominantly controlled by anthropogenic factors and natural activities including volcanoes, which can be modified by climate change. Climate change can influence groundwater quality because of the composition of recharge water, which is modified or because the proportions of water from a different origin change. Not only this but also it can influence groundwater temperature in opposite directions. For instance in areas dominated with infiltrating precipitation, the increase in air temperature will propagate to groundwater. However, if river water infiltration is a dominant recharge mechanism, the groundwater temperature can decrease due to higher fraction of recharge during winter.
Irrigation: Agroindustry and Sugar Plantation
Sugar industry being an important player in the foreign exchange earnings, also plays its part in polluting the vent with its discharges (Ansari, 2006). When sugar is made, a large amount of waste is produced, and this waste has a high pollution load, especially when it comes to organic matter, suspended particles, press mud, bagasse, heavy metals, and air pollutants (Poddar, P.K., Sahu, O., 2017). The sugar industry uses a variety of chemicals, mostly for the purposes of refining final goods (i.e., Sugar) and coagulating impurities. For instance, Ca(OH)2 is one of several compounds used for a variety of applications; it raises the pH and clarifies fluids. Large-scale canals and subterranean drains, sometimes known as tiles, are frequently used in irrigation to short circuit flow routes that connect surface water to ground water. Irrigation raises local groundwater recharge and water tables, which could lead to pollution as well. Pesticides and herbicides, which are agricultural chemicals, can contaminate groundwater in a variety of ways. One of the potential dangerous derived from the application of agrochemicals is the pollution of groundwater (Adeoye et al., 2013). After their application to crops, they are observed by soil and percolate through the soil after rain or floods, carry the chemical with it and are eventually leached to the underlying groundwater (Kulabako et al., 2007). Sugar mill effluents are as a source of groundwater contaminations (Deshmukh, K. K., 2014). High concentration of TDS, Alkalinity, DO, BOD, and COD above the permissible limit was observed in groundwater around sugar industries of India (Anoop & Renu, 2014). In agricultural landscapes, topography plays an important role in the transport of chemicals to ground water (Adeoye et al., 2013). Consequently, the concentrations of chemicals associated with agricultural practices, such as nitrate nitrogen (nitrate), chloride, sulfate, and atrazine are increasing at an alarming rate in water tables (Delin et al., 2000).
3. Material and Methods
To evaluate groundwater quality, a total of 32 samples from boreholes, piezometers, and wells were collected during the dry (June 2021) and wet (November 2021) seasons. These samples were sourced from 16 locations across the Middle Awash basin, including Addis Ketema, Merti camp, Abadir 4th camp, and Awash Sebat-kilo water supply (which includes two water supply wells), along with several piezometer sites (six piezometers) and one hot-spring well near the Awash Basin Office at Amibera Wereda, Ethiopia. While the bulk of sampled stations, eight, numbering 50%, are used for washing (clothes, kitchenware, and bathing uses) (near Merti & Addis Ketema), a fraction of sampling stations two, numbering 12.5%, are used for drinking (Awash Sebat-kilo town). A total of six (or 37.5%) of the sampled stations are piezometer stations (Amibera State Farm). Typically, half of the sample stations are located close to Lake Beseka. A total of eight sampled stations or 50% of the sampling sites are downstream of Lake Beseka (@Awash-Halidebi) while the other 50% are close to Lake Beseka (@Awash-Awash).
A short length of plastic tubing was inserted into the piezometer, and samples were collected by sucking on the tube, pulling it out of the piezometer, and emptying it after kinking off the tube. When it hits the water, an alarm will sound, and once the level is measured again, it will be recorded. Samples were stored in polyethylene bottles that had been acid-washed and rinsed with the water to be tested. Immediately after collection, the bottles were transported to the laboratory and stored at 4°C. On-site, parameters such as temperature, electrical conductivity, and pH were measured using a pre-calibrated portable digital EC, TDS, and pH meter. Major ions and other physicochemical parameters including total hardness, alkalinity, calcium (as CaCO3), magnesium (as MgCO3), bicarbonates, carbonates, fluoride, chloride, sulfate, phosphate, nitrate, nitrite, ammonia, and ammonium ions were analyzed using standard methodologies (APHA, 1995) with a Palintest 7100 (Multi-parameter photometer). Sodium (Na+) and potassium (K+) concentrations were determined by flame photometry (Perkin-Elmer model of Coleman) at the Melka-Werer Agriculture laboratory, Department of Water Quality, Afar, Ethiopia. Heavy metal samples, filtered and acidified, were analyzed for over 24 metals and metalloids (including Al, Li, Mo, Ti, V, Mn, Ba, Ni, Cu, Zn, Sr, As, Rb, Cr, Zr, Nb, Ge, Co, Fe, Ga, Ag, Cd, Sn, and Sb) using ICP-MS/OES at the Oxford Analytical Laboratory in the UK. Statistical analyses, including mean, standard deviation, and correlation, were conducted using SPSS version 26. The results were discussed using average values and 95% confidence intervals, with the least significant differences (LSD) employed to evaluate statistical differences between means at a significance level of α ≤ 0.05. Data visualization was enhanced using Origin software and Minitab 19.
4. Results and Discussion
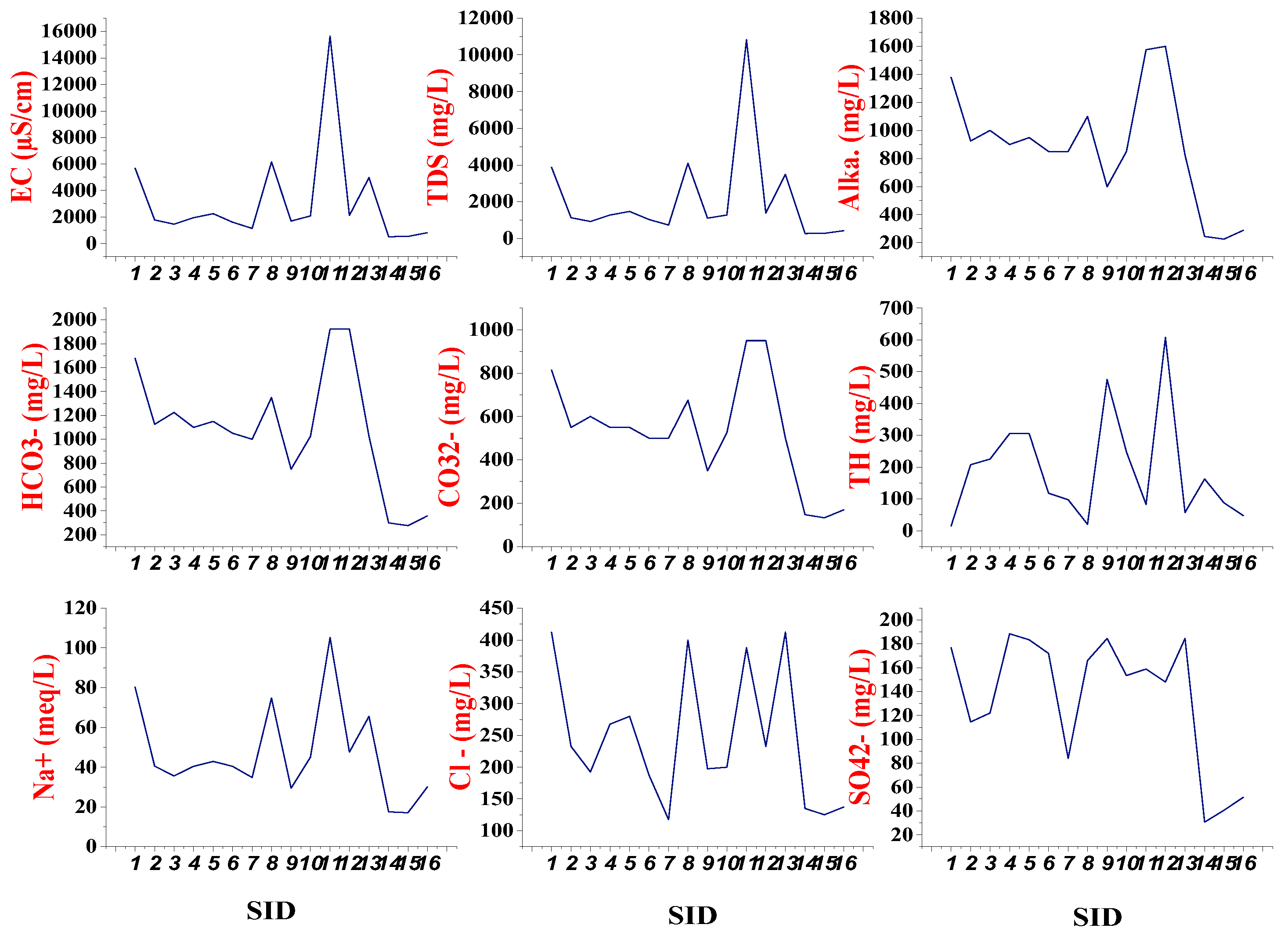
Understanding the main factors controlling groundwater chemistry is very important for sustainable groundwater development. Thus, for the purpose of evaluating the quality of groundwater and its suitability for drinking and agriculture, we have examined over twenty physicochemical parameters, including pH, electrical conductivity (EC), total dissolved solids (TDS), alkalinity, bicarbonate, carbonate, chloride, fluoride, sulfate, phosphate, nitrate, nitrite, ammonia, ammonium, and total hardness (TH) (
Table 1) in Middle Awash groundwater (
Figure 3 and
Figure 4). The study area’s groundwater resources have been subjected to an evaluation of their overall pollution level using twenty-four different heavy metals/metalloids (Al, Li, Ti, Co, Cu, As, Mo, Mn, Cr, Ba, Cr, Sr, Fe, Mn, Ni, Ba, and the like) (
Table 2). Multivariate statistical techniques (principal component analysis and Spearman’s correlation) are combined to determine the pollution status and likely sources of pollutants in the middle valley of Awash, thereby strengthening the results.
Distribution of Physicochemical Parameters
Turbidity and pH
Turbidity is a measure
of the degree to which the water loses its transparency due to the presence of suspended particulates (reference). One of the characteristics of groundwater that give it certain advantages over the surface water is its clearness and colorless unless tainted with humic materials. It usually contains no suspended matters. However, due to the presence of humic materials, colloidal and extremely fine dispersion of substances, some sampled stations show extremely high values of turbidity (NTU) were observed in GW8 (85.0), GW9 (28.8), GW10 (27.5), GW11 (183.0), GW12 (760.0), and GW13 (950). The very high values were recorded in piezometer stations (GW8-GW13), which might be due to organic matter such as the decay of plants, the presence of microorganisms, and/or mud formation. While the remaining ten stations (62.5%) show less than the WHO limit (5 NTU). Whereas, the power of hydrogen (pH) is a key indicator for water acidity and alkalinity. In the study, almost all of the water samples are alkaline due to the presence of carbonates and bicarbonates. As shown in
Table 1, the pH values of water samples varied between 8.2 to 10.1 and it was found above the limit prescribed by WHO (2011). A total of ten stations (62.5%) exceeded the drinking water standard limit.
Electrical Conductivity (EC) and Total Dissolved Solids (TDS)
The Electrical Conductivity (EC) in groundwater samples, which indicates its salinity, is a useful tool for assessing whether water is suitable for agriculture and drinking. With an average of 3152 μS/cm, EC values range from 517 to 15645 μS/cm. The influxes of saline lake water to groundwaters may increase the salinity (BGS, 2001). The maximum permissible limit of EC in drinking water, according to WHO standards, is 1500 μS/cm (WHO, 2011). According to the results of the analysis, about 70% of the samples, or 11 samples, had EC values that were higher than the standard value, these high salinity values in the groundwater are apparent as a result of the influence of saline geothermal waters. High concentrations of dissolved salts in the groundwater from the sedimentary formations are also common, as a result of the reaction of the often abundant evaporite minerals (BGS, 2001). The range of this sum is 269 to 10826 mg/L in the study area; the mean value is 2099 mg/L. The sum of dissolved solids was highest in sample GW11 (piezometer station). The median is lower in spring and river water than in the wells and higher in shallow wells, hot springs, and lakes.
Alkalinity, Bicarbonate (HCO3-), and Carbonate (CO32-)
In the study area, the highest concentrations of Alkalinity, carbonates, and bicarbonates were recorded at 1600 mg/L, 950 mg/L, and 1925 mg/L in the same station respectively. Alkalinity, bicarbonate (HCO
3-), and carbonates (CO
32-) range from 225 to 1600 mg/L; 275 to 1925 mg/L; and 132.5 to 950 mg/L, respectively. Perhaps, the source of the alkalinity, bicarbonates, and carbonates in the groundwater might be natural activities (near Lake Beseka) and various human activities (downstream of Beseka). As seen in
Table 1, the majority of sampled water exceeded the maximum allowable limit of Alkalinity (500 mg/L), bicarbonates (580 mg/L), and carbonates (250 mg/L). Bicarbonate (HCO
3) was the dominant anion; it might be due to the topology of the geology of the study area.
