1. Introduction
The rapid spread of African swine fever virus (ASFV), an enveloped, double-stranded DNA virus with a genome of 170-190 kpb, remains a threat for pig populations and economies world-wide [
1,
2]. Originally, ASFV circulated in an ancient sylvatic cycle among asymptomatic warthog and soft tick (genus:
Ornithodoros) populations in sub-Saharan Africa [
3]. Its introduction into domestic pigs or Eurasian wild boar, however, leads to severe, yet rather unspecific clinical signs resulting in high case fatality rates [
4,
5]. The disease is notifiable to the World Organization for Animal Health (WOAH). The current African swine fever (ASF) panzootic started in 2007, when ASFV was introduced into Georgia and subsequently into the Russian Federation and many Trans-Caucasian countries. In 2014, ASF entered the European Union [
6], in 2018 China [
7], and in 2021 the Caribbean [
8].
At present, 24 different genotypes, characterized based on variations within the p72 capsid protein encoded by the B464L gene, have been defined [
9,
10,
11,
12]. However, only genotype I and II strains have been found outside of Africa and the current panzootic involves genotype II strains only. Despite efforts to understand and restrict the disease, its ongoing spread emphasizes the need to evaluate alternative transmission routes and strengthen early warning systems. In a previous study, we demonstrated that artificial insemination is an efficient route to transmit ASFV from infected boars to naïve recipient gilts. Usually, domestic boar semen originates, with the exception of rural backyard farming, from boar studs [
13]. In these facilities, boars are kept individually and semen is collected regularly on demand. Collected semen has to pass mandatory quality control checks, e.g., count of spermatozoa, amount of abnormalities, and mobility. Since we showed that none of those criteria were affected by early ASFV infection or even acute viraemia, risk-based surveillance for the presence of ASFV in boar semen is of utmost importance and opens up the only possibility for a very early detection. Around the globe, real-time polymerase chain reaction (qPCR) is widely used as reliable, sensitive, and specific tool for detecting animal diseases, such as ASFV [
14,
15,
16,
17]. In addition to a robust qPCR system, a highly effective extraction method is key for correct laboratory diagnosis [
18]. Recommended extraction systems are listed in the WOAH guidelines [
19].
However, extraction methods and qPCR assays need to constantly adapt and meet putative evolutionary changes of the virus and/or the demands of the diagnostic field. This is especially true for such difficult matrices as semen. In recent years, several commercially available qPCR kits for ASFV genome detection have become available and performance comparisons have been done, e.g., Schoder et al. (2020, [
20]) and Pikalo et al. (2022, [
19]). To standardize conditions and facilitate data interpretation, regional reference laboratories should follow guidelines provided by the WOAH and regional (e.g., European Union) and national reference laboratories (NRLs). These guidelines include a list of registered or licensed diagnostic methods suited and permitted for routine diagnostics [
21].
As semen preparations for artificial insemination contain potentially qPCR inhibitory components [
22], such as sucrose [
23], it presents a complex and highly challenging matrix for routine diagnostic testing, also affecting ASFV diagnostic workflows. Hence, it is of utmost importance to define a suitable diagnostic pipeline to accurately detect ASFV genome in boar semen.
Here, we compared three methods for nucleic acid extraction, as well as five qPCR protocols to establish a suitable pipeline for efficient and early detection of ASFV genome in boar semen using standard methodology. Criteria applied for definition were (I) reliable amplification, (II) handling and time requirements and, (III) efficient detection of ASFV genome in critical samples, e.g., samples obtained early after inoculation, especially those presumed to contain only very few ASFV genome copies.
2. Materials and Methods
2.1. Samples
All samples used in this study were derived from breeding boars, which were intramuscularly inoculated with 10
4 HAD
50 of the ASFV strain ‘Estonia 2014’ [
24]. This trial included four adult breeding boars, two Large White and two Pietrain boars [
25]. Semen samples from all boars were collected regularly after experimental ASFV infection to assess an early ASFV screening in porcine semen. Since EDTA blood, already known for its accurate and early detection of ASFV genome [
26], was previously defined as the ‘
gold standard’ matrix, blood samples were used as comparison for the analytical performance with semen samples. In total,
n = 18 EDTA blood and
n = 17 semen samples (boar #4 could not be collected at 5 dpi) were obtained during the trial.
2.2. Extraction of viral DNA
All samples were frozen at -80°C upon collection to ensure full availability of cell-bound viral genome [
27]. For accurate detection of low ASFV genome loads in boar semen, performance of three commercially available, routinely used nucleic acid extraction kits was compared (
Table 1):
(I) the NucleoMag
® VET kit (Macherey-Nagel);
(II) the MagMAX™ Pathogen RNA/DNA kit (ThermoFisher) and;
(III) the MagMAX™ 96 Viral RNA isolation kit (ThermoFisher). All samples were extracted in triplicates (
n = 3) and all kits were used according to manufacturer’s instructions unless stated otherwise. Extraction was performed on the automated extraction platform KingFisher™ 96 flex (ThermoFisher) upon utilization of extraction protocols provided by the manufacturers.
To determine the best-suited extraction kit for subsequent detection of ASFV genome in boar semen, all extracted samples were further analyzed as described in
Section 2.3. Three criteria were employed to define assay efficiency and ultimately the best-suited extraction method:
(I) successful detection in all ‘true positive’ samples,
(II) early and accurate detection of ASFV genome in semen obtained early after inoculation (2-3 dpi), and
(III) low Cq values were preferred.
Because early detection of ASFV genome in semen is crucial, semen samples were divided into ‘early’ (2-3 dpi, n = 5 positive samples) and ‘late’ samples (> 4 dpi, n = 9 positive samples) prior to performance assessment. True positive samples among ‘early’ samples were defined by manual extraction and increasing the number of replicates, which was beneficiary for detection of samples with low amounts of ASFV genome. For manual extraction, DNA from 85 µl of semen was extracted using the QIAamp Viral RNA Mini kit (Qiagen). Manual extraction was performed in triplicates (n = 3) and samples were analyzed in three independent qPCR runs (using the VetAlert™ ASFV DNA Test Kit from Tetracore), where each sample was measured in triplicates (n = 3).