Fluoride (F-) and Chloride (Cl-)
As appeared in
Table 1, the highest level of fluoride (18.2 mg/L) more prominent than the WHO limit (1.5mg/L) was found in GW1. All inspecting stations including GW15 (1.96 mg/L) exceeded the WHO limit, which is subsequently a recognized major issue, particularly for the communities using the water for drinking (GW15 and GW16). Hundred percent of the tests exceed the standard, and the most noteworthy concentration was 18.2 mg/L, which is indeed higher than a few crack valley nations in Africa including Kenya and Tanzania (Demelash, H., et al., 2019). In fact, studies also revealed that about 30% of Ethiopia’s groundwater has naturally contaminated with high salinity and fluoride. It might be due to the weathering of the volcanic bed rocks interaction. In the research location, almost 37% of the groundwater samples have chloride WHO concentrations above the limit of 250 mg/L. The concentration of chloride ranges from 117.5 mg/L to 412.5 mg/L. The main source of chloride in the groundwater is the result of erosion and weathering of crystalline rocks. The anion is derived from minerals such as sodalite, apatite, micas, and hornblende in the rock matrix. In fact, chloride (Cl
−) is a highly mobile anionic species. The chloride limits were established mostly based on taste preferences. However, in this study, the highest concentration was found at 412.5 mg/L (GW1). However, using water with high chloride concentrations has not been found to have any negative health impacts on humans (Jain et al. 2010).
Total Hardness (TH), Calcium (Ca2+), and Magnesium (Mg2+)
The amount of dissolved calcium and magnesium in addition to a range of other metals in the water is the basic definition of water hardness (reference). There are a lot of dissolved minerals in hard water, mostly calcium and magnesium. One water-quality indicator that was investigated was water hardness; the results are displayed in the above
Figure 3 and
Figure 4. Since water dissolves trace amounts of naturally occurring minerals as it passes through soil and rock and transports them into the groundwater supply, water systems that use groundwater as a source are concerned about water hardness. Hard water may be seen around a water supply well if the minerals calcium and magnesium are present in the surrounding soil. Water hardness in the research area varies considerably and is categorized into four classes based on basic guidelines: less than 75 mg/L is classified as soft due to calcium carbonate content; 75 to 150 mg/L is considered moderately hard; 151 to 300 mg/L is categorized as hard; and more than 300 mg/L is classified as extremely hard. According to the study’s findings, the distribution of water hardness among the subsurface waters sampled was evenly spread, with 25% falling into each category: very hard, hard, moderately hard, and soft. As depicted in
Figure 2 and
Figure 3 and detailed in
Table 1, the highest recorded levels of calcium (Ca), magnesium (Mg), and total hardness (TH) were 200.5 mg/L, 69 mg/L, and 607.5 mg/L, respectively. These results suggest a common geological origin for these elements. The solubility of Ca and Mg in saline-alkaline groundwater is influenced by the presence of their respective carbonates and bicarbonates.
Sodium (Na+) and Potassium (K+)
Sodium (Na) is a naturally occurring element that is most frequently found in combination with chloride to produce salt. There are several reasons why groundwater may include sodium, including naturally occurring brackish water, sodium-bearing rock minerals, salt-containing geological formations, and the weathering of soil minerals. Actually, the salt content of groundwater is normally quite low, although it can rise in rift zones and locations close to volcanoes (reference). In the study area, the concentration of Na+ in groundwater ranges from 17.02 meq/L (GW15) to 105.2 meq/L (GW11). High concentrations of Na+ and Ca2+ in the groundwater were attributed to cation exchange capacity among minerals. The concentration of K in all groundwater samples is within the allowable limit. For instance, the highest (3.79 meq/L) and lowest (1.02 meq/L) concentration of K was observed in GW1 and GW13, respectively. The sources of K are probably the silicate minerals found in igneous and metamorphic rocks, such as orthoclase, microcline, hornblende, muscovite, and biotite, as well as evaporate deposits of sulphate and gypsum that release a significant amount of potassium into the groundwater. The primary cause of rising groundwater potassium levels: is an agricultural practice (Sayyed and Bhosle, 2011).
Sulfate (SO42-) and Phosphate (PO43-)
Higher concentration (above 100 mg/L) of sulfate was observed in 12 stations out of 16 sampled subsurface waters, but which did not exceed the WHO limits. Mostly sulfate can exist in groundwater in the form of calcium, magnesium, and sodium soluble salts. A study indicates that still little is known about the direct health impacts of elevated sulfate intake, particularly through drinking water. Sulphate does not contribute to toxicity (Sharma and Kumar, 2020). Elevated phosphate concentrations (0.3 to 2.4 mg/L) were detected in the groundwater. The highest value, which is 188.5 mg/L, was recorded in the GW4 station near LB. These values might come from the geology of the study area. While, the phosphate concentration ranged between 0.3 mg/L (GW7) and 2.4 mg/L (GW8). As an example, Awash Basin Agricultural nitrate is the most prevalent chemical pollution found in groundwater aquifers basin-wide. The increase in agricultural output worldwide has been made possible by the extensive use of chemicals, including chemical fertilizers and insecticides. The higher concentration of sulphate is observed in the downstream part which could be due to excessive use of fertilizers or use of soil amendments. Excess of phosphates and nitrogen can seep into groundwater or be carried into water bodies by surface runoff. Because phosphate is not as soluble as nitrate, it seeps into the soil and is subsequently carried there by soil erosion (Akhtar, N., et al., 2021).
Ammonia (NH3), Ammonium (NH4+), Nitrate (NO3-), and Nitrite (NO2-)
Ammonia is released into the atmosphere by industrial, agricultural, and metabolic processes as well as by using chloramine for disinfection. Surface and groundwater naturally occur at concentrations of less than 0.2 mg/l. Groundwater devoid of oxygen can have as much as 3 mg/L. Elevated amounts in surface water can result from intensive farm animals rising. Water containing ammonia may be contaminated by bacteria, sewage, or animal waste. Ammonium, nitrate, and nitrite play the most important role in biochemical processes, but some organic nitrogen compounds in water may also be of significance. Nitrate (NO
3−) is a very soluble anion, most mobile form of nitrogen, which is not easily absorbed by mineral soils (Chunli et al., 2023). Various sampling stations located both nearby and downstream of LB show high nitrate concentrations in the groundwater as a result of intensive farming practices that alter natural drainage patterns by using nitrogen fertilizers. When drinking water contains more than 45 mg/L of nitrate, it can cause harmful health effects in people, such as stomach cancer (Tank and Chandel, 2010) and methemoglobinemia, sometimes known as “blue baby syndrome,” which typically affects infants (Jain et al., 2010). All of the samples have nitrate contents between 1.1 mg/L (in stations GW11, GW13, & GW16) and 17.6 mg/L (GW1), which is under the WHO acceptable limit. As shown in
Figure 4, elevated level of turbidity was observed in GW13, EC and TDS in GW11, TH, alkalinity, bicarbonate and carbonate in GW9, Ca-CO
3, Mg-CO
3, Mg, and Ca in GW9, Na
+ in GW11, K
+ in GW1, NO
3-N in GW1, F
- in GW1, Cl
- in GW13, SO
42- in GW4, PO
43- in GW8, NH
3 and NH
4+ in GW14. The findings also revealed that relatively high concentrations of physicochemical parameters were observed in shallow GWs (Piezometer) than deep GWs (Lake Beseka catchment).
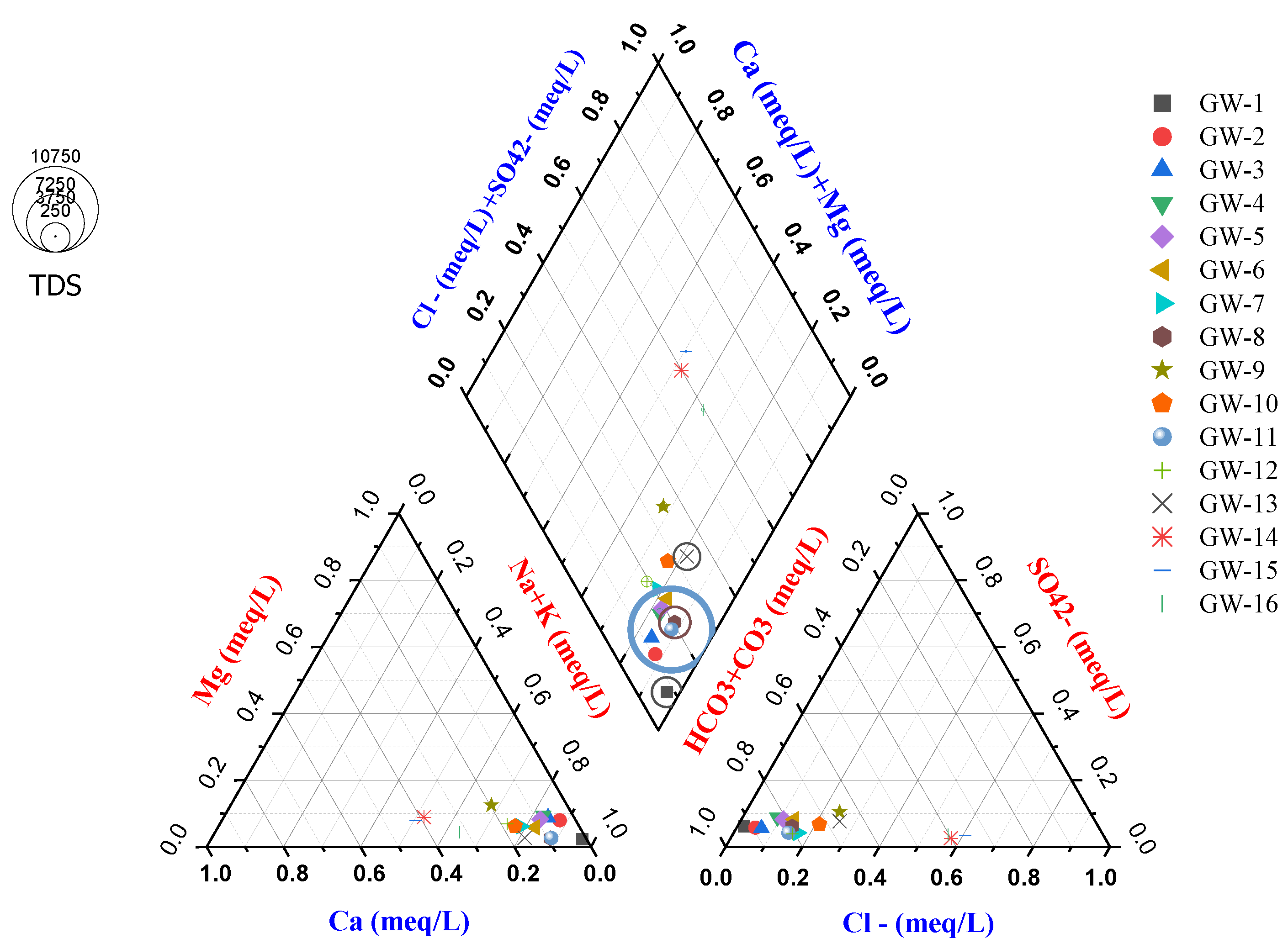
The water type in the study area is ascertained using the piper diagram, as seen in
Figure 5 above. As you are aware, one of the most widely used graphical techniques for interpreting with hydro-geochemical problems is the piper diagram (Piper, A.M., 1953). The flow diagram of the hydrochemical composition of the groundwater samples that were obtained in the study region, specifically from the Lake Catchment and Amibera irrigation field, is of the HCO
3-Na type, as per the analytical data. With the exception of drinking wells (GW14 & GW15), bicarbonate predominates when looking at the chemical kinds of groundwater in both sections. In general, sodium is a dominant cation and bicarbonate is a dominant anion. Over 81% of the sampled groundwater samples showing a HCO
3-Na type of water due to carbonate rocks, such as limestone and dolomite, and the weathering of carbonaceous rocks.