2.3. Molecular Assays – qPCR
Following extraction, all samples were compared using the VetAlert ASFV DNA assay (Tetracore). This assay is accredited in the German National Reference Laboratory (NRL) and was among the best commercial qPCR kits in previous studies when using various other sample matrices from ASFV infected pigs [
19]. The best performing extraction method was subsequently used to assess the analytical performance of five qPCR assays in ASFV detection (all certified for use in ASFV diagnostics).
The five qPCR protocols included in this study were (
Table 2):
(I) VetAlert™ ASFV DNA Test Kit;
(II) virotype ASFV 2.0 PCR Kit (Indical);
(III) VetMax™ ASFV Detection Kit (ThermoFisher),
(IV) the WOAH recommended protocol published by King et.al. (2003, [
15]) with slight modifications (accredited ASF System 1) and;
(V) RealPCR ASFV DNA Test (IDEXX). All protocols were utilized according to manufacturer’s/authors instructions.
To facilitate detection of low ASFV genome copy numbers in semen samples, the above-mentioned extracted DNA from semen samples (n = 3 per boar and time point) were evaluated in triplicates (a total of n = 9 per semen sample) for each qPCR assay. For qPCR assays, the same efficiency criteria as used for extraction methods were employed.
2.4. Data Analyses
All data generated by qPCR was visualized and analyzed for statistical relevance using GraphPad Prism 9 (GraphPad Software Inc.). Statistical analyses were performed using One-Way-ANOVA with Tukey’s post hoc testing and significant differences are depicted as follows: **p < 0.01, ***p < 0.001, ****p < 0.0001. Subsequently, all qPCR test results were compared to the best-performing protocol using the Bland-Altman test [
29]. Optimal performance was defined as number of wells with successful detection of ASFV genome in semen samples of 2-3 dpi. The Limit of Agreement (LoA) interval for each comparison was defined as mean difference ±1.96 standard deviation (SD) of Cq values. Furthermore, specificity (% of true negatives among total negative samples), sensitivity (% of true positive among total positive samples), and positive predictive value (% of true positives among true and false positives) was calculated for each qPCR method/matrix. Additionally, using the samples of day 2 and 3 pi, a repeated measures ANOVA, correcting for replicates, was performed to test for statistical differences in the performance of the five qPCR tests. However, the power of statistical calculations was limited due to the fact that correlated samples were used (repeated sampling of few individuals). Due to many samples not yielding Cq values upon analysis, the number of cycles minus Cq values was used for calculations. However, the power of statistical calculations was limited due to the fact that data of only few animals was available.
3. Results
3.1. Assessment of Preparation Time, Handling, and Time Requirements
3.1.1. Nucleic Acid Extraction Kits
Sample preparation time, which correlates directly with the number of mandatory pipetting steps, varied significantly between manufacturers. For example, the NucleoMag® VET kit required the sample to be only vortexed prior to adding the ready-to-use lysis buffer, while the MagMAX™ Pathogen RNA/DNA Kit instructs lysis buffer and bead mix preparation (2 and 3 steps, respectively) before DNA extraction. Differences were also found amongst matrices: EDTA blood could be added to the prepared solutions, while semen samples required preparation of the lysate in a separate plate. The subsequent semen-lysate was then added to the plate containing all necessary washing/elution solutions. An additional difference was notable for the MagMAX™-96 Viral RNA Isolation Kit which included the preparation of lysis/binding buffer and bead mix, however, EDTA blood and semen samples did not require varying preparation steps.
However, the steps required for nucleic acid extraction were comparable between all kits, as listed in
Table 1.
In terms of storing, all kits contained components that needed to be stored at different temperatures (-20°C/4°C/RT for kits by ThermoFisher, -20°C/RT for the NucleoMag® VET kit) and all kits allowed the use of automated nucleic acid extraction platforms.
3.1.2. Differences amongst Tested ASFV qPCR Assays
Required pipetting steps during preparation of qPCR reactions overall ranged from two to five steps. Two out of five kits included a ready-to-use mix that only needed mixing with the respective sample in the plate: the virotype ASFV 2.0 PCR Kit and VetMax™ ASFV Detection Kit. Furthermore, the VetAlert™ ASFV DNA Test Kit needed one extra step: adding the enzyme to the mix. Two methods, the WOAH King et.al. protocol and RealPCR ASFV DNA Test kit, required step-by-step mixing of all reagents, with five and three steps, respectively [
15].
In terms of qPCR cycles for target amplification, all assays included similar cycles, i.e., 45, with the exception of the virotype ASFV 2.0 PCR assay, which runs with 40 cycles. Duration of qPCR runs was also heterogenous, ranging from 1 h and 2 min (virotype ASFV 2.0 PCR Kit) to 2 h and 25 min (WOAH King et.al.).