Distribution of Heavy Metals
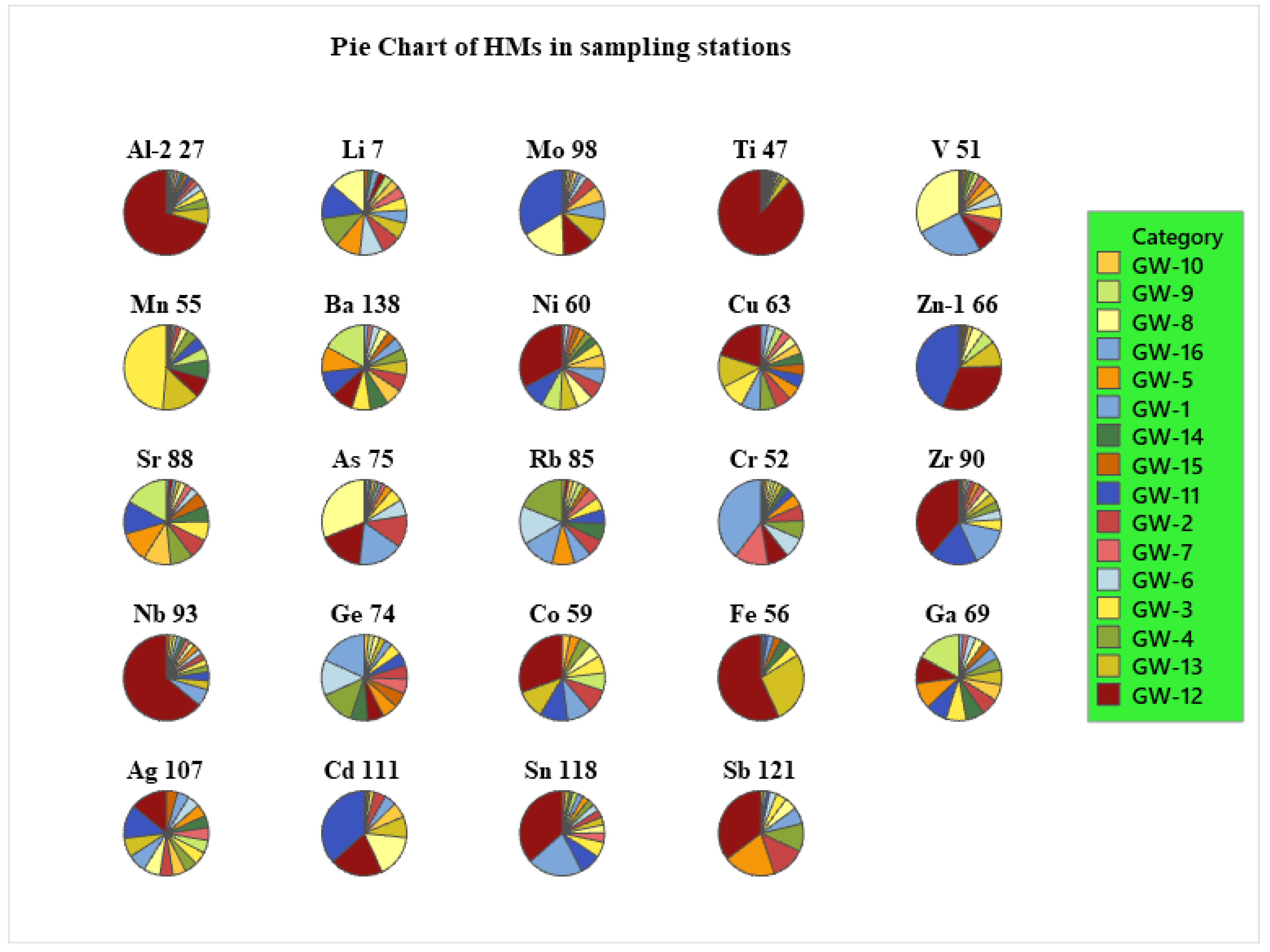
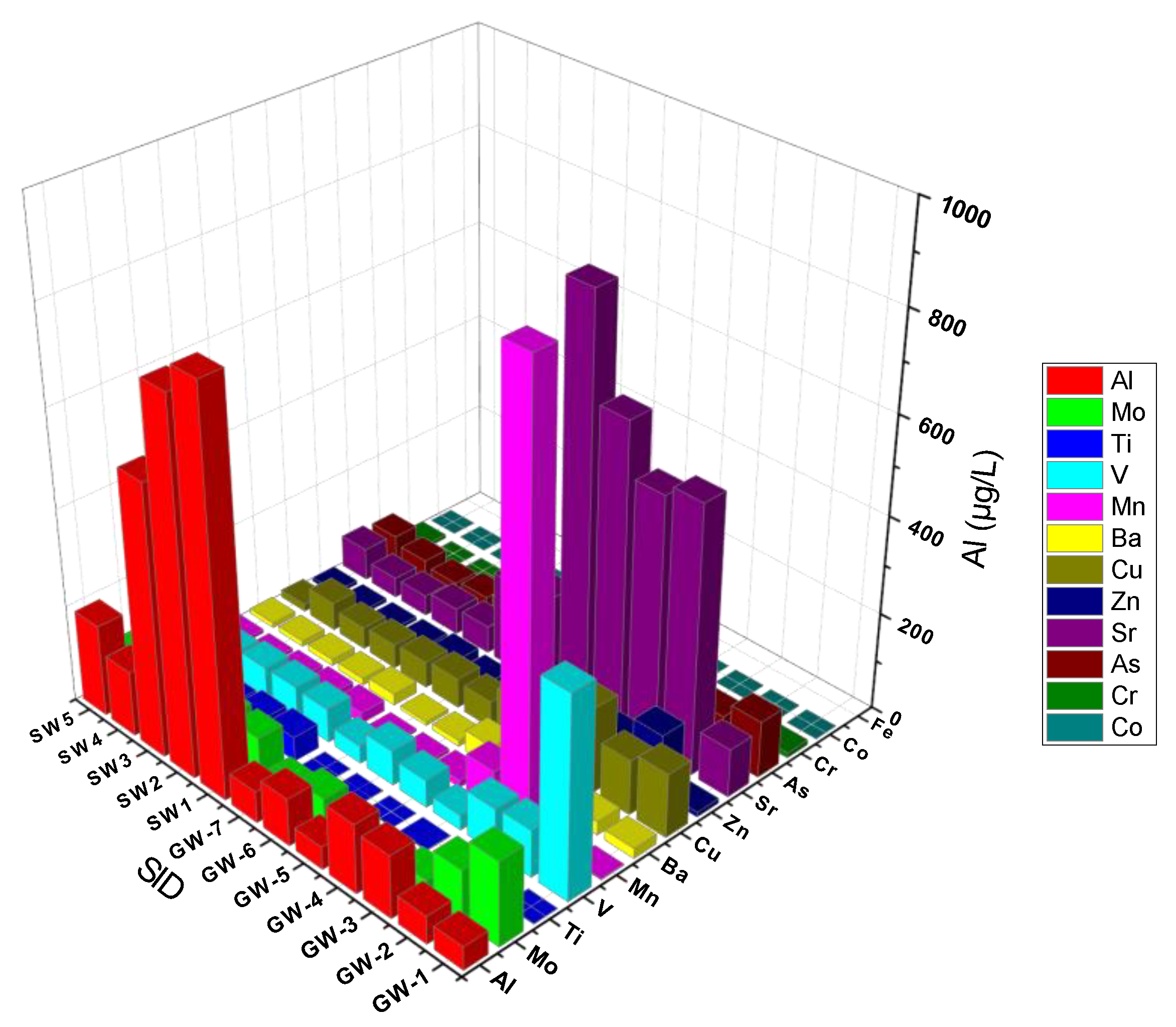
The summary statistics of heavy metals (metalloids) concentration in the study area is presented very well. For instance,
Table 1 and
Figure 6 illustrate that a high concentration of As above the permissible limits of WHO was observed in different stations (including GW1, GW2, GW3, GW5, & so on) in the groundwater samples. The high concentration of As was found in GW8 (near) 201 µg/L, which is 200 times higher than the permissible limit, which is 10 µg/L (WHO, 2011). Half (50%) of the samples revealed very high concentration of As (above 10 µg/L) recorded in stations GW7(11.1 µg/L) (Merti camp near Shibo-gebi), GW5(18.1 µg/L) (Merti Kera compound), GW3(34.3 µg/L) (Merti Beret house), GW6(41.9 µg/L) (Abadir 4th camp), GW2(83.1 µg/L) (Merti near Buret area), GW1(116.1 µg/L) (Addis Ketema Mosque), GW12(117.2 µg/L) (AIP-10.2) and GW8(210 µg/L) (AIP-41). About 6 sites are located around Lake Beseka. Almost all are deep wells, while, the remaining 2 sites are piezometers and were showing extremely high values. These stations are found in Amibera Irrigation (state) farms. Both are piezometer stations and their water levels at the time of sampling were 1232 cm (AIP-10.2) and 167 cm (AIP-41) meters. In fact, some researchers investigated the extent of As in the Rift Valley (MER), in which 50% of the samples exceeded the permissible limit of 10µg/L (WHO, 2011). Arsenic may also be present in some groundwater sites within the southern Rift where sodium-bicarbonate geothermal waters exert an influence on groundwater quality, although no data are available to substantiate this.
Aluminum (Al), Iron (Fe), and Manganese (Mn)
The concentration of Al varied from 20.3 to 2615.2 µg/L, a low concentration of Al was observed at site GW10 (20.3 µg/L), whereas the highest Al concentration was found at GW12 (2615.2 µg/L). The major source of Al in groundwater might be the volcanic ash, weathering of aluminum-containing rocks such as bauxite. The Al concentration of 2615.2 µg/L was found in groundwater samples collected below LB at station GW12 (AIP12). Aluminum ions in other compounds also hydrolyze, and this continues until the cationic charge has run out, ending the reaction by hydroxide formation. The beginning of the hydrolysis reaction is as follows:
Aluminum mainly occurs as Al3+ (aq) under acidic conditions and as Al(OH)4- (aq) under neutral to alkali conditions. Other forms include AlOH2+ (aq) and Al(OH)3 (aq). The regular aluminum concentration expected in groundwater is 4µg/L because it is present in soils as water-insoluble hydroxide. Depending on the variability of pH values the solubility of Al rapidly might increase, causing aluminum concentrations to rise above 5µg/L. However, elevated levels of aluminum (> 200µg/L) have an impact not only on fish but also on birds and other species that eat tainted fish and insects as well as on animals that inhale the metal through the air. When birds eat infected fish, their eggshells thin and their offspring are born with low birth weights. Animals that inhale aluminum through the air may experience respiratory issues, lose weight, and become less active. The effects of aluminum have drawn our attention, mainly due to the acidifying problems. Aluminum may accumulate in plants and cause health problems for animals that consume these plants.
Metals that are found naturally in rocks, minerals, and soils are iron (Fe) and manganese (Mn). When these solid materials enter the aquifer, groundwater in it starts to disintegrate them. This causes their contents, such as manganese and iron, to be released into the water. The concentration of Fe varied from 6.20 to 741.9 µg/L, the lowest concentration of Fe was observed at different sites including GW8 (6.20 µg/L), whereas the highest Fe concentration was found at GW12 (741.9 µg/L). As iron-bearing minerals and rocks weather, iron is naturally added to groundwater. The World Health Organization (WHO) establishes a 300 µg/L as the maximum permitted concentration of iron in drinking water. In the study high concentrations of iron above the maximum permitted limit were recorded in stations (
Table 2).
While, the concentration of Mn varied from 2.4 µg/L to 908.5 µg/L, a low concentration of Mn was observed at site GW1 (2.4 µg/L), whereas a high Mn concentration was found at GW3 (908.5 µg/L). As shown in
Table 2, for a particular well, the amount of dissolved iron and manganese in the groundwater changes seasonally. For instance, high concentrations of Fe (14 stations, or 87.5%) and Mn (in 11 stations, or 69%) were recorded in partly wet season (Nov 10, 2021). This variance is typically linked to the surface bringing in oxygenated water during times of strong recharge. The oxygenated water will keep the iron and manganese from dissolving, resulting in low quantities of these metals in the water drawn from the well. Iron and manganese will dissolve once more in the recharge water when the oxygen has been used up, giving the water dissolved iron and manganese properties.
Arsenic (As), Molybdenum (Mo), and Vanadium (V)
Heavy metals enter groundwater through numerous anthropogenic activities, including mining, agriculture, landfills, and urban developments (Matthew S Schuler, Rick A Relyea, 2018). As shown in
Table 1, a high concentration of chloride was observed. Accordingly, the presences of high concentrations of chloride affect the solubility and mobility of metals
. As metals are desorbed and transformed into a soluble phase, chloride complexes can increase the solubility of the metals, increasing their persistence in soil and freshwater ecosystems (Warren and Zimmerman 1994). Depending on the Redox conditions, and microbiological environment, As species can be present in water in solution or in a precipitated form, and they can also adsorb or desorb from the existing precipitates (Issa et al., 2010; Issa et al., 2011). When As species are soluble in water, they can be present in both inorganic and organic forms. For As species both As(III), arsenite, and As(V), arsenate, can be present (Ljubinka and Vladana, 2017). For oAs species, MMA, and DMA are soluble forms of organic As species (Ljubinka and Vladana, 2017). Water-soluble arsenic species existing in natural water are inorganic As(iAs) and organic As(oAs) species. As species exhibit different toxicity (Issa et al., 2011). The investigation of As species and their behavior in various samples, especially in natural waters and the environment is important for chemistry and environmental protection (Ljubinka and Vladana, 2017). From the value of the chemical equilibrium constants for each molecular or ionic form of As in water, the present species can be recognized (Rajaković et al., 2013).