3.2. Extraction Efficiency Evaluation of ASFV Genome from Boar Semen
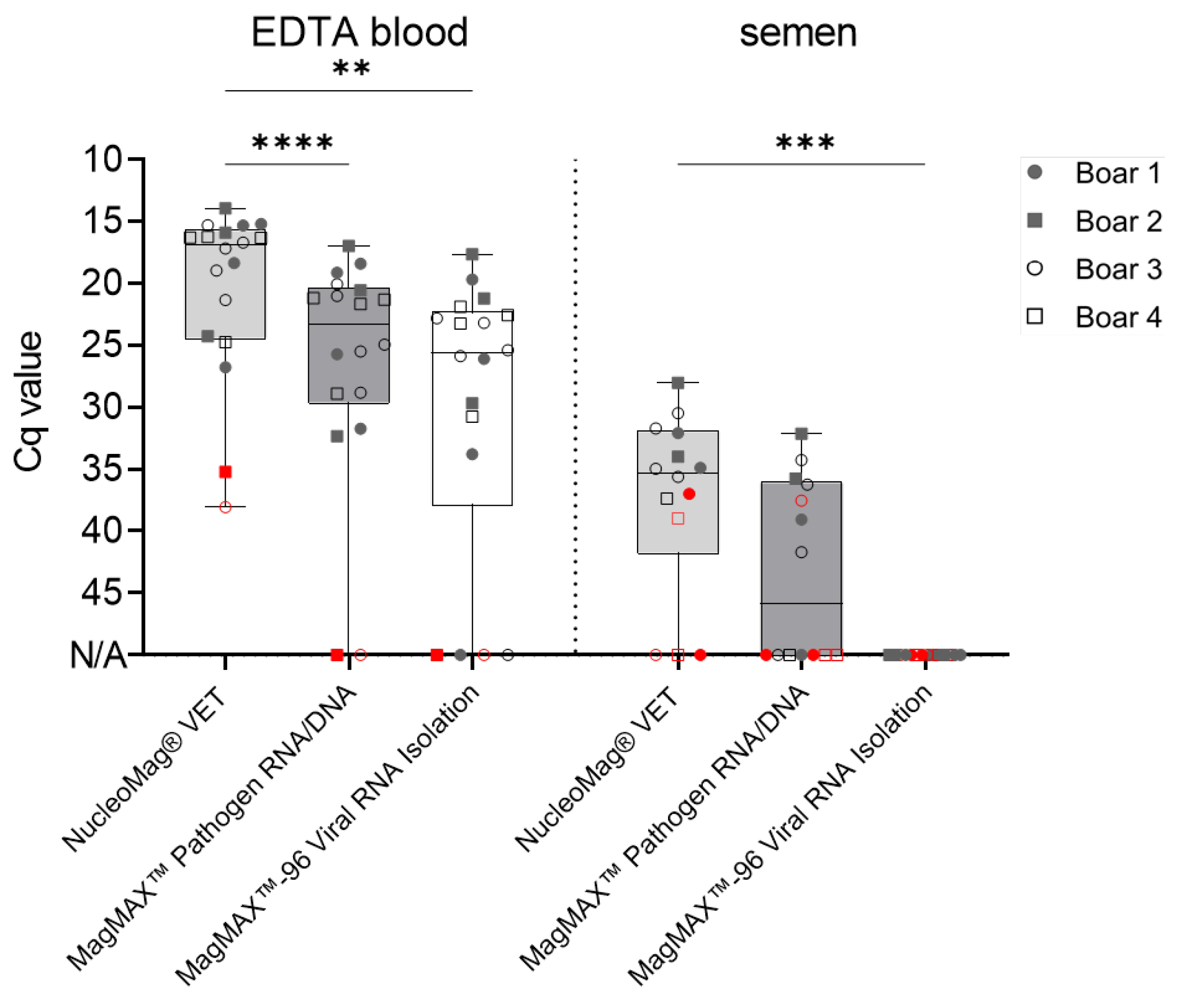
As shown in
Figure 1, sample extraction using the NucleoMag
® VET Kit resulted in 100% detection of positive EDTA blood samples (
n = 18/18) and 78.6% (
n = 11/14) of the total positive semen samples. Furthermore, the MagMAX™ Pathogen RNA/DNA Kit achieved detection of up to 88.9% (
n = 16/18) and 50.0% (
n = 7/14) of positive EDTA blood and semen samples, respectively. Similar results were obtained for EDTA blood samples (88.9%;
n = 16/18) extracted with the MagMAX™-96 Viral RNA Isolation Kit, however, no semen samples extracted with this kit yielded Cq values when analyzed with the VetAlert™ ASFV DNA assay (
Figure 1). Extraction of semen samples using the MagMAX™-96 Viral RNA Isolation Kit was carried out three times, further verifying these results.
Out of fourteen positive semen samples, five were positive at an early time point (2-3 dpi). Extraction with the NucleoMag® VET Kit detected 40.0% (n = 2/5) of true positive samples, followed by extraction with the MagMAX™ Pathogen RNA/DNA Kit (n = 1/5).
Differences in subsequent Cq values under the same cycling conditions were also observed amongst extraction kits. Extraction with the NucleoMag
® VET Kit rendered the lowest Cq values (EDTA blood: 20.3 ± 7 SD; Semen: 34.1 ± 3.3 SD), followed by the MagMAX™ Pathogen RNA/DNA Kit (Blood: 23.6 ± 4.8 SD; Semen: 36.7 ± 3.1 SD) and MagMAX™-96 Viral RNA Isolation Kit (Blood: 24.6 ± 4.4 SD) as shown in
Figure 1. Samples with low amounts of ASFV genome, e.g., blood samples of boars #2 (Cq 35.2 ± 0.2 SD) and #3 (Cq 38.1 ± 4.5 SD) at 2 dpi were not detected after extraction with the MagMAX™ kits.
3.3. Assessment of qPCR Performance in Detecting ASFV Genome in Boar Semen
Based on these results, the most suitable routine kit for extraction of viral genome copies from semen samples with an automated platform was the NucleoMag® VET Kit. Therefore, a comparison of the analytical performance of the five qPCR assays was carried out using nucleic acids extracted via this method.