When choosing and analyzing the most dominant form of As in water, the most present is inorganic As as As(V). If As(III) is present, there are two important things that need to be taken into account, As(III) is more poisonous (even at low concentrations) than As(V) (Ljubinka and Vladana, 2017). Besides, the severe toxic effect, As(III) is easily oxidized. Due to different reasons, the formation of complex solutions between As oxyanions and other elements is limited, however, even such limited interactions still influence As speciation (Redman et al., 2002). Arsenic species can be transformed into insoluble compounds in combination with other elements, such as iron and sulfur (Mandal and Suzuki, 2002). The adsorption of arsenate As (V) and arsenite As (III) to mineral surfaces is reduced in the presence of natural organic matter (NOM) (Grafe et al., 2001; Grafe et al., 2002; Redman et al., 2002). Surface complexation models suggest that dissolved carbonate should interfere with As adsorption on mineral surfaces at carbonate concentrations typically measured in-ground and soil waters (Appelo et al., 2002).
Molybdenum is a Group VIB HMs, Mo(IV) and Mo(VI) are predominant states in nature. The most common oxidation states are 4
+ and 6
+ (Pauline and David, 2017). It occurs as molybdenite (MoS
2) and moly dates (MoO
42) in igneous or sedimentary rocks. Even though most Mo compounds aren’t very soluble in water, the molybdate ion MoO
42- forms, when Mo-containing minerals come into contact with oxygen and water is soluble.
Strong alkaline molybdates have a high solubility for Mo(VI) oxides (MoO42-). Mo can exist in a wide variety of oxidation states, which are discussed in numerous Mo chlorides. Molybdenum (Mo) may be transformed in the LB catchment because of the hot weather and volcanic factors around the lake. In Lake Beseka, under physiological conditions (at pH > 6.5), the molybdate anion, [MoO4]2-, is the sole molybdenum species in aqueous media (Cruywagen, 2000; Cruywagen et al., 2002). Molybdenum compounds including molybdenum trioxide and polymolybdates transform rapidly to the [MoO4]2- ion under environmentally relevant test conditions. Protonated forms, such as [HMoO4]- and H2MoO4, are found at pH < 5 (Smedley and Kinniburgh, 2017). Molybdenum tends to be more mobile under alkaline conditions, but adsorption increases with decreasing pH (Goldberg et al., 2020). In the study we found that the highest concentration of Mo varied from 2.6 to 802.4 µg/L, a low concentration of Mo was observed at site GW15 (2.6 µg/L), whereas, the highest concentration was observed in GW11 (802.4 µg/L).
In water, V(IV) is commonly present as a vanadyl cation [VO
2+, VO(OH)
+], whereas V(V) exists as a vanadate oxyanion (H
2VO
4 −, HVO
4 2−) (Wanty and Goldhaber, 1992). VO
2+ is strongly adsorbed on solid phases, including organic and oxyhydroxide phases (Wehrli and Stumm, 1989). Vanadium varies in concentration around the world, and it is usually found associated with iron-bearing water (Kohl and Medlar, 2006). Vanadium was found in groundwater samples (Winter, 2004). The minimum and maximum vanadium concentrations found were 1.7 µg/L and 528 µg/L, respectively. Vanadium (V) is significantly correlated with other trace elements such as arsenic, fluoride, and boron (Khosravi, et al. 2021). As seen in
Table 4, it is mostly found associated with As (r=0.91).
Barium (Ba), lithium (Li), and Strontium
The concentration of barium in groundwater is regulated by barite (BaSO4) solubility (Elena Gimenez-Forcada and Marisol Vega-Alegre, 2015). The relationship between strontium and barium in groundwater is controlled by the dissolution of sulfate salts containing strontium and the precipitation of barite (Elena Gimenez-Forcada and Marisol Vega-Alegre, 2015). Surprisingly, Groundwater with low SO42- concentrations (or less than 10 mg/L) could be predicted to have high Ba contents (Reimann et al., 2002). As a result, only very low concentrations of Ba (ranging between 6.8 to 76.2 µg/L) were found in the investigated sites. This could be because the groundwater samples had significant concentrations of SO42- (188.5 mg/L). Although barium compounds are utilized in many industrial applications and can be found as trace elements in both igneous and sedimentary rocks, the majority of Ba in water comes from natural sources. Barium may be a connection between cardiovascular illnesses (WHO, 1993). Nearly all (100%) of the samples in the research area had results (Ba) below the WHO standards (700 µg/L).
Like all alkali metals, lithium reacts easily in water and does not occur freely in nature due to its activity. Lithium compounds such as lithium chloride, lithium carbonate, lithium phosphate, lithium fluoride, and lithium hydroxide are more or less water soluble. In the study, the highest concentration of Li 68 µg/L was observed in GW8. Most sampling stations do not exceed the WHO standard (WHO, 2011). However, too much lithium may be toxic.
In fact, strontium (Sr) and barium (Ba) are alkaline-earth metals that are a common trace element in most rocks, soils, sediments, and waters. Both rock weathering and soil erosion can lead to strontium contamination of water supplies. Because many strontium compounds react violently with water, they can travel across the environment rather easily. The groundwater sites that were analyzed showed Sr concentrations ranging from 71.1µg/L to 1236 µg/L, with the lowest concentration being seen there. Some stations including GW recorded above the 700 µg/L or Canadian water requirements, (Health Canada, 2019). In general, carbonates have higher strontium contents than ultrabasic rocks and sandstones. There are many sedimentary basins (which frequently contain evaporites), and carbonates that could be sources of sulfur or are probably connected to sulfur minerals (Musgrove, M., 2021).
Chromium (Cr), cadmium (Cd), and Zinc (Zn)
Cadmium (Cd) is expected in high concentration at low pH, however, not valid for the Rift Valley groundwater (Reimann et al., 2002). For instance, high pH wells (piezometer stations) showed also high Cd at station GW11 at 210 cm (2.1m). In the study at GW11, partly in dry and wet seasons, 4.6 µg/L (pH=8.44) and 0.62 µg/L (pH=9.06) were recorded respectively. The highest mean concentration of Cd-values is found in the alkaline groundwater samples at very high pH-values (Cd=2.62 µg/L). The source might be anthropogenic (might be fertilizers and pesticides). Chromium (Cr) is a well-known cancer-causing element. Anthropogenic sources like the tanning industry pollute water bodies in the Awash Basin (Abebe et al., 2023). While, the concentration of Cr exhibited in all samples was below the maximum allowable limit (50 µg/L) noted by WHO (WHO, 2008, 2011). Arguably, the previous studies done in the Rift Valley found the highest value of Cr in GW was 21 µg/L (Reimann et al., 2002). Similarly, the highest concentration of Cr recorded in this study was 10.7 µg/L in GW1 (at Addis Ketma, near LB).
Zinc (Zn), across all sampling stations, ranged from 10 to 6060 µg/L. In groundwater, the concentration of zinc (Zn) is usually expected between 10 to 40 µg/L. However, the highest concentration of Zn, 6060 µg/L was recorded in the groundwater sample collected in the piezometer station (GW11). Even higher Zn concentration (up to µg/L) was recorded in the rift valley of Ethiopia. In the study, the concentration of Zn was recorded between 871 µg/L to 6000 µg/L in the Amibera irrigation fields, or piezometer stations. Surprisingly the highest concentration of Zn was recorded in all piezometer stations, this might be associated with the fertilizers and chemicals (pesticides, herbicides, or else) used for cotton production or sugar cane plantation.
Silver (Ag), Antimony (Sb), and Tin (Sn)
Silver (Ag) is considered to be less toxic to humans. The main natural source of Ag is sulphidic ores. Its mobility is very low under alkaline conditions. Silver (Ag) ranged from 0.1 to 0.2 µg/L. The concentration of antimony (Sb) varies between ND to 5.3 µg/L across all sampling stations. Tin (Sn), across all sampling stations, ranged from 0.1 to 3.5 µg/L. The highest concentrations of Sb and Sn were recorded in GW11 with the values of 5.3 µg/L and 3.5 µg/L, respectively. It might be from organo-tin compounds or chemicals used for biocides in the Amibera state farm. Of the various tin-bearing minerals, cassiterite is an oxide, while the remainder is complex sulfides. The concentration of Sb in GW is expected 0.001µg/L, however, in the study, above 5 µg/L was recorded in station GW11 (WHO, 1993), but less than 20 µg/L the limit improved by WHO (WHO, 2011).
Copper (Cu), Cobalt (Co), and Nickel (Ni)
Copper (Cu) is a trace metal. According to the result of
Table 3, the minimum and maximum concentrations of Cu were recorded from 47.7 µg/L to 369.7 µg/L. The mobility of Co strongly depends on the geochemical conditions (Reimann et al., 2002). Cobalt concentrations observed in the Rift Valley drinking water range from less than 0.002 to 3.1 mg/L (Reimann et al., 2002). However, the highest Co value was recorded in station GW (1.6 µg/L). Studies indicate that Co-deficiency problems are more widespread than Co-toxicity (Reimann and Caritat, 1998).
Table 3.
Correlations between heavy metals (metalloids) and statistical summary of heavy metals concentration in groundwater (in µg/L).
Table 3.
Correlations between heavy metals (metalloids) and statistical summary of heavy metals concentration in groundwater (in µg/L).
| HMs |
Al |
Li |
Mo |
Ti |
V |
Mn |
Ba |
Ni |
Cu |
Zn |
Sr |
As |
Rb |
Cr |
Zr |
Nb |
Ge |
Co |
Fe |
Ga |
Ag |
Cd |
Sn |
Sb |
| Al |
1.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Li |
-0.22 |
1.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mo |
0.18 |
0.56 |
1.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Ti |
1.00
|
-0.23 |
0.19 |
1.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| V |
0.03 |
0.33 |
0.26 |
0.04 |
1.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Mn |
0.07 |
-0.09 |
-0.06 |
0.04 |
-0.08 |
1.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Ba |
0.17 |
-0.05 |
0.14 |
0.18 |
-0.19 |
0.15 |
1.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Ni |
0.94 |
-0.12 |
0.40 |
0.95 |
0.14 |
0.08 |
0.35 |
1.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cu |
0.86 |
-0.16 |
0.19 |
0.83 |
0.03 |
0.38 |
0.17 |
0.84 |
1.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Zn |
0.54 |
0.27 |
0.85 |
0.54 |
-0.09 |
0.01 |
0.35 |
0.68 |
0.47 |
1.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Sr |
-0.29 |
0.15 |
0.06 |
-0.28 |
-0.36 |
0.03 |
0.78 |
-0.13 |
-0.31 |
0.13 |
1.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| As |
0.31 |
0.33 |
0.32 |
0.33 |
0.91 |
-0.07 |
-0.16 |
0.40 |
0.25 |
0.08 |
-0.42 |
1.00 |
|
|
|
|
|
|
|
|
|
|
|
|
| Rb |
-0.24 |
0.36 |
-0.25 |
-0.26 |
0.01 |
-0.17 |
-0.26 |
-0.36 |
-0.23 |
-0.30 |
-0.03 |
-0.08 |
1.00 |
|
|
|
|
|
|
|
|
|
|
|
| Cr |
0.04 |
-0.04 |
-0.02 |
0.05 |
0.49 |
-0.22 |
-0.19 |
0.06 |
0.11 |
-0.11 |
-0.34 |
0.33 |
0.44 |
1.00 |
|
|
|
|
|
|
|
|
|
|
| Zr |
0.86 |
0.01 |
0.52 |
0.87 |
0.18 |
0.03 |
0.19 |
0.91 |
0.77 |
0.76 |
-0.23 |
0.37 |
-0.11 |
0.32 |
1.00 |
|
|
|
|
|
|
|
|
|
| Nb |
1.00 |
-0.22 |
0.21 |
1.00 |
0.09 |
0.03 |
0.17 |
0.95 |
0.84 |
0.55 |
-0.29 |
0.36 |
-0.23 |
0.12 |
0.89 |
1.00 |
|
|
|
|
|
|
|
|
| Ge |
0.01 |
0.02 |
-0.28 |
0.00 |
-0.34 |
-0.17 |
-0.44 |
-0.22 |
-0.19 |
-0.16 |
-0.29 |
-0.24 |
0.57 |
-0.11 |
-0.08 |
-0.01 |
1.00 |
|
|
|
|
|
|
|
| Co |
0.94 |
-0.35 |
0.29 |
0.94 |
-0.02 |
0.02 |
0.10 |
0.97 |
0.88 |
0.65 |
-0.46 |
0.28 |
-0.30 |
0.13 |
0.92 |
0.95 |
0.09 |
1.00 |
|
|
|
|
|
|
| Fe |
0.93 |
-0.26 |
0.06 |
0.91 |
-0.17 |
0.04 |
0.44 |
0.89 |
0.93 |
0.46 |
-0.37 |
0.14 |
-0.51 |
-0.05 |
0.73 |
0.90 |
-0.08 |
0.92 |
1.00 |
|
|
|
|
|
| Ga |
0.25 |
-0.11 |
0.06 |
0.26 |
-0.19 |
0.19 |
0.99 |
0.41 |
0.25 |
0.32 |
0.72 |
-0.13 |
-0.27 |
-0.18 |
0.23 |
0.26 |
-0.42 |
0.19 |
0.59 |
1.00 |
|
|
|
|
| Ag |
0.71 |
0.20 |
0.79 |
0.71 |
0.11 |
0.02 |
0.25 |
0.83 |
0.66 |
0.94 |
-0.10 |
0.29 |
-0.26 |
0.10 |
0.91 |
0.73 |
-0.19 |
0.82 |
0.64 |
0.25 |
1.00 |
|
|
|
| Cd |
0.32 |
0.48 |
0.97 |
0.33 |
0.06 |
-0.13 |
0.02 |
0.48 |
0.22 |
0.92 |
-0.03 |
0.18 |
-0.32 |
-0.14 |
0.60 |
0.34 |
-0.10 |
0.43 |
0.08 |
-0.05 |
0.86 |
1.00 |
|
|
| Sn |
0.85 |
-0.15 |
0.31 |
0.86 |
0.32 |
0.04 |
0.13 |
0.87 |
0.78 |
0.53 |
-0.35 |
0.47 |
-0.07 |
0.49 |
0.95 |
0.90 |
-0.11 |
0.89 |
0.72 |
0.19 |
0.76 |
0.35 |
1.00 |
|
| Sb |
0.83 |
-0.22 |
-0.08 |
0.83 |
-0.04 |
-0.08 |
0.06 |
0.73 |
0.82 |
0.27 |
-0.34 |
0.26 |
-0.09 |
0.07 |
0.65 |
0.83 |
0.17 |
0.75 |
0.99 |
0.17 |
0.47 |
0.12 |
0.69 |
1.00 |
| Min |
20 |
6.7 |
2.6 |
0.5 |
1.7 |
2.4 |
6.8 |
0.2 |
47.7 |
10 |
71.1 |
0.9 |
2.2 |
0.2 |
0.4 |
0.1 |
0.1 |
BD |
BD |
1.0 |
0.1 |
BD |
0.1 |
BD |
| Max |
2615 |
68 |
802 |
17.1 |
528 |
908 |
76.2 |
8.8 |
370 |
6060 |
1236 |
210 |
35 |
10.7 |
20.2 |
3.5 |
1.5 |
1.6 |
741.9 |
10.3 |
0.2 |
2.6 |
3.5 |
5.3 |
| Mean |
232 |
30.9 |
149 |
12.1 |
101 |
117 |
28 |
1.7 |
114 |
871 |
454 |
42.4 |
11.5 |
1.7 |
3.3 |
0.3 |
0.5 |
0.5 |
144.9 |
3.8 |
0.1 |
0.7 |
0.6 |
1.4 |
| WHO |
200 |
NG |
70 |
NG |
20 |
400 |
300 |
200 |
1300 |
5000 |
700 |
10 |
NG |
50 |
NG |
NG |
NG |
NG |
300 |
NG |
10 |
3 |
NG |
5 |
Table 4.