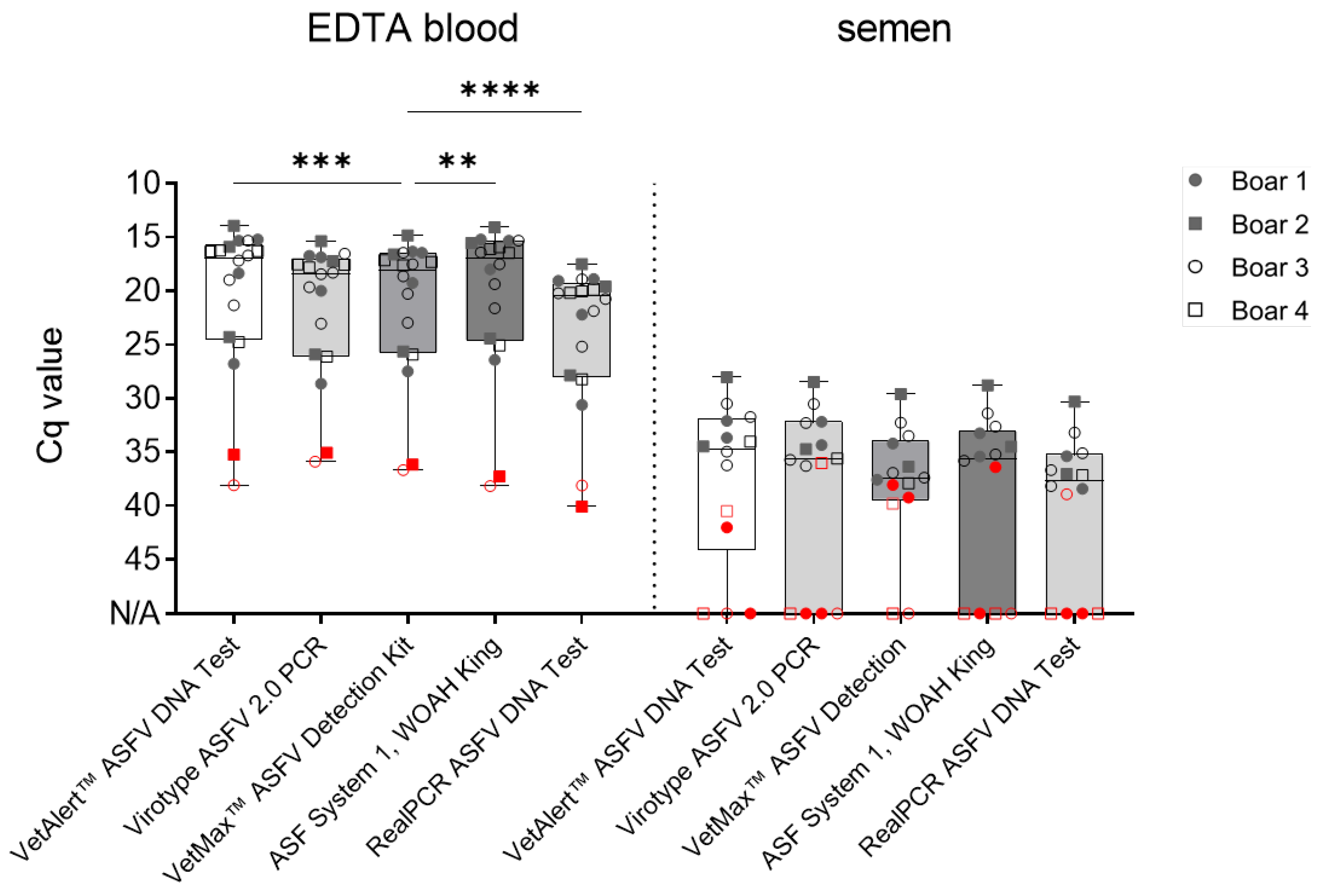
Differences amongst matrices were observed in all included assays. All positive EDTA blood samples (
n = 18) were detected by all assays, however, differences in Cq values were noted. The VetAlert™ ASFV DNA assay yielded similar values (20.3± 7 SD) as the ASF System 1 recommended by WOAH, King et.al. [
15], (20.4 ± 7.3 SD). Following, the virotype ASFV 2.0 (21.5 ± 6.3 SD) and VetMax™ ASFV Detection assays (21.3 ± 6.6 SD) rendered similar Cq values. Finally, the RealPCR ASFV DNA (23.8 ± 6.6 SD) showed higher Cq values for analogous samples (
Figure 2).
For the second matrix, semen, all mean Cq values were comparable and ranged from 33.3 ± 2.6 SD (virotype ASFV 2.0 PCR Kit) to 35.7 ± 2.6 SD (RealPCR ASFV DNA Test) as depicted in
Figure 2.
However, when samples were divided into ‘early’ and ‘late’ collected samples, results were more heterogenous (
Figure 2, Table 3). All qPCR assays successfully detected ASFV genome in ‘late’ boar semen samples obtained at 4−20 dpi (
n = 9). Nonetheless, differences were noted for ‘early’ semen samples (2−3 dpi;
n = 5 true positive). The VetMax™ ASFV Detection Kit was able to detect 60% of all true positive early samples (
n = 3), followed by the VetAlert™ ASFV DNA Test Kit detected 40.0 % (
n = 2). Finally, the ASF System 1, the virotype ASFV 2.0 PCR Kit, and the RealPCR ASFV DNA Test (20%;
n = 1).
As samples containing only low amounts of ASFV genome are prone to produce false negative results, DNA of ’early’ semen samples (2-3 dpi) were extracted manually, with an increased number of replicates in qPCR. Generally, no assay was able to detect all true positive samples. Specificity and precision reached up to 100% for all analyzed methods. However, differences in sensitivity amongst matrices were observed, as shown in
Table 3. Sensitivity reached 100% in EDTA Blood for all assays, while variations were observed for semen. The VetMax™ ASFV Detection Kit showed the highest sensitivity (60%, 38.1 ± 2.6 SD), followed by the VetAlert™ ASFV DNA Test Kit (40%, 39.6 ± 1.8 SD), the virotype ASFV 2.0 PCR Kit (20%, 36), ASF System 1 (20%, 33.6 ± 0.8 SD), and the RealPCR ASFV DNA Test (20%, 38.9 ± 1.2 SD).