Functional piezometer stations in Amibera irrigation schemes (SCD: Sample collected at the depth of).
Table 4.
Functional piezometer stations in Amibera irrigation schemes (SCD: Sample collected at the depth of).
|
No.
|
Location description
|
Piezometer
|
SID
|
SCD
|
Soil type
|
Field cover
|
|
1.
|
Shelko near new Asphalt Road
|
AIP-41
|
GW-8
|
167cm
|
Fluvisols
|
Sugarcane
|
|
2.
|
Ambash near new settlement DC
|
AIP-40
|
GW-9
|
135cm
|
Fluvisols
|
Sugarcane
|
|
3.
|
Near to Bolhamo village
|
AIP-62
|
GW-10
|
95cm
|
Fluvisols
|
Sugarcane
|
|
4.
|
Melka-Sedi farm near 4th camp
|
AIP-10-1
|
GW-11
|
210cm
|
Fluvisols
|
Trees & shrubs
|
|
5.
|
Melka-Sedi farm near 4th camp
|
AIP-10-2
|
GW-12
|
1232cm
|
Fluvisols
|
Trees & shrubs
|
|
6.
|
Melka-Sedi farm near 4th camp
|
AIP-10-4
|
GW-13
|
215cm
|
Fluvisols
|
Trees & shrubs
|
Niobium (Nb), Gallium (Ga) and Germanium (Ge)
Niobium (Nb) is a member of the fifth group of the periodic table. Niobium typically forms compounds with an oxidation state of +5. Niobium is closely associated with titanium in ores. Niobium can best be dissolved in a mixture nitric acid and hydrofluoric acids, completely miscible with iron. Its most common oxide is Nb2O5, which is produced by heating the metal in an oxygen environment. In a study of groundwater samples from LB and Amibera irrigation farms, Middle Awash, the typical range for naturally occurring Nb concentrations in groundwater was measured to be from 0.1 to 3.5 µg/L. High-level interaction between Al and Nb (r=1.0) was found in studied groundwater, which might be predominantly of a natural origin (Sutliff-Johansson, S., et al., 2021). Gallium (Ga) is the 34th most abundant element found in Earth’s crust (Emsley, 2001), and it is widely distributed in low amounts in many rock types. The results of this study showed that the minimum and maximum level of Ga was observed at 0.1 µg/L and 10.3 µg/L, respectively. The ecological effects of gallium may be similar to those of aluminum given their similar behavior in the environment. Germanium (Ge) is a non-essential and non-harmful element. An increased germanium level is mainly associated with thermal waters, waters with either very low or very high pH, and saline waters (Rosenberg, 2009). The highest concentration (1.5 µg/L) of Ge was recorded in GW16. Ge was found in increased amounts in CO2-rich thermal waters, and alkaline sodium-dominated thermal waters (Ivanov, 1996).
Rubidium (Rb), Titanium (Ti), and Zirconium (Zr)
The higher concentrations of Rb, Ti, and Zr were found in stations GW4, GW12, and GW12 with levels of 35 µg/L, 171.3 µg/L, and 20.2 µg/L, respectively. Titanium (Ti) is a chemical element and is found in nature only as an oxide. Titanium (Ti), across all sampling sites, ranged from 0.5 to 171.3 µg/L. Zirconium (Zr), ranged from 0.4 to 20.2 µg/L across all sampling stations. The concentration of Rb ranged from 2.2 to 35 µg/L. The concentrations of Ti do not exceed the standard limits of WHO.
Pearson Correlation (r) Analysis
Distinguishing between different types of groundwater samples can be achieved by applying multivariate statistical techniques to the interpretation of analytical results and Pearson’s correlation coefficient (r) between potential variables computed. To determine the inter-correlationship between the chosen parameters, a correction matrix consisting of twenty-two GWQ parameters was generated.
Table 1 illustrates that there was a significant correlation (r >0.9) between the Na, EC, and TDS. Even additional parameters show a good link (r>0.75), such as Na with Cl, F, Alkalinity, bicarbonate, and carbonate. Observed pH showed a weak correlation with all measures, with the exception of turbidity.
Table 3 revealed a positive correlation between heavy metals (metalloids) such as Al and Ni (r=0.94), Al and Cu (r=0.86), Al and Zr (r=0.86), Al and Co (r=0.94), Al and Fe (r=0.93), Al and Ag (r=0.71), Al and Sn (r=0.85), Al and Sb (r=0.83), and Al and Nb (r=1.0) while similar result was observed with Ti. For instance, Ti and Ni (r=0.95), Ti and Cu (r=0.83), Ti and Zr (r=0.87), Ti and Co (r=0.94), Ti and Fe (r=0.91), Ti and Ag (r=0.71), Ti and Sn (r=0.86), Ti and Sb (r=0.83), and Ti and Nb (r=1.0) were recorded due to similar geogenic source. This fact is supported by
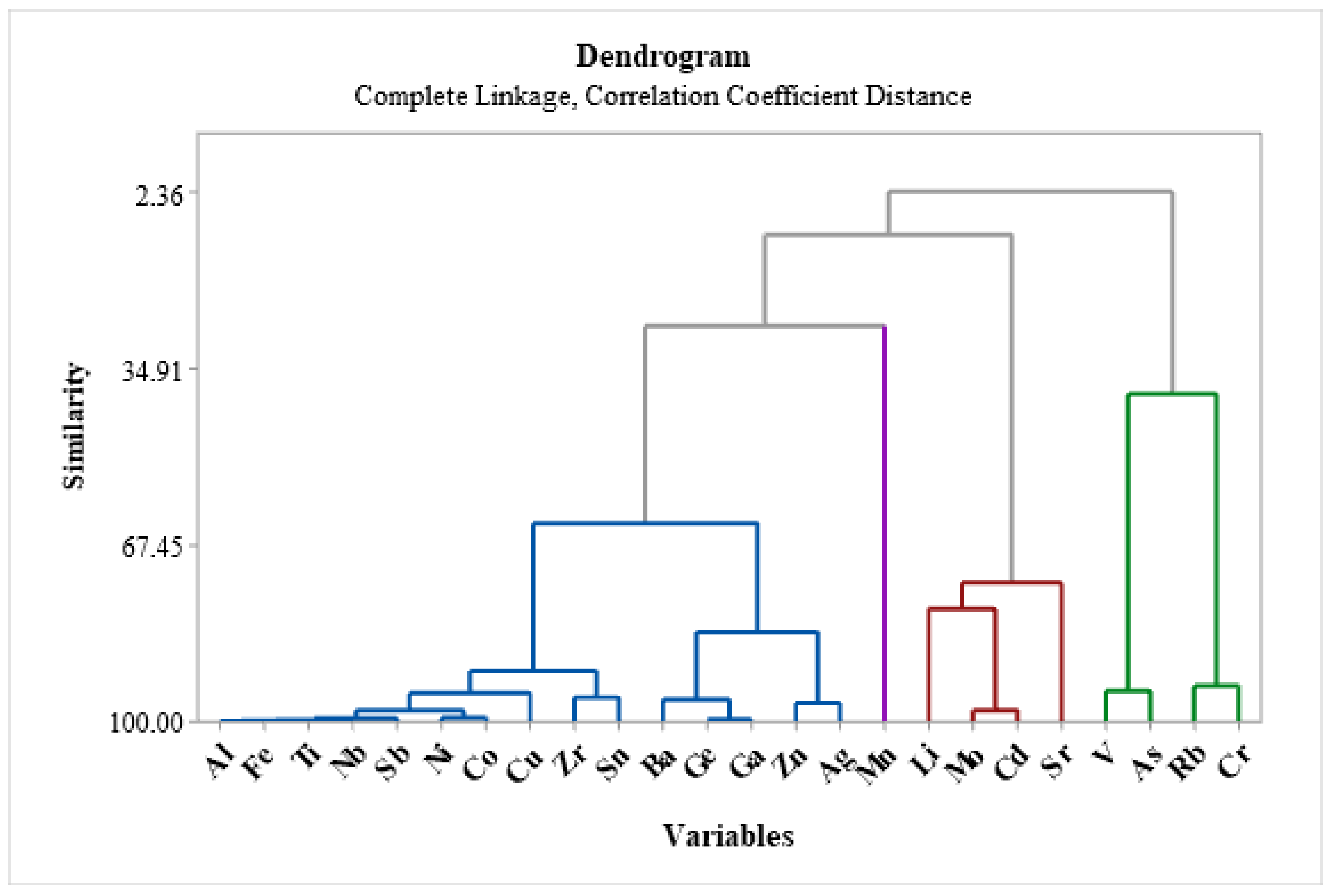
Figure 8 cluster 1 (Al, Ti, Ba, Ni, Cu, Zn, Zr, Nb, Ge, Co, Fe, Ga, Ag, Sn, and Sb might have the same origin). Surprisingly, Ni has strongly positive similarities with Al and Ti; Ti and Cu (r=0.84), Ni and Zr (r=0.91), Ni and Nb (r=0.95), Ni and Co (r=0.97), Ni and Fe (r=0.89), Ni and Ag (r=0.83), Ni and Sn (r=0.87), and Ni and Sb (r=0.73) arguably had a similar origin. While some other metals including As, Ba, Cd, Ba, Fe, Ga, Ag and others revealed strongly positive relations. For instance, if we compare cluster 2 heavy metals (Mo, Li, Sr, and Cd); Mo and Zn (r=0.85), Mo and Ag (r=0.79), and Mo and Cd (r=0.97) high positive correlation were recorded. In cluster 3 (V, As, Rb, and Cr), similarities in origin were recorded between V and As (r=0.91).
Multivariate Statistical Analysis
Principal component analysis (PCA): PCA is a linear analytical technique used to reduce a data set’s dimensionality while trying to guarantee that associations remain within the original data. Speaking to a large data set in less estimation may be interesting in data representation and compression tasks, where it is frequently used. In this study, the PCA analysis (
Figure 8) indicates that while the Amibera irrigation schemes (from GW9 – GW13) may be the result of anthropogenic factors such as excessive use of pesticides and agricultural inputs or groundwater salinization, the main reasons for the heavy metal pollution near the LB GW (from GW1- GW8) may be geogenic. Al, Ti, Ba, Ni, Cu, Zn, Zr, Nb, Ge, Co, Fe, and Ga, are included in Cluster 1; Mo, Li, Sr, and Cd are included in Cluster 2; V, As, Rb, and Cr are included in Cluster 3; and Mn is the solitary element in Cluster 4. The likelihood of a similar origin is demonstrated by these clusters, and associations between clustered samples were anticipated.