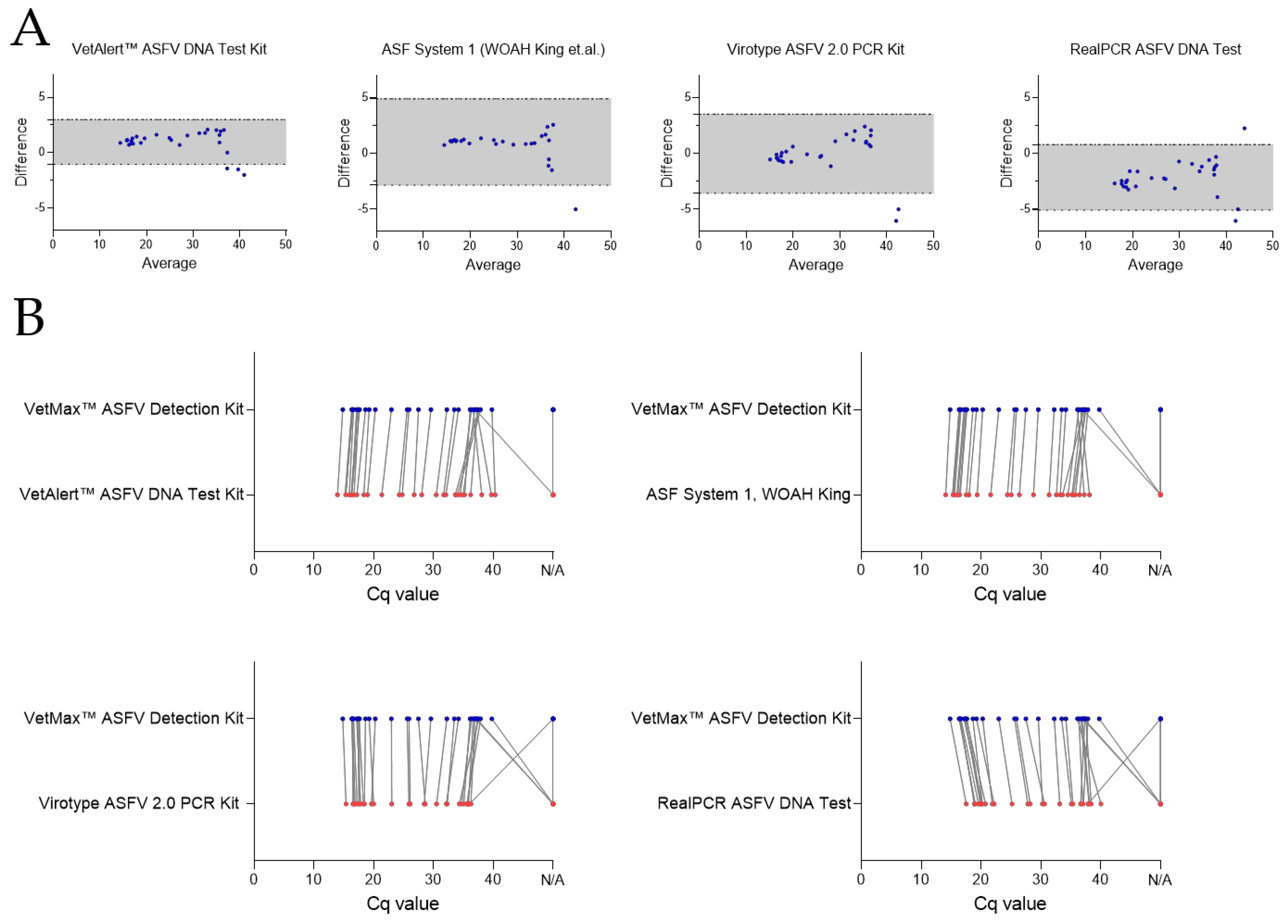
The analytical performance of the qPCR kits using all true positive EDTA blood and semen samples (
n = 32) was assesses by Bland-Altman Plots (
Figure 3A) and point-by-point evaluations (
Figure 3B). The best performing assay, VetMax™ ASFV Detection Kit, was used as reference for point-by-point-evaluations.
The VetAlert™ ASFV DNA Test Kit (bias 0.97) showed narrow LoA, indicating a high level of agreement. However, three samples were found outside the LoA, which means that the results of three samples vary beyond the calculated standard deviation. Two assays, ASF System 1 and the virotype ASFV 2.0 PCR Kit showed similar LoA, whereas one and two samples, respectively, were underestimated with these systems when compared with the VetMax™ ASFV Detection Kit. Finally, the RealPCR ASFV DNA test showed samples with Cq values to be both under- and overestimated, indicating higher variability and therefore disagreement in results between these kits. A manual in-detail comparison revealed once more that semen samples were those detected with a distinct shift in Cq values for the extraction of ASFV genome. This indicates that out of the tested assays only the VetAlert™ ASFV DNA Test Kit can be used interchangeably with the best-performing kit, VetMax™ ASFV Detection Kit. The point-by-point comparison revealed that the VetAlert™ ASFV DNA Test Kit overall had lower Cq values up to a Cq of ~37, where detection of weak positive samples was superior using the VetMax™ ASFV Detection Kit (
Figure 3B). The virotype ASFV 2.0 PCR Kit and ASF System 1 had comparable Cq values to the VetMax™ ASFV Detection Kit with EDTA blood samples, but failed to detect most of the weak positive semen samples. The RealPCR ASFV DNA Test had overall higher Cq values, but also failed to detect the weak positive semen samples.
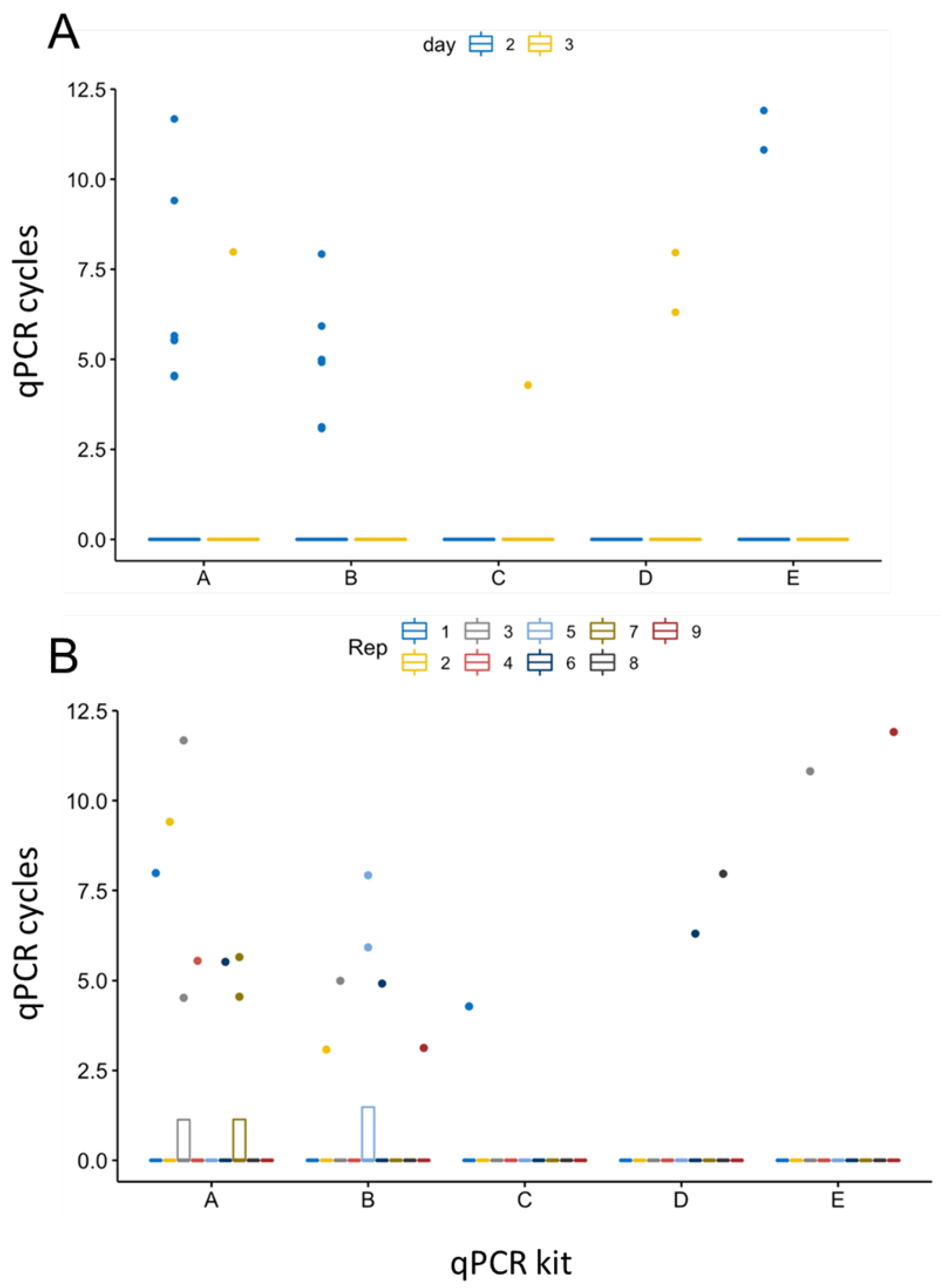
Furthermore, repeated measures ANOVA resulted in a statistically significant difference in the detection efficiency of the five different PCRs using semen samples from days 2 and 3 pi (p=0.0068,
Figure 4). While calculations revealed statistical significance between days 2 and 3 pi (p=0.0042,
Figure 4 A), no significant variations were observed among the replicates of each sample (n = 8 samples, each 9 replicates,
Figure 4 B). Of the two-way interactions, significance was confirmed between results of the five qPCR kits (p=0.0087), indicating differing efficiency in detecting samples with low amounts of ASFV genome correctly.
4. Discussion
As broadly protective vaccinations or reliable treatment options for ASF are still not available, accurate and early identification of infected individuals is key to prevent further spread of the disease. The modern pork industry mainly relies on artificial insemination with the boar semen acquired from boar studs. The semen is collected, diluted with nutrient-containing extenders and shipped on demand, often nationwide or even across borders. To prevent spread of ASFV-containing semen, surveillance of semen upon collection is needed. However, fast processing during quality management is essential to ensure high quality and viability of spermatozoa. Therefore, we compared various nucleic acid extraction kits as well as established and validated ASFV-specific qPCR assays to define a practicable diagnostic workflow without the need of special treatments or protocols for early detection of even low mounts of ASFV genome in boar semen in a high-throughput diagnostic laboratory. Similar studies were carried out, comparing the performance of WOAH approved qPCR assays on wild boar samples of varying degree of decay [
19]. Here, the best-suited kits were defined by the following criteria:
(I) successful definition of positive samples,
(II) early and accurate detection of ASFV genome in semen obtained on days 2 and 3 after inoculation, and
(III) yield of low Cq values. Since fresh boar semen typically is shipped less than 24 h after collection, sample preparation and qPCR run duration served as additional factor for efficiency determination.
Generally, no combination of nucleic acid extraction and qPCR kit was able to detect all true positive semen samples, as defined by manual DNA extraction. However, regarding nucleic acid extraction kits, considerable differences in the efficiency to extract ASFV genome from boar blood and semen were noted. While extraction with the NucleoMag
® VET Kit resulted in detection of all true positive blood samples, MagMAX™ kits gave two false negative results for the two samples with the lowest ASFV genome load (boar #2 and #3 at 2 dpi). Furthermore, differences were even much more striking regarding semen. Here, extraction with the NucleoMag
® VET Kit resulted in 11/14 true positive results, extraction with the MagMAX™ Pathogen RNA/DNA Kit obtained 7/14 true positive results. However, repeated extraction with the MagMAX™-96 Viral RNA Isolation Kit did not yield any positive results for semen. Conclusively, while performance on blood samples was largely comparable, extraction efficiency varied between the kits. Overall, reduced extraction efficiency from semen was noted for all extraction kits included in this study, indicating the presence of inhibitory components in this matrix, e.g., polysaccharides [
23,
30]. Additionally, low amounts of genome in the semen early after infection might result in false negative results, considering that only a tiny fraction of the whole ejaculate is sampled. Hence, detection variability is likely to occur in samples with low amounts of target genome. Based on detection efficiency in our study and the short preparation time, the NucleoMag
® VET Kit seems well-suited to facilitate ASFV monitoring in boar studs. Although manual extraction of DNA is considered as most sensitive method for ASFV diagnostics, an automated platform with similar detection efficiency is likely more suitable to monitor large pig herds. Given that we focused on the routine diagnostic workflows in high-throughput, we did not include special extraction protocols for semen (as can be found in the WOAH manual for e.g., Bovine herpesvirus 1 infection,
https://www.woah.org/fileadmin/Home/eng/Health_stanards/tahm/3.04.11_IBR_IPV.pdf, visited online May 6th 2024).
Additionally, similar observations were made for the studied qPCR assays. By extracting nucleic acids via the NucleoMag
® VET Kit, all qPCR methods successfully detected all true positive EDTA blood samples, even samples containing low amounts of ASFV genome, e.g., boar #2 and #3 at 2 dpi. This further indicated the unmatched suitability of blood samples for accurate and early detection of ASFV genome in pigs, as described previously [
26]. It is of note that boars tolerated blood sampling through saphenous veins without getting agitated during semen collection. Hence, collection of small amounts of blood during the procedure might be feasible to obtain blood for ASFV surveillance, as already routinely done for PRRSV [
31,
32]. However, although Cq values of EDTA blood samples were largely comparable, they were significantly higher using the RealPCR ASFV DNA Test, suggesting that there could be an impact on the detection of low positive samples. Furthermore, although the VetAlert™ ASFV DNA Test Kit and ASF System 1 rendered lower Cq values in general, Cq values of weak positive samples achieved with the VetMax™ ASFV Detection Kit were lower, indicating increased performance with weak / very weak positive samples as input matrix. Detection in semen benefited from increasing the number of replicates in qPCR, as described previously [
33].