Figure 7.
The similarity plot of heavy metals in the dendrogram.
Figure 7.
The similarity plot of heavy metals in the dendrogram.
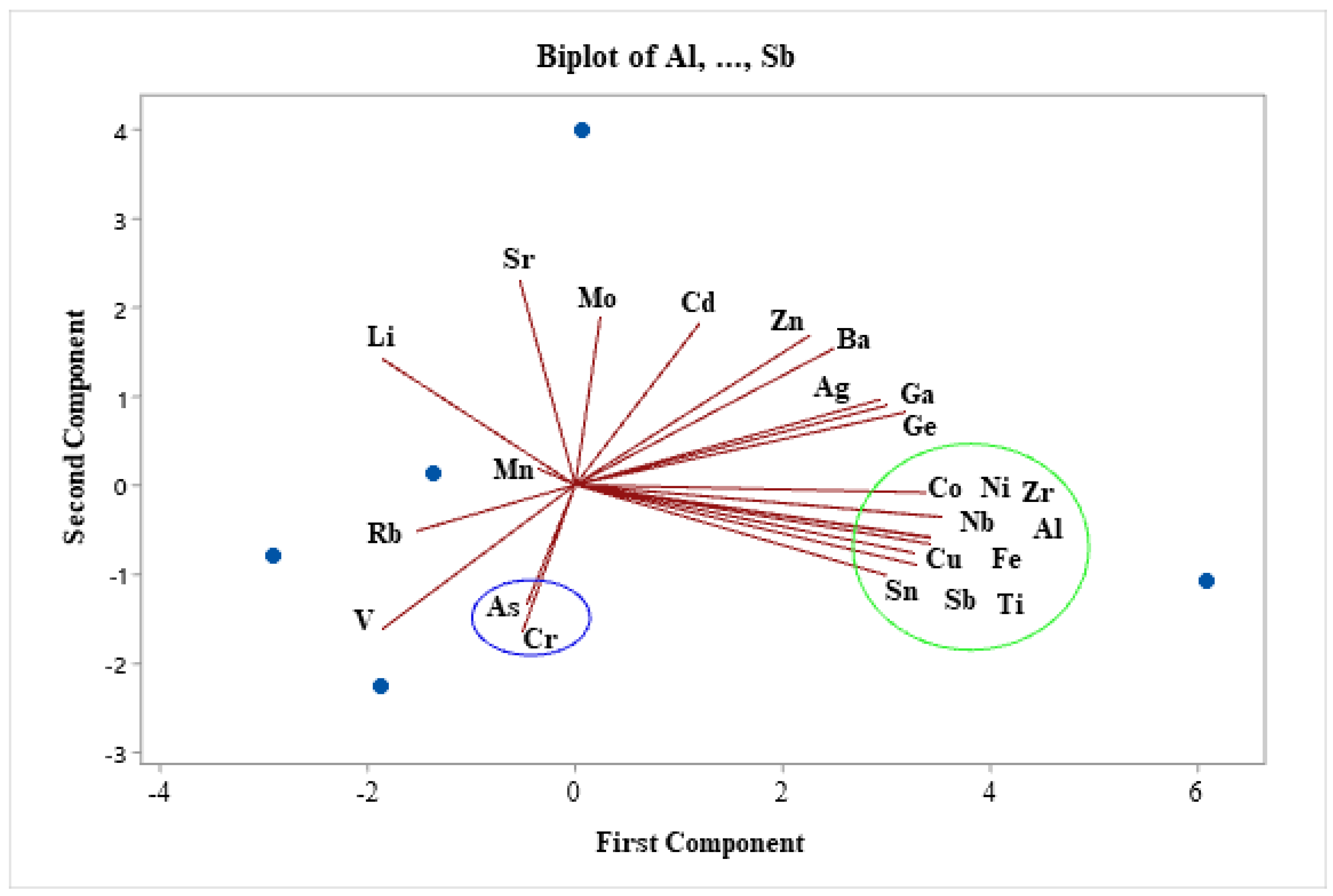
As illustrated in
Figure 8, the correlation type of matrix was chosen to weigh all parameters equally. As a result, the plot displayed the quantity of main components, or uncorrelated variables, in the heavy metals data sample. A short distance indicates the closure of two chemical parameters, while a high distance shows the dissimilarity in the parameters. For example, the first component has substantial positive loadings for Co, Ni, Zr, Nb, Cu, Sn, Sb, Ti, Fe, and Al. Conversely, the second component has significant negative loadings for As and Cr. All things considered, the biplot and dendrogram plots show that variations in the concentration of heavy metals in the groundwater aquifer under study may be caused by both geogenic (such as rock water interactions) and anthropogenic (such as surface runoff and fertilizers used in agriculture).
Figure 8.
The principal component analysis (PCA) biplot of heavy metals.
Figure 8.
The principal component analysis (PCA) biplot of heavy metals.
In the study, the words wells, boreholes, and piezometers are used interchangeably, however, they are not the same in functions, for instance, the drilled specifically to measure water level is an observation borehole. The level of water in an observation borehole will be a composite of all aquifers that are penetrated by the borehole. If it is drilled to measure water in a particular horizon and other horizons are cased off, it is called a piezometer. A piezometer, also known as a tube well, is primarily used for measuring subsurface water pressure. Since 1970s (Kebebe et al., 2021), more than fifty piezometer stations have been installed in the study area, with at least 25 of these used to monitor subsurface water quality and water level changes in the Middle Awash irrigation schemes. According to Mamo et al. (2019), all piezometers monitored in their studies have shallow water tables (Kebebe et al., 2021) and piezometers are mostly used to monitor shallow wells (Sprecher, S. W., 1993). Currently, over half of these piezometer stations are non-functional, primarily due to damage from machinery, while others suffer from lack of maintenance. This presents significant challenges due to limited information on their functionality, compounded by inadequate regulation and monitoring. The neglect of these valuable resources is notable. For this study, six sites were selected to assess the water quality and water level changes in the subsurface waters of Middle Awash.
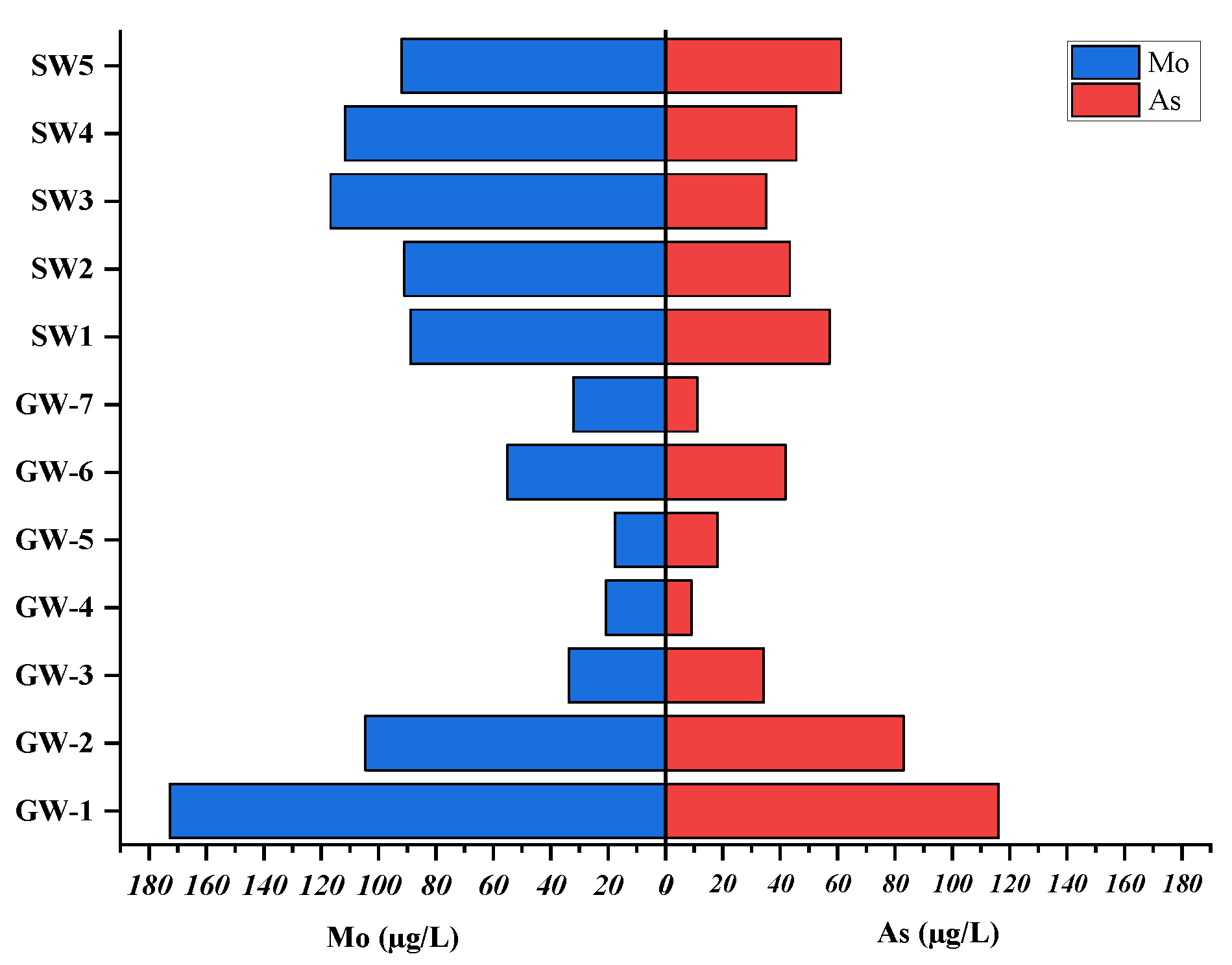
Comparison of Surface Water and Groundwater HMs Data in the Lake Beseka Catchment
In arrange to examine at the imaginable source of heavy metals both within the groundwater and surface water of LB catchment. Water tests analyzed within the display consider and past consider (abebe et al., 2023) was computed as takes after in
Table 5. In arrange to center around analyzing volcanic action crack and geography of the bowl influences the neighboring water bodies like Lake Beseka region was computed. The discoveries moreover uncovered that the tall concentration of HMs including As was watched in
Table 5. It may well be from the same sources influences both water bodies. Over the HMs counting Fe, Mo, V, Mn, Al, and also As in groundwater and surface water is non-potable and carries wellbeing chance on the off chance that devoured. Hence, we have to be creating remediation procedures for superior water supportability and security.
Comparing the HMs content of the two types of water and their potential sources was the goal of the above
Table 5 and the Figures below (
Figure 9 and
Figure 10). Moreover, to investigate the water chemistry of surface water interaction across catchments.
Table 5 illustrates that similar sources led to significant quantities of heavy metals and arsenic in both surface water and groundwater samples. In few cases, such as GW4, elevated levels of arsenic were observed exceeding the WHO endorsed threshold of 10 µg/L. Conversely, comparatively low concentrations of iron and aluminum were found in the Beseka catchment, whereas extraordinarily high concentrations of both were found in the surface water of Lake Beseka. It could be caused by man-made sources or by the catchment’s rocks deteriorating. In both cases, molybdenum concentrations were found to be quite similar, which may be because of similar sources.
Comparison of Water Level Change and Some Physicochemical Change
Although the terms well, borehole, and piezometer are used interchangeably throughout the study, they have different purposes. For example, an observation borehole is one that is drilled especially to record water level (
Table 4). An observation borehole’s water level is a combination of all the aquifers the drill has entered. It is referred to as a piezometer if it is drilled to measure the water in a certain horizon and other horizons are cased off. Where piezometric levels are greater than the lake stage area, groundwater flow is directed towards the lake (Kebede et al., 2021). Current piezometric values at Melka Worrer show that shallow groundwater flows mostly into the River Awash. In many areas of the irrigation area, groundwater levels are shallow typically less than three meters below the surface. The study by Kebede et al. (2021) indicates that the direction of GW flow is towards the Lake, suggesting that the groundwater surface interaction may be the source of the high concentration of heavy metals in the LB. Heavy metals from ground sources or water flows into the lake may pollute the water. Therefore, the interaction may be the cause of the change in water chemistry.