Although no qPCR assay was able to detect all true positive samples (EDTA blood and semen taken together), the VetMax™ ASFV Detection Kit rendered (I) the most positive results (3/5) and (II) overall lower Cq values which reached statistical significance for EDTA blood samples, further enhancing the suitability of this kit for accurate ASFV detection. It is of note that in-depth analyses of kit performances uncovered that the VetAlert™ ASFV DNA Test Kit can be used interchangeably with the VetMax™ ASFV Detection Kit due to similar performance. However, in terms of handling/preparation time and complexity, the VetMax™ ASFV Detection Kit provided a ready-to-use mix, while the VetAlert™ ASFV DNA Test Kit required preparation of said mix.
In addition, the virotype ASFV 2.0 PCR Kit only detected 1/5 true positive semen samples, which possibly results from the number of amplification cycles recommended for this kit. Considering that Cq values of true positive semen samples could exceed 40, the virotype ASFV 2.0 PCR Kit was likely at disadvantage due to being optimized for time (shortest run among all tested), not sensitivity.
In summary, based on our dataset, we identified the NucleoMag® VET Kit as most suitable kit for nucleic acid extraction to enable detection of even low amounts of ASFV genome in porcine blood and semen samples. Furthermore, the VetMax™ ASFV Detection Kit and, although to a lesser extent, the VetAlert™ ASFV DNA Test Kit provided paramount detection of weak positive blood and semen samples among all kits tested. However, suitability for diagnostic workflows of each kit has to be carefully assessed, e.g., handling, possibility to combine assays, time needed, and range of detectable pathogens with kits of one manufacturer. This is especially true when samples with expectedly high Cq values (obtained during early stages of infection without apparent clinical signs) are handled.
5. Conclusions
With this study, we present a suitable pipeline that enables efficient detection of ASFV genome in boar semen using routine protocols. We compared the performance of three widely used magnetic bead-based methods for nucleic acid extraction as well as WOAH-recommended qPCR methods and commercial qPCR kits. Among the tested options and based on our data set, the NucleoMag® VET Kit performed best in combination with the VetMax™ ASFV Detection Kit. Nevertheless, the limitations of this study have to be considered, as sample sizes are small (blood n = 18, semen n = 17) and samples were correlated, as they were derived from four individuals, not independent.
Author Contributions
Conceptualization, S.B., M.B., J.CH., D.R., and J.J.Z.; methodology, V.F., and S.B.; formal analysis, V.F., C.SL., J.CH., D.R., J.J.Z., S.B..; investigation, V.F.; data curation, V.F. and C.SL.; writing—original draft preparation, V.F. and S.B.; writing—review and editing, V.F., S.B., J.CH., D.R., E.A.N., C.SL.; visualization, V.F.; supervision, S.B., J.CH., D.R., J.J.Z., E.A.N., and M.B.; project administration, S.B., J.CH., D.R.; funding acquisition, S.B., J.CH., and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research has received funding through the Horizon 2020 ERA-NET Cofund International Coordination of Research on Infectious Animal Diseases (ICRAD), project “ASF-RASH”, the FLI ASF research network and in part by the National Pork Board, a program sponsored by the United States Department. of Agriculture).
Data Availability Statement
Data are available on request from the corresponding author.
Acknowledgments
We would like to thank all animal caretakers: Steffen Brenz, Matthias Jahn, Domenique Lux, and Patrice Mary for excellence in animal husbandry and during sampling procedures. Further, we would like to thank Ralf Henkel for preparing and assisting all necropsies. For excellent technical assistance during sample processing and experiments, we thank Ulrike Kleinert.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andres, G.; Charro, D.; Matamoros, T.; Dillard, R.S.; Abrescia, N.G.A. The cryo-EM structure of African swine fever virus unravels a unique architecture comprising two icosahedral protein capsids and two lipoprotein membranes. J Biol Chem 2020, 295, 1–12. [Google Scholar] [CrossRef]
- Alejo, A.; Matamoros, T.; Guerra, M.; Andres, G. A Proteomic Atlas of the African Swine Fever Virus Particle. J Virol 2018, 92. [Google Scholar] [CrossRef]
- Penrith, M.L.; Vosloo, W.; Jori, F.; Bastos, A.D. African swine fever virus eradication in Africa. Virus Res 2013, 173, 228–246. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, M.C.; Reoyo, A.T.; Fernandez-Pinero, J.; Iglesias, I.; Munoz, M.J.; Arias, M.L. African swine fever: a global view of the current challenge. Porcine Health Manag 2015, 1, 21. [Google Scholar] [CrossRef]
- Gallardo, C.; Nieto, R.; Soler, A.; Pelayo, V.; Fernandez-Pinero, J.; Markowska-Daniel, I.; Pridotkas, G.; Nurmoja, I.; Granta, R.; Simon, A.; et al. Assessment of African Swine Fever Diagnostic Techniques as a Response to the Epidemic Outbreaks in Eastern European Union Countries: How To Improve Surveillance and Control Programs. J Clin Microbiol 2015, 53, 2555–2565. [Google Scholar] [CrossRef] [PubMed]
- Chenais, E.; Depner, K.; Guberti, V.; Dietze, K.; Viltrop, A.; Stahl, K. Epidemiological considerations on African swine fever in Europe 2014-2018. Porcine Health Manag 2019, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, N.; Luo, Y.; Liu, Y.; Miao, F.; Chen, T.; Zhang, S.; Cao, P.; Li, X.; Tian, K.; et al. Emergence of African Swine Fever in China, 2018. Transbound Emerg Dis 2018, 65, 1482–1484. [Google Scholar] [CrossRef]
- Gonzales, W.; Moreno, C.; Duran, U.; Henao, N.; Bencosme, M.; Lora, P.; Reyes, R.; Nunez, R.; De Gracia, A.; Perez, A.M. African swine fever in the Dominican Republic. Transbound Emerg Dis 2021, 68, 3018–3019. [Google Scholar] [CrossRef]
- Galindo, I.; Alonso, C. African Swine Fever Virus: A Review. Viruses 2017, 9. [Google Scholar] [CrossRef]
- Achenbach, J.E.; Gallardo, C.; Nieto-Pelegrin, E.; Rivera-Arroyo, B.; Degefa-Negi, T.; Arias, M.; Jenberie, S.; Mulisa, D.D.; Gizaw, D.; Gelaye, E.; et al. Identification of a New Genotype of African Swine Fever Virus in Domestic Pigs from Ethiopia. Transbound Emerg Dis 2017, 64, 1393–1404. [Google Scholar] [CrossRef]
- Bastos, A.D.; Penrith, M.L.; Cruciere, C.; Edrich, J.L.; Hutchings, G.; Roger, F.; Couacy-Hymann, E.; G, R.T. Genotyping field strains of African swine fever virus by partial p72 gene characterisation. Arch Virol 2003, 148, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Franzke, K.; Beer, M. African swine fever - A review of current knowledge. Virus Res 2020, 287, 198099. [Google Scholar] [CrossRef] [PubMed]
- Broekhuijse, M.L.; Gaustad, A.H.; Bolarin Guillen, A.; Knol, E.F. Efficient Boar Semen Production and Genetic Contribution: The Impact of Low-Dose Artificial Insemination on Fertility. Reprod Domest Anim 2015, 50 Suppl 2, 103–109. [Google Scholar] [CrossRef]
- Tignon, M.; Gallardo, C.; Iscaro, C.; Hutet, E.; Van der Stede, Y.; Kolbasov, D.; De Mia, G.M.; Le Potier, M.F.; Bishop, R.P.; Arias, M.; et al. Development and inter-laboratory validation study of an improved new real-time PCR assay with internal control for detection and laboratory diagnosis of African swine fever virus. J Virol Methods 2011, 178, 161–170. [Google Scholar] [CrossRef] [PubMed]
- King, D.P.; Reid, S.M.; Hutchings, G.H.; Grierson, S.S.; Wilkinson, P.J.; Dixon, L.K.; Bastos, A.D.; Drew, T.W. Development of a TaqMan PCR assay with internal amplification control for the detection of African swine fever virus. J Virol Methods 2003, 107, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Pinero, J.; Gallardo, C.; Elizalde, M.; Robles, A.; Gomez, C.; Bishop, R.; Heath, L.; Couacy-Hymann, E.; Fasina, F.O.; Pelayo, V.; et al. Molecular diagnosis of African Swine Fever by a new real-time PCR using universal probe library. Transbound Emerg Dis 2013, 60, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Aguero, M.; Fernandez, J.; Romero, L.; Sanchez Mascaraque, C.; Arias, M.; Sanchez-Vizcaino, J.M. Highly sensitive PCR assay for routine diagnosis of African swine fever virus in clinical samples. J Clin Microbiol 2003, 41, 4431–4434. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; van der Poel, W.H.; Ponsart, C.; Cay, A.B.; Steinbach, F.; Zientara, S.; Beer, M.; Hoffmann, B. European interlaboratory comparison of Schmallenberg virus (SBV) real-time RT-PCR detection in experimental and field samples: The method of extraction is critical for SBV RNA detection in semen. J Vet Diagn Invest 2015, 27, 422–430. [Google Scholar] [CrossRef]
- Pikalo, J.; Carrau, T.; Deutschmann, P.; Fischer, M.; Schlottau, K.; Beer, M.; Blome, S. Performance Characteristics of Real-Time PCRs for African Swine Fever Virus Genome Detection-Comparison of Twelve Kits to an OIE-Recommended Method. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Schoder, M.E.; Tignon, M.; Linden, A.; Vervaeke, M.; Cay, A.B. Evaluation of seven commercial African swine fever virus detection kits and three Taq polymerases on 300 well-characterized field samples. J Virol Methods 2020, 280, 113874. [Google Scholar] [CrossRef]
- WOAH, W.O.f.A.H. Register of diagnostic kits certified by the OIE as validated as fit for purpose.
- Hoffmann, B.; Schulz, C.; Beer, M. First detection of Schmallenberg virus RNA in bovine semen, Germany, 2012. Vet Microbiol 2013, 167, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Louwrier, A.; van der Valk, A. Can sucrose affect polymerase chain reaction product formation? Biotechnology Letters 2001, 23, 175–178. [Google Scholar] [CrossRef]
- Zani, L.; Forth, J.H.; Forth, L.; Nurmoja, I.; Leidenberger, S.; Henke, J.; Carlson, J.; Breidenstein, C.; Viltrop, A.; Hoper, D.; et al. Deletion at the 5’-end of Estonian ASFV strains associated with an attenuated phenotype. Sci Rep 2018, 8, 6510. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, V.; Reicks, D.; Hasenfuss, T.; Gerstenkorn, E.; Zimmerman, J.J.; Nelson, E.A.; Carrau, T.; Deutschmann, P.; Sehl-Ewert, J.; Roszyk, H.; et al. Artificial Insemination as an Alternative Transmission Route for African Swine Fever Virus. Pathogens 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Pikalo, J.; Deutschmann, P.; Fischer, M.; Roszyk, H.; Beer, M.; Blome, S. African Swine Fever Laboratory Diagnosis-Lessons Learned from Recent Animal Trials. Pathogens 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Mallik, S. Identification Methods | Multilocus Enzyme Electrophoresis. In Encyclopedia of Food Microbiology; Elsevier, 2014; Volume 2, pp. 336–343. [Google Scholar]
- Van Engelenburg, F.A.; Van Schie, F.W.; Rijsewijk, F.A.; Van Oirschot, J.T. Excretion of bovine herpesvirus 1 in semen is detected much longer by PCR than by virus isolation. J Clin Microbiol 1995, 33, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.; Zardoya, R.; Santurde, G.; Solana, A.; Castro, J.M. Rapid and sensitive detection of the bovine viral diarrhea virus genome in semen. J Virol Methods 1995, 55, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Reicks, D.L.; Muñoz-Zanzi, C.; Rossow, K. Sampling of adult boars during early infection with porcine reproductive and respiratory syndrome virus for testing by polymerase chain reaction using a new blood collection technique (blood-swab method). JSHAP 2006, 14, 258–264. [Google Scholar]
- Pepin, B.J.; Kittawornrat, A.; Liu, F.; Gauger, P.C.; Harmon, K.; Abate, S.; Main, R.; Garton, C.; Hargrove, J.; Rademacher, C.; et al. Comparison of specimens for detection of porcine reproductive and respiratory syndrome virus infection in boar studs. Transbound Emerg Dis 2015, 62, 295–304. [Google Scholar] [CrossRef]
- Nybo, K. qPCR: Technical Replicate Variation. Biotechniques 2011, 50, 23. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).