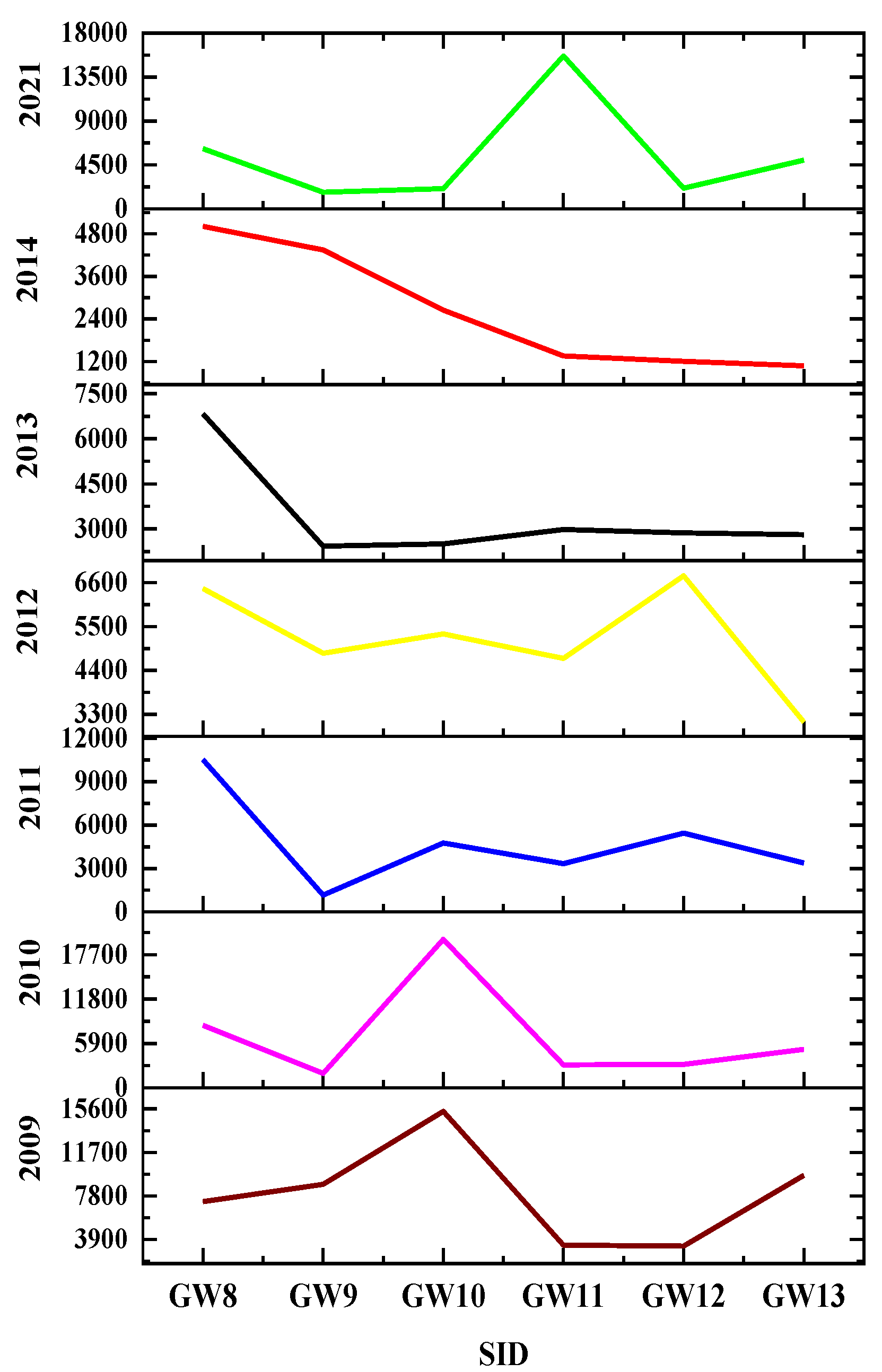
About six piezometers were used to compute the water quality of the groundwater in the Amibera irrigation farms in order to look at the patterns in changing water levels. The secondary data from the Awash Basin Administration Office, MoWE, the water level change, and the water quality were computed based on the study’s findings, as shown in Figs. 11i and 11ii. The results showed that the quality and level of the water had changed. For example, in 2009, a groundwater sample was taken at the following levels: 5.61 meters (GW10), 2.5 meters (GW11), and 2.75 meters (GW9). However, in 2021, the levels were 1.35 meters, 2.1 meters, and 0.95 meters, respectively. Electrical conductivity (EC) also changed in 2021, rising from 8850 µS/cm (GW9), 15390 µS/cm (GW10), and 9650 µS/cm (GW13) to 1702 µS/cm, 2074 µS/cm, and 4985 µS/cm, respectively.
Figure 11.
Spatiotemporal variability of wells EC in µS/cm
(Figure 11i) and water depth (in meter) increment (
Figure 11ii).
(N.B: The water depth, EC and pH raw data from 2009 to 2014 was took from the Awash Basin Administration office, MoWE)
Figure 11.
Spatiotemporal variability of wells EC in µS/cm
(Figure 11i) and water depth (in meter) increment (
Figure 11ii).
(N.B: The water depth, EC and pH raw data from 2009 to 2014 was took from the Awash Basin Administration office, MoWE)
5. Conclusions
This study aims to investigate the potential that boreholes in the Beseka watershed and Lake Beseka may have the same sources of arsenic and other heavy metals. Additionally, it offers a potential source of pollution due to volcanic activity in the rift valley and the basin’s geology, which may have an impact on nearby bodies of water like Lake Beseka. The results showed that the groundwater wells sampled and located near Lake Beseka are enriched in As (GW8), V (GW8), Ga (GW8), Li (GW8), Rb (GW4), Cr (GW1), and Mn (GW3). Interestingly, the piezometer stations located in the Amibera irrigation schemes show high concentrations of Cu, Sb, Sn, Co, Fe, Zr, Nb, Ni, Ti, and Al; Ba was found in GW9; Mo, Zn, Ag, and Cd were found in GW11; and Mo, Zn, Ag, and Cd were found in GW11. The results also showed that the highest values of certain elements, such as Al, As, Mo, Mn, Cu, and V, were above the WHO limits at different sampling locations.
The contamination of heavy metals in the Lake Beseka catchment’s groundwater is a significant issue. The presence of these pollutants is likely linked to the hydrological conditions and agricultural runoff, particularly from sugarcane plantations and previous agroindustry farms. Unfortunately, the residents living near the study area, particularly those in Addis Ketema and Werrer Office, face difficulties in accessing clean drinking water. As a result, they heavily depend on groundwater resources for their drinking needs. It is uncommon for them to utilize this water for drinking due to mechanical limitations and pipe issues that hinder access. However, the groundwater quality at certain sampled stations (GW15 & GW16) is suitable for drinking purposes. Conversely, the remaining 87.5% of sampled groundwater sources (both in Beseka catchment and Amibera farm states) are deemed unsuitable for drinking. Overall, more than half (eight sampling stations) of the sampled wells serve as primary sources of household water supply, mainly for washing and cleaning. Given the researcher’s objectives, these stations require a risk assessment test as they are frequently used by people and may pose a risk for dermal contact, although not for ingestion. Only twenty stations near Awash 7 town are designated for drinking purposes.
This study concentrated on a few of the most important and dangerous groundwater resource pollution problems brought on by human and natural activity. This study has also focused on anthropogenic activities, such as industrial applications, agricultural practices, sugar plantations, inadequate sewage facilities and hazardous pollutants. The primary natural sources of pollution are geological processes, natural activities including volcanic ash eruptions, climatic change, and interactions between surface and groundwater. Numerous case studies in the Middle Awash have been covering a wide range of topics, such as the expansion, sources, and effects of Lake Beseka, are accessible. However, only a small number of case studies on few elements including fluoride and physicochemical characteristics of groundwater quality and contaminating sources were discovered. The MoWE and other respected ministries (MoILLs, MoA), authorities (EPA), must assess how land use and other changes, such as river rejuvenation and climate change, affect groundwater systems and also to take into account how groundwater interacts with other hydrological systems, such as rivers and lakes, and also make sure that all groundwater services are provided. Additionally, protecting groundwater is crucial to preventing pollution from rendering those groundwater resources which are especially resistant to climate change unusable. The goal of this work is to examining the physicochemical and heavy metals extent and sources of groundwater quality and also partly comparing its interaction with the quality of the surface water bodies. Therefore, there is a great demand for in-depth research on the quality and quantity of ground water. In conclusion, more comprehensive data collection in subsequent studies is necessary to enhance the current research findings.
6. Future Works and Interventions
However, further assessment work pertaining to sugar plantations is also required. This will involve testing the wastewater quality using samples of leachates discharged from sugar factories in all sugar factories (Wonji, Methara, & Kesem Sugar factories) located within the study area. Following this research, the upcoming work will focus on developing remediation options including a promising technology for the removal of HMs including As from the water.
Author Contributions
Conceptualization, Yosef; methodology, Yosef, and Tena; validation, Yosef, Taye, Behailu, Tena, and Esayas; formal analysis, Yosef, Taye, and Behailu; writing original draft preparation, Yosef, Taye, and Esayas; writing review and editing, Behailu, Taye, Tena, and Esayas; supervision, Tena and Esayas; project administration, Tena. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article it is possible you can get it from OXFORD University REACH project Archive.
Acknowledgments
The authors wish to thank WLRC (REACH Program) for the useful contribution in the on-site activities and also laboratory support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Adeoye, P. A., Abubakar, K., and Adesiji, A. R. (2013) Effect of agrochemicals on groundwater quality: A review.
- Adimalla, N., Vasa, S.K. and Li, P. (2018) Evaluation of groundwater quality, Peddavagu in Central Telangana (PCT), South India: An insight of controlling factors of fluoride enrichment. Model. Earth Syst. Environ, vol. 4, pp#. 841–852. [CrossRef]
- Akhtar, N., Syakir Ishak, M. I., Bhawani, S. A., & Umar, K. (2021). Various natural and anthropogenic factors responsible for water quality degradation: A review. Water, vol. 13(19), 2660. [CrossRef]
- Anoop, Y., and Renu, D. (2014) Effect of sugar mill on physico-chemical characteristics of ground water of surrounding area. Int. Res. J. Env. Sci, vol. 3(6), pp#. 62-66.
- Ansari, A. K. (2006). Sugar industry effluent–characteristics and chemical analysis. Journal of Applied and Emerging Sciences, vol. 1(2), pp#.152-157.
- APHA, AWWA, WEF, (1995) Standard Methods for the Examination of Water and Waste Water, (nineteenth ed.), (Washington).
- Appelo, C. A. J., M. J. J. van Der Weiden, C. Tournassat and L. Charlet (2002) Surface Complexation of Ferrous Iron and Carbonate on Ferrihydrite and the Mobilization of Arsenic. Environmental Science and Technology, vol. 36: pp#. 3096-3103.
- BGS (2001) Groundwater quality: Ethiopia. British Geological Survey (BGS) https://www.scribd.com/document/542155484/Ethiopia.
- Bhatnagar, A., Kesari, K.K. & Shurpali, N. (2016) Multidisciplinary Approaches to Handling Wastes in Sugar Industries. Water Air Soil Pollut, vol.227, 11. [CrossRef]
- Bhubaneswar Pradhan, Sujata Chand, Sasmita Chand, Prangya Ranjan Rout, Sushanta Kumar Naik, (2023) Emerging groundwater contaminants: A comprehensive review on their health hazards and remediation technologies, Groundwater for Sustainable Development, vol 20. [CrossRef]
- Berhanu, B., Seleshi, Y., & Melesse, A. M. (2014). Surface water and groundwater resources of Ethiopia: Potentials and challenges of water resources development. Nile River Basin: Ecohydrological challenges, climate change and hydropolitics, pp#. 97-117.
- Birhanu, B., Kebede, S., Masetti, M., & Ayenew, T. (2018). WEAP-MODFLOW dynamic modeling approach to evaluate surface water and groundwater supply sources of Addis Ababa city. Acque Sotterranee-Italian Journal of Groundwater, vol. 7(2), pp#.15-24. [CrossRef]
- Bjorvatn, K., Reimann, C., Siewers, U., Melaku, Z., & Haimanot, R. T. (2002) Drinking water quality, Rift Valley, Ethiopia. Norges geologiske undersøkelse, Report, 132.
- Bonini, M., Corti, G., Innocenti, F., Manetti, P., Mazzarini, F., Abebe, T., Pecskay, Z., (2005) Evolution of the Main Ethiopian Rift in the frame of Afar and Kenya rifts propagation. Tectonics 24(1), pp#.1–21. [CrossRef]
- Chunli Su, Jiaqi Jiang, Xianjun Xie, Zhantao Han, Mengzhu Wang, Junxia Li, Huijian Shi, (2023) Sources and cycling processes of nitrogen revealed by stable isotopes and hydrochemistry in a typical agricultural lake basin, Applied Geochemistry, vol.156, 105662, ISSN 0883-2927. [CrossRef]
- Cruywagen JJ, Draaijer AG, Heyns JBB, (2002) Molybdenum (VI) equilibria in different ionic media. Formation constants and thermodynamic quantities. Inorg Chim Acta, vol. 331; pp#. 322-329. [CrossRef]
- Cruywagen JJ. (2000) Protonation, oligomerization, and condensation reactions of vanadate(V), molybdate(VI), and tungstate(VI), Advanced Inorganic Chemistry, vol. 49: pp#. 127-182. [CrossRef]
- Daba MH, Ayele GT, You S. (2020) Long-term homogeneity and trends of hydroclimatic variables in the upper Awash River basin, Ethiopia. Adv. Meteorol. [CrossRef]
- Delgado, C., Pacheco, J., Cabrera, A., Batllori, E., Orellana, R., & Bautista, F. J. A. W. M. (2010) Quality of groundwater for irrigation in tropical karst environment: The case of Yucatan, Mexico. Agricultural water management, vol.97 (10), pp#.1423-1433.
- Demelash, H., Beyene, A., Abebe, Z. et al. (2019) Fluoride concentration in ground water and prevalence of dental fluorosis in Ethiopian Rift Valley: Systematic review and meta-analysis. BMC Public Health, vol. 19, 1298. [CrossRef]
- Demlie, M., Wohnlich, S. (2006) Soil and groundwater pollution of an urban catchment by trace metals: Case study of the Addis Ababa region, central Ethiopia. Environ Geol, vol. 51, pp# 421–431. [CrossRef]
- Deshmukh, K. K. (2014) Environmental impact of sugar mill effluent on the quality of groundwater from Sangamner, Ahmednagar, Maharashtra, India. Research Journal of Recent Sciences, vol. 3, pp#385-392.
- Ekwueme BN, Agunwamba JC. (2021) Trend analysis and variability of air temperature and rainfall in regional river basins. Civ Eng J (Iran). Vol. 7: pp# 816–26. [CrossRef]
- Elena Gimenez-Forcada and Marisol Vega-Alegre (2015) Arsenic, barium, strontium and uranium geochemistry and their utility as tracers to characterize groundwaters from the Espadán–Calderona Triassic Domain, Spain. Science of the Environment, vol. 512-513, pp#. 599-612. [CrossRef]
- Fakayode, P. K. (2005) Alteration in physico-chemical characteristics of soil irrigated with sugar mill effluent. Journal of Environmental Biology, vol. 12, pp#. 103–109.
- Gasse, E., Street, F.A., (1978) Late Quaternary Lake-level fluctuations and environments of the northern Rift valley and Afar region (Ethiopia and Djibouti). Palaeogeogr. Palaeoclimatol. Palaeoecol. 24 (4). [CrossRef]
- Gebremicheal, E., Seyoum, W.M, Ishimwe, B., Sataer, G. (2022) Lake surface area expansion: Insights into the role of volcano-tectonic processes, Lake Beseka, East Africa. Journal of hydrology: Regional Studies, vol.41, pp#. [CrossRef]
- Giordano, M. (2009) Global Groundwater? Issues and Solutions. Annu. Rev. Environ. Resour., vol,34, pp#.153–178. [CrossRef]
- Goldberg S, Lesch SM, Suarez DL. (2020) Predicting molybdenum absorption by soils using soil chemical parameters in the constant capacitance model. Soil Sci Soc American Journal, vol.66, pp#. 1836-1842.
- Grafe, M., M. J. Eick and P. R. Grossl (2001) Adsorption of Arsenate (V) and Arsenite (III) on Goethite in the Presence and Absence of Dissolved Organic Carbon. Soil Science Society of America Journal, vol. 65, pp#. 1680-1687.
- Grafe, M., M. J. Eick, P. R. Grossl and A. M. Saunders (2002) Adsorption of Arsenate and Arsenite on Ferrihydrite in the Presence and Absence of Dissolved Organic Carbon. Journal of Environmental Quality, vol. 31: pp#.1115-1123.
- Hailu, K., Birhanu, B., Azagegn, T., & Kebede, S. (2023) Regional groundwater flow system characterization of volcanic aquifers in upper Awash using multiple approaches, central Ethiopia. Isotopes in Environmental and Health Studies, vol. 59(3), pp#. 269–289. [CrossRef]
- Health Canada, (2019) Guidelines for Canadian drinking water quality summary table. https://www.canada.ca/en/health-canada/services/environmental-workplace-health/ reports-publications/water-quality.html.
- Heluf G (1985) Investigation on Salt Affected Soils and Irrigation Water Quality in Melka Sedi-Amibara Plain, Rift Valley Zone of Ethiopia. M.Sc. Thesis, School of Graduate Studies, Addis Ababa University. Addis Ababa, Ethiopia 131 p.
- Issa N, Rajaković-Ognjanović V, Jovanović B, Rajaković L. (2010) Determination of inorganic arsenic species in natural waters-Benefits of separation and preconcentration on ion exchange and hybrid resins. Analytica Chimica Acta, vol. 673(2), pp#. 185-193. [CrossRef]
- Issa N, Rajaković-Ognjanović V, Marinković A, Rajaković L. (2011) Separation and determination of arsenic species in water by selective exchange and hybrid resins. Analytica Chimica Acta, vol 706(1): pp#. 191-198. [CrossRef]
- Italconsult (1969) Melka-Sadi Amibara proposed Irrigation Project feasibility study. Middle Awash, Ethiopia. http://documents.worldbank.org/curated/en/831001468250236544/pdf/multi-page.pdf.
- Ivanov VV. (1999) Rare p-elements. In: Burienkov EK, editor. Ecological geochemistry of elements 6 volumes set. Moscow: Nedra; pp# 1–352.
- Jain, C. K., Bandyopadhyay, A., & Bhadra, A. (2010) Assessment of ground water quality for drinking purpose, District Nainital, Uttarakhand, India. Environmental Monitoring and Assessment, vol.166,pp#663–676.
- Kebede, S., Charles, K., Godfrey, S., MacDonald, A., & Taylor, R. G. (2021) Regional-scale interactions between groundwater and surface water under changing aridity: Evidence from the River Awash Basin, Ethiopia. Hydrological Sciences Journal, vol. 66(3), pp#.450-463. [CrossRef]
- Kulabako NR, Nalubega M, Thunvik R. (2007) Study of the impact of land use and hydrogeological settings on the shallow groundwater quality in a peri-urban area of Kampala, Uganda. Journal of science of the total environment, vol. 381, pp#. 180-199. [CrossRef]
- Lemma Mamo, Bobe Bedadi, and Solomon Tamiru (2019) Spatial and temporal fluctuation of groundwater depth in Amibara, middle Awash, Ethiopia. Int. J. Water Res. Environ. Eng, vol. 11(8), pp. 159-169. [CrossRef]
- Ljubinka Rajakovic and Vladana Rajakovic-Ognjanovic (2017) Arsenic in Water: Determination and Removal. Chapter Two. [CrossRef]
- Malede, D.A., Andualem, T.G., Yibeltal, M. et al. (2024) Climate change impacts on hydroclimatic variables over Awash basin, Ethiopia: A systematic review. Discov Appl Sci vol. 6, pp#27. [CrossRef]
- Mall RK, Singh R, Gupta A, Srinivasan G, Rathore LS. (2006) Impact of climate change on Indian agriculture: A review. Clim Change, vol 78: pp# 445–78. [CrossRef]
- Mandal, B. K., and Suzuki, K. T. (2002) Arsenic round the world: A review. Talanta, vol. 58(1), pp#. 201–235.
- Marylynn Musgrove (2021) The occurrence and distribution of strontium in U.S. groundwater. Applied Geochemistry, vol. 126.104867. [CrossRef]
- Matthew S Schuler, Rick A Relyea, (2018) A Review of the Combined Threats of Road Salts and Heavy Metals to Freshwater Systems, BioScience, vol.68(5), PP# 327–335. [CrossRef]
- MoWR (2011) Ethiopia: Strategic Framework for Managed Groundwater Development. Ministry of water Resources, Ethiopia. Available on April 23 2024. https://www.waterethiopia.org/wp-content/uploads/2014/03/Ethiopia-Strategic-Framework-for-Managed-Groundwater-Development-DRAFT.pdf.
- Mukherjee I, Singh UK (2020) Fluoride abundance and their release mechanisms in groundwater along with associated human health risks in a geologically heterogeneous semi-arid region of East India. Microchem J. vol, 152:104304. [CrossRef]
- Pauline L. Smedley & David G, Kinniburgh (2017) Molybdenum in Natural Waters: A review of occurrence, Distribution and controls, Applied Geochemistry, vol. 84, pp#,387-432.
- Pawar, N., Pondhe, G. & Patil, S. (1998) Groundwater pollution due to sugar-mill effluent, at Sonai, Maharashtra, India. Environmental Geology, vol. 34, pp#. 151–158. [CrossRef]
- Piper, A.M. (1953) A Graphic Procedure in the Geochemical Interpretation of Water Analysis, USGS Groundwater Note, No.12.
- Poddar, P.K., Sahu, O. (2017) Quality and management of wastewater in sugar industry. Appl Water Sci, vol. 7, pp#. 461–468. [CrossRef]
- Rajaković L, Todorović Ž, Rajaković-Ognjanović V, Onjia A. (2013) Review: Analytical methods for arsenic speciation analysis. Journal of the Serbian Chemical Society, vol 78, pp#. 1-32. UDC JSCS-5666.
- Rosenberg E. (2009) Germanium: Environmental occurrence, importance and speciation. Reviews in Environmental Science and Biotechnology, vol. 8; pp#29–57. [CrossRef]
- Sayyed Juned A. and Bhosle Arjun B (2011) Analysis of Chloride, Sodium and Potassium in Groundwater Samples of Nanded City in Mahabharata, India. European Journal of Experimental Biology, vol, 1 (1), pp.#74-82. Available at www.pelagiaresearchlibrary.com.
- Sharma, M.K., Kumar, M. (2020) Sulphate contamination in groundwater and its remediation: An overview. Environ Monit Assess 192, 74. [CrossRef]
- Sophocleous, M. (2004) Global and Regional Water Availability and Demand: Prospects for the Future. Nat. Resour. Res., 13, pp# 61–75. [CrossRef]
- Sprecher, S. W. (1993) Installing monitoring wells/piezometers in wetlands.
- Sutliff-Johansson, S., Pontér, S., Engström, E. et al. (2021) Tracing anthropogenic sources of Tantalum and Niobium in Bothnian Bay sediments, Sweden. J Soils Sediments, vol. 21, pp#1488–1503. [CrossRef]
- Tank, D. K., & Chandel, C. P. S. (2010) A hydrochemical elucidation of the groundwater composition under domestic and irrigated land in Jaipur City. Environmental Monitoring and Assessment, vol. 166, pp#. 69–77. [CrossRef]
- Taye MT, Dyer E, Hirpa FA, Charles K. (2018) Climate change impact on water resources in the Awash basin, Ethiopia. Water (Switzerland).vol.10: pp#. 1–16.
- Taylor, R.G.; Scanlon, B.; Döll, P.; Rodell, M.; van Beek, R.; Wada, Y.; Longuevergne, L.; Leblanc, M.; Famiglietti, J.S.; Edmunds, M.; et al. (2013) Ground water and climate change. Nat. Clim. Chan, vol, 3, pp#. 322–329.
- Tefera, M., Chernet, T. and Haro, W. (1996) Explanation of the geological map of Ethiopia, scale 1:2,000,000. 2nd edition. Ethiopian Institute of Geological Surveys, Addis Ababa.
- USAID (2000) Ethiopian Water Resource Profile Overview. Winrock international, USAID’s Sustainable Water Partnership activity, Available at May 1, 2024 https://winrock.org/wp-content/uploads/2021/08/Ethiopia_Country_Profile-Final.pdf.
- Wanty, R.B., Goldhaber, M.B., (1992) Thermodynamics and kinetics of reactions involving vanadium in natural systems: Accumulation of vanadium in sedimentary rocks. Geochem. Cosmochim. Acta 56, pp#. 1471-1483. [CrossRef]
- Warren LA, Zimmerman AP. (1994) The influence of temperature and NaCl on cadmium, copper and zinc partitioning among suspended particulate and dissolved phases in an urban river. Water Research, vol, 28: pp#. 1921–1931. [CrossRef]
- Wehrli, B., Stumm, W., (1989) Vanadyl in natural waters: Adsorption and hydrolysis promote oxygenation. Geochem. Cosmochim. Acta 53, pp# 69–77. [CrossRef]
- WHO (1993) Guidelines for drinking water quality. World Health Organization, Geneva.
- WHO (2008) Guidelines for drinking water quality. Geneva: Switzerland, World Health Organization.
- 2011; 72. WHO (2011) Guidelines for drinking water quality, World Health Organization, 4th Ed, Geneva, Switzerland.
- Yosef Abebe and Abraha Geberkidan, (2020) The Pollution Status of Awash Rqiver Basin (Ethiopia) Using Descriptive Statistical Techniques. American Journal of Water Resources, vol. 8(2): pp# 56-68.
- Yousefi, M., Saleh, H. N., Yaseri, M., Mahvi, A. H., Soleimani, H., Saeedi, Z., & Mohammadi, A. A. (2018) Data on microbiological quality assessment of rural drinking water supplies in Poldasht County. Data in brief, vol.17, pp#763-769. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
 [Al(H2O)6]3+ (aq)
[Al(H2O)6]3+ (aq)
 2MoO3 + 4SO2
2MoO3 + 4SO2
 (NH2)2(MoO4)
(NH2)2(MoO4)